Abstract
Background:
Therapeutic applications of light emitting diode-red light (LED-RL) are expanding, yet data on its clinical effects are lacking.
Objectives:
To evaluate the safety of high fluence LED-RL (≥160 J/cm2).
Methods:
In two phase I, single-blind, dose escalation, randomized controlled trials, healthy subjects received LED-RL or mock irradiation to the forearm thrice weekly for three weeks at fluences of 160 to 640 J/cm2 for all skin types (STARS 1, n=60) and at 480 to 640 J/cm2 for non-Hispanic Caucasians (STARS 2, n=55). The primary outcome was the incidence of adverse events (AEs). The maximum tolerated dose was the highest fluence that did not elicit predefined AEs.
Results:
Dose-limiting AEs, including blistering and prolonged erythema, occurred at 480 J/cm2 in STARS 1 (n=1) and 640 J/cm2 in STARS 2 (n=2). AEs of transient erythema and hyperpigmentation were mild. No serious AEs occurred.
Conclusions:
LED-RL is safe up to 320 J/cm2 for skin of color and 480 J/cm2 for non-Hispanic Caucasian individuals. LED-RL may exert differential cutaneous effects depending on race and ethnicity, with darker skin being more photosensitive. These findings may guide future studies to evaluate the efficacy of LED-RL for the treatment of various diseases.
Trial Registration:
Keywords: Low-Level Light Therapy, Phototherapy, Skin Pigmentation, Randomized Controlled Trial
Graphical Abstract
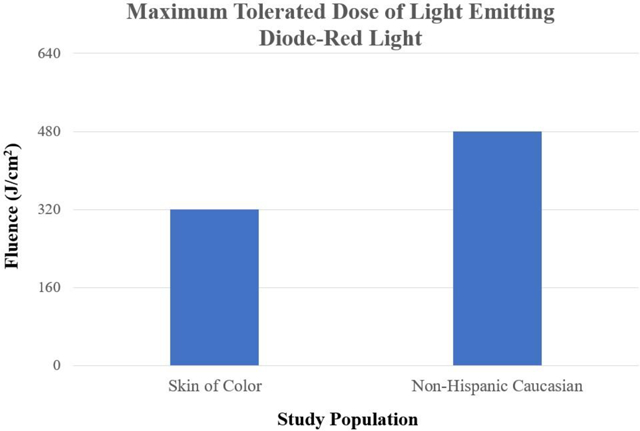
Light emitting diode (LED) technology has therapeutic applications for a wide range of medical conditions. These are the first clinical investigations into the safety of LED-red light (LED-RL) on human skin at fluences above 160 J/cm2. In two phase I, single-blind, dose escalation, randomized controlled trials, we found that LED-RL is safe up to 320 J/cm2 for skin of color and 480 J/cm2 for non-Hispanic Caucasian individuals. High fluence LED-RL may exert differential cutaneous effects depending on race and ethnicity.
Introduction
Medical light emitting diode (LED) technology has evolved rapidly since its introduction in the 1990s, paralleling increased research interest in photobiomodulation and patient demand for non-invasive treatment options [1,2]. LEDs are of increasing importance as a therapeutic modality in medical and cosmetic dermatology, as different wavelengths can alter skin physiology to provide beneficial cutaneous effects [1,3–5]. In 2017, dermatologic surgeons performed over 3.2 million procedures using lasers, lights, and energy-based devices, including LED phototherapy [6]. LED devices are commercially available for home use and have clearance by the U.S. Food and Drug Administration (FDA) for skin conditions including acne vulgaris and facial rhytides [4,7].
Visible light (400–700 nm) is ubiquitous in the environment and comprises 44% of the total solar radiation, yet its biological effects on the skin are not fully understood [8,9]. Red light (630–700 nm) has the greatest tissue penetration of the visible spectrum, reaching the entirety of the dermis at a depth of up to 6 mm [1,10,11]. Light emitting diode-red light (LED-RL) phototherapy has been demonstrated to be safe and effective for wound healing, hair regrowth, acne vulgaris, skin rejuvenation, oral mucositis, skin cancers, and premalignant skin lesions [1,4,12–18]. LED-RL activates cell signaling pathways that modulate skin cell function and translate to clinical benefits such as tissue regeneration and skin rejuvenation [19–21]. LED-RL has the advantages of being non-invasive with deep dermal penetration, non-ablative, cost-effective with no consumables, portable, easy to operate with customizable settings, and combinable with other treatments [4,22]. Furthmore, LED-RL is not associated with ultraviolet (UV)-related side effects including sunburn, premature photoaging, and DNA damage associated with increased risk of skin cancer [9,23–26]. The peak power output (power density) of LEDs is significantly lower than lasers, resulting in slower delivery of the same wavelengths of light and less potential harm to the skin [2,27].
Despite the expanding clinical applications of LED-RL, the safety profile is poorly characterized. There is a paucity of randomized controlled trials evaluating the effects of LED-RL administered at various treatment parameters, with studies being limited to assessments of LED-RL at fluences ≤126 J/cm2 [4]. The biological effects of LED-RL depend on irradiation parameters such as fluence (dose), power density, treatment time (duration of irradiation), delivery pattern (pulsed versus continuous), and treatment regimen (frequency of administration) [27]. To date, no clinical trials have been performed to assess the safety and tolerability of high-fluence LED-RL (HF-LED-RL) phototherapy on human skin [28].
Herein, we conducted two randomized controlled trials using a dose-escalation study design to evaluate the clinical cutaneous effects of HF-LED-RL. The aim of these two studies – Safety Trial Assessing Red-light on Skin (STARS) 1 and STARS 2 – was to characterize the safety profile of HF-LED-RL at fluences of 160 J/cm2 up to 640 J/cm2, as well as determine the maximum tolerated dose (MTD), in healthy subjects of diverse racial and ethnic backgrounds.
Methods
Study population.
Healthy subjects of any sex and age were recruited from the Sacramento VA Medical Center, Mather, CA. STARS 1 was conducted from December 1, 2015 to August 15, 2016. STARS 2 was conducted from February 1, 2018 to May 25, 2018. Major exclusion criteria included diabetes, history of skin cancer, lupus, light-sensitive conditions, skin disease at the treatment site, and photosensitizing medications. In STARS 2, subjects must have identified as non-Hispanic Caucasian according to the National Institute of Health’s definitions for racial and ethnic categories [29]. All subjects were screened for photosensitivity with a 20-minute LED-RL treatment session at a fluence of 106.7 J/cm2, followed by evaluation for signs and symptoms of photosensitivity (i.e., erythema, edema, rash, or pain/discomfort) 24 hours later.
Study design.
Two phase I, single-blind, dose escalation, randomized controlled trials were conducted to evaluate the safety of LED-RL administered three times weekly, a standard phototherapy regimen, for three consecutive weeks [30,31]. The complete study protocols are published elsewhere and available for reference [30,32]. The treatment site was the non-dominant proximal volar forearm. The maximum recommended starting dose (MRSD) of 160 J/cm2 was based on previously published maximum doses of LED-RL phototherapy that demonstrated safety without adverse events (AEs) [22,33]. The study endpoint of 640 J/cm2 was a practical limit based on the treatment duration required to deliver this fluence (two hours at a power density of 872 W/m2). Subjects were blinded to the treatment throughout the study.
Dose escalation protocol.
Following the dose escalation algorithm, subjects were enrolled sequentially in groups of five (Figure 1). Using a computer-based randomization program, subjects were randomly allocated in a 3:2 ratio to the experimental group (LED-RL phototherapy) or control group (mock therapy). In STARS 1, subjects in Group 1 received the MRSD of 160 J/cm2, equivalent to 30 minutes of irradiation. The dose was escalated in subsequent groups using the classical method for dose escalation as described by Spilker: starting dose (X) increased by an equal amount such that X=160 J/cm2, 2X=320 J/cm2, 3X=480 J/cm2, and 4X=640 J/cm2 [34]. In STARS 2, Group 1 received 480 J/cm2 and Group 2 received 640 J/cm2. Common expected post-treatment outcomes included mild warmth, erythema, and edema lasting less than 24 hours. The MTD was defined as the fluence level below the dose producing dose-limiting AEs. In these studies, a dose-limiting AE was defined as any of the following unacceptable but reversible AEs: second-degree or higher skin burning, blistering, erythema lasting more than 24 hours, severe swelling, pain, ulceration, change in sensation, and muscle weakness.
Figure 1.
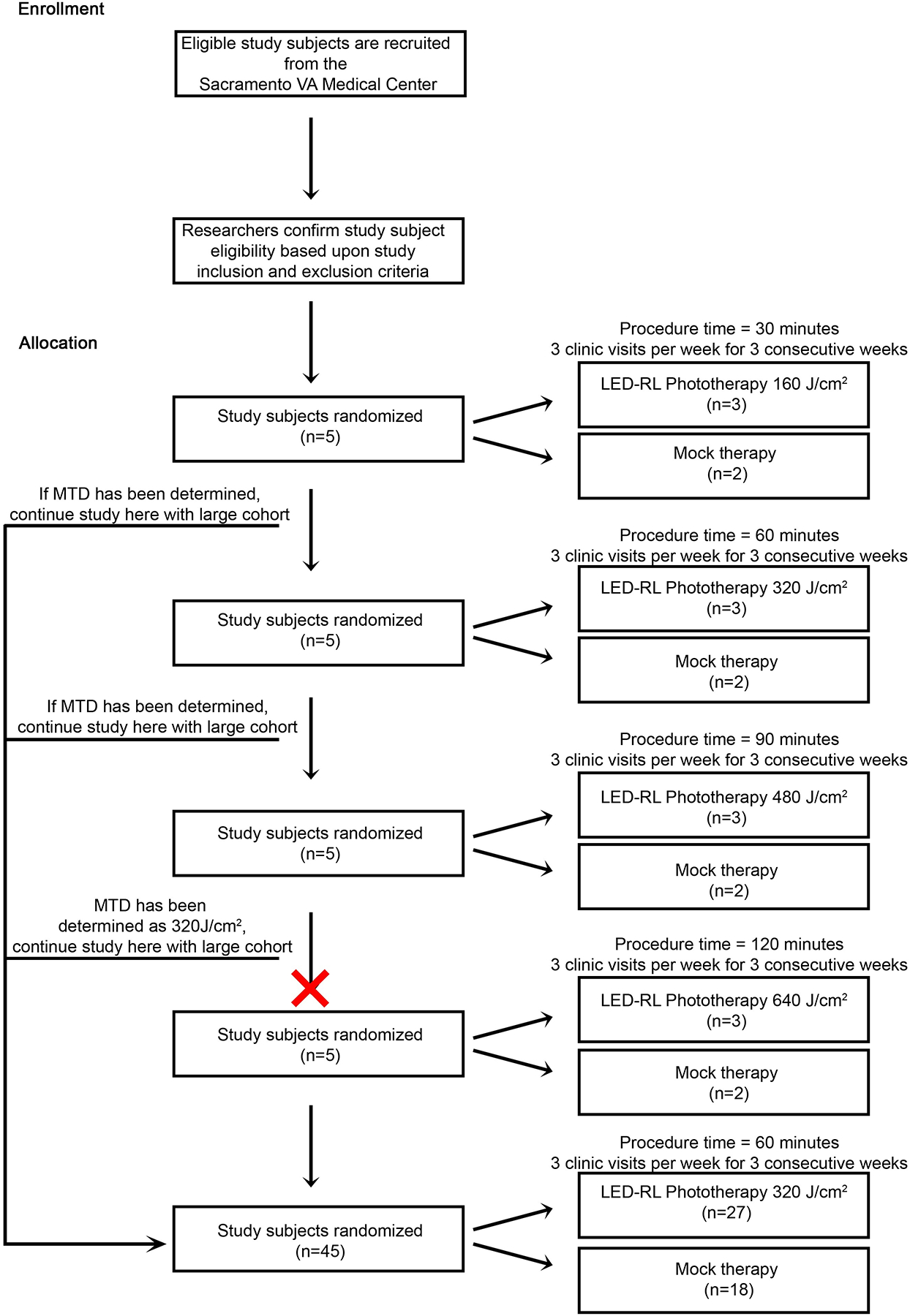
Study design schematic and dose escalation algorithm for STARS 1.
For STARS 1, if one or more subject experienced a dose-limiting AE, then this was the dose one level above the MTD and we did not proceed with dose escalation. To account for potential outlier effects in STARS 2, if two or more subjects experienced a dose-limiting AE, then this was the dose one level above the MTD, consistent with the conventional “3+3” dose escalation study design (Figure 2). However, if only one subject experienced a dose-limiting AE, this dose was repeated in a new cohort of five subjects.
Figure 2.
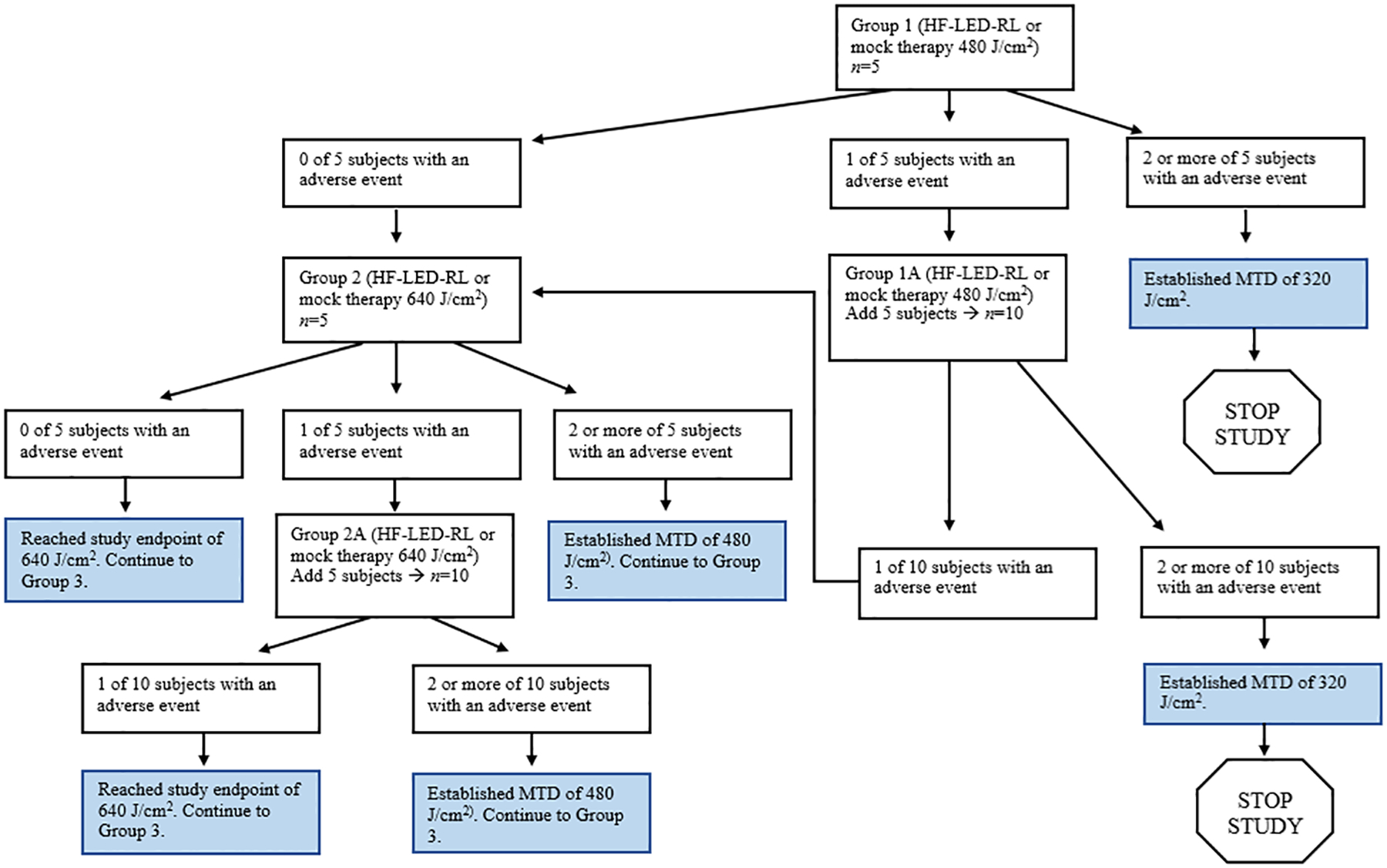
Study design schematic and dose escalation algorithm for STARS 2.
After either an MTD was established or the study endpoint of 640 J/cm2 was achieved, an additional 45 subjects (n=27 in the treatment group and n=18 in the control group) were enrolled and received the MTD. For this larger MTD cohort, the study would be halted if the incidence of AEs equaled or exceeded 30%.
Study intervention.
The source of LED-RL was the Omnilux new-U handheld LED device (GlobalMed Technologies, Glen Ellen, CA), which has a 4.7 cm × 6.1 cm rectangular aperture emitting red light (633±6 nm; average power density of 872 W/m2 measured 5 mm from the skin surface). It is FDA-cleared for the treatment of periorbital rhytides and may be placed in direct contact with the skin [35]. The mock therapy device (GlobalMed Technologies, Glen Ellen, CA) was identical-appearing and temperature-matched (i.e., generated thermal output comparable to the heat emitted by the LED-RL device), containing disabled LEDs that did not emit red light. Protective eyewear was provided during the treatment sessions.
Sample size calculation.
The MTD cohorts comprised of additional 27 LED-RL phototherapy subjects (for a total of 30) and 18 mock therapy subjects (for a total of 20) enrolled to satisfy Hanley’s “rule of three,” such that it can be concluded with 95% confidence that fewer than 1 person in 10 will experience an AE [36]. That is, if none of the 30 subjects in the treatment group experience an AE, we could be 95% confident that the risk of an AE is at most 10% (i.e., 3 over n=30).
Statistics.
Summary statistics of AEs were recorded. Summary statistics of age and average duration of erythema were reported as mean ± SD. All patients who met eligibility criteria and received any treatment during the trial were included in an intent-to-treat analysis. The Wilcoxon rank-sum test and t-test were used, respectively, to compare the age and resolution of erythema between the LED-RL and mock procedures. Fisher’s exact test was used to compare the frequency of a categorical variable between the LED-RL and mock procedures. Subgroup analysis was performed to identify potential differential effects with respect to gender, age, race, and ethnicity. Age subgroups consisted of <65 or ≥65 years. Race subgroups consisted of Caucasian, African-American, Asian, or Native Hawaiian/Pacific Islander. Ethnicity consisted of Hispanic and Latino or non-Hispanic. P-values ≤ 0.05 were considered statistically significant. Data analyses were performed in August 2016 and May 2018 using SAS, Version 9.4 (SAS Institute Inc., Cary, NC, USA).
Study approval.
The studies were conducted according to Declaration of Helsinki principles. The clinical trials were approved by the Institutional Review Board at the Sacramento VA Medical Center (reference #15-12-00756 and #18-01-00804). Written informed consent was obtained from study participants and compensation was provided for participation.
Results
Characteristics of the study population.
Summary demographic information of enrolled subjects is presented in Table 1. There were no significant differences in age, race/ethnicity, or sex between the treatment arms in both trials. In STARS 1, a total of 60 subjects were enrolled and 57 subjects completed the study (Figure 3). In STARS 2, a total of 55 non-Hispanic Caucasian subjects were enrolled and 42 subjects completed the study (Figure 4).
Table 1.
Demographics of enrolled subjects who received at least one treatment.
| STARS 1 | STARS 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Total (n = 60) | HF-LED-RL (n = 36) | Mock (n = 24) | P-value | Total (n = 54)† | HF-LED-RL (n = 33) | Mock (n = 21) | P-value |
| Age, years | 55.1 ± 13.0 | 54.7 ± 11.5 | 55.6 ± 15.2 | 0.63 | 56.2 ± 15.5 | 55.0 ± 16.0 | 58.0 ± 14.7 | 0.53 |
| Sex | ||||||||
| Male | 44 (73.3%) | 26 (72.2%) | 18 (75.0%) | 1.00 | 40 (74.0%) | 23 (69.7%) | 17 (81.0%) | 0.53 |
| Female | 16 (26.7%) | 10 (27.8%) | 6 (25.0%) | 14 (26.0%) | 10 (30.3%) | 4 (19.0%) | ||
| Race and ethnicity | ||||||||
| Caucasian, Not Hispanic or Latino | 36 (60.0%) | 19 (52.8%) | 17 (70.8%) | 0.75 | 54 (100%) | 33 (100%) | 21 (100%) | N/A |
| Caucasian, Hispanic or Latino | 4 (6.7%) | 3 (8.3%) | 1 (4.2%) | -- | -- | -- | ||
| Black or African American | 16 (26.7%) | 11 (30.6%) | 5 (20.8%) | -- | -- | -- | ||
| Asian | 3 (5.0%) | 2 (5.6%) | 1 (4.2%) | -- | -- | -- | ||
| Native Hawaiian or Other Pacific Islander | 1 (1.7%) | 1 (2.8%) | 0 (0.0%) | -- | -- | -- | ||
Age is presented as mean ± SD. Categorical variables are presented as n (%). P-values were calculated by two-sided Wilcoxon ranked sum test for age and Fisher’s exact test for categorical variables. P-values ≤ 0.05 were considered statistically significant.
HF-LED-RL: high fluence light-emitting diode-red light
A total of 55 subjects were enrolled in STARS 2, but 1 subject did not receive the assigned treatment since the intervention was halted per the study protocol.
Figure 3.
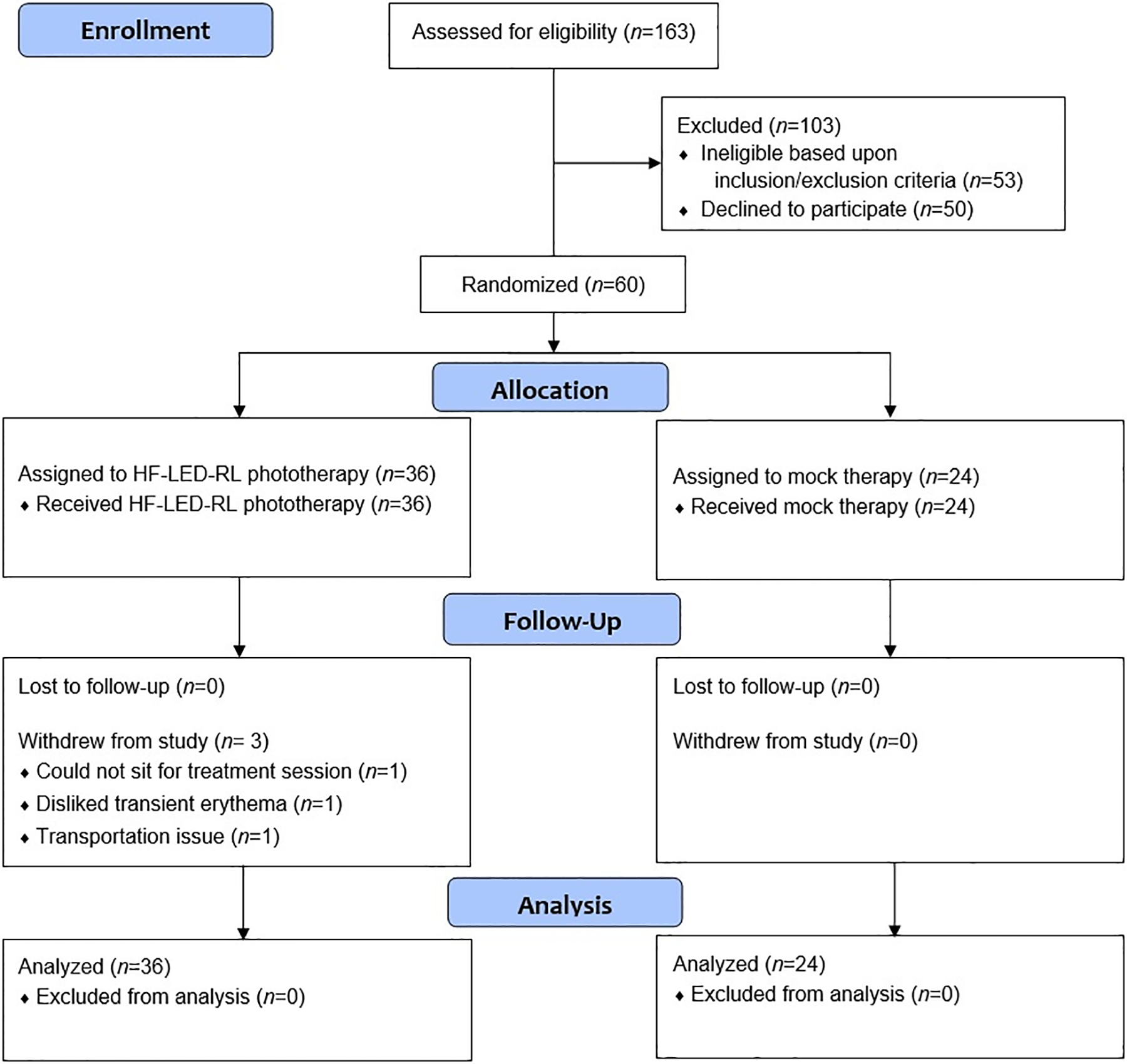
CONSORT flow diagram for STARS 1.
Figure 4.
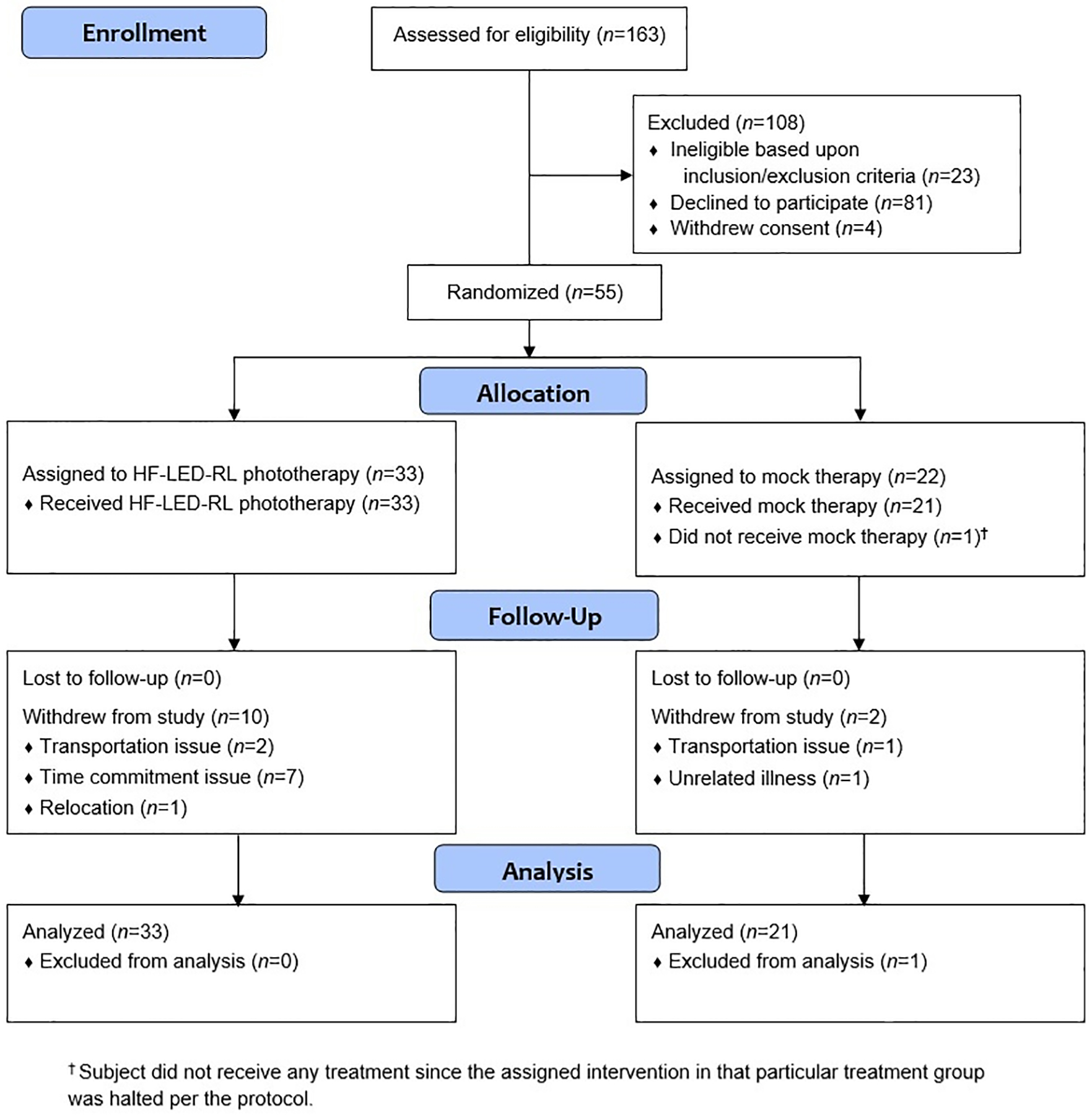
CONSORT flow diagram for STARS 2.
LED-RL at fluences up to 320 J/cm2 is safe for all races and ethnicities.
In STARS 1, we determined the MTD using a dose-escalation study design. All subjects in Group 1 (160 J/cm2) and Group 2 (320 J/cm2) completed the treatment with no dose-limiting AEs. In Group 3 (480 J/cm2), one African American subject experienced a dose-limiting AE, with the development of a 5 mm blister on the irradiated skin after the first HF-LED-RL phototherapy session (Figure 5A). A punch biopsy of the lesional and peri-lesional skin demonstrated a blister with focal subepidermal clefting and re-epithelization of the blister edges with no significant inflammatory dermal infiltrate (Figure 5B and 5C). The other non-Hispanic Caucasian subjects treated at 480 J/cm2 tolerated HF-LED-RL without AEs. Due to this dose-limiting AE, no subjects were enrolled to receive the escalated dose of 640 J/cm2. Therefore, in STARS 1, HF-LED-RL at 320 J/cm2 was the highest fluence to not elicit a dose-limiting AE and was deemed to be safe for all skin types, regardless of race and ethnicity.
Figure 5.
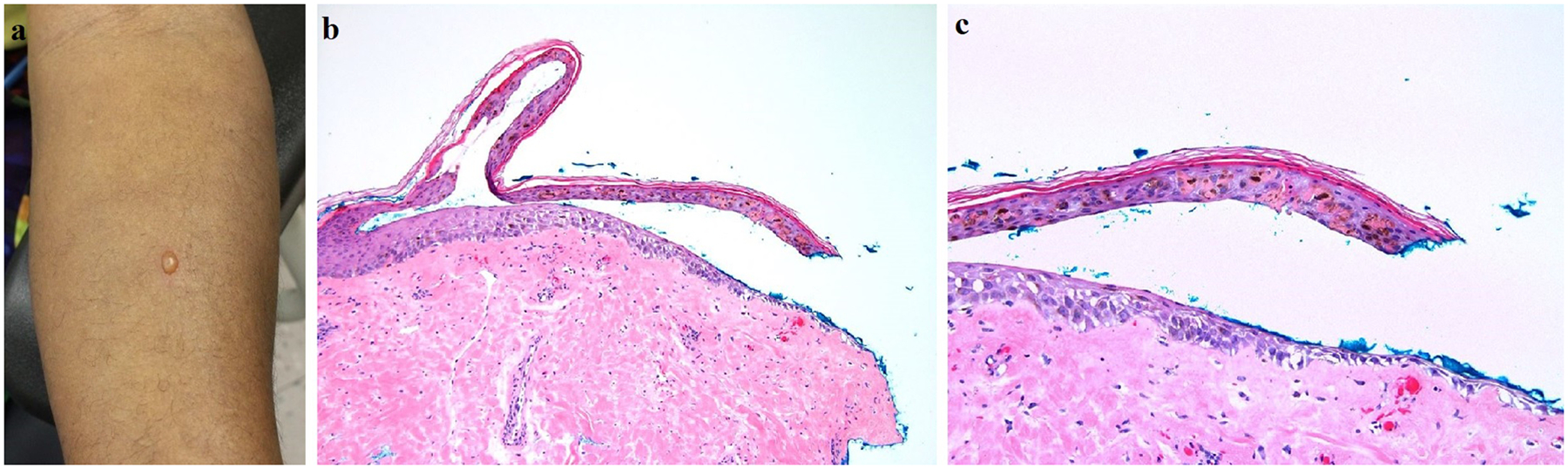
(A) Development of a 5 mm blister on the volar forearm of an African American subject after receiving the first HF-LED-RL treatment session (480 J/cm2). (B) Biopsy of the blister edge shows a subepidermal split with partial re-epithelialization and no significant underlying inflammatory infiltrate (hematoxylin & eosin, 100x). (C) On higher magnification, necrotic keratinocytes within the blister roof are evident, along with clumping of melanin pigment (hematoxylin & eosin, 200x).
Non-Hispanic Caucasian subjects have a higher maximum tolerated dose of 480 J/cm2.
We then sought to determine the MTD exclusively in non-Hispanic Caucasian individuals, since this population may be less photosensitive to HF-LED-RL than skin of color patients based on established clinical practice for visible light delivered by laser therapy [37]. In STARS 2, all subjects in Group 1 (480 J/cm2) completed the treatment period without the occurrence of a dose-limiting AE. In Group 2 (640 J/cm2), two subjects had dose-limiting AEs following the first HF-LED-RL phototherapy session; one subject had prolonged erythema (defined as skin redness lasting more than 24 hours) and the other subject developed two blisters (1.2 × 0.7 cm and 1.0 × 0.9 cm) at the treatment site in addition to prolonged erythema. Due to these dose-limiting AEs, further phototherapy sessions at 640 J/cm2 were halted. Given the occurrence of dose-limiting AEs at 640 J/cm2, the MTD for non-Hispanic Caucasian subjects was determined to be 480 J/cm2.
Subjects in the maximum tolerated dose cohorts experienced mild adverse events of hyperpigmentation and prolonged post-treatment erythema.
There were no serious or treatment-emergent AEs. In the MTD cohort of 320 J/cm2 in STARS 1, significantly more subjects who received HF-LED-RL developed treatment-site erythema at any visit than subjects who received mock therapy (P=0.002) (Table 2). However, all subjects in the MTD cohort of 480 J/cm2 in STARS 2 developed erythema immediately post-treatment, irrespective of the type of study intervention (HF-LED-RL versus mock therapy) (Table 2). The average duration of erythema following each treatment administration was significantly longer in subjects who received HF-LED-RL (P=0.007 and 0.0007 in STARS 1 and STARS 2, respectively), but was non-significant in subgroup analysis by sex, age, and race/ethnicity.
Table 2.
Summary of the adverse events in the HF-LED-RL and mock therapy groups in the MTD cohorts.
| STARS 1 (320 J/cm2) | STARS 2 (480 J/cm2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adverse event | HF-LED-RL (n = 30) | Mock (n = 20) | P-value | HF-LED-RL (n = 30) | Mock (n = 20) | P-value | ||||
| n | % | n | % | n | % | n | % | |||
| Any erythema | 0.002 | 1.000 | ||||||||
| + | 28 | 93.3 | 10 | 50.0 | 30 | 100.0 | 20 | 100.0 | ||
| − | 2 | 6.7 | 10 | 50.0 | 0 | 0.0 | 0 | 0 | ||
| Erythema lasting more than 24 hours | 1.000 | 0.075 | ||||||||
| + | 1 | 3.3 | 0 | 0.0 | 5 | 16.7 | 0 | 0.0 | ||
| − | 29 | 96.7 | 20 | 100.0 | 25 | 83.3 | 20 | 100.0 | ||
| Hyperpigmentation | 0.003 | 0.002 | ||||||||
| + | 10 | 33.3 | 0 | 0.0 | 11 | 36.7 | 0 | 0.0 | ||
| − | 20 | 66.7 | 20 | 100.0 | 19 | 63.3 | 20 | 100.0 | ||
All P-values were determined using Fisher’s exact test. P-values ≤ 0.05 were considered statistically significant.
(+): present; (−): absent; HF-LED-RL: high fluence light-emitting diode-red light; MTD: maximum tolerated dose
In STARS 1, cutaneous AEs were noted in 11 subjects (23%) treated with HF-LED-RL at a fluence of 320 J/cm2, all of which were considered grade 1 in severity and resolved without permanent sequelae. One Caucasian subject (2%) had painless erythema that lasted longer than 24 hours, but less than 48 hours after the first HF-LED-RL session. Ten skin of color subjects (21%) had hyperpigmentation, which resolved within three months after the last treatment session.
In STARS 2, the following grade 1 cutaneous AEs occurred in 14 subjects (28%) treated with HF-LED-RL at 480 J/cm2: one subject (2%) developed prolonged erythema, 8 subjects (16%) developed hyperpigmentation, 4 subjects (8%) developed both prolonged erythema and hyperpigmentation, and one subject (2%) developed prolonged erythema and an 8 mm blister. The subject who developed a blister in this cohort had unintentionally folded her arms during the treatment session and added pressure to the LED-RL device, which decreased the distance between the LED-RL source and her skin, thereby increasing the effective power density and dose of light delivered (i.e., greater than 480 J/cm2). All AEs had improved or completely resolved at follow-up one to three months after study completion.
In both trials, there was no significant difference in the incidence of prolonged erythema between the HF-LED-RL and mock irradiation groups (Table 2). However, subjects in the HF-LED-RL group had a significantly greater incidence of hyperpigmentation than control subjects (P=0.003 and 0.002 in STARS 1 and STARS 2, respectively) (Table 2). Hyperpigmentation was clinically more prominent in darker skin compared to lighter skin. Among subjects who received HF-LED-RL in both trials, the occurrence of prolonged erythema or hyperpigmentation was non-significant in subgroup analysis by sex, age, and race/ethnicity.
Discussion
STARS 1 and STARS 2 are the first randomized controlled trials investigating the safety of HF-LED-RL phototherapy on human skin. The results from these trials are significant and potentially paradigm-changing to current clinical practice as our findings highlight key safety features of visible red light for dermatological use.
Our results indicate that HF-LED-RL is safe at fluences up to 480 J/cm2 in non-Hispanic Caucasian skin (traditionally Fitzpatrick skin phototypes I-III) and at fluences up to 320 J/cm2 in skin of color (traditionally Fitzpatrick skin phototypes IV-VI), with fluences beyond the MTD eliciting dose-limiting AEs such as blistering [38,39]. Given the excellent safety profile of LED-RL established by prior clinical studies, it is surprising that our study identified the potential need to optimize LED-RL treatment parameters based upon race and ethnicity, due to the possible increased risk of side effects. Our finding is congruent with a recent study that investigated the cutaneous responses to broad-spectrum visible light, wherein photoprotected skin was irradiated with 480 J/cm2 of visible light daily for four days [40]. While visible light treatment induced no significant clinical or histological changes in lighter skin, darker skin types did not tolerate the full treatment regimen (i.e., blistering occurred in all darker skin subjects at total doses of 220–880 J/cm2) and the MTD correlated with individual typology angle, an objective measurement of melanization [40]. These results, combined with our observation, suggest that visible light including LED-RL exerts differential biological effects depending on cutaneous pigmentation, race and/or ethnicity.
The observed 50% difference in MTDs based on race/ethnicity (320 J/cm2 vs. 480 J/cm2) indicates that skin of color individuals are more photosensitive to red light than non-Hispanic Caucasians and is likely influenced by a variety of factors including pigmentation, race, and ethnicity [8,41,42]. It is well established that laser-delivered light at higher power densities generates differential effects based on pigmentation, with skin of color being more photosensitive and at increased risk of AEs such as blistering and hyperpigmentation [43]. Increased epidermal melanin content in darker skinned individuals may alter the absorption of visible light energy emitted by LEDs and lasers intended for another target, leading to hyperpigmentation of the irradiated area, thus requiring the use of more conservative treatment parameters and lower energy settings for skin of color [43]. Therefore, while increased skin pigmentation may offer protection against the deleterious effects of certain wavelengths (e.g., UV), it may confer an increased risk of cutaneous side effects at wavelengths in the visible light spectrum [23,44–46].
Clinical responses to LED-RL phototherapy may differ according to race and ethnicity more so than to pigmentation alone, as the biology of skin color is complex and genetic factors influence how light energy affects skin cells [39,47,48]. Few definitive conclusions about racial and ethnic differences in cutaneous photobiology can be made due to a historical lack of well-controlled studies in skin of color individuals. The published literature shows that racial and ethnic differences in human skin color are due to variations in epidermal melanin content and the quantity, quality (e.g., size and shape), and distribution of melanosomes [39,42,49]. For example, skin of color has been shown to have larger, individual dispersed melanosomes with higher melanin content compared to the aggregated, smaller melanosomes with less melanin content found in fair skin [39,47]. For these reasons, we categorized patients based upon race and ethnicity rather than by pigmentation as determined by Fitzpatrick skin phototype or colorimetry.
Visible light radiation exerts biologic effects on the skin including erythema, pigmentation, thermal damage, and free radical production [8,41,50]. Visible light from LED sources can induce immediate erythema and immediate, delayed and sustained pigmentation in exposed skin in a dose-dependent manner [23,41,51–54]. A study investigating the impact of visible light on melanocompetent skin (Fitzpatrick skin phototypes IV-VI) showed that pigmentation induced by visible light was darker and more sustained compared to long-wavelength UVA, and that the cutaneous response depends on skin type, as no pigmentation was induced on skin type II [51].
In both STARS1 and STARS 2, the majority of subjects (50–100% across all treatment groups) experienced post-treatment erythema, regardless of the intervention, fluence, and race/ethnicity. We also found a significantly higher incidence of hyperpigmentation in the HF-LED-RL group compared to mock therapy, and that more prominent hyperpigmentation was observed in darker skin compared to lighter skin. We propose that the blister formation observed in three study subjects developed as a result of LED-RL interaction with the skin, as the temperature-matched mock LED device did not induce a similar reaction in control subjects who received equivalent treatment parameters. Similar to our results, broad-spectrum visible light at cumulative doses of 220 J/cm2 to 880 J/cm2 induced pigmentation and blistering in all skin of color subjects in one recent study, but no significant clinical or histological changes occurred in lighter skin [40]. Thus, HF-LED-RL-induced phototoxicity that resulted in blistering may have been influenced by epidermal melanin content [40].
One strength of these clinical trials is that the study design incorporated patient-reported outcomes in addition to direct clinical assessment. The study subjects used a diary to characterize and quantify the frequency and severity of AEs as a safety endpoint, which is critically important in interpreting clinical outcomes, especially for studies evaluating therapies that are designed for at-home use [55]. Furthermore, it utilized stringent definitions for dose-limiting AEs, such as prolonged erythema and blistering. Since safety was the primary outcome measure and the incidence of AEs was previously unknown for the study intervention, the study was powered according to Hanley’s “rule of three”, which states that if none of n subjects (for this study, n=30 in the MTD treatment cohort in each trial) experiences an AE, then the upper 95% confidence limit of this rate is 3 in n (i.e., 10%) [36]. This concept is especially important in the interpretation of clinical trials because a finding of 0 events in a sample of n subjects can lead to a false and potentially dangerous underestimation of true risk in the general population. Since we observed AEs, Hanley’s rule of three was not applicable during the safety analysis.
Several limitations exist in this study. The true MTD may exist between 320 J/cm2 and 480 J/cm2 for skin of color and between 480 J/cm2 and 640 J/cm2 for non-Hispanic Caucasian skin. Another limitation is the single-center study design. Despite possible skewed representation of age, gender and race/ethnicity in the general veteran population, we still had a diverse group of study subjects [56]. Device limitations were also present as the LED output varied by up to 10% in power density (acceptable range considered 5–10% due to inherent variability in LED performance) [57,58].
Our findings may serve as the foundation for future photobiomodulation studies to evaluate the clinical efficacy of LED-RL for the treatment of skin diseases. Additional studies are warranted to evaluate the safety of HF-LED-RL administered at different treatment parameters (e.g., higher intensity or more frequent dosing) to establish comprehensive safety parameters, with particular attention to individual factors such as race/ethnicity and skin pigmentation. As LED-RL clinical indications and implementation expand, we envision that LED-RL will be well positioned for patients who seek non-invasive treatment in-office or at home.
Acknowledgments
We thank the study subjects at the Sacramento VA Medical Center for their support. The project was supported by the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under Award No. K23GM117309. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This material is the result of work supported with resources and facilities use at the VA Northern California Health Care System. The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government. Dr. Jagdeo, Dr. Mamalis, and Dr. Isseroff have a patent titled “Methods for in vitro inhibition of fibroblast proliferation” (US patent no. 9861832). All other authors have no financial disclosures relevant to the work reported in this manuscript.
References
- [1].Opel DR, Hagstrom E, Pace AK, Sisto K, Hirano-Ali SA, Desai S, Swan J, J. Clin. Aesthet. Dermatol 2015, 8, 36. [PMC free article] [PubMed] [Google Scholar]
- [2].Sorbellini E, Rucco M, Rinaldi F, Lasers Med. Sci 2018, 33, 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chung H, Dai T, Sharma S, Huang Y-Y, Carroll J, Hamblin M, Ann. Biomed Eng 2012, 40, 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jagdeo J, Austin E, Mamalis A, Wong C, Ho D, Siegel DM, Lasers Surg. Med 2018, 50, 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim W-S, Calderhead RG, Laser Ther 2011, 20, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].2017 ASDS Survey on Dermatologic Procedures. American Society for Dermatologic Surgery; [Cited October 12, 2018]. Available at: https://www.asds.net/Medical-Professionals/Practice-Resources/ASDS-Survey-on-Dermatologic-Procedures. [Google Scholar]
- [7].Hession MT, Markova A, Graber EM, Dermatologic Surg 2015, 41, 307. [DOI] [PubMed] [Google Scholar]
- [8].Mahmoud BH, Hexsel CL, Hamzavi IH, Lim HW, Photochem. Photobiol 2008, 84, 450. [DOI] [PubMed] [Google Scholar]
- [9].Liebel F, Kaur S, Ruvolo E, Kollias N, Southall MD, J. Invest. Dermatol 2012, 132, 1901. [DOI] [PubMed] [Google Scholar]
- [10].Sakamoto FH, Avram MM, Anderson RR, in: Bolognia J, Schaffer J, Cerroni L (Eds.), Dermatology, 4th ed., Elsevier, Philadelphia, 2018, pp. 2354–2363. [Google Scholar]
- [11].Li Y, Zhang J, Xu Y, Han Y, Jiang B, Huang L, Zhu H, Xu Y, Yang W, Qin C, PLoS One 2016, 11, e0157898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yadav A, Gupta A, Photodermatol. Photoimmunol. Photomed 2017, 33, 4. [DOI] [PubMed] [Google Scholar]
- [13].Wunsch A, Matuschka K, Photomed. Laser Surg 2014, 32, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim M-S, Cho Y-I, Kook M-S, Jung S-C, Hwang Y-H, Kim B-H, Int. J. Photoenergy 2015, 2015, 1. [Google Scholar]
- [15].Kim HS, Park WS, Baek JI, Lee BS, Yoo DS, Park SJ, Int. J. Mol. Med 2015, 35, 383. [DOI] [PubMed] [Google Scholar]
- [16].Ablon G, J. Clin. Aesthet. Dermatol 2018, 11, 21. [PMC free article] [PubMed] [Google Scholar]
- [17].Mignon C, Botchkareva NV, Uzunbajakava NE, Tobin DJ, Exp. Dermatol 2016, 25, 745. [DOI] [PubMed] [Google Scholar]
- [18].Myakishev-Rempel M, Stadler I, Brondon P, Axe DR, Friedman M, Nardia FB, Lanzafame R, Photomed. Laser Surg 2012, 30, 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Barolet D, Roberge CJ, Auger FA, Boucher A, Germain L, J. Invest. Dermatol 2009, 129, 2751. [DOI] [PubMed] [Google Scholar]
- [20].Almeida Issa MC, Piñeiro-Maceira J, Farias RE, Pureza M, Raggio Luiz R, Manela-Azulay M, Br. J. Dermatol 2009, 161, 647. [DOI] [PubMed] [Google Scholar]
- [21].Calderhead RG, Laser Ther 2007, 16, 97. [Google Scholar]
- [22].Sadick NS, J. Cosmet. Dermatol 2008, 7, 263. [DOI] [PubMed] [Google Scholar]
- [23].Randhawa M, Seo I, Liebel F, Southall MD, Kollias N, Ruvolo E, PLoS One 2015, 10, e0130949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mamalis A, Siegel D, Jagdeo J, Curr. Dermatol. Rep 2016, 5, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Del Bino S, Sok J, Bernerd F, Br. J. Dermatol 2013, 168, 1120. [DOI] [PubMed] [Google Scholar]
- [26].Matsumura Y, Ananthaswamy HN, Expert Rev. Mol. Med 2002, 4, 1. [DOI] [PubMed] [Google Scholar]
- [27].Barolet D, Semin. Cutan. Med. Surg 2008, 27, 227. [DOI] [PubMed] [Google Scholar]
- [28].Posadzki P, Car J, JAMA Dermatology 2018, 154, 597. [DOI] [PubMed] [Google Scholar]
- [29].Racial and Ethnic Categories and Definitions for NIH Diversity Programs and for Other Reporting Purposes. National Institutes of Health; [Cited February 21, 2019]. Available at: https://grants.nih.gov/grants/guide/notice-files/not-od-15-089.html. [Google Scholar]
- [30].Ho D, Kraeva E, Wun T, Isseroff RR, Jagdeo J, Trials 2016, 17, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Anderson KL, Feldman SR, J. Am. Acad. Dermatol 2015, 72, 868. [DOI] [PubMed] [Google Scholar]
- [32].Wang EB, Kaur R, Nguyen J, Ho D, Austin E, Maverakis E, Li C, Hwang ST, Isseroff RR, Jagdeo J, Trials 2019, 20, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sadick NS, J. Drugs Dermatol 2008, 7, 347. [PubMed] [Google Scholar]
- [34].Spilker B, Guide to Clinical Trials, Raven Press, New York, NY, 1991. [Google Scholar]
- [35].Photo Therapeutics Inc, Omnilux New-U User Guide, 2008.
- [36].Hanley JA, Lippman-Hand A, JAMA 1983, 249, 1743. [PubMed] [Google Scholar]
- [37].Anderson R, Parrish J, Science (80-.) 1983, 220, 524. [DOI] [PubMed] [Google Scholar]
- [38].He SY, McCulloch CE, Boscardin WJ, Chren M-M, Linos E, Arron ST, J. Am. Acad. Dermatol 2014, 71, 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Taylor SC, J. Am. Acad. Dermatol 2002, 46, 41. [DOI] [PubMed] [Google Scholar]
- [40].Lim HY, Kerns ML, Kang S, Chien AL, J. Invest. Dermatol 2018, 138, S194. [Google Scholar]
- [41].Sklar LR, Almutawa F, Lim HW, Hamzavi I, Photochem. Photobiol. Sci 2013, 12, 54. [DOI] [PubMed] [Google Scholar]
- [42].Alaluf S, Atkins D, Barrett K, Blount M, Carter N, Heath A, Pigment Cell Res 2002, 15, 112. [DOI] [PubMed] [Google Scholar]
- [43].Jackson BA, J. Am. Acad. Dermatol 2003, 48, 134. [DOI] [PubMed] [Google Scholar]
- [44].Brenner M, Hearing VJ, Photochem. Photobiol 2008, 84, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Del Bino S, Bernerd F, Br. J. Dermatol 2013, 169, 33. [DOI] [PubMed] [Google Scholar]
- [46].Del Bino S, Sok J, Bessac E, Bernerd F, Pigment Cell Res 2006, 19, 606. [DOI] [PubMed] [Google Scholar]
- [47].Rawlings AV, Int. J. Cosmet. Sci 2006, 28, 79. [DOI] [PubMed] [Google Scholar]
- [48].Berardesca E, Maibach H, J. Am. Acad. Dermatol 1996, 34, 667. [DOI] [PubMed] [Google Scholar]
- [49].Van Nieuwpoort F, Smit NPM, Kolb R, Van Der Meulen H, Koerten H, Pavel S, J. Invest. Dermatol 2004, 122, 1251. [DOI] [PubMed] [Google Scholar]
- [50].Kohli I, Chaowattanapanit S, Mohammad TF, Nicholson CL, Fatima S, Jacobsen G, Kollias N, Lim HW, Hamzavi IH, Br. J. Dermatol 2018, 178, 1173. [DOI] [PubMed] [Google Scholar]
- [51].Mahmoud BH, Ruvolo E, Hexsel CL, Liu Y, Owen MR, Kollias N, Lim HW, Hamzavi IH, J. Invest. Dermatol 2010, 130, 2092. [DOI] [PubMed] [Google Scholar]
- [52].Porges SB, Kaidbey KH, Grove GL, Photodermatol 1988, 5, 197. [PubMed] [Google Scholar]
- [53].Soleymani T, Cohen DE, Folan LM, Okereke UR, Elbuluk N, Soter NA, J. Drugs Dermatol 2017, 16, 1105. [PubMed] [Google Scholar]
- [54].Kollias N, Baqer A, Photochem. Photobiol 1984, 39, 651. [DOI] [PubMed] [Google Scholar]
- [55].U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health, Health Qual. Life Outcomes 2006, 4, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Veteran Population Projections 2017–2037. National Center for Veterans Analysis and Statistics; [Cited February 23, 2019]. Available at: https://www.va.gov/vetdata/docs/demographics/new_vetpop_model/vetpop_infographic_final31.pdf. [Google Scholar]
- [57].Bürmen M, Pernu F, Likar B, Meas. Sci. Technol 2008, 19,. [Google Scholar]
- [58].Variability in LED Production and the Impact on Performance. Luger Research e.U. [Cited March 8, 2019]. Available at: https://www.led-professional.com/resources-1/articles/variability-in-led-production-and-the-impact-on-performance. [Google Scholar]


