Abstract
Attack by the specialist herbivore, Manduca sexta, on its native host Nicotiana attenuata Torr. ex Wats. produces a dramatic ethylene release, a jasmonate burst, and a suppression of the nicotine accumulation that results from careful simulations of the herbivore's damage. Methyl-jasmonate (MeJA) treatment induces nicotine biosynthesis. However, this induction can be suppressed by ethylene as pretreatment of plants with 1-methylcyclopropene (1-MCP), a competitive inhibitor of ethylene receptors, restores the full MeJA-induced nicotine response in herbivore attacked plants (J. Kahl, D.H. Siemens, R.J. Aerts, R. Gäbler, F. Kühnemann, C.A. Preston, I.T. Baldwin [2000] Planta 210: 336–342). To understand whether this herbivore-induced signal cross-talk occurs at the level of transcript accumulation, we cloned the putrescine methyltransferase genes (NaPMT1 and NaPMT2) of N. attenuata, which are thought to represent the rate limiting step in nicotine biosynthesis, and measured transcript accumulations by northern analysis after various jasmonate, 1-MCP, ethephon, and herbivory treatments. Transcripts of both root putrescine N-methyltransferase (PMT) genes and nicotine accumulation increased dramatically within 10 h of shoot MeJA treatment and immediately after root treatments. Root ethephon treatments suppressed this response, which could be reversed by 1-MCP pretreatment. Moreover, 1-MCP pretreatment dramatically amplified the transcript accumulation resulting from both wounding and M. sexta herbivory. We conclude that attack from this nicotine-tolerant specialist insect causes N. attenuata to produce ethylene, which directly suppresses the nitrogen-intensive biosynthesis of nicotine.
Plants clearly respond differently to tissue damage caused by abiotic factors and pathogens, and the mechanisms responsible for this differential recognition and response may involve crosstalk among at least three different signal transduction pathways: JA, ethylene, and salicylates (SA; Dong, 1998; Reymond and Farmer, 1998; Maleck and Dietrich, 1999; Pieterse and van Loon, 1999; Paul et al., 2000). The particular combination of pathways activated in a response is often highly specific to the damaging agent but certain patterns are emerging. JA and ethylene frequently act synergistically, inducing defense responses that are distinct from, and often antagonized by those induced by SA (Dong, 1998; Reymond and Farmer, 1998; Pieterse and van Loon, 1999). Protease inhibitors (PI; O'Donnell et al., 1996; Koiwa et al., 1997), defensin, and certain pathogenesis-related (PR) proteins (Penninckx et al., 1998; Thomma et al., 1998) are examples of defense-oriented genes, which are synergistically induced through JA and ethylene signal cascades. The cloning of EIN2, which functions as a bifunctional transducer of ethylene and JA signal transduction, provides a molecular basis for the synergy between the two pathways (Alonso et al., 1999). However, not all responses are consistent with the JA-ethylene synergy and the JA/ethylene-SA antagonism paradigms, demonstrating that other combinations of signal crosstalk exist (Reymond and Farmer, 1998; Pieterse and van Loon, 1999). For example, the PR1b and PR5 genes of tobacco are induced more strongly by the combination of SA and JA than by SA alone (Xu et al., 1994), and in Arabidopsis, ethylene enhances the induction of PR1 by SA (Lawton et al., 1994). Ethylene antagonizes the local expression of the JA-induced GS-II lectin genes in Griffonia simplicifolia (Zhu-Salzman et al., 1998) and other JA-inducible defense genes in Arabidopsis (Rojo et al., 1999).
The interplay of JA, SA, and ethylene in plant-herbivore interactions is less studied than it is in plant-pathogen interactions, but it is known that herbivore-damage often results in different physiological, biochemical, and molecular responses in plants than does mechanical damage (Baldwin, 1990; Turlings et al., 1990; Hartley and Lawton, 1991; Tomlin and Sears, 1992; Stout et al., 1994; Korth and Dixon, 1997; Reymond et al., 2000; Schittko et al., 2000, 2001; Hermsmeier et al., 2001). For example, feeding by the larvae of Manduca sexta elicits responses in its Nicotiana host plants, which are clearly distinguishable from those of careful mechanical simulations of feeding damage. In Nicotiana sylvestris and its sibling species, Nicotiana attenuata, mechanical damage induces a rapid increase in JA in wounded leaves and a slightly delayed systemic increase in the roots, which results in a systemic, whole plant (WP) increase in the potent defense metabolite, nicotine, 5 d later (Baldwin et al., 1994a, 1994b, 1997). Moreover, strong positive relationships exist among the amount of wounding, leaf JA concentrations, and WP nicotine in N. sylvestris (Baldwin et al., 1997; Ohnmeiss et al., 1997), and inhibition of the wound-induced increase in leaf JA at the wound site with lipoxygenase inhibitors also inhibits nicotine induction (Baldwin et al., 1996, 1997).
It is interesting that feeding by M. sexta larvae or applying its oral secretions and regurgitants (R) to leaf wounds elicits higher endogenous leaf JA levels than does mechanical wounding (McCloud and Baldwin, 1997; Schittko et al., 2000). However, despite these higher JA concentrations, neither root JA nor WP nicotine levels increased above those induced by mechanical damage (McCloud and Baldwin, 1997; Kahl et al., 2000). A similar result was obtained by Baldwin (1988) who found higher nicotine responses in N. sylvestris plants subjected to a careful simulation of the larval feeding damage, than in plants damaged by the larvae themselves.
Clearly, M. sexta larvae interfere with the WP systemic defense response of its host plant and larval elicitation of ethylene production appears to be the mechanism responsible. Kahl et al. (2000) recently discovered that a single application of Manduca's R induced a short-lived burst in ethylene emission and that larval feeding resulted in a sustained ethylene release. Moreover, 2-chloroethylphosphonic acid (ethephon), which breaks down to release ethylene at cytoplasmic pH, inhibited MeJA-induced nicotine production when applied to plants in an amount comparable with that elicited by herbivore attack. Additionally, when 1-methylcyclopropene (1-MCP), a gaseous antagonist of ethylene receptors, was used to uncouple ethylene perception from its production in the plant, the MeJA- and wound-mediated nicotine induction in ethephon- and R-treated plants, respectively, could be fully restored (Kahl et al., 2000). In short, the JA accumulation induced by caterpillar feeding or its R does not elicit the expected proportionally larger increase in WP nicotine due to the ethylene burst, which is also specifically elicited by M. sexta herbivory.
Here we characterize at a transcriptional level the interplay between JA and ethylene on nicotine biosynthesis and accumulation in N. attenuata. Nicotine is synthesized from the polyamine, putrescine, and putrescine N-methyltransferase (PMT: EC 2.1.1.53) catalyzes the N-methylation of putrescine in the first committed, and likely regulatory step of nicotine biosynthesis (Hibi et al., 1994 and references therein). We clone the two PMTs of N. attenuata, find their transcripts only in root tissues, and monitor their accumulation in response to various JA, ethylene, wounding, and herbivore treatments.
RESULTS
Isolation and Sequence Analysis of N. attenuata PMT Genes
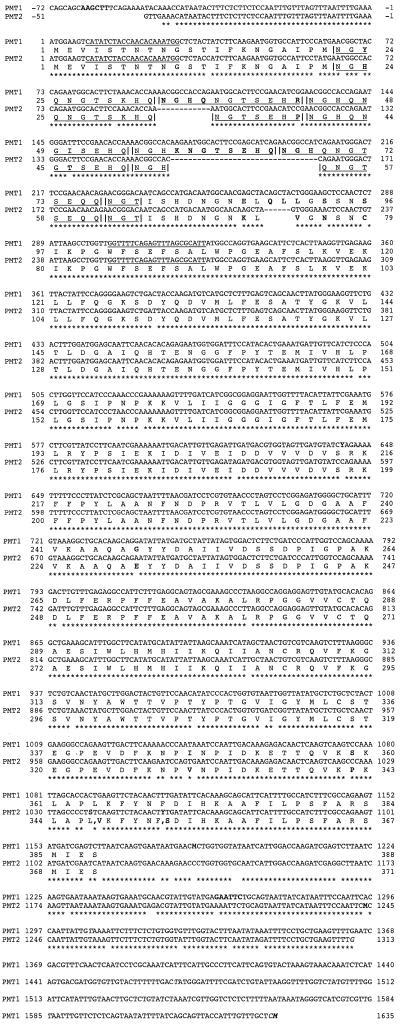
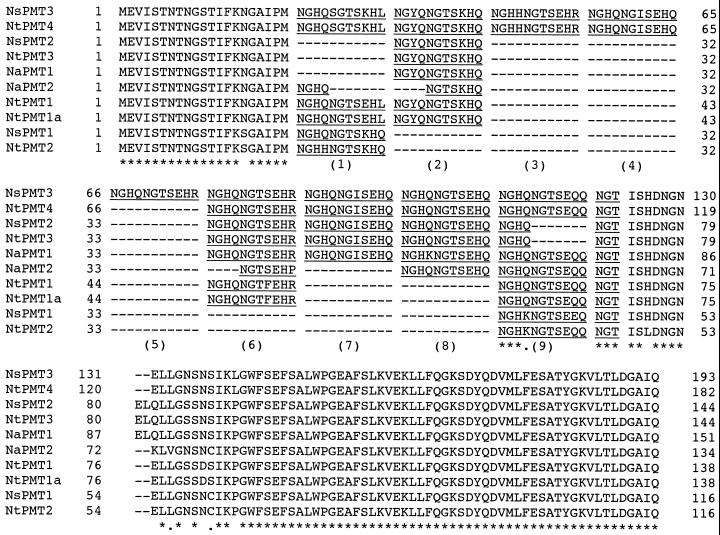
A partial 0.95-kb cDNA for N. attenuata PMT was isolated by reverse transcriptase (RT)-PCR using primers designed from the published sequence for Nicotiana tabacum PMT1, which is the NtPMT gene thought to have been derived from the ancestral N. tomentosiformis PMT gene (Riechers and Timko, 1999). This cDNA was used to probe a cDNA library, which was created from several pooled native populations of N. attenuata, resulting in 102 initial positives of the approximately 200,000 pfu screened. Of these initial positives, 43 phage clones were at least as long as the probe fragment, containing the amino-terminal tandem repeat zone. PCR-analysis of the repeat zone revealed that there were two types of PMT inserts, which varied in the length of their repeat zone. Of the 43 “full-length” clones, five had repeat zones of approximately 280 bp (NaPMT2), whereas the rest were approximately 350 bp (NaPMT1) long. Three NaPMT2 and eight NaPMT1 clones were sequenced (Fig. 1). NaPMT1 cDNAs ranged from 1,378 to 1,707 bp, corresponding to 388 amino acid residues, whereas NaPMT2 was 1,364 bp and 371 amino acid residues long. Start codons were assigned based on homology to the published PMT sequences from other Nicotiana species. All three NaPMT2 cDNA clones had the same polyadenylation sites, while the eight NaPMT1 clones possessed five different poly(A) sites among them, ranging from 139 to 468 bp after the stop codon. When the two N. attenuata PMT cDNAs were compared at the amino acid-level to PMTs from other Nicotiana species, major sequence differences were, not surprisingly, restricted to the amino-terminal tandem repeat zone (Fig. 2).
Figure 1.
Nucleotide and deduced amino acid sequences of NaPMT1 and NaPMT2 cDNA clones. The first Met residues, as well as the A of the ATG start codons, are numbered +1. Nucleotide identity is designated by a star. Amino acid differences and deletions, as well as nucleotide heterogeneity among individual clones for either NaPMT1 or NaPMT2, are highlighted in bold, as are the HindIII and EcoRI sites of NaPMT1. Polyadenylation sites are highlighted in italics. Individual amino-terminal repeat motifs are delineated by a vertical slash and underlined. PCR primers used to generate repeat-specific probes are underlined. Y, C or T; S, G or C; M, A or C. EMBL/GenBank/DDBJ accession numbers for NaPMT1 and NaPMT2 are AF280402 and AF280403, respectively.
Figure 2.
Divergence at the amino acid level among PMT genes from various Nicotiana species is mainly at the amino terminus. Amino acid sequences for the amino-terminal ends of the two N. attenuata (Na), three N. sylvestris (Ns), and four of the five N. tabacum (Nt; including two cultivar variants for NtPMT1) PMT genes were aligned. Identity is designated by a star and similarity by a period.
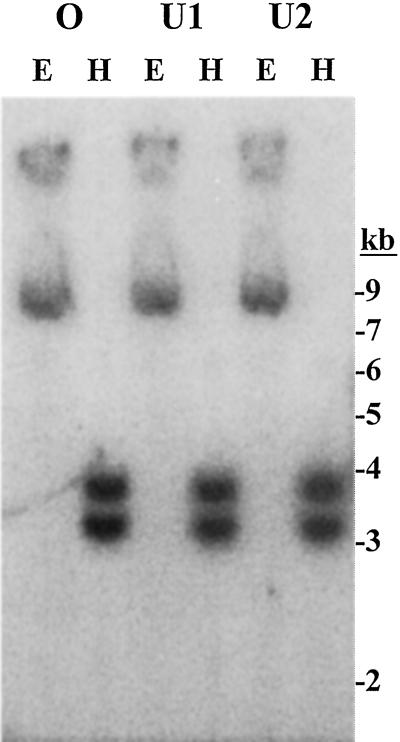
The partial 0.95-kb cDNA initially isolated by RT-PCR was derived from NaPMT1 and originated from sequence located well within the stretch from the HindIII site at the beginning of the 5′-UTR and the EcoRI site within all five 3′-UTRs (Fig. 1). NaPMT2 contained neither of these two restriction sites. When this RT-PCR fragment was used as a probe for a Southern blot of genomic DNA, which had been digested with either EcoRI or HindIII, the presence of two PMT genes was confirmed in three different native populations of N. attenuata (Fig. 3).
Figure 3.
N. attenuata has two PMT genes. Genomic DNA (10 μg) from N. attenuata plants from three different wild populations (one from Oregon [O] and two from Utah [U1, U2]) was digested with either EcoRI (E) or HindIII (H). The blot was analyzed using a 32P-labeled probe derived from full-length NaPMT1, i.e. a general PMT probe.
Effects of MeJA and Ethylene on Nicotine Biosynthesis
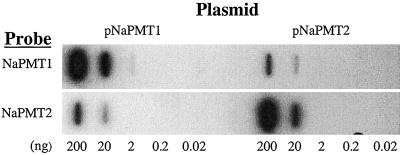
By using primers located within the conserved regions located on either side of the repeat zone (Fig. 1) in a PCR reaction with [α-32P]dCTP, we constructed probes specific for either NaPMT1 or NaPMT2. Their specificity was confirmed by probing two slot-blotted dilution series of plasmids, one containing an NaPMT1 insert and the other containing NaPMT2 (Fig. 4). In this manner, for each individual plant subjected to a specific treatment and harvested at a specific time, the mRNA levels of the two NaPMT transcripts in the roots could be monitored simultaneously with the corresponding nicotine concentration in the shoots. The slightly broader bands seen for NaPMT1 (Figs. 5–7) most likely reflect this gene's use of five different poly(A) sites. No NaPMT transcripts could be detected in any shoot tissues during any stage of ontogeny.
Figure 4.
Specific detection of the two PMT genes of N. attenuata. NaPMT probe specificity was verified by slot-blotting five different quantities (0.02–200 ng) of NaPMT plasmid (pNaPMT1 or pNaPMT2), and probing with either a NaPMT1- or NaPMT2-specific 32P-labeled PCR fragment.
Figure 5.
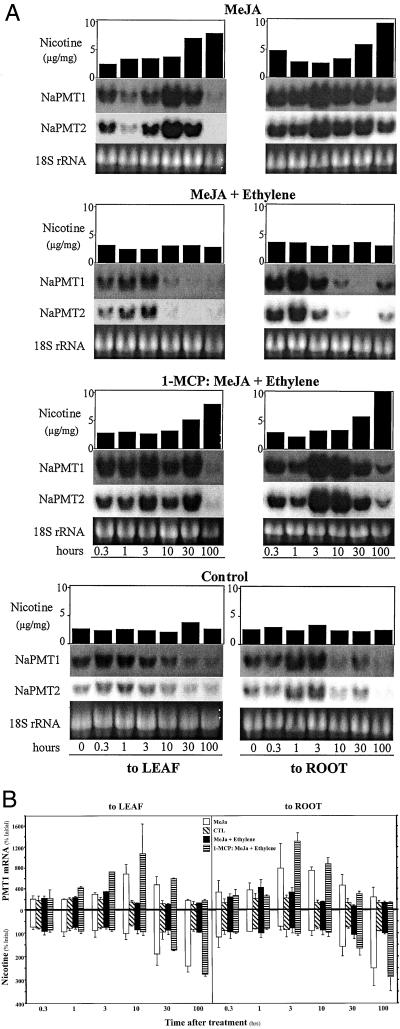
Ethylene suppresses MeJA-induced accumulation of NaPMT1 and NaPMT2 mRNA in roots and nicotine in shoots. A, All treatments were applied to individual (n = 1) hydroponically grown N. attenuata plants. MeJA treatments were applied either to the leaf (left, in lanolin paste) or roots (right, as an aqueous suspension): 50 μg of MeJA, 50 μg of MeJA plus 300 μg of ethephon to roots, 50 μg of MeJA plus 300 μg of ethephon to roots with 1-MCP pretreatment, lanolin leaf, or distilled water root controls. Roots and shoots were harvested separately at T = 0, 0.3, 1, 3, 10, 30, and 100 h after treatment (underlined). Leaf nicotine concentration (μg mg dry weight−1) was determined for each plant. Total root RNA (10 μg) was probed using either an NaPMT1- or NaPMT2-specific 32P-labeled PCR fragment. 18S rRNA bands served as loading control. A depicts one set of results from two replicate experiments. B, NaPMT1 mRNA (top, quantified by slot blot and phosphor imager) and nicotine (bottom) levels from plants treated as in A (left, MeJA to leaf; right, MeJA to root), are presented as percent of the initial (T = 0) level. B depicts the average values (±se) from two replicate experiments (n = 2).
Figure 7.
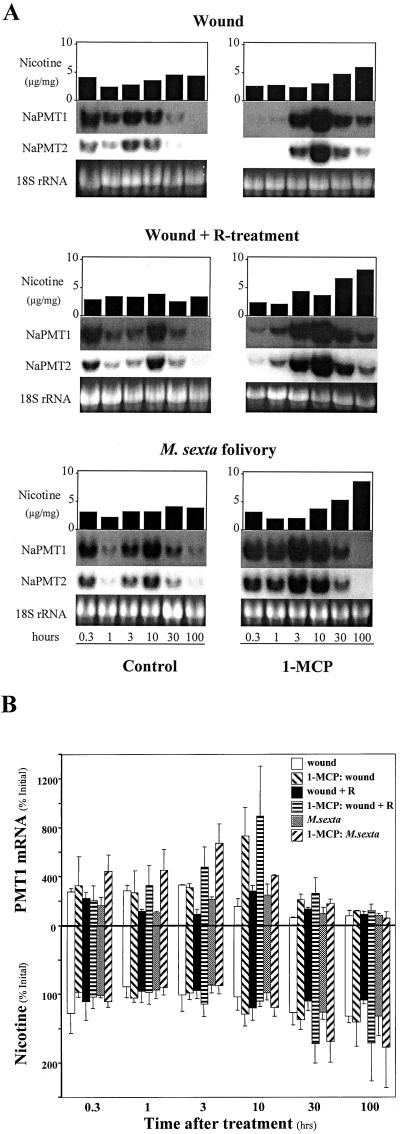
Simultaneous application of MeJA and ethylene mimics the effect of M. sexta herbivory on the accumulation of NaPMT1 and NaPMT2 mRNA in roots and nicotine in shoots of N. attenuata plants. A, Individual hydroponically grown plants (n = 1) received the following leaf wounding treatments without (left, control) and with (right) 1-MCP pretreatment: leaf wounding (wound); leaf wounding plus M. sexta R (wound + R-treatment); M. sexta larval feeding for 40 to 80 min (M. sexta folivory). Harvests, leaf nicotine determinations, and NaPMT1- and NaPMT2-specific RNA blotting were performed as in Figure 5. A depicts one set of results from three replicate experiments. B, NaPMT1 mRNA (top, quantified by slot blot and phosphor imager) and nicotine (bottom) levels from plants treated as in A, are presented as percent of the initial (T = 0) level. B depicts the average values (±se) from three replicate experiments (n = 3).
Figures 5, 6, and 7 each consist of two parts: part A and part B. The three A parts represent one entire experiment, depicting one typical set of results from two to three replicate experiments; therefore for each time point of every treatment in Figures 5A, 6A, and 7A, leaf nicotine (μg mg dry shoot−1), root NaPMT1, NaPMT2, and 18S rRNA levels all originate from the same, single plant (n = 1). Figures 5B, 6B, and 7B represent the average values (± se) for leaf nicotine (percentage of initial level) and root NaPMT1 rRNA levels (percentage of initial level) for the two to three replicate experiments performed for each time point and treatment (n = 2 or 3).
Figure 6.
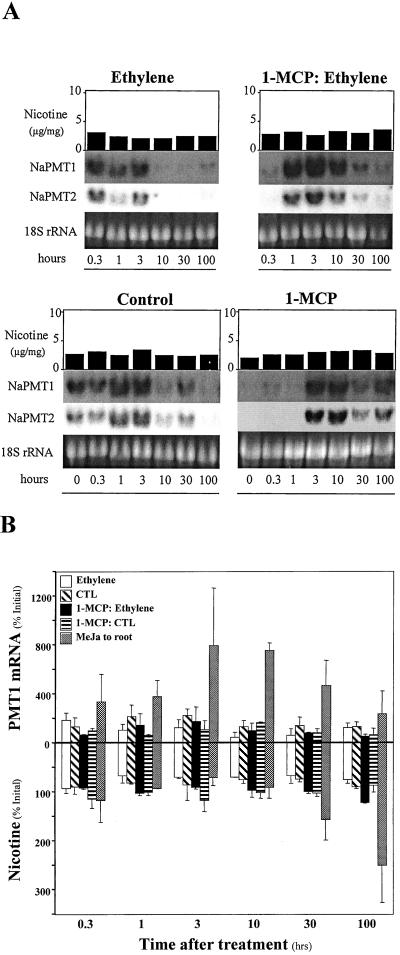
Ethylene does not affect baseline levels of NaPMT1 and NaPMT2 mRNA in roots and nicotine in shoots. A, All treatments were applied to individual (n = 1) hydroponically grown N. attenuata plants: 300 μg of ethephon to roots (ethylene), distilled water root control (control), 300 μg of ethephon to roots with 1-MCP pretreatment (ethylene + 1-MCP), 1-MCP pretreatment only (1-MCP). Harvests, leaf nicotine determinations, and NaPMT1- and NaPMT2-specific RNA blotting were performed as in Figure 5. A depicts one set of results from two replicate experiments. B, NaPMT1 mRNA (top, quantified by slot blot and phosphor imager) and nicotine (bottom) levels from plants treated as in A, are presented as percent of the initial (T = 0) level. For the sake of comparison, the MeJA to root treatment from Figure 5B has also been included. B depicts the average values (±se) from two replicate experiments (n = 2).
Northern analysis of both NaPMT mRNAs (Fig. 5) demonstrated that transcripts accumulated to maximum levels within 10 h after leaf MeJA application and 3 h after root application, consistent with the hypothesis that nicotine induction occurs at the level of PMT gene expression in roots. Increases in whole-shoot nicotine concentrations occurred within 30 h of leaf and root MeJA treatments (Fig. 5). In contrast, we found that ethylene, when added as the ethylene-releasing compound, ethephon, to the hydroponic medium of individual N. attenuata plants, effected neither the levels of nicotine nor NaPMT transcripts differently from distilled water control treatments (Fig. 6). In some experiments, pretreating plants with the ethylene receptor inhibitor, 1-MCP, may have delayed changes in NaPMT transcripts observed in control plants, and ethephon treatment of 1-MCP-pretreated plants counteracted this delay slightly, possibly even causing an increase in NaPMT transcript levels. However, none of these alterations in transcript abundance affected nicotine levels (Fig. 6A).
When ethephon is added simultaneously to the hydroponic medium of the same plant, as MeJA is added to either the roots or leaves, a dramatic reduction is observed in MeJAs ability to induce leaf nicotine and root NaPMT mRNA levels (Fig. 5). This suppression was most pronounced when MeJA was applied to the leaves. When plants were pretreated with 1-MCP prior to the addition of ethephon and MeJA, the suppressive effect of ethylene was inhibited and the induction of PMT transcripts and nicotine accumulation by MeJA was restored.
Ethylene-MeJA Signal Interaction Mimics Herbivory by M. sexta
We used three different leaf-wounding protocols to investigate the effects of endogenously produced JA and ethylene on nicotine biosynthesis: wounding, wounding plus M. sexta R, and M. sexta folivory. The three treatments therefore represent a series of increasingly realistic simulations of herbivory, and in all three treatments, plants were exposed to 1-MCP or left untreated to understand the role that ethylene plays in the accumulation of transcripts important for nicotine biosynthesis. Compared with untreated control plants (Fig. 5), simple leaf wounding produced modest increases in root NaPMT mRNA and shoot nicotine levels with NaPMT mRNA peaking around 3 h (Fig. 7). Once again, 1-MCP pretreatment may have delayed root NaPMT mRNA accumulation (peak at 10 h), however, increases in the levels of root NaPMT mRNA and, to a lesser extent, leaf nicotine were observed. When R was applied to plant wounds, the levels of NaPMT mRNA and nicotine accumulation were similar to or less than those found in wounded plants treated with water (Fig. 7). 1-MCP pretreatment of plants in the wound plus R treatment dramatically increased NaPMT mRNA and nicotine levels. Rapidly feeding M. sexta larvae similarly produced effects that are temporally and quantitatively similar to those found in the wound plus R treated plants (Fig. 7). Again, 1-MCP pretreatment drastically increased nicotine biosynthesis at the mRNA and enzymatic product level, however the characteristic delay in NaPMT transcript accumulation associated with 1-MCP treatment may have been decreased or absent (peak at 3 h).
DISCUSSION
N. attenuata PMT Genes, Nicotine Biosynthesis, and Their Regulation
As expected from the DNA sequence data for PMT from other Nicotiana species (Hibi et al., 1994; Hashimoto et al., 1998; Riechers and Timko, 1999), the two N. attenuata PMT cDNAs are almost identical except for their repeat zones, where NaPMT1 has five complete 11-amino acid repeats followed by one partial repeat (NGT). NaPMT2 may have been derived from an ancestral PMT1 gene, which has, based on DNA sequence identity, experienced two deletions in the repeat zone, as well as one deletion immediately 3′ to the repeat zone (Fig. 1). Because the N. attenuata cDNA library used in this study was constructed from tissues pooled from six geographically distinct native populations, it is highly likely that the three variable bases seen among NaPMT1 clones and the three variable bases among NaPMT2 clones reflect allelic variations among the different populations. Of these six variations, three occur within the coding regions, whereby the two from NaPMT2 lead to amino acid substitutions: Leu ↔ Val and Phe ↔ Ser (Fig. 1).
When the two N. attenuata PMT cDNAs were compared at the amino acid-level with PMTs from other Nicotiana species, both NaPMT1 and NaPMT2 possessed a relatively intermediate number of repeats, and NaPMT1 most resembled NsPMT2 and NtPMT3, where the major difference was a deletion in the last complete repeat (Fig. 2.). The Nicotiana species for which at least partial PMT sequence data is available are either progenitors of the natural amphidiploid, N. tabacum, i.e. N. sylvestris, N. tomentosiformis, and N. otophora, or N. tabacum itself (Hibi et al., 1994; Hashimoto et al., 1998; Riechers and Timko, 1999). Nicotiana species are grouped together into several sections, which are then grouped into the three subgenera of Tabacum, Rustica, and Petuniodes (Goodspeed, 1954). N. tomentosiformis and N. otophora have been grouped within the same section (Tomentosae) within the subgenus Tabacum, whereas N. sylvestris (Alatae) and N. attenuata (Acuminatae) have been placed into two different sections within the Petuniodes (Goodspeed, 1954). Contrary to this classification based on morphology, a classification based on RAPDs suggests that the relationship between N. sylvestris and N. attenuata is more distant with the Acuminatae being most closely related to the section Paniculatae of the subgenus Rustica (Bogani et al., 1997). The similarity, however, between the repeats of NaPMT1 and NsPMT2 may yet suggest a common PMT ancestor gene, as originally predicted by Goodspeed (1954).
Leaf nicotine pools have been previously and presently shown to increase dramatically after application of JA or JA-mimics to either the shoots or roots of Nicotiana species (Fig. 5; Baldwin et al., 1994a, 1996, 1997, 1998; Baldwin, 1996b; Zhang et al., 1997), reaching a maximum approximately 5 d (100 h) after elicitation. The accumulation to maximum levels of both NaPMT mRNAs within 10 h after leaf MeJA application and 3 h after root application (Fig. 5) is consistent with the hypothesis that nicotine induction occurs at the level of PMT gene expression in roots. Similar to previous 15NO3 pulse-chase experiments in which increases in de novo nicotine biosynthesis could be detected within 21 h of leaf wounding (Baldwin et al., 1994a), in this study increases in whole-shoot nicotine concentrations occurred within 30 h of leaf and root treatments (Fig. 5). These results confirm in planta the observations first made with N. tabacum cell cultures, that increases in levels of NtPMT transcripts precede increases in nicotine accumulation (Imanishi et al., 1998, 2000). In contrast, in Atropa belladonna, where PMT represents the first step in the formation of the tropane alkaloids, hyoscyamine and scopolamine, JA treatment does not increase transcript levels of AbPMT1 and AbPMT2 (Suzuki et al., 1999). However, neither the ecological functions nor the elicitors of these alkaloids are known.
Ethylene is known to suppress the JA-induced accumulation of nicotine but not influence constitutive nicotine levels (Kahl et al., 2000). We similarly found that ethephon affected neither the levels of nicotine nor NaPMT transcripts differently from control treatments (Fig. 6). We assume therefore that the level of NaPMT transcript accumulation observed under these conditions is responsible for the constitutive nicotine production that maintains the allometrically determined rate of nicotine production in concert with plant growth (Ohnmeiss and Baldwin, 1994; Baldwin, 1996b; Baldwin and Schmelz, 1996). However, the simultaneous addition of ethephon to the hydroponic medium and MeJA to either the roots or leaves resulted in a dramatic reduction in leaf nicotine and root NaPMT mRNA (Fig. 5). This suppression was most pronounced when MeJA was applied to the leaves, which represents the spatial relationship between induced JA and nicotine biosynthesis during folivory; JA is synthesized in the leaves of N. sylvestris and apparently transported to the roots via the phloem (Zhang and Baldwin, 1997). These spatial relationships also account for the more rapid accumulation of NaPMT mRNA when MeJA was added to the roots.
In this study three different leaf wounding protocols were used to investigate the effects of endogenously produced JA and ethylene on nicotine biosynthesis. First, we wounded the leaves of N. attenuata with a serrated pattern tracing wheel, which has been shown in previous work to produce a modest yet significant pulse of JA at 1 h and a transient increase in root nicotine biosynthesis within 1 d, which results in an increase in leaf nicotine pools in 5 d without increasing ethylene emissions (Baldwin et al., 1997, 1998; Kahl et al., 2000). This leaf wounding protocol produces modest induced increases in nicotine concentrations (Fig. 7; Baldwin et al., 1998) but has the advantage that it does not remove significant amounts of leaf area and does not influence plant growth (Baldwin et al., 1998). Wounding protocols that remove leaf area result in larger induced increases in nicotine concentrations but also influence plant growth and biomass accumulation (Baldwin and Schmelz, 1994; Baldwin et al., 1998), which confounds the quantification of the induced response. Hence this leaf wounding protocol functioned as a vehicle for R application without influencing the plant's growth allometry and served as a control for the wound plus R treatment. Second, we applied R to the puncture wounds, which has also been shown in our previous studies to cause JA accumulation at 1 h to increase by at least 10-fold and ethylene emissions by 5-fold and significantly reduce leaf nicotine accumulation in comparison with levels in equivalently wounded plants whose wounds are treated with water (Kahl et al., 2000). Third, we allowed several large (mainly fourth and fifth instar), hungry (3-h starvation period) M. sexta larvae to consume approximately half the shoot within 80 min. The rapid food intake of these larvae ensured that the kinetics of ethylene release from plants in this treatment were more comparable with those of the wounding plus R treatment. Constant 24-h feeding by two third instar M. sexta larvae has also been previously shown to produce a dramatic increase in ethylene emission for the duration of feeding activity, whereas R application to leaf wounds produces an ethylene burst that reaches a maximum at 2 h and rapidly declines to baseline levels (Kahl et al., 2000). The three treatments used in this study therefore represent a series of increasingly realistic simulations of herbivory.
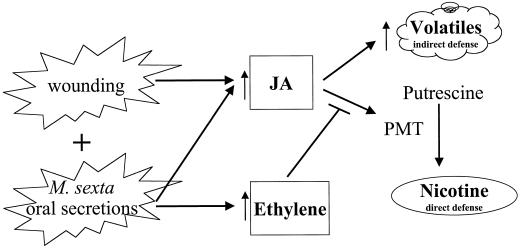
It is interesting that when 1-MCP-pretreated N. attenuata plants were subjected to simple leaf wounding, levels of root NaPMT mRNA increased when compared with plants that were not pretreated with 1-MCP (Fig. 7). Presumably, this is because 1-MCP inhibits the action of endogenously produced background levels of ethylene, which do not result in detectable increases in ethylene release into the atmosphere (Kahl et al., 2000). Other studies examining the synergistic effects of ethylene and JA on PI (O'Donnell et al., 1996) or PDF1.2 (Penninckx et al., 1998) induction with ethylene inhibitors, mutants, or plants grown in sterile agar culture, have reported results consistent with the model that plants produce small amounts of ethylene that are not normally detectable in the atmosphere, even with very sensitive photo-acoustic laser spectrometric detection techniques (Kahl et al., 2000). When R was applied to the wounds of 1-MCP pretreated N. attenuata plants or when 1-MCP pretreated plants were subjected to rapidly feeding M. sexta larvae, NaPMT mRNA and nicotine levels increased dramatically compared with plants not pretreated with 1-MCP (Fig. 7), presumably due to the increased JA biosynthesis that R application or M. sexta folivory has previously been shown to induce (Kahl et al., 2000; Schittko et al., 2000). However, the characteristic delay in NaPMT transcript accumulation associated with 1-MCP treatment may have been decreased or absent for M. sexta folivory (peak at 3 h) when compared with R application to wounds (peak at 10 h; Fig. 7). Since the addition of ethephon alone to 1-MCP-pretreated plants has also been observed to counteract this delay slightly (Fig. 6), the rapid M. sexta feeding may have produced more ethylene than did the wounding plus R treatment. M. sexta feeding resulted in damage to both leaf veins and the shoot apical meristem, whereas the wounding plus R treatment did not, and these differences in damage could be responsible for the differences in effects. In summary (Fig. 8), the proportional relationships between endogenous JA production and nicotine production that have been previously observed with mechanical wounding (Baldwin et al., 1997; Ohnmeiss et al., 1997), which are disrupted by Manduca feeding or Manduca R (Baldwin, 1988; McCloud and Baldwin, 1997; Kahl et al., 2000), are restored when the ability of N. attenuata to perceive its own ethylene production is inhibited by 1-MCP. We conclude that the Manduca-induced ethylene burst is responsible for inhibiting JA-induced nicotine production at the level of transcript accumulation.
Figure 8.
Signal cross-talk between JA and ethylene in N. attenuata differentially affects its induced defenses against herbivory by M. sexta. See “Discussion” for details.
Role of Ethylene in JA-Induced Defenses Against Herbivores
Because herbivores are frequently larger, more mobile and physiologically independent from their host plants than most pathogens, plant defense responses against herbivores occur at larger spatial scales within the plant (Baldwin and Preston, 1999). These differences in spatial scales may account for some of the differences observed in the effect of ethylene on JA-induced responses. JA is known to elicit strong induced resistance against a variety of insects (Howe et al., 1996; McConn et al., 1997; Baldwin, 1998) but is known to both synergize and antagonize putative defensive traits. On a small spatial scale and in plants with small statures (i.e. Arabidopsis), ethylene suppresses JA-inducible genes in the immediate area of the wound, thereby allowing the same genes to be induced by JA in adjacent undamaged tissues (Zhu-Salzman et al., 1998; Rojo et al., 1999). In several species, ethylene functions synergistically with JA in eliciting PI genes, the PDF1.2 defensin genes, and some PR proteins (Xu et al., 1994; O'Donnell et al., 1996; Koiwa et al., 1997; Penninckx et al., 1998), all of which are elicited in leaves. Here we demonstrate that attack to the leaves of N. attenuata by its specialist herbivore, M. sexta, elicits an ethylene burst that inhibits at the level of transcript accumulation one of the plant's major defense responses in the roots, namely nicotine induction. An understanding of the natural history of nicotine induction for both the plant and the herbivore provides a context for understanding why the plant-produced ethylene antagonizes this particular defense response, while synergizing other defense responses, even in related solanaceous plants.
Nicotine biosynthesis is sequestered below ground in root tissues, a location that has both liabilities and advantages for plant defense. Below-ground sequestration protects the induced defense response when plants are attacked by folivores, and allows plants to launch a full, induced nicotine response even after the loss of 88% of a plant's canopy (Baldwin and Schmelz, 1994). Root sequestration of nicotine biosynthesis allows plants to both defend the remaining tissues after an herbivore attack and allow these tissues to re-organize in preparation for regrowth of the canopy lost to the herbivores (Baldwin and Ohnmeiss, 1994b). If nicotine biosynthesis was located in the leaves, the loss of canopy area would likely compromise the plants ability to launch an induced defense response, particularly one that requires the de novo synthesis of defense compounds. Moreover, the biosynthetic demands of nicotine synthesis may interfere with regrowth processes. Once nicotine is synthesized in the roots, it is carried apoplastically in the xylem stream to the shoot (Baldwin, 1989). However, root sequestration of defense also demands a long-distance signal transduction cascade for its activation and, with it, time delays for its activation (3 d for WP changes and 5 d for maximal responses; Baldwin et al., 1998). Tailoring the output of this long-distance JA-induced response for the attack of this particular nicotine-resistant herbivore consequently may require a diffusible gaseous signal, such as ethylene, which can have different effects on different defenses produced in different tissues. These considerations beg the question, when M. sexta feeds on N. attenuata is ethylene biosynthesis activated in the leaves or roots or both? Both ACC synthase and ACC oxidase exist as multicopy genes and are known to be expressed in a tissue-specific manner (Johnson and Ecker, 1998).
Why would a plant repress one of its major defense responses when attacked by a specialist herbivore? The ethylene burst may allow N. attenuata to optimize the function of its putative indirect defense, the herbivore-induced release of volatile mono- and sesqui-terpenes (Halitschke et al., 2000; Kahl et al., 2000), which are thought to attract parasitoids to feeding larvae. Parasitoids of M. sexta are negatively affected by the nicotine in their hosts (Thorpe and Barbosa, 1986). Since the ethylene burst reduces the amount of nicotine that M. sexta larvae accumulate in their tissues through dietary intake, it may reduce the mortality of the third trophic level. The observation that the volatile release is not inhibited by ethylene (Kahl et al., 2000) is consistent with this adaptive scenario, and the ethylene burst may therefore be the mechanism by which N. attenuata switches from deploying direct defenses to indirect defenses (Fig. 8). However, it remains to be demonstrated that parasitoid and predator attraction by herbivore-induced plant volatiles can increase plant fitness in any plant-herbivore system.
Additionally, the ethylene mediated suppression of PMT genes may allow a plant to optimize its resource allocation among direct and indirect defenses and tolerance mechanisms after herbivore attack. Exogenous JA treatment dramatically increases the lifetime fitness of N. attenuata plants growing in natural populations that are being attacked but decreases the fitness of plants that are not attacked (Baldwin, 1998). Hence JA-induced defenses are beneficial when needed but costly when they are not. The costs are most pronounced when plants grow with strong intraspecific competitors (van Dam and Baldwin, 1998) and result in large part from a decreased ability to acquire nitrogen from the soil (Baldwin and Hamilton, 2000) and from using so much of their assimilated nitrogen for nicotine biosynthesis (8% of WP nitrogen; Baldwin et al., 1998), an investment that cannot be recouped by metabolism (Baldwin and Ohnmeiss, 1994a; Baldwin et al., 1994a, 1998). A recent experimental test of the impact of the ethylene burst on defense-related opportunity costs that are readily observed when plants are treated with MeJA and grown in competition with untreated plants, concluded that the ethylene burst reduced MeJA-induced opportunity costs and increased the competitive strength of R-treated plants (Voelckel et al., 2001). However, to assess why ethylene reduces fitness costs of JA-induced resistance, we need to determine how much of the fitness costs can be directly attributed to nicotine production and whether ethylene attenuates or intensifies other JA-induced defenses in N. attenuata as reported for other plant species (Xu et al., 1994; O'Donnell et al., 1996; Penninckx et al., 1998; Zhu-Salzman et al., 1998). In summary, M. sexta attack may elicit defense induction in the attacked leaves and adjacent tissues, which are synergistically activated by both JA and the ethylene burst, and suppress its nicotine defense in the roots, as a way of optimizing its defense response against this nicotine-tolerant herbivore.
The effects of ethylene on PMT transcripts are not common among the suite of N. attenuata genes whose transcripts are specifically induced by Manduca attack or the application of its R to plant wounds. In a northern analysis of transcripts of genes encoding R-specific expression patterns, namely, Thr deaminase, pathogen-induced oxygenase, a photosystem II light-harvesting protein (LHB C1), a retrotransposon homolog, and three unknown genes, ethylene was found not to be responsible for any of the specific patterns of expression (Schittko et al., 2001). Hence, it remains a possibility that the response of PMT to ethylene is a result of this herbivore's ability to eat “stealthily” to specifically suppress nicotine production.
Two research articles have been published recently demonstrating (a) jasmonate induction of root PMT genes (Shoji et al., 2000b), and (b) ethylene suppression of jasmonate-induced gene expression in nicotine biosynthesis (Shoji et al., 2000a). Both studies were done using N. sylvestris.
MATERIALS AND METHODS
Materials
The following chemicals were purchased commercially: methyl-JA (MeJA; lot no. 05310–068) and lanolin (Aldrich, St. Louis); ethephon (2-chloroethylphosphonic acid; Union Carbide, Research Triangle, NC); 1-MCP (1-methylcyclopropene; Biotechnologies for Horticulture, Burr Ridge, IL; sold as EthylBloc [0.43% (w/w) 1-MCP]); DNA and RNA size markers (Gibco/BRL, Rockville, MD). The software used for primer selection was Primer Premier (Premier Biosoft International, Palo Alto, CA), and MacVector (Oxford Molecular, Oxford) was used for sequence alignments. Seeds were collected from three native populations of Nicotiana attenuata Torr. ex Wats. in Utah, as well as from single populations in Oregon, California, and Arizona. DI 92 is an inbred line of N. attenuata originating from SW Utah (T40S R19W, section 10, 1988). Manduca sexta Linnaeus larvae were hatched from eggs (Carolina Biological Supply, Burlington, NC) and reared en masse on N. attenuata plants until the majority had attained the fourth or fifth instar. Oral secretions and R were collected from third to fifth instar larvae fed N. attenuata foliage, and stored under argon at −80°C until being centrifuged and diluted 50% (v/v) with distilled water before use (Schittko et al., 2000, 2001).
Isolation of PMT cDNA Clones
N. attenuata plants from six native populations were grown hydroponically and exposed to 24 h of feeding by second instar M. sexta larvae prior to root harvesting. Equal portions of root material from each population were pooled for total RNA isolation. mRNA was isolated using an oligo(dT)25 mRNA isolation kit (Dynabeads, Dynal, Oslo), from which a poly(T)-primed, induced root cDNA library was prepared commercially in Lambda ZAP II (Stratagene, LaJolla, CA) by directional cloning into EcoRI/XhoI. Two hundred thousand plaque-forming units (pfu) were plated, blotted, and screened using standard molecular biological techniques (Sambrook et al., 1989). A PMT cDNA fragment (0.95 kb) was synthesized by RT-PCR from N. attenuata total root RNA using primers (5′-ATGGAAGTCATATCTACCAACAC and 5′-GATATGTTGGAGCGGTTG) based on the N. tabacum PMT1 cDNA sequence (accession no. D28506). The RT-PCR product was blunt-ended with T4 DNA polymerase, ligated into the SmaI site of pUC19, and sequenced from both ends. The same primers were used to amplify the PMT insert, after which the resulting PCR fragment was subjected to 1% (w/v) agarose gel electrophoresis, excised, and purified using a gel extraction kit (Nucleospin Extract; Macherey-Nagel, Düren, Germany). The purified fragment was labeled with 32P using a random prime labeling kit (Rediprime II; Amersham-Pharmacia Biotech, Little Chalfont, UK), and after removing unincorporated radio-nucleotides by gel filtration, used as a probe for all PMT genes. Blots were washed five times at 65°C in 2× SSC, 0.1% (w/v) SDS before autoradiography. Initial positive phage clones were PCR-screened for full-length using primers based on the sequenced N. attenuata RT-PCR fragment (5′-CATATCTACC-AACACAAATGG and 5′-TAAAGACTTGACGACAGTTA-GC), as well as for the length of the amino-terminal tandem repeat zone (5′-CATATCTACCAACACAAATGG and 5′-AATGCGCTAAACTCTGAAAACC; Hashimoto et al., 1998). Plaque-pure phage clones of interest were excised in vivo into plasmids as described in the Lambda ZAP II protocol (Stratagene) and their DNA inserts sequenced.
Plant Treatment and Harvests
Seeds from N. attenuata inbred genotype DI 92 were germinated on smoke-treated soil and grown in 1-L hydroponic pots (Ohnmeiss and Baldwin, 1994) in walk-in growth rooms under conditions described in van Dam and Baldwin (1998). After 12 to 17 d of growth, six plants of similar size and appearance were assigned to each treatment group and placed into small 18.5-L growth chambers individually fitted with Plexiglas (UV-T) lids and 14.5-cm fans (described in Preston et al., 1999). Each individual plant's hydroponic solution was supplemented with 2 mm KNO3 the day before experimental treatments (T = 0) commenced. Where required, exposure to 1-MCP took place the night before T = 0. The six plants within each treatment were harvested at six different time points after treatment application: 0.3 (18 min), 1, 3, 10, 30, and 100 h. Roots and shoots were harvested and weighed separately before flash-freezing in N2(L). Additional groups of two plants per chamber, with or without 1-MCP pretreatment, were harvested at T = 0; these represent duplicate untreated zero time points. In this experimental design, with the exception of the zero time points, there was only one plant (n = 1) for any given treatment at any given harvest time within each experiment. The entire experiment was replicated twice in its entirety and three times for the treatments shown in Figure 7. Plants within each experiment were most comparable, but less so between experiments because of size differences and the allometry of nicotine induction (Baldwin, 1996a).
1-MCP pretreatment consisted of adding 10 mL of aqueous 0.134 n KOH, 0.187 n NaOH to 500 mg 1-MCP in a scintillation vial in the six-plant chambers scheduled to receive 1-MCP. The addition of the base liberated 1-MCP as a gas into the chamber, and chambers were sealed and fans turned off for at least 6 h during the dark period before T = 0. A fresh vial of 1-MCP was placed in each chamber after treatment application but with the chamber's fan turned on to provide flow-through ventilation.
Treatments were as follows: (a) 300 μg of ethephon freshly dissolved in 1 mL of distilled water and applied to the roots of each plant; (b) 300 μg of ethephon applied to the roots of each plant with 1-MCP pretreatment; (c) 50 μg of MeJA applied in 20 μL of lanolin paste to the adaxial surfaces of two fully expanded leaves of each plant; (d) 50 μg of MeJA applied to two fully expanded leaves, plus 300 μg of ethephon applied to the roots of each plant; (e) 50 μg of MeJA applied to two fully expanded leaves, plus 300 μg of ethephon applied to the roots of each plant with 1-MCP pretreatment; (f) 50 μg of MeJA applied in 1 mL of distilled water (stock solution kept in sonic water bath to keep MeJA in suspension) to the roots of each plant; (g) 50 μg of MeJA applied to the roots, plus 300 μg of ethephon applied to the roots of each plant; (h) 50 μg of MeJA applied to the roots, plus 300 μg of ethephon applied to the roots of each plant, with 1-MCP pretreatment; (i) 1 mL of distilled water applied to the roots of each plant; (j) 20 μL of lanolin paste applied to the adaxial surfaces of two fully expanded leaves; (k) leaf wounding, which entailed applying 40 to 60 μL of distilled water to eight rows of puncture wounds made by rolling a serrated pattern tracing wheel parallel with the midrib over the six oldest leaves of each plant; (l) leaf wounding as in treatment 11, with 1-MCP pretreatment; (m) leaf wounding, but applying 40 to 60 μL of a 50% (v/v) dilution of M. sexta R to the wounds of the six oldest leaves of each plant; (n) leaf wounding plus R with 1-MCP pretreatment; (o) three to six M. sexta larvae (third to fifth instar, and starved for 3 h) actively feeding on each plant for the first 40 to 80 min after T = 0, resulting in the removal of approximately half the shoot mass; (p) three to six actively feeding M. sexta larvae with 1-MCP pretreatment; (q) no treatment; and (r) no treatment with 1-MCP pretreatment.
Isolation and Blotting of Genomic DNA
Plant genomic DNA was prepared from young leaves of N. attenuata using cetyltrimethylammonium bromide (Reichardt and Rogers, 1994). DNA samples (10 μg) were restriction digested, size-fractionated by 0.8% (w/v) agarose gel electrophoresis, and Southern blotted onto nylon membrane with high-salt buffer (Brown, 1995). The blot was analyzed with the same 32P-labeled general PMT probe used to isolate the PMT cDNA clones.
Isolation and Blotting of Total RNA
Root and shoot tissues were pulverized in N2(L) using a mortar and pestle. From this powder, total RNA was extracted using the acid guanidinium thiocyanate-phenol-chloroform method (Chomczynski and Sacchi, 1987), which was modified by Ogawa (1999) as follows: after the first isopropanol precipitation, the pellet was instead dissolved in diethyl pyrocarbonate-treated distilled water, extracted twice with an equal volume of phenol-chloroform (25:24:1:: phenol equilibrated with 10 mm Tris-HCl, pH 8.0, 1 mm EDTA: chloroform: isoamyl alcohol), precipitated with one-third volume of 8 m LiCl, and incubated at 4°C overnight before centrifuging. The resulting pellet was washed with 70% (v/v) ethanol, dried for 5 min in a Speed-Vac (Savant, Holbrook, NY) without heat, and dissolved in diethyl pyrocarbonate-treated distilled water. RNA was quantitated spectrophotometrically at 260, 280, and 320 nm.
RNA samples (10 μg) were size-fractionated by 1.2% (w/v) agarose formaldehyde gel electrophoresis and northern blotted onto nylon membrane (GeneScreenPlus; NEN-DuPont, Boston) as described in the manufacturer's protocol. Ethidium bromide staining of the gel prior to blotting revealed rRNA bands, which served as the loading control. After blotting and UV-crosslinking, the end lanes of the blot containing the RNA size markers were excised, washed in 5% (v/v) acetic acid, stained with 0.04% (w/v) methylene blue (in 0.5 m Na-phosphate, pH 5.2), and washed in distilled water. 32P-labeled probes specific for either NaPMT1 or NaPMT2 were prepared by PCR using [α-32P]dCTP in the reaction with the corresponding isolated PMT plasmid as template and the previously-mentioned primer pair that flanks the amino-terminal tandem repeat zone of the PMT genes. Northern blots were washed twice at 42°C in 2× sodium chloride/sodium phosphate/EDTA (SSPE), followed by once at 65°C in 2× SSPE, 2% (w/v) SDS, and once at 42°C in 0.1× SSPE, before being subjected to autoradiography. Where necessary, blots were re-used after stripping at 85°C in 0.1× SSC, 70% (v/v) formamide, followed by boiling in 0.1× SSC, 5% (w/v) SDS.
NaPMT probe specificity was verified by slot-blotting (model PR 648; Hoefer, San Francisco) a dilution series (200, 20, 2 ng, 200, 20 pg) of NaPMT plasmids (pNaPMT1 or pNaPMT2) onto nylon membrane (GeneScreenPlus; NEN-DuPont) as described in the manufacturer's protocol. Probing with either an NaPMT1- or NaPMT2-specific 32P-labeled PCR fragment demonstrated the required specificity above a minimal level of background cross-reactivity (Fig. 4).
RNA samples (10 μg) alternatively were slot-blotted onto nylon membrane (GeneScreenPlus; NEN-DuPont) as described in the manufacturer's protocol, probed with an NaPMT1-specific 32P-labeled probe, and then quantitated using a Storage Phosphor Molecular Imaging System (model GS-525; Bio-Rad Laboratories, Hercules, CA).
Nicotine Analysis
Leaf nicotine concentrations were determined by RP-HPLC analysis (Baldwin and Schmelz, 1994) of methanol: 1.7 mm HCl (1:1.5, v/v, pH 5.0–5.5) extracts of shoots, which had previously been pulverized in N2(L) with a mortar and pestle and freeze-dried.
Sequence data were submitted to GenBank and are on hold until publication.
ACKNOWLEDGMENTS
We thank Dr. Vaka Reddy for initial work on NaPMT1, Natasha Sandoval for cDNA library construction, Domenica Schnabelrauch for DNA sequencing, Rayko Halitschke for assistance in RNA extraction and nicotine analysis, Evelyn Claussen for assistance with the figures, and Dr. Carlos Ballaré for Figure 8.
Footnotes
This work was supported by the Max Planck Gesellschaft.
Part III in the series is: Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata: III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol 125: 711–717.
LITERATURE CITED
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Baldwin IT. The alkaloidal responses of wild tobacco to real and simulated herbivory. Oecologia. 1988;77:378–381. doi: 10.1007/BF00378046. [DOI] [PubMed] [Google Scholar]
- Baldwin IT. The mechanism of damaged-induced alkaloids in wild tobacco. J Chem Ecol. 1989;15:1661–1680. doi: 10.1007/BF01012392. [DOI] [PubMed] [Google Scholar]
- Baldwin IT. Herbivory simulations in ecological research. Trends Ecol Evol. 1990;5:91–93. doi: 10.1016/0169-5347(90)90237-8. [DOI] [PubMed] [Google Scholar]
- Baldwin IT. Allometric limits to the induced accumulation of nicotine in native tobacco. Plant Species Biol. 1996a;11:107–114. [Google Scholar]
- Baldwin IT. Methyl jasmonate-induced nicotine production in Nicotiana attenuata: inducing defenses in the field without wounding. Ent Exp Appl. 1996b;80:213–220. [Google Scholar]
- Baldwin IT. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc Natl Acad Sci USA. 1998;95:8113–8118. doi: 10.1073/pnas.95.14.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT, Gorham D, Schmelz EA, Lewandowski C, Lynds GY. Allocation of nitrogen to an inducible defense and seed production in Nicotiana attenuata. Oecologia. 1998;115:541–552. doi: 10.1007/s004420050552. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Hamilton W. Jasmonate-induced responces of Nicotiana sylvestris results in fitness costs due to impaired competitive ability for nitrogen. J Chem Ecol. 2000;26:915–952. [Google Scholar]
- Baldwin IT, Karb MJ, Ohnmeiss TE. Allocation of 15N from nitrate to nicotine: production and turnover of a damage-induced mobile defense. Ecology. 1994a;75:1703–1713. [Google Scholar]
- Baldwin IT, Ohnmeiss TE. Swords into plowshares? Nicotiana sylvestris does not use nicotine as a nitrogen source under nitrogen-limited growth. Oecologia. 1994a;98:385–392. doi: 10.1007/BF00324228. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Ohnmeiss TE. Coordination of photosynthetic and alkaloidal responses to leaf damage in uninducible and inducible Nicotiana sylvestris. Ecology. 1994b;75:1003–1014. [Google Scholar]
- Baldwin IT, Preston CA. The eco-physiological complexity of plant responses to insect herbivores. Planta. 1999;208:137–145. [Google Scholar]
- Baldwin IT, Schmelz EA. Constraints on an induced defense: the role of the leaf area. Oecologia. 1994;97:424–430. doi: 10.1007/BF00317335. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Schmelz EA. Immunological “memory” in the induced accumulation of nicotine in wild tobacco. Ecology. 1996;77:236–246. [Google Scholar]
- Baldwin IT, Schmelz EA, Ohnmeiss TE. Wound-induced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana sylvestris Spegazzini and Comes. J Chem Ecol. 1994b;20:2139–2157. doi: 10.1007/BF02066250. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Schmelz EA, Zhang Z-P. Effects of octadecanoid metabolites and inhibitors on induced nicotine accumulation in Nicotiana sylvestris. J Chem Ecol. 1996;22:61–73. doi: 10.1007/BF02040200. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Zhang Z, Diab N, Ohnmeiss TE, McCloud ES, Lynds GY, Schmelz EA. Quantification, correlations and manipulations of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta. 1997;201:397–404. [Google Scholar]
- Bogani P, Lio P, Intrieri MC, Buiatti M. A physiological and molecular analysis of the genus Nicotiana. Mol Phylogenet Evol. 1997;7:62–70. doi: 10.1006/mpev.1996.0356. [DOI] [PubMed] [Google Scholar]
- Brown T. Southern blotting. In: Ausubel F, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Short Protocols in Molecular Biology. New York: John Wiley & Sons; 1995. pp. 2–28–2–32. [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dong X. SA, JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol. 1998;1:316–323. doi: 10.1016/1369-5266(88)80053-0. [DOI] [PubMed] [Google Scholar]
- Goodspeed TH. The Genus Nicotiana. Waltham, MA: Chronica Botanica; 1954. [Google Scholar]
- Halitschke R, Keβler A, Kahl J, Lorenz A, Baldwin IT. Ecophysiological comparison of direct and indirect defenses in Nicotiana attenuata. Oecologia. 2000;124:408–417. doi: 10.1007/s004420000389. [DOI] [PubMed] [Google Scholar]
- Hartley SE, Lawton JH. Biochemical aspects and significance of the rapidly induced accumulation of phenolics in birch foliage. In: Tallamy DW, Raupp MJ, editors. Phytochemical Induction by Herbivores. New York: John Wiley & Sons; 1991. pp. 105–132. [Google Scholar]
- Hashimoto T, Shoji T, Mihara T, Oguri H, Tamaki K, Suzuki K, Yamada Y. Intraspecific variability of the tandem repeats in Nicotiana putrescine N-methyltrans-ferases. Plant Mol Biol. 1998;37:25–37. doi: 10.1023/a:1005961122814. [DOI] [PubMed] [Google Scholar]
- Hermsmeier DH, Schittko U, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata: I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiol (in press) [DOI] [PMC free article] [PubMed]
- Hibi N, Higashiguchi S, Hashimoto T, Yamada Y. Gene expression in tobacco low-nicotine mutants. Plant Cell. 1994;6:723–735. doi: 10.1105/tpc.6.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA. An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell. 1996;8:2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi S, Hashizume K, Nakakita M, Kojima H, Matsubayashi Y, Hashimoto T, Sakagami Y, Yamada Y, Nakamura K. Differential induction by methyl jasmonate of genes encoding ornithine decarboxylase and other enzymes involved in nicotine biosynthesis in tobacco cell cultures. Plant Mol Biol. 1998;38:1101–1111. doi: 10.1023/a:1006058700949. [DOI] [PubMed] [Google Scholar]
- Imanishi S, Nakakita M, Yamashita K, Furuta A, Utsuno K, Muramoto N, Kojima H, Nakamura K. Aspirin and salicylic acid do not inhibit methyl jasmonate-inducible expression of a gene for ornithine decarboxylase in tobacco BY-2 cells. Biosci Biotechnol Biochem. 2000;64:125–133. doi: 10.1271/bbb.64.125. [DOI] [PubMed] [Google Scholar]
- Johnson PR, Ecker JR. The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet. 1998;32:227–254. doi: 10.1146/annurev.genet.32.1.227. [DOI] [PubMed] [Google Scholar]
- Kahl J, Siemens DH, Aerts RJ, Gäbler R, Kühnemann F, Preston CA, Baldwin IT. Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta. 2000;210:336–342. doi: 10.1007/PL00008142. [DOI] [PubMed] [Google Scholar]
- Koiwa H, Bressan RA, Hasegawa PM. Regulation of protease inhibitors and plant defense. Trends Plant Sci. 1997;2:379–384. [Google Scholar]
- Korth KL, Dixon RA. Evidence for chewing insect-specific molecular events distinct from a general wound response in leaves. Plant Physiol. 1997;115:1299–1305. doi: 10.1104/pp.115.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton KA, Potter SL, Uknes S, Ryals J. Acquired resistance signal transduction in Arabidopsis is ethylene independent. Plant Cell. 1994;6:581–588. doi: 10.1105/tpc.6.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleck K, Dietrich RA. Defense on multiple fronts: how do plants cope with diverse enemies? Trends Plant Sci. 1999;4:215–219. doi: 10.1016/s1360-1385(99)01415-6. [DOI] [PubMed] [Google Scholar]
- McCloud ES, Baldwin IT. Herbivory and caterpillar regurgitants amplify the wound-induced increases in jasmonic acid but not nicotine in Nicotiana sylvestris. Planta. 1997;203:430–435. [Google Scholar]
- McConn M, Creelman RA, Bell E, Mullet JE, Browse J. Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA. 1997;4:5473–5477. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Kusano T, Koizumi N, Katsumi M, Sano H. Gibberellin-responsive genes: high level of transcript accumulation in leaf sheath meristematic tissue from Zea mays L. Plant Mol Biol. 1999;40:645–657. doi: 10.1023/a:1006291917591. [DOI] [PubMed] [Google Scholar]
- Ohnmeiss T, Baldwin IT. The allometry of nitrogen allocation to growth and an inducible defense under nitrogen-limited growth. Ecology. 1994;75:995–1002. [Google Scholar]
- Ohnmeiss T, McCloud ES, Lynds GY, Baldwin IT. Within-plant relationships among wounding, jasmonic acid, and nicotine: implications for defense in Nicotiana sylvestris. New Phytol. 1997;137:441–452. doi: 10.1046/j.1469-8137.1997.00845.x. [DOI] [PubMed] [Google Scholar]
- Paul ND, Hatcher PE, Taylor JE. Coping with multiple enemies: an integration of molecular and ecological perspectives. Trends Plant Sci. 2000;5:220–225. doi: 10.1016/s1360-1385(00)01603-4. [DOI] [PubMed] [Google Scholar]
- Penninckx IAMA, Thomma BPHJ, Buchala A, Métraux JP, Broekaert WF. Concomitant activation of jasmonte and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, van Loon LC. Salicylic acid-independent plant defense pathways. Trends Plant Sci. 1999;4:52–58. doi: 10.1016/s1360-1385(98)01364-8. [DOI] [PubMed] [Google Scholar]
- Preston CA, Lewandowski C, Enyedi AJ, Baldwin IT. Tobacco mosaic virus inoculation inhibits wound-induced jasmonic acid-mediated responses within but not between plants. Planta. 1999;209:87–95. doi: 10.1007/s004250050609. [DOI] [PubMed] [Google Scholar]
- Reichardt M, Rogers S. Preparation of plant DNA using CTAB. In: Ausubel F, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1994. pp. 2.3.3–2.3.7. [Google Scholar]
- Reymond P, Farmer EE. Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol. 1998;1:404–411. doi: 10.1016/s1369-5266(98)80264-1. [DOI] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell. 2000;12:707–719. doi: 10.1105/tpc.12.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechers DE, Timko MP. Structure and expression of the gene family encoding putrescine N-methyltransferase in Nicotiana tabacum: new clues to the evolutionary origin of cultivated tobacco. Plant Mol Biol. 1999;41:387–401. doi: 10.1023/a:1006342018991. [DOI] [PubMed] [Google Scholar]
- Rojo E, León J, Sánchez-Serrano JJ. Cross-talk between wound signalling pathways determines local versus systemic gene expression in Arabidopsis thaliana. Plant J. 1999;20:135–142. doi: 10.1046/j.1365-313x.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schittko U, Hermsmeier D, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata: II. Accumulation of plant mRNAs in response to insect-derived cues. Plant Physiol. 2001;125:701–710. doi: 10.1104/pp.125.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittko U, Preston CA, Baldwin IT. Eating the evidence: Manduca sexta larvae can not disrupt specific jasmonate induction in Nicotiana attenuata by rapid consumption. Planta. 2000;210:343–346. doi: 10.1007/PL00008143. [DOI] [PubMed] [Google Scholar]
- Shoji T, Nakajima K, Hashimoto T. Ethylene suppresses jasmonate-induced gene expression in nicotine biosynthesis. Plant Cell Physiol. 2000a;41:1072–1076. doi: 10.1093/pcp/pcd027. [DOI] [PubMed] [Google Scholar]
- Shoji T, Yamada Y, Hashimoto T. Jasmonate induction of putrescine N-methyltransferase genes in the root of Nicotiana sylvestris. Plant Cell Physiol. 2000b;41:831–839. doi: 10.1093/pcp/pcd001. [DOI] [PubMed] [Google Scholar]
- Stout MJ, Workman J, Duffey SS. Differential induction of tomato foliar proteins by arthropod herbivores. J Chem Ecol. 1994;20:2575–2594. doi: 10.1007/BF02036193. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Yamada Y, Hashimoto T. Expression of Atropa belladonna putrescine N-methyltransferase gene in root pericycle. Plant Cell Physiol. 1999;40:289–297. doi: 10.1093/oxfordjournals.pcp.a029540. [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe KW, Barbosa P. Effects of consumption of high and low nicotine tobacco by Manduca sexta on the survival of the gregarious endoparasitoid Cotesia congregata. J Chem Ecol. 1986;12:1329–1327. doi: 10.1007/BF01012352. [DOI] [PubMed] [Google Scholar]
- Tomlin ES, Sears MK. Indirect competition between the Colorado potato beetle (Coleoptera: Chrysomelidae) and the potato leafhopper (Homoptera: Cicadellidae) on potato: laboratory study. Environ Entomol. 1992;21:787–792. [Google Scholar]
- Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- van Dam NM, Baldwin IT. Costs of jasmonate-induced responses in plants competing for limited resources. Ecol Lett. 1998;1:30–33. [Google Scholar]
- Voelckel C, Schittko U, Baldwin IT. January 19, 2001. Herbivore-induced ethylene burst reduces fitness costs of jasmonate- and oral secretion-induced defenses in Nicotiana attenuata. Oecologia. 10.1007/s004420000581 [DOI] [PubMed]
- Xu Y, Chang PL, Liu D, Narasimhan ML, Raghothama KG, Hasegawa PM, Bressan RA. Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell. 1994;6:1077–1085. doi: 10.1105/tpc.6.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZP, Baldwin IT. Transport of [2-14C]jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta. 1997;203:436–441. [Google Scholar]
- Zhang ZP, Krumm T, Baldwin IT. Structural requirements of jasmonates and mimics for nicotine induction in Nicotiana sylvestris. J Chem Ecol. 1997;23:2777–2789. [Google Scholar]
- Zhu-Salzman K, Salzman RA, Koiwa H, Murdock LL, Bressan RA, Hasegawa PM. Ethylene negatively regulates local expression of plant defense lectin genes. Physiol Plant. 1998;104:365–372. [Google Scholar]