Abstract
Cutaneous photobiology studies have focused primarily on the UV portion of the solar spectrum. However, VL comprises 50% of EMR that reaches the earth’s surface, and, as discussed in Part I of this CME, VL has cutaneous biologic effects such as pigment darkening and erythema. Photoprotection against VL includes sun avoidance, seeking shade, and the use of photoprotective clothing. Organic and inorganic UV filters used in sunscreens do not protect against VL; only tinted sunscreens do. In the US, these filters are regulated by the FDA as an over-the-counter drug and are subjected to more stringent regulations than in Europe, Asia, and Australia. There are no established guidelines regarding VL photoprotection. Alternative measures to confer VL photoprotection are being explored. These novel methods include topical, oral, and subcutaneous agents. Further development should focus on better protection in the range of UVA1 (340–400nm) and VL while enhancing the cosmesis of the final products.
Keywords: visible light, ultraviolet light, visible light photoprotection, photoprotection, organic filter, inorganic filter, photolyase, light filter, sunscreen, sunblock
I. TYPES OF PHOTOPROTECTION
Key points.
VL (400–700 nm) accounts for 50% of electromagnetic radiation that reaches the earth’s surface.
Photoprotective measures against VL include avoiding the sun, seeking shade, and using photoprotective clothing.
Tinted sunscreens are the only currently available topical photoprotection products for VL.
Electromagnetic radiation.
The adverse effects of sun exposure on the skin are well-established.1 The sun emits broad-spectrum electromagnetic radiation (EMR) with a peak in the visible light (VL) (400–700 nm) range.2–7 The majority of EMR that reaches the earth’s surface is composed of UVB (290–320 nm), UVA2 (320–340 nm), UVA1 (340–400 nm), VL (400–700 nm), and infrared (IR) (700 nm-1 mm) radiation.2–8 Cutaneous photobiology studies have focused primarily on the ultraviolet (UV) portion of the solar spectrum, as the erythema peak is around 295nm.3,9–11 However, VL compromises 50% of EMR that reaches the earth’s surface (versus UV, which is responsible for only 5%) and has been shown to induce pigment darkening and erythema as discussed in Part I.3,11–19,17,20–24 Environmental exposure to VL is primarily from the sun, but also from electronic devices such as smartphones, tablets, and computer screens.25–28 However, the cumulative dose of blue light emitted by these low-intensity sources is not relevant for VL biologic effects as it does not reach the dose demonstrated to induce hyperpigmentation.28
Photoprotection modalities.
Photoprotection is critical to maintain skin health, minimize post-inflammatory hyperpigmentation, and prevent photoaging and photocarcinogenesis. Photoprotective measures include avoiding the sun, seeking shade, using photoprotective clothing, wearing wide-brimmed hats and sunglasses, and applying broad-spectrum sunscreens.26,29 UV filters used in sunscreens may be either organic (i.e., chemical) or inorganic (i.e., mineral) (Table 1).2,17,22,23,30 While these terms are used interchangeably, organic and inorganic filters are the terms recommended by the US Food and Drug Administration (FDA).2,17,22,23,30 All UV filters, including mineral filters [zinc oxide (ZnO) and titanium dioxide (TiO2)] are “chemicals”.2,17,22,23,30 It is a misconception that mineral filters are “physical blockers” as they absorb UV photons, especially in nanosized form.2,17,22,23,30 Nanosized inorganic filters (i.e., ZnO, TiO2) do not have VL photoprotective properties, but non-nanosized inorganic filters do.15,17,24,31–33 For proper photoprotection, sunscreens should be combined with measures outlined above.33–38
Table 1.
Approved UV filters listed in the 1999 United States Food and Drug Administration sunscreen monograph.30,46,73
| Light filter | Maximum Approved Concentration (%) | Peak Absorption (nm) | Action spectrum |
|---|---|---|---|
| Organic Filters | |||
| PABA derivatives | |||
| PABA | 15% | 283 | UVB |
| Padimate O | 8% | 311 | UVB |
| Benzophenones | |||
| Dioxybenzone | 3% | 352 | UVB, UVA2 |
| Oxybenzone | 6% | 288, 325 | UVB, UVA2 |
| Sulisobenzone | 10% | 366 | UVB, UVA2 |
| Salicylates | |||
| Homosalate | 15% | 306 | UVB |
| Octisalate | 5% | 307 | UVB |
| Trolamine salicylate | 12% | 260–355 | UVB |
| Cinnamates | |||
| Cinoxate | 3% | 289 | UVB |
| Octinoxate | 7.5% | 311 | UVB |
| Other | |||
| Avobenzone | 3% | 360 | UVA2, UVA1 |
| Ensulizole | 4% | 310 | UVB |
| Meradimate | 5% | 340 | UVA2 |
| Octocrylene | 10% | 303 | UVB, UVA2 |
| Inorganic | |||
| Titanium dioxide | 25% | UVB, UVA2, UVA1 | |
| Zinc oxide | 25% | UVB, UVA2, UVA1 |
PABA (para-aminobenzoic acid), Padimate O (octyl dimethyl PABA), Dioxybenzone (benzophenone-8), Oxybenzone (benzophenone-3), sulisobenzone (benzophenone-4), Homosalate (homomethyl salicylate), Octisalate (octyl salicylate), Trolamine salicylate (triethanolamine salicylate), Cinoxate (2-ethyoxyethyl p-methoxycinnamate), Octinoxate (octyl methoxycinnamate), Avobenzone (butyl methoxydibenzoyl methane), Ensulizole (phenylbenzimidazole sulfonic acid), Meradimate (menthyl anthranilate)
Organic filters.
Organic filters are composed of an aromatic ring and functional groups of electron donors and acceptors that delocalize electrons upon UV irradiation and absorption.6,16,22,39–41 There are five main types of organic filters: para-aminobenzoic acid (PABA) derivatives, benzophenones, salicylates, cinnamates, and other.6 Oxybenzone is the most commonly used benzophenone and absorbs UVB and short UVA.6 UV filters are often combined to increase photostability and spectral performance.2,4,42 The structure of organic filters allow for UVR, but not VL, to be absorbed, resulting in molecular conformational changes.43–45 As the molecule returns from the excited to the ground state, energy is released as heat (Figure 1).22,43–45
Figure 1. Mechanism of action of organic and inorganic UV filters.
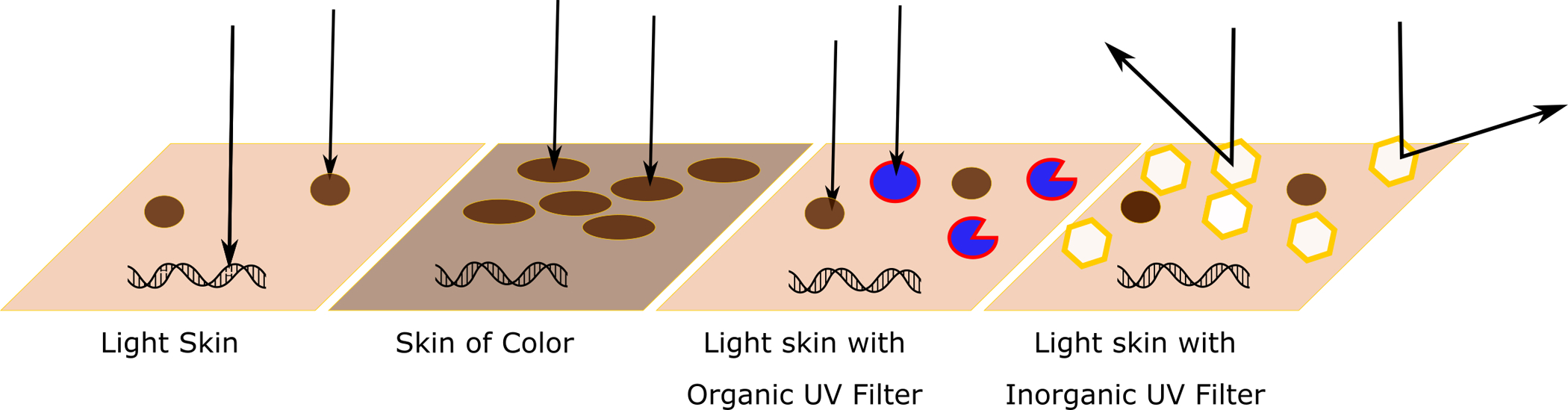
Note that as the diameter of the inorganic filters decreases, they do absorb UVB.
Inorganic filters.
The two FDA-approved inorganic filters, ZnO and TiO2, are metal oxides that effectively absorb, reflect, or scatter EMR.2,3,7,22,25,32,39,40,46,47 Inorganic filters are nontoxic, nonallergenic, and largely unaffected by light-induced reactions, unlike organic filters.2,3,47 Products with inorganic filters might give skin a chalky white appearance that limits usage due to cosmesis, especially in skin of color (SOC).12,15–17,24,26,32,37,51–53 Micronized formulations make inorganic filters more cosmetically appealing, but less protective for UVA and VL.2,3,7,12,20,29,30,32,35,39,46,47,54–57 Larger opaque pigments confer superior protection against photodermatoses induced by VL, such as erythropoietic protoporphyria (EPP).2,3,7,12,20,21,29,30,32,35,38,39,46,47,54–57
Tinted (colored) sunscreens:
Since neither organic nor inorganic UV filters used in sunscreens protect against VL, tinted (colored) sunscreens are available to protect against VL.48,58 Tinted sunscreens consist of a blend of iron oxides (Fe2O3) and TiO2 pigments that function as VL and UV blockers.24,58 Depending on the oxidation state, Fe2O3 may appear yellow, red, or black.24 Yellow Fe2O3 protects melanocompetent subjects from VL-induced pigmentation.59 Tinted sunscreens reduce VL transmission by 93–98%.2,3,7,22,24,25,32,39,40,46,47
Daily application of tinted sunscreens reduced the appearance of cutaneous hyperchromias after 60 days.58,60 One study compared a combination of Fe2O3 and TiO2 to a non-tinted mineral SPF 50+ sunscreen with ZnO and TiO2 for protection against VL-induced pigmentation.60,61 Expert grading and colorimetry demonstrated that the Fe2O3-containing formulations better protected against VL-induced pigmentation than non-tinted mineral sunscreen in Fitzpatrick IV individuals.60,61
Fe2O3-containing formulations in women’s facial products, including foundations, have a dual function in covering pigmentary blemishes and reducing the development of further pigmentation induced by sunlight.60 The availability of foundations in multiple shades and tones can offer daily, customized protection beyond the UV spectrum for individuals of all skin phototypes.60 Foundations that contain Fe2O3 to even skin tone and cover blemishes have been demonstrated to protect against blue light.59
Photoprotection against VL is relevant for SOC, as VL may contribute to melasma and post-inflammatory hyperpigmentation.9,12,13,20,24,26,27,32,33,36,47–49 Tinted sunscreens which include mineral pigments improve the Melasma Area and Severity Index (MASI) score.50 A study compared the use of broad-spectrum UV protection that contained Fe2O3 as a VL-absorbing pigment (UV-VL) and a regular UV-only broad-spectrum sunscreen in 61 patients with melasma, receiving 4% hydroquinone as a depigmenting treatment.62 At 8 weeks, UV-VL protection showed a 15%, 28%, and 4% greater improvement in MASI, colorimetric values, and melanin assessments, respectively.62 In addition to improving melasma lesions after 8 weeks, broad-spectrum sunscreens containing Fe2O3 alone or in combination with ZnO and TiO2 prevented relapses after 6 months.60
Application of sunscreens.
Sunscreen efficacy is measured by the sun protection factor (SPF), an assessment of the ratio of the minimal erythemal dose (MED) of UVR on filter protected skin compared to unprotected skin (MED protected/MED unprotected).25,63 For SPF testing, sunscreen is applied at 2 mg/cm2, which corresponds to 30 mL (1 oz) for the entire body surface.44 SPF is a measure of the erythemogenic effect of UVB, and to a lesser extent, UVA2.33 In the US, sunscreens labeled as “broad-spectrum” must have a critical wavelength (CW) of ≥370 nm.1,3,9,29,46,64 To meet this criterion, at least 90% of the product’s total absorbance must be at or above this CW value when measured using UV wavelengths ranging from 290 to 400 nm.29,57 Broad-spectrum sunscreens with an SPF >15 may claim protection from skin cancer and early skin aging.3,35,46 Theoretically, for someone who burns typically after 10 minutes, wearing SPF 15 would allow them to stay outside 15 times longer (2.5 hours) without burning if sun exposure is constant.65,66 However, most individuals tend to underapply sunscreens.2,3,44,46,64,67,68 Therefore, the in-use SPF is significantly lower than the labeled SPF. Furthermore, SPF alone does not indicate protection against UVA nor VL.65,66,69,70
The FDA and US Preventive Services Task Force recommend the use of a broad-spectrum filter with SPF>15, while the American Academy of Dermatology (AAD) recommends SPF>30.46,71 Products with SPF 15, 30, and 60 allow 6.7%, 3.3%, and 1.7% UVR to be transmitted to the skin surface, respectively, based on topical application at 2 mg/cm2; however, consumers usually apply 0.5–1.0 mg/cm.2,3,44,46,64,67,68 While the difference between SPF 30 and 60 (3.3% vs. 1.7% transmission) is relatively minimal for a single acute exposure, with daily application over time, the more than 2-fold difference might significantly affect chronic UV effects on the skin.72 The “Teaspoon Rule of Applying Sunscreen,” which advises 1 teaspoon of sunscreen to the face/head/neck, 1 teaspoon to each upper extremity, 2 teaspoons to the torso, and 2 teaspoons to each lower extremity, was proposed to help achieve 2 mg/cm2 of density.23,35,67
Other photoprotective strategies.
Pollutants, clouds, and fog may reduce the intensity of UVR, VL, and IR; ozone absorbs UVC (99%), some UVB (90%), but little to no UVA or VL (50%).5,73 The US National Weather Service calculation of the UV index assumes that clear skies allow 100% of UV transmission, scattered clouds 89%, broken clouds 73%, and overcast skies 31%.5,73 Clear glass allows up to 90% of VL (assessed from 400–780 nm), 72% of UV (from 300–400 nm), and 83% of solar heat to penetrate.74,75 Tinted or reflective glass transmits less VL, UV, and IR radiation; however, US federal standards mandate at least 70% VL transmission through the windshield.5,47,74 All types of glass block transmission of UVB (280–315 nm).5,47,74 Darkly tinted sunglasses may block UVA and VL but can obscure vision.47 UVB may damage the cornea and lens, whereas VL can affect the retina.11,47,74 Glasses with blue lenses absorb VL between 400–500nm.74,76–80 Orange and yellow lenses provide the best protection against both UV and VL.81 Wide-brimmed hats may offer an SPF of up to 7.5,64 The UV protection factor (UPF) is a measure of protection against UV through clothing.5,23,73,82 A UPF of 15–24 indicates good protection, 25–39 very good protection, and 40–50 excellent protection, with tightly woven and dark fabrics being superior.5,46 The pigments in makeup and tanning preparations (e.g., dihydroxyacetone) protect against UVA and VL by their oxidation effects that change skin color to orange-brown.32,47 The color remains adherent to the stratum corneum and confers an SPF of 2.5,47 Systemic agents may also protect against VL as discussed later.
II. GLOBAL DIFFERENCES IN REGULATIONS OF UV FILTERS
Key points.
In the US, the FDA regulates UV filters as an over-the-counter drug.
The FDA categorize inorganic filters as generally recognized as safe and effective.
VL photoprotection regulations and guidelines are lacking in the US and globally.
United States FDA regulations.
In the US, the FDA regulates UV filters as an over-the-counter drug.3,6,16,29,30,42,83,84 The FDA proposed its first set of rules regarding UV filters in 1978, and the original FDA monograph listed 16 approved UV filters (Table 1).1,30,46,47 Another UV filter available in the US, ecamsule, was approved as part of New Drug Application in 2006.30,47 From 1997 to 2009, the percentage of low SPF products (SPF 4–14) decreased from 27% to 6%, the number of products that filter against UVA increased from 5% to 70%, and 68% of the products tested in 2009 attained CW >370 nm.85 There is a trend toward more broad-spectrum coverage in the UVA and VL range. The FDA proposed rule, released on February 2019, classified the 16 approved UV filters in the monograph into 3 categories: Category 1 (zinc oxide and titanium dioxide): generally recognized as safe and effective (GRASE), Category 2 (PABA, trolamine salicylate): not GRASE, and Category 3 (remaining 12 UV filters): insufficient data to determine GRASE.25,31,86 However, guidelines addressing VL are insufficient.7,20
Other countries.
Compared to Europe, Asia, Central, and South America, Canada, and Australia, the US has fewer available UV filters, which offer less superior UVA protection due to stringent requirements.3,6,29,31,35,87,88 UV filters are regulated as cosmetics in Europe, quasi-drugs in Japan, and therapeutic drugs in Australia.3,6,16,30,35,42,47,89,90 Currently, there are 29 UV filters approved in Europe.3,47 Several UV filters with broadband UV protection have been approved in Europe and other parts of the world since the 1990s; however, they are not yet approved by the US FDA.1,3,6,34,88,89
III. SAFETY OF UV FILTERS
Key points.
Safety concerns regarding UV filters include photoallergic reactions and potential systemic absorption.
The use of sunscreens has been associated with frontal fibrosing alopecia, b no causal relationship has been established.
The AAD and the National Council on Skin Cancer Prevention recommend receiving vitamin D through the diet and oral supplementation and avoiding intentional UV radiation exposure to induce production.
Data currently support the regular use of UV filters as the benefits greatly outweigh the limited data regarding its risks.
Photoallergic reactions.
UV filters may induce irritant and allergic contact reactions, photoallergy, and phototoxic effects.3,16,35,46,47,73 In a 10-year retrospective analysis of almost 24,000 patients patch-tested, 0.9% had a sunscreen allergy, and 70% of those were due to oxybenzone.15,34,35,47,91 The American Contact Dermatitis Society named oxybenzone contact allergen of the year, and the European Scientific Committee on Consumer Safety has recommended its replacement with other broad-spectrum filters.14,15,34,73,92 However, its use continues in the US because the FDA has not yet approved alternative filters.6,15 The Centers for Disease Control and Prevention estimate that 96.8% of the US population has been exposed to oxybenzone since its first use in 1978.15,52,92–94ut Thus, in regards to its widespread use, the development of photoallergic reactions are uncommon.47,73
Systemic absorption of UV filters.
In vitro and animal studies report endocrinologic effects of UV filters, but the results are equivocal in human studies.6,15,30,34,47,73,93,95–97 Metal oxide nanoparticles do not penetrate the stratum corneum but are deposited in the openings of pilosebaceous follicles, sweat glands, and skin folds.30,34,35,39,40,51,54,55,98,99 In vitro, nanoparticles generate reactive oxygen species (ROS) when exposed to UVA and UVB light.39,40,73,98 However, their safety in sunscreen products is well established.39,40,73,98 In sunscreens, nanoparticles are coated with silica or aluminum hydroxide that minimize ROS formation and cytotoxicity.25,31,34,35,57,100 Additionally, endogenous antioxidants in the skin can neutralize ROS.35
Possible link to frontal fibrosing alopecia.
Survey data demonstrates an association between sunscreen use and frontal fibrosing alopecia (FFA) in men and women.33,101–106 TiO2 has been found in the hair shafts of FFA patients, and the dominant putative mechanism for the association is that UV filters penetrate the follicular infundibulum and elicit a lichenoid reaction.104,106 Overall incidence of FFA remains low compared to the prevalence of sunscreen use, and not all patients with FFA endorse exposure to UV filters.106 Thus, there is insufficient evidence to establish a direct causal relationship.106
Vitamin D.
While questions exist about UV filters reducing the production of UVB-induced vitamin D synthesis, this is not a concern due to the underapplication of sunscreen by the public.3,33,35,73,89,107–111 The AAD and the National Council on Skin Cancer Prevention recommend receiving vitamin D through the diet and oral supplementation and avoiding intentional UV radiation exposure to induce production.33,36,107,109–113 The benefits of regular sunscreen use outweigh the risks.6,16,83
IV. ENVIRONMENTAL IMPACT OF UV FILTERS
Key points.
UV filters may enter the aquatic environment and cause coral reef bleaching and death.
Hawaii became the first state to ban the use of two light filters, oxybenzone and octinoxate.
Except for the US, oxybenzone is no longer commonly used in sunscreens in many parts of the world.
Damage to coral reefs.
It is estimated that as much as 14,000 tons of UV filters are released into the coral reefs annually.92,94,114–115 Oxybenzone was added to the Environmental Protection Agency High Production Volume Challenge Program, which identifies ingredients manufactured or imported into the US in amounts equal to or greater than one million pounds per year.92 Organic filters cause coral bleaching and death.31,52,92,94,115,117–121 However, a study done in Oahu, Hawaii, showed that the concentrations detected in seawater were 1000th-fold lower than those reported to be cytotoxic to coral reefs in vitro.115 Multiple studies have concluded that ocean water warming is a major contributing factor in coral bleaching.6,31,52,94,122–128 In many parts of the world, because of the availability of other filters, oxybenzone is no longer commonly used in sunscreens; this is not the case in the US due to the limited availability of filters to replace oxybenzone.14,25,33,46,68,92,94,129
Environmental regulations.
Hawaii became the first state to ban the sale of two UV filters, oxybenzone, and octinoxate, by January 1, 2021.14,25,33,46,68,92,94,129 The US Virgin Islands, Palau, Bonaire, the nature reserves of Mexico, and Key West enacted similar bans, while active discussions are occurring in Brazil and Europe.31,52,94,117
V. ROLE OF ANTIOXIDANTS AND OTHER AGENTS IN PHOTOPROTECTION AND PHOTOREPAIR
Key points.
Emerging evidence exists for the beneficial effects of sunscreen containing photolyases, enzymes that repair DNA damage.
Early studies show that oral and topical antioxidants might confer VL photoprotection.
Photorepair with photolyase.
There is emerging evidence for the beneficial effects of sunscreen containing photolyases.21,130–132 Photolyases are enzymes that repair cyclobutane pyrimidine dimers (CPDs) upon exposure to blue light.15,131,133–139 After photodynamic therapy (PDT), daily application of a sunscreen with photolyases was associated with a reduction in the number of new AK lesions compared to conventional sunscreen.15,21,130,140 The combination of topical antioxidants and photolyases may have a synergistic effect.15,132 However, photolyases are not effective when used with VL blockers as they are activated by blue light.15,133–135
Antioxidant mechanism of photoprotection.
UVB-induced erythema results from direct DNA damage (i.e., CPDs), while UVA effects are largely mediated by ROS.33 VL exposure may contribute up to one-half of ROS generated in the skin.7,11,25,133,136 The addition of antioxidants to sunscreens reduces ROS formation by an additional 1.7-fold for SPF 4 and 2.4-fold for SPF 15 products.130 Antioxidants modulate the effects of VL on a molecular level by reducing interleukin-1a and matrix metalloproteinase expression.5,6,11,15,17,18,25,32,34,35,141,142 In one study, topical application of a sunscreen containing an antioxidant complex reduced immediate erythema and pigmentation in subjects with skin phototypes I-III and IV-VI, respectively, after irradiation with VL and UVA1.60 However, this protective effect was not observed at day 7, indicating that antioxidants may be more effective at reducing pigmentation mediated by photo-oxidation than pigmentation caused by de novo melanin synthesis.60
Topical, oral, and systemic agents for VL photoprotection.
In addition to offering superior VL protection, topical antioxidants are non-toxic.5,22,81,143–149 However, in an analysis of sunscreens with antioxidant ingredients, 10 of 12 sunscreens had no antioxidant activity, and the other 2 had only low activity.34,35 More research is needed to determine how to stabilize antioxidants into a biologically active product.34,35
Oral photoprotective agents (Table 2)11,15,21,22,25,33,53,142,147–155 offer the advantage of protecting the skin surface without being affected by external factors such as washing, perspiration, or rubbing.12,21,73,81 Oral Polypodium leucotomos extract has photoprotective, chemoprotective, anti-inflammatory, and immunomodulatory properties that mitigate VL-induced effects, including persistent pigment darkening and delayed tanning.154 Subcutaneous afamelanotide, an analog of α-melanocyte stimulating hormone, stimulates melanin production and has antioxidative properties, and is approved for the management of EPP, a VL-induced photodermatosis.32,150,156,157
Table 2.
Non-topical forms of photoprotection15
| Photoprotective agent | Source | Mechanism | Clinical Use |
|---|---|---|---|
| Polypodium leucotomos extract | Tropical fern of the Polypodiaceae family |
|
|
| Nicotinamide | Active form of vitamin B3 (niacin) |
|
|
| Afamelanotide | Analogue of alpha-melanocyte-stimulating hormone |
|
|
COX-2 (cyclooxygenase-2), CPDs (cyclobutene pyrimidine dimers), UV (ultraviolet), UVR (UV radiation), ATP (adenosine triphosphate), ROS (reactive oxygen species), EPP (erythropoietic protoporphyria), XLPP (X-linked protoporphyria)
VI. RECOMMENDATIONS, FUTURE DIRECTIONS, AND CONCLUSION
Key points.
Sunscreens have undergone fundamental improvements, but there is still a need for additional research on VL photoprotection.
Further development should focus on better protection in the range of UVA1 and VL, enhancing cosmesis, and incorporating antioxidants to enhance photoprotection.
Photoprotection against VL includes seeking shade during peak hours, wearing photoprotective clothing and accessories, and applying tinted broad-spectrum sunscreens.1,52,67,85,94 While sunscreens have undergone significant improvements, there is still a need for additional VL photoprotection research. Further development should focus on better protection in the range of UVA1 and VL, enhancing cosmesis of these filters (especially for susceptible SOC individuals), and the use of effective antioxidants and other agents to enhance photoprotection.29,48,85,87,94,158
Supplementary Material
Funding Source:
Dr. Jagdeo is supported by a National Institute of General Medical Sciences of the National Institute of Health (NIH) award (K23GM1173090).
Conflict of Interest Disclosure(s):
Henry W. Lim is an investigator for Incyte, L’Oreal, Pfizer, and PCORI, has served as a consultant for Pierre Fabre, ISDIN, Ferndale, Galderma, and La Roche-Posay, and has participated as a speaker in general educational session for Johnson and Johnson, Ra Medical System, La Roche-Posay, and Cantabria labs. Indermeet Kohli is an investigator (grant funding received by the institution) for Ferndale, Estee Lauder, L’Oreal, Unigen, Johnson and Johnson, Allergan, and Bayer and is a consultant (fee and equipment received by the institution) for Pfizer, Johnson and Johnson, and Bayer. Jared Jagdeo is a member of the GlobalMed Scientific advisory board and a consultant for UV Biotek. Iltefat Hamzavi is an investigator for Estee Lauder, Ferndale Laboratories, Galderma, Bayer, Loreal, Lenicura, and Unigen.
ABBREVIATIONS
- AK
actinic keratoses
- AAD
American Academy of Dermatology
- CW
critical wavelength
- CPD
cyclobutene pyrimidine dimer
- EMR
electromagnetic radiation
- EPP
erythropoietic protoporphyria
- FAD
flavin adenine dinucleotide
- FDA
Food and Drug Administration
- FFA
frontal fibrosing alopecia
- GRASE
generally recognized as safe and effective
- IR
infrared
- MASI
Melasma Area and Severity Index
- MED
minimal erythemal dose
- PABA
para-aminobenzoic acid
- PDT
photodynamic therapy
- ROS
reactive oxygen species
- SOC
skin of color
- SPF
sun protection factor
- TiO2
titanium dioxide
- UV
ultraviolet
- UPF
UV protection factor
- UVR
UV radiation
- WWTP
wastewater treatment plant
- VL
visible light
- ZnO
zinc oxide
Footnotes
Prior presentation(s): The contents of this manuscript are not under consideration for publication elsewhere, have not been copyrighted or published previously, and will not be copyrighted, submitted, or published elsewhere while acceptance by your journal is under consideration.
IRB Approval: CME article; not required.
References
- 1.Wang SQ, Lim HW. Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration’s final rule on labeling and effectiveness testing. J Am Acad Dermatol. 2011;65(4):863–869. [DOI] [PubMed] [Google Scholar]
- 2.Gasparro FP, Mitchnick M, Nash JF. A review of sunscreen safety and efficacy. Photochem Photobiol. 1998;68(3):243–256. [PubMed] [Google Scholar]
- 3.Bens G Sunscreens. Adv Exp Med Biol. 2014;810:429–463. [DOI] [PubMed] [Google Scholar]
- 4.Hojerova J, Medovcikova A, Mikula M. Photoprotective efficacy and photostability of fifteen sunscreen products having the same label SPF subjected to natural sunlight. Int J Pharm. 2011;408(1–2):27–38. [DOI] [PubMed] [Google Scholar]
- 5.Jansen R, Wang SQ, Burnett M, Osterwalder U, Lim HW. Photoprotection: part I. Photoprotection by naturally occurring, physical, and systemic agents. J Am Acad Dermatol. 2013;69(6):853. e851–812; quiz 865–856. [DOI] [PubMed] [Google Scholar]
- 6.Mancuso JB, Maruthi R, Wang SQ, Lim HW. Sunscreens: An Update. Am J Clin Dermatol. 2017;18(5):643–650. [DOI] [PubMed] [Google Scholar]
- 7.Martini APM, Maia Campos P. Influence of visible light on cutaneous hyperchromias: Clinical efficacy of broad-spectrum sunscreens. Photodermatol Photoimmunol Photomed. 2018;34(4):241–248. [DOI] [PubMed] [Google Scholar]
- 8.Diffey BL. Sources and measurement of ultraviolet radiation. Methods. 2002;28(1):4–13. [DOI] [PubMed] [Google Scholar]
- 9.Kohli I, Braunberger TL, Nahhas AF, et al. Long-wavelength Ultraviolet A1 and Visible Light Photoprotection: A Multimodality Assessment of Dose and Response. Photochem Photobiol. 2019;96(1):208–214. [DOI] [PubMed] [Google Scholar]
- 10.Kohli I, Lim HW, Hamzavi IH. Caution regarding testing for long wavelength ultraviolet A1 and visible light effects on human skin in vivo. Photodermatol Photoimmunol Photomed. 2019;36(1):58–60. [DOI] [PubMed] [Google Scholar]
- 11.Mann T, Eggers K, Rippke F, et al. High-energy visible light at ambient doses and intensities induces oxidative stress of skin-Protective effects of the antioxidant and Nrf2 inducer Licochalcone A in vitro and in vivo. Photodermatol Photoimmunol Photomed. 2020;36(2):135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmoud BH, Hexsel CL, Hamzavi IH, Lim HW. Effects of visible light on the skin. Photochem Photobiol. 2008;84(2):450–462. [DOI] [PubMed] [Google Scholar]
- 13.Kohli I, Nahhas AF, Braunberger TL, et al. Spectral characteristics of visible light-induced pigmentation and visible light protection factor. Photodermatol Photoimmunol Photomed. 2019;35(6):393–399. [DOI] [PubMed] [Google Scholar]
- 14.Siller A, Blaszak SC, Lazar M, Olasz Harken E. Update About the Effects of the Sunscreen Ingredients Oxybenzone and Octinoxate on Humans and the Environment. Plast Surg Nurs. 2018;38(4):158–161. [DOI] [PubMed] [Google Scholar]
- 15.Yeager DG, Lim HW. What’s New in Photoprotection: A Review of New Concepts and Controversies. Dermatol Clin. 2019;37(2):149–157. [DOI] [PubMed] [Google Scholar]
- 16.Maier T, Korting HC. Sunscreens - which and what for? Skin Pharmacol Physiol. 2005;18(6):253–262. [DOI] [PubMed] [Google Scholar]
- 17.Peres G, Miot HA. Transmittance of UVB, UVA, and visible light (blue-violet) among the main Brazilian commercial opaque sunscreens. An Bras Dermatol. 2020;95(1):108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liebel F, Kaur S, Ruvolo E, Kollias N, Southall MD. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132(7):1901–1907. [DOI] [PubMed] [Google Scholar]
- 19.Frederick JE, Snell HE, Haywood EK. SOLAR ULTRAVIOLET RADIATION AT THE EARTH’S SURFACE. Photochemistry and Photobiology. 1989;50(4):443–450. [Google Scholar]
- 20.Schalka S, de Paula Correa M, Sawada LY, Canale CC, de Andrade TN. A novel method for evaluating sun visible light protection factor and pigmentation protection factor of sunscreens. Clin Cosmet Investig Dermatol. 2019;12:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sondenheimer K, Krutmann J. Novel Means for Photoprotection. Front Med (Lausanne). 2018;5:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geoffrey K, Mwangi AN, Maru SM. Sunscreen products: Rationale for use, formulation development and regulatory considerations. Saudi Pharm J. 2019;27(7):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rai R, Srinivas CR. Photoprotection. Indian J Dermatol Venereol Leprol. 2007;73(2):73–79. [DOI] [PubMed] [Google Scholar]
- 24.Lyons AB, Trullas C, Kohli I, Hamzavi IH, Lim HW. Photoprotection beyond ultraviolet radiation: A review of tinted sunscreens [published online ahead of print, 2020 April 23]. J Am Acad Dermatol. 2020;S0190–9622(20)30694–0. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein EF, Sarkas HW, Boland P, Bouche D. Beyond sun protection factor: An approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19(2):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen L, Brodsky MA, Zubair R, Kohli I, Hamzavi IH, Sadeghpour M. Cutaneous Interaction with Visible Light: What Do We Know [published online ahead of print, 2020 April 11]. J Am Acad Dermatol. 2020;S0190–9622(20)30551-X. [DOI] [PubMed] [Google Scholar]
- 27.Austin E, Huang A, Adar T, Wang E, Jagdeo J. Electronic device generated light increases reactive oxygen species in human fibroblasts. Lasers Surg Med. 2018;50(6):689–69. [DOI] [PubMed] [Google Scholar]
- 28.Duteil L, Queille-Roussel C, Lacour JP, Montaudié H, Passeron T. Short-term exposure to blue light emitted by electronic devices does not worsen melasma. J Am Acad Dermatol. 2020;83(3):913–914.. [DOI] [PubMed] [Google Scholar]
- 29.Burnett ME, Hu JY, Wang SQ. Sunscreens: obtaining adequate photoprotection. Dermatol Ther. 2012;25(3):244–251. [DOI] [PubMed] [Google Scholar]
- 30.Hexsel CL, Bangert SD, Hebert AA, Lim HW. Current sunscreen issues: 2007 Food and Drug Administration sunscreen labelling recommendations and combination sunscreen/insect repellent products. J Am Acad Dermatol. 2008;59(2):316–323. [DOI] [PubMed] [Google Scholar]
- 31.Narla S, Lim HW. Sunscreen: FDA regulation, and environmental and health impact. Photochem Photobiol Sci. 2020;19(1):66–70. [DOI] [PubMed] [Google Scholar]
- 32.Narla S, Kohli I, Hamzavi IH, Lim HW. Visible light in photodermatology. Photochem Photobiol Sci. 2020;19(1):99–104. [DOI] [PubMed] [Google Scholar]
- 33.Krutmann J, Passeron T, Gilaberte Y, et al. Photoprotection of the future: challenges and opportunities. Journal of the European Academy of Dermatology and Venereology. 2020;34(3):447–454. [DOI] [PubMed] [Google Scholar]
- 34.Lim HW, Arellano-Mendoza MI, Stengel F. Current challenges in photoprotection. J Am Acad Dermatol. 2017;76(3s1):S91–s99. [DOI] [PubMed] [Google Scholar]
- 35.Jansen R, Osterwalder U, Wang SQ, Burnett M, Lim HW. Photoprotection: part II. Sunscreen: development, efficacy, and controversies. J Am Acad Dermatol. 2013;69(6):867. e1–882. [DOI] [PubMed] [Google Scholar]
- 36.Lim HW. Challenges in photoprotection: Introduction. J Am Acad Dermatol. 2017;76(3s1):S89–s90. [DOI] [PubMed] [Google Scholar]
- 37.Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70(4):748–762. [DOI] [PubMed] [Google Scholar]
- 38.Teramura T, Mizuno M, Asano H, et al. Prevention of photosensitivity with action spectrum adjusted protection for erythropoietic protoporphyria. J Dermatol. 2018;45(2):145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulson B, McCall MJ, Bowman DM, Pinheiro T. A review of critical factors for assessing the dermal absorption of metal oxide nanoparticles from sunscreens applied to humans, and a research strategy to address current deficiencies. Arch Toxicol. 2015;89(11):1909–1930. [DOI] [PubMed] [Google Scholar]
- 40.Filipe P, Silva JN, Silva R, et al. Stratum corneum is an effective barrier to TiO2 and ZnO nanoparticle percutaneous absorption. Skin Pharmacol Physiol. 2009;22(5):266–275. [DOI] [PubMed] [Google Scholar]
- 41.Kim S, Choi K. Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: a mini-review. Environ Int. 2014;70:143–157. [DOI] [PubMed] [Google Scholar]
- 42.Nohynek GJ, Schaefer H. Benefit and risk of organic ultraviolet filters. Regul Toxicol Pharmacol. 2001;33(3):285–299. [DOI] [PubMed] [Google Scholar]
- 43.Shaath NA. Ultraviolet filters. Photochemical & Photobiological Sciences. 2010;9(4):464–469. [DOI] [PubMed] [Google Scholar]
- 44.Gabros S, Nessel TA, Zito PM. Sunscreens and Photoprotection. [Updated 2020 July 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537164/. [PubMed] [Google Scholar]
- 45.Manaia EB, Kaminski RCK, Corrêa MA, Chiavacci LA. Inorganic UV filters. Brazilian Journal of Pharmaceutical Sciences. 2013;49:201–209. [Google Scholar]
- 46.Sunscreens. Med Lett Drugs Ther. 2018;60(1553):129–132. [PubMed] [Google Scholar]
- 47.Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet. 2007;370(9586):528–537. [DOI] [PubMed] [Google Scholar]
- 48.Boukari F, Jourdan E, Fontas E, et al. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol. 2015;72(1):189–190. e181. [DOI] [PubMed] [Google Scholar]
- 49.Jagdeo J, Nguyen JK, Ho D, et al. Safety of light emitting diode-red light on human skin: Two randomized controlled trials. J Biophotonics. 2020;13(3):e201960014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moyle D S S Prevention of melasma intensification with sunscreen combining protection against UV and short visible light. AAD Virtual Meeting. 2020. [Google Scholar]
- 51.Monteiro-Riviere NA, Wiench K, Landsiedel R, Schulte S, Inman AO, Riviere JE. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in UVB sunburned skin: an in vitro and in vivo study. Toxicol Sci. 2011;123(1):264–280. [DOI] [PubMed] [Google Scholar]
- 52.Schneider SL, Lim HW. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol Photoimmunol Photomed. 2019;35(6):442–446. [DOI] [PubMed] [Google Scholar]
- 53.Fatima S, Braunberger T, Mohammad TF, Kohli I, Hamzavi IH. The Role of Sunscreen in Melasma and Postinflammatory Hyperpigmentation. Indian J Dermatol. 2020;65(1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newman MD, Stotland M, Ellis JI. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J Am Acad Dermatol. 2009;61(4):685–692. [DOI] [PubMed] [Google Scholar]
- 55.Cross SE, Innes B, Roberts MS, Tsuzuki T, Robertson TA, McCormick P. Human skin penetration of sunscreen nanoparticles: in-vitro assessment of a novel micronized zinc oxide formulation. Skin Pharmacol Physiol. 2007;20(3):148–154. [DOI] [PubMed] [Google Scholar]
- 56.Botta C, Di Giorgio C, Sabatier AS, De Meo M. Genotoxicity of visible light (400–800 nm) and photoprotection assessment of ectoin, L-ergothioneine and mannitol and four sunscreens. J Photochem Photobiol B. 2008;91(1):24–34. [DOI] [PubMed] [Google Scholar]
- 57.Mancebo SE, Hu JY, Wang SQ. Sunscreens: a review of health benefits, regulations, and controversies. Dermatol Clin. 2014;32(3):427–438. [DOI] [PubMed] [Google Scholar]
- 58.Lyons AB, Trullas C, Kohli I, Hamzavi IH, and Lim HW. Photoprotection Beyond Ultraviolet Radiation: A Review of Tinted Sunscreens. J Am Acad Dermatol. 2020. Apr. doi: 10.1016/j.jaad.2020.04.079. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 59.Ruvolo E, et al. , Photoprotection against visible light-induced pigmentation. International Journal of Cosmetic Science, 2018. 40(6): p. 589–595. [DOI] [PubMed] [Google Scholar]
- 60.Dumbuya H, et al. , Impact of Iron-Oxide Containing Formulations Against Visible Light-Induced Skin Pigmentation in Skin of Color Individuals. J Drugs Dermatol, 2020. 19(7): p. 712–717. [DOI] [PubMed] [Google Scholar]
- 61.Duteil L, Esdaile J, Maubert Y, et al. A method to assess the protective efficacy of sunscreens against visible light-induced pigmentation. Photodermatol Photoimmunol Photomed. 2017;33(5):260–266. [DOI] [PubMed] [Google Scholar]
- 62.Castanedo-Cazares JP, et al. , Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed, 2014. 30(1): p. 35–42. [DOI] [PubMed] [Google Scholar]
- 63.Reinau D, Osterwalder U, Stockfleth E, Surber C. The meaning and implication of sun protection factor. Br J Dermatol. 2015;173(5):1345. [DOI] [PubMed] [Google Scholar]
- 64.Porto DA, Wang SQ, Lim HW. Counseling Patients on Photoprotection: What the Dermatologist Needs to Know. JAMA Dermatol. 2017;153(1):110. [DOI] [PubMed] [Google Scholar]
- 65.Dennis LK, Vanbeek MJ, Beane Freeman LE, Smith BJ, Dawson DV, Coughlin JA. Sunburns and risk of cutaneous melanoma: does age matter? A comprehensive meta-analysis. Ann Epidemiol. 2008;18(8):614–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bech-Thomsen N, Wulf HC. Sunbathers’ application of sunscreen is probably inadequate to obtain the sun protection factor assigned to the preparation. Photodermatol Photoimmunol Photomed. 1992;9(6):242–244. [PubMed] [Google Scholar]
- 67.Isedeh P, Osterwalder U, Lim HW. Teaspoon rule revisited: proper amount of sunscreen application. Photodermatol Photoimmunol Photomed. 2013;29(1):55–56. [DOI] [PubMed] [Google Scholar]
- 68.He T, Tsui MMP, Tan CJ, et al. Toxicological effects of two organic ultraviolet filters and a related commercial sunscreen product in adult corals. Environ Pollut. 2019;245:462–471. [DOI] [PubMed] [Google Scholar]
- 69.McKenzie R, Bodeker G, Scott G, Slusser J, Lantz K. Geographical differences in erythemally-weighted UV measured at mid-latitude USDA sites. Photochem Photobiol Sci. 2006;5(3):343–352. [DOI] [PubMed] [Google Scholar]
- 70.Allen M, McKenzie R. Enhanced UV exposure on a ski-field compared with exposures at sea level. Photochem Photobiol Sci. 2005;4(5):429–437. [DOI] [PubMed] [Google Scholar]
- 71.Kohli I, Nicholson CL, Williams JD, et al. Greater efficacy of SPF 100+ sunscreen compared with SPF 50+ in sunburn prevention during 5 consecutive days of sunlight exposure: A randomized, double-blind clinical trial. J Am Acad Dermatol. 2020;82(4):869–877. [DOI] [PubMed] [Google Scholar]
- 72.Herzog SM, Lim HW, Williams MS, de Maddalena ID, Osterwalder U, Surber C. Sun Protection Factor Communication of Sunscreen Effectiveness: A Web-Based Study of Perception of Effectiveness by Dermatologists. JAMA Dermatol. 2017;153(3):348–350. [DOI] [PubMed] [Google Scholar]
- 73.Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol. 2005;52(6):937–962. [DOI] [PubMed] [Google Scholar]
- 74.Tuchinda C, Srivannaboon S, Lim HW. Photoprotection by window glass, automobile glass, and sunglasses. J Am Acad Dermatol. 2006;54(5):845–854. [DOI] [PubMed] [Google Scholar]
- 75.Choi D, Kannan S, Lim HW. Evaluation of patients with photodermatoses. Dermatol Clin. 2014;32(3):267–275. [DOI] [PubMed] [Google Scholar]
- 76.Downie LE, Wormald R, Evans J, et al. Analysis of a Systematic Review About Blue Light-Filtering Intraocular Lenses for Retinal Protection: Understanding the Limitations of the Evidence. JAMA Ophthalmol. 2019;137(6):694–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mainster MA. Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br J Ophthalmol. 2006;90(6):784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mainster MA, Turner PL. Blue-blocking IOLs decrease photoreception without providing significant photoprotection. Surv Ophthalmol. 2010;55(3):272–289. [DOI] [PubMed] [Google Scholar]
- 79.Cuthbertson FM, Peirson SN, Wulff K, Foster RG, Downes SM. Blue light-filtering intraocular lenses: review of potential benefits and side effects. J Cataract Refract Surg. 2009;35(7):1281–1297. [DOI] [PubMed] [Google Scholar]
- 80.Davison JA, Patel AS, Cunha JP, Schwiegerling J, Muftuoglu O. Recent studies provide an updated clinical perspective on blue light-filtering IOLs. Graefes Arch Clin Exp Ophthalmol. 2011;249(7):957–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Skotarczak K, Osmola-Mankowska A, Lodyga M, Polanska A, Mazur M, Adamski Z. Photoprotection: facts and controversies. Eur Rev Med Pharmacol Sci. 2015;19(1):98–112. [PubMed] [Google Scholar]
- 82.Morison WL. Photoprotection by clothing. Dermatol Ther. 2003;16(1):16–22. [DOI] [PubMed] [Google Scholar]
- 83.Matta MK, Zusterzeel R, Pilli NR, et al. Effect of Sunscreen Application Under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial. JAMA. 2019;321(21):2082–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paul SP. Ensuring the Safety of Sunscreens, and Their Efficacy in Preventing Skin Cancers: Challenges and Controversies for Clinicians, Formulators, and Regulators. Front Med (Lausanne). 2019;6:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang SQ, Tanner PR, Lim HW, Nash JF. The evolution of sunscreen products in the United States--a 12-year cross sectional study. Photochem Photobiol Sci. 2013;12(1):197–202. [DOI] [PubMed] [Google Scholar]
- 86.Wang SQ, Lim HW. Highlights and implications of the 2019 proposed rule on sunscreens by the US Food and Drug Administration. J Am Acad Dermatol. 2019;81(2):650–651. [DOI] [PubMed] [Google Scholar]
- 87.Wang SQ, Virmani P, Lim HW. Consumer acceptability and compliance: the next frontier in sunscreen innovation. Photodermatol Photoimmunol Photomed. 2016;32(1):55–56. [DOI] [PubMed] [Google Scholar]
- 88.Fourtanier A, Moyal D, Seite S. Sunscreens containing the broad-spectrum UVA absorber, Mexoryl SX, prevent the cutaneous detrimental effects of UV exposure: a review of clinical study results. Photodermatol Photoimmunol Photomed. 2008;24(4):164–174. [DOI] [PubMed] [Google Scholar]
- 89.Loden M, Beitner H, Gonzalez H, et al. Sunscreen use: controversies, challenges and regulatory aspects. Br J Dermatol. 2011;165(2):255–262. [DOI] [PubMed] [Google Scholar]
- 90.Osterwalder U, Herzog B. Sun protection factors: world wide confusion. Br J Dermatol. 2009;161 Suppl 3:13–24. [DOI] [PubMed] [Google Scholar]
- 91.Warshaw EM, Wang MZ, Maibach HI, et al. Patch test reactions associated with sunscreen products and the importance of testing to an expanded series: retrospective analysis of North American Contact Dermatitis Group data, 2001 to 2010. Dermatitis. 2013;24(4):176–182. [DOI] [PubMed] [Google Scholar]
- 92.DiNardo JC, Downs CA. Dermatological and environmental toxicological impact of the sunscreen ingredient oxybenzone/benzophenone-3. J Cosmet Dermatol. 2018;17(1):15–19. [DOI] [PubMed] [Google Scholar]
- 93.Wang SQ, Burnett ME, Lim HW. Safety of oxybenzone: putting numbers into perspective. Arch Dermatol. 2011;147(7):865–866. [DOI] [PubMed] [Google Scholar]
- 94.Schneider SL, Lim HW. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J Am Acad Dermatol. 2019;80(1):266–271. [DOI] [PubMed] [Google Scholar]
- 95.Pollack AZ, Buck Louis GM, Chen Z, et al. Bisphenol A, benzophenone-type ultraviolet filters, and phthalates in relation to uterine leiomyoma. Environ Res. 2015;137:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kunisue T, Chen Z, Buck Louis GM, et al. Urinary concentrations of benzophenone-type UV filters in U.S. women and their association with endometriosis. Environ Sci Technol. 2012;46(8):4624–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schlumpf M, Cotton B, Conscience M, Haller V, Steinmann B, Lichtensteiger W. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect. 2001;109(3):239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gulson B, McCall M, Korsch M, et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol Sci. 2010;118(1):140–149. [DOI] [PubMed] [Google Scholar]
- 99.Mohammed YH, Holmes A, Haridass IN, et al. Support for the Safe Use of Zinc Oxide Nanoparticle Sunscreens: Lack of Skin Penetration or Cellular Toxicity after Repeated Application in Volunteers. J Invest Dermatol. 2019;139(2):308–315. [DOI] [PubMed] [Google Scholar]
- 100.Osterwalder U, Sohn M, Herzog B. Global state of sunscreens. Photodermatol Photoimmunol Photomed. 2014;30(2–3):62–80. [DOI] [PubMed] [Google Scholar]
- 101.Cranwell WC, Lai VW, Photiou L, et al. Treatment of alopecia areata: An Australian expert consensus statement. Australas J Dermatol. 2019;60(2):163–170. [DOI] [PubMed] [Google Scholar]
- 102.Aldoori N, Dobson K, Holden CR, McDonagh AJ, Harries M, Messenger AG. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175(4):762–767. [DOI] [PubMed] [Google Scholar]
- 103.Debroy Kidambi A, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177(1):260–261. [DOI] [PubMed] [Google Scholar]
- 104.Iorizzo M, Tosti A. Frontal Fibrosing Alopecia: An Update on Pathogenesis, Diagnosis, and Treatment. Am J Clin Dermatol. 2019;20(3):379–390. [DOI] [PubMed] [Google Scholar]
- 105.Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case-control study. Clin Exp Dermatol. 2019;44(4):404–410. [DOI] [PubMed] [Google Scholar]
- 106.Robinson G, McMichael A, Wang SQ, Lim HW. Sunscreen and frontal fibrosing alopecia: A review. J Am Acad Dermatol. 2020;82(3):723–728. [DOI] [PubMed] [Google Scholar]
- 107.Kannan S, Lim HW. Photoprotection and vitamin D: a review. Photodermatol Photoimmunol Photomed. 2014;30(2–3):137–145. [DOI] [PubMed] [Google Scholar]
- 108.Lim HW, Gilchrest BA, Cooper KD, et al. Sunlight, tanning booths, and vitamin D. J Am Acad Dermatol. 2005;52(5):868–876. [DOI] [PubMed] [Google Scholar]
- 109.Lim HW, Schneider SL. Sun Safety Practices-Progress Made, More to Go. JAMA Dermatol. 2017;153(5):379–380. [DOI] [PubMed] [Google Scholar]
- 110.LoPiccolo MC, Lim HW. Vitamin D in health and disease. Photodermatol Photoimmunol Photomed. 2010;26(5):224–229. [DOI] [PubMed] [Google Scholar]
- 111.Vanchinathan V, Lim HW. A dermatologist’s perspective on vitamin D. Mayo Clin Proc. 2012;87(4):372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sage RJ, Lim HW. Therapeutic Hotline: Recommendations on photoprotection and vitamin D. Dermatol Ther. 2010;23(1):82–85. [DOI] [PubMed] [Google Scholar]
- 113.Lim HW, Carucci JA, Spencer JM, Rigel DS. Commentary: A responsible approach to maintaining adequate serum vitamin D levels. J Am Acad Dermatol. 2007;57(4):594–595. [DOI] [PubMed] [Google Scholar]
- 114.Horricks RA, Tabin SK, Edwards JJ, Lumsden JS, Marancik DP. Organic ultraviolet filters in nearshore waters and in the invasive lionfish (Pterois volitans) in Grenada, West Indies. PLoS One. 2019;14(7):e0220280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mitchelmore CL, He K, Gonsior M, et al. Occurrence and distribution of UV-filters and other anthropogenic contaminants in coastal surface water, sediment, and coral tissue from Hawaii. Sci Total Environ. 2019;670:398–410. [DOI] [PubMed] [Google Scholar]
- 116.Zhang Z, Ren N, Li YF, Kunisue T, Gao D, Kannan K. Determination of benzotriazole and benzophenone UV filters in sediment and sewage sludge. Environ Sci Technol. 2011;45(9):3909–3916. [DOI] [PubMed] [Google Scholar]
- 117.Danovaro R, Bongiorni L, Corinaldesi C, et al. Sunscreens cause coral bleaching by promoting viral infections. Environ Health Perspect. 2008;116(4):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch Environ Contam Toxicol. 2016;70(2):265–288. [DOI] [PubMed] [Google Scholar]
- 119.Corinaldesi C, Marcellini F, Nepote E, Damiani E, Danovaro R. Impact of inorganic UV filters contained in sunscreen products on tropical stony corals (Acropora spp.). Sci Total Environ. 2018;637–638:1279–1285. [DOI] [PubMed] [Google Scholar]
- 120.Sanchez-Quiles D, Tovar-Sanchez A. Sunscreens as a source of hydrogen peroxide production in coastal waters. Environ Sci Technol. 2014;48(16):9037–9042. [DOI] [PubMed] [Google Scholar]
- 121.Sendra M, Sanchez-Quiles D, Blasco J, et al. Effects of TiO2 nanoparticles and sunscreens on coastal marine microalgae: Ultraviolet radiation is key variable for toxicity assessment. Environ Int. 2017;98:62–68. [DOI] [PubMed] [Google Scholar]
- 122.Hughes TP, Kerry JT, Baird AH, et al. Global warming impairs stock-recruitment dynamics of corals. Nature. 2019;568(7752):387–390. [DOI] [PubMed] [Google Scholar]
- 123.Fisher R, Bessell-Browne P, Jones R. Synergistic and antagonistic impacts of suspended sediments and thermal stress on corals. Nat Commun. 2019;10(1):2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Slattery M, Pankey MS, Lesser MP. Annual Thermal Stress Increases a Soft Coral’s Susceptibility to Bleaching. Scientific reports. 2019;9(1):8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheng L, Abraham J, Hausfather Z, Trenberth KE. How fast are the oceans warming? Science. 2019;363(6423):128–129. [DOI] [PubMed] [Google Scholar]
- 126.Tsui MMP, Lam JCW, Ng TY, Ang PO, Murphy MB, Lam PKS. Occurrence, Distribution, and Fate of Organic UV Filters in Coral Communities. Environ Sci Technol. 2017;51(8):4182–4190. [DOI] [PubMed] [Google Scholar]
- 127.Wu MH, Xie DG, Xu G, et al. Benzophenone-type UV filters in surface waters: An assessment of profiles and ecological risks in Shanghai, China. Ecotoxicol Environ Saf. 2017;141:235–241. [DOI] [PubMed] [Google Scholar]
- 128.Sirois J Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211–212. [DOI] [PubMed] [Google Scholar]
- 129.Raffa RB, Pergolizzi JV Jr., Taylor R Jr., Kitzen JM. Sunscreen bans: Coral reefs and skin cancer. J Clin Pharm Ther. 2019;44(1):134–139. [DOI] [PubMed] [Google Scholar]
- 130.Singer S, Karrer S, Berneburg M. Modern sun protection. Curr Opin Pharmacol. 2019;46:24–28. [DOI] [PubMed] [Google Scholar]
- 131.Kabir Y, Seidel R, McKnight B, Moy R. DNA repair enzymes: an important role in skin cancer prevention and reversal of photodamage--a review of the literature. J Drugs Dermatol. 2015;14(3):297–303. [PubMed] [Google Scholar]
- 132.Emanuele E, Spencer JM, Braun M. An experimental double-blind irradiation study of a novel topical product (TPF 50) compared to other topical products with DNA repair enzymes, antioxidants, and growth factors with sunscreens: implications for preventing skin aging and cancer. J Drugs Dermatol. 2014;13(3):309–314. [PubMed] [Google Scholar]
- 133.Wang DY, Fu B, Tong SM, Ying SH, Feng MG. Two Photolyases Repair Distinct DNA Lesions and Reactivate UVB-Inactivated Conidia of an Insect Mycopathogen under Visible Light. Appl Environ Microbiol. 2019;85(4):e02459–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weber S Light-driven enzymatic catalysis of DNA repair: a review of recent biophysical studies on photolyase. Biochim Biophys Acta. 2005;1707(1):1–23. [DOI] [PubMed] [Google Scholar]
- 135.Faraji S, Dreuw A. Physicochemical mechanism of light-driven DNA repair by (6–4) photolyases. Annu Rev Phys Chem. 2014;65:275–292. [DOI] [PubMed] [Google Scholar]
- 136.Faraji S, Dreuw A. Insights into Light-driven DNA Repair by Photolyases: Challenges and Opportunities for Electronic Structure Theory. Photochem Photobiol. 2017;93(1):37–50. [DOI] [PubMed] [Google Scholar]
- 137.Komori H, Masui R, Kuramitsu S, et al. Crystal structure of thermostable DNA photolyase: pyrimidine-dimer recognition mechanism. Proc Natl Acad Sci U S A. 2001;98(24):13560–13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Essen LO. Photolyases and cryptochromes: common mechanisms of DNA repair and light-driven signaling? Curr Opin Struct Biol. 2006;16(1):51–59. [DOI] [PubMed] [Google Scholar]
- 139.Kavakli IH, Ozturk N, Gul S. DNA repair by photolyases. Adv Protein Chem Struct Biol. 2019;115:1–19. [DOI] [PubMed] [Google Scholar]
- 140.Bhatia N, Berman B, Ceilley RI, Kircik LH. Understanding the Role of Photolyases: Photoprotection and Beyond. J Drugs Dermatol. 2017;16(5):61–66. [PubMed] [Google Scholar]
- 141.Edlich RF, Winters KL, Lim HW, et al. Photoprotection by sunscreens with topical antioxidants and systemic antioxidants to reduce sun exposure. J Long Term Eff Med Implants. 2004;14(4):317–340. [DOI] [PubMed] [Google Scholar]
- 142.Duteil L, Esdaile J, Maubert Y, et al. A method to assess the protective efficacy of sunscreens against visible light-induced pigmentation. Photodermatol Photoimmunol Photomed. 2017;33(5):260–266. [DOI] [PubMed] [Google Scholar]
- 143.Saewan N, Jimtaisong A. Natural products as photoprotection. J Cosmet Dermatol. 2015;14(1):47–63. [DOI] [PubMed] [Google Scholar]
- 144.Santoro FA, Lim HW. Update on photodermatoses. Semin Cutan Med Surg. 2011;30(4):229–238. [DOI] [PubMed] [Google Scholar]
- 145.Nahhas AF, Abdel-Malek ZA, Kohli I, Braunberger TL, Lim HW, Hamzavi IH. The potential role of antioxidants in mitigating skin hyperpigmentation resulting from ultraviolet and visible light-induced oxidative stress. Photodermatol Photoimmunol Photomed. 2019;35(6):420–428. [DOI] [PubMed] [Google Scholar]
- 146.Lim HW. Pathogenesis of photosensitivity in the cutaneous porphyrias. J Invest Dermatol. 2005;124(1):xvi–xvii. [DOI] [PubMed] [Google Scholar]
- 147.Grether-Beck S, Marini A, Jaenicke T, Krutmann J. Photoprotection of human skin beyond ultraviolet radiation. Photodermatol Photoimmunol Photomed. 2014;30(2–3):167–174. [DOI] [PubMed] [Google Scholar]
- 148.Jagdeo J, Brody N. Complementary antioxidant function of caffeine and green tea polyphenols in normal human skin fibroblasts. J Drugs Dermatol. 2011;10(7):753–761. [PubMed] [Google Scholar]
- 149.Jagdeo J, Adams L, Lev-Tov H, Sieminska J, Michl J, Brody N. Dose-dependent antioxidant function of resveratrol demonstrated via modulation of reactive oxygen species in normal human skin fibroblasts in vitro. J Drugs Dermatol. 2010;9(12):1523–1526. [PubMed] [Google Scholar]
- 150.Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for Erythropoietic Protoporphyria. New England Journal of Medicine. 2015;373(1):48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Grimes PE, Hamzavi I, Lebwohl M, Ortonne JP, Lim HW. The efficacy of afamelanotide and narrowband UV-B phototherapy for repigmentation of vitiligo. JAMA Dermatol. 2013;149(1):68–73. [DOI] [PubMed] [Google Scholar]
- 152.Kohli I, Shafi R, Isedeh P, et al. The impact of oral Polypodium leucotomos extract on ultraviolet B response: A human clinical study. J Am Acad Dermatol. 2017;77(1):33–41. e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Solano F Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules. 2020;25(7):1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mohammad TF, Kohli I, Nicholson CL, et al. Oral Polypodium Leucotomos Extract and Its Impact on Visible Light-Induced Pigmentation in Human Subjects. J Drugs Dermatol. 2019;18(12):1198–1203. [PubMed] [Google Scholar]
- 155.Parrado C, Mascaraque M, Gilaberte Y, Juarranz A, Gonzalez S. Fernblock (Polypodium leucotomos Extract): Molecular Mechanisms and Pleiotropic Effects in Light-Related Skin Conditions, Photoaging and Skin Cancers, a Review. Int J Mol Sci. 2016;17(7):1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nahhas AF, Oberlin DM, Braunberger TL, Lim HW. Recent Developments in the Diagnosis and Management of Photosensitive Disorders. Am J Clin Dermatol. 2018;19(5):707–731. [DOI] [PubMed] [Google Scholar]
- 157.Dawe R An overview of the cutaneous porphyrias. F1000Res. 2017;6:1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pourciau CY, Eide MJ, Mahan M, Lim HW. Photoprotection counseling of non-white ethno-racial groups: a survey of the practice of expert dermatologists. Photodermatol Photoimmunol Photomed. 2012;28(6):335–337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


