Abstract
Introduction
There is tremendous need for efficacious and accessible interventions for smoking cessation among American Indians and Alaska Natives. We tested the efficacy of an Acceptance and Commitment Therapy (ACT)-based smartphone application (iCanQuit) versus US Clinical Practice Guidelines-based smartphone application (QuitGuide) for smoking cessation among American Indians and Alaska Natives.
Aims and Methods
We compared cessation, changes in ACT-based processes, engagement and satisfaction between American Indian and Alaska Native iCanQuit (n = 89) and QuitGuide (n = 80) participants enrolled in the iCanQuit trial. The primary outcome was self-reported, complete-case, 30-day point-prevalence abstinence. Follow-up timepoints were 12, 6, and 3 months.
Results
Randomized American Indians and Alaska Natives from 31 US states (70% urban, 30% rural, with 25% of participants residing on tribal land). The outcome data retention rates were 93%, 92%, and 90% at the 12-, 6-, and 3-month follow-ups, respectively, with no differential retention between arms. The 30-day point-prevalence abstinence for iCanQuit versus QuitGuide was 30% versus 18% at 12 months (odds ratio [OR] = 1.96; 95% confidence interval [CI]: 0.90 to 4.26) 25% versus 11% at 6 months (OR = 2.62; 95% CI: 1.06 to 6.45), and 15% versus 6% at 3 months (OR = 2.93; 95% CI: 0.90 to 9.59). Increases in acceptance of internal cues to smoke mediated the effect of treatment on smoking cessation at 12 months. iCanQuit arm participants were also significantly more engaged and satisfied with their assigned application.
Conclusions
In a nationwide sample with high data retention and participant engagement, this is the first study to show that a digital intervention may be efficacious for helping American Indians and Alaska Natives quit smoking.
Implications
This is the first study to provide evidence of an efficacious, accessible, and engaging treatment for helping American Indians and Alaska Natives quit smoking. Compared to a US Clinical Practice Guidelines-based smartphone application (QuitGuide), an ACT-based smartphone application (iCanQuit) was more efficacious, engaging, and satisfactory among American Indians and Alaska Natives nationwide. Our results will inform the tailoring of the iCanQuit smartphone application for American Indian and Alaska Native tribal communities and organizations with potential for broad dissemination and high impact.
Introduction
American Indians and Alaska Natives in the United States (US) are disproportionally affected by high rates of cigarette smoking (24%) and low quit rates (38%).1,2 Cigarette smoking disparities in this population may be due to barriers to smoking cessation at the physiological (high nicotine dependence), social (eg, discrimination, racism, historical trauma, and acceptability of smoking), and cultural levels (eg, ceremonial use of tobacco in certain tribes and cultural insensitivity in existing smoking cessation programs).3–5 Furthermore, nicotine dependence among American Indians and Alaska Natives is associated with an increased prevalence of comorbid mood and anxiety as well as alcohol and other substance use disorders.6 Lack of medical care, travel costs, and living in remote areas further contribute to the disproportionally high rates of cigarette smoking and poor cessation outcomes among American Indians and Alaska Natives.5,7,8
Despite these known disparities, only four randomized controlled trials (RCTs) for smoking cessation with follow-ups at 6 months or longer have been conducted among American Indians and/or Alaska Natives, of which only one has demonstrated efficacy.9–12 Most trials have tested culturally tailored smoking cessation interventions delivered either by community doctors, one-on-one counseling, group-based therapy or a combination thereof. Specifically, the GAINS Project tested the effect of receiving tailored messages to quit smoking from doctors among 601 American Indians and Alaska Natives recruited from health clinics serving urban American Indian and Alaska Native sites in Seattle, Milwaukee, Minneapolis, and Spokane. The study found no differences in cessation between treatment and control groups.9 Similarly, a study that tested culturally tailored one-on-one counseling plus nicotine replacement therapy among 103 American Indians and Alaska Natives recruited from one tribal community in Wisconsin found no differences in cessation between treatment and control groups.10 The Walking Forward study tested 15 combinations of an intervention program among 254 American Indians recruited from three sites in South Dakota, but results were limited by very low retention (6%) at the 18-month follow-up.12 Finally, the All Nations Breath of Life trial tested the efficacy of a culturally tailored group-based program for smoking cessation among 463 American Indians and Alaska Natives recruited from several tribal urban communities in five states.11 Quit rates were significantly higher among those receiving the culturally tailored program (27.9% vs.17.4%) at the 6-month follow-up but results are limited by differential attrition rates between treatment arms (57.5% intervention group; 43.6% control group).11
The combination of both higher efficacy and reach has the greatest potential for making a high population-level impact for American Indians and Alaska Natives. Regarding the need for more efficacious cessation interventions among American Indians and Alaska Natives who smoke, a newer theory-based behavioral intervention holds promise. Specifically, Acceptance and Commitment Therapy (ACT) is a behavioral change approach that addresses internal cues to smoke with acceptance rather than avoidance and uses life values rather than expectations as motivation to quit smoking.13,14 ACT could be particularly helpful to American Indians and Alaska Natives who smoke by targeting physiological barriers through acceptance of internal cues to smoke (eg, cravings) and cultural barriers through living consistently with one’s values such as spirituality, self-reliance, and honoring life events as lessons.15,16 There is congruity between ACT core processes and several American Indian and Alaska Native cultural principles.15 For example, acceptance in ACT is culturally congruent with the American Indian and Alaska Native principle of “events in life can best be understood as lessons,” while commitment in ACT aligns with “actions having broad consequences, so we should act deliberately and thoughtfully.” Furthermore, the commitment component of ACT can prompt the American Indian and Alaska Native value of relationship-oriented interdependence (eg, family, clanship) as a motivation to quit smoking.16
The emergence of accessible and affordable digital interventions for smoking cessation has strong potential for greater reach to American Indians and Alaska Natives.1 For example, smartphone applications can be used to deliver smoking cessation interventions while providing more direct access to otherwise hard-to-reach populations. Recent reports show that at least 86% of American Indians and Alaska Natives living in rural communities owned cell phones and 68%–78% of these had smartphones with Internet access.17–19 Given this widespread availability, there are about 500 available smartphone applications for smoking cessation20 but only one has been shown efficacious in a full-scale RCT with long-term follow-up.
In a recent RCT, the use of an ACT-based smartphone application (iCanQuit) compared to a US Clinical Practice Guidelines (USCPG)-based smartphone application (QuitGuide) resulted in 1.5 times higher odds of quitting smoking (28% vs. 21%) at the 12-month follow-up among a racially diverse sample of 2415 smokers, including American Indians and Alaska Natives, from all 50 US states.21 To date, the efficacy of iCanQuit among American Indian and Alaska Native trial participants remains unknown as the topic was not the focus of the main trial. To address this gap, we conducted a secondary analysis of this RCT to determine the efficacy of iCanQuit versus QuitGuide for smoking cessation among American Indians and Alaska Natives enrolled in the parent trial. We hypothesized that compared to the QuitGuide arm, American Indians and Alaska Natives in the iCanQuit arm would have higher quit rates, engagement and satisfaction, and greater changes in measures of ACT-based processes.
Methods
Overview
The iCanQuit trial was a 12-month blinded, parallel, 2-arm RCT testing the efficacy of iCanQuit versus QuitGuide for smoking cessation. Interested and eligible adults who smoke were recruited online and randomized 1:1 to receive either iCanQuit or QuitGuide for a period of 12 months. Randomization was stratified by daily smoking frequency (≤20 vs. ≥21 cigarettes per day), minority race or ethnicity (non-Hispanic White race vs. any other race or Hispanic ethnicity), education level (≤high school vs. some college or more), and positive screening for depression (CESD-20 scale,22 score ≤15 vs. ≥16). All trial activities were approved by the Fred Hutchinson Cancer Research Center Institutional Review Board. All participants provided informed consent online. Full details of the parent trial have been previously published.21
Population and Recruitment
The parent trial enrolled a total of 2415 adults who smoke daily from all 50 US states between May 2017 and September 2018. Of those, 169 (7%) self-identified as American Indians or Alaska Natives either alone or in combination with other races or ethnicities. The primary sources of recruitment for the 169 American Indians and Alaska Natives enrolled in the trial were Facebook ads (155/169, 92%), a survey sampling company (8/169, 5%), search engine results (4/169, 2%), and referrals from friends and family (2/169, 1%). A donation from the Snoqualmie Tribe in Washington State funded Facebook ads specifically tailored to American Indians and Alaska Natives who smoke. Tribal oversight was not required for the design and implementation of the parent trial since it was designed for the general US population. These ads were tailored in two ways. First, three different images from American Indian and Alaska Native community members were used in each of the tailored ads. Recruitment ads were closely monitored and modified as needed based on recruitment success. For example, an ad portraying a young man led to 539 comments and 828 shares compared to eight comments and 11 shares for an ad portraying a young woman and one comment and two shares for an ad portraying an older woman with a child. Therefore, the ad of the young man was posted more frequently than the other two. To ensure representation of men, enrollment was limited to no more than 70% women. Second, in addition to using interests among Facebook users associated with commercial cigarette smoking (eg, Marlboro, e-cigarette) and tobacco control (eg, smoking ban, health effects of tobacco), an array of interests thought to be associated with American Indian and/or Alaska Native communities were selected. These included native languages, news, magazines, national health, and religious organizations (eg, Native American Times, National Congress of American Indians, Native American Church). For the American Indian and Alaska Native tailored recruitment campaign, the Facebook ad cost per click was $0.36 and cost per randomized participant was $25.73. The total impressions were 494 837. The ad language was the same for all participants, regardless of race/ethnicity: “Quit now with a free study from the Fred Hutchinson Cancer Research Center! Earn up to $105 to quit smoking! You’re ready to quit smoking.”
Eligibility Criteria
Interested participants were directed to the study website to provide consent to complete the screening questionnaire to assess eligibility (see the Supplementary Material for more details). Eligibility criteria included: (1) 18 years old or older, (2) smokes five or more cigarettes per day for the past year, (3) wants to quit smoking within the next 30 days, (4) if concurrently using other tobacco products, wants to quit all tobacco products within 30 days, (5) wants to learn skills to quit smoking and is willing to be randomized to either treatment condition, (6) has daily access to their own smartphone, (7) knows how to download smartphone applications, (8) can read English, (9) has never used QuitGuide and is not currently using another smoking cessation treatment, (10) has never participated in our prior studies, (11) has no household members enrolled in the study, (12) is willing to complete outcome surveys, and (13) can provide contact information for themselves and two relatives.
Interventions
iCanQuit
The iCanQuit smartphone application (version 1.2.1) teaches ACT skills for coping with smoking urges, staying motivated, and preventing relapse.21 The content is delivered in eight levels, including on-demand help in coping with smoking urges, tracking daily number of cigarettes smoked, and urges passed without smoking. The program is self-paced, and content is unlocked in a sequential manner. If a participant lapses, the program encourages (but does not require) them to set a new quit date and return to the first five levels for preparation (see the Supplementary Material for more details). iCanQuit targeted two core processes of ACT: acceptance and values. The acceptance component of the application teaches skills to accept sensations, emotions, and thoughts that trigger smoking via distancing from thoughts about smoking, mindfulness skills, and flexible perspective taking. This teaching of acceptance is conceptually distinct from USCPG-based standard approaches that teach avoidance of internal cues to smoke. The values component of the application teaches skills for determining the core life domains that motivate quitting smoking (eg, family, health, spirituality) and taking repeated small actions within these domains (eg, playing with grandchildren) to develop a smoke-free life. This focus on motivation by appealing to values is conceptually distinct from USCPG-based standard approaches that motivate by focusing on reasons for change.21 iCanQuit did not contain content specifically tailored to American Indians and Alaska Natives, nor was it previously tested for acceptability in this group.
QuitGuide
Participants randomized to the QuitGuide treatment arm received access to download the USCPG-based QuitGuide smartphone application (version 1.2.2). QuitGuide content is delivered in four main sections: (1) “Thinking about quitting,” which focuses on motivations to quit by using reason and logic and providing information on the health consequences of smoking and quitting; (2) “Preparing to Quit,” which helps users develop a quit plan, identify smoking behaviors, triggers, and reasons for being smoke-free, and social support for quitting; (3) “Quitting,” which teaches skills for avoiding cravings to smoke; and (4) “Staying Quit,” which presents tips, motivations, and actions to stay smoke-free and skills for coping with slips. Full details on both interventions have been previously published.21 No quit smoking medications or coaching was provided in either intervention arm.
Measures
Socio-Demographic Characteristics
Data collected at baseline included age, gender, ethnicity, education, employment, income, zip code, marital status, and sexual orientation. Zip codes were tied to geographic location using the R library “zipcode” 23 and categorized as urban or rural using sub-county level Rural-Urban Commuting Area (RUCA) codes.24 Guided by literature, RUCA codes of 1–3 were considered urban, while RUCA codes of 4–10 were considered rural.25–29 Zip codes were also categorized as tribal lands using US Environmental Protection Agency data.30 Study participants completed validated positive screening tools to assess depression, panic, and posttraumatic stress disorders.22,31,32
Smoking Behaviors and Alcohol Use
Participants were queried on the frequency of smoking cigarettes, the number of cigarettes, the timing of their first cigarette, and the last time they smoked any tobacco products, including e-cigarettes and vaping. Participants were not specifically asked about the use of ceremonial tobacco. Nicotine dependence was assessed with the Fagerström Test for Nicotine Dependence.33 Alcohol consumption was assessed via the Quick Drinking Screen.34
Smoking Cessation Outcomes
The primary smoking cessation outcome was self-reported complete-case 30-day point prevalence abstinence (PPA) at the 12-month follow-up. Secondary smoking cessation outcomes were: 7- and 30-day PPA at the 3-, 6-, and 12-month follow-up, and intent-to-treat missing as smoking 30-day PPA, prolonged abstinence, and cessation of all nicotine/tobacco products at the 12-month follow-up.
ACT-Based Processes
Acceptance of internal cues to smoke and valued living were measured at baseline and 3-month follow-up only since we expected acceptance and commitment to increase the most by 3 months post-randomization, consistent with previous ACT-based smoking cessation studies.35,36 Acceptance of internal cues to smoke was measured via a 27-item adaptation of the Avoidance and Inflexibility Scale37 which assesses one’s willingness to experience physical sensations (9-items), emotions (9-items), and thoughts (9-items) that cue smoking. The 27 items are rated on a 5-point scale from (1) “Not at all” to (5) “Very willing” with higher scores indicating greater acceptance. Scores were derived by averaging the items. Valued living was measured via the 10-item Valuing Questionnaire designed to assess the extent of personal values enactment.38 Items are intended to capture the quality of life of valued action in everyday language and without reference to specific life domains. Each item is rated on a 7-point scale ranging from (0) “Not at all true” to (6) “Completely true.” Scores were derived by averaging the items in progress and obstruction subscales with higher scores indicating either greater progress or greater obstruction toward valued living, respectively.
Engagement and Satisfaction
Engagement data were objectively collected via Google Analytics. Data included the number of times the application was opened, the time spent per session, and the number of unique days of use. Data on satisfaction was self-reported and collected at 3 months via an 11-item treatment satisfaction scale. Each item is rated on a 5-point scale ranging from (1) “not at all” to (5) “very much.” Satisfaction scores were dichotomized at a threshold of (3) “somewhat” or higher.
Statistical Analysis
Baseline socio-demographic characteristics, smoking behaviors, and ACT-based processes were compared between arms using t tests for continuous variables and Fisher’s exact tests for categorical variables. To assess study outcomes, we first used logistic regression models to compare binary cessation and retention measures between arms. Second, we compared changes in measures of ACT-based processes, and treatment engagement and satisfaction between arms. Generalized linear models were used for continuous outcomes (ie, changes in ACT-based processes from baseline to 3 months), negative binomial models were used to compare arms on right-skewed count outcomes (eg, number of application openings), and logistic regression models were used for binary satisfaction outcomes. All regression models were adjusted for the factors used in stratified randomization to avoid losing power and obtaining incorrect confidence intervals (CIs).39 Finally, we used Hayes’ PROCESS macro for SAS40 to assess mediation of the primary 12-month 30-day PPA smoking cessation outcome by changes from baseline to 3 months in each of the three acceptance subscales and total mean acceptance scale. We estimated indirect effects with 5000 bootstrapped samples and bias-corrected 95% CIs. Mediation effects were considered statistically significant when CIs did not include zero. All statistical tests were two-sided, with α = .05. Regression analyses were completed using R version 4.0.3,41 and library “MASS” 42 for negative binomial regression.
Results
Recruitment and Enrollment
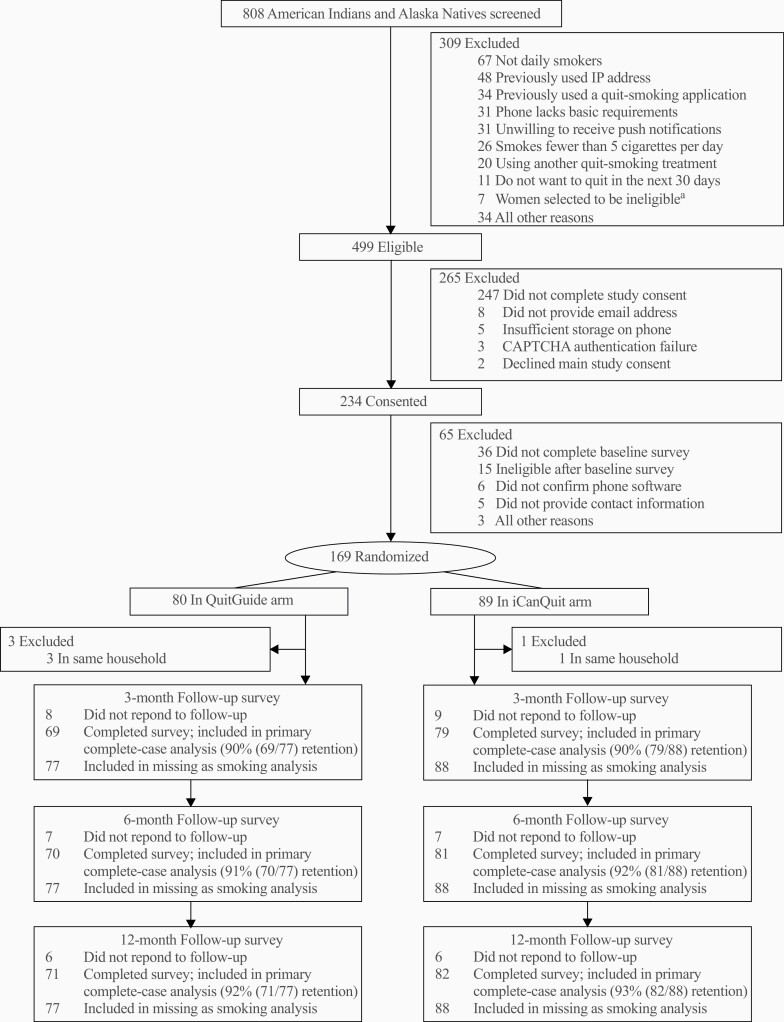
Eight hundred and eight self-identified American Indians and Alaska Natives were screened for eligibility (Figure 1). The three most common reasons for ineligibility were not completing the study consent or baseline questionnaire (35.0%), not being daily smokers (8.3%) and previously having used a quit-smoking application (4.2%). A total of 169 (20.9%) American Indian and Alaska Native adults who smoke were eligible to participate and were randomized to either the iCanQuit arm (n = 89) or QuitGuide arm (n = 80). Of the 169 American Indian and Alaska Native participants randomized, four were excluded post-randomization due to living in the same household as another enrollee. Data retention was high, with 93%, 92%, and 90% of participants retained at the 12-, 6-, and 3-month follow-ups, respectively, and no differential retention rates between treatment arms (all p > .05).
Figure 1.
CONSORT diagram.aTo increase enrollment of men, some women who were eligible for study enrollment were randomly selected to be excluded.
Baseline Characteristics
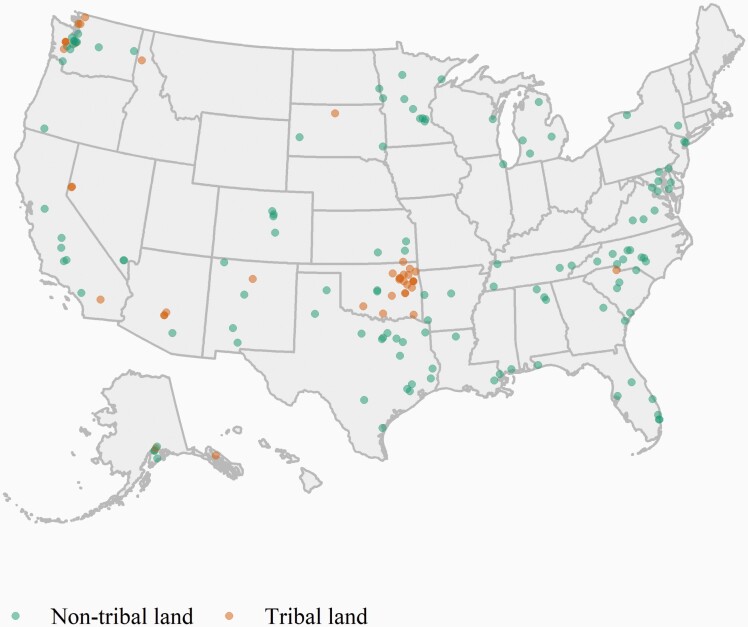
Participants’ baseline characteristics were balanced between treatment arms (Table 1). On average, study participants were 38.9 years old, 26% male, 15% Hispanic or Latino. Most (70%) of participants resided in an urban area, 30% in a rural area, with 25% of participants residing on tribal land. More than half (53%) of participants screened positive for depression, and 14% were heavy drinkers (4 or more drinks per typical drinking day for women and 5 or more drinks per typical drinking day for men within the past 30 days). The majority (85%) had smoked for a long time (≥10 years), and more than half (59%) had high nicotine dependence. The geographic representation of participants from 31 US states, is illustrated in Figure 2. Each point represents one of the 165 participants included in the analyses. Visual geographic clusters for high concentrations of participants with residence on tribal land can be seen in Alaska, Oklahoma, Utah, and Washington.
Table 1.
Baseline Characteristics of American Indian and Alaska Native Trial Participants
| Characteristic | No. (%) or Mean (SD) | p | ||
|---|---|---|---|---|
| Total (N = 165) |
QuitGuide (n = 77) |
iCanQuit (n = 88) |
||
| Age, mean (SD), years | 38.9 (10.4) | 39.4 (10.9) | 38.4 (10.0) | .544 |
| Men | 43 (26%) | 24 (31%) | 19 (22%) | .222 |
| Hispanic or Latino ethnicity | 25 (15%) | 11 (14%) | 14 (16%) | .942 |
| High school or less education | 67 (41%) | 34 (44%) | 33 (38%) | .478 |
| Employment | .555 | |||
| Employed | 76 (46%) | 39 (51%) | 37 (42%) | |
| Unemployed | 29 (18%) | 13 (17%) | 16 (18%) | |
| Homemaker | 32 (19%) | 12 (16%) | 20 (23%) | |
| Disabled | 19 (12%) | 7 (9%) | 12 (14%) | |
| Retired | 6 (4%) | 4 (5%) | 2 (2%) | |
| Other | 3 (2%) | 2 (3%) | 1 (1%) | |
| Income | .932 | |||
| <$20,000/year | 72 (44%) | 33 (43%) | 39 (44%) | |
| $20,000–$54,499/year | 67 (41%) | 31 (40%) | 36 (41%) | |
| ≥$55,000/year | 26 (16%) | 13 (17%) | 13 (15%) | |
| Geographic locationa | ||||
| Urban | 114 (70%)b | 49 (64%)c | 65 (74%) | .257 |
| Tribal land | 41 (25%) | 18 (23%) | 23 (26%) | .819 |
| Married | 46 (28%) | 23 (30%) | 23 (26%) | .719 |
| LGBT | 30 (18%) | 15 (19%) | 15 (17%) | .840 |
| Mental health positive screening results | ||||
| Depressiond | 87 (53%)e | 42 (55%)c | 45 (52%)f | .768 |
| Panicg | 59 (36%)b | 28 (36%) | 31 (36%)f | >.99 |
| PTSDh | 80 (49%)e | 39 (52%)i | 41 (47%) | .595 |
| Alcohol use | ||||
| No. of daily drinks, mean (SD) | 1.8 (3.6)j | 1.9 (3.9)k | 1.6 (3.3)l | .634 |
| Heavy drinker, No. (%)m | 23 (14%)j | 11 (15%)k | 12 (14%)l | >.99 |
| Smoking behavior | ||||
| FTND score, mean (SD) | 5.6 (2.2) | 5.7 (2.4) | 5.5 (2.0) | .659 |
| High nicotine dependence (FTND score ≥ 6) | 98 (59%) | 48 (62%) | 50 (57%) | .575 |
| Smokes more than one-half pack/day | 113 (68%) | 55 (71%) | 58 (66%) | .553 |
| Smokes more than 1 pack/day | 26 (16%) | 13 (17%) | 13 (15%) | .875 |
| First cigarette within 5 min of waking | 83 (50%) | 40 (52%) | 43 (49%) | .811 |
| Smoked for ≥10 years | 140 (85%) | 64 (84%) | 75 (85%) | >.99 |
| Used e-cigarettes at least once in past month | 46 (28%) | 24 (31%) | 22 (25%) | .479 |
| Quit attempts in past 12 months, mean (SD) | 2.3 (8.6)j | 3.1 (12.2)k | 1.6 (3.5)l | .336 |
| Confidence to quit smoking, mean (SD)n | 66.0 (27.6) | 61.9 (28.8) | 69.7 (26.1) | .071 |
| Friend and partner smoking | ||||
| No. of close friends who smoke, mean (SD) | 2.7 (1.8) | 2.8 (1.8) | 2.6 (1.8) | .403 |
| No. of housemates who smoke, mean (SD) | 1.6 (0.8) | 1.6 (0.9) | 1.6 (0.7) | .960 |
| Living with partner who smokes, No (%) | 59 (36%) | 31 (40%) | 28 (32%) | .334 |
| ACT-based measures, mean (SD) | ||||
| Acceptanceo | ||||
| Sensations | 3.1 (0.6)p | 3.1 (0.7)q | 3.1 (0.6)f | .927 |
| Emotions | 2.9 (0.5) | 2.9 (0.5) | 2.9 (0.5) | .650 |
| Thoughts | 2.9 (0.5) | 2.9 (0.5) | 2.9 (0.5) | .915 |
| Acceptance mean score | 3.0 (0.5)p | 3.0 (0.5)q | 3.0 (0.4)f | .981 |
| Valued livingr | ||||
| Progresss | 18.5 (7.6)t | 18.4 (7.3)i | 18.5 (8.0)f | .941 |
| Obstructionu | 12.0 (8.8)e | 12.4 (8.6)c | 11.7 (8.9)f | .625 |
ACT = Acceptance and Commitment Therapy; FTND = Fagerström Test for Nicotine Dependence; LGBT = lesbian, gay, bisexual, or transgender; PTSD = Posttraumatic Stress Disorder.
aZip codes were tied to geographic location and categorized as urban or rural using Rural-Urban Commuting Area (RUCA) codes. Zip codes were also categorized as tribal lands using US Environmental Protection Agency data.
b n = 164.
c n = 76.
dPositive screening results for depression via the Center for Epidemiological Studies Depression Scale (CESD-20; cutoff ≥ 16).
e n = 163.
f n = 87.
gPanic disorder via the Autonomic Nervous System Questionnaire (ANSQ; reporting ≥1 panic attack within the past month indicates a positive screen).
hPTSD via the 6-item PTSD Checklist (PCL-6; scores of ≥14 indicate a positive screen).
i n = 75.
j n = 159.
k n = 73.
l n = 86.
mHeavy drinking is defined as four or more drinks per typical drinking day for women and five or more drinks per typical drinking day for men within the past 30 days.
nRange, 0–100, where 0 indicates not at all confident and 100 indicates extremely confident.
oAvoidance and Inflexibility Scale. Range is 1–5. Higher scores indicate greater acceptance.
p n = 161.
q n = 74.
rValuing Questionnaire.
sRange is 0–30. Higher scores indicate greater progression toward one’s values.
t n = 162
uRange is 0–30. Higher scores indicate greater obstruction of one’s values.
Figure 2.
Geographic location of American Indian and Alaska Native trial participants.
Smoking Cessation Outcomes
As shown in Table 2, participants in the iCanQuit arm had greater odds of quitting smoking compared with those in the QuitGuide arm, across all timepoints. The 30-day PPA for iCanQuit versus QuitGuide was 30% versus 18% at the 12-month follow-up (odds ratio [OR] = 1.96; 95% CI: 0.90 to 4.26) 25% versus 11% at 6 months (OR = 2.62; 95% CI: 1.06 to 6.45), and 15% versus 6% at 3 months (OR = 2.93; 95% CI: 0.90 to 9.59). Other secondary outcomes followed a similar pattern. The missing as smoking 30-day PPA at 12 months was 28% for iCanQuit versus 17% for QuitGuide (OR = 1.97; 95% CI: 0.92 to 4.25). The 7-day PPA for iCanQuit versus QuitGuide was 35% versus 21% at the 12-month follow-up (OR = 2.02; 95% CI: 0.96 to 4.24), 33% versus 19% at 6 months (OR = 2.18; 95% CI: 1.02 to 4.70), and 32% versus 10% at 3 months (OR = 4.10; 95% CI: 1.64 to 10.27).
Table 2.
Smoking Cessation Outcomes by Follow-up Time Point and Change in ACT-Based Processesa
| Variable | No. (%) or Mean (SD) | p | |||
|---|---|---|---|---|---|
| Overall (n = 165) |
QuitGuide (n = 77) |
iCanQuit (n = 88) |
Point estimate or OR (95% CI) | ||
| Smoking cessation outcomesb | |||||
| 12-Month outcomes | |||||
| 30-d PPA | 38/153 (25%) | 13/71 (18%) | 25/82 (30%) | 1.96 (0.90 to 4.26) | .089 |
| 30-d PPA, missing as smokingc | 38/165 (23%) | 13/77 (17%) | 25/88 (28%) | 1.97 (0.92 to 4.25) | .083 |
| 7-d PPA | 44/153 (29%) | 15/71 (21%) | 29/82 (35%) | 2.02 (0.96 to 4.24) | .063 |
| Prolonged abstinenced | 15/126 (12%) | 5/59 (8%) | 10/67 (15%) | 1.89 (0.59 to 6.01) | .282 |
| 30-d PPA of all tobacco productse | 28/153 (18%) | 10/71 (14%) | 18/82 (22%) | 1.66 (0.71 to 3.92) | .245 |
| 6-Month outcomes | |||||
| 30-d PPA | 28/151 (19%) | 8/70 (11%) | 20/81 (25%) | 2.62 (1.06 to 6.45) | .036 |
| 7-d PPA | 40/151 (26%) | 13/70 (19%) | 27/81 (33%) | 2.18 (1.02 to 4.70) | .045 |
| 3-Month outcomes | |||||
| 30-d PPA | 16/148 (11%) | 4/69 (6%) | 12/79 (15%) | 2.93 (0.90 to 9.59) | .075 |
| 7-d PPA | 32/148 (22%) | 7/69 (10%) | 25/79 (32%) | 4.10 (1.64 to 10.27) | .003 |
| Change from baseline to 3-month post-randomization in ACT-based processesf | |||||
| Acceptance of internal cues to smokeg | |||||
| Sensations | 0.2 (0.8)h | −0.1 (0.8)i | 0.4 (0.8)j | 0.4 (0.2 to 0.7) | <.001 |
| Emotions | 0.2 (0.7)k | 0.0 (0.5)l | 0.3 (0.7)m | 0.4 (0.2 to 0.6) | <.001 |
| Thoughts | 0.1 (0.6)k | 0.0 (0.5)l | 0.2 (0.6)m | 0.3 (0.1 to 0.4) | .005 |
| Mean score | 0.2 (0.6)h | 0.0 (0.5)n | 0.3 (0.6)j | 0.4 (0.2 to 0.5) | <.001 |
| Valued living | |||||
| Progressp | −0.2 (6.9)q | −0.5 (6.9)r | 0.0 (7.0)s | 0.5 (−1.5 to 2.6) | .599 |
| Obstructiont | −0.6 (7.9)q | −0.5 (7.0)r | −0.7 (8.7)s | −0.7 (−2.9 to 1.4) | .510 |
ACT = Acceptance and Commitment Therapy; CI = confidence interval; OR = odds ratio; PPA = point prevalence abstinence.
aAll models include the following covariates: education (high school diploma or less), heavy smoking (>20 cigarettes/day) and depression symptoms (CESD-20 ≥ 16).
bAll outcomes are complete case (ie, exclusion of participants lost to follow-up) was specified a priori as the primary outcome, except where noted.
cIntent-to-treat missing as smoking analysis was specified a priori as a secondary outcome.
dDefined as no smoking since 3-month post-randomization, using self-reported data of last cigarette.
eIncluding e-cigarettes.
fAll changes in acceptance scores calculated as follow-up minus baseline. Negative score indicates measure was higher at baseline.
gAvoidance and Inflexibility Scale. Range is −4 to 4. Positive scores indicate higher acceptance at follow-up.
h n = 139.
i n = 63.
j n = 76.
k n = 145.
l n = 67.
m n = 78.
n n = 63.
oValuing Questionnaire.
pRange is −30 to 30. Positive scores indicate improvement.
q n = 142.
r n = 65.
s n = 77.
tRange is −30 to 30. Positive scores indicate worse condition.
ACT-Based Processes as Mediators of iCanQuit Effects on Quit Rates
Compared to QuitGuide participants, iCanQuit participants had greater baseline to 3-month increases in acceptance of physical sensations (p < .001), emotions (p < .001), and thoughts (p = .005) that cue smoking (Table 2). The increase in mean acceptance was greater for iCanQuit than QuitGuide participants (point estimate: 0.4; 95% CI: 0.2 to 0.5). Changes from baseline to 3 months in valued living measures of progress and obstruction were not significantly different between treatment arms (all p > .05).
In mediation analyses, every acceptance measure was a significant mediator of the treatment effect on 12-month 30-day PPA: acceptance of physical sensations (indirect effect: 0.13; 95% CI: 0.02 to 0.29), emotions (indirect effect: 0.16; 95% CI: 0.04 to 0.34), thoughts (indirect effect: 0.34; 95% CI: 0.10 to 0.78), and mean acceptance (indirect effect: 0.57; 95% CI: 0.21 to 1.16).
Engagement and Satisfaction
Engagement with iCanQuit was greater than with QuitGuide (Supplementary Table S1). On average, iCanQuit participants opened the application 32.4 times compared to 8.5 times among QuitGuide participants (p < .001). Time spent per session was significantly higher among iCanQuit participants (3.5 vs. 2.1 min per session; p < .001). Compared to QuitGuide participants, iCanQuit participants reported greater overall satisfaction with their assigned application (92% vs. 65%, p < .001), were much more likely to recommend the assigned application (88% vs. 63%, p = .001), and were more likely to report they felt the application was made for them (80% vs. 56%, p < .001).
Discussion
In a nationwide sample with high data retention and participant engagement, this is the first study to show that a digital intervention may be efficacious for helping American Indian and Alaska Native adults quit smoking. The 30-day PPA rates were significantly higher in the iCanQuit arm than the QuitGuide arm at the 6-month follow-up. There were suggestive higher cessation rates at the 3- and 12-month follow-ups with ORs ranging from 1.96 to 2.93 across all timepoints. Greater acceptance of internal cues to smoke mediated the effect of treatment on smoking cessation among iCanQuit participants. iCanQuit arm participants were also significantly more engaged and satisfied with the assigned application.
This study addresses several of the methodological concerns of previous trials that were limited by high and differential attrition rates as well as lack of geographic diversity.9,11,12 Compared to previous trials with attrition ranging between 20% and 94%, this study had a very low 7% attrition rate at the 12-month follow-up, with no differential attrition between arms. Our group’s methods for obtaining high retention rates are described elsewhere.43 Reach of interventions has also been a limitation of previous trials and a large barrier for treatment access among American Indians and Alaska Natives. This study recruited American Indian and Alaska Native adults from 31 US states (70% urban, 30% rural, with 25% of participants residing on tribal land), demonstrating accessibility and reach of the trial among those living in remote areas.
To date, only one trial has demonstrated efficacy for American Indians who smoke. That study tested a culturally tailored nine-session group-based program delivered by American Indian coaches versus a non-tailored current best practices program delivered by non-American Indian coaches.11 All participants, regardless of intervention assignment, were offered a choice of free medication to aid cessation. The reported 7-day PPA in the intervention was 27.9% versus 17.4% in the control group at the 6-month follow-up. By contrast, the current study showed a 7-day PPA of 33% in the iCanQuit versus 19% in the QuitGuide arm without the provision of coaching or pharmacotherapy.
In line with our hypotheses, mediation analyses confirmed that changes in acceptance mediated the effect of the treatment on smoking cessation at the 12-month follow-up. These results suggest that the efficacy of ACT-based iCanQuit for smoking cessation among American Indians and Alaska Natives may operate, in part, through improving acceptance of internal cues to smoke. Another reason why iCanQuit might be efficacious and engaging among American Indians and Alaska Natives could be the testimonials and personal stories that are part of the iCanQuit application content. Storytelling reinforces traditional American Indian and Alaska Native knowledge systems, promotes cross-generational learning, and builds social connections.44 Therefore, these features may be more acceptable and culturally congruent with the American Indian and Alaska Native community (Supplementary Table S2). Finally, as shown in our past research with the SmartQuit application45 that preceded iCanQuit, it is possible that the high engagement rates due to the appeal of the ACT skills contributed to increased acceptance of internal smoking cues, which in turn impacted the high quit rates.
On the other hand, valued living changes from baseline to 3 months did not differ between arms. There are several potential explanations for these findings. The Valuing Questionnaire38 used in the study is not a smoking-specific measure and as such may lack the specificity to capture changes in values related to quitting smoking. Furthermore, population-specific values may not be accurately captured by this scale since it was not designed to be culturally sensitive to American Indians and Alaska Natives. Another possibility is that this sample of American Indians and Alaska Natives may have had strong cultural values to begin with and not much room for improvement. Future larger trials should evaluate cultural values with a validated tool that is specific to both the population and the behaviors evaluated.
There are several strengths of this study. This is the first study, to our knowledge, that has tested and demonstrated the potential efficacy of a digital intervention for smoking cessation among American Indians and Alaska Natives. Second, we obtained the highest retention rates ever reported in this population with no differential rates between arms. Third, we showed that smartphone applications for smoking cessation have great potential for high reach, utilization, and satisfaction among American Indians and Alaska Natives nationwide.
The study also has limitations. First, results are based on a secondary analysis of the parent trial that was not tailored to American Indians and Alaska Natives, and therefore the results should be interpreted with caution. Second, only 20.9% (169/808) of people screened were randomized into the trial. This level of selection bias11 is consistent with prior smartphone application-delivered smoking cessation trials,46,47 and more favorable than prior telephone-delivered cessation trials.48 Third, the cessation outcomes were self-reported and not biochemically confirmed. However, expert consensus suggests that biochemical verification of abstinence is impractical and unnecessary in population-based studies that do not involve in-person contact.49 Furthermore, previous studies have demonstrated strong agreement between self-reported and biochemically verified smoking status.50,51 Additionally, there is no reason to believe that the validity of self-reported abstinence would differ by treatment arm. Fourth, we reported engagement data up to 6 months and not 12 months due to a technical error by Google Analytics. Because participants were unaware of the error and the majority stopped using their application by 6 months, the missing data is unlikely to change the validity of the results.52 Finally, the use of commercial tobacco products for ceremonial use was not ascertained which can differ widely across different tribal populations.
Despite these limitations, this study provides necessary preliminary data for the cultural adaptation and testing of smartphone applications that are evidence-based for American Indians and Alaska Natives nationwide. Culturally adapted behavioral interventions for priority populations are four times more efficacious than non-adapted clinical practice guidelines-based treatments.53 Therefore, to maximize the effect on cessation outcomes that we observed in this study, a cultural adaptation of the iCanQuit smartphone application for American Indians and Alaska Natives is the next logical step for broad dissemination and high public health impact. Future larger trials testing the potential synergistic interaction between cultural adaptation and an ACT-based smartphone application for American Indians and Alaska Natives is warranted. Each one of the six ACT core processes taught in iCanQuit could be tailored to American Indian and Alaska Native cultural principles (Supplementary Table S2).15 For example, the ACT process of being present, which means being nonjudgmental and fully aware of the present moment with openness, interest and receptiveness, could be tailored to the American Indian and Alaska Native principle of “this current moment is part of the lesson of who we were, who we are, and who we will be become.” 15 Finally, to ensure increased enrollment and participation of American Indians and Alaska Natives nationwide, partnerships with tribes and tribal organizations would be invaluable for the future cultural adaptation of iCanQuit.
This is the first study to show that a digital intervention may be efficacious for helping American Indian and Alaska Native adults quit smoking. The study represents an important step in developing behavioral interventions that can reach and engage American Indians and Alaska Natives who smoke nationwide. Our results will inform the future testing of iCanQuit tailored to American Indians and Alaska Natives, with potential for broad dissemination and high public health impact in this population.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
We gratefully acknowledge the philanthropic donation from the Snoqualmie Tribe in Washington State and their review of the results presented here. We appreciate the tireless contributions of the entire study staff, most notably, Eric Meier, Eric Strand, Carolyn Ehret, Alanna Boynton, the design services of Ayogo, Inc., and the development services of Moby, Inc. We are very appreciative of the American Indian and Alaska Native study participants.
Funding
This study was funded by grant R01CA192849, awarded to Dr. Bricker, from the National Cancer Institute. A donation from the Snoqualmie Tribe in Washington State funded Faceboougk ads specifically tailored to American Indians and Alaska Natives who smoke.
Declaration of Interests
None of the authors have declarations. None of the authors have a financial interest in the iCanQuit app.
Code Availability Statement
The code for the data analysis underlying this article will be shared on reasonable request to Jonathan B. Bricker at jbricker@fredhutch.org.
References
- 1. U.S. Department of Health and Human Services. Smoking Cessation: A Report of the Surgeon General—Executive Summary. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2020. [Google Scholar]
- 2. Sakuma KK, Pierce JP, Fagan P, et al. WORKING racial/ethnic disparities across indicators of cigarette smoking in the era of increased tobacco control, 1992–2019. Nicotine Tob Res. 2021;23(6):909–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heart MY, Chase J, Elkins J, Altschul DB. Historical trauma among Indigenous Peoples of the Americas: concepts, research, and clinical considerations. J Psychoactive Drugs. 2011;43(4):282–290. [DOI] [PubMed] [Google Scholar]
- 4. Daley CM, Faseru B, Nazir N, et al. Influence of traditional tobacco use on smoking cessation among American Indians. Addiction. 2011;106(5):1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burgess D, Fu SS, Joseph AM, Hatsukami DK, Solomon J, van Ryn M. Beliefs and experiences regarding smoking cessation among American Indians. Nicotine Tob Res. 2007;9(suppl 1):S19–S28. [DOI] [PubMed] [Google Scholar]
- 6. Moghaddam JF, Dickerson DL, Yoon G, Westermeyer J. Nicotine dependence and psychiatric and substance use disorder comorbidities among American Indians/Alaska Natives: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2014;144:127–133. [DOI] [PubMed] [Google Scholar]
- 7. Twyman L, Bonevski B, Paul C, Bryant J. Perceived barriers to smoking cessation in selected vulnerable groups: a systematic review of the qualitative and quantitative literature. BMJ Open. 2014;4(12):e006414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gryczynski J, Feldman R, Carter-Pokras O, Kanamori M, Chen L, Roth S. Contexts of tobacco use and perspectives on smoking cessation among a sample of urban American Indians. J Health Care Poor Underserved. 2010;21(2):544–558. [DOI] [PubMed] [Google Scholar]
- 9. Johnson KM, Lando HA, Schmid LS, Solberg LI. The GAINS project: outcome of smoking cessation strategies in four urban Native American clinics. Giving American Indians No-smoking Strategies. Addict Behav. 1997;22(2):207–218. [DOI] [PubMed] [Google Scholar]
- 10. Smith SS, Rouse LM, Caskey M, et al. Culturally-tailored smoking cessation for adult American Indian smokers: a clinical trial. Couns Psychol. 2014;42(6):852–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi WS, Beebe LA, Nazir N, et al. All Nations Breath of Life: a randomized trial of smoking cessation for American Indians. Am J Prev Med. 2016;51(5):743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dignan MB, Jones K, Burhansstipanov L, et al. A randomized trial to reduce smoking among American Indians in South Dakota: the walking forward study. Contemp Clin Trials. 2019;81:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bricker JB, Bush T, Zbikowski SM, Mercer LD, Heffner JL. Randomized trial of telephone-delivered acceptance and commitment therapy versus cognitive behavioral therapy for smoking cessation: a pilot study. Nicotine Tob Res. 2014;16(11):1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayes SC, Levin ME, Plumb-Vilardaga J, Villatte JL, Pistorello J. Acceptance and commitment therapy and contextual behavioral science: examining the progress of a distinctive model of behavioral and cognitive therapy. Behav Ther. 2013;44(2):180–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia MA, Tehee M.. Society of Indian Psychologists Commentary on the American Psychological Association’s (APA) Ethical Principles of Psychologists and Code of Conduct; 2014. [Google Scholar]
- 16. Teufel-Shone NI, Tippens JA, McCrary HC, Ehiri JE, Sanderson PR. Resilience in American Indian and Alaska Native public health: an underexplored framework. Am J Health Promot. 2018;32(2):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Orr MF, Burduli E, Hirchak KA, et al. Culturally-tailored text-messaging intervention for smoking cessation in rural American Indian communities: rationale, design, and methods. Contemp Clin Trials Commun. 2019;15:100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sinicrope PS, Koller KR, Prochaska JJ, et al. Social media intervention to promote smoking treatment utilization and cessation among Alaska native people who smoke: protocol for the connecting Alaska native people to quit smoking (CAN Quit) pilot study. JMIR Res Protoc. 2019;8(11):e15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dotson JA, Nelson LA, Young SL, Buchwald D, Roll J. Use of cell phones and computers for health promotion and tobacco cessation by American Indian college students in Montana. Rural Remote Health. 2017;17(1):4014. [DOI] [PubMed] [Google Scholar]
- 20. Abroms LC, Lee Westmaas J, Bontemps-Jones J, Ramani R, Mellerson J. A content analysis of popular smartphone apps for smoking cessation. Am J Prev Med. 2013;45(6):732–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bricker JB, Watson NL, Mull KE, Sullivan BM, Heffner JL. Efficacy of smartphone applications for smoking cessation: A randomized clinical trial. JAMA Intern Med. 2020;180(11):1472–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 23. Breen J. zipcode: U.S. ZIP Code Database for Geocoding. R Package Version 1.0. https://cran.r-project.org/package=zipcode. Accessed February 21, 2021.
- 24. US Department of Agriculture, Economic Research Service. Rural-Urban Commuting Area Codes; 2020. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/. Accessed February 21, 2021.
- 25. Larson EH, Andrilla CHA, Garberson LA, Evans DV.. Geographic Access to Health Care for Rural Medicare Beneficiaries in Five States: An Update. Policy Brief. Seattle, WA: WWAMI Rural Health Research Center, University of Washington; 2021. [Google Scholar]
- 26. Ratcliffe M, Burd C, Holder K, Fields A.. Defining Rural at the U.S. Census Bureau, Report Number ACSGEO-1. Washington, DC: U.S. Census Bureau; 2016. [Google Scholar]
- 27. Brooks MM, Mueller JT, Thiede BC. County reclassifications and rural-urban mortality disparities in the United States (1970–2018). Am J Public Health. 2020;110(12):1814–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen X, Orom H, Hay JL, et al. Differences in rural and urban health information access and use. J Rural Health. 2019;35(3):405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Unger JM, Moseley A, Symington B, Chavez-MacGregor M, Ramsey SD, Hershman DL. Geographic distribution and survival outcomes for rural patients with cancer treated in clinical trials. JAMA Netw Open. 2018;1(4):e181235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. US Environmental Protection Agency, Office of Environmental Information. Zip Code to Tribal Lands Lookup Table ; 2016. https://www.arcgis.com/home/item.html?id=241b01b48db1409381a4e42fe6477821. Accessed February 25, 2021.
- 31. Stein MB, Roy-Byrne PP, McQuaid JR, et al. Development of a brief diagnostic screen for panic disorder in primary care. Psychosom Med. 1999;61(3):359–364. [DOI] [PubMed] [Google Scholar]
- 32. Sawchuk CN, Roy-Byrne P, Noonan C, et al. ; AI-SUPERPFP Team . The association of panic disorder, posttraumatic stress disorder, and major depression with smoking in American Indians. Nicotine Tob Res. 2016;18(3):259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 34. Roy M, Dum M, Sobell LC, et al. Comparison of the quick drinking screen and the alcohol timeline followback with outpatient alcohol abusers. Subst Use Misuse. 2008;43(14):2116–2123. [DOI] [PubMed] [Google Scholar]
- 35. Bricker J, Wyszynski C, Comstock B, Heffner JL. Pilot randomized controlled trial of web-based acceptance and commitment therapy for smoking cessation. Nicotine Tob Res. 2013;15(10):1756–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schuck K, Otten R, Kleinjan M, Bricker JB, Engels RC. Self-efficacy and acceptance of cravings to smoke underlie the effectiveness of quitline counseling for smoking cessation. Drug Alcohol Depend. 2014;142:269–276. [DOI] [PubMed] [Google Scholar]
- 37. Gifford EV, Kohlenberg BS, Hayes SC, et al. Acceptance-based treatment for smoking cessation. Behav Ther. 2004;35:689–705. [Google Scholar]
- 38. Smout M, Davies M, Burns N, Christie A. Development of the Valuing Questionnaire (VQ). J Contextual Behav Sci. 2014;3:164–72. [Google Scholar]
- 39. Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomization for clinical trials. J Clin Epidemiol. 1999;52(1):19–26. [DOI] [PubMed] [Google Scholar]
- 40. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. 2nd ed. New York, NY: Guilford Press; 2018. [Google Scholar]
- 41. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. https://www.R-project.org/. [Google Scholar]
- 42. Venables WN, Ripley BD.. Modern Applied Statistics With S. 4th ed. New York, NY: Springer; 2002. [Google Scholar]
- 43. Watson NL, Mull KE, Heffner JL, McClure JB, Bricker JB. Participant recruitment and retention in remote eHealth intervention trials: methods and lessons learned from a large randomized controlled trial of two web-based smoking interventions. J Med Internet Res. 2018;20(8):e10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cochran PA, Marshall CA, Garcia-Downing C, et al. Indigenous ways of knowing: implications for participatory research and community. Am J Public Health. 2008;98(1):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zeng EY, Heffner JL, Copeland WK, Mull KE, Bricker JB. Get with the program: adherence to a smartphone app for smoking cessation. Addict Behav. 2016;63:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Danaher BG, Tyler MS, Crowley RC, Brendryen H, Seeley JR. Outcomes and device usage for fully automated internet interventions designed for a smartphone or personal computer: the MobileQuit smoking cessation randomized controlled trial. J Med Internet Res. 2019;21(6):e13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baskerville NB, Struik LL, Dash D. Crush the crave: Development and formative evaluation of a smartphone app for smoking cessation. JMIR mHealth uHealth. 2018;6(3):e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews. 2013;8:CD002850. [DOI] [PubMed] [Google Scholar]
- 49. Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob Res. 2020;22(7):1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van der Aalst CM, de Koning HJ. Biochemical verification of the self-reported smoking status of screened male smokers of the Dutch-Belgian randomized controlled lung cancer screening trial. Lung Cancer. 2016;94:96–101. [DOI] [PubMed] [Google Scholar]
- 51. Wong SL, Shields M, Leatherdale S, Malaison E, Hammond D. Assessment of validity of self-reported smoking status. Health Rep. 2012;23(1):47–53. [PubMed] [Google Scholar]
- 52. Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–576. [DOI] [PubMed] [Google Scholar]
- 53. Griner D, Smith TB. Culturally adapted mental health intervention: a meta-analytic review. Psychotherapy (Chic). 2006;43(4):531–548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.