In this Outlook, Yoon and Shi discuss two papers in this issue of Genes & Development from Boreikaite et al. and Schmidt et al., who report their independent reconstitution of human pre-mRNA 3′ end processing.
Keywords: 3′ processing, CPSF, poly(A) polymerase, RBBP6, RNA cleavage, RNA processing, RNA, endonuclease, gene expression, polyadenylation
Abstract
It is every biochemist's dream to reconstitute a biological process in vitro using defined components, because doing so not only reduces a biological phenomenon to one or a series of biochemical reactions, but also defines the minimal list of essential components. In this issue of Genes & Development, Boreikaite and colleagues (pp. 210–224) and Schmidt and colleagues (pp. 195–209) report their independent reconstitution of human pre-mRNA 3′ end processing.
Eukaryotic pre-mRNA 3′ end processing typically takes place in two tightly coupled steps: an endonucleolytic cleavage of the pre-mRNA and the addition of a poly(A) tail. One exception to this general mechanism is the 3′ end formation of replication-dependent histone mRNAs in metazoans, which only involves a cleavage step. In vitro pre-mRNA 3′ processing assays were established in the 1980s and, similar to transcription and splicing, this was accomplished first in mammalian cell extracts and later in budding yeast extracts (Moore and Sharp 1985; Butler and Platt 1988). Using these assays, many essential 3′ processing factors were purified and identified. For the mammalian system, the poly(A) polymerase (PAP) was the first pre-mRNA 3′ processing factor to be characterized followed by several multiprotein complexes such as the cleavage and polyadenylation specificity factor (CPSF), cleavage stimulation factor (CstF), and mammalian cleavage factors I (CFIm) and II (CFIIm) (Chan et al. 2011). Several additional 3′ processing factors, including Rbbp6, were discovered by characterizing the human pre-mRNA 3′ processing complexes assembled on RNA substrates (Shi et al. 2009). Meanwhile, in yeast, biochemical and genetic analyses have identified the CPF, CFIA, and CFIB complexes as pre-mRNA 3′ processing factors. Most 3′ processing factors are conserved between yeast and humans, but both systems have unique factors as well. Finally, many histone pre-mRNA 3′ processing factors have been identified, including SLBP, FLASH, U7 snRNP, and a number of canonical pre-mRNA 3′ processing factors, including CPSF and CstF subunits (Sun et al. 2020).
After nearly four decades of research, it was widely believed that most, if not all, essential pre-mRNA 3′ processing factors had been identified. Therefore, the stage was set for reconstituting pre-mRNA 3′ processing in vitro with recombinant components. Yeast pre-mRNA 3′ processing was the first to be successfully reconstituted. To do so, the Passmore group (Hill et al. 2019) used 13 recombinant proteins, including the eight-subunit CPF core complex, the four-subunit CFIA, and the single protein CFIB. In 2020, the Tong, Dominski, and Walz laboratories (Sun et al. 2020) used, coincidentally, 13 recombinant proteins and one snRNA to recapitulate human histone pre-mRNA 3′ processing in vitro. Now, Boreikaite et al. (2022) and Schmidt et al. (2022) add another exciting chapter by reconstituting human pre-mRNA 3′ processing using 13 proteins, including CPSF, CstF, CFIIm, PAP, and Rbbp6 (Fig. 1; Boreikaite et al. 2022; Schmidt et al. 2022).
Figure 1.
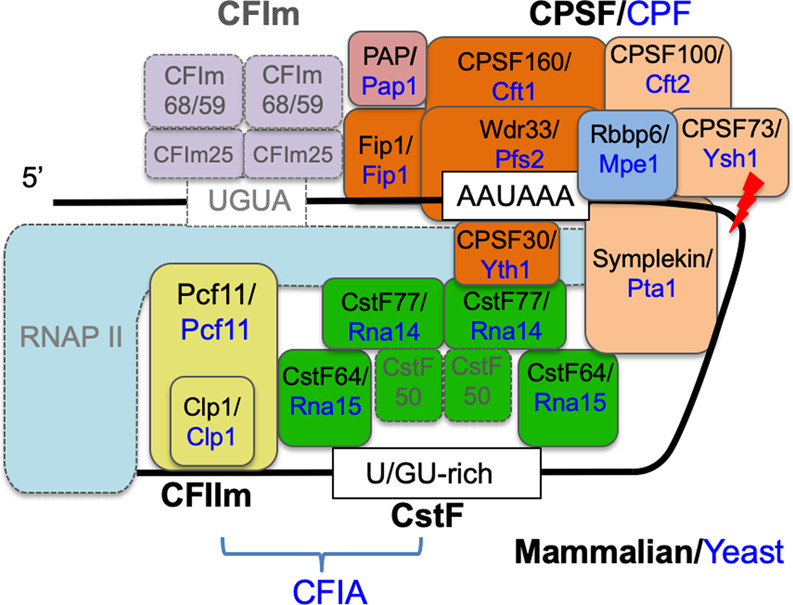
A model for the core human pre-mRNA 3′ processing machinery. The essential pre-mRNA 3′ processing factors are shown as boxes with solid outlines, and the nonessential ones are shown as boxes with dotted outlines. The names of the essential mammalian factors and their corresponding yeast homologs are shown in black and blue, respectively.
One of the most important insights from these studies is that the core pre-mRNA 3′ processing machinery is remarkably conserved (Boreikaite et al. 2022; Schmidt et al. 2022). First, all 13 proteins necessary for reconstituting human pre-mRNA 3′ processing are homologs of yeast 3′ processing factors (Fig. 1). With the exception of symplekin, the yeast homologs of all essential human pre-mRNA 3′ processing factors are also required for yeast pre-mRNA 3′ end formation (Fig. 1). Second, several mammalian factors that were previously thought to be essential for pre-mRNA 3′ processing, but lack yeast homologs, were found to be dispensable. These include CFIm, which consists of CFIm25 and CFIm68/59, and CstF50, a subunit of CstF. They may play regulatory roles instead. For example, CFIm acts as an enhancer-dependent activator of pre-mRNA 3′ processing (Zhu et al. 2018). Third, Rbbp6/Mpe1 plays a key and conserved role in stimulating the cleavage step in both humans and yeast. Previous studies identified the CPSF subunit CPSF73 (or Ysh1 in yeast) as the endonuclease for both canonical and histone pre-mRNA 3′ processing. CPSF73/Ysh1 alone has low endonuclease activities, as it adopts a closed conformation, and therefore must be activated within the pre-mRNA 3′ processing complex. Recent studies in yeast suggest that Mpe1 activates Ysh1 through direct interactions near its active site (Hill et al. 2019). Now, the current studies provide strong evidence that Rbbp6, the Mpe1 homolog, plays a similar role in humans. Originally identified as an Rb- and p53-binding protein, Rbbp6 was first implicated in pre-mRNA 3′ processing when it was detected in the human pre-mRNA 3′ processing complex (Shi et al. 2009). Follow-up studies provided evidence that Rbbp6 is required for pre-mRNA cleavage (Di Giammartino et al. 2014). However, its role in pre-mRNA 3′ processing remained underappreciated, which was due at least in part to the fact that the homology between Rbbp6 and Mpe1 is limited to its N-terminal region and that, unlike Mpe1, Rbbp6 does not stably associate with the human pre-mRNA 3′ processing complex (Shi et al. 2009). Using purified recombinant proteins and transient overexpression assays, the current studies provide evidence that Rbbp6 directly interacts with CPSF73 near its active site, and this interaction is likely to activate the nuclease activity of CPSF73 (Boreikaite et al. 2022; Schmidt et al. 2022). This mechanism may be similar to histone pre-mRNA 3′ processing, in which a U7 snRNP protein, Lsm10, binds to CPSF73 and induces the latter to shift to an open conformation that allows RNA binding and cleavage (Sun et al. 2020).
While the findings from both studies are highly consistent, some differences do exist. First, Schmidt et al. (2022) found that PAP was essential for pre-mRNA cleavage, consistent with previous studies using purified factors from HeLa nuclear extract. However, Boreikaite et al. (2022) managed to reconstitute cleavage in the absence of PAP. Second, Schmidt et al. (2022) found an essential role for ATP in cleavage and provided evidence that ATP serves as a cofactor for Clp1, but no such requirement was observed by Boreikaite et al. (2022). The exact reasons for such discrepancies remain unclear for now but could reflect differences in the exact polypeptides and conditions used. Usage of truncated proteins at high concentration in vitro may overcome some barriers that exist under native conditions. Further studies are needed to clarify these issues.
The establishment of reconstituted yeast and human pre-mRNA 3′ processing systems represents an important milestone for the field. In turn, these systems provide unprecedented opportunities for in-depth structural and functional dissection of the core machinery and regulatory factors. We look forward to seeing new advances building on these studies in the coming years, including the structures of the pre-mRNA 3′ processing complexes.
Acknowledgments
We thank our team members for their discussion and comments. Work in the Shi laboratory is currently supported by National Institutes of Health grants R01GM090056, R01GM12844, R21AI166703, R01MH122556, and R01AI155962.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.349453.122.
References
- Boreikaite V, Elliot TS, Chin JW, Passmore LA. 2022. RBBP6 activates the pre-mRNA 3′ end processing machinery in humans. Genes Dev (this issue). 10.1101/gad.349223.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JS, Platt T. 1988. RNA processing generates the mature 3′ end of yeast CYC1 messenger RNA in vitro. Science 242: 1270–1274. 10.1126/science.2848317 [DOI] [PubMed] [Google Scholar]
- Chan S, Choi EA, Shi Y. 2011. Pre-mRNA 3'-end processing complex assembly and function. Wiley Interdiscip Rev RNA 2: 321–335. 10.1002/wrna.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giammartino DC, Li W, Ogami K, Yashinskie JJ, Hoque M, Tian B, Manley JL. 2014. RBBP6 isoforms regulate the human polyadenylation machinery and modulate expression of mRNAs with AU-rich 3′ UTRs. Genes Dev 28: 2248–2260. 10.1101/gad.245787.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CH, Boreikaitė V, Kumar A, Casañal A, Kubík P, Degliesposti G, Maslen S, Mariani A, von Loeffelholz O, Girbig M, et al. 2019. Activation of the endonuclease that defines mRNA 3′ ends requires incorporation into an 8-subunit core cleavage and polyadenylation factor complex. Mol Cell 73: 1217–1231.e11. 10.1016/j.molcel.2018.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CL, Sharp PA. 1985. Accurate cleavage and polyadenylation of exogenous RNA substrate. Cell 41: 845–855. 10.1016/S0092-8674(85)80065-9 [DOI] [PubMed] [Google Scholar]
- Schmidt M, Kluge F, Sandmeir F, Kühn U, Schäfer P, Tüting C, Ihling C, Conti E,Wahle E. 2022. Reconstitution of 3′ processing of mammalian pre-mRNA reveals a central role of RBBP6. Genes Dev (this issue). 10.1101/gad.349217.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Di Giammartino DC, Taylor D, Sarkeshik A, Rice WJ, Yates JR III, Frank J, Manley JL. 2009. Molecular architecture of the human pre-mRNA 3′ processing complex. Mol Cell 33: 365–376. 10.1016/j.molcel.2008.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang Y, Aik WS, Yang XC, Marzluff WF, Walz T, Dominski Z, Tong L. 2020. Structure of an active human histone pre-mRNA 3'-end processing machinery. Science 367: 700–703. 10.1126/science.aaz7758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Wang X, Forouzmand E, Jeong J, Qiao F, Sowd GA, Engelman AN, Xie X, Hertel KJ, Shi Y. 2018. Molecular mechanisms for CFIm-mediated regulation of mRNA alternative polyadenylation. Mol Cell 69: 62–74.e4. 10.1016/j.molcel.2017.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]


