Abstract
Background
Ovarian cancer remains the most deadly gynecologic cancer with the majority of patients relapsing within 3 years of diagnosis. Traditional treatment paradigms linked to platinum sensitivity or resistance are currently being questioned in the setting of new diagnostic methods and treatment options.
Design
Authors carried out review of the literature on key topics in treatment of recurrent epithelial ovarian cancer (EOC) when platinum is still an option; including secondary surgical cytoreduction, chemotherapy, novel treatment options, and maintenance therapy. A treatment algorithm is proposed.
Results
Molecular characterization of EOC is critical to help guide treatment decisions. The role of secondary cytoreductive surgery is currently being evaluated with results from Gynecologic Oncology Group (GOG) 213 and anticipated results from DESKTOP III clinical trials. Chemotherapy backbone has remained relatively unchanged but utilizing non-platinum-based regimens is under investigation. In addition, maintenance therapy with anti-angiogenic therapy and Poly (ADP-ribose) Polymerase (PARP) inhibitors has emerged as the standard of care. Novel combinations, including immunotherapy and anti-angiogenesis agents, may further change the current landscape.
Conclusions
The treatment of recurrent EOC is rapidly changing. Clinical trial design will need to continue to evolve as many novel therapies move to the upfront setting. Ultimately, the treatment of patients with recurrent EOC must incorporate individual patient and tumor factors.
Keywords: ovarian cancer, platinum, maintenance therapy, molecular characterization
Key Message
The traditional treatment paradigm for recurrent epithelial ovarian cancer is changing. Treatment options including cytoreductive surgery, platinum-based chemotherapy, and maintenance therapy are all evolving based on new clinical trial results. Molecular characterization of patient’s tumors will continue to help guide treatment decision making in the era of targeted treatment options.
Introduction
Epithelial ovarian cancer (EOC) remains the most deadly cancer of the female genital tract with ∼22 000 new cases and 14 000 deaths expected in 2018 in the USA [1]. While an initial disease-free interval is achieved in many patients, most will eventually relapse. This treatment free interval (TFI) has traditionally labeled patients as either platinum sensitive (relapse ≥6 months since last platinum agent) or platinum resistant (relapse <6 months since last platinum agent). While we understand that length of TFI from last platinum (TFIp) does correlate with response to subsequent platinum-based chemotherapy, this classification system likely needs adaptation as our understanding of EOC develops.
Alvarez et al. challenged this classic paradigm. They proposed a multi-step classification system based on two primary principles. First, EOC surveillance is not standardized. The utilization of CA-125 monitoring and more advanced imaging techniques allows for detection of recurrence sooner than traditional physical exam/symptom surveillance. Patients that would have previously been deemed platinum sensitive may now fall into the platinum resistant category and be excluded from further platinum therapy or clinical trials based merely on earlier identification of disease recurrence. Secondly, improved molecular understanding of the pathogenesis of EOC has altered the understanding of response to platinum-based chemotherapy and newer targeted therapies [2].
With these factors in mind, the classic rigid paradigm no longer seems applicable to patients with a platinum sensitive recurrence. With this in mind, the Gynecologic Cancer Intergroup proposed utilizing new nomenclature to describe patients in their disease course which will be utilized in this review [3]. Patients will be defined by their TFI from last platinum (TFIp), from last non-platinum (TFInp), or biologic agent (TFIb). Patients are subsequently characterized as those for whom platinum re-treatment is an option and those for whom it is not. In this article, we review the recent data on secondary cytoreductive surgery (CRS), molecular characterization and applications to treatment, agents for treatment of recurrent EOC for whom platinum is an option and evidence for maintenance therapy following treatment and finally, a discussion of the challenges in developing new treatment strategies given recent and ongoing clinical trial activity in front line therapy.
Secondary cytoreductive surgery
In a patient with first recurrence, one of the initial questions is whether additional surgical intervention is warranted. Multiple retrospective cohort studies have shown increased survival if optimal CRS is achieved [4–7]. Data to inform patient selection for secondary CRS were presented in the original DESKTOP OVAR trial (Descriptive evaluation of Preoperative Selection Kirteria for Operability in Recurrent Ovarian Cancer). This multi-institutional retrospective cohort study analyzed demographic, surgical, and treatment variables in 267 patients. Patients with completely resected tumors had significantly longer overall survival (OS) (45.2 versus 19.7 months). The size of residual tumor impacted OS and factors independently associated with complete resection were Eastern Cooperative Oncology Group performance status (PS) of 0, no residual tumor following initial surgery, and ascites <500 ml [5].
DESKTOP II prospectively validated the score developed in the first trial and predicted complete resection in 75% of patients who were score ‘positive’ [5, 8]. DESKTOP III, randomized patients with a ‘positive’ score to either second line chemotherapy alone or CRS followed by chemotherapy. OS data are still immature but progression free survival (PFS) was improved in those patients who underwent surgery (19.6 versus 14 months; hazard ratio (HR): 0.66 CI 0.52–0.83 P < 0.0001). In addition, 72% of patients achieved complete resection with acceptable toxicity in both arms [9]. Gynecologic Oncology Group (GOG) protocol 213, had two primary objectives [10]. The first assessed the impact of bevacizumab administered concomitantly with paclitaxel and carboplatin as well as in maintenance on OS (primary end point) among patients with one prior line of treatment. The second evaluated the impact of secondary CRS on OS in this same cohort. Patients who were deemed appropriate for secondary CRS were randomized to surgery followed by chemotherapy versus chemotherapy alone. Eligibility included a complete response (CR) to front line chemotherapy, no evidence of bowel obstruction, or need for parenteral nutrition and no evidence of carcinomatosis or parenchymal organ disease that was felt to be unresectable. Complete resection was achieved in 67% of patients. Despite this high level of complete resection, OS was, in fact, negatively impacted by secondary CRS with a median of 53.6 versus 65.7 months (HR = 1.28; 0.92–1.78) in favor of no surgery [11]. Subgroup analysis of GOG 213 patients with oligometastatic disease also found no benefit from resection [12].
While the final OS results from DESKTOP III are pending, the results of the two studies are thus far very consistent when comparing PFS. In GOG 213, the median PFS of the completely resected patients versus those patients who underwent no surgery was 21.4 versus 16.5 months (HR = 0.68; 0.51–0.90)—very similar to that reported in DESKTOP III. In both trials, target lesions were resected in the surgery arm making PFS difficult to interpret. The OS findings from DESKTOP III are therefore of critical importance, for if they confirm the surgical end points of GOG 213, recommendations for secondary CRS in this setting may be abandoned.
Molecular characterization
Role of tumor profiling
Tumor profiling ideally identifies molecular alterations that can be therapeutically manipulated to induce an anticancer effect. These actionable mutations can be identified with next generation sequencing or immunohistochemistry among other techniques. The challenge in solid tumors, in general, and in EOC specifically, is pinpointing the driver mutations, which are critical to successful application of targeted therapies. Additional challenges include spatial and temporal tumor heterogeneity, redundant pathways, and a relatively low identification of molecular alterations that have an available, regulatory-approved, and clinically validated therapeutic agents.
BRCA identification
Clearly, the best current example of tailored therapy selection for EOC has been the therapeutic effect demonstrated with poly (ADP-ribose) polymerase inhibitors (PARPi) in patients with germline or somatic BRCA (g or s BRCA) mutations. BRCA testing is recommended for all patients with EOC, and this information can trigger cascade testing in relatives, provide important prognostic information for patients, and inform treatment planning. Currently, most clinicians are performing germline testing, but there is growing interest in tumor testing with reflexing to germline determination. This approach may help us focus germline testing; however, current tumor testing for BRCA is less reliable than germline testing and the presence of reversion mutations may lead to false negatives [13]. The utility and uptake of tumor testing and high throughput panel testing will continue to grow as additional targeted agents are developed that depend upon specific genetic alterations beyond just BRCA mutations. With the recent publication of SOLO-1 demonstrating an unprecedented improvement in PFS with use of the PARPi, olaparib following front line platinum-based induction chemotherapy among patients with g or sBRCA mutation, the imperative to identify BRCA mutations close to the time of diagnosis will become standard of care so this molecular characterization should be known for recurrent EOC in the future [14].
Homologous recombination deficiency status
An important concept discovered during the development of PARPi was that there are genetic or epigenetic alterations other than BRCA that confer sensitivity to PARPi and that prevent high fidelity double strand DNA break repair via homologous recombination.
Understanding homologous recombination deficiency (HRD) status may potentially prioritize between competing PARPi and non-PARPi regimens. For example, one current clinical quandary is whether to use a PARPi or bevacizumab as maintenance therapy in the patients with recurrent EOC for whom induction platinum is an option. The surprising finding across all PARPi maintenance trials (discussed below) is that there is a statistically significant treatment effect in those who are BRCA wild type and HRD negative. Nonetheless, the magnitude of effect is not nearly as sizeable as seen for the BRCA or HRD+ cohorts, which calls to question whether other strategies potentially would be superior for patients who lack demonstrable DNA repair deficiencies.
Currently, the only validated biomarker for patient selection of PARPi is germline or somatic BRCA. This may change as results of ongoing studies evaluate alternative biomarkers.
Options for treatment
Chemotherapy backbone
The standard of care for patients with recurrent EOC for whom platinum is an option has been a platinum-containing regimen. When considering which chemotherapy backbone to use, there are four options with differences in schedule, toxicity profile, and in the case of single agent versus doublet chemotherapy, efficacy. These are summarized in Table 1.
Table 1.
Summary of randomized trials on chemotherapy backbone
| Trial | Treatment | RR (%) | PFS (months) | HR | OS (months) | HR |
|---|---|---|---|---|---|---|
| ICON4/AGO-OVAR-2.2 [81] (n = 802) | Carboplatin or cisplatin | 54 | 10 | 0.76 P = 0.0004 | 24 | 0.82 P = 0.02 |
| Carboplatin or cisplatin + paclitaxel | 66 | 13 | 29 | |||
| Pfisterer et al. [82] (n = 356) | Carboplatin | 31 | 6 | 0.72 P = 0.0031 | 17 | 0.96 P = 0.7349 |
| Carboplatin + gemcitabine | 47 | 9 | 18 | |||
| CALYPSO [83] (n = 976) | Carboplatin + pegylated Liposomal doxorubicin | – | 11 | 0.821 P = 0.005 | 31 | 0.99 P = 0.94 |
| Carboplatin + paclitaxel | – | 9 | 33 |
RR, objective response rate; PFS, progression free survival; HR, hazard ratio; OS, overall survival.
Substitution of other standard cytotoxics does not provide superior or even equivalent oncologic outcomes when compared with platinum-based regimens. This concept was demonstrated in MITO 8, a study of patients with TFIp of 6–12 months who were randomized to platinum-based chemotherapy versus non-platinum-based chemotherapy with an end point of OS which found no improvement in OS by delaying platinum-based therapy (21.8 versus 24.5 months; HR = 1.38; 95% CI 0.99–1.94; P = 0.06) [15].
The combination of pegylated liposomal doxorubicin (PLD) and trabectedin, a novel cytotoxic derived from the marine tunicate Ecteinascidia Turbinatanata, that acts as a DNA minor groove binder that leads to double strand DNA breaks, has been studied as a platinum-based substitution in patients with a TFIp of 6–12 months [16–18]. An improvement of PFS of 4 months of PLD alone was seen [19]. The phase III ORCHYD trial evaluated the efficacy of trabectedin and PLD versus PLD in patients with PSR EOC in the third line (NCT01846611). This study was closed early for futility and results have not yet been presented. The results of the INOVATYON trial (NCT01379989) are still awaited. This is a phase III randomized trial in patients with TFIp of 6–12 months to trabectedin and PLD versus carboplatin and PLD.
Maintenance therapy
Continuance of some form of active therapy in patients demonstrating a response to induction therapy is highly desired since eventual progression is an expected vicissitude [20]. Several agents and strategies have been reported, including anti-angiogenics (AA) and PARPi, which have provided strong evidence of a treatment effect. Studies evaluating immune-oncology agents such as anti-programmed cell death protein-1 (PD-1) or anti-programmed cell death ligand-1 (PD-L1) are ongoing.
There are essentially two broad concepts of maintenance therapy: secondary maintenance and switch maintenance. Fundamentally, these terms could apply to maintenance treatment in the primary setting as well. Studies that are evaluating secondary maintenance are generally designed as a treatment combination, commonly chemotherapy and a biologic, where one or more agents, but not the induction regimen, is continued until progression or some defined duration of therapy [10, 21, 22]. In contrast, switch maintenance therapy involves a therapy that is new to the treatment plan following a desired response to induction therapy. An example would be administration of a novel agent following treatment with 4–6 cycles of platinum-based chemotherapy [23–26]. In addition, many maintenance trials allow for the initiation of a maintenance regimen after both a CR and ‘partial’ response (PR). This arguably is a ‘switch’ of therapeutic strategies after a PR and not a maintenance therapy by the purest definitions. Since most patients in this setting ultimately develop progressive disease, continuance of treatment to progression or unacceptable toxicity is often prescribed. Utilization of this trial design mandates the incorporation of OS end points to help elucidate true benefit from this strategy.
The anti-angiogenesis strategy
The relationship between factors driving the development of tumor-associated vasculature and outcomes in EOC has been well documented [27–41]. Table 2 outlines the AA agents that have undergone formal evaluation in large prospective randomized trials [10, 21, 22, 42, 43]. As can be seen, agents targeting the vascular endothelial growth factor (VEGF) isoforms (e.g. bevacizumab) or its receptors (e.g. cediranib) have undergone the most intensive investigation.
Table 2.
Summary of randomized trials with maintenance anti-angiogenesis therapy in platinum sensitive EOC
| Trial | Treatment | RR (%) | PFS (months) | HR | OS (months) | HR |
|---|---|---|---|---|---|---|
| TRINOVA-1 [42] (n = 435a) | Paclitaxel weekly | – | 5.6 | 0.66 Subgroup 95% CI 0.52–0.84 | – | – |
| Paclitaxel weekly + trebananib | – | 7.6 | – | |||
| OCEANS [21] (n = 484) | Gemcitabine + carboplatin | 57 | 8.4 | 0.48 P < 0.0001 | 33.6 | 0.96 P = 0.65 |
| Gemcitabine + carboplatin + bevacizumab | 79 | 12.4 | 32.9 | |||
| GOG 213 [10] (n = 674) | Paclitaxel + carboplatin | 59 | 10.4 | 0.61 P < 0.0001 | 37.3 | 0.82 P = 0.045 |
| Paclitaxel + carboplatin + bevacizumab | 79 | 13.8 | 42.2 | |||
| ICON6 [22] (n = 456) | Platinum + paclitaxel | – | 8.7 | 0.67 P = 0.0003 | 21.0 | |
| Chemotherapy + concurrent cediranib | – | 9.9 | 25.0 | |||
| Chemotherapy + cediranib + maintenance cediranib | – | 11.0 | 0.56 P < 0.0001 | 26.3 | 0.77 P = 0.11 | |
| AGO-OVAR 2.21 [43] (n = 682) | Gemcitabine + carboplatin + bevacizumab | – | 11.7 | 0.807 P = 0.0128 | 28.2 | 0.833 P = 0.787 |
| Pegylated liposomal doxorubicin + carboplatin + bevacizumab | – | 13.3 | 33.5 |
EOC, epithelial ovarian cancer; RR, objective response rate; PFS, progression free survival; HR, hazard ratio; OS, overall survival; PFI, platinum free interval.
Platinum sensitive (PFI 6–12 months).
The remaining trial evaluating trebananib evaluated this novel AA agent as a secondary maintenance strategy—that is, once chemotherapy induction was complete, continuance on the AA agent was assessed for survival end points [42].
Recently, a direct comparison of carboplatin, bevacizumab (concurrent and maintenance) with either gemcitabine or PLD demonstrated superior PFS of 11.7 versus 13.3 months respectively (HR = 0.807; 95% CI 0.681–0.956; P = 0.0128) and a trend towards improvement in OS (HR = 0.833; 95% CI 0.680–1.022) for the PLD doublet [43]. These data support prioritization of a PLD/carboplatin backbone when a bevacizumab based approach is selected in recurrent EOC when platinum is an option.
The PARPi strategy
There are currently five PARP inhibitors in use or under study in EOC: olaparib, niraparib, rucaparib, talazoparib, and veliparib. PARPi are under investigation both as monotherapy treatment in the recurrent setting as well as under study and approved as switch maintenance. The results from three randomized phase III (NOVA, SOLO2, ARIEL3) trials and one phase II (Study 19) trial are surprisingly consistent when looking at PARPi in the switch maintenance setting [23–26]. However, significant differences in trial design, eligibility, patient populations, and assessment techniques distinguish them (Table 3). Each of these trials follows a placebo-controlled, switch maintenance design, in which eligible patients are first treated with a platinum-based induction regimen, and if they demonstrate a response, they are randomized to PARPi versus placebo. Notably, patients with a best response of stable disease to induction chemotherapy were not included in these studies whereas they were included in studies of bevacizumab. Similar to the trial designs mentioned above for secondary maintenance, response to induction therapy in these trials did not need to be complete before randomization.
Table 3.
Summary of randomized switch maintenance clinical trials in patients receiving PARP inhibitors for recurrent platinum sensitive EOC
| PFS (inv review—primary) | Rucaparib | Placebo | HR | P |
|---|---|---|---|---|
| sBRCA | 16.8 months | 5.4 months | 0.23 | P < 0.0001 |
| sBRCA + HRD | 13.6 months | 5.4 months | 0.32 | P < 0.0001 |
| ITT | 10.8 months | 5.4 months | 0.37 | P < 0.0001 |
|
| ||||
| PFS (BICR—primary) | Niraparib | Placebo | HR | P |
|
| ||||
| sBRCA | 21.0 months | 5.5 months | 0.26 | P < 0.0001 |
| sBRCA + HRD | NA | NA | NA | |
| All non-gBRCA (sBRCA+ HRD + HRC) | 9.3 months | 3.9 months | 0.45 | P < 0.001 |
| ITT (FDA analysis) | 11.3 months | 4.7 months | 0.42 | Not Given |
|
| ||||
| PFS (inv review—primary) | Olaparib | Placebo | HR | P |
|
| ||||
| SOLO-2—gBRCA | 19.1 months | 5.5 months | 0.30 | P < 0.0001 |
| Study 19—ITT | 8.4 months | 4.8 months | 0.35 | P < 0.0001 |
| Study 19—gBRCA | 11.2 months | 4.3 months | 0.18 | P < 0.0001 |
EOC, epithelial ovarian cancer; sBRCA, somatic BRCA mutation; gBRCA, germline BRCA mutation; HR, hazard ratio; HRD, homologous recombination deficiency; HRC, homologous recombination compliant; ITT, intent-to-treat; BICR, blinded independent central review (imaging and clinical); NA, not applicable.
Unique to ARIEL3, ORR was collected from those patients entering the trial with measurable disease in each of the step-down cohorts. As might have been expected, patients with g or sBRCA cancers achieved further response (38%) when treated with rucaparib [26].
Review of these secondary versus switch maintenance and the outcomes from well-controlled phase III clinical trials suggest that both have merit in patients with recurrent EOC for whom platinum is an option. Only one trial to date has demonstrated an OS advantage (GOG-0213) and the current availability of both classes of agents will challenge attaining this goal in subsequent trials due to crossover. Ongoing trials are looking into combinations of both AA agents and PARPi as maintenance strategies and others will assess the impact of either or both of these agents in combination with various immune-oncology agents such as anti PD-1/PD-L1 or CTLA-4 inhibitors. For example, ICON 9 (NCT03278717) is a randomized phase III trial of recurrent EOC for whom platinum is an option. This study randomizes patients who have had a PR or CR platinum-based chemotherapy to either olaparib alone or olaparib plus cedirinib. The primary end point is PFS with secondary end points to include OS, safety, health related quality of life and PFS2. Stratification includes HRD but eligibility is not biomarker based.
The immune-oncology strategy
For now, a bevacizumab-containing regimen or PARPi represents new standards of care for patients with recurrent EOC for whom platinum remains an option with improved PFS (both) and OS (bevacizumab). The challenge remains to further improve PFS and OS outcomes among a broader biomarker population of EOC patients. Immunotherapy appears potentially promising, but the ideal sequencing and combinations of immunotherapy with chemotherapy and other novel agents have yet to be determined.
Evasion of immune surveillance is one of the established hallmarks of cancer [44, 45]. EOC is a valid potential target for immuno-oncology agents given that over 50% of patients have tumor infiltrating lymphocytes (TILs) at the time of diagnosis and the presence or absence of TILs is prognostic [46]. EOC also carries a significant mutational load [47]; however, we see a very modest response seen to immune checkpoint inhibitors thus far, with response rates from 11% to 25%, when used as a single agent in the recurrent, platinum resistant setting [48–50].
Combinations of immune checkpoint inhibitors with other agents are likely to improve the efficacy and durability of responses seen with monotherapy use of any tested agent. Rational combination partners with immune checkpoint inhibitors include molecules that cause DNA damage, chemotherapy, and AA agents.
Chemotherapy combined with an immune checkpoint inhibitor capitalizes on the complimentary effects on the immune system imparted by each component. Chemotherapy alone may modulate dendritic cells, enhance MHC1 presentation on tumor cells, and lead to immunogenic cell death. In addition, studies have demonstrated that the percentage of TILS increased in immunocompetent mouse models when treated with both platinum and taxane agents [51, 52].
There is mechanistic rationale for combining AA with immunotherapy. VEGF is a highly immunosuppressive molecule with both direct and indirect inhibition of T-cell function, stimulation of regulatory T-cells, inhibition of dendritic function, and induction of abnormal tumor vasculature which decreases tumor T-cell trafficking [44, 53–57]. There is already demonstration of the safety and efficacy for this combination in non-squamous, non-small-cell lung cancer (NSCLC). In the IMpower150 study, patients with chemotherapy naïve, stage IV or recurrent non-squamous NSCLC were randomized to carboplatin, paclitaxel, and atezolizumab followed by atezolizumab maintenance, the same plus bevacizumab with and following chemotherapy versus carboplatin, paclitaxel and bevacizumab followed by bevacizumab maintenance. In comparing the second two arms, the PFS favored combination atezolizumab and bevacizumab (HR = 0.617; 0.517–0.737; P < 0.0001) as did the OS (HR = 0.780; 0.636–0.956; P = 0.0164). Patients were allowed to participate regardless of PD-L1 status and all patients showed benefit, but subset analysis of those with higher PD-L1 benefited more (HR = 0.44 for PD-L1 high, 0.50 for PD-L1 low, and 0.77 for PD-L1 negative) [58]. ATALANTE (GINECO-OV236b/NCT 02891824) is a phase III randomized trial enrolling patients with recurrent EOC and randomizes to platinum-based chemotherapy, bevacizumab, and placebo followed by bevacizumab and placebo maintenance versus platinum-based chemotherapy, bevacizumab and atezolizumab followed by bevacizumab and atezolizumab maintenance until progression. The primary end point is PFS with secondary end points inclusive of time to second subsequent therapy or death, OS, and safety. This study is actively accruing patients and results are expected in 2023.
Combination of PARPi and chemotherapy
Combinations of PARPi and chemotherapy have proved challenging with dose modifications required on both sides. Oza reported on the combination of paclitaxel and carboplatin with olaparib compared with chemotherapy alone in the PSR setting. Overlapping toxicity of myelosuppression required dose reduction of the carboplatin to AUC4 and olaparib to 200 mg b.i.d. (capsule formulation). This randomized phase II trial demonstrated no significant difference in ORR based on the addition of olaparib to chemotherapy; however, there was a clear improvement in PFS for maintenance olaparib versus placebo supporting development of olaparib for switch maintenance [59]. Even single agent chemotherapy combinations with PARP inhibition has proven difficult, as significant bone marrow toxicity was observed limiting adequate doses of either the PARPi or the cytotoxic chemotherapy [60–63].
Non-cytotoxic treatments instead of platinum
A number of PARPi have been evaluated as monotherapy treatment in recurrent EOC. In this setting, indications have been specific to patients harboring g or s BRCA mutations. Table 4 outlines the various trials investigating PARPi in recurrent EOC. Olaparib was the first PARPi to obtain approval in gBRCA EOC with ≥3 lines of therapy. This was based on a phase II study which demonstrated a 33% ORR (n = 137) and a 45% ORR among PS EOC. Importantly, 56% of patients were progression free at 6 months [64].
Table 4.
Summary of randomized of clinical trials in women receiving PARP inhibitors for recurrent Platinum sensitive EOC
| ORR (%) | PFS (months) | |
|---|---|---|
| Rucaparib | ||
| Study 10 [65] | 59.5 | NA |
| ITT | ||
| ARIEL 2 [67] | 12.8 | |
| BRCAmut | 80 | |
| LOH High | 29 | 5.7 |
| LOH Low | 10 | 5.2 |
| Niraparib | ||
| Phase 1 [24] | NA | |
| BRCAmut | 40 (50 in PSen) | |
| BRCAwt | 16 (33 in PSen) | |
| Quadra II [68] | ||
| BRCAmut | 39 | NA |
| BRCAwt | 21 | |
| Olaparib | ||
| Study 42 [84] | 34 (46 in PSen) | 6.7 (9.4 in PSen) |
| Velaparib | ||
| Coleman et al. [72] | 26 (35 in PSen) | 8.2 |
EOC, epithelial ovarian cancer; BRCAmut, somatic or germline BRCA mutation; BRCAwt, BRCA wild type; LOH, loss of heterozygosity; ITT, intent-to-treat; ORR, objective response rate; PFS, progression free survival; PSen: platinum sensitive); NA, not applicable.
Based on data from two large open label trials (Study 10 and ARIEL2) in recurrent EOC, rucaparib was the second PARPi to receive an indication in recurrent EOC [65–67]. This approval was in patients with ≥2 prior therapies in the setting of either g or s BRCA. In the integrated analysis of both trials, rucaparib achieved an ORR of 54% [66]. ARIEL2 was designed to identify and curate the cut-off of the test to identify a set of ‘BRCA-like’ tumors that garnered more benefit from PARPi compared with BRCAwt tumors. Tumors identified as ‘BRCA-like’ achieved ORR of 30% and a median PFS of 7.1 months, indicating success of the test to predict efficacy. This test was then used to stratify patients in the ARIEL-3 maintenance study noted above [67].
The phase II QUADRA study (open label, single arm) enrolled 463 patients evaluating niraparib (300 mg po qd) until progression in patients with relapsed EOC who have received ≥3 prior regimens. The primary end point for this study was overall response rate (ORR) among EOC patients who were HRD+ and for whom platinum therapy was still an option. This study demonstrated the efficacy of monotherapy nirparib in heavily pretreated, HRD+ recurrent EOC with ORR of 29% and duration of response (DOR) was 9.2 months [68].
The unanswered question is the performance of PARPi compared with platinum-based chemotherapy in a patient for whom platinum therapy is still an option. A randomized phase II trial of single agent olaparib compared with PLD in gBRCA mutated EOC demonstrated superior ORR but no difference in PFS. The trial did not meet the primary end point of superiority, which was unexpected, but this is postulated to be due to higher than expected efficacy of PLD in gBRCA EOC [69]. There are two ongoing studies of PARPi monotherapy compared with physician’s choice chemotherapy, SOLO3 (olaparib), and ARIEL4 (rucaparib) (NCT02282020, NCT02855944).
Across a number of targeted therapies, there are growing data to indicate that single agents will not be adequate to achieve durable efficacy. PARP inhibition is no exception given the existence of innate, acquired, and adaptive resistance to therapy [70]. Certainly, there are a proportion of tumors with g or BRCA mutation that do not respond to PARPi. Further, the median DOR to PARPi when used as monotherapy, even in BRCA tumors, is ∼8 months [64, 67, 71, 72]. This supports the development of adaptive resistance to PARPi. Thus, the exploration of combination therapies with PARP inhibition has been of great interest.
The combination of PARPi with AA therapies developed out of the concept that synthetic lethality may occur in the tumor microenvironment when hypoxia from AA therapy leads to development of HRD [73]. This would provide a mechanism of sensitivity to PARPi outside of the presence of BRCA mutation. Phase I studies of PARPi have been successfully completed with bevacizumab and cediranib [73, 74]. A phase II study of olaparib and cediranib compared with olaparib alone demonstrated improved PFS in the combination arm (17.7 versus 9.0 months) irrespective of BRCA mutational status with the benefit of combination therapy greatest in the BRCAwt status cohorts [75]. A randomized phase II trial (OTOVA, NCT03117933) is comparing weekly taxol versus olaparib versus olaparib/cediranib in platinum resistant EOC; however, this includes patients with TFIp of <12 months. Phase III trials in two settings have launched to assess this combination: (i) NRG-GY004 recurrent EOC compared with standard platinum-based chemotherapy and (ii) NRG-GY005 recurrent EOC compared with physician’s choice chemotherapy for patients in which platinum is no longer an option (NCT02446600; NCT02889900). The combination of olaparib and bevacizumab was explored in a phase I trial which demonstrated acceptable toxicity and promising efficacy [73]. This combination is being explored in the first line maintenance setting in the PAOLA-1 trial (NCT02477644). Rucaparib in combination with bevacizumab as a maintenance strategy is also being investigated in a phase I followed by a randomized phase II trial of upfront ovarian cancer in MITO25 (NCT03462212).Niraparib has also been successfully combined with bevacizumab in a phase I trial. Impressively, this combination achieved a 50% response rate [76]. The AVANOVA2 trial (NCT02354131) will assess the impact on PFS of three options: (i) niraparib alone, (ii) combination of bevacizumab and niraparib, and (iii) sequential bevacizumab followed by niraparib all in patients with recurrent disease for whom platinum is still an option. This study is still recruiting patients at the time of this publication.
As described previously, combinations of immune-oncology agents (anti PD-1 and PD-L1 currently) with either PARPi or AA agents are of great interest as maintenance strategies or as doublet (or triplet) therapies, and studies are under way to best evaluate how and when to use these combinations.
Discussion
Conclusions
In summary, the landscape of treatment options for patients with recurrent EOC is changing. First, the role for secondary CRS is in question given the recent data from GOG 213. As we await mature data from DESKTOP III we must carefully contemplate the role surgery plays in this patient population.
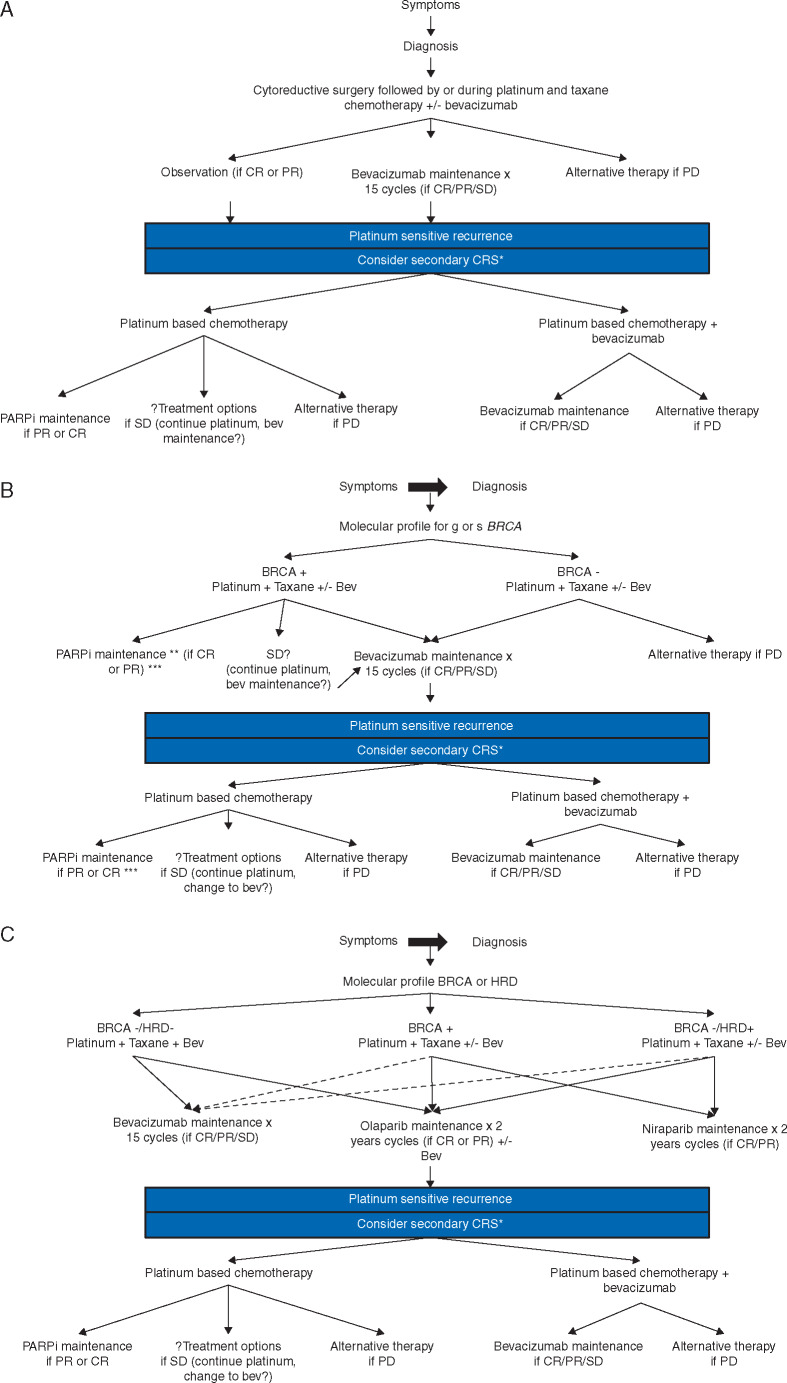
Secondly, while our chemotherapy backbone has remained relatively unchanged for the past decade, newer therapies both as maintenance following and instead of traditional cytotoxic therapy are being developed. Indications may be specific to molecular or genetic subsets of patients; therefore, molecular and genetic profiling should be confirmed at the time of recurrent disease but ideally identified at time of diagnosis. When discussing maintenance therapy in the recurrent setting following induction platinum-based therapy, both AA and PARPi are viable options; however, as the indications for use of these agents in primary treatment changes, so will their use in recurrent disease. For example, the FDA recently approved the use of bevacizumab in upfront EOC. While we now have data for use of bevacizumab in multiple treatment lines, we must still consider how its routine use in upfront treatment may alter our decision to incorporate it into later lines [77] (Figure 1A). Similarly, the positive results of SOLO1 and recent FDA approval of Olaparib maitenance in gBRCA patients in the upfront setting will change the standard of care for gBRCA patients and may impact how we consider treatment of those who do later recur [78, 79]. Unlike bevacizumab, there is not yet data for re-treatment with a PARPi after prior exposure and therefore, selection of treatment plans in the setting of recurrence may be complicated (Figure 1B). Does it make sense to re-use a PARPi in the setting of prior maintenance use if (i) a patient recurred remote from use of the PARPi or (ii) recurred while taking the PARPi? Is there a difference? The ongoing OReO trial (NCT03106987) will attempt to address this question. This is a randomized phase III trial, which enrolls patients who have progressed or relapsed following PARPi maintenance have responded to platinum-based therapy and are then randomized to olaparib or placebo maintenance. With the current landscape, the sequence and combination of treatments will need to be individualized. Figure 1B attempts to help readers weigh the possible options. For BRCA positive patients, the authors recommend PARPi maintenance in the absence of previous PARPi therapy. Beyond this, patient clinical factors (e.g. disease distribution, patient performance status, ongoing toxicities, etc.), molecular profile, and previous treatments must be taken into account when choosing optimal treatment.
Figure 1.
Treatment algorithm for patients diagnosed with epithelial ovarian cancer. CR, complete response; PR, partial response; SD, stable disease; CRS, cytoreductive surgery; Bev, bevacizumab. (A) Current treatment algorithm. (B) Algorithm incorporating results of SOLO-1. (C) Hypothetical algorithm incorporating the potential positive results of PRIMA and PAOLA-1.*Controversial, see text. **At the time of publication Olaparib is the only approved PARPi for this indication. ***preferred method if BRCA positive. Solid lines, preferred treatment option, dashed lines, allowable treatment option.
Finally, as molecular understanding of EOC continues to progress, so will the development of new therapeutics. For example, immune modulators including PD-1/PD-L1 inhibitors are currently under investigation and will likely play a role in treatment of recurrent EOC. While JAVELIN Ovarian 100 (a phase III RTC of chemotherapy naïve advanced EOC comparing chemotherapy versus chemotherapy followed by avelumab maintenance versus chemotherapy plus avelumab plus avelumab maintenance for up to 2 years) was terminated early at a planned interim analysis for failure to meet its primary end point, multiple studies combining immune modulators in both the upfront and recurrent setting are still under way [80]. In this changing landscape, we can no longer base treatment decisions solely on the traditional paradigm of ‘platinum sensitive’ versus ‘platinum resistant’. A multi-step treatment approach that incorporates multiple patient factors including molecular and genetic profiles will be necessary as we advance the care of patients with recurrent EOC. A lack of understanding of the most appropriate sequence of many of these therapeutics remains. As in a biomarker agnostic setting, how will clinicians choose which targeted agent to add to front line chemotherapy to obtain highest benefit and how will this impact what is selected at the time of recurrence? While the results of JAVELIN Ovarian 100 were negative, PRIMA (NCT02655016) and PAOLA-1 (NCT02477644), which, if positive, will dramatically change both front line and recurrent treatment paradigms. PRIMA is a randomized phase III trial evaluating niraparib switch maintenance following front line platinum-based chemotherapy among those patients with a response. The primary end point is PFS. PAOLA-1 is a randomized phase III trial that enrolled patients following response to front line platinum-based chemotherapy containing bevacizumab and randomized them to receive continued bevacizumab maintenance plus placebo versus bevacizumab and olaparib maintenance. The end point here is PFS and the primary assessment will be in the intent-to-treat population. In addition to these, in the recurrent setting, NRG-GY004 is expected to report as well and will provide the first analysis of PARPi versus chemotherapy. If all of these studies meet their primary end points, the treatment paradigm for patients with recurrent EOC becomes very complicated (Figure 1C).
Moving forward, clinical trial design will be imperative. Traditional drug development has started in the ‘platinum resistant’ (PR) patient population and sequentially progressed to the ‘platinum sensitive’ (PS) and then eventually upfront settings; however, in the era of new targeted therapies and novel trial design utilizing molecular characteristics, this traditional paradigm may not always be necessary. Table 5 illustrates PFS and HR for the development of both bevacizumab and olaparib. The table demonstrates the similar hazard ratios in both the PS and upfront patient settings leading to the question: should we focus trial design initially in the upfront patient setting? Should we be expediting drug development of compounds with known efficacy to the population they are likely to benefit the most and focus our use of already scarce patient resources? However, this shift would leave a gap in knowledge for what to do in the recurrent setting. As discussed above, additional trials will be needed demonstrating efficacy of repeat exposure of newer regimens as our upfront treatment of EOC shifts. The landscape of treating EOC is changing at a rapid pace and our ability to adjust our methodology and treatment paradigms must continue to change as well in order to provide optimal care for our patients with recurrent ovarian cancer.
Table 5.
PFS and HR of bevacizumab and olaparib in sequential ovarian cancer patient populations
| Platinum resistant | Platinum sensitive | Upfront | |
|---|---|---|---|
| Bevacizumab | AURELIA [85] | GOG 213 [10] | GOG 218 [86] |
| PFS | 3.4 versus 6.7 months | 10.4 versus 13.8 months | 11.2 versus 14.1 months |
| HR | 0.42 (0.32–0.53 P < 0.001) | 0.628 (0.534–0.739 P < 0.0001) | 0.908 (0.795–1.040 P = 0.16) |
| Olaparib | Study 42 [84] | SOLO 2 [25] | SOLO 1 [78] |
| PFS | 6.7 months | 19.1 versus 5.5 months | Not Reached versus 13.8 months |
| HR | NA | 0.30 (0.22–0.41 P < 0.0001) | 0.30 (0.23–0.41 P > 0.0001) |
PFS, progression free survival; HR, hazard ratio; GOG, Gynecologic Oncology Group; NA, not applicable.
Table 6.
Current platinum sensitive studies
| Name | N | Population | Arms | Primary end point | NCT |
|---|---|---|---|---|---|
| AGO-OVAR 2.21 ENGOT ov18 | 682 | PFI > 6 months | Gem/carbo/bev versus PLD/carbo/bev | PFS | NCT01837251 |
| INOVATYON | 618 | PFI 6–12 months, 1–2 priors | PLD 30 mg/m2 / carbo AUC 5 versus PLD 30 mg/m2 / trabectedin 1.1 mg/m2 q 21 days | OS | NCT01379989 |
| MITO 23/ENGOT Ov-32 | 244 | Recurrent EOC with BRCA or BRCAness | PC chemo versus trabectedin 1.3 mg/mg d1 q 21 days | OS | NCT02903004 |
| ICON9 | 618 | 1st recurrence, PFI > 6 months, CR or PR to induction therapy | Olaparib 300 mg b.i.d. + cediranib 20 mg qd versus olaparib 300 mg po b.i.d. | PFS and OS | NCT03278717 |
| AVANOVA part 2 | 103 | PFI > 6 months | Niraparib versus niraparib + bevacizumab | PFS | NCT02354131 |
| AVANOVA part 3 | 72 | PFI > 6 months | Niraparib + bev + TSR042 | PFS | Pending |
| ATALANTE | 405 | PFI > 6 months, 1–2 prior lines | Carboplatin doublet + atezolizumab + bev versus carboplatin doublet + bev + placebo | PFS | NCT02891824 |
| OREO/ENGOT Ov38 | 136 BRCA 280 BRCAwt | Response to induction platinum therapy in recurrent setting; prior PARPi exposure | Olaparib 300 mg po bid versus placebo switch maintenance | PFS | NCT03106987 |
| ANITA/ENGOT Ov 41/GEICO 69-O | 414 | TFIp > 6 months, <2 prior lines | Platinum doublet + placebo followed by niraparib + placebo versus platinum doublet + atezolizumab followed by niraparib + atezo | PFS | NCT03598270 |
CR, complete response; PR, partial response; PLD, pegylated liposomal doxorubicin; PFS, progression free survival; OS, overall survival; TFIp, treatment free interval from last platinum NCT, National Clinical Trial; PFI, platinum free interval.
Funding
None declared.
Disclosure
MB has no disclosures. TJH reports personal fees from J & J, personal fees from Clovis, personal fees from AstraZeneca, personal fees from Tesaro, personal fees from Roche, personal fees from Caris. SNW reports grants and personal fees from AstraZeneca, grants and personal fees from Clovis, grants and personal fees from Tesaro, grants and personal fees from Roche/Genentech, grants from Cotinga Pharmaceuticals, personal fees from Merck, personal fees from Pfizer, grants from Novartis, personal fees from Takeda, grants from ArQule, outside the submitted work. RLC reports grants from AstraZeneca, Roche/Genentech, Janssen, OncoMed, Merck, Clovis Oncology, Esperance, and AbbVie and reports serving as an advisor to AstraZeneca, Roche/Genentech, Janssen, OncoMed, Agenus, Incyte, Merck, Clovis Oncology, Esperance, Tesaro, GamaMabs, Pfizer, Genmab, and AbbVie. BJM reports honorarium/consulting for Abbvie, Advaxis, Amgen, AstraZeneca, Biodesix, Clovis, Conjupro, Genmab, Gradalis, Immunogen, Immunomedics, Incyte, Janssen/Johnson & Johnson, Mateon, Merck, Myriad, Perthera, Pfizer, Precision Oncology, Puma, Roche/Genetech, Samumed, Takeda, Tesaro, and VBL. KNM reports advisory board participation and compensation from Astra Zeneca, Genentech/Roche, Merck, Pfizer, Janssen, Immunogen, Clovis, Tesaro, VBL Therapeutics, Aravive, OncoMed, Incyte, Genmab. She reports research grants from PTC Therapeutics, Lilly and Genentech.
References
- 1.American Cancer Society. Cancer Facts and Figures 2018. Atlanta, GA 2018. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html.
- 2. Alvarez RD, Matulonis UA, Herzog TJ. et al. Moving beyond the platinum sensitive/resistant paradigm for patients with recurrent ovarian cancer. Gynecol Oncol 2016; 141(3): 405–409. [DOI] [PubMed] [Google Scholar]
- 3. Wilson MK, Pujade-Lauraine E, Aoki D. et al. Fifth ovarian cancer consensus conference of the gynecologic cancer intergroup: recurrent disease. Ann Oncol 2017; 28: 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chi DS, McCaughty K, Diaz JP. et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer 2006; 106(9): 1933–1939. [DOI] [PubMed] [Google Scholar]
- 5. Harter P, du Bois A, Hahmann M. et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol 2006; 13(12): 1702–1710. [DOI] [PubMed] [Google Scholar]
- 6. Lee CK, Lord S, Grunewald T. et al. Impact of secondary cytoreductive surgery on survival in patients with platinum sensitive recurrent ovarian cancer: analysis of the CALYPSO trial. Gynecol Oncol 2015; 136(1): 18–24. [DOI] [PubMed] [Google Scholar]
- 7. Zang RY, Harter P, Chi DS. et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer 2011; 105(7): 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harter P, Sehouli J, Reuss A. et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer 2011; 21(2): 289–295. [DOI] [PubMed] [Google Scholar]
- 9. Bois AD, Vergote I, Ferron G. et al. Randomized controlled phase III study evaluating the impact of secondary cytoreductive surgery in recurrent ovarian cancer: AGO DESKTOP III/ENGOT ov20. J Clin Oncol 2017; 35: 5501. [Google Scholar]
- 10. Coleman RL, Brady MF, Herzog TJ. et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2017; 18(6): 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coleman R, Enserro D, Spirtos N. et al. A phase III randomized controlled trial of secondary surgical cytoreduction (SSC) followed by platinum-based combination chemotherapy (PBC), with or without bevacizumab (B) in platinum-sensitive, recurrent ovarian cancer (PSOC): a NRG Oncology/Gynecologic Oncology Group (GOG) study. J Clin Oncol 2018; 36: 5501. [Google Scholar]
- 12. Coleman R, Enserro D, Herzog TJ. et al. A phase III randomized controlled trial of secondary cytoreductive surgery (SCS) followed by platinum-based chemotherapy (PC) platinum-sensitive, recurrent ovarian cancer (PSOC)—surgical parameters. In International Gynecologic Cancer Society. Kyoto, Japan: 2018. [Google Scholar]
- 13. Capoluongo E, Ellison G, Lopez-Guerrero JA. et al. Guidance statement on BRCA1/2 tumor testing in ovarian cancer patients. Semin Oncol 2017; 44(3): 187–197. [DOI] [PubMed] [Google Scholar]
- 14. Moore K, Colombo N, Scambia G. et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018; 379(26): 2495–2505. [DOI] [PubMed] [Google Scholar]
- 15. Pignata S, Scambia G, Bologna A. et al. Randomized controlled trial testing the efficacy of platinum-free interval prolongation in advanced ovarian cancer: the MITO-8, MaNGO, BGOG-Ov1, AGO-Ovar2.16, ENGOT-Ov1, GCIG study. J Clin Oncol 2017; 35(29): 3347–3353. [DOI] [PubMed] [Google Scholar]
- 16. Ventriglia J, Paciolla I, Cecere SC. et al. Trabectedin in ovarian cancer: is it now a standard of care? Clin Oncol (R Coll Radiol) 2018; 30(8): 498–503. [DOI] [PubMed] [Google Scholar]
- 17. Takebayashi Y, Pourquier P, Zimonjic DB. et al. Antiproliferative activity of ecteinascidin 743 is dependent upon transcription-coupled nucleotide-excision repair. Nat Med 2001; 7(8): 961–966. [DOI] [PubMed] [Google Scholar]
- 18. Cuevas C, Francesch A.. Development of Yondelis (trabectedin, ET-743). A semisynthetic process solves the supply problem. Nat Prod Rep 2009; 26(3): 322–337. [DOI] [PubMed] [Google Scholar]
- 19. Poveda A, Vergote I, Tjulandin S. et al. Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer: outcomes in the partially platinum-sensitive (platinum-free interval 6-12 months) subpopulation of OVA-301 phase III randomized trial. Ann Oncol 2011; 22(1): 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coleman RL, Monk BJ, Sood AK, Herzog TJ.. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat Rev Clin Oncol 2013; 10(4): 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aghajanian C, Blank SV, Goff BA. et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 2012; 30(17): 2039–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ledermann JA, Embleton AC, Raja F. et al. Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016; 387(10023): 1066–1074. [DOI] [PubMed] [Google Scholar]
- 23. Ledermann J, Harter P, Gourley C. et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012; 366(15): 1382–1392. [DOI] [PubMed] [Google Scholar]
- 24. Mirza MR, Monk BJ, Herrstedt J. et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 2016; 375(22): 2154–2164. [DOI] [PubMed] [Google Scholar]
- 25. Pujade-Lauraine E, Ledermann JA, Selle F. et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18(9): 1274–1284. [DOI] [PubMed] [Google Scholar]
- 26. Coleman RL, Oza AM, Lorusso D. et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390(10106): 1949–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schoenfeld A, Levavi H, Breslavski D. et al. Three-dimensional modelling of tumor-induced ovarian angiogenesis. Cancer Lett 1994; 87(1): 79–84. [DOI] [PubMed] [Google Scholar]
- 28. Hollingsworth HC, Kohn EC, Steinberg SM. et al. Tumor angiogenesis in advanced stage ovarian carcinoma. Am J Pathol 1995; 147(1): 33–41. [PMC free article] [PubMed] [Google Scholar]
- 29. Gasparini G, Bonoldi E, Viale G. et al. Prognostic and predictive value of tumour angiogenesis in ovarian carcinomas. Int J Cancer 1996; 69(3): 205–211. [DOI] [PubMed] [Google Scholar]
- 30. Nakanishi Y, Kodama J, Yoshinouchi M. et al. The expression of vascular endothelial growth factor and transforming growth factor-beta associates with angiogenesis in epithelial ovarian cancer. Int J Gynecol Pathol 1997; 16(3): 256–262. [DOI] [PubMed] [Google Scholar]
- 31. Yoneda J, Kuniyasu H, Crispens MA. et al. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst 1998; 90(6): 447–454. [DOI] [PubMed] [Google Scholar]
- 32. Alvarez AA, Krigman HR, Whitaker RS. et al. The prognostic significance of angiogenesis in epithelial ovarian carcinoma. Clin Cancer Res 1999; 5: 587–591. [PubMed] [Google Scholar]
- 33. Sood AK, Seftor EA, Fletcher MS. et al. Molecular determinants of ovarian cancer plasticity. Am J Pathol 2001; 158(4): 1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bamberger ES, Perrett CW.. Angiogenesis in epithelian ovarian cancer. Mol Pathol 2002; 55(6): 348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paley PJ. Angiogenesis in ovarian cancer: molecular pathology and therapeutic strategies. Curr Oncol Rep 2002; 4(2): 165–174. [DOI] [PubMed] [Google Scholar]
- 36. Ueda M, Hung YC, Terai Y. et al. Vascular endothelial growth factor-C expression and invasive phenotype in ovarian carcinomas. Clin Cancer Res 2005; 11(9): 3225–3232. [DOI] [PubMed] [Google Scholar]
- 37. Buckanovich RJ, Sasaroli D, O'Brien-Jenkins A. et al. Tumor vascular proteins as biomarkers in ovarian cancer. J Clin Oncol 2007; 25(7): 852–861. [DOI] [PubMed] [Google Scholar]
- 38. Martin L, Schilder R.. Novel approaches in advancing the treatment of epithelial ovarian cancer: the role of angiogenesis inhibition. J Clin Oncol 2007; 25(20): 2894–2901. [DOI] [PubMed] [Google Scholar]
- 39. Merritt WM, Sood AK.. Markers of angiogenesis in ovarian cancer. Dis Markers 2007; 23(5–6): 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosa DD, Clamp AR, Collinson F, Jayson GC.. Antiangiogenic therapy for ovarian cancer. Curr Opin Oncol 2007; 19(5): 497–505. [DOI] [PubMed] [Google Scholar]
- 41. Spannuth WA, Sood AK, Coleman RL.. Angiogenesis as a strategic target for ovarian cancer therapy. Nat Clin Pract Oncol 2008; 5(4): 194–204. [DOI] [PubMed] [Google Scholar]
- 42. Monk BJ, Poveda A, Vergote I. et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2014; 15(8): 799–808. [DOI] [PubMed] [Google Scholar]
- 43. Pfisterer J, Dean AP, Baumann K. et al. Carboplatin/pegylated liposomal doxorubicin/bevacizumab (CD-BEV) vs. carboplatin/gemcitabine/bevacizumab (CG-BEV) in patients with recurrent ovarian cancer. Ann Oncol 2018; 29: viii3332–viii3358. [Google Scholar]
- 44. Chen DS, Mellman I.. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 45. Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell 2011; 144(5): 646–674. [DOI] [PubMed] [Google Scholar]
- 46. Hwang WT, Adams SF, Tahirovic E. et al. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol 2012; 124(2): 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zehir A, Benayed R, Shah RH. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10, 000 patients. Nat Med 2017; 23(6): 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Infante JR, Braiteh F, Emens LA. et al. Safety, clinical activity and biomarkers of atezolizumab (atezo) in advanced ovarian cancer (OC). Ann Oncol 2016; 27: 296–312 (abstr 871P). [Google Scholar]
- 49. Varga A, Piha-Paul SA, Ott PA. et al. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: interim results from a phase Ib study. J Clin Oncol 2015; 33: 5510–5510. [Google Scholar]
- 50. Hamanishi J, Mandai M, Ikeda T. et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 2015; 33(34): 4015–4022. [DOI] [PubMed] [Google Scholar]
- 51. Chang CL, Hsu YT, Wu CC. et al. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer Res 2013; 73(1): 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lo CS, Sanii S, Kroeger DR. et al. Neoadjuvant chemotherapy of ovarian cancer results in three patterns of tumor-infiltrating lymphocyte response with distinct implications for immunotherapy. Clin Cancer Res 2017; 23(4): 925–934. [DOI] [PubMed] [Google Scholar]
- 53. Terme M, Pernot S, Marcheteau E. et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 2013; 73(2): 539–549. [DOI] [PubMed] [Google Scholar]
- 54. Coukos G, Benencia F, Buckanovich RJ, Conejo-Garcia JR.. The role of dendritic cell precursors in tumour vasculogenesis. Br J Cancer 2005; 92(7): 1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gavalas NG, Tsiatas M, Tsitsilonis O. et al. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br J Cancer 2012; 107(11): 1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bouzin C, Brouet A, De Vriese J. et al. Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J Immunol 2007; 178(3): 1505–1511. [DOI] [PubMed] [Google Scholar]
- 57. Shrimali RK, Yu Z, Theoret MR. et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res 2010; 70(15): 6171–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Socinski MA, Jotte RM, Cappuzzo F. et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378(24): 2288–2301. [DOI] [PubMed] [Google Scholar]
- 59. Oza AM, Cibula D, Benzaquen AO. et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol 2015; 16(1): 87–97. [DOI] [PubMed] [Google Scholar]
- 60. Samol J, Ranson M, Scott E. et al. Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: a phase I study. Invest New Drugs 2012; 30(4): 1493–1500. [DOI] [PubMed] [Google Scholar]
- 61. Khan OA, Gore M, Lorigan P. et al. A phase I study of the safety and tolerability of olaparib (AZD2281, KU0059436) and dacarbazine in patients with advanced solid tumours. Br J Cancer 2011; 104(5): 750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dent RA, Lindeman GJ, Clemons M. et al. Phase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first- or second-line treatment of patients with metastatic triple-negative breast cancer. Breast Cancer Res 2013; 15(5): R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rajan A, Carter CA, Kelly RJ. et al. A phase I combination study of olaparib with cisplatin and gemcitabine in adults with solid tumors. Clin Cancer Res 2012; 18(8): 2344–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Audeh MW, Carmichael J, Penson RT. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 2010; 376(9737): 245–251. [DOI] [PubMed] [Google Scholar]
- 65. Kristeleit R, Shapiro GI, Burris HA. et al. A phase I-II study of the oral PARP inhibitor rucaparib in patients with germline BRCA1/2-mutated ovarian carcinoma or other solid tumors. Clin Cancer Res 2017; 23(15): 4095–4106. [DOI] [PubMed] [Google Scholar]
- 66. Oza AM, Tinker AV, Oaknin A. et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: integrated analysis of data from Study 10 and ARIEL2. Gynecol Oncol 2017; 147(2): 267–275. [DOI] [PubMed] [Google Scholar]
- 67. Swisher EM, Lin KK, Oza AM. et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol 2017; 18(1): 75–87. [DOI] [PubMed] [Google Scholar]
- 68. Moore KN, Secord AA, Geller MA. et al. QUADRA: a phase 2, open-label, single-arm study to evaluate niraparib in patients (pts) with relapsed ovarian cancer (ROC) who have received ≥3 prior chemotherapy regimens. J Clin Oncol 2018; 36(Suppl 15): 5514. [Google Scholar]
- 69. Kaye SB, Lubinski J, Matulonis U. et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly(ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol 2012; 30(4): 372–379. [DOI] [PubMed] [Google Scholar]
- 70. Lord CJ, Ashworth A.. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat Med 2013; 19(11): 1381–1388. [DOI] [PubMed] [Google Scholar]
- 71. Sandhu SK, Schelman WR, Wilding G. et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol 2013; 14(9): 882–892. [DOI] [PubMed] [Google Scholar]
- 72. Coleman RL, Sill MW, Bell-McGuinn K. et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation—an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 2015; 137: 386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dean E, Middleton MR, Pwint T. et al. Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours. Br J Cancer 2012; 106(3): 468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu JF, Tolaney SM, Birrer M. et al. A phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer. Eur J Cancer 2013; 49(14): 2972–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu JF, Barry WT, Birrer M. et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol 2014; 15(11): 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mirza MR, Wang J, Mau-Sørensen M. et al. 953PA phase 1 study to evaluate the safety and tolerability of bevacizumab-niraparib combination therapy and determine the recommended phase 2 dose (RP2D) in women with platinum-sensitive epithelial ovarian cancer (ENGOT-OV24/AVANOVA1). Ann Oncol 2017; 28: mdx372.024. [Google Scholar]
- 77. Pignata S, Lorusso D, Joley F. et al. Chemotherapy plus or minus bevacizumab for platinum-sensitive ovarian cancer patients recurring after a bevacizumab containing first line treatment: the randomized phase 3 trial MITO16B-MaNGO OV2B-ENGOT OV17. J Clin Oncol 2018; 36(Suppl 15): 5506-5506. [Google Scholar]
- 78. Moore KN, Colombo N, Scambia G. et al. Maintenance olaparib following platinum-based chemotherapy in newly diagnosed patients (pts) with advanced ovarian cancer (OC) and a BRCA1/2 mutation (BRCAm): phase III SOLO1 trial. Ann Oncol 2018; 29(Suppl 9): mdy483.002. [Google Scholar]
- 79.FDA approves olaparib for first-line maintenance of BRCA-mutated advanced ovarian cancer. 2018. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm628876.htm.
- 80.Merck KGaA, Darmstadt, Germany, and Pfizer provide update on JAVELIN ovarian 100 trial of avelumab in previously untreated advanced ovarian cancer. 2018. https://investors.pfizer.com/investor-news/press-release-details/2018/Merck-KGaA-Darmstadt-Germany-and-Pfizer-Provide-Update-on-JAVELIN-Ovarian-100-Trial-of-Avelumab-in-Previously-Untreated-Advanced-Ovarian-Cancer/default.aspx.
- 81. Parmar MK, Ledermann JA, Colombo N. et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet 2003; 361(9375): 2099–2106. [DOI] [PubMed] [Google Scholar]
- 82. Pfisterer J, Plante M, Vergote I. et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol 2006; 24(29): 4699–4707. [DOI] [PubMed] [Google Scholar]
- 83. Wagner U, Marth C, Largillier R. et al. Final overall survival results of phase III GCIG CALYPSO trial of pegylated liposomal doxorubicin and carboplatin vs paclitaxel and carboplatin in platinum-sensitive ovarian cancer patients. Br J Cancer 2012; 107(4): 588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Domchek SM, Aghajanian C, Shapira-Frommer R. et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol 2016; 140(2): 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pujade-Lauraine E, Hilpert F, Weber B. et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol 2014; 32(13): 1302–1308. [DOI] [PubMed] [Google Scholar]
- 86. Burger RA, Brady MF, Bookman MA. et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011; 365(26): 2473–2483. [DOI] [PubMed] [Google Scholar]