Abstract
Ethnicity might be associated with treatment outcomes in advanced prostate cancer. This study aimed to evaluate the efficacy and safety of androgen deprivation therapy (ADT) combined with apalutamide in East Asians with metastatic castration-sensitive prostate cancer (mCSPC). The original phase 3 Targeted Investigational Treatment Analysis of Novel Anti-androgen (TITAN) trial was conducted at 260 sites in 23 countries. This subgroup analysis included patients enrolled in 62 participating centers in China, Japan, and Korea. Radiographic progression-free survival (PFS), time to prostate-specific antigen (PSA) progression, and PSA changes from baseline were compared between groups in the East Asian population. The intent-to-treat East Asian population included 111 and 110 participants in the apalutamide and placebo groups, respectively. The 24-month radiographic PFS rates were 76.1% and 52.3% in the apalutamide and placebo groups, respectively (apalutamide vs placebo: hazard ratio [HR] = 0.506; 95% confidence interval [CI], 0.302–0.849; P = 0.009). Median time to PSA progression was more favorable with apalutamide than placebo (HR = 0.210; 95% CI, 0.124–0.357; P < 0.001). Median maximum percentages of PSA decline from baseline were 99.0% and 73.9% in the apalutamide and placebo groups, respectively. The most common adverse event (AE) was rash in the apalutamide group, with a higher rate than that in the placebo group (37.3% vs 9.1%). The most common grade 3 or 4 AEs were rash (12 [10.9%]) and hypertension (12 [10.9%]) for apalutamide. The efficacy and safety of apalutamide in the East Asian subgroup of the TITAN trial are consistent with the global results.
Keywords: apalutamide, East Asia, metastasis, prostatic neoplasm, survival
INTRODUCTION
Globally, prostate cancer is the second most frequent cancer and the fifth leading cause of cancer death in men.1 In East Asia, while the incidence rate is lower compared with those of Western countries, an increase in incidence has been observed in the past decades, and mortality and incidence rates remain high, with a high proportion of cases diagnosed at an advanced disease stage.2,3,4,5 Novel treatment options are therefore urgently needed for East Asian patients.
The conventional standard of care for metastatic prostate cancer is androgen-deprivation therapy (ADT).6,7,8 Combination therapy of ADT plus either docetaxel or abiraterone acetate plus prednisone/prednisolone is currently recommended for high-risk/high-volume disease in patients with metastatic castration-sensitive prostate cancer (mCSPC).6,7,8 Recently, next-generation androgen receptor (AR) inhibitors, including enzalutamide and apalutamide, have emerged as alternative therapeutics that could improve survival regardless of disease volume compared with ADT monotherapy in prostate cancer.9,10 These new agents are also recommended in the guidelines.6,11
Apalutamide is an oral competitive inhibitor of AR.12,13 A phase I study in castration-resistant prostate cancer (CRPC) examined the safety, tolerability, pharmacokinetics, and pharmacodynamics of apalutamide in the dose range of 30–480 mg.14 Later, the phase 3 Selective Prostate Androgen Receptor Targeting with ARN-509 (SPARTAN) trial showed that metastasis-free survival was more than 2 years longer with apalutamide compared with placebo in nonmetastatic CRPC at a dose of 240 mg per day.15 Apalutamide was firstly approved for nonmetastatic CRPC in February 2018.16,17 The phase 3 Targeted Investigational Treatment Analysis of Novel Anti-androgen (TITAN) trial was designed to determine whether apalutamide combined with ADT would prolong survival in patients with mCSPC compared to ADT monotherapy. Finally, at 260 sites in 23 countries, 525 participants received apalutamide plus ADT, and 527 received a placebo plus ADT. The results indicated that addition of apalutamide to ADT results in significantly longer radiographic progression-free survival (PFS) and overall survival (OS) compared with placebo plus ADT in patients with mCSPC.18 Radiographic progression is defined by the Prostate Cancer Clinical Trials Working Group 2 (PCWG2) as an endpoint of choice for prostate cancer.19 Nevertheless, previous studies demonstrated that ethnicity may be associated with treatment outcomes in advanced prostate cancer,20,21,22 and no data is available for East Asian patients with mCSPC treated with ADT plus apalutamide.
Therefore, the present study aimed to evaluate the efficacy and safety of ADT in combination with apalutamide in East Asian patients with mCSPC by performing a subgroup analysis of the multinational phase 3 TITAN trial, which has already been published.18,23 The results could provide evidence for the management of East Asian patients with mCSPC.
PATIENTS AND METHODS
Study design
The original phase 3 TITAN trial was designed by Janssen Research and Development (Spring House, PA, USA) and conducted at 260 sites in 23 countries.18 The present substudy included patients enrolled in the 62 participating centers in East Asia (China, Japan, and Korea). The original study and this sub-study were approved by the ethics committee of each participating center (No. 2016-YW-008-003 from Sun Yat-Sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China; No. [2016](31)-10 from Wuhan Tongji Hospital, Tongji Medical College, Wuhan, China; No. XJTU1AF2016LSY-37—10 from First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China; and No. 1604159-9-2010J from Fudan University Shanghai Cancer Center, Shanghai, China). All the patients provided written informed consent. The entire description of the original trial and the related protocol are available from the published report18 at NEJM.org.
Patients
Inclusion criteria were: (1) documented prostate adenocarcinoma; (2) documented distant metastasis, with at least one lesion on bone scanning, irrespective of visceral or lymph node metastases; (3) Eastern Cooperative Oncology Group (ECOG) performance status of 0–1; and (4) castration-sensitive, i.e., not receiving ADT at the time of progression.6,24 Permitted previous treatments were limited to docetaxel (≤6 cycles, without progression before randomization), ADT for no more than 6 months in the metastatic setting or no more than 3 years for localized disease, a maximum of one course of radiation therapy or surgery for metastatic disease-related symptoms, and other localized treatments completed at least 12 months before randomization. The patients administered a gonadotropin-releasing hormone agonist within 28 days had to take a first-generation antiandrogen agent for at least 14 days before randomization.25 The first-generation antiandrogen therapy had to be discontinued before randomization. Exclusion criteria were: (1) severe angina, myocardial infarction, or congestive heart failure within 6 months of randomization; (2) arterial or venous thromboembolic event within 6 months of randomization; (3) a history of or predisposition to seizure; or (4) recent ventricular arrhythmias.
Treatment
The patients were randomized 1:1 to the ADT plus apalutamide and ADT plus placebo groups. Apalutamide (240 mg) or placebo was administered orally once a day. The original randomization scheme stratified the patients according to the Gleason score (≤7 vs >7), geographic region (North America and European Union vs all other countries), and previous docetaxel use (yes vs no).
Endpoints
The primary endpoints in the original study were radiographic PFS and OS. As the published article is an interim analysis of the phase 3 TITAN trial, median OS was not reached in both groups.18 Besides the primary endpoint of radiographic PFS, time to prostate-specific antigen (PSA) progression and PSA changes from baseline were compared between the two groups. Radiographic PFS was defined as the time from randomization to the first documented radiographic disease progression or death from any cause, whichever occurred first. Time to PSA progression was defined as the time from randomization to date of PSA progression based on PCWG2 criteria.19
Assessments
Radiographic progression of soft-tissue lesions was assessed according to modified Response Evaluation Criteria in Solid Tumors, version 1.1, using computed tomography (CT) or magnetic resonance imaging (MRI).19 PSA progression was determined based on PCWG2 criteria.19 Baseline images were acquired within 6 weeks before randomization. Bone scanning combined with CT or MRI was performed on cycles 3 and 5 and every four cycles thereafter. Adverse events (AEs) were recorded and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.3 (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm).
Statistical analyses
The original trial was adequately powered.18 A post hoc power analysis showed that this subgroup analysis had a >95% power based on the 24-month radiographic PFS rate. Baseline characteristics were summarized by descriptive statistics. Continuous variables were tested for normality of distribution by the Kolmogorov–Smirnov test. Those with normal distribution were presented as mean ± standard deviation; otherwise, they were presented as median (range). Categorical variables were presented as number (percentage). Time-to-event variables were estimated by the Kaplan–Meier method and compared by the stratified log-rank test and the Cox proportional-hazards model. Stratification was performed according to the Gleason score at diagnosis (≤7 vs >7), geographic region (North America and European Union vs all other countries), and previous docetaxel use (yes vs no). Two-sided P < 0.05 was considered statistically significant.
RESULTS
Characteristics of the East Asian participants
The intent-to-treat East Asian population included 111 participants in the apalutamide group and 110 in the placebo group. Median follow-up was 21.2 (range: 1.0–33.0) months in the apalutamide group, versus 20.3 (range: 4.6–32.1) months in the placebo group. The median numbers of treatment cycles were 21 (range: 1–33) and 19 (range: 1–33) in the apalutamide and placebo groups, respectively. The median treatment durations were 19.2 (range: 0.2–33.0) months for apalutamide and 17.3 (range: 0.1–32.1) months for the placebo. At the data cutoff date (November 23, 2018), 76 patients (68.5%) in the apalutamide group and 52 (47.3%) in the placebo group were still on treatment (Figure 1).
Figure 1.
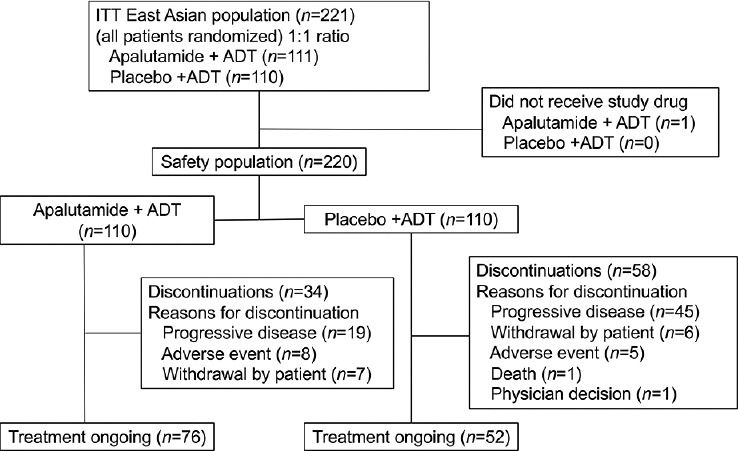
Study flowchart. ITT: intent-to-treat; ADT: androgen-deprivation therapy.
The median age of the overall East Asian patient population was 70 years. A total of 12 (5.4%) patients had undergone prostatectomy or received radiotherapy, and 14 (6.3%) had received previous docetaxel therapy. A total of 146 (66.1%) patients had a high-volume disease, while 75 (33.9%) had a low-volume disease (Table 1). The proportion of patients with Gleason scores >7 was 86.4% in East Asians, versus 66.3% in the overall population. The proportions of M1 disease at diagnosis were 94.6% and 81.0% in East Asians and the overall population, respectively.
Table 1.
Baseline characteristics (intent-to-treat population)
| Characteristic | East Asian (n=221) | Overall (n=1052) | ||
|---|---|---|---|---|
|
|
|
|||
| Apalutamide (n=111) | Placebo (n=110) | Apalutamide (n=525) | Placebo (n=527) | |
| Age (year), median (range) | 70 (47–89) | 70 (50–85) | 69 (45–94) | 68 (43–90) |
| ECOG performance status, n (%) | ||||
| 0 | 74 (66.7) | 77 (70.0) | 328 (62.5) | 348 (66.0) |
| 1 | 37 (33.3) | 32 (29.1) | 197 (37.5) | 178 (33.8) |
| 2 | 0 (0) | 1 (0.9) | 0 (0) | 1 (0.2) |
| Gleason score at diagnosis, n (%) | ||||
| ≤7 | 15 (13.5) | 15 (13.6) | 174 (33.1) | 169 (32.1) |
| >7 | 96 (86.5) | 95 (86.4) | 348 (66.9) | 349 (67.9) |
| Metastatic stage at diagnosis, n (%) | ||||
| M0 | 3 (2.7) | 3 (2.7) | 85 (16.2) | 59 (11.2) |
| M1 | 104 (93.7) | 105 (95.5) | 411 (78.3) | 441 (83.7) |
| Mx | 4 (3.6) | 2 (1.8) | 29 (5.5) | 27 (5.1) |
| Metastasis site at baseline, n (%) | ||||
| Bone | 111 (100) | 110 (100) | 525 (100) | 527 (100) |
| Bone only | 59 (53.2) | 48 (43.6) | 289 (55.0) | 269 (51.0) |
| Lymph node | 46 (41.4) | 52 (47.3) | 199 (37.9) | 219 (41.6) |
| Visceral | 8 (7.2) | 11 (10.0) | 56 (10.7) | 72 (13.7) |
| Lung | 7 (6.3) | 10 (9.1) | 47 (9.0) | 64 (12.1) |
| Liver | 0 (0) | 1 (0.9) | 12 (2.3) | 13 (2.5) |
| Soft tissue | 5 (4.5) | 8 (7.3) | 22 (4.2) | 27 (5.1) |
| Disease volume, n (%) | ||||
| Low | 37 (33.3) | 38 (34.5) | 200 (38.1) | 192 (36.4) |
| High | 74 (66.7) | 72 (65.5) | 325 (61.9) | 335 (63.6) |
| Prior prostate cancer treatment, n (%) | ||||
| Prostatectomy or radiotherapy | 4 (3.6) | 8 (7.3) | 94 (17.9) | 79 (15.0) |
| Hormonal therapy | 111 (100) | 110 (100) | 525 (100) | 527 (100) |
| Docetaxel | 7 (6.3) | 7 (6.4) | 58 (11.0) | 55 (10.4) |
| Vandetanib | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) |
| PSA level (µg l−1), median (range) | 10.24 (0–2682) | 3.77 (0–803) | 5.97 (0–2682) | 4.02 (0–2229) |
PSA: prostate-specific antigen; ECOG: Eastern Cooperative Oncology Group
Efficacy
At the data cutoff date (November 23, 2018), 63 radiographic progression events were observed in the East Asian population (23 and 40 in the apalutamide and placebo groups, respectively). The 24-month radiographic PFS rates were 76.1% and 52.3% in the apalutamide and placebo groups, respectively (apalutamide vs placebo: hazard ratio [HR] = 0.506; 95% confidence interval [CI], 0.302–0.849; P = 0.009). There was a 49.4% lower risk of radiographic progression in the apalutamide group. In the overall population, the 24-month radiographic PFS rates were 68.2% and 47.5% in the apalutamide and placebo groups, respectively (apalutamide vs placebo: HR = 0.484, 95% CI, 0.391–0.600; P < 0.001). Similar trends of radiographic PFS were observed between the East Asian and overall populations (Figure 2a).
Figure 2.
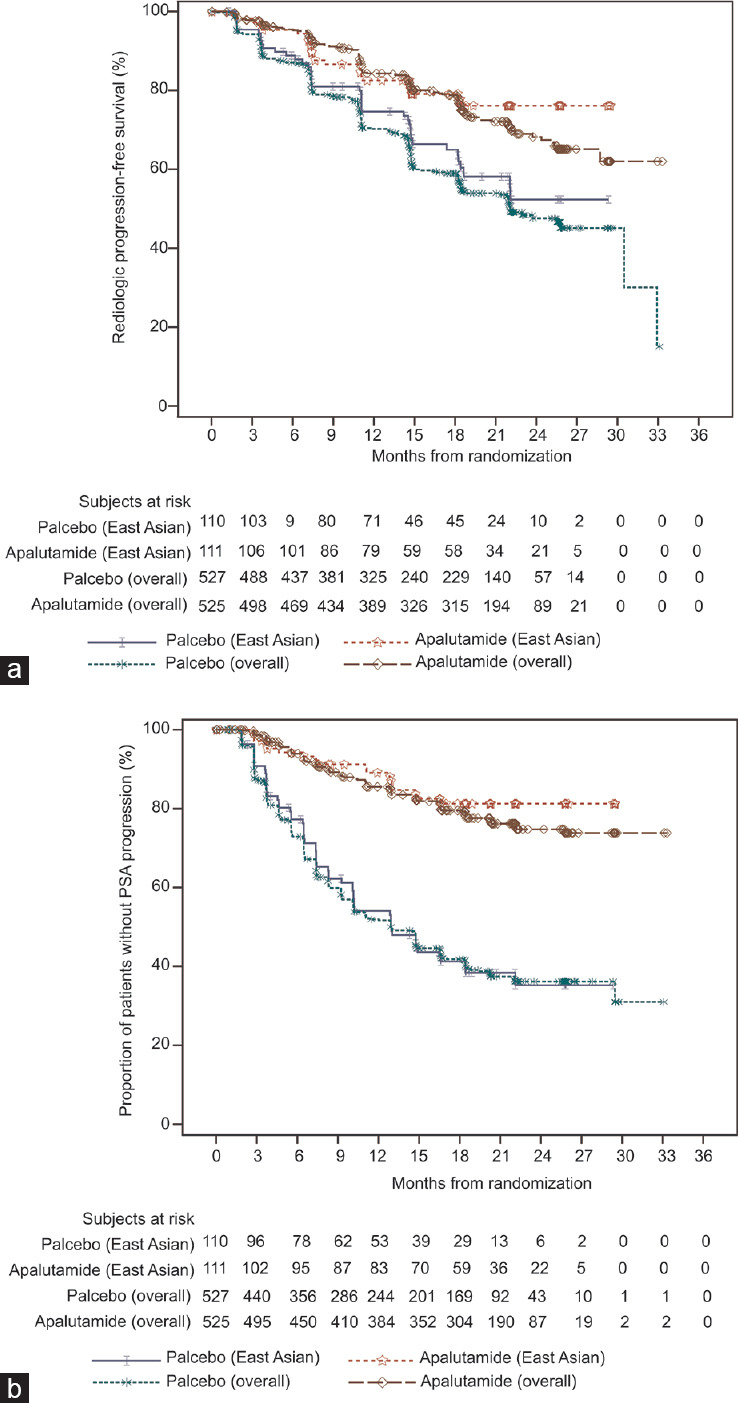
Kaplan–Meier curves. (a) Radiographic progression-free survival in East Asian and overall populations (East Asian: HR = 0.506; 95% confidence interval [CI], 0.302–0.849; P = 0.009; overall: HR = 0.484; 95% CI, 0.391–0.600; P < 0.001). (b) Time to PSA progression in East Asian (HR = 0.210; 95% CI, 0.124–0.357; P < 0.001) and overall (HR = 0.259; 95% CI, 0.207–0.323; P < 0.001) populations. HR: hazard ratio; PSA: prostate-specific antigen.
There were 80 PSA progression events (18 in the apalutamide group and 62 in the placebo group) at the data cutoff date. The PSA progression-free rates at 24 months were 81.2% and 35.2% in the apalutamide and placebo groups, respectively. The median time to PSA progression was more favorable with apalutamide than with placebo (HR = 0.210; 95% CI, 0.124–0.357; P < 0.001). There was a 79.0% lower risk of PSA progression in the apalutamide group. In the overall population, the PSA progression-free rates at 24 months were 74.7% and 36.2% in the apalutamide and placebo groups, respectively (apalutamide vs placebo: HR = 0.259; 95% CI, 0.207–0.323; P < 0.001). Similar trends of time to PSA progression were observed between the East Asian and overall populations (Figure 2b). Regarding PSA change in the East Asian population, median percentages of decline at 12 weeks from baseline were 96.1% and 55.4% in the apalutamide and placebo groups, respectively (Figure 3a and 3b). Median maximum percentages of PSA decline at any time point from baseline were 99.0% and 73.9% for the apalutamide and placebo groups, respectively (Figure 3c and 3d).
Figure 3.
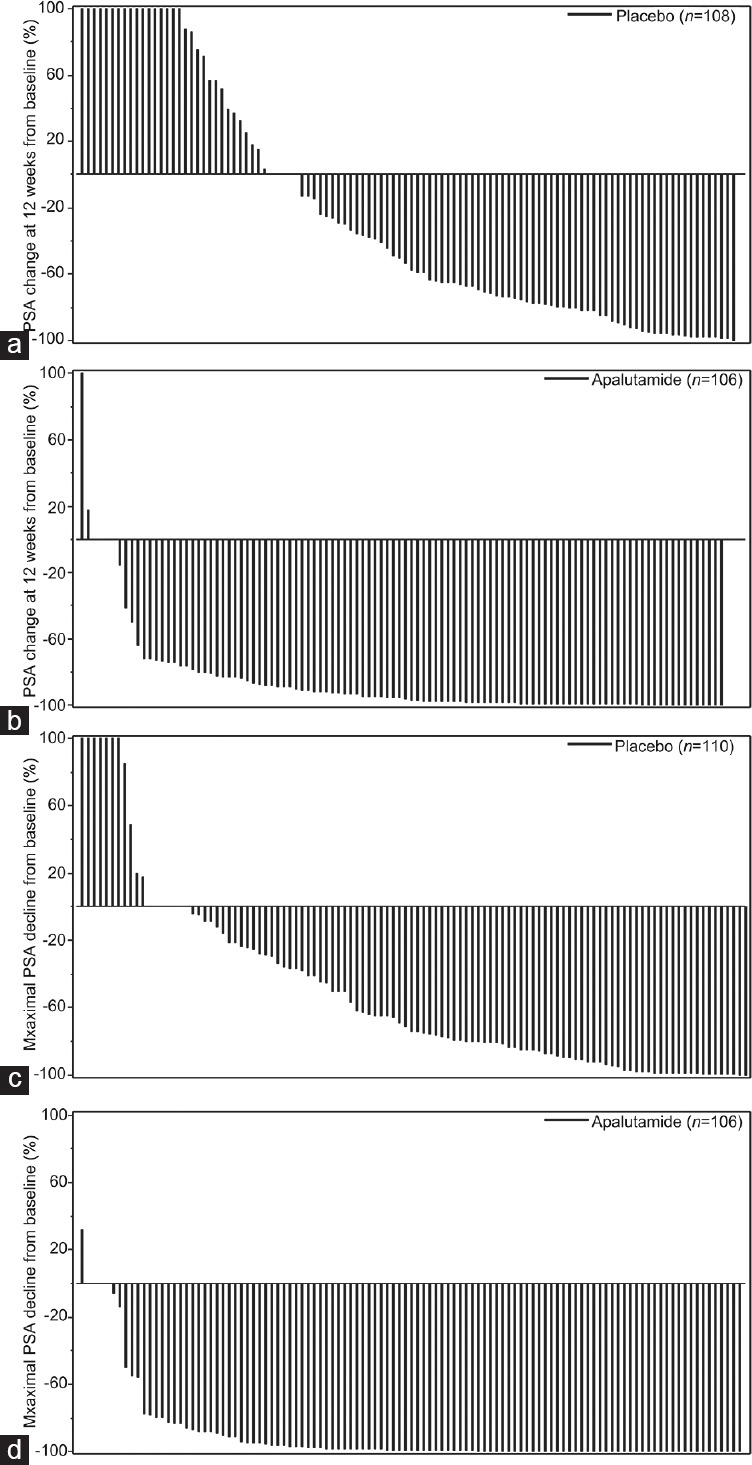
PSA changes from baseline. (a) PSA change at 12 weeks from baseline in the placebo group. (b) PSA change at 12 weeks from baseline in the apalutamide group. (c) Maximum PSA change at any time point from baseline in the placebo group. (d) Maximum PSA change at any time point from baseline in the apalutamide group. PSA: prostate-specific antigen.
Supplementary Table 1 presents the radiographic PFS data of East-Asians (Japan, China, and Korea) and other participants. The HR for radiographic progression with apalutamide was 0.511 (95% CI, 0.305–0.856) in East Asians, which was similar to 0.485 (95% CI, 0.383–0.613) found in other participants.
Supplementary Table 1.
Radiographic progression-free survival in East Asians and the other subjects
| East Asians | Overall but excluding East Asians | |||
|---|---|---|---|---|
|
|
|
|||
| Placebo (n=110) | Apalutamide (n=111) | Placebo (n=417) | Apalutamide (n=414) | |
| Events, n (%) | 40 (36.4) | 23 (20.7) | 191 (45.8) | 111 (26.8) |
| Median rPFS, months (95% CI) | NE (18.40–NE) | NE (NE–NE) | 21.98 (18.33–32.92) | NE (28.71–NE) |
| 6 months rPFS rate (95% CI) | 0.888 (0.811–0.935) | 0.954 (0.892–0.980) | 0.866 (0.829–0.895) | 0.955 (0.929–0.971) |
| 12 months rPFS rate (95% CI) | 0.746 (0.650–0.820) | 0.825 (0.737–0.886) | 0.691 (0.643–0.734) | 0.848 (0.808–0.880) |
| 24 months rPFS rate (95% CI) | 0.523 (0.393–0.638) | 0.761 (0.660–0.836) | 0.463 (0.403–0.521) | 0.665 (0.603–0.720) |
| P a | 0.0094 | <0.0001 | ||
| HR (95% CI)b | 0.511 (0.305–0.856) | 0.485 (0.383–0.613) | ||
aP value from the log-rank test, bHR from the proportional hazards model. HR <1 favors active treatment. The East Asian sub-population included the subjects from Japan, China, and South Korea. rPFS: radiographic progression-free survival; NE: not estimable; HR: hazard ratio; CI: confidence interval
Tolerance and safety
One patient in the apalutamide group did not receive the study drug. The safety population included 110 participants in the apalutamide group and 110 in the placebo group. The proportions of East Asian patients with compliance in taking >95% prescribed drugs were 71.8% and 91.8% in the apalutamide and placebo groups, respectively. Tables 2 and 3 present the AE profiles. The frequencies of grade 3 or 4 AEs (45 [40.9%] in the apalutamide group and 42 [38.2%] in the placebo group) and serious AEs (23 [20.9%] in the apalutamide group and 26 [23.6%] in the placebo group) were similar in both groups. There were eight (7.3%) and five (4.5%) patients with AE leading to treatment discontinuation in the apalutamide and placebo groups, respectively. There were 16 patients (14.5%) with AE leading to dose reduction in the apalutamide group, versus two (1.8%) in the placebo group. Treatment interruption was required in 35 (31.8%) and 10 (9.1%) participants in the apalutamide and placebo groups, respectively. No patient died due to AEs in the apalutamide group, while three (2.7%) died as a result of AEs in the placebo group.
Table 2.
Adverse events in the East Asian population of the targeted investigational treatment analysis of novel anti-androgen trial (safety population)
| Event | Apalutamide (total=110), n (%) | Placebo (total=110), n (%) |
|---|---|---|
| Any AE | 106 (96.4) | 108 (98.2) |
| Drug-related | 77 (70.0) | 55 (50.0) |
| Grade 3 or 4 AE | 45 (40.9) | 42 (38.2) |
| Drug-related | 20 (18.2) | 10 (9.1) |
| Any SAE | 23 (20.9) | 26 (23.6) |
| Drug-related | 3 (2.7) | 2 (1.8) |
| AE leading to treatment discontinuation | 8 (7.3) | 5 (4.5) |
| Drug-related | 4 (3.6) | 1 (0.9) |
| AE leading to death | 0 (0) | 3 (2.7) |
| Drug-related | 0 (0) | 0 (0) |
AE: adverse event; SAE: serious adverse event
Table 3.
Individual adverse events in the East Asian population of the targeted investigational treatment analysis of novel anti-androgen trial (safety population)
| Event | Apalutamide (total=110), n (%) | Placebo (total=110), n (%) | ||
|---|---|---|---|---|
|
|
|
|||
| Any grade | Grade 3 or 4 | Any grade | Grade 3 or 4 | |
| AE reported in ≥10% of patients | ||||
| Hypertension | 25 (22.7) | 12 (10.9) | 16 (14.5) | 9 (8.2) |
| Weight increased | 24 (21.8) | 4 (3.6) | 40 (36.4) | 4 (3.6) |
| Weight decreased | 20 (18.2) | 1 (0.9) | 5 (4.5) | 0 (0) |
| Pruritus | 19 (17.3) | 0 (0) | 9 (8.2) | 0 (0) |
| Hot flush | 18 (16.4) | 0 (0) | 11 (10.0) | 0 (0) |
| Upper respiratory tract infection | 16 (14.5) | 1 (0.9) | 11 (10.0) | 1 (0.9) |
| Viral upper respiratory tract infection | 14 (12.7) | 0 (0) | 13 (11.8) | 0 (0) |
| Arthralgia | 14 (12.7) | 0 (0) | 12 (10.9) | 0 (0) |
| Constipation | 13 (11.8) | 0 (0) | 10 (9.1) | 0 (0) |
| Pain in arm or leg | 12 (10.9) | 0 (0) | 16 (14.5) | 1 (0.9) |
| Rash, generalized | 11 (10.0) | 4 (3.6) | 1 (0.9) | 0 (0) |
| AE of special interest | ||||
| Rash | 41 (37.3) | 12 (10.9) | 10 (9.1) | 1 (0.9) |
| Fall | 3 (2.7) | 0 (0) | 5 (4.5) | 0 (0) |
| Fracture | 6 (5.5) | 1 (0.9) | 7 (6.4) | 2 (1.8) |
| Hypothyroidism | 5 (4.5) | 0 (0) | 0 (0) | 0 (0) |
AE: adverse event
The most common AE in the apalutamide group was rash, with a higher rate compared with that of the placebo group (37.3% vs 9.1%). The most common grade 3 or 4 AEs of apalutamide were rash (12 [10.9%]) and hypertension (12 [10.9%]).
DISCUSSION
Compared with the overall population in the TITAN trial, there were more patients with newly diagnosed mCSPC (without prior prostatectomy, radiotherapy, and docetaxel therapy), more individuals with a more advanced stage (M1 stage) at initial diagnosis, and more high-risk cancer cases (Gleason score >7) in the East Asian population. These findings were consistent with the characteristics of prostate cancer observed in the East Asian population compared with Westerners. Indeed, even though the incidence of prostate cancer is lower in East Asian countries compared with Western nations, disease stage at presentation is more advanced in the East Asian population.2,3,4,5
As an endpoint not affected by postprotocol treatment, radiographic PFS can directly reflect the efficacy of study drugs before discontinuation. The present subgroup analysis showed that the addition of apalutamide to ADT prolonged PFS compared with placebo plus ADT (24-month radiographic PFS rate: 76.1% vs 52.3%) in the East Asian population with mCSPC. The HR (apalutamide vs placebo) for radiographic PFS was 0.506 and similar to that reported in the global TITAN trial (HR = 0.48).18 The TITAN trial demonstrated impressive OS improvements with apalutamide plus ADT compared with placebo plus ADT in patients with mCSPC.18 The prespecified interim analysis limited the follow-up time, and death events were not enough for subgroup analysis. Thus, in the present subgroup analysis, OS was not selected for efficacy analysis. The OS benefit will be verified in the final results of the TITAN trial.
For the tumor marker PSA, the PCWG2 recommended reporting time to PSA progression and PSA changes from baseline. In the East Asian subgroup, the PSA progression-free rate at 24 months was significantly higher with apalutamide plus ADT compared with placebo plus ADT (81.2% vs 35.2%). A similar risk of PSA progression was observed in time to PSA progression in the East Asian and overall populations (HR = 0.210 and 0.259, respectively).18 As there were not enough events in either group regarding other secondary (time to cytotoxic chemotherapy, time to pain progression, time to chronic opioid use, and time to skeletal-related event) and exploratory (time to symptomatic local progression and second PFS) endpoints in the interim analysis of the TITAN trial, the clinical benefits of apalutamide addition to ADT in other aspects will be evaluated in the future.
The frequencies of grade 3 or 4 and serious AEs were similar in the apalutamide and placebo groups. Low discontinuation rates due to AEs were observed in both groups. The safety profile in the East Asian population was consistent with that of the overall population, with the most common AE being rash.18 Higher frequencies of increased weight and rash were reported in East Asian patients compared with the overall population. Grade 3 or 4 rash was the main reason for dose reduction and treatment interruption in East Asian patients, and was managed with supportive measures and/or pharmacological interventions (systemic and/or topical corticosteroids and antihistamines). No new safety signal was identified specifically in the East Asian population, and AEs were similar to those reported previously.14,15
Although the TITAN trial was not powered to compare the different populations and ethnicities of the participants, HRs for radiographic progression after apalutamide administration appeared to be similar in East Asians and other patients. In addition, longer follow-up is indeed necessary. A study by Fukagai et al.26 showed marked differences in overall and cause-specific survival between Japanese American and Caucasian men with prostate cancer treated by hormonal therapy. Further investigation is required to clarify racial differences in clinical outcomes after apalutamide treatment.
Beyond the limitations of the original TITAN trial,18 the present sub-study was limited by the relatively small sample size and short follow-up time. Importantly, this sub-study was not preplanned in the original TITAN trial but necessary owing to differences in prostate cancer characteristics between Asian and Western populations. Further analyses will be performed in East Asian populations to verify the benefits of apalutamide in OS and pre-specified secondary and exploratory endpoints of the original TITAN trial after the final results are obtained.
In conclusion, the efficacy and safety of apalutamide addition to ADT in the East Asian subgroup of the TITAN trial are consistent with the global results. In the Asia-Pacific region, apalutamide was granted approval for mCSPC in China and Japan. Therefore, apalutamide appears to be a valid treatment option for East Asian patients with mCSPC.
AUTHOR CONTRIBUTIONS
All the authors assume responsibility for the completeness and accuracy of the data and analyses as well as for the fidelity of the trial to the protocol. DWY designed and checked the protocol of this subgroup analysis, led the implement of the study in Chinese centers, and was responsible for enrolling, treating and evaluating the participants. BHC designed and checked the protocol of this subgroup analysis, led the implement of the study in Korean centers, and was responsible for enrolling, treating and evaluating the participants. The other authors were responsible for the enrolment, treatment and evaluating the participants. All the authors had full access to the data, participated in data interpretation, read and approved the final manuscript, and agree with the order of presentation of the authors.
COMPETING INTERESTS
DWY, JH, ZQY, DLH, HU, BHC, CSK, and KNC report receiving research grants from Janssen Pharmaceutical Ltd. The following authors declare a conflict of interest on the basis that they are full-time employees of the Janssen medical affairs department (YYZ), and Janssen Research and Development (ST and SM).
ACKNOWLEDGMENTS
The authors thank all the participants and their families as well as the investigational site staff for their contributions. This study was funded by Janssen Pharmaceutical Ltd., which designed the study.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Kimura T. East meets West: ethnic differences in prostate cancer epidemiology between East Asians and Caucasians. Chin J Cancer. 2012;31:421–9. doi: 10.5732/cjc.011.10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen R, Ren S, Yiu MK, Fai NC, et al. Chinese Prostate Cancer Consortium. Prostate cancer in Asia: a collaborative report. Asian J Urol. 2014;1:15–29. doi: 10.1016/j.ajur.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baade PD, Youlden DR, Cramb SM, Dunn J, Gardiner RA. Epidemiology of prostate cancer in the Asia-Pacific region. Prostate Int. 2013;1:47–58. doi: 10.12954/PI.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ha Chung B, Horie S, Chiong E. The incidence, mortality, and risk factors of prostate cancer in Asian men. Prostate Int. 2019;7:1–8. doi: 10.1016/j.prnil.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NCCN Clinical Practice Guildeines in Oncology (NCCN Guidelines). Prostate Cancer. Version 2.2020. Fort Washington: National Comprehensive Cancer Network. 2020 [Google Scholar]
- 7.Droz JP, Balducci L, Bolla M, Emberton M, Fitzpatrick JM, et al. Management of prostate cancer in older men: recommendations of a working group of the International Society of Geriatric Oncology. BJU Int. 2010;106:462–9. doi: 10.1111/j.1464-410X.2010.09334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker C, Gillessen S, Heidenreich A, Horwich A, Committee EG. Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v69–77. doi: 10.1093/annonc/mdv222. [DOI] [PubMed] [Google Scholar]
- 9.Ritch C, Cookson M. Recent trends in the management of advanced prostate cancer. F1000es. 2018;7:1513. doi: 10.12688/f1000research.15382.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nevedomskaya E, Baumgart SJ, Haendler B. Recent advances in prostate cancer treatment and drug discovery. Int J Mol Sci. 2018;19:1359. doi: 10.3390/ijms19051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowrance WT, Murad MH, Oh WK, Jarrard DF, Resnick MJ, et al. Castration-resistant prostate cancer: AUA guideline amendment 2018. J Urol. 2018;200:1264–72. doi: 10.1016/j.juro.2018.07.090. [DOI] [PubMed] [Google Scholar]
- 12.Clegg NJ, Wongvipat J, Joseph JD, Tran C, Ouk S, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koukourakis MI, Kakouratos C, Kalamida D, Mitrakas A, Pouliliou S, et al. Comparison of the effect of the antiandrogen apalutamide (ARN-509) versus bicalutamide on the androgen receptor pathway in prostate cancer cell lines. Anticancer Drugs. 2018;29:323–33. doi: 10.1097/CAD.0000000000000592. [DOI] [PubMed] [Google Scholar]
- 14.Rathkopf DE, Morris MJ, Fox JJ, Danila DC, Slovin SF, et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2013;31:3525–30. doi: 10.1200/JCO.2013.50.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408–18. doi: 10.1056/NEJMoa1715546. [DOI] [PubMed] [Google Scholar]
- 16.Al-Salama ZT. Apalutamide: first global approval. Drugs. 2018;78:699–705. doi: 10.1007/s40265-018-0900-z. [DOI] [PubMed] [Google Scholar]
- 17.Koshkin VS, Small EJ. Apalutamide in the treatment of castrate-resistant prostate cancer: evidence from clinical trials. Ther Adv Urol. 2018;10:445–54. doi: 10.1177/1756287218811450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381:13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- 19.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell IJ, Banerjee M, Bianco FJ, Wood DP, Jr, Dey J, et al. The effect of race/ethnicity on prostate cancer treatment outcome is conditional: a review of Wayne State University data. J Urol. 2004;171:1508–12. doi: 10.1097/01.ju.0000118906.16629.8c. [DOI] [PubMed] [Google Scholar]
- 21.Bernard B, Muralidhar V, Chen YH, Sridhar SS, Mitchell EP, et al. Impact of ethnicity on the outcome of men with metastatic, hormone-sensitive prostate cancer. Cancer. 2017;123:1536–44. doi: 10.1002/cncr.30503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman RM, Gilliland FD, Eley JW, Harlan LC, Stephenson RA, et al. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2001;93:388–95. doi: 10.1093/jnci/93.5.388. [DOI] [PubMed] [Google Scholar]
- 23.Kwon DH, Friedlander T. A TITAN step forward: apalutamide for metastatic castration-sensitive prostate cancer. Ann Transl Med. 2019;7:S364. doi: 10.21037/atm.2019.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, et al. Management of patients with advanced prostate cancer: the report of the advanced prostate cancer consensus conference APCCC 2017. Eur Urol. 2018;73:178–211. doi: 10.1016/j.eururo.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukagai T, Namiki TS, Carlile RG, Yoshida H, Namiki M. Comparison of the clinical outcome after hormonal therapy for prostate cancer between Japanese and Caucasian men. BJU Int. 2006;97:1190–3. doi: 10.1111/j.1464-410X.2006.06201.x. [DOI] [PubMed] [Google Scholar]


