Abstract
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) receptor, angiotensin-converting enzyme 2 (ACE2), has been identified in the human testis, but the risk of transmission of SARS-CoV-2 through sexual intercourse still needs to be defined. The goal of our study was to determine if SARS-CoV-2 is detectable in the semen of patients suffering or recovering from coronavirus disease-19 (COVID-19), still testing positive at nasopharyngeal swabs but showing mild or no symptoms at the time of sampling. Detection of SARS-CoV-2 RNA in semen was performed by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and nested PCR targeting open reading frame (ORF) 1ab. Medical history of the enrolled patients was taken, including COVID-19-correlated symptoms, both at the time of diagnosis and at the time of interview. Results of real-time RT-PCR and nested PCR in semen showed no evidence of SARS-CoV-2 RNA in the 36 patients suffering or recovering from COVID-19 but still positive in a nasopharyngeal swab, from over 116 patients enrolled in the study. SARS-CoV-2 detection and persistence in semen would have an impact on both clinical practice and public health strategies, but our results would suggest that SARS-CoV-2 is not present in the semen of men recovering from COVID-19.
Keywords: coronavirus, COVID-19, Italy, SARS-CoV-2, semen, sexual transmission
INTRODUCTION
According to the World Health Organization (WHO) statement and guideline,1 droplets and fomites are the main transmission routes for coronavirus disease-19 (COVID-19), but the likelihood of sexual transmission of the virus is of major interest, specifically to those working within the field of reproductive medicine.2,3 Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) enters human cells by using a receptor, angiotensin-converting enzyme 2 (ACE2),4 which is also present in many cell types in the human testis, for instance, in Leydig cells and Sertoli cells, and in the seminiferous tubules.5,6 Furthermore, some findings also suggest expression in spermatocytes.7
In the past, several viruses that were not initially thought to be sexually transmissible have been found to be able to pass the blood–testis barrier and enter semen. Salam and Horby8 found evidence in literature that at least 27 viruses, including Ebola, Zika, human immunodeficiency virus (HIV), hepatitis B virus (HBV), and human herpes viruses, could be found in human semen. Among viruses involved in previous recent pandemics, both Ebola and Zika have shown evidence of sexual transmission.9,10 The SARS-CoV-1 causing the early 2000s epidemic has also been involved in viral orchitis.11 Contradictory conclusions have been presented in literature over the presence of SARS-CoV-2 in human semen.12,13,14,15,16 While several studies showed the presence of SARS-CoV-2 RNA in semen during the acute phase of severe symptomatic infections,17,18,19 only a few data support the idea that the virus may be present in the spermatozoa of COVID-19 patients with milder symptoms or in asymptomatic cases, or during recovery or convalescence. Our main goal in this study was to determine if SARS-CoV-2 is detectable in the semen of Italian patients testing positive in nasopharyngeal swab tests and showing mild symptoms of COVID-19, or being asymptomatic at the time of sampling, and to determine if the sexual transmission can be considered a possible route of spread of SARS-CoV-2.
PATIENTS AND METHODS
Inclusion criteria and patients’ data collection
This study was approved by the ethical commission of the University of Palermo Hospital, Palermo, Italy (approval No. 4/2020-22/04/2020). Only patients with positive nasopharyngeal swab PCR tests for SARS-CoV-2 (performed according to WHO statement and guideline1) and isolated in nonhospital settings (e.g., houses or hotels) were eligible for this study. Of the 116 patients who were asked, 36 (31.0%) accepted to participate in the study. In line with the Declaration of Helsinki, informed consent was gained. Participants were interviewed in details to obtain their medical history, including information about age, weight, comorbidities, therapy, and urological conditions, and collect additional information, such as SARS-CoV-2 tests on nasopharyngeal swabs (dates and results), computed tomography (CT) scans (dates and results), and symptoms related to COVID-19, both at the time of diagnosis and at the time of the interview. None of the patients had a history of previous COVID-19 exposure or positive testing. In this study, we defined patients as “acute” up to 14 days from first positive nasopharyngeal swab, regardless of the severity of symptoms. The patients who had their first positive swab more than 14 days before semen sampling were defined as “convalescent”, provided that the improvement in symptoms allowed for their home isolation, eventually after hospital discharge, waiting for negative nasopharyngeal swabs.
Semen sampling and SARS-CoV-2 real-time reverse transcriptase-polymerase chain reaction (RT-PCR) testing
Semen specimens were collected in sterile containers by patients, who were given specific collection instructions. Specimens were sent to the laboratory within one hour from collection. When immediate transfer to the laboratory was not possible, samples were refrigerated at 4°C–6°C for up to 12 h from collection. Following the manufacturer's instructions, nucleic acids were extracted from 0.5 ml of semen samples using the MagNA Pure 24 automatic total nucleic acids extraction system (Roche Diagnostics, Rotkreuz, Switzerland), and eluted in 50 μl elution buffer. SARS-CoV-2 RNA was detected by real-time RT-PCR protocols targeting open reading frame (ORF) 1ab of SARS-CoV-2 genome, using AgPath-ID™ One-step RT-PCR Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA).20 All PCR tests were performed in triplicate, and a cycle threshold value less than 40 cycles was defined as a positive test. All semen samples were also tested in triplicate by a qualitative nested PCR protocol targeting ORF 1ab, using Platinum™ SuperFI™ II Green PCR Master Mix (Thermo Fisher Scientific Inc.) to ensure maximum sensitivity.
RESULTS
In our study, we did not detect SARS-CoV-2 RNA in the semen samples collected from 36 adult Italian males isolated in non-hospital settings while recovering from COVID-19.
From the data collected at the recruitment interviews, all the 36 patients enrolled for semen testing had at least one SARS-CoV-2-positive nasopharyngeal swab, and 21 of them (mostly convalescent from mild or severe COVID-19) had more than one positive test in their records (Table 1). On their first positive SARS-CoV-2 test, 33 of 36 patients (91.7%) showed symptoms compatible with COVID-19, including fever, cough, fatigue, muscle aches, headache, loss of taste or smell, diarrhea, and conjunctivitis (Figure 1). However, 12 of these 33 symptomatic patients (36.4%) had required hospitalization having a chest CT at admission, indicating the presence of bilateral interstitial pneumonia. For the purpose of this study, severe COVID-19 patients were further subdivided into acute cases (four patients) when the diagnosis based on the first positive nasopharyngeal swab lasted less than 15 days from semen sampling, and convalescent cases (eight patients) when the first positive swab was older than 14 days (Table 1). The remaining 21 symptomatic patients had a mild COVID-19, not requiring hospitalization, that had been diagnosed from less than 15 days in eleven cases (acute mild COVID-19 patients) and from more than 14 days in the other ten cases (convalescent mild COVID-19 patients). Only three patients were asymptomatic at diagnosis, with their first positive swab within the last 14 days of semen sampling. The median age and body mass index (BMI) of the 36 patients enrolled in this study were 41 years and 26.7 kg m−2, respectively. Furthermore, 25 of 36 patients (69.4%) were classified as overweight (BMI >25 kg m−2). Regarding their pathological history, 13.8% (5/36) of the patients had hyperlipidemia, 22.2% (8/36) had hypertension, 13.8% (5/36) had cardiovascular diseases, and 25.0% (9/36) had no pathological history whatsoever. In addition, 22.2% (8/36) of the patients declared themselves to be under therapy with statins, angiotensin II receptor blockers (ARBs), β-blockers, or aspirin, and 41.6% (15/36) were smokers.
Table 1.
Timeline to semen sampling for 36 male patients included in the study, divided into “acute” and “convalescent” groups
| Group | Patients (n) | Time from first positive swab (day), median (range) | Time from last positive swab (day), median (range) | ORF1ab RT-PCR results (positive/tested) | ORF1ab nested-PCR results (positive/tested) |
|---|---|---|---|---|---|
| Total acute patients | 18 | 10.0 (2.0–14.0) | 7.0 (2.0–14.0) | 0/18 | 0/18 |
| Acute asymptomatic | 3 | 5.0 (5.0–7.0) | 5.0 (5.0–7.0) | 0/3 | 0/3 |
| Acute mild COVID-19 | 11 | 10.0 (2.0–14.0) | 10.0 (2.0–14.0) | 0/11 | 0/11 |
| Acute severe COVID-19 | 4 | 11.0 (4.0–14.0) | 5.5 (4.0–9.0) | 0/4 | 0/4 |
| Total convalescent patients | 18 | 27.0 (16.0–88.0) | 5.0 (2.0–10.0) | 0/18 | 0/18 |
| Convalescent from mild COVID-19 | 10 | 29.0 (16.0–88.0) | 5.0 (2.0–8.0) | 0/10 | 0/10 |
| Convalescent from severe COVID-19 | 8 | 27.0 (17.0–48.0) | 7.5 (4.0–10.0) | 0/8 | 0/8 |
| Total study population | 36 | 15.0 (2.0–88.0) | 6.5 (2.0–14.0) | 0/36 | 0/36 |
Patients were defined as “acute” when semen sample was obtained up to 14 days from the first positive nasopharyngeal swab, regardless of the severity of symptoms; we defined “convalescent” patients who had their first positive swab >14 days before semen sampling, provided that the improvement in symptoms allowed for their home isolation, eventually after hospital discharge, waiting for negative nasopharyngeal swabs. All PCR tests were performed in triplicate to ensure maximum sensitivity. COVID-19: coronavirus disease-2019; ORF: open reading frame; RT-PCR: real-time polymerase chain reaction
Figure 1.
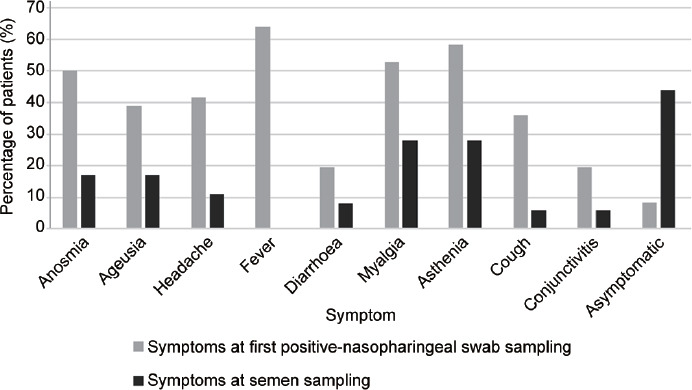
Clinical characteristics of 36 patients positive at SARS-CoV-2 nasopharyngeal swab and included in the study of SARS-CoV-2 shedding in semen. SARS-CoV-2: severe acute respiratory syndrome coronavirus-2.
At semen sampling, 44.4% (16/36) of patients showed no symptoms. The remaining patients who were still symptomatic at semen sampling complained of muscle aches and asthenia (Figure 1). Semen samples were collected within 2.0–14.0 days (median: 6.5 days) of the last PCR-positive nasopharyngeal swab (Table 1). In particular, for the 18 patients who were still considered to be in the acute phase of their infection (three asymptomatic, 11 mild COVID-19, and four severe COVID-19), having generally a single SARS-CoV-2-positive swab before semen sampling and being in their 14-day protocol isolation period, the interval between the last positive nasal swab and the collection of a sperm sample was 2.0–14.0 days (median: 7.0 days). While for the 18 convalescent patients (ten recovering from mild COVID-19 and eight from severe COVID-19), who had more than one positive swab result at the time of semen sampling and were still kept in isolation at home or at a COVID-hotel waiting for negative nasopharyngeal swabs, the semen samples were collected 16.0–88.0 days (median: 27.0 days) after the first positive nasopharyngeal swab and 2.0–10.0 days (median: 5.0 days) after the last positive nasopharyngeal swab.
Both the real-time RT-PCR and the nested PCR in semen showed no evidence of SARS-CoV-2 RNA in the 36 patients enrolled, their samples being negative for the detection of the ORF 1ab molecular target used in all triplicate repetitions.
DISCUSSION
It is well known that the SARS-CoV-2 virus affects the lungs and heart, but also the kidneys and liver. Conversely, little information is available on the pathogenesis of the virus in the testes. Sexual transmission of SARS-CoV-2 has been under investigation from the early time of the pandemic, but to date, discordant data have been described in literature. In our study, SARS-CoV-2 RNA was not detected in semen of 36 Italian males. The study population included 15 symptomatic infections (diagnosed within the last 14.0 days from semen sampling), including four severe cases having required hospitalization for pneumonia. Eighteen of the enrolled volunteers were recovering from severe (eight patients) and mild (ten patients) COVID-19, respectively, and three showed recent asymptomatic infection. In a previous survey, we failed to detect any SARS-CoV-2 viral RNA in nine patients during the first wave of the pandemic in Italy.21 Pan et al.22 first reported that there was no evidence of SARS-CoV-2 in semen from 34 Chinese men recovering from COVID-19 with mild-moderate symptoms at the time of disease confirmation, including six patients (19%) referring for testicular discomfort attributable to a viral orchitis. Similarly, in many studies conducted on patients in the acute stage of SARS-CoV-2 infection, with respiratory symptoms and positive nasopharyngeal swab test, or on men who were in various stages of recovery from COVID-19, the SARS-CoV-2 RNA could not be detected in semen samples.14,22,23,24,25,26 Conversely, Li et al.15 found SARS-CoV-2 genome in the semen of 26.7% of patients with COVID-19 in the acute stage of infection and in 8.7% of those who were recovering, and Achua et al.17 showed SARS-CoV-2 virus could be detected in testis tissue of a fatal COVID-19 infection using transmission electron microscopy. Machado et al.18 and Gacci et al.19 showed that SARS-CoV-2 RNA could be found in semen samples, although only in a small percentage of males who recovered from COVID-19. Although acknowledging from the review of previous literature that the presence of SARS-CoV-2 in semen cannot be excluded, Gonzalez et al.27 concluded for an extremely low risk of the presence of SARS-CoV-2 in semen and a negligible risk to sexually transmit SARS-CoV-2 in men recovered from COVID-19. The discordant results of SARS-CoV-2 RNA detection in semen could be connected to the use of different bio-molecular assays, targeting diverse viral RNA regions, that not necessarily allow to have a comparable detection sensitivity. However, the discrepancy could also derive from the study population sampled since the stage or severity of SARS-CoV-2 infection at semen samples collection could deeply influence the likelihood or the amount of viral shedding.
Cytokine production that occurs during SARS-CoV-2 infection, as well as corticosteroid treatments or high fever, may be capable of altering the blood–testis barrier, allowing the virus to reach testis via the blood,28 but this does not necessarily imply shedding through semen. Paoli et al.29 highlighted the implications of viral shedding concerning sperm cryopreservation, noting that the viruses stored in liquid nitrogen could maintain their pathogenic properties. However, Huang et al.30 could not find any traces of SARS-CoV-2 RNA in semen cryopreserved at a sperm bank in Hunan Province during and after the COVID-19 pandemic wave.
Despite the limited size of the population sampled, the results of our study would exclude the possibility that SARS-CoV-2 may be present in the seminal fluid of men suffering or recovering from COVID-19. Therefore, sexual intercourse would be highly unlikely to allow SARS-CoV-2 transmission. Nonetheless, larger studies possibly including more patients having suffered from severe acute COVID-19 would be necessary to discard the option of sexual transmission after clinical recovery. The presence of SARS-CoV-2 in semen of patients with severe infections is difficult to assess owing to the severity of illness preventing to provide a semen specimen. However, further larger scale studies are needed to monitor the sexual transmission of SARS-CoV-2, including serial samples taken from the onset of symptoms to complete certified recovery and possibly extending over time after recovery, focusing on long-term SARS-CoV-2 shedders and on long COVID-19 conditions.
AUTHOR CONTRIBUTIONS
CP, GMG, SDG and FB worked to research the topic and draft the manuscript. APC, DB, and MP carried out patients enrolling, while CF and GS performed technical approaches. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
The authors wish to thank Leonardo Mangiaracina and Giuseppa Sanfilippo (Section of Microbiology, Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, University of Palermo, Palermo) for technical assistance.
REFERENCES
- 1.World Health Organization. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance. World Health Organization. 2020. [Last accessed on 2021 Jun15]. Available from: https://apps.who.int/iris/handle/10665/331329 .
- 2.Mohseni AH, Taghinezhad SS, Xu Z, Fu X. Body fluids may contribute to human-to-human transmission of severe acute respiratory syndrome coronavirus 2: evidence and practical experience. Chin Med. 2020;15:58. doi: 10.1186/s13020-020-00337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turban JL, Keuroghlian AS, Mayer KH. Sexual health in the SARS-CoV-2 era. Ann Intern Med. 2020;173:387–9. doi: 10.7326/M20-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, leydig and sertoli cells. Cells. 2020;9:920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen Q, Xiao X, Aierken A, Yue W, Wu X, et al. The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. J Cell Mol Med. 2020;24:9472–7. doi: 10.1111/jcmm.15541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vishvkarma R, Rajender S. Could SARS-CoV-2 affect male fertility? Andrologia. 2020;52:e13712. doi: 10.1111/and.13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salam AP, Horby PW. The breadth of viruses in human semen. Emerg Infect Dis. 2017;23:1922–4. doi: 10.3201/eid2311.171049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Payne K, Kenny P, Scovell JM, Khodamoradi K, Ramasamy R. Twenty-first century viral pandemics: a literature review of sexual transmission and fertility implications in men. Sex Med Rev. 2020;8:518–30. doi: 10.1016/j.sxmr.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mate SE, Kugelman JR, Nyenswah TG, Ladner JT, Wiley MR, et al. Molecular evidence of sexual transmission of Ebola virus. N Engl J Med. 2015;373:2448–54. doi: 10.1056/NEJMoa1509773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Qi L, Chi X, Yang J, Wei X, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS) Biol Reprod. 2006;74:410–6. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song C, Wang Y, Li W, Hu B, Chen G, et al. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients. Biol Reprod. 2020;103:4–6. doi: 10.1093/biolre/ioaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paoli D, Pallotti F, Colangelo S, Basilico F, Mazzuti L, et al. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J Endocrinol Invest. 2020;43:1819–22. doi: 10.1007/s40618-020-01261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, et al. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114:233–8. doi: 10.1016/j.fertnstert.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3:e208292. doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry MJ, Arrington S, Neumann LM, Carrell D, Mores CN. It is currently unknown whether SARS-CoV-2 is viable in semen or whether COVID-19 damages spermatozoa. Andrology. 2020;9:30–2. doi: 10.1111/andr.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Achua JK, Chu KY, Ibrahim E, Khodamoradi K, Delma KS, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections on testis. World J Mens Health. 2021;39:65–74. doi: 10.5534/wjmh.200170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machado B, Barcelos Barra G, Scherzer N, Massey J, Dos Santos Luz H, et al. Presence of SARS-CoV-2 RNA in semen-cohort study in the United States COVID-19 positive patients. Infect Dis Rep. 2021;13:96–101. doi: 10.3390/idr13010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gacci M, Coppi M, Baldi E, Sebastianelli A, Zaccaro C, et al. Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum Reprod. 2021;36:1520–9. doi: 10.1093/humrep/deab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.La Rosa G, Iaconelli M, Mancini P, Bonanno Ferraro G, Veneri C, et al. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavone C, Giammanco GM, Baiamonte D, Pinelli M, Bonura C, et al. Italian males recovering from mild COVID-19 show no evidence of SARS-CoV-2 in semen despite prolonged nasopharyngeal swab positivity. Int J Impot Res. 2020;32:560–2. doi: 10.1038/s41443-020-00344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan F, Xiao X, Guo J, Song Y, Li H, et al. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113:1135–9. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rawlings SA, Ignacio C, Porrachia M, Du P, Smith DM, et al. No evidence of SARS-CoV-2 seminal shedding despite SARS-CoV-2 persistence in the upper respiratory tract. Open Forum Infect Dis. 2020;7:ofaa325. doi: 10.1093/ofid/ofaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo L, Zhao S, Li W, Wang Y, Li L, et al. Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology. 2021;9:42–7. doi: 10.1111/andr.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kayaaslan B, Korukluoglu G, Hasanoglu I, Kalem AK, Eser F, et al. Investigation of SARS-CoV-2 in semen of patients in the acute stage of COVID-19 infection. Urol Int. 2020;104:678–83. doi: 10.1159/000510531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paoli D, Pallotti F, Nigro G, Mazzuti L, Hirsch MN, et al. Molecular diagnosis of SARS-CoV-2 in seminal fluid. J Endocrinol Invest. 2021;44:2675–84. doi: 10.1007/s40618-021-01580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez DC, Khodamoradi K, Pai R, Guarch K, Connelly ZM, et al. A systematic review on the investigation of SARS-CoV-2 in semen. Res Rep Urol. 2020;12:615–21. doi: 10.2147/RRU.S277679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian Y, Zhou LQ. Evaluating the impact of COVID-19 on male reproduction. Reproduction. 2021;161:R37–44. doi: 10.1530/REP-20-0523. [DOI] [PubMed] [Google Scholar]
- 29.Paoli D, Pallotti F, Turriziani O, Mazzuti L, Antonelli G, et al. SARS-CoV-2 presence in seminal fluid: myth or reality. Andrology. 2021;9:23–6. doi: 10.1111/andr.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C, Wang Y, Li X, Ren L, Zhao J, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


