Abstract
BACKGROUND:
E5103 was a study designed to evaluate the efficacy and safety of bevacizumab. It was a negative trial for the end points of invasive disease–free survival and overall survival. The current work examines the tolerability of bevacizumab and other medication exposures with respect to clinical outcomes and patient-reported outcomes (PROs).
METHODS:
Adverse events (AEs) collected from the Common Terminology Criteria for Adverse Events were summarized to form an AE profile at each treatment cycle. All-grade and high-grade events were separately analyzed. The change in the AE profile over the treatment cycle was delineated as distinct AE trajectory clusters. AE-related and any-reason early treatment discontinuations were treated as clinical outcome measures. PROs were measured with the Functional Assessment of Cancer Therapy–Breast + Lymphedema. The relationships between the AE trajectory and early treatment discontinuation as well as PROs were analyzed.
RESULTS:
More than half of all AEs (57.5%) were low-grade. A cluster of patients with broad and mixed AE (all-grade) trajectory grades was significantly associated with any-reason early treatment discontinuation (odds ratio [OR], 2.87; P = .01) as well as AE-related discontinuation (OR, 4.14; P = .001). This cluster had the highest count of all-grade AEs per cycle in comparison with other clusters. Another cluster of patients with primary neuropathic AEs in their trajectories had poorer physical well-being in comparison with a trajectory of no or few AEs (P < .01). A high-grade AE trajectory did not predict discontinuations.
CONCLUSIONS:
A sustained and cumulative burden of across-the-board toxicities, which were not necessarily all recognized as high-grade AEs, contributed to early treatment discontinuation. Patients with neuropathic all-grade AEs may require additional attention for preventing deterioration in their physical well-being.
Keywords: adverse events, breast cancer, drug treatment, early treatment discontinuation, patient-reported outcome, peripheral neuropathy
INTRODUCTION
The ECOG-ACRIN Cancer Research Group E5103 trial was a randomized, phase 3, double-blind, clinical trial for patients with human epidermal growth factor receptor 2 (HER2)–negative breast cancer.1 Previous research has suggested that the most successful clinical application of angiogenesis inhibitors is likely to be in patients with micrometastatic disease in the adjuvant setting.2–8 E5103 was a study to test that hypothesis and evaluate the efficacy and safety of bevacizumab, a humanized monoclonal antibody that targets vascular endothelial growth factor. Results from E5103 showed that the incorporation of bevacizumab into sequential anthracycline- and taxane-containing adjuvant therapy did not improve invasive disease–free survival or overall survival in targeted patients.1 The negative study result was partly attributed to early drug modification and discontinuation, which resulted in severely limited bevacizumab exposure. As such, it is possible that even if bevacizumab is beneficial, the effect could be attenuated because many of the bevacizumab-specific toxicities have a constant, cumulative risk over time that leads to drug intolerance, early adverse events (AEs), and eventual nonadherence.1,9
In this article, instead of examining invasive disease–free survival or overall survival, we turn our focus to the tolerability of bevacizumab and other medication exposures. Operationally, we use discontinuation of treatment as a clinical outcome to indicate (in)tolerability. Because tolerability is critical to patients’ adherence to the protocol treatment, its management needs to address and prioritize the many dimensions of AEs, including multiplicity, severity, and persistence. Consider the following 2 competing scenarios: Patient A has a high-grade but episodic, isolated case of diarrhea, and patient B has a grade 2 facial rash and grade 2 joint pain, both of which are persistent. Current clinician practice would regard only patient A’s episodic diarrhea as severe, not patient B’s constant rash and pain. However, patient B may be more unwilling to continue treatment because the low-grade toxicity severely limits her daily activities and negatively affects her health-related quality of life (HRQOL). In this article, we use a trajectory-based method that captures the full range of AE manifestation, including the multiplicity, severity (grade), and pattern of occurrence over time. With a comprehensive representation of AE manifestation, our objective is to seek answers to the following questions (objectives 1–3). First, what are the patterns of AE trajectories from bevacizumab and other medication exposure? Second, are AEs related to early discontinuation of treatment, and if so, what patterns in AE trajectories would likely lead to such an outcome? Third, how are AE trajectories related to patient-reported outcomes (PROs), particularly the different aspects of well-being (eg, physical and mental)?
MATERIALS AND METHODS
Study Design and Sample
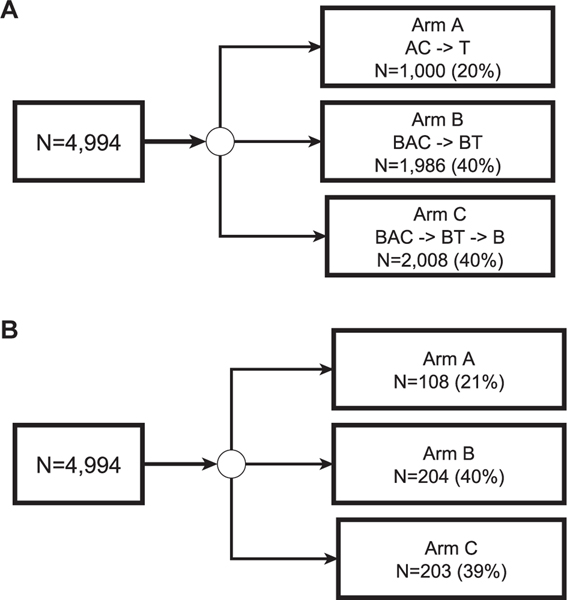
This is a substudy of E5103, a phase 3 adjuvant breast cancer trial that randomly assigned 4994 patients with node-positive or high-risk node-negative breast cancer to standard chemotherapy plus bevacizumab or a placebo; it was conducted by the ECOG-ACRIN Cancer Research Group (Fig. 1A). Detailed eligibility criteria have been reported.1,10 The current tolerability study includes 515 patients who completed PROs1,10 (Fig. 1B). PRO participants were representative of the original sample1 with respect to demographic and clinical characteristics and randomization assignment (Table 1). Institutional review boards for participating sites approved the protocol, and patients provided written informed consent before screening.
Figure 1.
(A) Randomization for the original sample and (B) selection for the subsample for ECOG-ACRIN E5103. AC indicates intravenous doxorubicin and cyclophosphamide; B, bevacizumab; BAC, bevacizumab concurrent with intravenous doxorubicin and cyclophosphamide; BT, bevacizumab concurrent with paclitaxel; T, paclitaxel.
TABLE 1.
Descriptive Sample Statistics
| Characteristic | Arm A (n = 108) | Arm B (n = 204) | Arm C (n = 203) | Total (n = 515) |
|---|---|---|---|---|
|
| ||||
| Age, mean (SD), y | 51.19 (10.65) | 51.69 (10.07) | 52.00 (10.07) | 51.71 (10.28) |
| Patient weight, mean (SD), kg | 79.72 (21.99) | 78.57 (19.58) | 80.21 (19.58) | 79.48 (19.11) |
| Sex, No. (%) | ||||
| Female | 106 (98.1) | 204 (100) | 202 (99.5) | 512 (99.4) |
| Male | 2 (1.9) | 1 (0.5) | 3 (0.6) | |
| Race, No. (%) | ||||
| Non-White | 14 (13.0) | 26 (12.8) | 34 (16.7) | 74 (14.4) |
| White | 94 (87.0) | 178 (87.2) | 169 (83.3) | 441 (85.6) |
| Primary tumor size, No. (%) | ||||
| ≤2 cm | 43 (39.8) | 63 (30.9) | 77 (37.9) | 183 (35.5) |
| >2 to 5 cm | 53 (49.1) | 114 (55.9) | 101 (49.8) | 268 (52.1) |
| >5 cm | 12 (11.1) | 27 (13.2) | 25 (12.3) | 64 (12.4) |
| Histologic grade, No. (%) | ||||
| 1 | 12 (11.1) | 16 (7.8) | 14 (6.9) | 42 (8.2) |
| 2 | 34 (31.5) | 70 (34.3) | 71 (35.0) | 175 (34.0) |
| 3 | 61 (56.5) | 114 (55.9) | 111 (54.7) | 286 (55.5) |
| Missing | 1 (0.9) | 4 (2.0) | 7 (3.4) | 12 (2.3) |
| ER status, No. (%) | ||||
| Negative | 43 (39.8) | 74 (36.1) | 78 (38.4) | 195 (37.9) |
| Positive | 64 (59.3) | 131 (63.9) | 124 (61.1) | 318 (61.7) |
| Missing | 1 (0.9) | 1 (0.5) | 2 (0.4) | |
| PGR status, No. (%) | ||||
| Negative | 49 (45.4) | 82 (40.2) | 93 (45.8) | 224 (43.5) |
| Positive | 58 (53.7) | 122 (59.8) | 109 (53.7) | 289 (56.1) |
| Missing | 1 (0.9) | 1 (0.5) | 2 (0.4) | |
| HER2/neu chromosome, No. (%) | ||||
| 0 | 37 (34.3) | 62 (30.4) | 85 (41.9) | 184 (35.7) |
| 1 + | 36 (33.3) | 71 (34.8) | 56 (27.6) | 163 (31.7) |
| 2+ | 16 (14.8) | 35 (17.2) | 27 (13.3) | 78 (15.1) |
| Missing | 19 (17.6) | 36 (17.7) | 35 (17.2) | 90 (17.5) |
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PGR, progesterone receptor.
All patients received doxorubicin and cyclophosphamide (AC) followed by paclitaxel weekly for 12 weeks. AC could be administered according to a classic schedule (every 3 weeks) or a dose-dense schedule (every 2 weeks) according to the investigator’s discretion; the bevacizumab dose was adjusted for the AC schedule choice (patients receiving classic AC received bevacizumab at 15 mg/kg; patients receiving dose-dense AC received bevacizumab at 10 mg/kg). A placebo (arm A) or bevacizumab (arms B and C) was administered concurrently with chemotherapy. All patients were unblinded at week 10 of paclitaxel therapy—the first day of the last cycle in the first phase (8 cycles in total) of E5103. In the second (maintenance) phase, patients in arm C continued bevacizumab monotherapy (15 mg/kg every 3 weeks) for an additional 10 cycles unless the patients did not consent to continue. The current analyses focused on the first phase of the trial. We excluded participants who died or experienced disease progression in this phase from the analysis.
Measures
Treatment-related variables for the purpose of this analysis are described next.
Bevacizumab exposure
Arm A participants were categorized as non–bevacizumab-exposed, and both arm B and arm C participants were categorized as bevacizumab-exposed.
Clinical outcomes
Early treatment discontinuation.
Discontinuation was a dichotomous variable in which 1 indicated discontinuation before the completion of the planned treatment and 0 indicated otherwise. Discontinuation due to AEs and discontinuation for any reason (including AEs) were analyzed separately as distinct outcomes.
PROs
Functional Assessment of Cancer Therapy–Breast + Lymphedema.
The Functional Assessment of Cancer Therapy–Breast + Lymphedema (FACT-B+4) questionnaire11 was used to measure cancer-related well-being. The FACT-B+4 consists of the Functional Assessment of Cancer Therapy–General (FACT-G) subscales (27 items) and the Additional Concerns subscale (14 items). The FACT-G consists of 4 subscales that assess physical, social/family, emotional, and functional well-being. The Additional Concerns subscale measures breast cancer–specific concerns. Items were based on a 5-point scale and referred to the past 7 days. After the reverse coding of items that were negatively worded, responses were summed within a domain to derive a domain-level score, and higher scores reflected better well-being.
Demographic and other cancer-related variables.
In addition to routine demographic (age, sex, and race) and anthropometric (weight) variables, cancer-related variables included primary tumor size, histologic grade, estrogen receptor status, and progesterone receptor status. Factors related to genetic ancestry were reported elsewhere and were not included in this analysis.1,10
Common Terminology Criteria for Adverse Events and AE trajectory.
Data on AEs were collected according to the Common Terminology Criteria for Adverse Events (CTCAE; version 3.0). The analysis included a total of 53 different AE events reported during the first phase of treatment in E5103. Because of the complexity and sparsity of the AE data, summary measures were derived to facilitate analysis. First, the AEs were grouped under 11 domains: cardiac, febrile, reaction-related, gastrointestinal, hemorrhage, edema, metabolic, neuropathic, pain, breathing-related, and other. Because many AEs were sparse, instead of using individual event-level data, we treated the presence of an AE within a domain in a specific cycle as a domain-level indicator. We curated data both for 1) the presence of any-grade AEs (representing what a patient experienced) and 2) the presence of a severe AE (grade 3 or higher; representing what a clinician typically recorded). When discontinuation occurred, no AE data were collected afterward. For each type of AE data, 2 summary measures were further derived from the AE domain-level indicators. The first was derived from an analysis of the 11 domain indicators such that individuals with similar profiles across domains were clustered into a specific AE state or latent class12,13 at a given cycle. For example, “no or few AEs” formed a state. The second summary measure used the collection of AE states over cycles to identify distinct groups of trajectories, which represented how patient AE profiles changed over time. An example of a trajectory group was patients belonging to the “no or few AEs” state for all cycles. The AE trajectory group was used to represent the patient burden from AEs in the subsequent analysis. Specific details for the derivation of AE states and trajectories are provided in the following section. The any-grade AE trajectory formed the basis for the primary analysis, and the same methodology was applied to high-grade (severe) AEs for a sensitivity analysis.
Statistical Analysis
The statistical analysis plan used pertinent tools to achieve each objective. We operationalized the AE trajectory variable to capture the entire AE profile over all 8 cycles. Although AEs could be summarized by measures such as the total AE count over cycles, the approach had limitations such as not differentiating AEs in different domains, and this was important for understanding the patterns of toxicity. Instead, we used the multivariate hidden Markov model (MHMM) to capture the manifestation of AE patterns in the form of different states and trajectories.12–14 Briefly, the MHMM used multiple variables (in this case, the domain-level AE) to form an AE profile. On the basis of the pattern of the profile variables, the MHMM determined the appropriate number of states K for capturing both intraindividual and interindividual variation. A state represented a statistically distinguishable patient cluster within which the patients had similar profiles. Each individual was classified as exclusively belonging to one of the K states at a given time point, and state membership could change over time. The Bayesian information criterion, a goodness-of-fit statistical index, determined the value of K. In other words, a range of models with different numbers of states were fitted, and the model with the lowest Bayesian information criterion was selected. The MHMM states thus represented the heterogeneity in multiple AE measures. Accordingly, an individual’s change in states over time constituted an AE trajectory. On the basis of an inspection of the individual trajectories, we further identified a number of distinct AE trajectory groups, which were treated as categorical variables in the subsequent analysis.
Missing values were treated as missing at random. For the MHMM analysis, if a patient missed an observation at a specific time point, the state was imputed from information from the previous states under the model assumptions.13
Depending on the objective, an AE trajectory group was treated as either a dependent variable or an independent variable in the analysis. In objective 1, the AE trajectory group was the dependent variable in a multinomial logistic model, and the primary predictor was bevacizumab exposure (model 1). For the selection of covariates, univariate logistic analysis was first used to determine the significance of the individual variables: age, race, weight, primary tumor size, estrogen receptor status, and progesterone receptor status. Significant variables (P < .1) were then entered into the final multivariable logistic model. A 2-way interaction term between each included variable and bevacizumab exposure was also tested for significance.
In objective 2, the dependent variables were early treatment discontinuations (due to AEs and for any reason), and the primary predictor was the AE trajectory group. Independent logistic regression models were used to analyze the early treatment discontinuation outcomes (model 2). The same set of covariates and interactions used in model 1 were included in model 2. For objective 3, the multiple dependent variables were PROs, all measured at the end of phase 1 of the study (cycle 8). PROs were treated as continuous, and linear regression models were fit with the AE trajectory group as the primary predictor, with adjustments made for baseline PRO values (model 3). A direct effect of bevacizumab on PROs was plausible, so a mediational model was used to explore the effect between bevacizumab and PROs, for which the AE trajectory group was the mediator. All analyses used 2-sided testing with a significance level of .05, and 95% confidence intervals (CIs) were reported for key predictor variables. MHMM and regression analyses were conducted with MATLAB and SAS v9.4, respectively.
RESULTS
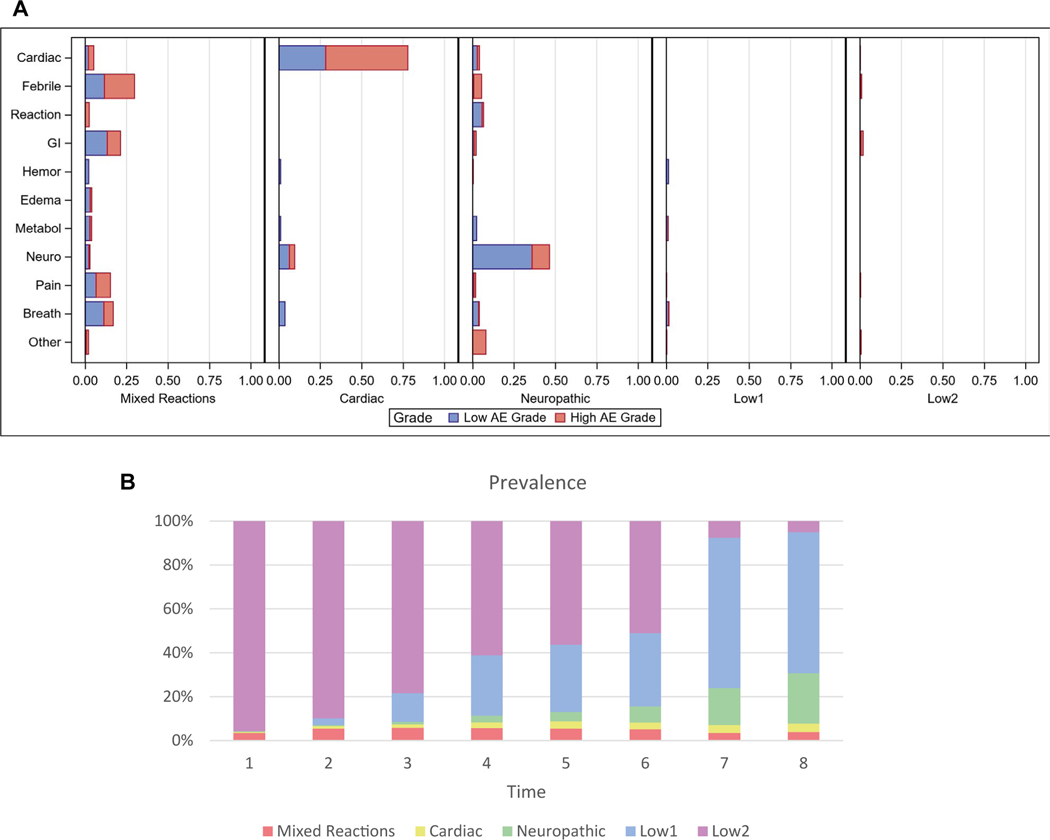
Table 1 shows descriptive statistics of the sample, and Supporting Table 1 shows the corresponding statistics of the original full sample. Five distinct AE states were identified from the data such that each state represented a subgroup of patients with similar AE profiles (Fig. 2A). The profiles of the 5 states were labeled Mixed Reactions (state 1), Cardiac (state 2), Neuropathic (state 3), First Low AE (state 4; labeled Low 1), and Second Low AE (state 5; labeled Low 2). For example, a patient in the Cardiac state had a 78% chance on average of having a positive indicator of a cardiac AE in a given cycle. The most prevalent manifestation of a cardiac AE was hypertension. Others included left ventricular systolic dysfunction, cardiac ischemia/infarction, and ventricular diastolic dysfunction. The most prevalent neuropathic AE was sensory neuropathy. Others included dizziness, mood alteration/depression, central nervous system cerebrovascular ischemia, and ataxia (incoordination). A patient in the Mixed Reactions state was likely to experience multiple AEs across domains, including fever, pain, and gastrointestinal and breathing-related issues. Both the Low 1 AE state and the Low 2 AE state had a low incidence of AEs. In our subsequent trajectory analysis, a decision was made to collapse these 2 statistical states into a single Low AE state. Figure 2B shows the prevalence of the states over time (in cycle). The 2 Low AE states together constituted the most prevalent state. Both the Cardiac and Neuropathic states increased in prevalence over time, whereas the Mixed Reactions state appeared early and persisted into the treatment cycles. Note that some states (eg, Cardiac) emerged only after the initial cycle.
Figure 2.
(A) Profiles of the AE states. Each bar indicates the probability of having the specific AE and grade (1–2 = low; 3–4 = high). (B) Prevalence of AE states over 8 cycles. The order for the states from top to bottom is as follows: Low 2, Low 1, Neuropathic, Cardiac, and Mixed Reactions. AE indicates adverse event; breath, breathing-related; GI, gastrointestinal; hemor, hemorrhage; metabol, metabolic; neuro, neuropathic; reaction, reaction-related.
Individuals’ trajectories were categorized into the following 4 groups: the Low AE Traj group, within which only the Low 1 or Low 2 AE state was present throughout the cycles; the Cardiac Traj group, within which the Cardiac state was present at some point in the cycles; the Neuro Traj group, within which the Neuropathic state was present at some point in the cycles; and the Mixed Reactions Traj group, within which the Mixed Reactions state was present at some point in the cycles. Because there was very little movement from one state to another—with the exception of a transition between the Low AE states and other states—all the trajectories could be readily classified into one of the aforementioned trajectory groups. Table 2 shows a sample of the most prevalent trajectories and how the trajectories were classified. The Mixed Reactions Traj group shows a pattern distinct from the Cardiac Traj and Neuro Traj groups. Table 2 also shows that for the Mixed Reactions Traj group, the Mixed Reactions state tended to persist throughout the cycles, whereas for the Cardiac Traj and Neuro Traj groups, the respective Cardiac and Neuropathic states tended to be episodic and appeared only toward later cycles. The overall prevalences of the Cardiac Traj, Neuro Traj, Mixed Reactions Traj, and Low AE Traj groups were 7.7%, 25.1%, 8.7%, and 58.6%, respectively.
TABLE 2.
Sample of Top 10 Trajectories (Ordered by Prevalence) With Corresponding Group Labels
| Rank | Count | % | Cycle | Trajectory Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||
|
| |||||||||||
| 1 | 302 | 58.6 | Low | Low | Low | Low | Low | Low | Low | Low | Low AE Traj |
| 2 | 41 | 8.0 | Low | Low | Low | Low | Low | Low | Neuro | Neuro | Neuro Traj |
| 3 | 24 | 4.7 | Low | Low | Low | Low | Low | Low | Low | Neuro | Neuro Traj |
| 4 | 13 | 2,5 | Low | Low | Low | Low | Low | Neuro | Neuro | Neuro | Neuro Traj |
| 5 | 6 | 1.2 | Low | Low | Low | Low | Neuro | Neuro | Neuro | Neuro | Neuro Traj |
| 6 | 5 | 1.0 | Mixed | Mixed | Mixed | Mixed | Mixed | Mixed | Mixed | Mixed | Mixed Reactions Traj |
| 7 | 3 | 0.6 | Low | Mixed | Mixed | Mixed | Mixed | Mixed | Mixed | Mixed | Mixed Reactions Traj |
| 8 | 3 | 0.6 | Low | Low | Low | Low | Cardiac | Cardiac | Low | Low | Cardiac Traj |
| 9 | 3 | 0.6 | Low | Low | Low | Low | Low | Low | Cardiac | Cardiac | Cardiac Traj |
| 10 | 3 | 0.6 | Low | Low | Low | Low | Low | Low | Low | Cardiac | Cardiac Traj |
Abbreviations: Cardiac, Cardiac state; Low, Low 1 or Low 2 AE state (collapsed); Mixed, Mixed Reactions state; Neuro, Neuropathic state.
Objective 1 Result (Bevacizumab Exposure and AE Trajectory)
The final model (model 1) included bevacizumab exposure, age, and weight as independent variables. None of the interaction terms among the selected independent variables were significant, so no interaction term was included in the model. Table 3 shows the multinomial logistic regression results for which the AE trajectory group was the outcome. Compared with the Low AE Traj group, the Cardiac Traj group was significantly related to bevacizumab exposure (odds ratio [OR], 3.58; 95% CI, 1.06–12.1; P = .04). Descriptive statistics showed that 92.3% of the participants in the Cardiac Traj group were exposed to bevacizumab, whereas 77.6% were exposed in the Low AE Traj group. Older participants had higher odds of belonging to the Mixed Reactions Traj group, and participants with heavier body weights had significantly higher odds of belonging to the other trajectory groups in comparison with the Low AE Traj group (Table 3).
TABLE 3.
Parameter Estimates in Model 1 for the Analysis of the Relationship Between Bevacizumab Exposure and the AE Trajectory (n = 510)
| Parameter | AE Trajectory Groupa | Odds Ratio | 95% CI | P |
|---|---|---|---|---|
|
| ||||
| BV exposure | Cardiac Traj | 3.58 | 1.06–12.1 | <.05 |
| Neuro Traj | 1.08 | 0.65–1.80 | .77 | |
| Mixed Reactions Traj | 1.11 | 0.50–2.46 | .79 | |
| Age (10 y) | Cardiac Traj | 1.29 | 0.92–1.82 | .14 |
| Neuro Traj | 1.17 | 0.95–1.44 | .13 | |
| Mixed Reactions Traj | 1.60 | 1.15–2.22 | <.01 | |
| Weight (10 | Cardiac Traj | 1.26 | 1.07–1.49 | <.01 |
| kg) | Neuro Traj | 1.14 | 1.02–1.27 | <.05 |
| Mixed Reactions Traj | 1.17 | 1.00–1.38 | .05 | |
Abbreviations: AE, adverse event; BV, bevacizumab; CI, confidence interval.
The Low AE Traj group is the reference group for the odds ratios.
Objective 2 Results (AE Trajectory and Discontinuation)
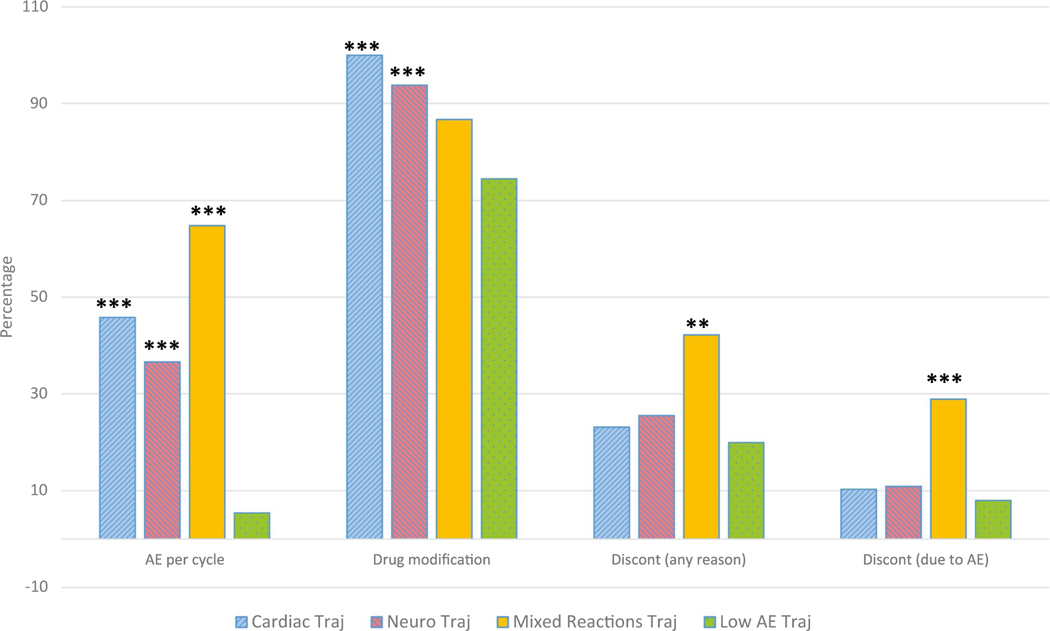
Dependent variables for this objective included treatment discontinuation due to any reason and discontinuation due to AEs. Five participants—1 who died and 4 who experienced disease progression—were excluded from the analysis. In the sample, the percentages of discontinuations due to any reason and due to AEs were 22.8% (116 of 510) and 10.8% (55 out of 510), respectively. In other words, 47% of the patients who discontinued treatment (55 of 116) did so because of AEs. Figure 3 shows, by trajectory group, the percentages of AEs (incidence) per cycle, drug modification, treatment discontinuation due to any reason, and discontinuation due to AEs.
Figure 3.
Percentages of treatment intolerability outcomes by AE trajectory groups. The 4 clusters of bar graphs from left to right indicate the incidence rate of AEs per cycle, drug modification in any cycle, early treatment discontinuation due to any reason, and discontinuation due to an AE. **P < .01, and ***P < .001 for all pairwise comparisons with the Low AE Traj group. AE indicates adverse event; Discont, discontinuation.
The Mixed Reactions Traj group was highly significant in predicting early treatment discontinuation due to any reason (OR, 2.87; 95% CI, 1.47–5.58; P = .01) and due to AEs (OR, 4.14; 95% CI, 1.90–9.06; P = .001). None of the other trajectory groups were found to be significantly associated with treatment discontinuation. Additionally, no other covariate was significant.
Objective 3 Results (AE Trajectory and PROs)
For the PRO analysis by domain of well-being, participants with missing PRO values were excluded, and the resulting range of sample sizes was 445 to 447. Neuro Traj was significant in predicting physical well-being (P < .01), whereas Cardiac Traj was marginally significant (P = .055). The regression coefficients in model 3 were such that being in the Neuro Traj group decreased the physical well-being score by 1.51 points (95% CI, –2.61 to –0.40), and being in the Cardiac Traj group decreased the score by 1.78 points (95% CI, –3.60 to 0.04); both implied worse physical well-being. There was no detectable direct effect from exposure to bevacizumab on physical well-being (P = .81). Because no direct effect was found, no mediational model was further tested. In all the other domains of well-being (social, emotional, functional, and additional concerns), none of the AE trajectory groups showed a significant predictive effect, nor did exposure to bevacizumab.
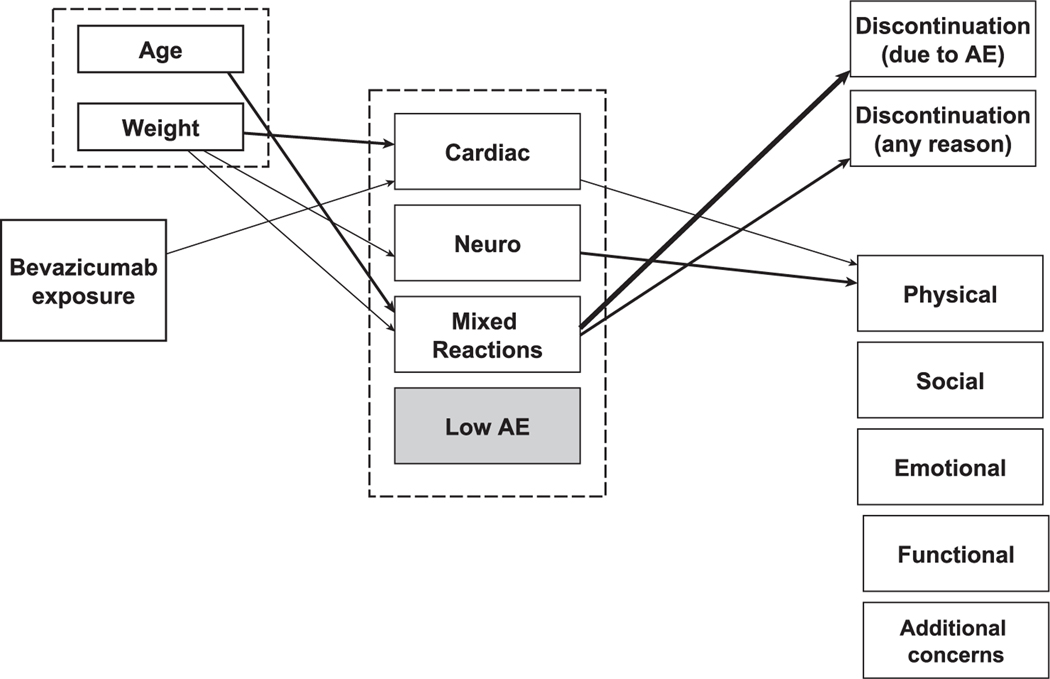
Figure 4 visualizes the results for the relationships between bevacizumab exposure, AE trajectory, and clinical and PRO outcomes.
Figure 4.
Schematic diagram summarizing significant relationships between bevacizumab exposure, the AE trajectory group, clinical outcomes, and patient-reported outcomes. The thickest arrow indicates P < .001, medium arrows indicate P < .01, and thin arrows indicate P < .05. For Cardiac Traj group → physical well-being domain, P = .054. AE indicates adverse event; Cardiac, Cardiac Traj group; Neuro, Neuro Traj group; Mixed Reactions, Mixed Reactions Traj group, Low AE, Low AE Traj group.
Sensitivity Analysis
The analysis of the severe AE trajectory identified 3 states (Supporting Fig. 1). Besides a Low AE state, the 2 other states were labeled Cardiac/Neuropathic state and Other AE state. Three corresponding high-grade AE trajectories were derived from the data. The 3 trajectories were called the Cardiac/Neuro High-Grade AE Traj group (8.2%), the Other High-Grade AE Traj group (14.7%), and the Low High-Grade AE Traj group (77.1%). For objective 1, bevacizumab exposure was not significantly related to any of the high-grade AE trajectory groups. For objective 2, none of the high-grade trajectory groups were related to early discontinuation due to AEs or any reason (all P values > .1). For objective 3, the Cardiac/Neuro High-Grade AE Traj group was significantly associated only with the patient’s emotional well-being (P = .03).
DISCUSSION
Although the efficacy of bevacizumab in addition to chemotherapy has been demonstrated in randomized clinical oncology trials, published studies have also revealed AEs attributed to the agent. The majority of such AEs are considered manageable, but others are severe and life-threatening.15 The current retrospective study confirms previous findings showing that toxicity, specifically cardiac toxicity, was induced by bevacizumab in a study of patients with HER2–negative breast cancer.1,16 Our finding is also consistent with the literature on the presence of classic toxicities likely due to other drugs used in the study (eg, peripheral neuropathy as induced by paclitaxel, although we only distinguished bevacizumab from other medications [objective 1]), and the analysis does not specifically tease out different toxicities due to these drugs.
It is important to understand the study findings regarding tolerability in the context of 2 unique features of our analysis. First, toxicities across multiple domains were simultaneously analyzed with a profile-based method. Unlike approaches based on counting the total number of AEs such that a low score in one domain may compensate for a high score in another, the profile-based approach allows a more comprehensive representation of the multiplicity of AE grouping and changes exhibited in longitudinal AE data.
The profile-based method identified 4 distinct and clinically meaningful types of all-grade AE trajectories in the analyzed patients. More than half of the patients had a trajectory type that either did not show any AE or had very low levels of AEs over the 8 treatment cycles. For the neuropathy- and cardiac-dominated trajectory types, we found respective AEs that appeared later in the treatment cycles and tended to be episodic. In contrast, the group of patients labeled as Mixed Reactions Traj experienced a broad range of toxicity burden that was persistent over treatment cycles as well as AEs that began early in the cycle. One possible explanation is that this group of patients had underlying health issues or was more sensitive to the treatment than other patients. The profile-based method captured salient features of AE patterns in the longitudinal data that were unlikely to be captured with traditional methods such as separate domain-level AE counts or overall AE counts.
A second important feature of the current study is the inclusion of low-grade AEs in capturing the overall patient burden. In addition to the standard high-grade events (grade 3 or higher), grade 1 and 2 AEs were also included. It has been shown that the high-grade approach may miss important details of how patients experience the cumulative toxicity burden that contributes to overall treatment tolerability and HRQOL.17,18 Our findings from the sensitivity analysis indeed suggest that the high-grade-only approach is not predictive of outcomes such as early treatment discontinuation. In the current data, a substantial proportion of the reported AEs (57.5%) were low-grade. Importantly, patients belonging to the Mixed Reactions Traj group were significantly related to early discontinuation of treatment that was both related to AEs and for any other reason. For example, the rate of discontinuation for any reason was 42.3% for the Mixed Reactions Traj group versus 23.1% and 25.6% for the Cardiac Traj and Neuro Traj groups, respectively (Fig. 3). An inspection of AE counts confirmed that the Mixed Reactions Traj group had a higher AE incidence (any grade) per patient cycle (0.638) than the Cardiac Traj group (0.458), the Neuro Traj group (0.366), and the Low AE Traj group (0.054). It is thus possible that simultaneous AEs, regardless of type or grade, that are sustained over time create a high toxicity burden, perhaps even more than a specific type of episodic, high-grade AE (eg, a cardiac AE). The cumulative burden of such across-the-board toxicities, which were not necessarily all recognized as high-grade AEs, contributed to early treatment discontinuation. The sensitivity analysis lends further evidence to contrast the contribution to the predictive power (or lack thereof) of high-grade AEs only.
With the wide development and adoption of molecularly targeted agents (MTAs) and immunotherapies for cancer treatment, accounting for sustained low- and moderate-grade AEs may be more important than ever before. Traditional cytotoxic agents are generally administered intermittently in 3- or 4-week cycles, and they often result in immediate, acute AEs that resolve in a relatively short period of time. An acute grade 2 AE that resolves itself within a week may be of low concern. However, MTAs are often administered daily and may be taken indefinitely. A grade 2 AE experienced chronically as a result of an MTA may present a significantly higher burden to a patient than the same AE if it were experienced only temporarily for a short period of time.
The Cardiac Traj group had the highest percentage of high-grade AEs (59.1%) among all patient-cycle AEs in comparison with the Neuro Traj group (31.1%), the Mixed Reactions Traj group (46.8%), and the Low AE Traj group (50.0%). A further examination of the data regarding dosage modification by the trajectory groups confirmed that severe toxicities were more prevalent in the Cardiac Traj group. Per study protocol, the drug dosage was modified whenever specific severe AEs were reported; thus, dosage modification was primarily driven by high-grade AEs. We found no one (0%) in the Cardiac Traj group who did not modify the dosage, whereas 6.2% in the Neuro Traj group and 13.3% in the Mixed Reactions Traj group did not modify the dosage (Fig. 3).
We noted that the Low AE Traj group had nontrivial dosage modification and discontinuation percentages, although the rates were substantially lower than those for the other trajectory groups. Only 25.5% of this group did not experience any dosage modification, and 19.9% discontinued early for any reason. This suggests that other factors, such as personal reasons or individual differences in tolerability, also contributed to early discontinuation.
This report also examines treatment tolerability via PROs.19,20 Although clinician-centered assessment information such as that captured by CTCAE is useful, it indicates proximal effects due to treatment and provides a specific and likely limited perspective on tolerability.21 Studies have shown that with the CTCAE approach, the symptom burden is underdetected by as much as one-half of AEs in comparison with patient self-reports.22,23 Thus, incorporating PROs and perspectives on the treatment burden can provide unique information and higher precision on how patients experience treatment at both the initial and later stages as well as which symptoms and AEs might affect treatment decisions. This information could lead to better management of chemotherapy to enhance treatment tolerability, optimize treatment outcomes, and enhance patient HRQOL.24,25
The findings from this study indicate that the physical well-being domain of PROs, measured at the end of the first trial phase, is significantly associated with the Neuro Traj group and marginally associated with the Cardiac Traj group. This suggests that neuropathic and cardiac toxicities may have a longer and lasting negative effect on a patient’s physical well-being. At the end of cycle 8, treatment toxicity did not appear to have negative effects on other patient well-being domains such as social, emotional, functional, and breast cancer–related concerns. In comparison with the Low AE Traj group, differences in physical well-being scores for the Neuro Traj and Cardiac Traj groups were 1.51 and 1.78 points lower, respectively. Although a minimal importance difference (MID) has not been established for physical well-being, the MID for the Trial Outcome Score, which combines physical, functional, and breast cancer scores, was 5 to 6 points.26 With 5 points used as a benchmark, the proportionally derived MID for physical well-being is approximately 1.46. Therefore, the previously reported differences appear to be clinically meaningful. This finding points to the need for additional care for patients who experience neuropathic and/or cardiac toxicities during treatment even if the experience might be only episodic.
Our sensitivity analysis revealed that although high-grade AEs were not predictive of early discontinuation, AE-related or otherwise, they may exact a higher toll on some HRQOL domains than all-grade AEs do. Specifically, high-grade cardiac and neuropathic toxicities tend to contemporaneously occur within a small proportion of patients (8.2% in the current sample) and affect their emotional and functional well-being.
There are limitations to the study. We only retrospectively examined bevacizumab and chemotherapy exposure and did not disentangle toxicities due to other specific medications because E5103 was not designed to study other medications. The CTCAE form for this study required clinicians to report only all-grade AEs for toxicities related to the treatment. Low-grade AEs were likely underreported in this study.
Finally, the results regarding the prediction of early treatment discontinuation by AE trajectory may not be readily generalizable to other populations. The reported methodology for summarizing the multidimensional, multicycle AE data, however, should remain applicable to other applications.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
This work is funded by the National Cancer Institute (grant U01CA233169; principal investigators Lynne I. Wagner and Robert J. Gray). Edward H. Ip is also partly funded by the National Cancer Institute (grant U10 CA081851; principal investigator Glenn Lesser). The E5103 trial was supported by the National Cancer Institute of the National Institutes of Health under award numbers U01CA233169, U10CA081851, U10CA180820, U10CA180794, UG1CA189828, UG1CA232760, UG1CA233320, UG1CA233160, and UG1CA233331. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support for E5103 was provided by Genentech.
CONFLICT OF INTEREST DISCLOSURES
Ruth C. Carlos reports salary support paid to her institution from the Journal of the American College of Radiology; reimbursement for travel from GERRAF, ECOG-ACRIN, and the Academy for Radiology and Biomedical Imaging Research (ARBIR); participation on a data safety monitoring board for ECOG-ACRIN; and leadership or fiduciary roles with ARBIR, the Association of University Radiologists, and GE-Radiology Research Academic Fellowship. Kathy D. Miller reports participation on boards for Merck, Roche, and AstraZeneca. Lynne I. Wagner reports consulting fees from Celgene (now Bristol-Myers Squibb) and Athenex. The other authors made no disclosures.
Footnotes
This study is registered at ClinicalTrials.gov (NCT00433511).
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Miller KD, O’Neill A, Gradishar W, et al. Double-blind phase III trial of adjuvant chemotherapy with and without bevacizumab in patients with lymph node–positive and high-risk lymph node–negative breast cancer (E5103). J Clin Oncol. 2018;36:2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2–negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. [DOI] [PubMed] [Google Scholar]
- 3.Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2–negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. [DOI] [PubMed] [Google Scholar]
- 4.Miles D, Cameron D, Bondarenko I, et al. Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): a double blind placebo-controlled randomised phase III trial with prospective biomarker evaluation. Eur J Cancer. 2017;70:146–155. [DOI] [PubMed] [Google Scholar]
- 5.von Minckwitz G, Puglisi F, Cortes J, et al. Bevacizumab plus chemotherapy versus chemotherapy alone as second-line treatment for patients with HER2-negative locally recurrent or metastatic breast cancer after first-line treatment with bevacizumab plus chemotherapy (TANIA): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1269–1278. [DOI] [PubMed] [Google Scholar]
- 6.Brufsky AM, Hurvitz S, Perez E, et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2–negative metastatic breast cancer. J Clin Oncol. 2011;29:4286–4293. [DOI] [PubMed] [Google Scholar]
- 7.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. [DOI] [PubMed] [Google Scholar]
- 8.Jubb AM, Miller KD, Rugo HS, et al. Impact of exploratory biomarkers on the treatment effect of bevacizumab in metastatic breast cancer. Clin Cancer Res. 2011;17:372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roviello G, Generali D, Sobhani N. The combination of bevacizumab with chemotherapy is more beneficial in the metastatic setting rather than in the adjuvant setting for the treatment of HER2-negative breast cancer—a commentary on the E5103 randomized phase III clinical study. Transl Cancer Res. 2019;8(suppl 2):S94–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider BP, Shen F, Jiang G, et al. Impact of genetic ancestry on outcomes in ECOG-ACRIN-E5103. JCO Precis Oncol. 2017;2017:PO.17.00059. doi: 10.1200/PO.17.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coster S, Poole K, Fallowfield LJ. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res Treat. 2001;68:273–282. [DOI] [PubMed] [Google Scholar]
- 12.Ip EH, Zhang Q, Rejeski WJ, Harris TB, Kritchevsky S. Partially ordered mixed hidden Markov model for the disablement process of older adults. J Am Stat Assoc. 2013;108:370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ip EH, Zhang Q, Schwartz R, et al. Multi-profile hidden Markov model for mood, dietary intake, and physical activity in an intervention study of childhood obesity. Stat Med. 2013;32:3314–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rejeski WJ, Ip EH, Bertoni A, et al. Lifestyle change and mobility in aging obese adults with type 2 diabetes. N Engl J Med. 2012;366:1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gressett SM, Shah SR. Intricacies of bevacizumab-induced toxicities and their management. Ann Pharmacother. 2009;43:490–501. [DOI] [PubMed] [Google Scholar]
- 16.Gullo G, Eustace AJ, Canonici A, et al. Pilot study of bevacizumab in combination with docetaxel and cyclophosphamide as adjuvant treatment for patients with early-stage HER-2 negative breast cancer, including analysis of candidate circulating markers of cardiac toxicity: ICORG 08–10 trial. Ther Adv Med Oncol. 2019;11:1–9. doi: 10.1177/1758835919864236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong ML, Gao J, Thanarajasingam G, et al. Expanding beyond maximum grade: chemotherapy toxicity over time by age and performance status in advanced non–small cell lung cancer in CALGB 9730 (Alliance A151729). Oncologist. Published online September 20, 2020. doi: 10.1002/onco.13527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thanarajasingam G, Leonard JP, Witzig TE, et al. Longitudinal toxicity over time (ToxT) analysis to evaluate tolerability: a case study of lenalidomide in the CALGB 50401 (Alliance) trial. Lancet Haematol. 2020;7:e490–e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101:1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basch E, Abernethy AP, Mullins D, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30(34):4249–4255. [DOI] [PubMed] [Google Scholar]
- 21.Basch E, Rogak LJ, Dueck AC. Methods for implementing and reporting patient-reported outcome (PRO) measures of symptomatic adverse events in cancer clinical trials. Clin Ther. 2016;38:821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Maio M, Gallo C, Leighl NB, et al. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol. 2015;33:910–915. [DOI] [PubMed] [Google Scholar]
- 23.Basch E, Iasonos A, McDonough T, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol. 2006;7:903–909. [DOI] [PubMed] [Google Scholar]
- 24.Basch E, Wilfong L, Schrag D. Adding patient-reported outcomes to Medicare’s oncology value-based payment model. JAMA. 2020;323:213–214. [DOI] [PubMed] [Google Scholar]
- 25.Kluetz PG, Kanapuru BK, Lemery S, et al. Informing the tolerability of cancer treatments using patient-reported outcome measures: summary of an FDA and Critical Path Institute workshop. Value Health. 2018;21:742–747. [DOI] [PubMed] [Google Scholar]
- 26.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57:898–910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.