Abstract
Introduction
This study explores how the emergence of FDA-funded Tobacco Regulatory Science (TRS) research complements and perhaps influenced the direction of tobacco research supported by NIH.
Aims and Methods
New NIH- and FDA-funded tobacco projects awarded in fiscal years (FY) 2011–2020 were identified using internal NIH databases of awarded grants. Project abstracts and research aims were coded by the authors to characterize research domains and tobacco products studied.
Results
Between FY 2011 and 2020, NIH funded 1032 and FDA funded 322 new tobacco projects. For the years and grant activity codes studied, the number of new NIH tobacco projects declined while FDA’s increased; combined the number of new projects held steady. Much of NIH research included smoking combustibles (43.7%). The most common products in FDA research were cigarettes (74.8%) and e-cigarettes/ENDS (48.1%). Most NIH (58.6%) and FDA (67.7%) projects included research on the determinants of tobacco use. Another area of apparent overlap was health effects (29.5% NIH and 30.1% FDA). Projects unique to NIH included treatment interventions (33.3%), disease pathology/progression (17.8%) and neurobiology (18.9%). A minority of both NIH and FDA projects included populations particularly vulnerable to tobacco product use.
Conclusions
In total, support for new tobacco research supported by NIH and FDA combined remained steady for the time period covered, though there was a concomitant decline in NIH tobacco projects with the increase in FDA-funded TRS projects for the activity codes studied. Despite the apparent overlap in some areas, both NIH and FDA support research that is unique to their respective missions.
Implications
NIH continues to support tobacco research that falls within and outside of FDA’s regulatory authorities. This research still is needed not only to bolster the evidence base for regulatory decisions at the national and state levels, but also to advance a comprehensive scientific agenda that can inform multiple levels of influence on tobacco control, use and addiction. It will be important to continue monitoring FDA-funded TRS and NIH-funded tobacco research portfolios to ensure that the level of support for and focus of the research is sufficient to address the burden of tobacco-related morbidity and mortality.
Introduction
The National Institutes of Health (NIH) has a decades-long history of advancing research to prevent and control tobacco use and addiction.1 This extensive body of research has been supported by multiple NIH Institutes2–5 and spans scientific domains from understanding tobacco’s detrimental effects on health6 to the development of interventions to prevent uptake of tobacco products and treat nicotine addiction.7
In 2009, The Family Smoking Prevention and Tobacco Control Act (FSPTCA)8 granted the Food and Drug Administration (FDA) regulatory authority over the manufacture, marketing and distribution of tobacco products. The law provided FDA with the resources necessary to ensure that its regulatory decisions are based on the best available science, limiting its use of funding to support only research that falls within FDA’s regulatory authorities (as specified in Section 919 (c) (2) (A) of the FSPTCA).8 Given NIH’s history in tobacco control and its existing infrastructure to develop, support and advance new science, FDA partnered with the NIH to support research to inform regulation of tobacco products, i.e., Tobacco Regulatory Science (TRS). Bound by FDA’s regulatory authorities, TRS is an area of research more narrowly focused than the wide breadth of tobacco research funded by the NIH.9 While there may be some overlap in research supported by both agencies, such as studies on addiction, toxicity and appeal of tobacco products, FDA does not support research typically funded by NIH, such as studies investigating the underlying mechanisms of disease and progression associated with tobacco products, diagnosis of tobacco-related disease, treatment of disease or tobacco addiction, or testing interventions to improve clinical practice.10
It is important to consider how this unique collaboration to establish the field of TRS meets the goals of both agencies. The goal of research supported by FDA is solely to establish a solid evidence-base to inform potential regulatory decisions to protect the public health. This contrasts with the goal of NIH-supported research that seeks innovative approaches to advance fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health. Though these goals clearly differ, together they are needed to implement evidence-based policy and practice to improve public health.10
Preliminary evaluation of FDA’s portfolio of tobacco research suggests that the program has been achieving its goal to support regulation and communication about tobacco products, as well as growing a cadre of investigators trained in TRS.11 However, assessing how the emergence of TRS as a field of tobacco control and the infusion of funds to support it complements the focus and continued support for tobacco research at NIH is another important metric for evaluating the NIH-FDA collaboration outcomes. Here we address how NIH-funded tobacco research compares to the NIH administered FDA-funded TRS research portfolio.
Methods
We used the NIH’s internal iSearch portfolio analysis platform for this analysis, which allows the user to search and code grants on a particular topic. The portfolio analysis was restricted to new extramural tobacco research (types 1 = new, 2 = renewal, and 9 = change of institute at renewal)12 funded between fiscal years (FY) 2011–2020. We searched for new research grants focusing on ‘Tobacco’ across all 27 NIH Institutes and Centers (ICs) (N = 1871). We then identified all new awarded research grants (N = 261) within the FDA-funded TRS portfolio for these fiscal years using another internal NIH database, Query, View, Report (QVR). For purposes of comparing the research supported by both agencies, we only included NIH grant activity codes (i.e., grant mechanisms)13 that FDA primarily used to support TRS: R01, R03, R21, P50, U01, U54, K01, and K99. These activity codes comprise about 66% of the NIH extramural tobacco portfolio for the years examined. A total of 1227 NIH grants and 255 FDA grants with these activity codes were identified. We conducted a trend analysis of NIH and FDA support for these grant activity codes, as well as all NIH activity codes that supported tobacco research during this time period.
Multi-component grants (P50, U54), which include three or more distinct research projects, were disaggregated into separate research projects. The grant title and abstract were reviewed to identify and exclude cores and other infrastructure projects within multi-component grants (N = 207 for NIH and N = 18 for FDA) to focus the analyses specifically on research projects. We also excluded projects without tobacco-focused aims (N = 268 for NIH and N = 0 for FDA). For example, we excluded projects using tobacco exposure to generate a disease of interest (e.g., COPD in mice) but did not study tobacco-related mechanisms or pathophysiology. This resulted in 1032 NIH projects and 322 FDA projects for analysis. Appendix A displays a flow diagram of included/excluded grants and projects.
Projects were coded manually using a taxonomy developed by the authors to characterize the type of products and the focus of research within the research projects (Appendix B). The codebook was developed through an iterative process based on a sampling of tobacco research grant abstracts and the authors’ knowledge of the NIH and FDA tobacco research portfolios to ensure that all relevant characteristics were included. We prepared a comprehensive list of tobacco products to capture studies with aims falling within FDA regulatory authority (e.g., e-cigarette use among youth), as well as studies that may use products that fall outside of FDA authority (e.g., use of cigars with cannabis). We prepared a list of research domains to capture the aims of research that would likely be supported by both NIH and FDA (e.g., health effects of tobacco products), as well as study domains that may be more likely funded through NIH (e.g., mechanisms of tobacco-related disease and treatment) versus FDA (e.g., analysis of specific tobacco product constituents). We pilot tested the taxonomy on a sample of projects to assess its utility. Once finalized, the taxonomy included two categories—Tobacco Product and Research Domain, with a ‘select all that apply’ response option. The coding process involved reading of the title, abstract, specific aims and public health relevance sections of each project and assigning appropriate codes within the iSearch database. One author coded all projects using the taxonomy and a 20% sample of the coded projects was also coded independently by other authors. A weekly discussion was held with team members and coding consensus was reached in case of discrepancies.
We attempted to capture certain populations of interest within the FDA and NIH research portfolios, including rural, pregnant persons, Lesbian, Gay, Bisexual, Transgender or Queer (LGBTQ), racial/ethnic minority and youth. We used categories in the FDA portfolio from the population of interest codes that are assigned by FDA to these grants internally. The NIH has its own internal coding system, Research, Condition, and Disease Categorization (RCDC), to capture scientific categorical spending and includes codes for certain populations of interest (https://report.nih.gov/funding/categorical-spending#/). A crosswalk between the two coding systems gave us an opportunity to study certain comparable population codes: Rural Health; Pregnant Women/Maternal Health; LGBTQ; and Minority Health, including African American or Black, Asian American (including East, Southeast and South Asian regions, but not the Middle East or Western Asia), American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander or Hispanic. Since the NIH system does not capture ‘Youth’, we used Medical Subject Headings (MeSH) terms for this category in the NIH portfolio. We were only able to examine populations of interest for projects within the most recent 5 years, i.e. between FY 2016–2020, as NIH did not code for all these populations in prior years. Research projects that included only humans as study subjects were used as the denominator when calculating percentages.
Descriptive data (e.g., abstract, specific aims) and administrative details (e.g., funding, institute/agency, activity code, year awarded) for the projects were extracted from QVR, whenever required. Frequency and descriptive statistics were used to characterize the overall sample of grants within the two portfolios using STATA statistical software Version 1614 and Microsoft Excel.
Results
Between FY 2011 and 2020, NIH funded 1032 new tobacco research projects and FDA funded 322 new tobacco research projects. Within this sample of projects, NIH used the R01 (53%) and R21 (25%) activity code more often than FDA (36% and 16%, respectively), while FDA used the P50 (15%) and U54 (13%) activity code more often than NIH (2% and 2%, respectively) to support new projects.
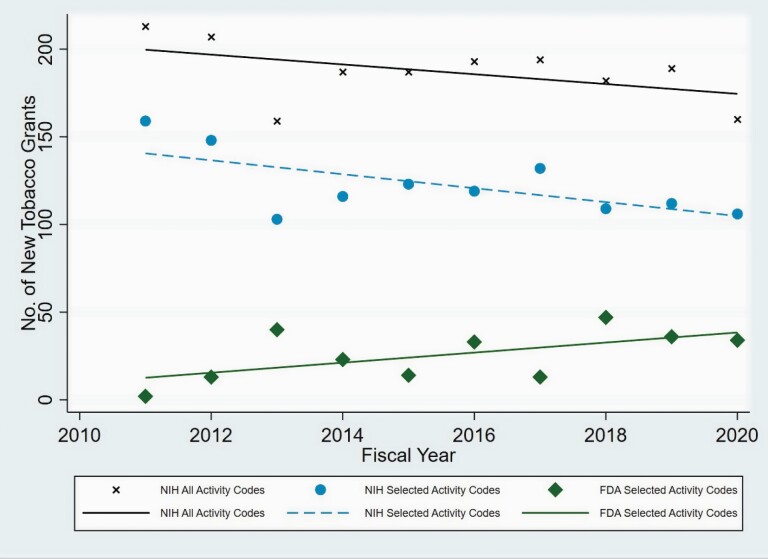
Of the grant activity codes studied (R01, R03, R21, P50, U01, U54, K01, and K99), the number of new tobacco research grants funded by NIH from FY11 to FY20 declined over the study period while the number for FDA increased (Figure 1). This interaction between agency and time was found to be significant (p = 0.006). Within agencies, the positive FDA trend of 2.9 grants/year was not significant (p = 0.076) while the NIH trend of −4.0 grants/year was (p = 0.018). Spearman’s rho values for FDA- and NIH-specific associations were 0.5593 and −0.5394, respectively, indicating associations of moderate strength. To determine whether the decline in NIH funding of new tobacco research grants was due to an overall decline in NIH tobacco research funding or just among the NIH activity codes studied, we investigated the trend for NIH grants overall. While the number of total NIH grants declined by an average of −2.8 grants per year, this decline was not significant (p = 0.152). Thus, there was no significant decline overall in NIH support for non-TRS tobacco research, but there was a significant decline among the set of selected activity codes that were used to support TRS projects.
Figure 1.
Number of new NIH-and FDA-funded Tobacco Research Grants FY2011–2020.
A comparison of FDA and NIH-funded projects shows some differences in tobacco products studied (Table 1). NIH projects were significantly more likely to include research on unspecified combustible products; secondhand or thirdhand smoke exposure; nicotine replacement therapy (NRT) and use of tobacco products with other non-tobacco substances, including cannabis, THC and alcohol. In contrast, FDA projects were significantly more likely to specify the product and include cigarettes, smokeless tobacco, and newly deemed tobacco products including e-cigarettes, cigars, hookah, cigarillos, heated tobacco, dissolvables, as well as polyuse of tobacco products.
Table 1.
Number and percentage of research projects within the NIH portfolio (n = 1032) and the FDA portfolio (n = 322) by tobacco product and research domain
| Project focus | Projects within the NIH Portfolio, N = 1032 | Projects within the FDA Portfolio, N = 322 | ||
|---|---|---|---|---|
| n | % | n | % | |
| Tobacco product | ||||
| Cigarette | 355 | 34.4 | 242 | 75.2 |
| E-Cigarette | 116 | 11.2 | 162 | 50.3 |
| Nicotine | 286 | 27.7 | 67 | 20.8 |
| Smoking/Combustible unspecified | 451 | 43.7 | 66 | 20.5 |
| Tobacco products unspecified | 182 | 17.6 | 58 | 18.0 |
| Smokeless products | 20 | 1.9 | 39 | 12.1 |
| Cigar | 12 | 1.2 | 35 | 10.9 |
| Waterpipe/Hookah | 19 | 1.8 | 33 | 10.3 |
| Cigarillo | 4 | 0.4 | 21 | 6.5 |
| Polyuse of tobacco products | 7 | 0.7 | 13 | 4.0 |
| Heated or heat not burned | 0 | – | 7 | 2.2 |
| Dissolvables | 0 | – | 7 | 2.2 |
| Secondhand/Thirdhand smoke | 70 | 6.8 | 4 | 1.2 |
| Nicotine Replacement Therapy (NRT) | 107 | 10.4 | 2 | 0.6 |
| Tobacco use with other products (Cannabis, Alcohol etc.) | 59 | 5.7 | 0 | – |
| Other/unclear | 6 | 0.58 | 4 | 1.2 |
| Research Domain | ||||
| Determinants of use and addiction | 606 | 58.7 | 219 | 68.0 |
| Treatment or cessation intervention | 345 | 33.4 | 2 | 0.6 |
| Health effects | 304 | 29.5 | 97 | 30.1 |
| Neurobiology | 195 | 18.9 | 11 | 3.4 |
| Mechanisms of disease pathology | 184 | 17.8 | 21 | 6.5 |
| Policy, economics, impact analysis | 131 | 12.7 | 38 | 11.8 |
| Genetics and epigenetics | 124 | 12.0 | 11 | 3.4 |
| Tobacco-related disparities | 87 | 8.4 | 26 | 8.1 |
| Epidemiology and surveillance | 82 | 7.9 | 35 | 10.9 |
| Communications and marketing | 69 | 6.7 | 91 | 28.3 |
| Methods research | 52 | 5.0 | 19 | 5.9 |
| Toxicity | 49 | 4.8 | 101 | 31.4 |
| Screening and diagnostic tests | 40 | 3.9 | 14 | 4.4 |
| Tobacco prevention interventions | 36 | 3.5 | 5 | 1.6 |
| Tobacco product analysis | 3 | 0.3 | 54 | 16.8 |
| Global tobacco control | 11 | 1.1 | 0 | – |
| Tobacco industry documents | 6 | 0.6 | 0 | – |
| Other/unclear | 16 | 1.5 | 0 | – |
Note: Estimates for each category are not mutually exclusive. Percentages may sum to more than 100%.
A comparison of research domains shows differences between the aims of FDA and NIH funded research (Table 1). NIH supported more research than FDA on treatment or cessation interventions; neurobiology; genetics and epigenetics; and mechanisms of disease pathology and/or progression. FDA funded proportionately more projects that included research on etiology or determinants of tobacco use; toxicology; health effects; communications research, including advertising and marketing of products; and tobacco product analysis.
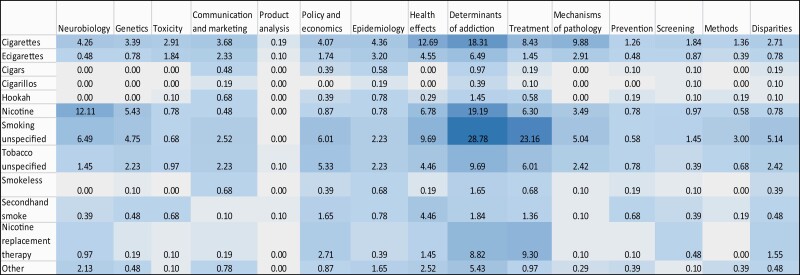
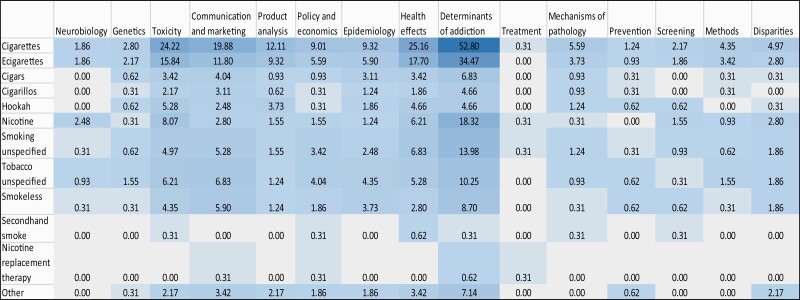
A heat map display of the NIH projects (Figure 2) shows that much of the research focused on the determinants of smoking and treatment interventions; NRT; health effects of cigarettes/smoking; neurobiology of nicotine; and mechanisms of disease pathology related to use of cigarettes. In contrast, the FDA portfolio (Figure 3) shows that much of the research on cigarettes and e-cigarettes focused on the determinants of use/addiction; toxicity; health effects; and communication/marketing of these products.
Figure 2.
NIH portfolio (N = 1032): distribution of projects focusing on tobacco products by research domain. Note: Box values are percentages over the total number of new projects (N = 1032). Darker shades depict higher percentage of projects for a particular category. ‘Other’ product category in the leftmost column includes Heated or Heat Not Burned, Dissolvables, Polyuse of tobacco products, Tobacco use with other products (Cannabis, Alcohol etc.) and Other/unclear in the product categories.
Figure 3.
FDA portfolio (N = 322): distribution of projects focusing on tobacco product by research domain. Note: Box values are percentages over the total number of new projects (N = 322). Darker shades depict higher percentage of projects for a particular category. ‘Other’ product category in the leftmost column includes Heated or Heat Not Burned, Dissolvables, Polyuse of tobacco products, Tobacco use with other products (Cannabis, Alcohol etc.) and Other/unclear in the product categories.
Of the 192 new FDA projects and 501 new NIH projects related to tobacco between FY2016 and FY2020, human subjects were studied in 180 and 366 projects, respectively. Of this total, only a minority of new projects funded by NIH and FDA focused on populations particularly vulnerable to tobacco product use, including those residing in rural areas (3% NIH; 2% FDA), pregnant persons (3% NIH and FDA), LGBTQ (1% NIH; 4% FDA) and underrepresented minorities (40% NIH; 6% FDA). However, 40% of FDA’s new projects included research involving youth (vs. 25% NIH)—a primary target population for tobacco prevention efforts, although not defined by NIH as a population experiencing healthcare disparities.
Discussion
The emergence of TRS as a discipline within tobacco control offers a unique opportunity to examine how a partnership between a regulatory agency and a research agency might shape the focus of and investment in an existing research grant program. In total, support for new tobacco research supported by NIH and FDA combined remained steady for the period of time covered. However, our data indicate a concomitant decline in NIH tobacco projects with the increase in FDA-funded TRS projects for the activity codes and years included in this analysis. The cause for this is unknown. While it is possible that the infusion of new support for tobacco research by FDA allowed NIH to divert funds to other areas, it also is possible that tobacco researchers took advantage of FDA solicitations for TRS and increasingly focused their research applications on this new area of tobacco control. Examining trends in new tobacco applications prior to the NIH-FDA partnership might provide more clarity regarding this finding. Examination of all activity codes for NIH tobacco grants shows that the number of new grants awarded between 2011 and 2020 did not experience the same significant decline as the activity codes that were included in this study.
Our result demonstrating a decline in NIH-funded tobacco research for the activity codes studied contrasts with the analysis reported by Merianos et al.,15 who examined NIH funding for tobacco control between 2006 and 2016. They reported an increase in the number of grant awards during this time period. However, using the publicly available RePORTER database, they would not have been able to separate NIH- from FDA-funded/NIH administered grants. Also, the time span reviewed included 5 years before FDA started to fund tobacco grants at the NIH, so the increase in tobacco grants observed is understandable given that FDA started to fund tobacco research during the later half of the time period examined. Examination of publicly available data on NIH grants, contracts and other sources of funding from 2008 to 2021 also suggests a moderate decline in funding for tobacco research during this time period.16
The differences identified in tobacco products and research domains investigated in FDA- and NIH-supported research projects reflect differences in FDA and NIH missions. There were few or no FDA grants that included research on secondhand/thirdhand smoke, NRT, or use of tobacco products with other non-tobacco substances. This reflects the fact that FDA authorities do not extend to indoor air policies, regulating substances other than tobacco or treatment of nicotine dependence. A majority of projects in the FDA portfolio include combustible cigarettes as one of the products under study. This is consistent with FDA’s comprehensive plan for tobacco and nicotine regulation, which includes lowering nicotine in cigarettes to a minimally or non-addictive level, as well as studies comparing the toxicity of newly deemed products with cigarettes. The FDA focus on specific tobacco products reflects its mission to inform regulatory decision-making about tobacco products, in contrast to the NIH mission which seeks fundamental knowledge that can be used to enhance health. FDA projects also reflect that the agency actively solicited research on newly deemed tobacco products, such as e-cigarettes and hookah.
Some areas of research remain uniquely in the interests of NIH, including neurobiology, genetics and mechanisms of disease pathology or progression. In contrast, some areas reflect FDA’s goal of informing regulation of tobacco products, such as tobacco product analysis, assessing toxicity of products, as well as advertising and marketing of tobacco products. Examining determinants of tobacco use, nicotine addiction and cessation is frequently included as a focus of both NIH and FDA research—even if not the primary study aim.
The fact that FDA projects focused heavily on the toxicity and health effects of cigarettes and e-cigarettes during the years examined is not surprising. The prevalence of e-cigarette use during this time increased rapidly among youth17 and the agency needed to assess the health impact of this product, which is often compared with the harms of combustible cigarettes. FDA also focused its efforts on understanding the marketing of these products and developed public education campaigns particularly aimed at warning youth about the dangers of tobacco product use.18 In contrast, the NIH projects were often focused on interventions for smoking cessation and the neurobiology of nicotine addiction.
Our ability to elaborate on populations of special interest for tobacco control was limited by what and when NIH started to code population focus. Given the epidemic of e-cigarette use among youth in recent years, it is not surprising that this population was an important study group in FDA projects. That said, it is striking that about 40% of NIH projects and only six percent of FDA projects focused on racial and ethnic minorities who share a disproportionate burden of tobacco-related disease and mortality19 and only about 8% of both NIH and FDA projects had a health disparities focus. NIH’s recent move to more actively pursue research to address inequalities in health20 will hopefully increase the number of projects including these populations.
There are a number of limitations to the analysis we present here. We did not examine the NIH tobacco research portfolio in the years prior to FDA funding of TRS and therefore cannot infer whether a decline in NIH tobacco research was already occurring. A recent analysis of substance use prevention research from 2012 to 2017 did not find a statistically significant change in grant funding for research on nicotine during this time period.21 Regardless, given the fact that tobacco use is a leading cause of morbidity and mortality,19 more support for this research seems warranted. Hughes22 found only 1.3% of the NIH budget was dedicated to tobacco in 2014—a level of support unchanged since 1995.
Another limitation is that we limited our analysis to the grant activity codes that FDA uses to fund TRS administered by NIH to provide a more direct comparison between the two funding agencies. Our data, therefore, do not capture the entirety of tobacco research supported through other activity codes at NIH nor through other agencies supported by FDA. Some of these other activity codes represent a smaller percentage of NIH funding that may provide ancillary or non-traditional support to tobacco research such as planning grants and small business awards. Nevertheless, this analysis captures a majority of tobacco research projects supported by both agencies and includes the main research activity codes used by NIH (R activity codes). Despite its limitations, this is the first study to compare the focus and scope of tobacco research supported by the two agencies and how the emergence of TRS complements and perhaps has influenced the direction of tobacco research unique to NIH.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Funding
This work was supported by the Office of Disease Prevention, National Institutes of Health.
Declaration of Interests
The authors declare no conflicts of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Department of Health and Human Services or any of its affiliated institutions or agencies.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Hughes JR, Liguori A. A critical view of past NIH research funding on tobacco and nicotine. Nicotine Tob Res. 2000;2(2):117–120. [DOI] [PubMed] [Google Scholar]
- 2. Glynn TJ, Manley MW, Mills SL, Shopland DR. The United States National Cancer Institute and the science of tobacco control research. Cancer Detect Prev. 1993;17(4-5):507–512. [PubMed] [Google Scholar]
- 3. Wu LT, McNeely J, Subramaniam GA, Sharma G, VanVeldhuisen P, Schwartz RP. Design of the NIDA clinical trials network validation study of tobacco, alcohol, prescription medications, and substance use/misuse (TAPS) tool. Contemp Clin Trials. 2016;50:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janoff A, Pryor WA, Bengali ZH. NHLBI workshop summary. Effects of tobacco smoke components on cellular and biochemical processes in the lung. Am Rev Respir Dis. 1987;136(4):1058–1064. [DOI] [PubMed] [Google Scholar]
- 5. NIH State-of-the-Science Panel. National Institutes of Health State-of-the-Science conference statement: tobacco use: prevention, cessation, and control. Ann Intern Med. 2006;145(11):839–844. [DOI] [PubMed] [Google Scholar]
- 6. National Cancer Institute. Respiratory Health Effects of Passive Smoking: Lung Cancer and Other Disorders. Tobacco Control Monograph No 4. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. NIH Pub. No. 93-3605, August 1993. [Google Scholar]
- 7. National Cancer Institute. Population Based Smoking Cessation. Tobacco Control Monograph No 12. Bethesda, MD: U.S.Department of Health and Human Services, National Institutes of Health, National Cancer Institute. NIH Pub. No. 00-4892, November 2000. [Google Scholar]
- 8. Family Smoking Prevention and Tobacco Control Act. (2009). Public Law 111–31, June 22, 2009. https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/family-smoking-prevention-and-tobacco-control-act-table-contents. [Google Scholar]
- 9. Ashley DL, Backinger CL, van Bemmel DM, Neveleff DJ. Tobacco regulatory science: research to inform regulatory action at the Food and Drug Administration’s Center for Tobacco Products. Nicotine Tob Res. 2014;16(8):1045–1049. [DOI] [PubMed] [Google Scholar]
- 10. Backinger CL, Meissner HI, Ashley DL. The FDA “Deeming Rule” and Tobacco Regulatory Research. Tob Regul Sci. 2016;2(3):290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frechtling JA, Dunderdale, T, Meissner, HI, et al. Establishing a Research Base to Inform Tobacco Regulation: Overview. Tob Regul Sci. 2021;7(2):144–154. [Google Scholar]
- 12. National Institutes of Health. Grants and funding: types of applications. https://grants.nih.gov/grants/how-to-apply-application-guide/prepare-to-apply-and-register/type-of-applications.htm. Accessed April 15, 2021.
- 13. National Institutes of Health. Grants and funding: activity codes search results. https://grants.nih.gov/grants/funding/ac_search_results.htm. Accessed September 10, 2021.
- 14. StataCorp. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC; 2019. [Google Scholar]
- 15. Merianos AL, Gordon JS, Wood KJ, Mahabee-Gittens EM. National institutes of health funding for tobacco control: 2006 and 2016. Am J Health Promot. 2019;33(2):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC). https://report.nih.gov/funding/categorical-spending#/ Accessed June 2, 2021.
- 17. Cullen KA, Ambrose BK, Gentzke AS, Apelberg B, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students – United States, 2011-2018. MMWR MorbMortal Wkly Rep. 2018;67:1276–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeller M. Evolving “the real cost” campaign to address the rising epidemic of youth e-cigarette use. Am J Prev Med. 2019;56(2 Suppl 1):S76–S78. [DOI] [PubMed] [Google Scholar]
- 19. The health consequences of smoking—50 years of progress: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. https://www.ncbi.nlm.nih.gov/books/NBK179276/. [Google Scholar]
- 20. Pérez-Stable EJ, Collins FS. Science visioning in minority health and health disparities. Am J Public Health. 2019;109(S1):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Villani J, Ganoza L, Sims BE, et al. Substance use prevention research funded by the NIH. Drug Alcohol Depend. 2020;206:107724. [DOI] [PubMed] [Google Scholar]
- 22. Hughs JR. National Institutes of Health funding for tobacco versus harm from tobacco. Nicotine Tob Res. 2016;18(5):1299–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.