Abstract
Mesenchymal stem cells (MSCs) secrete various cytokines with angiogenic and neuroprotective effects. This study aimed to assess the effects of human umbilical cord Wharton's jelly-derived MSCs (hWJ-MSCs) on diabetes-related intracavernosal pressure (ICP) impairment in rats. hWJ-MSCs were isolated from human umbilical cord Wharton's jelly and transplanted into the corpus cavernosum of streptozotocin (STZ)-induced diabetic rats by unilateral injection. The erectile function was evaluated at 4 weeks, as well as the expression levels of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), endothelial nitric oxide synthase (eNOS), and insulin-like growth factor 1 (IGF1). STZ-induced diabetic rats showed impaired ICP, which was significantly improved by hWJ-MSC treatment. VEGF, eNOS, IGF1, and bFGF expression levels were higher in hWJ-MSC injection sites than those in control ones in STZ-induced diabetic rats. These results suggest that hWJ-MSC transplantation might improve diabetic erectile dysfunction through increased production of paracrine growth factors, highlighting a novel potential therapeutic option for erectile dysfunction.
Keywords: diabetes, endothelial nitric oxide synthase, erectile dysfunction, mesenchymal stem cells, vascular endothelial growth factor
INTRODUCTION
Erectile dysfunction (ED) is the inability to attain and/or maintain a penile erection sufficient for satisfactory sexual performance.1 ED is highly prevalent and affects the quality of life of affected men and their partners.2 A large number of men suffer from ED,3 which might affect 322 million individuals by 2025.4 ED is a complex neurovascular physiological process that involves neural, vascular, hormonal, and psychological factors.5 The integrity of the vascular bed of the penis also plays a crucial role in ED.5
Type 2 diabetes is a common endocrine disorder with a worldwide prevalence of 6.1% or 6059 cases per 100 000 individuals.6 ED is a common complication of diabetes, affecting up to 75% of diabetic men.7 Diabetic ED is associated with increased extracellular matrix (ECM) deposition and reduced smooth muscle cell number in the corpus cavernosum.7 The increased intracavernosal pressure (ICP) is mainly responsible for the quality of a penile erection.8 Therefore, damage or injury to penile cavernous smooth muscle cells and sinus endothelial cells due to various metabolic conditions or mechanical manipulations, including diabetes, hypertension, atherosclerosis, trauma, and surgery, leads to ED.9,10
Stem cells promote tissue repair and have various functions to achieve this goal. Indeed, stem cells have paracrine functions that promote cell survival via stromal cell-derived factor 1 (SDF-1), hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF1), endothelial growth factor (EGF), neural growth factor (NGF), and transforming growth factor (TGF)-α, as well as tissue angiogenesis through vascular endothelial growth factor (VEGF). Stem cells also migrate to the injury site, where adverse conditions, such as hypoxia, cytokines, and inflammation, can activate their functions to attract additional stem cells. Stem cells can also mitigate fibrosis.11,12,13 Transplanted stem cells differentiate into various cell types or stimulate mature cells to restore normal organ function after injury or degeneration.14 Since oral drugs, such as phosphodiesterase-5 inhibitors, have temporary effects and are relatively costly,15 stem cells could be an alternative therapeutic strategy for ED.16,17,18 Compared with other options, stem cell-based therapy for ED is relatively new.19 Human umbilical cord Wharton's jelly-derived mesenchymal stem cells (hWJ-MSCs) might be used in future to treat retinal degeneration,20 but their effects on ED remain unknown.
Therefore, this study aimed to evaluate the effects of hWJ-MSCs on diabetes-related ED by transplanting hWJ-MSCs in the corpus cavernosum of diabetic rat models and assessing ICP. ICP assessment after cavernosal nerve stimulation in rodents is a well-established animal model of ED.21 The results could pave the way for developing novel therapies for diabetic ED.
MATERIALS AND METHODS
Animals
Eight-week-old male Sprague–Dawley (SD) rats (200–220 g) with normal mating abilities were randomized to the normal control, diabetes, and diabetes + hWJ-MSCs groups. The animals were housed at 20°C–24°C and 40%–70% humidity, under a 12-h/12-h light cycle, with free access to rat chow and water. Diabetes was induced by intraperitoneal injection of streptozocin (STZ; 60 mg kg−1; Sigma-Aldrich Chemical Co., St. Louis, MO, USA). Control rats received an equal volume of citrate buffer. Seventy-two hours after STZ injection, the animals with plasma glucose concentrations >16.7 mmol l−1 were considered to be diabetic and examined in subsequent experiments.
Isolation and expansion of hWJ-MSCs
Umbilical cord samples were obtained from women delivering full-term infants by cesarean section at Shanghai General Hospital (Shanghai, China). After the removal of arteries and veins, Wharton's jelly was transferred into a sterile dish containing Dulbecco's modified essential medium (DMEM)/F12 (1:1; Gibco, Gaithersburg, MD, USA) and minced. The explants were transferred into 24-well plates in fresh culture medium (DMEM/F12 supplemented with 10% fetal bovine serum [FBS], 100 mg ml−1 penicillin, and 100 mg ml−1 streptomycin) and incubated for 5–7 days at 37°C in a humidified incubator with 5% CO2, to allow the migration of cells from the explants. The culture medium was replaced every 2 days. At 80%–90% confluency, the cells were harvested with a 0.05% trypsin/0.53 mmol l−1 ethylene diaminetetraacetic acid (EDTA) solution and seeded into larger culture flasks. The protocols for human umbilical cord sampling were approved by the Institutional Review Board of Shanghai General Hospital (No. 2017KY101), and written informed consent was obtained from each donor.
Flow cytometry
A total of 1 × 105 cells were washed with 2% FBS in phosphate-buffered saline (PBS; washing buffer) and resuspended in washing buffer containing mouse antihuman CD90, CD44, CD105, CD14, CD19, CD34, CD45, and human leukocyte antigen DR (HLA-DR) antibodies (1:100 dilution; eBioscience, Waltham, MA, USA). After two washes with the washing buffer, the cells were analyzed by flow cytometry on a CytoFLEX Platform (Beckman Coulter, Indianapolis, IN, USA), with the ExpoADCXL4 software (Beckman Coulter). Positive staining was defined as the emission of fluorescence signals exceeding the levels found in >99% of control cells stained with matched isotype antibodies (fluorescein isothiocyanate isomer [FITC]-conjugated and P-phycoerythrin [PE]-conjugated mouse IgG1κ monoclonal isotype standards).
hWJ-MSC transplantation
The hWJ-MSCs were labeled with 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (DiI; Molecular Probes Inc., Eugene, OR, USA) according to the manufacturer's instructions, with minor modifications. Briefly, 2 × 107 hWJ-MSCs were labeled with 2 × 105 mmol l−1 to 2 × 106 mmol l−1 DiI for 30 min at room temperature. After terminating the reaction with 2 ml of FBS, the cells were washed twice with 5 ml of DMEM/F12 (1:1) and transplanted. Eight weeks after diabetes induction, 1 × 106 hWJ-MSCs in 100 μl of saline were injected into the corpus cavernosum of the STZ-induced diabetic rats. The control group was administered saline alone.
ICP measurement
ICP changes after electrical stimulation of the cavernous nerve were evaluated as previously described.15 Briefly, the animals were submitted to anesthesia by intraperitoneal injection of thiopental (50 mg kg−1). After penile skin incision and prepuce removal, the corpus cavernosum was exposed. A 26-gauge needle containing heparinized saline was inserted into the corpus cavernosum to assess ICP.22 Through a lower abdominal incision, the lateral prostate was dissected, and the major pelvic ganglion was identified.23,24 The cavernous nerve was stimulated electrically as previously described,25 for 1 min at 5 V and 0.3–5.0 Hz. A 15-min rest followed each stimulation session. A frequency response curve was generated, with maximum ICP increase during stimulation assessed at each frequency.
Observation of the transplanted hWJ-MSCs
Immunofluorescence was performed to detect the transplanted MSCs. Cryostat sections of soleus muscle samples were fixed with 4% formaldehyde and blocked with 5% normal goat serum for 1 h. Then, MSCs were labeled with DiI (Thermo Fisher, Waltham, MA, USA), counterstained with 4’,6-diamidino-2-phenylindole (DAPI; Wako, Osaka, Japan), and analyzed under a fluorescence microscope (Leica, Osaka, Japan).
Quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR)
qRT-PCR was performed as previously described.26 RNA was extracted from corpus cavernosum samples with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Reverse transcription was performed on an AMV Reverse Transcription System (Promega, Madison, WI, USA). SYBR Green (Takara, Dalian, China) was used for qRT-PCR, and amplification conditions were one cycle at 95°C for 10 min; 45 cycles at 95°C for 30 s, 57°C for 30 s, and 72°C for 30 s. Relative gene expression levels were assessed by the 2−DDCt method, with β-actin as the reference gene. The specific primers used for VEGF, basic fibroblast growth factor (bFGF), endothelial nitric oxide synthase (eNOS), and IGF1 are shown in Supplementary Table 1.
Supplementary Table 1.
Primers used for quantitative reverse transcription-polymerase chain reaction
| Forward (5’–3’) | Reverse (5’–3’) | |
|---|---|---|
| VEGF | TGACGGACAGACAGACAGACACC | AGAGCCCAGAAGTTGGACGAA |
| bFGF | GGCTGCTGGCTTCTAAGTGT | CCAACTGGAGTATTTCCGTGA |
| eNOS | ACAGGCATCACCAGGAAGAAG | CTCAGAGCCATACAGGATAGTCG |
| IGF1 | GGCACTCTGCTTGCTCACCTT | ACGAACTGAAGAGCGTCCACC |
VEGF: vascular endothelial growth factor; bFGF: basic fibroblast growth factor; eNOS: endothelial nitric oxide synthase; IGF1: insulin-like growth factor 1
Immunohistochemistry
The protein expression levels of VEGF, eNOS, IGF1, and bFGF in corpus cavernosum samples were analyzed by immunohistochemistry at four weeks posttransplantation. Paraffin-embedded sections were deparaffinized and heat treated with citrate buffer (pH 6.0) for 10 min. After endogenous peroxidase quenching with 1% hydrogen peroxide for 30 min, nonspecific binding sites were blocked with 5% goat serum for 1 h. The sections were successively incubated at room temperature with primary antibodies against VEGF, bFGF, eNOS, and IGF1 (1:200 dilution; PeproTech, Rocky Hill, NJ, USA) for 1 h, respectively, and biotinylated secondary antibodies for 30 min. Avidin-biotin-peroxidase (Beyotime, Nantong, China) was added for detection, with the signals enhanced using 3-3’-diaminobenzidine. Counterstaining was performed with hematoxylin.
Statistical analyses
Data are shown as mean ± standard deviation (s.d.) and were analyzed by one-way analysis of variance (ANOVA), with Bonferroni correction for multiple comparisons. Two-tailed P < 0.05 was considered statistically significant.
RESULTS
Elevated blood glucose levels and decreased body weight after diabetes establishment
Compared with the control group, diabetic rats showed a 14% weight loss within 12 weeks of STZ injection (Table 1). Meanwhile, blood glucose levels in diabetic rats were elevated compared with control values. Meanwhile, there was no significant difference in blood glucose levels between diabetic rats administered hWJ-MSCs and saline (Table 1).
Table 1.
Serum glucose levels and body weights
| Variable | NDM | DM | MSC | P |
|---|---|---|---|---|
| Body weight (g) | ||||
| Initial | 214.5±9.85 | 218.0±7.52 | 217.0±6.75 | 0.25 |
| 12 weeks | 401.4±20.86 | 190.7±18.95 | 188.6±21.34 | <0.0001 |
| Blood glucose level (mmol l−1) | ||||
| 72 h | 6.76±0.26 | 23.85±1.62 | 24.59±2.32 | <0.0001 |
| 8 weeks | 6.75±0.31 | 24.26±2.11 | 25.02±2.18 | <0.0001 |
| 12 weeks | 7.01±0.28 | 22.46±2.31 | 23.64±3.22 | <0.0001 |
Each group included 10 animals, and values are shown as mean±s.d. MSCs: mesenchymal stem cells; NDM: normal rat; DM: diabetes rat; MSC: diabetes rat injected with mesenchymal stem cells; s.d.: standard deviation
Primary culture and characterization of hWJ-MSCs
Using bone marrow-derived MSCs as positive controls, flow cytometry showed that hWJ-MSCs expressed high levels of matrix markers (CD44, CD90, and CD105) but low amounts of hematopoietic lineage markers (CD14, CD34, CD19, and CD45) and HLA-DR (Figure 1). These findings indicated that the isolated hWJ-MSCs possessed stem properties.
Figure 1.
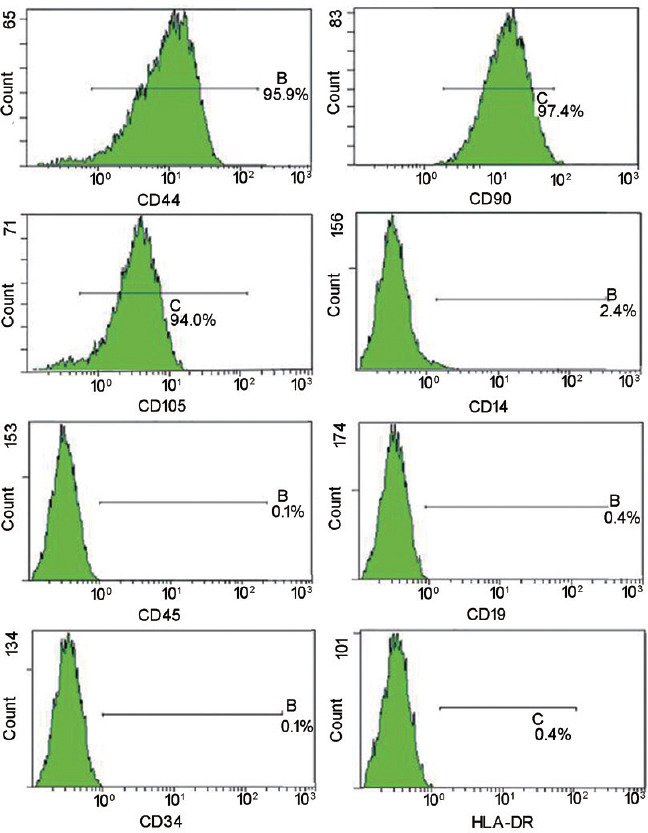
Validation of hWJ-MSCs by flow cytometry. Flow cytometry showed that hWJ-MSCs expressed high levels of matrix markers (CD44, CD90, and CD105) but low amounts of hematopoietic lineage markers (CD14, CD34, CD45, and CD19) and HLA-DR. hWJ-MSCs: human umbilical cord Wharton's jelly-derived mesenchymal stem cells; HLA-DR: human leukocyte antigen DR.
ICP is improved after hWJ-MSC transplantation
Four weeks after transplantation, hWJ-MSCs with red fluorescence were detected in corpus cavernosum specimens (Figure 2a). ICP measurements were performed during unilateral cavernous nerve (CN) stimulation at 4 weeks after hWJ-MSC injection (12 weeks after diabetes induction). Mean ICP in diabetic rats treated with saline was significantly lower than that of normal nondiabetic rats (29.7 ± 8.5 cmH2O vs 95.2 ± 10.6 cmH2O, P < 0.01; Table 2). Meanwhile, the mean ICP was significantly higher in diabetic rats administered hWJ-MSCs compared with diabetic animals after saline injection (65.4 ± 14.5 cmH2O vs 29.7 ± 8.5 cmH2O, P < 0.01; Table 2).
Figure 2.
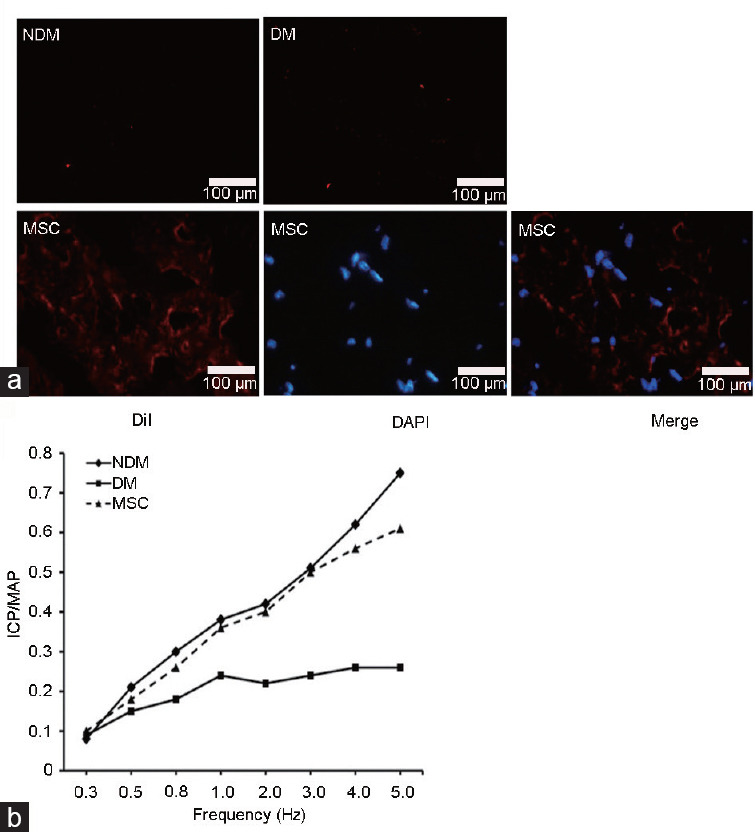
hWJ-MSC transplantation improves ICP. The NDM (normal rats with PBS injected into the corpus cavernosum), DM (diabetic rats with PBS injected into the corpus cavernosum), and MSC (diabetic rats with DiI-positive hWJ-MSCs injected into the corpus cavernosum) groups were assessed. (a) Four weeks after transplantation, DiI-positive hWJ-MSCs were mainly observed within the corpus cavernosum. (b) The ICP/MAP curve showed the effect of hWJ-MSC transplantation. hWJ-MSCs: human umbilical cord Wharton's jelly-derived mesenchymal stem cells; ICP: impaired intracavernosal pressure; DiI: 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate; DAPI: 4’,6-diamidino-2-phenylindole; MAP: mean arterial pressure; PBS: phosphate-buffered saline.
Table 2.
Intracavernosal pressure levels and intracavernosal pressure increase/mean arterial pressure were measured before treatment and 4 weeks after treatment
| Variable | NDM | DM | MSC | P |
|---|---|---|---|---|
| ICP (cmH2O) | ||||
| Pretreatment | 96.6±8.7 | 30.5±7.9 | 31.3±7.5 | 0.41 |
| Posttreatment | 95.2±10.6 | 29.7±8.5 | 65.4±14.5 | <0.01 |
| ICP/MAP | 0.76±0.21 | 0.26±0.14 | 0.61±0.18 | <0.01 |
Each group included 10 animals, and values are shown as mean±s.d. MSCs: mesenchymal stem cells; NDM: normal rat; DM: diabetes rat; MSC: diabetes rat injected with mesenchymal stem cells; s.d.: standard deviation; ICP: intracavernosal pressure; MAP: mean arterial pressure
Mean arterial pressure (MAP) values were similar in the control (126.7 ± 19.6 cmH2O) and treatment (119.4 ± 9.2 cmH2O) groups (P = 0.30). The ICP/MAP ratios were significantly higher in diabetic rats administered hWJ-MSCs (0.61 ± 0.18) than control animals (0.26 ± 0.14; P = 0.0004), as shown in Table 2 and Figure 2b. These findings suggested that hWJ-MSC transplantation could ameliorate diabetes-related ED.
hWJ-MSC transplantation increases the expression levels of growth factors
The expression levels of angiogenic and/or neurotrophic factors were evaluated to explore the mechanisms by which hWJ-MSCs improved diabetes-related ED. qRT-PCR showed that angiogenic and neurotrophic gene expression levels were significantly increased after hWJ-MSC transplantation (DM-hWJ-MSCs) compared with the DM-saline group, as determined for VEGF (2.3 ± 0.33 folds), bFGF (2.1 ± 0.46 folds), eNOS (1.2 ± 0.18 folds), and IGF1 (20.1 ± 0.56 folds) (all P < 0.01; Figure 3). A similar trend was observed at the protein level (Figure 4).
Figure 3.
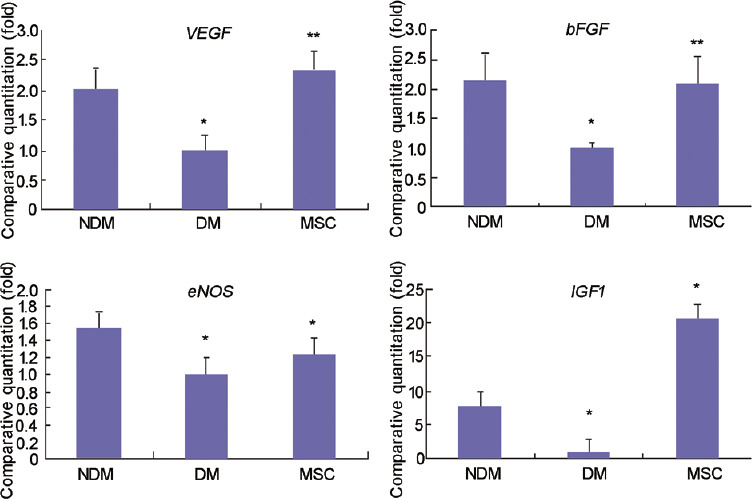
Gene expression levels of growth factors after hWJ-MSC transplantation. VEGF, bFGF, eNOS, and IGF1mRNA levels after saline or MSC injection into the corpus cavernosum. Data are mean ± standard deviation. *P<0.01, **P<0.05. hWJ-MSCs: human umbilical cord Wharton's jelly-derived mesenchymal stem cells; NDM: normal rat; DM: diabetes rat; MSC: diabetes rat injected with mesenchymal stem cells; VEGF: vascular endothelial growth factor; bFGF: basic fibroblast growth factor; eNOS: endothelial nitric oxide synthase; IGF1: insulin-like growth factor 1.
Figure 4.
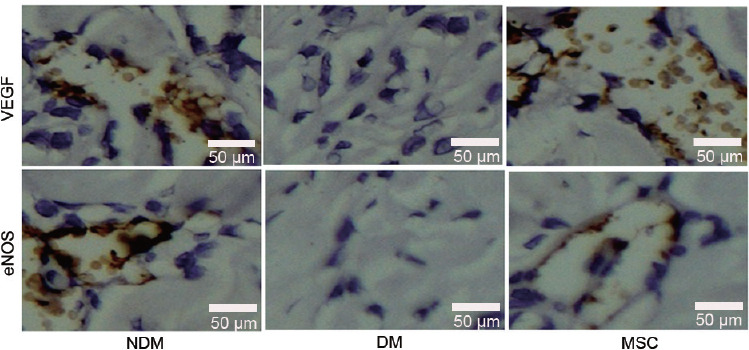
Protein expression levels of VEGF and eNOS after hWJ-MSC transplantation. Immunohistochemistry data showing VEGF and eNOS protein expression levels in various groups. hWJ-MSCs: human umbilical cord Wharton's jelly-derived mesenchymal stem cells; NDM: normal rat; DM: diabetes rat; MSC: diabetes rat injected with mesenchymal stem cells; VEGF: vascular endothelial growth factor; eNOS: endothelial nitric oxide synthase.
DISCUSSION
This study demonstrated that hWJ-MSC transplantation improves diabetic intracavernosal pressure, likely by increasing the production of paracrine growth factors, indicating hWJ-MSC transplantation might be an efficient therapeutic strategy for ED management.
MSCs attract increasing attention for tissue and organ regeneration because of their plasticity, easy isolation from bone marrow specimens, and expansion by repeated passage.19 MSCs can differentiate into multiple mesenchymal cell types,27 myelin-forming cells,28 neuron-like cells expressing nestin and neuronal nuclei (NeuN),29 neural cells,30 and the cellular components of vascular structures.31 MSCs are currently under investigation for the regeneration of various organs such as the spinal cord, heart, liver, and kidney.32–34
Subtle differences among MSCs from distinct tissue sources would probably influence transplantation efficacy.35,36 Due to Wharton's jelly's young age, MSCs obtained from this fetal tissue yield markedly more proliferative, immunosuppressive, and even therapeutically active stem cells compared with those from adult tissues.37 Other advantages of hWJ-MSCs over other stem cells include their pluripotency and the possibility to differentiate into bone, cartilage, fat, muscle, heart, and nerve cells.38,39 hWJ-MSC isolation is noninvasive with no related moral or ethical issues since umbilical cords are usually discarded, which is not the case for MSCs from other sources, including embryos and the bone marrow.40 Nevertheless, WJ-MSCs are necessarily considered to be allogeneic for transplantation in any patients, and the safety of the procedure requires further assessment. The efficacy of WJ-MSCs and autologous bone marrow MSCs for the management of diabetes-related ED could also be compared in future studies.
Flow cytometry showed that umbilical cord MSCs expressed high matrix markers (CD44, CD90, and CD105) ex-vivo but not hematopoietic lineage markers (CD14, CD34, and CD45) or HLA-DR,41,42 confirming the MSC nature of stroma cells from Wharton's jelly. The isolated hWJ-MSCs were transplanted into the corpus cavernosum of STZ-induced diabetic rats to alleviate ED. In this study, diabetic rats showed impaired ICP, which was significantly improved by treatment with hWJ-MSCs, indicating alleviated ED. Recent findings demonstrated that hWJ-MSCs are immunosuppressive and well tolerated in animals,43 avoiding immunological rejection.44 Corroborating the above results, these findings demonstrate the potential use of hWJ-MSCs for regenerative medicine, e.g., in ED treatment. A review published in 2020 identified 27 phase 1 and 2 trials using stem cells for the treatment of ED,45 highlighting the growing interest in this promising field.
The effects of hWJ-MSCs on growth factor production were evaluated to explore the possible mechanism by which they normalize erectile function, both at the gene and protein levels. VEGF, eNOS, IGF1, and bFGF levels were increased at the sites of hWJ-MSC injection in STZ-induced diabetic rats. Hence, hWJ-MSCs probably mediated ED improvement by increasing the production of growth factors that participate in tissue regeneration. Notably, the transplanted hWJ-MSCs had no overt adverse effects on nerve function and remained at the transplantation sites, suggesting the safety of this procedure, in agreement with previous findings.20
Of course, this study is limited because it was performed in animals, and translation of results to humans is uncertain. In addition, only four genes and the respective encoded proteins were examined, and future studies should explore the comprehensive mechanisms of ICP improvement by hWJ-MSCs. Besides, a dose–response or time–response relationship was not examined in this study. Finally, hWJ-MSCs were used in rats. Although these cells do not raise any immunological response in the host, the species barrier might remain. Stem cells act in different ways, and the paracrine action investigated in this study is only one of them, which could underestimate the total effects. Use of homologous cells might produce more impressive results and reveal more complex pathways of hWJ-MSC effects.
Overall, successively isolated hWJ-MSCs transplanted into the corpus cavernosum of STZ-induced diabetic rats alleviate diabetes-induced ED, as assessed by ICP changes. This is associated with increased production of growth factors, which might participate in tissue regeneration. These findings highlight a novel potential therapeutic option for ED. Nevertheless, further studies are needed to comprehensively determine the effects of hWJ-MSCs on ED.
AUTHOR CONTRIBUTIONS
JHW carried out the studies, participated in collecting data, and drafted the manuscript. DYW and LS performed the statistical analysis and participated in its design. WQQ participated in acquisition, analysis, and interpretation of data and draft the manuscript. SJX and QJ participated in conception and design the study. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No. 81400683).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Bella AJ, Lee JC, Carrier S, Benard F, Brock GB. 2015 CUA practice guidelines for erectile dysfunction. Can Urol Assoc J. 2015;9:23–9. doi: 10.5489/cuaj.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huri HZ, Mat Sanusi ND, Razack AH, Mark R. Association of psychological factors, patients’ knowledge, and management among patients with erectile dysfunction. Patient Prefer Adherence. 2016;10:807–23. doi: 10.2147/PPA.S99544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z, Li Z, Yu Q, Wu C, Lu Z, et al. The prevalence of and risk factors for prostatitis-like symptoms and its relation to erectile dysfunction in Chinese men. Andrology. 2015;3:1119–24. doi: 10.1111/andr.12104. [DOI] [PubMed] [Google Scholar]
- 4.Maiorino MI, Bellastella G, Esposito K. Lifestyle modifications and erectile dysfunction: what can be expected? Asian J Androl. 2015;17:5–10. doi: 10.4103/1008-682X.137687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yafi FA, Jenkins L, Albersen M, Corona G, Isidori AM, et al. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:16003. doi: 10.1038/nrdp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan MA, Hashim MJ, King JK, Govender RD, Mustafa H, et al. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107–11. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castela A, Costa C. Molecular mechanisms associated with diabetic endothelial-erectile dysfunction. Nat Rev Urol. 2016;13:266–74. doi: 10.1038/nrurol.2016.23. [DOI] [PubMed] [Google Scholar]
- 8.Jung DC, Park SY, Lee JY. Penile Doppler ultrasonography revisited. Ultrasonography. 2018;37:16–24. doi: 10.14366/usg.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, He WY, Lin F, Gou X. Panax notoginseng saponins improve erectile function through attenuation of oxidative stress, restoration of Akt activity and protection of endothelial and smooth muscle cells in diabetic rats with erectile dysfunction. Urol Int. 2014;93:92–9. doi: 10.1159/000354878. [DOI] [PubMed] [Google Scholar]
- 10.Pereira VA, Abidu-Figueiredo M, Pereira-Sampaio MA, Chagas MA, Costa WS, et al. Sinusoidal constriction and vascular hypertrophy in the diabetes-induced rabbit penis. Int Braz J Urol. 2013;39:424–31. doi: 10.1590/S1677-5538.IBJU.2013.03.17. [DOI] [PubMed] [Google Scholar]
- 11.Ayala-Cuellar AP, Kang JH, Jeung EB, Choi KC. Roles of mesenchymal stem cells in tissue regeneration and immunomodulation. Biomol Ther (Seoul) 2019;27:25–33. doi: 10.4062/biomolther.2017.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. doi: 10.3389/fimmu.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nourian Dehkordi A, Mirahmadi Babaheydari F, Chehelgerdi M, Raeisi Dehkordi S. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. 2019;10:111. doi: 10.1186/s13287-019-1212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol. 2016;2016:1–24. doi: 10.1155/2016/6940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ismail EA, El-Sakka AI. Innovative trends and perspectives for erectile dysfunction treatment: a systematic review. Arab J Urol. 2016;14:84–93. doi: 10.1016/j.aju.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangir N, Akbal C, Tarcan T, Simsek F, Turkeri L. Mesenchymal stem cell therapy in treatment of erectile dysfunction: autologous or allogeneic cell sources? Int J Urol. 2014;21:1280–5. doi: 10.1111/iju.12585. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang B, Sun X, Han D, Chen S, Yao B, et al. Human urine-derived stem cells alone or genetically-modified with FGF2 improve type 2 diabetic erectile dysfunction in a rat model. PLoS One. 2014;9:e92825. doi: 10.1371/journal.pone.0092825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu G, Sun X, Bian J, Wu R, Guan X, et al. Correction of diabetic erectile dysfunction with adipose derived stem cells modified with the vascular endothelial growth factor gene in a rodent diabetic model. PLoS One. 2013;8:e72790. doi: 10.1371/journal.pone.0072790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M, Li H, Ruan Y, Wang T, Liu J. Stem cell therapy for diabetic erectile dysfunction in rats: a meta-analysis. PLoS One. 2016;11:e0154341. doi: 10.1371/journal.pone.0154341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leow SN, Luu CD, Hairul Nizam MH, Mok PL, Ruhaslizan R, et al. Safety and efficacy of human Wharton's jelly-derived mesenchymal stem cells therapy for retinal degeneration. PLoS One. 2015;10:e0128973. doi: 10.1371/journal.pone.0128973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cellek S, Bivalacqua TJ, Burnett AL, Chitaley K, Lin CS. Common pitfalls in some of the experimental studies in erectile function and dysfunction: a consensus article. J Sex Med. 2012;9:2770–84. doi: 10.1111/j.1743-6109.2012.02916.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Ko M, Chae MR, Lee SJ, Kam SC, et al. Radiotelemetric assessment of intracavernosal pressure in apomorphine-induced erection: hypercholesterolemic rats vs normal control. Int J Impot Res. 2014;26:41–4. doi: 10.1038/ijir.2013.32. [DOI] [PubMed] [Google Scholar]
- 23.You D, Jang MJ, Kim BH, Song G, Lee C, et al. Comparative study of autologous stromal vascular fraction and adipose-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Stem Cell Transl Med. 2015;4:351–8. doi: 10.5966/sctm.2014-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufmann O, Claro J, Cury J, Andrade E, Longo B, et al. The development of a rat model of erectile dysfunction after radical prostatectomy: preliminary findings. BJU Int. 2008;102:1026–8. doi: 10.1111/j.1464-410X.2008.07760.x. [DOI] [PubMed] [Google Scholar]
- 25.Abdel Aziz MT, El-Haggar S, Mostafa T, Atta H, Fouad H, et al. Effect of mesenchymal stem cell penile transplantation on erectile signaling of aged rats. Andrologia. 2010;42:187–92. doi: 10.1111/j.1439-0272.2009.00977.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhao R, Bei X, Yang B, Wang X, Jiang C, et al. Endothelial cells promote metastasis of prostate cancer by enhancing autophagy. J Exp Clin Cancer Res. 2018;37:221. doi: 10.1186/s13046-018-0884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35:e00191. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mimura T, Dezawa M, Kanno H, Sawada H, Yamamoto I. Peripheral nerve regeneration by transplantation of bone marrow stromal cell-derived Schwann cells in adult rats. J Neurosurg. 2004;101:806–12. doi: 10.3171/jns.2004.101.5.0806. [DOI] [PubMed] [Google Scholar]
- 29.Abouelfetouh A, Kondoh T, Ehara K, Kohmura E. Morphological differentiation of bone marrow stromal cells into neuron-like cells after co-culture with hippocampal slice. Brain Res. 2004;1029:114–9. doi: 10.1016/j.brainres.2004.07.092. [DOI] [PubMed] [Google Scholar]
- 30.Keilhoff G, Goihl A, Stang F, Wolf G, Fansa H. Peripheral nerve tissue engineering: autologous Schwann cells vs. transdifferentiated mesenchymal stem cells. Tissue Eng. 2006;12:1451–65. doi: 10.1089/ten.2006.12.1451. [DOI] [PubMed] [Google Scholar]
- 31.Al-Khaldi A, Al-Sabti H, Galipeau J, Lachapelle K. Therapeutic angiogenesis using autologous bone marrow stromal cells: improved blood flow in a chronic limb ischemia model. Ann Thorac Surg. 2003;75:204–9. doi: 10.1016/s0003-4975(02)04291-1. [DOI] [PubMed] [Google Scholar]
- 32.Han Y, Li X, Zhang Y, Han Y, Chang F, et al. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8:886. doi: 10.3390/cells8080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohban R, Pieber TR. Mesenchymal stem and progenitor cells in regeneration: tissue specificity and regenerative potential. Stem Cells Int 2017. 2017:1–16. doi: 10.1155/2017/5173732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoogduijn MJ, Betjes MG, Baan CC. Mesenchymal stromal cells for organ transplantation: different sources and unique characteristics? Curr Opin Organ Transplant. 2014;19:41–6. doi: 10.1097/MOT.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 36.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–92. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 37.Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, et al. Wharton's jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14:11692–712. doi: 10.3390/ijms140611692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, et al. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 39.Karahuseyinoglu S, Kocaefe C, Balci D, Erdemli E, Can A. Functional structure of adipocytes differentiated from human umbilical cord stroma-derived stem cells. Stem Cells. 2008;26:682–91. doi: 10.1634/stemcells.2007-0738. [DOI] [PubMed] [Google Scholar]
- 40.Romano G. Stem cell transplantation therapy: controversy over ethical issues and clinical relevance. Drug News Perspect. 2004;17:637–45. doi: 10.1358/dnp.2004.17.10.873915. [DOI] [PubMed] [Google Scholar]
- 41.Ramos TL, Sanchez-Abarca LI, Muntion S, Preciado S, Puig N, et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal. 2016;14:2. doi: 10.1186/s12964-015-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan SL, Ahmad TS, Selvaratnam L, Kamarul T. Isolation, characterization and the multi-lineage differentiation potential of rabbit bone marrow-derived mesenchymal stem cells. J Anat. 2013;222:437–50. doi: 10.1111/joa.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, et al. Immune properties of human umbilical cord Wharton's jelly-derived cells. Stem Cells. 2008;26:2865–74. doi: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- 44.Cho PS, Messina DJ, Hirsh EL, Chi N, Goldman SN, et al. Immunogenicity of umbilical cord tissue derived cells. Blood. 2008;111:430–8. doi: 10.1182/blood-2007-03-078774. [DOI] [PubMed] [Google Scholar]
- 45.He M, von Schwarz ER. Stem-cell therapy for erectile dysfunction: a review of clinical outcomes. Int J Impot Res. 2020;33:271–7. doi: 10.1038/s41443-020-0279-8. [DOI] [PubMed] [Google Scholar]


