Abstract
Involvement of an oxidative burst, usually related to incompatible plant/pathogen interactions leading to hypersensitive reactions, was investigated with Erwinia amylovora, the causal agent of fire blight of Maloideae subfamily of Rosaceae, in interaction with pear (Pyrus communis; compatible situation) and tobacco (Nicotiana tabacum; incompatible situation). As expected, this necrogenic bacterium induced in tobacco a sustained production of superoxide anion, lipid peroxidation, electrolyte leakage, and concomitant increases of several antioxidative enzymes (ascorbate peroxidases, glutathion reductases, glutathion-S-transferases, and peroxidases), in contrast to the compatible pathogen Pseudomonas syringae pv tabaci, which did not cause such reactions. In pear leaves, however, inoculations with both the disease- and the hypersensitive reaction-inducing bacteria (E. amylovora and P. syringae pv tabaci, respectively) resulted in superoxide accumulation, lipid peroxidation, electrolyte leakage, and enzyme induction at similar rates and according to equivalent time courses. The unexpected ability of E. amylovora to generate an oxidative stress even in compatible situation was linked to its functional hrp (for hypersensitive reaction and pathogenicity) cluster because an Hrp secretion mutant of the bacteria did not induce any plant response. It is suggested that E. amylovora uses the production of reactive oxygen species as a tool to provoke host cell death during pathogenesis to invade plant tissues. The bacterial exopolysaccharide could protect this pathogen against the toxic effects of oxygen species since a non-capsular mutant of E. amylovora induced locally the same responses than the wild type but was unable to further colonize the plant.
Erwinia amylovora is a necrogenic bacteria causing fire blight of Maloideae subfamily of Rosaceae such as apple (Malus spp.) and pear (Pyrus spp.) (Billing, 1983). It provokes progressive necrosis in aerial parts of susceptible host plants and the typical hypersensitive reaction (HR, rapid and localized plant cell death at the infection site) in non-host plants.
Molecular genetic studies of the bacteria lead to the identification of various genes involved in the establishment of compatibility and incompatibility. An hrp cluster (for HR and pathogenicity) has been identified on its chromosome and encodes components of an Hrp secretion apparatus (type III secretion system), common to several necrogenic bacteria (for review, see Lindgren, 1997). At least three proteins are known to be secreted through this apparatus: (a) harpin, a major HR elicitor also involved in pathogenicity (Wei et al., 1992; Barny, 1995; Dong et al., 1999); (b) DspA, an essential pathogenicity determinant (Gaudriault et al., 1997); (c) HrpW, which shares similarities with harpin but acts as a negative effector of the HR mechanisms (Gaudriault et al., 1998). Besides, exopolysaccharides, whose synthesis is controlled by an ams cluster (for amylovoran synthesis), is another major pathogenicity determinant of E. amylovora, which may protect the bacteria against host defense reactions (Bugert and Geider, 1995). However biochemical mechanisms leading to disease (in compatible situations) or HR (in incompatible situations) are not well understood yet.
The oxidative burst, a rapid production of reactive oxygen species (ROS) released into the apoplast, is described as one of the earliest responses to pathogen infection and is generally associated with HR (for review, see Lamb and Dixon, 1997). Among the ROS produced during plant/pathogen interactions, the first detectable oxidants are superoxide anion (O2·−) and hydrogen peroxide (H2O2), which is produced by spontaneous or enzymatic dismutation of superoxide. These compounds are moderately reactive, but they can be converted into more reactive species (especially the hydroxyl radical OH·). ROS have direct antimicrobial activities and can therefore reduce pathogen viability. They have been also implicated in the destruction of the challenged plant cells, either through lipid peroxidation or through initiation of programmed cell death (for review, see Greenberg, 1997). However, in at least two cases it has been demonstrated that ROS by themselves are not sufficient to cause cell death (Glazener et al., 1996; Dorey et al., 1999). H2O2 has been shown to play a central role in the expression of disease resistance in several plant/pathogen systems. It serves as substrate for oxidative cross-linking of various plant cell wall components leading to the reinforcement of cell walls and as a diffusible signal for the induction of defense-related genes in healthy adjacent tissues (Lamb and Dixon, 1997).
Several works based on the interaction between pathogenic bacteria (mainly pathovars of Pseudomonas syringae) and suspension-cultured plant cells described the oxidative burst as a two-phase phenomenon (Keppler et al., 1989; Baker et al., 1991; Levine et al., 1994; Glazener et al., 1996). Phase I is an immediate and very transient ROS production, non-specifically stimulated by compatible, incompatible, and even saprophytic bacteria. In contrast, phase II is a delayed (1–3 h after the addition of bacteria) and prolonged ROS production that is specifically stimulated by incompatible HR-causing bacteria and is therefore characteristic of the HR.
E. amylovora induces both ROS phases when co-cultured with tobacco (Nicotiana tabacum) cell suspensions (incompatible situation), whereas a mutant of the same strain that does not produce the HR elicitor harpin induces only the ROS phase I (Baker et al., 1993). Furthermore cell-free preparation of harpin is able to elicit ROS production when incubated with tobacco cells. These results strongly suggest that harpin may be the bacterial elicitor of ROS production at least during incompatible situations.
In this work, we examine the possible involvement of ROS in the initiation of infection of pear by E. amylovora. To compare with previous works dealing with pathovars of P. syringae, we studied different interactions involving E. amylovora and Pseudomonas syringae pv tabaci either in compatible (fire blight on pear and wild fire on tobacco, respectively) or in incompatible situations (HR on tobacco and on pear, respectively). Oxidative burst (O2·− generation) and its consequences, i.e. lipid peroxidation, electrolyte leakage, change in activity of antioxidative enzymes, were investigated in leaves of pear and tobacco challenged with these bacteria. A secretory hrp mutant and an ams mutant of E. amylovora were also included in the study to determine the role of different bacterial pathogenicity determinants.
RESULTS
Symptoms Obtained after Infiltration of the Different Strains
Infiltration of both wild types E. amylovora CFBP1430 (Ea 1430) and P. syringae pv tabaci CFBP2106 (Pst 2106), as well as of the non-capsular ams mutant E. amylovora PMV6089 (Ea 6089), at a bacterial concentration of 108 cfu (colony forming unit) mL−1, into young leaves of pear provoked within 24 h generalized necrotic lesions of the entire infiltrated area. At this stage no noticeable differences could be detected between the three strains. The following days, only plants infiltrated with Ea 1430 showed progressive necrosis reaching the petiole of the infiltrated leaves and the stem. One week after infiltration, these plants were almost completely necrosed when symptoms on plants infiltrated with Ea 6089 or Pst 2106 did not show any evolution. No symptoms at all were recorded with the Hrp secretory mutant E. amylovora PMV6023 (Ea 6023). Attempts to re-isolate the different strains in stems of inoculated plants several days after infiltration proved to be successful only in plants inoculated with Ea 1430 in which high bacterial populations were found (results not shown).
Infiltration of Ea 1430 and Ea 6089 (108 cfu mL−1) into tobacco leaves induced the typical HR within 24 h, i.e. brown collapsed areas corresponding exactly to the infiltrated zones. For Pst 2106 at the same concentration, the infiltrated areas showed a partial collapse of tissues without brown discoloration within 24 h. These green collapsed areas were surrounded with a dark-green margin. In the case of Pst 2106 only, a blackening of veins in the infiltrated zones was observed the following days, as well as a yellow halo surrounding the collapsed area. No symptoms were recorded after infiltration of the Hrp secretory mutant Ea 6023.
Production of O2·−
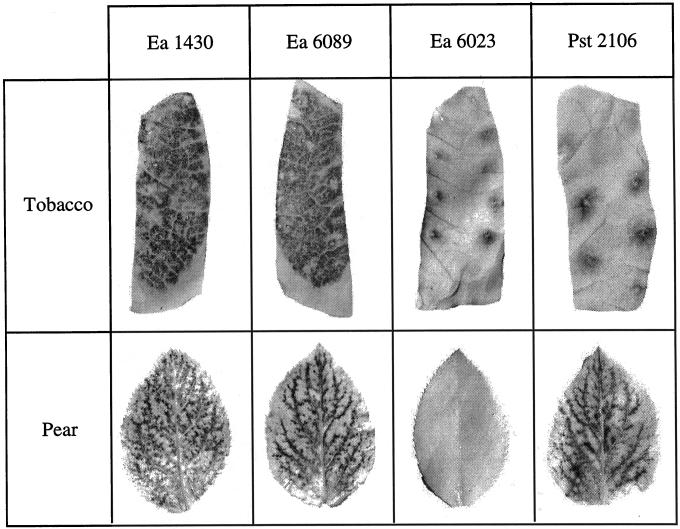
Leaves were sampled from pear and tobacco seedlings at various times following infiltration of bacteria at a concentration of 108 cfu mL−1, and the leaf samples were infiltrated with nitroblue tetrazolium (NBT). Accumulation of insoluble blue-colored formazan complex (reduced NBT) is an indicator of generation of ROS, in particular O2·− (Doke, 1983). This accumulation was observed in pear leaves after infiltration of the wild-type Ea 1430, the non-capsular ams mutant Ea 6089, or the wild-type Pst 2106, and in tobacco leaves after infiltration of the two HR-inducing bacteria Ea 1430 and Ea 6089 (Fig. 1). No reaction was detectable after infiltration of the Hrp secretory mutant Ea 6023 in leaves of both species and infiltration of Pst 2106 in leaves of its host plant tobacco (except some blue spots due to wounding during infiltration).
Figure 1.
NBT staining in pear and tobacco leaves 12 and 8 h, respectively, after infiltration of Ea 1430, Ea 6089, Ea 6023, and Pst 2106. Bacterial suspensions were adjusted to 108 cfu mL−1. The black staining indicates the presence of O2·−.
NBT reduction was a transient phenomenon. In pear leaves, the reaction began to be detectable 6 h after inoculation, whatever the bacteria, and reached a maximum of intensity on average 12 h after inoculation. After this period of time, staining declined rapidly, preceding the apparition of necrosis. In tobacco leaves, formazan was detectable earlier (4 h after inoculation), and the maximum of staining intensity was reached approximately 8 h after inoculation.
Lipid Peroxidation
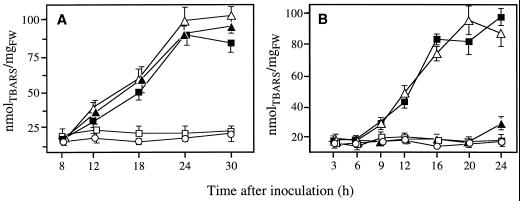
Induction of lipid peroxidation was assessed by determining the accumulation of thiobarbituric acid reactive species (TBARS) at various time following infiltration of bacterial suspensions adjusted to a concentration of 108 cfu mL−1 in intact leaves of pear or tobacco. Increasing levels of TBARS were detected only in such plant/bacteria combinations where NBT reduction was observed, i.e. Ea 1430/pear or tobacco, Ea 6089/pear or tobacco, Pst 2106/pear (Fig. 2). No significant lipid peroxidation was recorded after infiltration of the Hrp secretory mutant Ea 6023 in pear or tobacco and of Pst 2106 in tobacco for the whole duration of the experiment (24 or 30 h according to the plant species).
Figure 2.
Changes in lipid peroxidation expressed as equivalents of TBARS in pear (A) and tobacco (B) leaf tissues after infiltration of Ea 1430 (▪), Pst 2106 (▴), Ea 6089 (▵), Ea 6023 (□), and water (·). Bacterial suspensions were adjusted to 108 cfu mL−1. Data are means ± se of at least nine repetitions from three independent experiments.
When detected, accumulation of TBARS began between 8 and 12 h after bacterial infiltration in pear leaves and between 6 and 9 h after bacterial infiltration in tobacco leaves. This coincided exactly with the maximum O2·− production observed in equivalent and similarly treated leaves of both plant species. Time-course of lipid peroxidation were similar for the three inducing bacteria in pear and the two inducing bacteria in tobacco (Fig. 2).
AsPOX, GR, GST, and POX Activities
Intact leaves of pear and tobacco were infiltrated with bacterial suspensions adjusted to a concentration of 107 cfu mL−1 and sampled at various times for enzyme extractions. The lower bacterial concentration used in these experiments (107 as compared with 108 cfu mL−1) caused only small necrotic lesions in the infiltrated leaves at the end of the sampling period. Extracted proteins originated mainly from apparently healthy tissues surrounding the dying cells. At a concentration of 108 cfu mL−1, generalized necrosis occurred too rapidly to allow the thorough assessment and comparison of enzyme activities (high variability, drastic decrease in protein contents, which artificially increased enzyme activities if any).
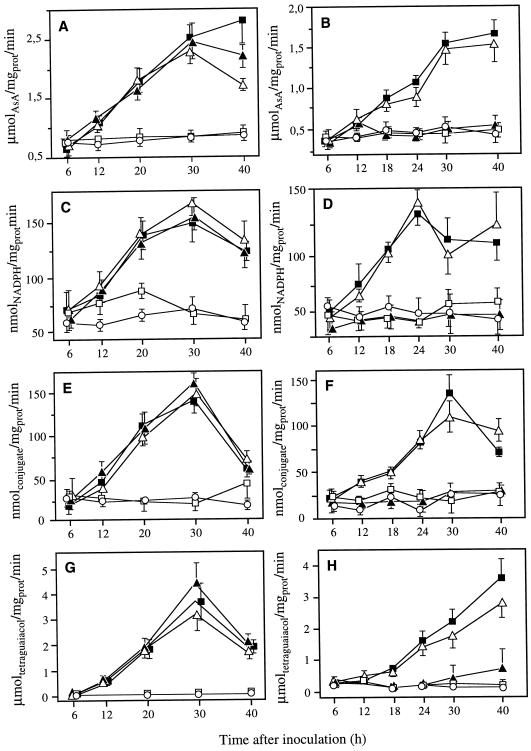
In pear leaves, ascorbate peroxidase (AsPOX), glutathione reductase (GR), glutathione-S-transferase (GST), and peroxidase (POX) were similarly activated following infiltration with both compatible and incompatible wild-type strains Ea 1430 and Pst 2106, or with the non-capsular ams mutant Ea 6089 (Fig. 3). The maximum of activation was generally reached approximately 30 h after bacterial infiltration. The level of enzyme activities remained low and not significantly different from the control when leaves were infiltrated with the Hrp secretory mutant Ea 6023. In tobacco leaves, the two HR-inducing bacteria Ea 1430 and Ea 6089 only were able to identically activate the four families of enzymes. The tobacco compatible strain Pst 2106 as well as the Hrp secretory mutant Ea 6023 had no effect on the level of these enzymes that remained identical to the level of enzymes extracted from control plants.
Figure 3.
Changes in the activities of AsPOX (A and B), GR (C and D), GST (E and F), and POX (G and H) in pear (A, C, E, and G) and tobacco (B, D, F, and H) leaf tissues after infiltration of Ea 1430 (▪), Pst 2106 (▴), Ea 6089 (▵), Ea 6023 (□), and water (·). Bacterial suspensions were adjusted to 107 cfu mL−1. Data are means ± se of at least nine repetitions from three independent experiments.
Electrolyte Loss Assessment
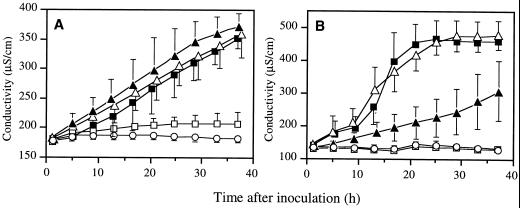
The two wild-type strains, Ea 1430 and Pst 2106 and the non-capsular ams mutant Ea 6089 showed the same ability to induce electrolyte leakage from pear discs (Fig. 4). In contrast with tobacco discs a significant difference was recorded between the response induced by the compatible strain Pst 2106 and the responses induced by the incompatible strains Ea 1430 and Ea 6089. The latter induced a fast and high electrolyte loss when the first one caused a progressive and weak leakage. The Hrp secretory mutant Ea 6023 did not induce any leakage from pear discs as well as from tobacco discs.
Figure 4.
Electrolyte leakage induced in pear (A) and tobacco (B) leaf disks after infiltration of Ea 1430 (▪), Pst 2106 (▴), Ea 6089 (▵), Ea 6023 (□), and water (·). Bacterial suspensions were adjusted to 108 cfu mL−1. Data are means ± se of six repetitions from two independent experiments.
DISCUSSION
Our results show that a sustained production of ROS and its immediate consequences in plant tissues, usually ascribed to incompatible plant-pathogen interactions, can also be a feature of a compatible interaction in the case of E. amylovora. This has been previously demonstrated in three compatible plant-fungi interactions (Phaseolus vulgaris/Botrytis cinerea [Tiedemann, 1997], Capsicum annuum/Botrytis cinerea [Deighton et al., 1999], and Avena sativa/Drechslera spp. [Gönner and Schlösser, 1993]) only. To our knowledge, this is the first report of such a phenomenon during a compatible plant/bacteria interaction. However it can be noticed that this particular interaction is associated with the rapid necrosis of invaded tissues. Such a necrosis is not usual for every compatible plant/bacteria interaction.
E. amylovora induced the production of superoxide anion O2·− for several hours both in the compatible (pear) and the incompatible (tobacco) situations, in contrast to P. syringae pv tabaci, which induced this production in the incompatible situation (pear) only. Although we did not quantify the production of O2·−, no macroscopic differences could be detected between the accumulation of formazan induced by the two wild-type bacteria in pear tissues, in terms of intensity as well as the time course of this coloration. The absence of any accumulation of formazan after infiltration of the Hrp secretory mutant of E. amylovora in pear or tobacco tissues or after infiltration of P. syringae pv tabaci in tobacco tissues suggests that the non-specific ROS phase I is not detectable with this method and that the production of O2·− observed with both wild-type strains corresponds to the ROS phase II, usually ascribed to incompatible situations leading to HR.
Lipid peroxidation, leading to the loss of membrane integrity, has been described as consequence of oxidative burst in numerous systems (Adam et al., 1989; Keppler and Baker, 1989; May et al., 1996; Rusterucci et al., 1996). This phenomenon can be initiated directly by ROS (Keppler and Baker, 1989) and indirectly through the involvement of lipoxygenase (Buonaurio and Servili, 1999). As for ROS phase II, it has been generally ascribed to HR-producing combinations. The results obtained in this work with P. syringae pv tabaci are in accordance with previous works involving various pathovars of P. syringae (Keppler and Novacky, 1986; Adam et al., 1989; Keppler and Baker, 1989). Lipid peroxidation and electrolyte leakage occurred only during the HR (or O2·−)-producing combination. No lipid peroxidation and a delayed and slight electrolyte leakage were recorded in the compatible situation where no O2·− was detected. As suggested by Keppler and Novacky (1986) for P. syringae pv lachrymans, P. syringae pv tabaci could provoke cellular damages in its host plant through a mechanism different from lipid peroxidation.
Concerning E. amylovora, its ability to provoke electrolyte leakage from host and non-host tissues has been previously shown (Brisset and Paulin, 1991), but we demonstrate here a close correlation between O2·− accumulation, lipid peroxidation, and electrolyte leakage in non-host as well as in susceptible host tissues. This suggests that in the case of this bacterium, O2·−-initiated lipid peroxidation is the mechanism through which membranes are altered, even during disease reaction. Furthermore, the identical patterns obtained with both parameters after infiltration of E. amylovora (disease) or P. syringae pv tabaci (HR) in pear tissues confirm the similar generation of O2·− induced by both the compatible and the incompatible bacteria in this plant.
AsPOX, GR, POX, and GST are part of the repertoire of anti-oxidative defense system of the plant. AsPOX and GR are constituents of the ascorbate-glutathione cycle that detoxifies H2O2 (Foyer and Halliwell, 1976). Various POX use H2O2 as a substrate (i.e. extracellular POX for the generation of phenoxyl radicals during lignin formation) and thus participate to the disappearance of ROS (Bestwick et al., 1998). GST detoxifies lipid hydroperoxides deriving from lipid peroxidation by conjugation with reduced glutathione (Mars, 1996). Enzymes of antioxidant metabolism are usually found to be co-regulated and their activities increase in response to stress (Mullineaux and Creissen, 1997). This is in accordance with our results showing the concommitant activation of these enzymes in each situation where an oxidative stress was recorded. Besides Levine et al. (1994) demonstrated the direct induction of several cellular protectant genes in soybean cells by exogenous H2O2. This induction was correlated with increasing concentrations of H2O2 up to 2 mm (lower doses than required for cell death), higher concentrations giving a weaker response (but triggering cell death). In this work and for enzymatic studies, we used lower bacterial concentrations than those required for confluent cell death of the infiltrated areas. According to Levine et al. (1994), the similar patterns of activation obtained in the case of each enzyme after infiltration of E. amylovora or P. syringae pv tabaci in pear tissues are an additional confirmation of an identical level of production of ROS in both compatible and incompatible situations.
The Hrp secretory mutant of E. amylovora used in this study was unable to induce any response, in pear as well as in tobacco tissues. These observations confirm the essential role of Hrp-secreted proteins in O2·− generation, as already demonstrated for one of them, harpin, on tobacco cell suspensions (Baker et al., 1993). However the relative role of each Hrp-secreted proteins (e.g. harpin, DspA, and HrpW) in this phenomena remains to be determined, especially during the compatible interaction. On the other hand, the bacterial exopolysaccharide does not seem to play any role in O2·− generation because the non-capsular ams mutant of E. amylovora induced a similar oxidative stress than the wild-type strain. But it could play a protective role against the toxic ROS, since the ams mutant conversely to the wild type was unable to colonize the plant.
In conclusion, our results show that E. amylovora seems to behave like an HR-inducing bacteria in its host plant, conversely to P. syringae pv tabaci. As suggested for Dreschselera spp. (Gönner and Schlösser, 1993) and Botrytis cinerea (Tiedemann, 1997), this bacteria could take advantage of the generation of active oxygen species as a tool to kill the host cells during pathogenesis and to invade the plant. It remains to be determined how the bacteria can overcome the plant defenses usually elicited in tissues surrounding cells undergoing an oxidative burst.
MATERIAL AND METHODS
Plant Material
Experiments were performed on pear seedlings (six to eight leaves) from open-pollinated pear (Pyrus communis cv Kirchensaller) and on tobacco (Nicotiana tabacum cv Xanthi) plants (10–12 leaves). Plants were grown in individual pots in the greenhouse at 20°C to 25°C under natural photoperiod.
Bacteria and Inoculation Procedures
Bacterial strains used in this study are listed with their main characteristics in Table I. For inoculum preparation, bacteria were grown on solid King's medium B (King et al., 1954) supplemented with chloramphenicol (20 mg L−1) for the transposon mutants at 27°C for 24 h. Except for assessment of electrolyte leakage (see below), bacterial suspensions were prepared in sterile distilled water to yield a concentration of 107 or 108 cfu mL−1 according to the experiments. Inoculation was performed by vacuum infiltration of the three youngest fully expanded leaves of pear seedlings and by infiltration with a syringe of young leaves of tobacco.
Table I.
Bacterial strains used in this study
| Straina | Relevant Characteristicsb | Reference or Source |
|---|---|---|
| Erwinia amylovora | ||
| CFBP1430 | Wild-type, isolated from Crataegus | Paulin and Samson (1973) |
| PMV6023 | hrcV ∷ MudIIPR13, Path−, HR−, CmR | Barny (1995) |
| PMV6089 | ams ∷ MudIIPR13, Path−, HR+, EPS−, CmR | Tharaud et al. (1994) |
| Pseudomonas syringae pv tabaci | ||
| CFBP2106 | Wild-type, pathotype strain, isolated from Nicotiana tabacum | CFBP |
CFBP, Collection Française de Bactéries Phytopathogènes, Institut National de la Recherche Agronomique-Angers, France; PMV, Pathologie Moléculaire et Végétale, Institut National de la Recherche Agronomique-Institut National Agronomique Paris-Grignon, Paris, France.
hrc, Hypersensitive response conserved (Hrp secretion mutant); Path−, non-pathogenic; HR, hypersensitive response; CmR, chloramphenicol resistance; EPS, exopolysaccharides.
NBT Staining
O2·− was detected in situ as described by Doke (1983) with some modifications. Leaves were vacuum-infiltrated with 0.05 m sodium phosphate buffer (pH 7.5) containing 0.05% NBT. After 15 min of staining at room temperature under light, the NBT-treated tissues were placed in 96% (v/v) ethanol to stop reaction, remove chlorophyll, and preserve tissue integrity.
Lipid Peroxidation Analysis
Lipoperoxidation was monitored with the spectrophotometric determination of malondialdehyde using thiobarbituric acid according to Popham and Novacky (1991). Plant material (1 g fresh weight) was homogenized in 2 mL of trichloroacetic acid (10% [w/v]) and centrifugated at 15,000g for 20 min. To 250-μL aliquot of crude extract was added 250 μL of trichloroacetic acid (10% [w/v]) plus 1 mL of thiobarbituric acid (0.2% [w/v] in trichloroacetic acid 10%). The mixture was boiled at 95°C for 30 min and cooled in ice for 5 min. After centrifugation at 10,000g for 10 min, the absorbance of supernatant was determined at 532 nm. The value of nonspecific A600 was measured and substracted. Due to the limited specificity of the method, the concentration of TBARS was calculated by using the extinction coefficient of 155 mm−1 cm−1, and results were expressed as nanomols TBARS per milligram FW.
Electrolyte Leakage Measurements
Electrolyte loss was determined according to Dellagi et al. (1998) with some modifications. Pear and tobacco leaf discs (5-mm diameter) were vacuum infiltrated with bacterial suspensions adjusted to 108 cfu mL−1 in appropriate assay medium (5 mm morpholinoethanesulfonic acid pH 6 for pear and 0.5 mm morpholinoethanesulfonic acid + 0.5 mm CaCl2, pH 6, for tobacco). Discs were then blotted dry for 30 min and incubated in vials with fresh pear or tobacco assay medium under light conditions at 25°C with continuous stirring. One experiment consisted in three assays of seven pear or five tobacco leaf discs in 2.5 mL of medium per bacterial treatment. Conductivity probes (Tacussel XE 120) were permanently immersed in each vial and connected to a computer-monitored conductivimeter. Measurements were automatically performed every 2 h for at least 40 h.
Enzyme Extractions
Leaf tissues (0.5 g fresh weight) were homogenized in 1 mL of ice-cold 50 mm sodium phosphate buffer (pH 7.5) containing 1 mm polyethyleneglycol, 1 mm phenylmethylsulfonyl fluoride, 8% (w/v) polyvinylpolypyrolydone, and 0.01% (v/v) Triton X-100. Homogenates were centrifugated at 16,000g for 20 min at 4°C and supernatants were immediately assayed for enzyme activities.
Measurements of Enzyme Activities
Spectrophotometric methods were used to determine the various total enzyme activities.
AsPOX (EC 1.11.1.11) activity was assayed by following the oxidation of ascorbic acid at 290 nm (extinction coefficient of 2.8 mm−1 cm−1) according to the method of Nakano and Asada (1981). Ten microliters of leaf extract was added to 1 mL of the reaction mixture. The mixture consisted of a solution of 0.2 m Tris/HCl buffer (pH 7.8), 0.25 mm ascorbic acid, and 0.5 mm H2O2.
GR (EC 1.6.4.2) activity was determined by following the oxidation of NADPH at 340 nm (extinction coefficient of 6.2 mm−1 cm−1) according to Halliwell and Foyer (1978). Fifty microliters of leaf extract was added to 1 mL of the reaction mixture. The mixture consisted of a solution of 0.2 mm Tris/HCl buffer (pH 7.8) containing 3 mm EDTA, 0.2 mm NADPH, and 0.5 mm oxidized glutathione.
GST (EC 2.5.1.18) activity was determined by measuring the formation of the conjugate reaction product (extinction coefficient of 9.6 mm−1 cm−1) at 340 nm using 1-chloro-2,4-dinitrobenzene and glutathione as substrates (Mauch and Dudler, 1993). Fifty microliters of leaf extract was added to 1 mL of the reaction mixture. The mixture consisted of a solution of 0.1 m potassium phosphate (pH 6.5), 3.6 mm reduced glutathione, and 1 mm 1-chloro-2,4-dinitrobenzene.
POX (EC 1.11.1.7) activity was measured by following the increasing A470 due to the formation of tetraguaiacol (extinction coefficient of 26.6 mm−1 cm−1) as described by Chance and Maehly (1955). Fifty microliters of enzyme source (leaf extract diluted in 50 mm sodium phosphate 1:5 for pear extract or 1:10 for tobacco extract) was added to 2 mL of the reaction mixture. The mixture consisted of a solution of 50 mm sodium acetate buffer (pH 7), 25 mm guaiacol, and 25 mm H2O2.
Protein content in the extracts was determined according to the method of Bradford (1976) using the Coomassie Protein Assay Reagent (Pierce, Rockford, IL).
Experimental Design and Statistical Analysis
All experiments were performed with a minimum of three tissue sample replicates per treatment and per time point. Each experiment was realized at least three times and data are expressed as the means (± se).
ACKNOWLEDGMENT
The authors wish to thank Jean-Pierre Paulin for excellent technical assistance, helpful discussions, and critical reading of the manuscript.
Footnotes
This work was supported in part by the Etablissement Public Régional Pays de la Loire, France.
LITERATURE CITED
- Adam A, Farkas T, Somlyai G, Hevesi M, Kiraly Z. Consequence of O2·− generation during a bacterially induced hypersensitive reaction in tobacco: deterioration of membrane lipids. Physiol Mol Plant Pathol. 1989;34:13–26. [Google Scholar]
- Baker CJ, O'Neill NR, Keppler LD, Orlandi EW. Early responses during plant-bacteria interactions in tobacco cell suspensions. Phytopathology. 1991;81:1504–1507. [Google Scholar]
- Baker CJ, Orlandi EW, Mock NM. Harpin, an elicitor of the hypersensitive response in tobacco caused by Erwinia amylovora, elicits active oxygen production in suspension cells. Plant Physiol. 1993;102:1341–1344. doi: 10.1104/pp.102.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barny MA. Erwinia amylovora hrpN mutants, blocked in harpin synthesis, express a reduced virulence on host plants and elicit variable hypersensitive reactions on tobacco. Eur J Plant Pathol. 1995;101:333–340. [Google Scholar]
- Bestwick CS, Brown IR, Mansfield JW. Localized changes in peroxidase activity accompany hydrogen peroxide generation during the development of a nonhost hypersensitive reaction in lettuce. Plant Physiol. 1998;118:1067–1078. doi: 10.1104/pp.118.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billing E. Fire blight. J R Hortic Soc. 1983;108:206–210. [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brisset MN, Paulin JP. Relationships between electrolyte leakage from host cells and virulence of strains in the interaction between Erwinia amylovora and Pyrus communis. Physiol Mol Plant Pathol. 1991;39:443–453. [Google Scholar]
- Bugert P, Geider K. Molecular analysis of the ams operon required for exopolysaccharide synthesis of Erwinia amylovora. Mol Microbiol. 1995;15:917–933. doi: 10.1111/j.1365-2958.1995.tb02361.x. [DOI] [PubMed] [Google Scholar]
- Buonaurio R, Servili M. Involvement of lipoxygenase, lipoxygenase pathway volatiles, and lipid peroxidation during the hypersensitive reaction of pepper leaves to Xanthomonas campestris pv. vesicatoria. Physiol Mol Plant Pathol. 1999;54:155–169. [Google Scholar]
- Chance B, Maehly AC. Assay of catalases and peroxidases. Methods Enzymol. 1955;2:764–775. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- Deighton N, Muckenschnabel I, Goodman BA, Williamson B. Lipid peroxidation and the oxidative burst associated with infection of Capsicum annuum by Botrytis cinerea. Plant J. 1999;20:485–492. doi: 10.1046/j.1365-313x.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- Dellagi A, Brisset MN, Paulin JP, Expert D. Dual role of desferrioxamine in Erwinia amylovora pathogenicity. Mol Plant-Microbe Interact. 1998;11:734–742. doi: 10.1094/MPMI.1998.11.8.734. [DOI] [PubMed] [Google Scholar]
- Doke N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol. 1983;23:345–357. [Google Scholar]
- Dong H, Delaney TP, Bauer DW, Beer SV. Harpin induces disease resistance in Arabidopsis through the systemic acquired resistance pathway mediated by salicylic acid and the NIM1 gene. Plant J. 1999;20:207–215. doi: 10.1046/j.1365-313x.1999.00595.x. [DOI] [PubMed] [Google Scholar]
- Dorey S, Kopp M, Geoffroy P, Fritig B, Kauffmann S. Hydrogen peroxide from the oxidative burst is neither necessary nor sufficient for hypersensitive cell death induction, phenylalanine ammonia lyase stimulation, salicylic acid accumulation, or scopoletin consumption in cultured tobacco cells treated with elicitin. Plant Physiol. 1999;121:163–171. doi: 10.1104/pp.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Gaudriault S, Brisset MN, Barny MA. HrpW of Erwinia amylovora, a new Hrp-secreted protein. FEBS Lett. 1998;428:224–228. doi: 10.1016/s0014-5793(98)00534-1. [DOI] [PubMed] [Google Scholar]
- Gaudriault S, Malandrin L, Paulin JP, Barny MA. DspA, an essential pathogenicity factor of Erwinia amylovora showing homology with AvrE of Pseudomonas syringae, is secreted via Hrp secretion pathway in a DspB dependent way. Mol Microbiol. 1997;26:1057–1069. doi: 10.1046/j.1365-2958.1997.6442015.x. [DOI] [PubMed] [Google Scholar]
- Glazener JA, Orlandi EW, Baker CJ. The active oxygen response of cell suspensions to incompatible bacteria is not sufficient to cause hypersensitive cell death. Plant Physiol. 1996;110:759–763. doi: 10.1104/pp.110.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönner MV, Schlösser E. Oxidative stress in interactions between Avena sativa L. and Drechslera spp. Physiol Mol Plant Pathol. 1993;42:221–234. [Google Scholar]
- Greenberg JT. Programmed cell death in plant-pathogen interactions. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:524–545. doi: 10.1146/annurev.arplant.48.1.525. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Foyer CH. Properties and physiological function of a glutathion reductase purified from spinach leaves by affinity chromotography. Planta. 1978;139:9–17. doi: 10.1007/BF00390803. [DOI] [PubMed] [Google Scholar]
- Keppler LD, Baker CJ. O2·−-initiated lipid peroxidation in a bacteria-induced hypersensitive reaction in tobacco cells suspensions. Phytopathology. 1989;79:555–562. [Google Scholar]
- Keppler LD, Baker CJ, Atkinson MM. Active oxygen production during a bacteria-induced hypersensitive reaction in tobacco suspension cells. Phytopathology. 1989;79:974–978. [Google Scholar]
- Keppler LD, Novacky A. Involvement of membrane lipid peroxidation in the development of a bacterially induced hypersensitive reaction. Phytopathology. 1986;76:104–108. [Google Scholar]
- King EO, Ward MK, Raney DE. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Lindgren PB. The role of hrp genes during plant-bacterial interactions. Annu Rev Phytopathol. 1997;35:129–152. doi: 10.1146/annurev.phyto.35.1.129. [DOI] [PubMed] [Google Scholar]
- Mars KA. The functions and regulation of glutathione-S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:127–158. doi: 10.1146/annurev.arplant.47.1.127. [DOI] [PubMed] [Google Scholar]
- Mauch F, Dudler R. Differential induction of distinct glutathione-S-transferase of wheat by xenobiotics and by pathogen attack. Plant Physiol. 1993;102:1193–1201. doi: 10.1104/pp.102.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Hammond-Kosack KE, Jones JDG. Involvement of reactive oxygen species, glutathione metabolism, and lipid peroxidation in the Cf-gene-dependent defense response of tomato cotyledons induced by race-specific elicitors of Cladosporium fulvum. Plant Physiol. 1996;110:1367–1379. doi: 10.1104/pp.110.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux PM, Creissen GP. Glutathione reductase: regulation and role in oxidative stress. In: Scandalios JG, editor. Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 667–713. [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloropasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Paulin JP, Samson R. Le feu bactérien en France: II. Caractères des souches d'Erwinia amylovora (Burrill) Winslow et al., 1920, isolées du foyer franco-belge. Ann Phytopathol. 1973;5:389–397. [Google Scholar]
- Popham PL, Novacky A. Use of dimethyl sulfoxide to detect hydroxyl radical during bacteria-induced hypersensitive reaction. Plant Physiol. 1991;96:1157–1162. doi: 10.1104/pp.96.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusterucci C, Stallaert V, Milat ML, Pugin A, Ricci P, Blein JP. Relationship between active oxygen species, lipid peroxidation, necrosis, and phytoalexin production induced by elicitins in Nicotiana. Plant Physiol. 1996;111:885–891. doi: 10.1104/pp.111.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharaud M, Menggad M, Paulin JP, Laurent J. Virulence, growth and surface characteristics of Erwinia amylovora mutants with altered pathogenicity. Microbiology. 1994;140:659–669. [Google Scholar]
- Tiedemann AV. Evidence for a primary role of active oxygen species in induction of host cell death during infection of bean leaves with Botrytis cinerea. Physiol Mol Plant Pathol. 1997;50:151–166. [Google Scholar]
- Wei ZM, Laby RJ, Zumoff CH, Bauer DW, He SY, Collmer A, Beer SV. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science. 1992;257:85–88. doi: 10.1126/science.1621099. [DOI] [PubMed] [Google Scholar]