Abstract
Background
Intestinal dysbiosis may contribute to the pathogenesis of necrotising enterocolitis (NEC) in very preterm or very low birth weight (VLBW) infants. Dietary supplementation with synbiotics (probiotic micro‐organisms combined with prebiotic oligosaccharides) to modulate the intestinal microbiome has been proposed as a strategy to reduce the risk of NEC and associated mortality and morbidity.
Objectives
To assess the effect of enteral supplementation with synbiotics (versus placebo or no treatment, or versus probiotics or prebiotics alone) for preventing NEC and associated morbidity and mortality in very preterm or VLBW infants.
Search methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, the Maternity and Infant Care database and CINAHL, from earliest records to 17 June 2021. We searched clinical trials databases and conference proceedings, and examined the reference lists of retrieved articles.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs comparing prophylactic synbiotics supplementation with placebo or no synbiotics in very preterm (< 32 weeks' gestation) or very low birth weight (< 1500 g) infants.
Data collection and analysis
Two review authors separately performed the screening and selection process, evaluated risk of bias of the trials, extracted data, and synthesised effect estimates using risk ratio (RR), risk difference (RD), and mean difference, with associated 95% confidence intervals (CIs). We used the GRADE approach to assess the level of certainty for effects on NEC, all‐cause mortality, late‐onset invasive infection, and neurodevelopmental impairment.
Main results
We included six trials in which a total of 925 infants participated. Most trials were small (median sample size 200). Lack of clarity on methods used to conceal allocation and mask caregivers or investigators were potential sources of bias in four of the trials. The studied synbiotics preparations contained lactobacilli or bifidobacteria (or both) combined with fructo‐ or galacto‐oligosaccharides (or both).
Meta‐analyses suggested that synbiotics may reduce the risk of NEC (RR 0.18, 95% CI 0.09 to 0.40; RD 70 fewer per 1000, 95% CI 100 fewer to 40 fewer; number needed to treat for an additional beneficial outcome (NNTB) 14, 95% CI 10 to 25; six trials (907 infants); low certainty evidence); and all‐cause mortality prior to hospital discharge (RR 0.53, 95% CI 0.33 to 0.85; RD 50 fewer per 1000, 95% CI 120 fewer to 100 fewer; NNTB 20, 95% CI 8 to 100; six trials (925 infants); low‐certainty evidence). Synbiotics may have little or no effect on late‐onset invasive infection, but the evidence is very uncertain (RR 0.84, 95% CI 0.58 to 1.21; RD 20 fewer per 1000, 95% CI 70 fewer to 30 more; five trials (707 infants); very low‐certainty evidence). None of the trials assessed neurodevelopmental outcomes. In the absence of high levels of heterogeneity, we did not undertake any subgroup analysis (including the type of feeding).
Authors' conclusions
The available trial data provide only low‐certainty evidence about the effects of synbiotics on the risk of NEC and associated morbidity and mortality for very preterm or very low birth weight infants. Our confidence in the effect estimates is limited; the true effects may be substantially different from these estimates. Large, high‐quality trials would be needed to provide evidence of sufficient validity and applicability to inform policy and practice.
Plain language summary
Do synbiotics prevent necrotising enterocolitis in very preterm or very low birth weight infants?
Background
Very preterm (born more than eight weeks early) and very low birth weight (less than 1.5 kg) infants are at risk of developing necrotising enterocolitis, a severe condition where some of the lining of the infant's bowel becomes inflamed, and the cells of this tissue die. This condition is associated with death, serious infection, and long‐term disability, as well as developmental problems.
What did we want to find out?
One way to help prevent necrotising enterocolitis may be to add synbiotics (combinations of probiotic bacteria or yeasts plus non‐digestible sugars to support probiotic growth and colonisation) to milk feeds. We wanted to find out whether synbiotics might benefit very preterm and very low birth weight infants. Our outcomes of interest included necrotising enterocolitis, death from any cause, serious infection, duration of hospitalisation since birth and neurodevelopmental outcomes.
What did we do?
For our Cochrane Review, we searched several important databases to identify randomized controlled trials that investigated the use of synbiotics for preventing necrotising enterocolitis in very preterm and very low birth weight infants. We used standard Cochrane methods to conduct our review and perform our analyses. We used the GRADE approach to assess the certainty of the evidence for each outcome.
What did we find?
We found six trials with a total of 925 infant participants. Trials were mostly small, and most had design flaws that might have biased their findings.
Main results
Combined analyses showed that giving synbiotics to very preterm or very low birth weight infants may reduce the risk of necrotising enterocolitis and death. Synbiotics may have little or no effect in reducing the risk of serious infection, but the evidence is very uncertain. None of the studies that we identified assessed the effect of synbiotics on disability or developmental outcomes.
What are the limitations of this evidence?
Although the evidence from randomised controlled trials can potentially be of high certainty, the methods used in our review's included trials may have introduced biases that exaggerated the benefits of giving synbiotics to very preterm and very low birth weight infants. Also, because most of the trials were small, the effect estimates for some outcomes were imprecise. For these reasons, we graded down the certainty of the evidence. All evidence in our review is of low or very low certainty.
There is low‐certainty evidence that synbiotics prevent necrotising enterocolitis and death from any cause. There is very low‐certainty evidence that synbiotics may have little or no effect in preventing serious infection.
How up to date is this evidence?
We conducted our database searches on 17 June 2021.
Summary of findings
Summary of findings 1. Synbiotics compared to control in very preterm or very low birth weight infants.
| Synbiotics compared to control in very preterm or very low birth weight infants | ||||||
| Patient or population: very preterm or very low birth weight infants Setting: neonatal care centres globally Intervention: synbiotics (typically Bifidobacterium spp., Lactobacillus spp., plus fructo‐ or galacto‐oligosaccharides (or both) Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Risk ratio (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with control | Risk with Probiotics | |||||
| Necrotising enterocolitis (before hospital discharge) | 83 per 1000 | 15 per 1000 (7 to 33) |
0.18 (0.09 to 0.40) | 70 per 1000 fewer (100 fewer to 40 fewer per 1000) | 907 (6 studies) | ⊕⊕⊝⊝ Lowa |
| Mortality (all‐cause before hospital discharge) | 93 per 1000 | 50 per 1000 (31 to 79) | 0.53 (0.33 to 0.85) | 50 per 1000 fewer (120 fewer to 100 fewer per 1000) | 925 (6 studies) | ⊕⊕⊝⊝ Low,b,c |
| Late‐onset Invasive infection (before hospital discharge) | 134 per 1000 | 113 per 1000 (78 to 162) | 0.84 (0.58 to 1.21) | 20 per 1000 fewer (70 fewer to 30 more per 1000) | 707 (5 studies) | ⊕⊝⊝⊝ Very lowc,d |
| Neurodevelopmental impairment (assessed beyond infancy) | None of the included trials reported neurodevelopmental outcomes. | |||||
| *The risk in the intervention group (and its 95% confidence interval (CI) is based on the assumed risk in the comparison group and the relative effect of the intervention and its 95% CI. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Methodological limitations in trials (high risk of bias due to uncertainty about methods used to generate random sequence, conceal allocation, and mask outcome assessment): downgraded two levels for NEC because of subjectivity in ascertainment and diagnosis.
b Methodological limitations in trials (high risk of bias due to uncertainty about methods used to generate random sequence, conceal allocation, and mask outcome assessment): downgraded one level for all‐cause mortality before discharge because of objectivity in ascertainment and diagnosis.
c Serious imprecision of effect estimate (low number of events).
d Methodological limitations in trials (high risk of bias due to uncertainty about methods used to generate random sequence, conceal allocation, and mask outcome assessment): downgraded two levels for late‐onset invasive infection because of subjectivity in ascertainment and diagnosis.
Background
This review assesses the randomised controlled trial (RCT) evidence for the effect of enteral synbiotics (combinations of probiotic micro‐organisms and prebiotic oligosaccharides) for preventing necrotising enterocolitis (NEC) in very preterm or very low birth weight (VLBW) infants. Other Cochrane Reviews assess the evidence for probiotics alone (Sharif 2020) or prebiotics alone (Sharif 2021a).
Description of the condition
Necrotising enterocolitis is a syndrome of acute intestinal necrosis that affects about one in twenty very preterm (< 32 weeks' gestation) or VLBW (< 1500 g) infants (Horbar 2012). The risk factors for NEC include being extremely preterm (< 28 weeks' gestation) or extremely low birth weight (ELBW) (< 1000 g), and intrauterine growth restriction or compromise indicated by absent or reversed end‐diastolic flow velocities in antenatal Doppler studies of the umbilical artery or fetal aorta (Samuels 2017). Infants who develop NEC experience more infections, have lower levels of nutrient intake, grow more slowly, and have longer durations of intensive care and hospital stay than gestation‐comparable infants who do not (Battersby 2018; Berrington 2012). The mortality rate of infants with NEC is about 20%. Compared with their peers, infants who survive NEC have a higher risk of neurodevelopmental problems and disability, especially if it is associated with bloodstream infections (Hickey 2018).
The pathogenesis of NEC is not fully understood but is postulated to involve intestinal dysbiosis, infection and inflammation (Eaton 2017; Mara 2018; Stewart 2016). Evidence exists that the pattern, diversity and stability of the intestinal microbiome is associated with the risk of developing NEC (Masi 2019; Olm 2019; Stewart 2012; Warner 2016). Compared with cow milk formula, feeding with human milk reduces the risk of NEC in very preterm or VLBW infants (Quigley 2019). A putative mechanism for this protective effect is that prebiotic human milk oligosaccharides promote the growth of non‐pathogenic probiotic micro‐organisms, such as lactobacilli and bifidobacteria, which modulate the intestinal microbiome and promote mucosal barrier functions (Embleton 2017; Granger 2020; Walsh 2019). Compared with human milk‐fed term infants, however, very preterm or VLBW infants tend to harbour fewer intestinal probiotic micro‐organisms, and more potential pathogens, which might be due to dysbiotic effects of enteral fasting and antibiotic exposure (Stewart 2017).
Description of the intervention
Synbiotics (probiotic‐prebiotic combinations)
Synbiotics are combinations of probiotics and prebiotics. The prebiotic content is intended to enhance probiotic growth and intestinal colonisation (Nolan 2020).
Probiotics
Probiotics are live micro‐organisms (predominantly bifidobacteria and lactobacilli) that benefit the host by modulating the intestinal microbiome and promoting mucosal barrier functions and resistance to pathogens (Berrington 2019; Esaiassen 2018). Preterm infants supplemented enterally with bifidobacteria and lactobacilli establish an intestinal microbiome that is dominated by probiotics and contains fewer potential pathogens, compared with non‐supplemented infants (Alcon‐Giner 2020). Meta‐analysis of data from more than 50 RCTs using a variety of probiotic strains and multi‐organism combinations suggests that in very preterm or VLBW infants, probiotic supplementation may reduce the risk of NEC, and probably reduces all‐cause mortality before hospital discharge as well as late‐onset invasive infection (Sharif 2020). The certainty of this evidence is low, however, because of concerns that effect estimates are inflated by methodological weaknesses in the trials, which were mainly small, and by publication bias. Consequently, and because of ongoing issues about safety and the availability of regulated products, probiotic supplementation has not become established as a common practice in most neonatal care facilities (Duffield 2019; Fleming 2019; Pell 2019; Vermeulen 2020).
Prebiotics
Prebiotics are a diverse family of complex glycans that promote intestinal colonisation with probiotic micro‐organisms. Human milk contains numerous prebiotic substances, predominantly galacto‐oligosaccharides and fructo‐oligosaccharides, which influence the intestinal microbiome in preterm infants (Boehm 2008; Nolan 2020). Natural human milk oligosaccharides vary markedly between individual women, and vary temporally (depending on the stage of lactation) within individual women (Aakko 2017; Smilowitz 2013). Newborn infants do not digest human milk oligosaccharides. Rather, these are primarily nutrient sources for intestinal probiotic micro‐organisms, particularly bifidobacteria (Alcon‐Giner 2020; Jost 2015). Feeding with human milk, rather than cow milk‐derived formula, may reduce intestinal dysbiosis, and there is emerging evidence about how specific human milk oligosaccharides promote probiotic predominance in very preterm infants (Lyons 2020; Masi 2020; Underwood 2015).
Manufactured or plant‐based (e.g. inulin) prebiotic oligosaccharides are less heterogeneous than natural human milk oligosaccharides, and typically consist of short chains of galactose or fructose, usually with a terminal glucose monomer (Johnson‐Henry 2016). Evidence exists that giving supplemental synthetic prebiotic oligosaccharides to formula‐fed very preterm infants stimulates the growth of an intestinal microflora that is similar to that found in infants fed with maternal milk (Autran 2018; Boehm 2008; Kapiki 2007; Veereman‐Wauters 2011). RCTs have not, however, provided evidence of their effectiveness in preventing NEC, NEC‐associated morbidity or all‐cause mortality before hospital discharge (Chi 2019; Johnson‐Henry 2016; Srinivasjois 2013).
How the intervention might work
It is postulated that administering supplemental prebiotic oligosaccharides enhances both exogenous and endogenous probiotic growth and intestinal colonisation (Nolan 2020; Underwood 2019). Probiotic bacteria and fungi use prebiotic oligosaccharides as a major nutrient source (Alcon‐Giner 2020). Bifidobacteria and lactobacilli ferment prebiotic oligosaccharides to produce short‐chain fatty acids that inhibit adhesion of pathogenic bacteria and modulate intestinal epithelial integrity and barrier function (Johnson‐Henry 2016). Synbiotic combinations, therefore, may be more effective than supplementation with either a probiotic or prebiotic alone (Zmora 2018). A recent RCT involving more than 4500 newborn infants (birth weight at least 2000 grams or gestation greater than 34 weeks) in rural India showed that enteral synbiotic supplementation (Lactobacillus plantarum plus fructo‐oligosaccharide) was associated with a reduced risk of neonatal sepsis (Panigrahi 2017).
Why it is important to do this review
Necrotising enterocolitis and associated complications, particularly infection, are the most common causes of mortality and serious morbidity in very preterm or VLBW infants beyond the early neonatal period (Berrington 2012). A 2019 Cochrane Review found only low‐certainty evidence that probiotic or prebiotic supplementation alone reduce the risk of NEC (Quigley 2019). Given the plausibility that synbiotics might have an advantage over probiotics or prebiotics alone, appraising and synthesising the trial evidence about the benefits and harms of synbiotics supplementation could inform practice, policy and research.
Objectives
To assess the effect of enteral supplementation with synbiotics (versus placebo or no treatment, or versus probiotics or prebiotics alone) for preventing NEC and associated morbidity and mortality in very preterm or VLBW infants.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised (predictable allocation) controlled trials, including cluster‐RCTs.
Cross‐over studies were not eligible for inclusion.
Types of participants
Very preterm (< 32 weeks' gestation) or VLBW (< 1500 g) infants.
Types of interventions
Prophylactic enteral synbiotics: any combination or dose of probiotic organisms and prebiotic oligosaccharides, commenced within 14 days of birth and continued daily (or more frequently) for at least one week. Probiotics and prebiotics need not be given simultaneously, but should be given on the same day.
Types of outcome measures
We focused on assessing effects on infant‐ and family‐important outcomes, principally neonatal morbidities that plausibly affect rates of mortality or neurodisability. We did not include surrogate outcomes such as stool colonisation patterns.
Primary outcomes
-
NEC before discharge from hospital, confirmed at surgery or autopsy or using standardized clinical and radiological criteria (VON 2020):
at least one of: bilious gastric aspirate or emesis; or abdominal distention; or blood in stool; and
at least one of: abdominal radiograph showing pneumatosis intestinalis; or gas in the portal venous system; or free air in the abdomen
All‐cause mortality before discharge from hospital
Secondary outcomes
Late‐onset invasive infection, as determined by the culture of bacteria or fungus from blood or cerebrospinal fluid or from a normally sterile body space (> 48 hours after birth until discharge from hospital)
Invasive infection with the supplemented probiotic micro‐organism until discharge from hospital
Duration of hospitalisation since birth
Neurodevelopmental impairment assessed by a validated test after 12 months' post‐term: neurological evaluations, developmental scores, and classifications of disability, including cerebral palsy and auditory and visual impairment
Search methods for identification of studies
We used the criteria and standard methods of Cochrane Neonatal.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 6) in the Cochrane Library; MEDLINE Ovid (1946 to June 2020), Embase Ovid (1974 to June 2021), Maternity and Infant Care Database Ovid (1971 to May 2021), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to June 2021) using a combination of text words and Medical Subject Heading (MeSH) indexing terms described in Appendix 1. We limited the search outputs with filters for clinical trials as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). We did not apply any language restrictions.
We searched for ongoing or recently completed trials at the US National Institutes of Health's trials registry (clinicaltrials.gov), the World Health Organization's International Clinical Trials Registry Platform (trialsearch.who.int), and the ISRCTN Registry (www.isrctn.com).
Searching other resources
We examined the reference lists of any articles selected for inclusion in this review.
Data collection and analysis
We used the standard methods of Cochrane Neonatal.
Selection of studies
Two review authors (SS, PTH or SO) independently screened the titles and abstract of all studies and assessed full‐text articles for all potentially relevant trials. We excluded those reports that did not meet all of the inclusion criteria, and we stated the reasons for exclusion. We discussed disagreements until consensus was achieved, with referral to another review author (WM) for final decision as necessary.
Data extraction and management
Two review authors (SS, SO or WM) extracted data independently, using a form to aid extraction of information on design, methodology, participants, interventions, outcomes and treatment effects from each included study. We discussed disagreements until we reached a consensus. If data from the study reports were insufficient, we contacted the report authors for further information.
Assessment of risk of bias in included studies
Two review authors (SS, SO or WM) independently assessed the risk of bias (low, high or unclear) of all included trials using the original version of Cochrane's ‘Risk of bias’ tool (Higgins 2011). We assessed the following domains:
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
any other bias (including baseline imbalance).
Had any disagreements occurred, we planned to resolve these through discussion or by involving the third review author. See Appendix 2 for a description of risk of bias for each domain.
For cluster‐RCTs, where groups of individuals were randomized to the different interventions, we additionally planned to assess bias arising from prior knowledge of cluster‐allocation (identification/recruitment bias, suggested by baseline imbalances in characteristics of participants rather than of clusters) and bias arising from the timing of identification and recruitment of participants (Higgins 2020).
Measures of treatment effect
We analysed the treatment effects in the individual trials and reported risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference (MD) for continuous data, with respective 95% confidence intervals (CI). We planned to determine the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH) for analyses with a statistically significant difference in the RD.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials and the neonatal unit (or sub‐unit) for cluster‐randomised trials. For cluster‐randomised trials, we planned to undertake analyses at the level of the individual while accounting for the clustering in the data using the methods recommended in the Cochrane Handbook (Higgins 2020).
Dealing with missing data
We planned to request additional data from trial investigators when data on important outcomes were missing or reported unclearly. If unavailable, we planned to undertake sensitivity analyses to assess the potential impact on outcomes by excluding those trials with > 20% missing data.
Assessment of heterogeneity
We examined the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We calculated the I² statistic for each analysis to quantify inconsistency across studies and describe the percentage of variability in effect estimates that may be due to heterogeneity rather than to sampling error. If we detected high levels of heterogeneity (I² > 75%), we planned to explore the possible sources in subgroup analyses.
Assessment of reporting biases
If at least 10 trials were included in a meta‐analysis, we planned to examine a funnel plot for asymmetry visually and with Harbord's modification of Egger's test (Harbord 2006).
Data synthesis
We used a fixed‐effect model inverse variance meta‐analysis for combining data where trials examined the same intervention and the populations and methods of the trials were judged to be similar.
Subgroup analysis and investigation of heterogeneity
Where data were available, we planned subgroup analyses for the primary outcomes by:
genus of probiotics or combinations (Bifidobacterium spp., Lactobacillus spp., Sacchromyces spp., Streptococcal spp., others, and combinations thereof);
type of prebiotic oligosaccharide: natural versus synthetic;
type of enteral feeding permitted for participating infants: human milk versus formula versus mixed;
trials in which most (> 50%) participants were extremely low birth weight (ELBW; < 1000 g) or extremely preterm (< 28 weeks' gestation at birth) versus trials in which most infants were ≥ 28 weeks' gestation at birth of birth weight ≥ 1000 g;
trials which restricted participation to infants with intrauterine growth restriction or absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery versus trials that did not do so.
Sensitivity analysis
We planned to undertake sensitivity analyses to determine how estimates are affected by including only studies at low risk of bias: (i) selection bias (adequate randomization and allocation concealment), (ii) detection or performance bias (adequate masking of intervention and measurement), (iii) attrition bias (< 20% loss to follow‐up for primary outcome assessment), and (iv) reporting bias (selective reporting).
Summary of findings and assessment of the certainty of the evidence
Two review authors (PTH, SO or WM) used the GRADE approach to assess the certainty of the evidence for effects on NEC, all‐cause mortality before hospital discharge, late‐onset invasive infection, and neurodevelopmental impairment (Schünemann 2013; Walsh 2021). We initially considered evidence from RCTs to be of high certainty, but downgraded the evidence certainty by one level for serious limitations (or by two levels for very serious limitations), based upon the following domains: risk of bias (study limitations), inconsistency across studies, indirectness of the evidence, imprecision of estimates, and presence of publication bias. This approach results in an assessment of the certainty of a body of evidence for a given outcome as one of four grades (Appendix 3).
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
We used the GRADEpro GDT software to create a 'Summary of findings' table to report the certainty of the evidence.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
Results of the search
After the removal of duplicates from the search results, we screened 2382 titles and abstracts, which included forward and backward citation searches, clinical trials registers and grey literature. We evaluated 13 articles sourced as full‐text reports (Figure 1).
1.
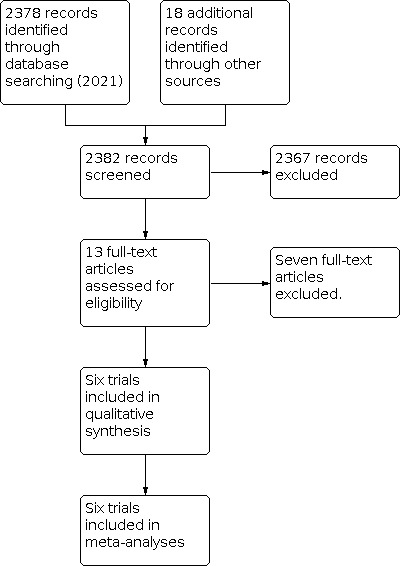
Study flow diagram (June 2021)
Included studies
We included six trials (See: Characteristics of included studies). These were conducted during the past 20 years in neonatal centres in Turkey (Dilli 2015; El 2017; Guney‐Varal 2017), India (Nandhini 2016; Sreenivasa 2015), and the USA (Underwood 2009). Most trials were conducted in single centres. One was a multi‐centre trial (Dilli 2015). Individual infants were allocated randomly to intervention or control groups in all of the trials. No trial used a cluster design.
Population
In total, 925 infants participated in the trials. The median number of participants was 200 (range 90 to 220). Two trials enrolled only very preterm or VLBW infants (Guney‐Varal 2017; Nandhini 2016). The other trials enrolled infants of gestational age up to 34 or 35 weeks' (El 2017; Nandhini 2016; Sreenivasa 2015; Underwood 2009). Because the average gestation at birth was < 32 weeks', or the average birth weight < 1500 g, we included these trials. In one trial, participants were preterm infants with evidence of feed intolerance (El 2017). None of the trials excluded infants born 'small for gestational age' or with evidence of absent or reversed end‐diastolic flow velocities in antenatal Doppler studies of the umbilical artery or fetal aorta.
Infants received an exclusive human milk diet in two of the trials (Nandhini 2016; Sreenivasa 2015). In the other trials, participating infants could be fed with human milk or formula or both (Dilli 2015; El 2017; Guney‐Varal 2017; Underwood 2009), but reporting of outcome data was not stratified by these subgroups.
Interventions
The studied synbiotics preparations contained lactobacilli or bifidobacteria (or both) combined with fructo‐oligosaccharides (FOS) and galacto‐oligosaccharides (GOS) or inulin (naturally occurring plant oligosaccharides).
Bifidobacterium spp. plus inulin (Dilli 2015; El 2017)
Bifidobacterium spp. and Lactobacillus spp. plus FOS/GOS (Guney‐Varal 2017; Nandhini 2016)
Bifidobacterium spp. and Lactobacillus spp. and Streptococcus thermophiles plus FOS (Sreenivasa 2015)
Bifidobacterium spp. andLactobacillus spp or Lactobacillus spp. plus inulin (Underwood 2009)
Comparisons
Two trials used placebo:
Maltodextrin (Dilli 2015)
Dilute elemental formula (Underwood 2009)
The other trials were unmasked and did not use a placebo (El 2017; Guney‐Varal 2017; Nandhini 2016; Sreenivasa 2015).
Outcomes
All trials reported the number of infants who developed NEC and all reported all‐cause mortality prior to hospital discharge. Five trials reported the number of infants with at least one episode of culture‐confirmed infection. In one trial, stool colonisation with the supplemented probiotic species was the primary outcome (Underwood 2009). Other in‐hospital outcomes reported included time to establish full enteral feeding and duration of hospital stay. None of the trials reported long‐term growth or neurodevelopmental outcomes.
Excluded studies
We excluded seven reports of studies (Bering 2018; Dasopoulou 2015; Dilli 2013; Panigrahi 2008; Serce Pehlevan 2020; Underwood 2014; Vakiliamini 2020). The reasons for exclusion were ineligible population (three trials), wrong intervention (one trial), co‐intervention (one trial), and non‐randomised design (one trial). See Characteristics of excluded studies.
Risk of bias in included studies
'Risk of bias' assessments and judgements are described in Characteristics of included studies and are summarised in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three trial reports described methods to generate random sequences (typically computer‐generated) and to ensure adequate allocation concealment (typically sealed opaque envelopes) (Dilli 2015; Nandhini 2016; Underwood 2009). Two other reports did not describe these methods (El 2017; Sreenivasa 2015). One trial was quasi‐randomised, employing alternate allocation (Guney‐Varal 2017).
Blinding
Two of the trials were placebo‐controlled (Dilli 2015; Underwood 2009). The other trials did not mask parents, caregivers, or clinical investigators (El 2017; Guney‐Varal 2017; Nandhini 2016; Sreenivasa 2015).
Incomplete outcome data
All trials reported complete or near‐complete assessments of primary outcomes.
Selective reporting
Although trial protocols were not available for most trials, selective reporting bias was not considered a major threat given that all relevant clinical outcomes were reported.
Other potential sources of bias
We did not find evidence of between‐group baseline differences in participant characteristics or demographics in four of the trials (Dilli 2015; Nandhini 2016; Sreenivasa 2015; Underwood 2009). In two trials, including the quasi‐randomised trial, the average birth weight (but not average gestational age) differed substantially between the groups (El 2017; Guney‐Varal 2017).
Effects of interventions
See: Table 1
Primary outcomes
Necrotising enterocolitis
Meta‐analysis of data from six trials (907 infants) suggests that synbiotics (compared to no synbiotics) may reduce the risk of NEC prior to hospital discharge (Analysis 1.1; Figure 3):
1.1. Analysis.

Comparison 1: Synbiotics versus control, Outcome 1: Necrotising enterocolitis
3.

Forest plot of comparison: 1 Synbiotics versus control, outcome: 1.1 Necrotising enterocolitis.
RR 0.18, 95% CI 0.09 to 0.40
RD 70 fewer per 1000, 95% CI 100 fewer to 40 fewer per 1000
NNTB 14; 95% CI 10 to 25
Subgroup analysis for heterogeneity
In the absence of high levels of heterogeneity (I² = 0%), we did not undertake the pre‐specified subgroup analyses (Subgroup analysis and investigation of heterogeneity).
Using the GRADE approach, we assessed the certainty of the evidence to be 'low'. We downgraded evidence certainty by two levels for very serious study limitations (Table 1).
All‐cause mortality before hospital discharge
Meta‐analysis of data from six trials (925 infants) suggests that synbiotics (compared to no synbiotics) may reduce mortality prior to hospital discharge (Analysis 1.2; Figure 4).
1.2. Analysis.

Comparison 1: Synbiotics versus control, Outcome 2: All‐cause mortality
4.

Forest plot of comparison: 1 Synbiotics versus control, outcome: 1.2 All‐cause mortality.
RR 0.53, 95% CI 0.33 to 0.85
RD 50 per 1000 fewer, 95% CI 120 fewer to 100 fewer per 1000
NNTB 20, 95% CI 8 to 100
Subgroup analysis for heterogeneity
In the absence of high levels of heterogeneity (I² = 48%), we did not undertake the pre‐specified subgroup analyses (Subgroup analysis and investigation of heterogeneity).
We assessed the certainty of evidence to be 'low'. We downgraded evidence certainty by one level (because of objectivity of the outcome) for serious study limitations and by one level for imprecision (Table 1).
Secondary outcomes
Late‐onset invasive infection
Meta‐analysis of data from five trials (707 infants) suggests that synbiotics (compared to no synbiotics) may result in little or no difference in risk of late‐onset invasive infection, but the evidence is very uncertain (Analysis 1.3; Figure 5).
1.3. Analysis.

Comparison 1: Synbiotics versus control, Outcome 3: Invasive infection
5.

Forest plot of comparison: 1 Synbiotics versus control, outcome: 1.3 Infection.
RR 0.84, 95% CI 0.58 to 1.21
RD 20 per 1000 fewer, 95% CI 70 fewer to 30 more per 1000
Subgroup analysis for heterogeneity
In the absence of high levels of heterogeneity (I² = 50%), we did not undertake the pre‐specified subgroup analyses (Subgroup analysis and investigation of heterogeneity).
We assessed the certainty of evidence to be 'very low'. We downgraded by two levels for very serious study limitations and by one level for serious imprecision (Table 1).
Invasive infection with the supplemented probiotic micro‐organism
None of the trials reported any episodes of infection with the supplemented probiotic micro‐organisms.
Duration of hospitalisation
Two trials showed a reduced duration of hospitalizations with synbiotics compared to no synbiotics (median difference eight to nine days).
Dilli 2015: median 42 versus 50 days
Guney‐Varal 2017: Median 41 versus 50 days
Three trials did not show a difference.
El 2017: median 34 versus 31 days
Nandhini 2016: mean 8.3 versus 8.4 days
Sreenivasa 2015: mean 13.7 versus 13.6 days
One trial did not report duration of hospitalisation (Underwood 2009).
Neurodevelopmental outcomes
No trial assessed neurodevelopmental outcomes.
Sensitivity analyses of trials at low risk of bias
We undertook sensitivity meta‐analyses of data from the two trials (290 infants) that were assessed as having low overall risk of bias (Dilli 2015; Underwood 2009). These showed similar results compared with the primary analyses.
NEC: RR 0.27, 95% CI 0.11 to 0.69; RD 100 fewer per 1000, 95% CI 170 fewer to 40 fewer; NNTB 10, 95% CI 6 to 25 (Analysis 2.1)
All‐cause mortality prior to hospital discharge: RR 0.25, 95% CI 0.07 to 0.86; RD 60 fewer per 1000, 95% CI 120 fewer to 10 fewer; NNTB 17, 95% CI 8 to 100 (Analysis 2.2)
Late‐onset invasive infection: RR 0.64, 95% CI 0.33 to 1.28; RD 50 fewer per 1000, 95% CI 120 fewer to 30 more (Analysis 2.3)
2.1. Analysis.

Comparison 2: Synbiotics versus control (trials at low risk of bias), Outcome 1: Necrotising enterocolitis
2.2. Analysis.

Comparison 2: Synbiotics versus control (trials at low risk of bias), Outcome 2: All cause mortality
2.3. Analysis.

Comparison 2: Synbiotics versus control (trials at low risk of bias), Outcome 3: Invasive infection
Neither trial assessed neurodevelopmental outcomes.
Discussion
Summary of main results
Meta‐analyses of data from six trials, with 925 participants in total, show that enteral supplementation with synbiotics may reduce the risk of NEC and all‐cause mortality prior to hospital discharge in very preterm or VLBW infants (low certainty evidence). Meta‐analysis of data from five trials, with 707 participants, shows that synbiotics may have little or no impact on the risk of late‐onset invasive infection, but the evidence is of very low certainty. No trial assessed neurodevelopmental outcomes.
Overall completeness and applicability of evidence
These data are likely to be relevant to current practice since all of the included trials were conducted during the past 20 years in neonatal care facilities in a variety of settings (India, Turkey, USA). The risk of developing NEC amongst the control infants was about 8% (compared with 1% in the intervention group), similar to incidence estimates from recent observational studies (Battersby 2018; Horbar 2012). While most participants were very preterm or VLBW infants, few were extremely preterm or ELBW. One trial recruited infants with feeding intolerance (El 2017). Although it is unclear whether feeding intolerance is independently predictive of NEC, findings in this selected population may not be generalisable to all very preterm or VLBA infants. None of the trials specifically excluded infants born 'small for gestational age' or with evidence of absent or reversed end‐diastolic flow velocities in antenatal Doppler studies of the umbilical artery or fetal aorta. Consequently, the applicability of the review findings to the population of preterm infants at highest risk of NEC and associated mortality and morbidity is uncertain.
The type of milk feeds that infants receive might influence the effects of synbiotics supplementation. Evidence exists that human milk rather than formula feeding reduces the risk of NEC (Quigley 2019). Two trials permitted only human milk feeding, while in the other four trials infants could be fed with human milk or formula or both. In the absence of high levels of heterogeneity, we did not undertake any subgroup analyses by type of milk feeding. Any such analysis, furthermore, would need to be interpreted cautiously as the data available were insufficient to define subgroups at an infant (rather than trial) level. The possibility remains that infants who receive human milk as their predominant source of nutrition might not gain added benefit from synbiotics supplementation since their milk is already rich in prebiotic human milk oligosaccharides that enhance probiotic growth and colonisation. Conversely, it is feasible that exclusively formula‐fed infants may experience less benefit than human milk‐fed infants because their diet lacks natural prebiotics that can enhance the growth and colonisation of the exogenous probiotics in the synbiotics supplement. A better understanding of the mechanisms and events occurring at the intestinal epithelial and mucosal level may help to determine which combinations of probiotics and prebiotics optimally supports a putatively beneficial microbiome in very preterm or VLBW infants receiving different types of milk feeds (Nolan 2020).
Quality of the evidence
We used GRADE methods to assess the certainty of the evidence for effects on NEC, all‐cause mortality prior to hospital discharge, late‐onset invasive infection, and neurodevelopmental impairment (Table 1). The certainty of the evidence was downgraded because of methodological weaknesses (risk of bias) in four of the six trials. These included lack of masking measures for parents, caregivers, and clinical assessors that may have introduced performance and detection biases and caused an overestimation of effect estimates, particularly for NEC, given the subjectivity of this diagnosis. Pre‐specified sensitivity analyses of the two trials at overall low risk of bias showed effects consistent with those in the primary meta‐analyses that included all the trials. These analyses, however, included data from only 290 infants (with fewer than 25 episodes of NEC and 15 deaths) and consequently generated imprecise estimates of effect.
The other reason for downgrading the certainty of the evidence was the existence of substantial imprecision in estimates of effect, with meta‐analyses generating 95% CI that included large benefit as well as small or no benefit or harm. Although the total number of participants in the included trials was more than 900, not all trials contributed data to all outcome estimates. Estimates of effect were consequently imprecise, especially for less common outcomes, including all‐cause mortality prior to hospital discharge, where the 95% CI ranged from an NNTB of 8 to 100 infants. Such imprecise estimates of effect are unlikely to meaningfully inform decision‐making in this context.
Potential biases in the review process
An important concern with the review process is the possibility that the findings are subject to publication and other reporting biases (Hopewell 2009). Data from trials which show statistically significant or potentially important effects tend to be more readily available for inclusion in meta‐analyses (Gale 2020). Publication bias, as well as other sources of small‐study bias, is an important contributor to inflation of effect size estimates in meta‐analyses of interventions to improve outcomes in very preterm or VLBW infants (Young 2021). For example, the Cochrane Review of probiotics to prevent NEC in very preterm or VLBW infants shows a large reduction in the risk of NEC, but the funnel plot and regression analysis indicate that publication bias is likely to have inflated the pooled effect size estimate (Sharif 2020).
In this review, we could not assess whether publication bias (or related small study biases) exaggerated the effect size since the meta‐analyses contained insufficient data points (fewer than 10) to make funnel plot inspection and regression analysis valid and reliable; that is, able to distinguish real asymmetry from chance asymmetry (Higgins 2020). Although we attempted to minimise the threat of publication bias by screening the reference lists of included trials and related reviews and searching the proceedings of the major international perinatal conferences to identify trial reports that are not published in full form in academic journals, we cannot be sure that other trials have been undertaken but not reported.
Agreements and disagreements with other studies or reviews
We are not aware of any other systematic reviews that have assessed the trial evidence for synbiotics supplementation in very preterm or VLBW infants. Other Cochrane Reviews address whether probiotics alone or prebiotics alone affect the risk of NEC (Sharif 2020; Sharif 2021a). Meta‐analyses of data from trials of probiotic supplementation suggest a reduction in the risk of NEC and associated morbidity and all‐cause mortality for very preterm or very low birth weight infants. Similar to the findings in this review, however, concerns about trial quality, heterogeneity of interventions, and publication bias, as well as the paucity of data for extremely preterm or ELBW infants, means that these findings are of low certainty, and should be interpreted and applied cautiously.
A large cluster‐randomised controlled trial of a synbiotics supplementation for newborn infants was not eligible for inclusion in this review because participants were not very preterm or VLBW (Panigrahi 2017). This community‐based trial, undertaken in rural India between 2008 to 2016, enrolled newborn infants who were at least 2000 g and at least 35 weeks' gestation at birth (N = 4556). Infant participants were randomly allocated (by village clusters) to receive a synbiotics preparation of Lactobacillus plantarum plus fructo‐oligosaccharides or placebo. Analysis showed a 40% reduction in the primary outcome, which was a composite of “sepsis or death." Necrotising enterocolitis was not an outcome of interest, given its rarity in this population. The trial was of high methodological quality, although substantially more infants in the synbiotics group were lost to follow‐up (outcomes not assessed) than in the placebo group. Given the differences in population, setting, and types of morbidity, the applicability of this trial's findings to very preterm or VLBW infants is limited.
Authors' conclusions
Implications for practice.
The available trial data provide only low‐certainty evidence about the effects of synbiotics on the risk of necrotising enterocolitis (NEC) and associated morbidity and all‐cause mortality for very preterm or very low birth weight infants. Considerable uncertainty exists about how to interpret and apply these trial data because our confidence in the effect estimates is limited; the true effects may be substantially different from these estimates. In addition to concern that effect size estimates are inflated by biases in the existing trials, a major barrier to implementing the findings is that existing analyses are not able to determine reliably the optimal constitution of synbiotics (strains, doses, timing of introduction, duration of use) for routine prophylactic use. A variety of commercially available synbiotics preparations are in use in a minority of neonatal units internationally, but widespread use is limited by availability and regulatory and licensing issues. Furthermore, important safety concerns persist, given that probiotic bacteraemia or fungaemia have been reported in preterm infants receiving probiotic supplements (Bertelli 2015; Esaiassen 2016; Jenke 2012; Zbinden 2015).
Implications for research.
Given the low level of certainty about whether (and which) synbiotics affect important outcomes in very preterm or very low birth weight (VLBW) infants, further assessment in high‐quality randomised, placebo‐controlled trials is needed to inform policy and practice. Such trials are likely to need to recruit at least 2000 participants to reliably detect plausible effects on uncommon outcomes such as NEC and mortality prior to hospital discharge (Gale 2020). Ideally, any planned trials should attempt to ensure that caregivers and assessors are masked to the intervention, as investigation and diagnosis of NEC, late‐onset invasive infection and neurodevelopmental impairment can be subjective, and can be associated with the inter‐rater variation. While it may be appropriate to be broadly inclusive of very preterm and VLBW infant participants, trials should ensure sufficient power to assess effects in extremely preterm or extremely low birth weight (ELBW) infants, infants born 'small for gestational age', or with evidence of absent or reversed end‐diastolic flow velocities in antenatal Doppler studies of the umbilical artery or fetal aorta. Trials should also explore interactions with the type of enteral feed received (Griffiths 2018).
A key concern in planning any trial is choosing the appropriate intervention to assess (Poindexter 2021). Investigators could consider whether trials using synbiotics are merited alongside trials of probiotics and prebiotics alone, including as part of a factorial or an adaptive design (Underwood 2019). Unit of randomization and analysis is another consideration. Although individual infant randomisation is preferred for statistical and analytical reasons, concern exists that cross‐contamination of the probiotic micro‐organisms to infants in the control group would limit the power of the trial to detect an effect. Randomising at the neonatal care centre level (cluster‐randomised controlled trial) obviates this problem, although it inflates the sample size requirement considerably due to the inter‐cluster correlation of outcomes (Gale 2020).
History
Protocol first published: Issue 5, 2021
Acknowledgements
Cochrane Neonatal supported the authors in the development of this review. We thank Colleen Ovelman and Jane Cracknell (former Managing Editors), Michelle Fiander, (Information Specialist and current Managing Editor), and Roger Soll (Coordinating editor).
We thank Melissa Harden (Information Specialist, Centre for Reviews and Dissemination, University of York, UK) for the electronic search strategy and database management.
William McGuire is a member of the Cochrane Neonatal group but was not involved in the editorial process or decision‐making for this review.
Editorial and peer‐review contribution:
Sign‐off Editor (final editorial decision): Robert Boyle, Imperial College London
Managing editor (selected peer reviewers, provided comments, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Lara Kahale, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Hacsi Horváth, Cochrane Copy Edit Support
Peer reviewers (provided comments and recommended an editorial decision): William W. Hay, Jr., University of Colorado, Denver, Colorado, USA; Rosemary D. Higgins, George Mason University, Fairfax, Virginia, USA (clinical review); Mara Marongiu, IRGB‐CNR, Sardinia‐ Italy (consumer review); Sarah Hodgkinson, Evidence Production and Methods Directorate, Cochrane (methods review); Samantha Cox, Information Specialist Cochrane ENT (search review)
Appendices
Appendix 1. Electronic search strategy
Search date: 17 June 2021
Cochrane Register of Controlled Trials (CENTRAL)
#1 [mh Probiotics]
#2 (probiotic*):ti,ab,kw
#3 [mh Bifidobacterium]
#4 (bifidobacterium*):ti,ab,kw
#5 [mh Lactobacillus]
#6 (lactobacill*):ti,ab,kw
#7 ([mh ^Saccharomyces] or [mh ^"Saccharomyces boulardii"] or [mh ^"Saccharomyces cerevisiae"])
#8 [mh ^"Saccharomyces boulardii"]
#9 (Saccharomyces):ti,ab,kw
#10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9
#11 [mh Prebiotics]
#12 (prebiotic*):ti,ab,kw
#13 [mh Oligosaccharides]
#14 (oligosaccharide*):ti,ab,kw
#15 [mh Inulin]
#16 (inulin*):ti,ab,kw
#17 ((fructooligosaccharide* or fructo NEXT oligosaccharide* or FOS or FOSs or galacto NEXT oligosaccharide* or galactooligosaccharide*)):ti,ab,kw
#18 [mh Lactoferrin]
#19 (lactoferrin*):ti,ab,kw
#20 [mh Lactulose] 439
#21 (lactulose*):ti,ab,kw
#22 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 or #20 or #21
#23 [mh Synbiotics]
#24 (synbiotic*):ti,ab,kw
#25 (((probiotic* and prebiotic*) NEAR/4 combin*)):ti,ab,kw
#26 #23 OR #24 OR #25
#27 #10 OR #22 OR #26
#28 [mh "Infant, Newborn"]
#29 [mh "Premature Birth"]
#30 neonat*:ti,ab,kw
#31 neo NEXT nat*:ti,ab,kw
#32 newborn or new NEXT born* or newly NEXT born*:ti,ab,kw
#33 preterm or preterms or pre NEXT term or pre NEXT terms:ti,ab,kw
#34 preemie* or premie or premies:ti,ab,kw
#35 prematur* NEAR/3 (birth* or born or deliver*):ti,ab,kw
#36 low NEAR/3 (birthweight* or birth NEXT weight*):ti,ab,kw
#37 lbw or vlbw or elbw:ti,ab,kw
#38 infan* or baby or babies:ti,ab,kw
#39 #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38
#40 #27 AND #39 in Trials
CINAHL via EBSCO
S35 S31 AND S34
S34 S32 OR S33
S33 TX ( (neonat* or neo nat*) ) OR TX ( (newborn* or new born* or newly born*) ) OR TX ( (preterm or preterms or pre term or pre terms) ) OR TX ( (preemie$ or premie or premies) ) OR TX ( (prematur* N3 (birth* or born or deliver*)) ) OR TX ( (low N3 (birthweight* or birth weight*)) ) OR TX ( (lbw or vlbw or elbw) ) OR TX infan* OR TX ( (baby or babies) )
S32 (MH "Infant, Newborn+")
S31 S22 AND S30
S30 S28 not S29
S29 ( MH animals+ OR MH (animal studies) OR TI (animal model*) ) NOT MH (human) 194,413
S28 S23 OR S24 OR S25 OR S26 OR S27
S27 AB (cluster W3 RCT)
S26 MH placebos OR PT randomized controlled trial OR AB control W5 group OR MH crossover design OR MH comparative studies
S25 MH sample size AND AB ( (assigned OR allocated OR control) )
S24 TI ( (randomised OR randomized) ) OR AB random* OR TI trial
S23 MH Randomized Controlled Trials OR MH double‐blind studies OR MH single‐blind studies OR MH random assignment OR MH pretest‐posttest design OR MH cluster sample
S22 S9 OR S18 OR S21
S21 S19 OR S20
S20 TI ( (probiotic* and prebiotic*) N4 combin* ) OR AB ( (probiotic* and prebiotic*) N4 combin* )
S19 TI Synbiotic* OR AB Synbiotic*
S18 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17
S17 TI Lactoferrin OR AB Lactoferrin
S16 TI fructooligosaccharide* OR AB fructooligosaccharide* OR TI fructo‐oligosaccharide* OR AB fructo‐oligosaccharide* OR TI galactooligosaccharide* OR AB galactooligosaccharide* OR TI galacto‐oligosaccharide* OR AB galacto‐oligosaccharide*
S15 TI Inulin OR AB Inulin
S14 TI lactulose* OR AB lactulose*
S13 TI Oligosaccharides OR AB Oligosaccharides
S12 MH "Oligosaccharides"
S11 TI Prebiotic* OR AB Prebiotic*
S10 MH "Prebiotics"
S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8
S8 TI Saccharomyces OR AB Saccharomyces
S7 MH "Saccharomyces"
S6 TI lactobacillus OR AB lactobacillus
S5 (MH "Lactobacillus") OR (MH "Lactobacillus Acidophilus")
S4 TI bifidobacterium* OR AB bifidobacterium*
S3 MH "Bifidobacterium"
S2 TI probiotic* OR AB probiotic*
S1 MH "Probiotics"
Embase via OVID <1974 to 2021 June 16>
1 Probiotic Agent/
2 probiotic$.ti,ab,kw.
3 exp bifidobacterium/
4 bifidobacterium$.ti,ab,kw.
5 exp lactobacillus/
6 lactobacill$.ti,ab,kw.
7 Saccharomyces/ or Saccharomyces boulardii/ or Saccharomyces cerevisiae/
8 Saccharomyces$.ti,ab,kw.
9 or/1‐8
10 Prebiotic Agent/
11 prebiotic$.ti,ab,kw.
12 exp Oligosaccharide/
13 oligosaccharide$.ti,ab,kw.
14 Galactose oligosaccharide/
15 (galacto‐oligosaccharide$ or galactooligosaccharide$).ti,ab,kw.
16 Fructose Oligosaccharide/
17 (fructooligosaccharide$ or fructo‐oligosaccharide$ or FOS or FOSs).ti,ab,kw.
18 Lactulose/
19 lactulose$.ti,ab,kw.
20 Inulin/
21 inulin$.ti,ab,kw.
22 Lactoferrin/
23 lactoferrin$.ti,ab,kw.
24 or/10‐23
25 Synbiotic Agent/
26 synbiotic$.ti,ab,kw.
27 ((probiotic$ and prebiotic$) adj4 combin$).ti,ab,kw.
28 25 or 26 or 27
29 9 or 24 or 28
30 Newborn/
31 Prematurity/
32 (neonat$ or neo nat$).ti,ab.
33 (newborn$ or new born$ or newly born$).ti,ab.
34 (preterm or preterms or pre term or pre terms).ti,ab.
35 (preemie$ or premie or premies).ti,ab.
36 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab.
37 (low adj3 (birthweight$ or birth weight$)).ti,ab.
38 (lbw or vlbw or elbw).ti,ab.
39 infan$.ti,ab.
40 (baby or babies).ti,ab.
41 or/30‐40
42 Randomized controlled trial/
43 Controlled clinical study/
44 Random$.ti,ab.
45 randomization/
46 intermethod comparison/
47 placebo.ti,ab.
48 (compare or compared or comparison).ti.
49 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
50 (open adj label).ti,ab.
51 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
52 double blind procedure/
53 parallel group$1.ti,ab.
54 (crossover or cross over).ti,ab.
55 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
56 (assigned or allocated).ti,ab.
57 (controlled adj7 (study or design or trial)).ti,ab.
58 (volunteer or volunteers).ti,ab.
59 human experiment/
60 trial.ti.
61 or/42‐60
62 (random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)
63 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)
64 (((case adj control$) and random$) not randomi?ed controlled).ti,ab.
65 (Systematic review not (trial or study)).ti.
66 (nonrandom$ not random$).ti,ab.
67 "Random field$".ti,ab.
68 (random cluster adj3 sampl$).ti,ab.
69 (review.ab. and review.pt.) not trial.ti.
70 "we searched".ab. and (review.ti. or review.pt.)
71 "update review".ab.
72 (databases adj4 searched).ab.
73 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/
74 Animal experiment/ not (human experiment/ or human/)
75 or/62‐74
76 61 not 75
77 29 and 41 and 76
Maternity & Infant Care Database (MIDIRS) via OVID <1971 to May 11, 2021>
1 probiotic$.ti,ab,de.
2 bifidobacterium$.ti,ab,de.
3 lactobacill$.ti,ab,de.
4 Saccharomyces$.ti,ab,de.
5 or/1‐4
6 prebiotic$.ti,ab,de.
7 oligosaccharide$.ti,ab,de.
8 inulin$.ti,ab,de.
9 (fructooligosaccharide$ or fructo‐oligosaccharide$ or FOS or FOSs).ti,ab,de.
10 (galactooligosaccharide$ or galacto‐oligosaccharide$).ti,ab,de.
11 lactoferrin$.ti,ab,de.
12 lactulose$.ti,ab,de.
13 or/6‐12
14 synbiotic$.ti,ab,de.
15 ((probiotic$ and prebiotic$) adj4 combin$).ti,ab,de.
16 14 or 15
17 5 or 13 or 16
18 (neonat$ or neo nat$).ti,ab.
19 (newborn$ or new born$ or newly born$).ti,ab.
20 (preterm or preterms or pre term or pre terms).ti,ab.
21 (preemie$ or premie or premies).ti,ab.
22 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab.
23 (low adj3 (birthweight$ or birth weight$)).ti,ab.
24 (lbw or vlbw or elbw).ti,ab.
25 infan$.ti,ab.
26 (baby or babies).ti,ab.
27 or/18‐26
28 17 and 27
29 limit 28 to randomised controlled trial
Ovid MEDLINE(R) ALL <1946 to June 16, 2021>
1 Probiotics/
2 probiotic$.ti,ab,kw.
3 exp bifidobacterium/
4 bifidobacterium$.ti,ab,kw.
5 exp lactobacillus/
6 lactobacill$.ti,ab,kw.
7 Saccharomyces/ or Saccharomyces boulardii/ or Saccharomyces cerevisiae/
8 Saccharomyces$.ti,ab,kw.
9 or/1‐8
10 Prebiotics/
11 prebiotic$.ti,ab,kw.
12 Oligosaccharides/
13 oligosaccharide$.ti,ab,kw.
14 (galactooligosaccharides or galacto‐oligosaccharides).ti,ab,kw.
15 (fructooligosaccharide$ or fructo‐oligosaccharide$ or FOS or FOSs).ti,ab,kw.
16 Lactulose/
17 lactulose$.ti,ab,kw.
18 Inulin/
19 inulin$.ti,ab,kw.
20 Lactoferrin/
21 lactoferrin$.ti,ab,kw.
22 or/10‐21
23 Synbiotics/
24 synbiotic$.ti,ab,kw.
25 ((probiotic$ and prebiotic$) adj4 combin$).ti,ab,kw. (374)
26 or/23‐25
27 9 or 22 or 26
28 exp Infant, Newborn/
29 Premature Birth/
30 (neonat$ or neo nat$).ti,ab.
31 (newborn$ or new born$ or newly born$).ti,ab.
32 (preterm or preterms or pre term or pre terms).ti,ab.
33 (preemie$ or premie or premies).ti,ab.
34 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab.
35 (low adj3 (birthweight$ or birth weight$)).ti,ab.
36 (lbw or vlbw or elbw).ti,ab.
37 infan$.ti,ab.
38 (baby or babies).ti,ab.
39 or/28‐38
40 randomized controlled trial.pt.
41 controlled clinical trial.pt.
42 randomized.ab.
43 placebo.ab.
44 drug therapy.fs.
45 randomly.ab.
46 trial.ab.
47 groups.ab.
48 or/40‐47
49 exp animals/ not humans.sh.
50 48 not 49
51 27 and 39 and 50
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Clinical Trials.gov (via https://clinicaltrials.gov/)
infant OR baby OR babies OR premature or neonate OR new born OR preterm OR low birth weight OR low birthweight OR LBW OR VLBW or ELBW
AND
fructooligosaccharide OR fructo‐oligosaccharide$ OR FOS OR Lactulose OR Inulin OR Lactoferrin OR Synbiotics
Appendix 2. 'Risk of bias' tool
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Appendix 3. GRADE
GRADE considers that evidence from randomised controlled trials is of high certainty, but that assessment may be downgraded based on consideration of any of five areas.
Design (risk of bias).
Consistency across studies.
Directness of evidence.
Precision of estimates.
Presence of publication bias.
Usually, the certainty rating will be downgraded by one level for each factor, up to a maximum of three levels for all factors. If there are very severe problems for any one factor (e.g. when assessing limitations in design and implementation, all trials were unconcealed, unmasked, and lost over 50% of their participants to follow‐up), trial evidence may be downgraded by two levels due to that factor alone.
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Data and analyses
Comparison 1. Synbiotics versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Necrotising enterocolitis | 6 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.09, 0.40] |
| 1.2 All‐cause mortality | 6 | 925 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.33, 0.85] |
| 1.3 Invasive infection | 5 | 707 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.58, 1.21] |
Comparison 2. Synbiotics versus control (trials at low risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Necrotising enterocolitis | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.11, 0.69] |
| 2.2 All cause mortality | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.07, 0.86] |
| 2.3 Invasive infection | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.33, 1.28] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dilli 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 200 very preterm or VLBW infants | |
| Interventions | Synbiotics (N = 100): Bifidobacterium lactis (5 x 109 colony‐forming units (cfu)) plus inulin (900 mg) added to human milk or formula once daily for 8 weeks (or until hospital discharge) Control (N = 100): maltodextrin powder placebo |
|
| Outcomes |
|
|
| Notes | Setting: Turkey (5 centres: 2011 to 14) Funding: not stated NB. This was a 4‐arm RCT‐ 2 other groups were probiotic only (N = 100) and prebiotic only (N = 100) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete reporting |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
El 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 98 preterm (< 35 weeks) or low birth weight (< 2500 g) infants with feed intolerance | |
| Interventions | Synbiotics (N = 52): Bifidobacterium lactis (5 × 109 cfu) plus inulin (900 mg) diluted in distilled water and given 3 times a day with human milk or formula for 10 days. Control (N = 46): no synbiotic supplement |
|
| Outcomes |
|
|
| Notes | Turkey (single centre: 2010 to 13) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on randomization method |
| Allocation concealment (selection bias) | Low risk | Quote: “by balanced blocks using sealed envelopes” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete reporting for primary outcomes |
| Selective reporting (reporting bias) | Low risk | No access to protocol but unlikely |
| Other bias | High risk | Differences in baseline birth weight (1270 versus 1410 g), no difference in gestational age at birth (31 versus 31 weeks) |
Guney‐Varal 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 110 preterm (< 33 weeks) or VLBW infants | |
| Interventions | Synbiotics (N = 76): Lactobacillus rhamnosus (4 × 10⁸ cfu) + L. casei (8 × 10⁸ cfu) + L. plantorum (4 x10⁸ cfu) + Bifidobacterium animalis (4 × 10⁸ cfu) plus FOS (383 mg) and GOS (100 mg) added to human milk or formula daily until hospital discharge Control (N = 43): no synbiotic supplement |
|
| Outcomes |
|
|
| Notes | Turkey (single centre: 2013 to 2016) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote. “Alternate randomization” (quasi‐randomised) |
| Allocation concealment (selection bias) | High risk | Quote: “Alternate randomization” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete reporting for primary outcomes |
| Selective reporting (reporting bias) | Low risk | No access to protocol but unlikely |
| Other bias | High risk | Differences in baseline birth weight (1728 versus 1228 g), but no difference in gestational age at birth (29.7 versus 29.3 weeks) |
Nandhini 2016.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 220 preterms (28 to 34 weeks) infants (most participants very preterm or VLBW) | |
| Interventions | Synbiotics (N = 110): Lactobacillus casei (3 × 10⁸ cfu) + L. rhamnosus (4 x10⁸ cfu) + L. plantaris (3 x10⁸ cfu) + L. bulgaricus (3 x10⁸ cfu) + Bifidobacterium infantis (3 × 10⁸ cfu) + B. breve (3 x10⁸ cfu) + B. longum (4 × 10⁸ cfu) plus FOS (100 mg) added to maternal milk twice daily for 7 days Control (N= 43): no synbiotic supplement |
|
| Outcomes |
|
|
| Notes | India (single centre: study dates not stated) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete reporting for primary outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in protocol reported |
| Other bias | Low risk | No evidence baseline imbalance |
Sreenivasa 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 200 preterm (< 34 weeks) infants (most participants very preterm or VLBW) | |
| Interventions | Synbiotics (N = 100): Lactobacillus acidophilus (3 × 10⁸ cfu) +Bifidobacterium longum (1.5 × 10⁸ cfu) +B. bifidum (1.5 × 10⁸ cfu) +Streptococcus thermophiles (1.5 × 10⁸ cfu) plus FOS (100 mg) added to maternal milk twice daily from onset of enteral feeding until infant accepted full enteral feeds Control (N= 100): no synbiotic supplement |
|
| Outcomes |
|
|
| Notes | India (single centre: 2012 to 2014) Funding: (quote:) “No funding sources” Investigators contacted for unpublished data (June 2021) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on random generation (quote: “simple random sampling”) |
| Allocation concealment (selection bias) | Unclear risk | No details on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete reporting for primary outcomes |
| Selective reporting (reporting bias) | Low risk | No access to protocol but unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
Underwood 2009.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 90 preterm (< 35 weeks) or low birth weight (750 to 2000 g) infants (most participants very preterm or VLBW) | |
| Interventions | [3‐arm trial] Synbiotics (N = 61): (i) Lactobacillus rhamnosus (5 × 10⁸ cfu) OR (ii) L. acidophilus (1 × 1010 cfu) + Bifidobacterium longum (5 × 10⁸ cfu) + B. bifidum (cfu) +B. infantis (5 × 10⁸ cfu) plus inulin (dose not stated) given twice daily dissolved in saline for 28 days or until hospital discharge Control (N = 29): placebo (1:30 dilute preparation of elemental formula) Participating infants were fed with human milk or formula (or both). |
|
| Outcomes |
|
|
| Notes | USA (single centre: 2004‐ 06) Funding: NIH UC Davis K30 Program (#UL1RR024146, MAU) and the Children's Miracle network (CLB). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐assigned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete reporting |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
cfu: colony‐forming units;FOS: fructo‐oligosaccharides;GOS: galacto‐oligosaccharides; NEC: necrotising enterocolitis; NIH:National Institutes of Health; RCT: randomized controlled trial; VLBW: very low birth weight
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bering 2018 | Not an RCT |
| Dasopoulou 2015 | RCT of prebiotics |
| Dilli 2013 | Participants were not very preterm or VLBW |
| Panigrahi 2008 | Participants were not very preterm or VLBW |
| Serce Pehlevan 2020 | RCT ‐ the intervention group also received lactoferrin (co‐intervention) |
| Underwood 2014 | RCT comparing supplementation of formula with either prebiotic oligosaccharides or (quote:) “a pooled concentrated donor human milk product” |
| Vakiliamini 2020 | Most participants were not very preterm or VLBW (subgroup data not available) |
RCT: randomized controlled trial; VLBW: very low birth weight
Differences between protocol and review
Title changed from "Synbiotics for preventing necrotising enterocolitis in preterm infants" to "Synbiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants".
The protocol specified the population of interest as very preterm and VLBW infants in order to enhance applicability to those infants at high risk of developing NEC and associated complications. Some included trials included infants born up to 34 to 35 weeks' gestation. We included these trials provided most participants were very preterm and VLBW infants.
Contributions of authors
All authors contributed equally to the development and completion of the review.
Sources of support
Internal sources
-
Centre for Reviews and Dissemination, University of York, UK
Logistical support.
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
National Institute for Health Research (NIHR), UK
This report is independent research funded by a UK NIHR Cochrane Programme Grant (133131). The views expressed in this publication are those of the review authors and are not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
Declarations of interest
SS has declared that she has no conflict of interest.
PTH has declared that he has no conflict of interest.
SJO has declared that he has no conflict of interest.
WM is a co‐ordinating editor of Cochrane Neonatal but was not involved in the editorial process or decision‐making for this review.
New
References
References to studies included in this review
Dilli 2015 {published data only}
- Aydin B, Dilli D, Erol S, Sorguc NH, Beken S, Ilarslan NC, et al.The effects of synbiotic use on morbidity and mortality in premature infants: a prospective randomized controlled trial. In: Archives of Disease in Childhood. Vol. 97. 2012:A462. [DOI: 10.1136/archdischild-2012-302724.1634] [DOI]
- Dilli D, Aydin B, Fettah ND, Ozyazıcı E, Beken S, Zenciroglu A, et al.The propre-save study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis on very low birth weight infants. Journal of Pediatrics 2015;166:545-51. [DOI: 10.1016/j.jpeds.2014.12.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
El 2017 {published data only}
- El Ç, Satar M, Yıldızdaş H, Özlü F, Asker H.Evaluation of influence of Bifidobacterium lactis and Hindiba inulin on feeding intolerance and weight gain in premature babies [Prematüre bebeklerde beslenme intoleransında Bifidobakteriyum laktis ve Hindiba inülinin beslenme intoleransı ve ağırlık artışı üzerine etkilerinin değerlendirilmesi]. Cukurova Medical Journal 2017;42:419-26. [DOI: 10.17826/cutf.323371] [DOI] [Google Scholar]
- El C, Satar M, Yildizdas H, Özlü F, Asker HS.Evaluation of the influence of Bifidobakterium lactis 2011 and hindiba inulin on feeding intolerance and necrotising enterocolitis in premature babies. Archives of Disease in Childhood 2014;99(Suppl 2):A110. [DOI: 10.1136/archdischild-2014-307384.292] [DOI] [Google Scholar]
Guney‐Varal 2017 {published data only}
- Güney-Varal İ, Köksal N, Özkan H, Bağcı O, Doğan P.The effect of early administration of combined multi-strain and multi-species probiotics on gastrointestinal morbidities and mortality in preterm infants: a randomized controlled trial in a tertiary care unit. Turkish Journal of Pediatrics 2017;59(1):13-9. [DOI: 10.24953/turkjped.2017.01.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Guney Varal I, Koksal N, Ozkan H, Bagci O, Dogan P.Potential use of multi-strain synbiotics for improving postnatal head circumference. Pakastan Journal of Medical Sciences 2018;34(6):1502-6. [DOI: 10.12669/pjms.346.16107] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köksal N, Varal İ, Özkan H, Bagcı O, Doğan P.Effect of probiotic support on feeding intolerance and mortality at preterm infantshttps://doi.org/10.1515/jpm-2015-2003. In: Journal of Perinatal Medicine. Vol. 43. 2015:P-0612. [CENTRAL: CN-01198916] [DOI: 10.1515/jpm-2015-2003] [DOI]
Nandhini 2016 {published data only}
- Nandhini LP, Biswal N, Adhisivam B, Mandal J, Bhat BV, Mathai B.Synbiotics for decreasing incidence of necrotizing enterocolitis among preterm neonates - a randomized controlled trial. Journal of Maternal-Fetal & Neonatal Medicine 2016;29(5):821-5. [DOI: 10.3109/14767058.2015.1019854] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sreenivasa 2015 {published data only}
- Sreenivasa B, Sunil Kumar P, Suresh Babu MT, Ragavendra K.Role of synbiotics in the prevention of necrotizing enterocolitis in preterm neonates: a randomized controlled trial. International Journal of Contemporary Pediatrics 2015;2(2):127-30. [DOI: 10.1097/INF.0b013e3182620e52] [PMID: ] [DOI] [PubMed] [Google Scholar]
Underwood 2009 {published data only}
- Underwood MA, Salzman NH, Bennett SH, Barman M, Mills D, Marcobal A, et al.A randomized placebo-controlled comparison of two prebiotic/probiotic combinations in preterm infants: impact on weight gain, intestinal microbiota, and fecal short chain fatty acids. Journal of Pediatric Gastroenterology and Nutrition 2009;48(2):216–25. [DOI: 10.1097/MPG.0b013e31818de195] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bering 2018 {published data only}
- Bering SB.Human milk oligosaccharides to prevent gut dysfunction and necrotizing enterocolitis in preterm neonates. Nutrients 2018;10(10):1461. [DOI: 10.3390/nu10101461] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dasopoulou 2015 {published data only}
- Dasopoulou M, Briana DD, Boutsikou T, Karakasidou E, Roma E, Costalos C, et al.Motilin and gastrin secretion and lipid profile in preterm neonates following prebiotics supplementation: a double-blind randomized controlled study. Journal of Parenteral and Enteral Nutrition 2015;39(3):359-68. [DOI: 10.1177/0148607113510182] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dilli 2013 {published data only}
- Dilli D, Aydin B, Zenciroğlu A, Özyazici E, Beken S, Okumuş N.Treatment outcomes of infants with cyanotic congenital heart disease treated with synbiotics. Pediatrics 2013;132(4):e932-8 . [DOI: 10.1542/peds.2013-1262] [DOI] [PubMed] [Google Scholar]
Panigrahi 2008 {published data only}
- Panigrahi P, Parida S, Pradhan L, Mohapatra SS, Misra PR, Johnson JA, et al.Long-term colonization of a Lactobacillus plantarum synbiotic preparation in the neonatal gut. Journal of Pediatric Gastroenterolpgy and Nutrition 2008;47(1):45-53. [DOI: 10.1097/MPG.0b013e31815a5f2c] [PMID: ] [DOI] [PubMed] [Google Scholar]
Serce Pehlevan 2020 {published data only}
- Serce Pehlevan O, Benzer D, Gursoy T, Karatekin G, Ovali F.Synbiotics use for preventing sepsis and necrotizing enterocolitis in very low birth weight neonates: a randomized controlled trial. Clinical and Experimental Pediatrics 2020;63(6):226-31. [DOI: 10.3345/cep.2019.00381] [PMID: 32023397] [DOI] [PMC free article] [PubMed] [Google Scholar]
Underwood 2014 {published data only}
- Underwood MA, Kalanetra KM, Bokulich NA, Mirmiran M, Barile D, Tancredi DJ, et al.Prebiotic oligosaccharides in premature infants. Journal of Pediatric Gastroenterology and Nutrition 2014;58(3):352-60. [DOI: 10.1097/MPG.0000000000000211] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vakiliamini 2020 {published data only}
- Vakiliamini M, Babaei H, Mohammadi M, Habibi R, Motamed H.Intestinal colonization rate of Candida albicans among low birth weight neonates after using oral synbiotic supplementation: a randomized placebo-controlled trial. Iranian Journal of Neonatology 2020;11(3):51-6. [DOI: 10.22038/IJN.2020.40131.1651] [DOI] [Google Scholar]
Additional references
Aakko 2017
- Aakko J, Kumar H, Rautava S, Wise A, Autran C, Bode L, et al.Human milk oligosaccharide categories define the microbiota composition in human colostrum. Beneficial Microbes 2017;8(4):563-7. [DOI: 10.3920/BM2016.0185]] [PMID: ] [DOI] [PubMed] [Google Scholar]
Alcon‐Giner 2020
- Alcon-Giner C, Dalby MJ, Caim S, Ketskemety J, Shaw A, Sim K, et al.Microbiota supplementation with Bifidobacterium and Lactobacillus modifies the preterm infant gut microbiota and metabolome: an observational study. Cell Reports Medicine 2020;1(5):100077. [DOI: 10.1016/j.xcrm.2020.100077] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Autran 2018
- Autran CA, Kellman BP, Kim JH, Asztalos E, BloodAB, Spence EC, et al.Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 2018;67(6):1064-70. [DOI: 10.1136/gutjnl-2016-312819] [PMID: ] [DOI] [PubMed] [Google Scholar]
Battersby 2018
- Battersby C, Santhalingam T, Costeloe K, Modi N.Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Archives of Disease in Childhood. Fetal and Neonatal Edition 2018;103(2):F182-9. [DOI: 10.1136/archdischild-2017-313880] [PMID: ] [DOI] [PubMed] [Google Scholar]
Berrington 2012
- Berrington JE, Hearn RI, Bythell M, Wright C, Embleton ND.Deaths in preterm infants: changing pathology over 2 decades. Journal of Pediatrics 2012;160(1):49-53.e1. [DOI: 10.1016/j.jpeds.2011.06.046] [PMID: ] [DOI] [PubMed] [Google Scholar]
Berrington 2019
- Berrington JE, Zalewski S.The future of probiotics in the preterm infant. Early Human Development 2019;135:75-81. [DOI: 10.1016/j.earlhumdev.2019.05.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bertelli 2015
- Bertelli C, Pillonel T, Torregrossa A, Prod'hom G, Fischer CJ, Greub G, et al.Bifidobacterium longum bacteremia in preterm infants receiving probiotics. Clinical Infectious Diseases 2015;60(6):924-7. [DOI: 10.1093/cid/ciu946] [PMID: ] [DOI] [PubMed] [Google Scholar]
Boehm 2008
- Boehm G, Moro G.Structural and functional aspects of prebiotics used in infant nutrition. Journal of Nutrition 2008;138(9):1818S-28S. [DOI: 10.1093/jn/138.9.1818S] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chi 2019
- Chi C, Buys N, Li C, Sun J, Yin C.Effects of prebiotics on sepsis, necrotizing enterocolitis, mortality, feeding intolerance, time to full enteral feeding, length of hospital stay, and stool frequency in preterm infants: a meta-analysis. European Journal of Clinical Nutrition 2019;73(5):657-70. [DOI: 10.1038/s41430-018-0377-6] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffield 2019
- Duffield SD, Clarke P.Current use of probiotics to prevent necrotising enterocolitis. Archives of Disease in Childhood. Fetal and Neonatal Edition 2019;104(2):F228. [DOI: 10.1136/archdischild-2018-316199] [PMID: ] [DOI] [PubMed] [Google Scholar]
Eaton 2017
- Eaton S, Rees CM, Hall NJ.Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology 2017;111(4):423-30. [DOI: 10.1159/000458462] [PMID: ] [DOI] [PubMed] [Google Scholar]
Embleton 2017
- Embleton ND, Berrington JE, Dorling J, Ewer AK, Juszczak E, Kirby JA, et al.Mechanisms affecting the gut of preterm infants in enteral feeding trials. Frontiers in Nutrition 2017;4:14. [DOI: 10.3389/fnut.2017.00014] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Esaiassen 2016
- Esaiassen E, Cavanagh P, Hjerde E, Simonsen GS, Stoen R, Klingenberg C.Bifidobacterium longum subspecies infantis bacteremia in 3 extremely preterm infants receiving probiotics. Emerging Infectious Diseases 2016;22(9):1664-6. [DOI: 10.3201/eid2209.160033] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Esaiassen 2018
- Esaiassen E, Hjerde E, Cavanagh JP, Pedersen T, Andresen JH, Rettedal SI, et al.Effects of probiotic supplementation on the gut microbiota and antibiotic resistome development in preterm infants. Frontiers in Pediatrics 2018;6:347. [DOI: 10.3389/fped.2018.00347] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleming 2019
- Fleming PF, Berrington JE, Jacobs SE.Addressing safety concerns of probiotic use in preterm babies. Early Human Development 2019;135:72-4. [DOI: 10.1016/j.earlhumdev.2019.05.016] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gale 2020
- Gale C, McGuire W, Juszczak E.Randomised controlled trials for informing perinatal care. Neonatology 2020;117(1):8-14. [DOI: 10.1159/000499881] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT.Version accessed 11th July 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Granger 2020
- Granger CL, Embleton ND, Palmer JM, Lamb CA, Berrington JE, Stewart CJ.Maternal breast milk, infant gut microbiome, and the impact on preterm infant health. Acta Paediatrica 2020;110(2):450-7. [DOI: 10.1111/apa.15534] [PMID: ] [DOI] [PubMed] [Google Scholar]
Griffiths 2018
- Griffiths J, Jenkins P, Vargova M, Bowler U, Juszczak E, King A, et al.Enteral lactoferrin to prevent infection for very preterm infants: the ELFIN RCT.. Health Technol Assessment 2018;22(74):1-60. [DOI: 10.3310/hta22740] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA.A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. [DOI: 10.1002/sim.2380] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hickey 2018
- Hickey M, Georgieff M, Ramel S.Neurodevelopmental outcomes following necrotizing enterocolitis. Seminars in Fetal and Neonatal Medicine 2018;23(6):426-32. [DOI: 10.1016/j.siny.2018.08.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group.Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors).Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Hopewell 2009
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K.Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: MR000006. [DOI: 10.1002/14651858.MR000006.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horbar 2012
- Horbar JH, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al.Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 2012;129(6):1019-26. [DOI: 10.1542/peds.2011-3028] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jenke 2012
- Jenke A, Ruf EM, Hoppe T, Heldmann M, Wirth S.Bifidobacterium septicaemia in an extremely low-birthweight infant under probiotic therapy. Archives of Disease in Childhood-Fetal and Neonatal Edition 2012;97(3):F217-8. [DOI: 10.1136/archdischild-2011-300838] [PMID: ] [DOI] [PubMed] [Google Scholar]
Johnson‐Henry 2016
- Johnson-Henry KC, Abrahamsson TR, Wu RY, Sherman PM.Probiotics, prebiotics, and synbiotics for the prevention of necrotizing enterocolitis. Advances in Nutrition 2016;7(5):928-37. [DOI: 10.3945/an.116.012237] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jost 2015
- Jost T, Lacroix C, Braegger C, Chassard C.Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutrition Reviews 2015;73(7):426-37. [DOI: 10.1093/nutrit/nuu016] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kapiki 2007
- Kapiki A, Costalos C, Oikonomidou C, Triantafyllidou A, Loukatou E, Pertrohilou V.The effect of a fructo-oligosaccharide supplemented formula on gut flora of preterm infants. Early Human Development 2007;83:335-9. [DOI: 10.1016/j.earlhumdev.2006.07.003]] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lyons 2020
- Lyons KE, Ryan CA, Dempsey EM, Ross RP, Stanton C.Breast milk, a source of beneficial microbes and associated benefits for infant health. Nutrients 2020;12(4):1039. [DOI: 10.3390/nu12041039] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mara 2018
- Mara MA, Good M, Weitkamp JH.Innate and adaptive immunity in necrotizing enterocolitis. Seminars in Fetal Neonatal Medicine 2018;23(6):394-9. [DOI: 10.1016/j.siny.2018.08.002] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Masi 2019
- Masi AC, Stewart CJ.The role of the preterm intestinal microbiome in sepsis and necrotising enterocolitis. Early Human Development 2019;138:104854. [DOI: 10.1016/j.earlhumdev.2019.104854] [PMID: ] [DOI] [PubMed] [Google Scholar]
Masi 2020
- Masi AC, Embleton ND, Lamb CA, Young G, Granger CL, Najera J, et al.Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut [Epub ahead of print] 2020. [DOI: 10.1136/gutjnl-2020-322771] [PMID: ] [DOI] [PMC free article] [PubMed]
Nolan 2020
- Nolan LS, Rimer JM, Good M.The role of human milk oligosaccharides and probiotics on the neonatal microbiome and risk of necrotizing enterocolitis: a narrative review. Nutrients 2020;12(10):3052. [DOI: 10.3390/nu12103052]] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olm 2019
- Olm MR, Bhattacharya N, Crits-Christoph A, Firek BA, Baker R, Song YS, et al.Necrotizing enterocolitis is preceded by increased gut bacterial replication, Klebsiella, and fimbriae-encoding bacteria. Science Advances 2019;5(12):eaax5727. [DOI: 10.1126/sciadv.aax5727] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Panigrahi 2017
- Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS, et al.A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 2017;548(7668):407-12. [DOI: 10.1038/nature23480] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pell 2019
- Pell LG, Loutet MG, Roth DE, Sherman PM.Arguments against routine administration of probiotics for NEC prevention. Current Opinions in Pediatrics 2019;31(2):195-201. [DOI: 10.1097/MOP.0000000000000730] [PMID: 30624281] [DOI] [PubMed] [Google Scholar]
Poindexter 2021
- Poindexter B, Committee on Fetus and Newborn.Use of probiotics in preterm infants. Pediatrcs 2021;147(6):e2021051485. [DOI: 10.1542/peds.2021-051485] [PMID: ] [DOI] [PubMed] [Google Scholar]
Quigley 2019
- Quigley M, Embleton ND, McGuire W.Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD002971. [DOI: 10.1002/14651858.CD002971.pub5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samuels 2017
- Samuels N, Van de Graaf RA, Jonge RC, Reiss IK, Vermeulen MJ.Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatrics 2017;17(1):105. [DOI: 10.1186/s12887-017-0847-3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s).Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sharif 2020
- Sharif S, Meader N, Oddie SJ, Rojas-Reyes MX, McGuire W.Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD005496. [DOI: 10.1002/14651858.CD005496.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharif 2021a
- Sharif S, Oddie SJ, Heath PT, McGuire W.Prebiotics for the prevention of necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database of Systematic Reviews 2021, Issue 7. Art. No: CD015133. [DOI: 10.1002/14651858.CD015133] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smilowitz 2013
- Smilowitz JT, O'Sullivan A, Barile D, German JB, Lönnerdal B, Slupsky CM.The human milk metabolome reveals diverse oligosaccharide profiles. Journal of Nutrition 2013;143(11):1709-18. [DOI: 10.3945/jn.113.178772] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Srinivasjois 2013
- Srinivasjois R, Rao S, Patole S.Prebiotic supplementation in preterm neonates: updated systematic review and meta-analysis of randomised controlled trials. Clinical Nutrition 2013;32(6):958-65. [DOI: 10.1016/j.clnu.2013.05.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stewart 2012
- Stewart CJ, Marrs EC, Magorrian S, Nelson A, Lanyon C, Perry JD, et al.The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta Paediatrica 2012;101(11):1121-7. [DOI: 10.1111/j.1651-2227.2012.02801.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stewart 2016
- Stewart CJ, Embleton ND, Marrs EC, Smith DP, Nelson A, Abdulkadir B, et al.Temporal bacterial and metabolic development of the preterm gut reveals specific signatures in health and disease. Microbiome 2016;4(1):67. [DOI: 10.1186/s40168-016-0216-8] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2017
- Stewart CJ, Embleton ND, Marrs EC, Smith DP, Fofanova T, Nelson A, et al.Longitudinal development of the gut microbiome and metabolome in preterm neonates with late onset sepsis and healthy controls. Microbiome 2017;5(1):75. [DOI: 10.1186/s40168-017-0295-1] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Underwood 2015
- Underwood MA, Gaerlan S, De Leoz ML, Dimapasoc L, Kalanetra KM, Lemay DG, et al.Human milk oligosaccharides in premature infants: absorption, excretion, and influence on the intestinal microbiota. Pediatric Research 2015;78(6):670-7. [DOI: 10.1038/pr.2015.162] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Underwood 2019
- Underwood MA.Probiotics and human milk oligosaccharides in premature infants. Neoreviews 2019;20(1):e1-1. [DOI: 10.1542/neo.20-1-e1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Veereman‐Wauters 2011
- Veereman-Wauters G, Staelens S, Van de Broek H, Plaskie K, Wesling F, Roger LC, et al.Physiological and bifidogenic effects of prebiotic supplements in infant formulae. Journal of Pediatric Gastroenterology and Nutrition 2011;52(6):763-71. [DOI: 10.1097/MPG.0b013e3182139f39] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vermeulen 2020
- Vermeulen MJ, Luijendijk A, Van Toledo L, Van Kaam AH, Reiss IK.Quality of probiotic products for preterm infants: contamination and missing strains. Acta Paediatrica 2020;109(2):276-9. [DOI: 10.1111/apa.14976] [PMID: ] [DOI] [PubMed] [Google Scholar]
VON 2020
- Vermont Oxford Network.Manual of Operations. Data Definitions & Infant Data Booklets 2020;Part 2(Release 25.0).
Walsh 2019
- Walsh V, McGuire W.Immunonutrition for preterm infants. Neonatology 2019;115(4):398-405. [DOI: 10.1159/000497332] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 2021
- Walsh V, McGuire W, Halliday HL.Evaluation of the quality of perinatal trials: making the GRADE. Neonatology 2021;118(3):378-83. [DOI: 10.1159/000516239] [PMID: ] [DOI] [PubMed] [Google Scholar]
Warner 2016
- Warner BB, Deych E, Zhou Y, Hall-Moore C, Weinstock GM, Sodergren E, et al.Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 2016;387(10031):1928-36. [DOI: 10.1016/S0140-6736(16)00081-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2021
- Young L, McGuire W, Fowlie PW.Commentary on "enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants". Neonatology 2021;118(2):139-42. [DOI: 10.1159/000512988] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zbinden 2015
- Zbinden A, Zbinden R, Berger C, Arlettaz R.Case series of Bifidobacterium longum bacteremia in three preterm infants on probiotic therapy. Neonatology 2015;107(1):56-9. [DOI: 10.1159/000367985] [PMID: 25402825] [DOI] [PubMed] [Google Scholar]
Zmora 2018
- Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, et al.Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018;174(6):1388-405. [DOI: 10.1016/j.cell.2018.08.041] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sharif 2021b
- Sharif S, Heath PT, Oddie SJ, McGuire W.Synbiotics for preventing necrotising enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD014067. [DOI: 10.1002/14651858.CD014067] [DOI] [PMC free article] [PubMed] [Google Scholar]


