Abstract
Background:
Recent data have demonstrated that overall mortality and adverse events are not significantly different for primary (PR) and staged repair (SR) approaches to management of neonates with symptomatic tetralogy of Fallot (sTOF). Cost data can be used to compare the relative value (cost for similar outcomes) of these approaches and are a potentially more sensitive measure of morbidity.
Objectives:
To compare the economic costs associated with PR and SR in neonates with sTOF.
Methods:
Data from a multicenter retrospective cohort study of neonates with sTOF were merged with administrative data to compare total costs and cost-per-day-alive over the first 18 months-of-life in a propensity-score-adjusted analysis. A secondary analysis evaluated differences in department-level costs.
Results:
In total, 324 subjects from 6 centers from 1/2011 – 11/2017 were studied (40% PR). 18-month cumulative mortality (p=0.18), procedural complications (p=0.10), hospital complications (p=0.94), and reinterventions (p=0.22) did not differ between PR and SR. Total 18-month costs for PR (median: $179,494, IQR:121,760–310,721) were less than for SR (median: $222,799, IQR:167,581–327,113, p<0.001). Cost-per-day-alive (p=0.005) and department-level costs were also all lower for PR. In propensity score adjusted analyses, PR was associated with lower total cost (cost ratio: 0.73, p<0.001) and lower department-level costs.
Conclusion:
In this multicenter study of neonates with sTOF, PR was associated with lower costs. Given similar overall mortality between treatment strategies, this finding suggests that PR provides superior value.
Keywords: Pediatrics, heart catheterization, economic analysis, heart surgery
Condensed abstract:
In a multicenter, retrospective cohort study combining data from the Congenital Cardiac Research Collaborative Tetralogy of Fallot study and the Pediatric Health Information Systems database, total 18-month costs and cost-per-day-alive were compared for 324 infants undergoing primary (PR; 40%) or staged repair (SR) at 6 centers from 1/2011–11/2017. Eighteen-month mortality (p=0.18) did not differ significantly. In propensity score adjusted analyses, PR was associated with lower total cost (cost ratio: 0.73, p<0.001) and lower department-level cost. We conclude that PR provides superior value to SR in symptomatic neonates with tetralogy of Fallot.
INTRODUCTION:
Infants with tetralogy of Fallot (TOF) typically undergo operative correction in their first year of life. Elective anatomic repair outside of early infancy is associated with a low risk of mortality(1–3). However, a subset with unfavorable anatomy experience symptomatic cyanosis as neonates, prompting early intervention. Controversy surrounding the optimal management strategy for neonatal symptomatic TOF (sTOF) persists, with treatment options including primary repair (PR) or staged repair (SR), in which an initial palliation (IP; e.g. aortopulmonary shunt) is followed by complete repair (CR). Recent expansion in transcatheter options for palliation (balloon pulmonary valvuloplasty, right ventricular outflow tract stent, and/or ductal stent) have only added to the controversy(4–7).
The potential advantages of PR are that it requires a single exposure to general anesthesia and cardiopulmonary bypass, reduces the duration of cyanosis, and avoids the risks of a potentially unstable circulation in the period between IP and CR. By delaying complete operative correction, SR reduces exposure to general anesthesia and cardiopulmonary bypass in the neonatal period, during which neurological development may be especially vulnerable. Allowing for interval growth of the patient and their pulmonary arteries may also reduce the risks of perioperative adverse outcomes and post-operative reintervention(s). The recent multicenter retrospective cohort study of neonates with sTOF from the Congenital Cardiac Research Collaborative (CCRC) demonstrated no significant difference in overall mortality or major adverse events between PR and SR, after adjusting for measurable confounders(8). In this analysis, SR was associated with greater odds of reintervention and longer cumulative hospital and intensive care unit length of stay (LOS)(8).
Comparing the healthcare costs for both PR and SR can complement previous analyses and aid in decision-making for neonates with sTOF in several ways. When traditional outcomes are similar, lower costs support pursuing a strategy, since it represents good value relative to the more costly alternative. Also, on average, sicker patients consume more medical care (labs, imaging, and medication along with longer duration of care) and in so doing, incur more costs. Total medical costs can provide a composite measure of morbidity, combining the range of adverse events into a single metric that quantifies morbidity not captured in disparate traditional outcome measures. Finally, converting dichotomous outcomes of myriad severity into a single continuous metric is also statistically expedient(6, 9–12), which is especially important in congenital cardiology where statistical power is frequently a limitation.
Therefore, we sought to link clinical data from the multicenter CCRC TOF study with individual patient-level inpatient costs from the Pediatric Health Information Systems (PHIS) database to determine if there were significant differences in healthcare costs for PR and SR over the first 18 months of life. We hypothesized that increased total LOS, increased resource utilization, and other morbidity associated with SR would be reflected in higher economic costs.
METHODS:
Data Sources:
The CCRC is a multicenter collaborative currently comprised of investigators from 12 congenital heart disease programs in the US, which at the time of the study included 9 contributing centers(13). The CCRC TOF study was a multicenter cohort study of consecutive neonates with TOF undergoing intervention at ≤30 days of age between 1/1/2005 and 11/30/2017, evaluating for differences in clinical outcome between those undergoing PR and SR(8). Each center reviewed subject medical records to collect clinical data. De-identified data were transferred securely to a web-based central database using Research Electronic Data Capture tools hosted by Children’s Healthcare of Atlanta, the data-coordinating center for the CCRC. Data quality and reliability were assured through remote auditing of submitted data(14). This study was approved by the Institutional Review Board at Cincinnati Children’s Hospital, which acted as the single institutional review board, with a waiver of the need for informed consent. A data-use agreement governed center participation.
PHIS is an administrative database including inpatient, emergency department, ambulatory surgery, and observation encounters from 45 not-for-profit, tertiary care pediatric hospitals in the US affiliated with the Children’s Hospital Association (Overland Park, KS). Participating hospitals provide discharge/encounter data including demographics, diagnoses, and procedures as well as utilization data (e.g. pharmacy products, radiologic studies, and laboratory studies). Data are de-identified at the time of submission and subject to checks of reliability and validity. Due to the data-use agreements within the CCRC and between individual centers and PHIS, data from this study will not be shared.
Study design/Study population:
A retrospective multicenter cohort study was performed combining clinical data from the CCRC TOF study cohort and cost data from PHIS. To create a contemporaneous cohort, only data from the 6 hospitals contributing to both the CCRC TOF study and PHIS from 1/1/2011–12/31/2017 were included. Potential subjects were excluded if they were alive and had not undergone operative correction by 18 months of age or if their total hospital costs were missing. This linkage was accomplished as described previously (6)..Accuracy of the match was evaluated by ensuring that the age at operation and first hospital discharge was correct to ±2 days. Subjects who could not be matched to PHIS correctly, and they were excluded. No significant differences in the cohort characteristics and treatment strategies were seen between included and excluded subjects (data not shown).
Study measures:
Data collected as part of the CCRC TOF study have been described previously(8), and include baseline characteristics, treatment strategy and outcomes (mortality, reintervention, and LOS). PR was defined receiving operative correction of TOF as the index procedure. SR was defined as surgical or transcatheter palliation as the index procedure.
Cost data (adjusted for regional wage-price indices) were extracted and cleaned by the Healthcare Analytics Unit at the Children’s Hospital of Philadelphia, before analysis at the CCRC data-coordinating center. Inflation-adjustment to 2017 US Dollars was performed using the consumer price index for medical care goods and services. Given the 18-month time horizon, no adjustment was made for discounting later costs. Total costs for each inpatient and observation encounter were included. Costs were calculated from recorded line-item charges by PHIS using internal cost-to-charge ratios. The primary outcome was the total hospital cost over the first 18 months of life (birth to day-of-life 540). Cost-per-day-alive was chosen as a complementary primary outcome, to mitigate bias due to subject deaths or loss to follow-up. The 18-month end point was selected because it provided a reasonable time window to capture clinically significant differences, while balancing a timespan in which most subjects would have undergone CR and for which most subjects would have complete follow-up, avoiding attrition of subjects who underwent later follow-up remote from participating centers. If the subject remained an inpatient on the 540th day of life, the costs from that entire hospitalization were included in total costs. The analysis of cost was performed from the perspective of the health system (the magnitude of goods and services utilized) because this provides a measure of value and morbidity as described. Measurement of out-of-pocket cost to families (with or without lost wages and other costs) is not possible..
Alternative measurements of 18-month costs were calculated:1) starting at the index hospital admission and 2) starting from the index procedure (PR or IP) to determine if they introduced differential bias between PR and SR. In each of these time-windows, we also evaluated whether subjects who were inpatients on the 540th day affected our estimates of relative cost by measuring total costs including the rest of that hospitalization and comparing it to costs excluding these additional costs. A hospital stay including the 540th day of life was expected to be more likely in recipients of SR, given their older age at CR. None of these changes affected the reported asssoications (data not shown).
Secondary outcomes included all department-level (clinical, imaging, supply, laboratory, pharmacy, and other) costs, which were collected to provide detail about potentially differential drivers of cost. Department-level costs were calculated from PHIS-reported department-level charges as previously described(6).
While PHIS records inpatient and observation encounters, short-stay hospitalizations vary in reporting by hospital. For this analysis, differential reporting of cardiac catheterizations are potentially important because they incur significant cost and because the rate at which they are might differ between PR and SR. To mitigate this, catheterization procedures in CCRC TOF study records without a matching PHIS encounter were identified. The cost of these encounters was imputed as the median cost of successfully-matched catheterization encounters with total LOS ≤2 days ($21,189). These encounters reflect a high-bound estimate of the unmatched encounter cost as they include stays of >1 day. Department-level costs for unmatched catheterizations were calculated by taking the proportion of the sum of the encounter costs of matched catheterizations with LOS ≤2 days for each department. Pre-specified sensitivity analyses comparing costs were performed 1) if imputed costs were excluded or 2) if imputing a range of estimates, specifically 25th ($14,710) or 75th ($28,373) percentiles, with no significant difference in the observed findings (data not shown).
Statistical analysis:
Demographic and clinical characteristics and outcomes were summarized and compared between PR and SR to evaluate whether they differed between the current study population and the CCRC TOF cohort. Continuous data were compared between PR- and SR-treated patients using Wilcoxon rank-sum tests. Comparisons in the distributions of categorical variables were performed using chi-square tests or the Fisher exact test.
The primary exposure was treatment strategy (PR vs. SR). The primary outcomes were total inpatient and observation costs over 18 months of life and cost-per-day-alive. Patient characteristics associated with the choice between PR and SR have been identified previously(8), and were likely to be associated with clinical outcomes and cost. Confounding by indication was addressed through propensity score adjustment(5, 6, 8–10). The propensity score included center, prematurity, pre-intervention invasive ventilation, 22q11.2 micro deletion (DiGeorge) syndrome, and the presence of antegrade pulmonary blood flow. Inverse probability of treatment weighting using propensity scores was used in all comparisons of primary and secondary outcomes to adjust for potential confounders between groups. To reduce the influence of extreme weights, inverted propensity scores were stabilized and then truncated at the first and 99th percentiles. Stabilized weights were computed by multiplying the inverse probability of treatment weight by the marginal probability of receiving the given treatment resulting in a mean weight of 1 and standard deviation <0.5 in both treatment groups. The standardized mean difference was used to quantify the relative imbalance in a covariate between the 2 treatment groups. All adjusted models included the main effect of treatment and were weighted by stabilized propensity score to achieve balance between treatment groups (Supplementary Table 1). Elements with adjusted standardized mean difference <0.10 were considered to have achieved satisfactory balance(15). This approach addresses potential confounding by indication at the center level and attempts to evaluate costs across the entire population, but makes measurement of between-hospital variation in cost challenging.
As expected, cost data followed a right-skewed distribution. As a result, prior to statistical modeling, data were log-transformed, and analysis was conducted on the transformed data(7). Estimated differences in cost, on the log scale, were exponentiated resulting in a ratio of estimated cost between the two treatment groups. Cost ratios (CR) were calculated along with 95% confidence intervals (CI) with CR<1 favoring PR and CR>1 favoring SR.
As a sensitivity analysis, untransformed costs were also analyzed using a gamma distribution, which in other settings has been used to analyze cost data(10, 16–18). The gamma distribution was heavily influenced by outliers and failed to provide a better fit to the data when compared to the log-transformation. However, resulting estimates of ratios of costs did not differ when compared to the log-transformed results (data not shown). Similar analyses were used to compare department-specific costs. Bootstrapping using patients with propensity scores between 0.4 and 0.6 was used to provide estimates of outcomes and 95% CI for a hypothetical subject with a propensity score of 0.5. Appropriate balance in the study sample was evaluated prior to analysis.
Several additional pre-specified secondary analyses were also performed. First, department-level total costs were analyzed using the same techniques as in the primary analyses. Second, the costs of the index hospitalization for PR subjects were compared to the sum of the IP and CR hospitalization costs in SR subjects, thereby excluding costs outside of the PR, IP or CR hospitalizations. This analysis was performed to determine the degree to which reinterventions, which occur outside of these hospitalizations, influenced cost-differences between treatment strategies. Third, within the SR cohort, we compared the total 18-month costs and cost-per-day-alive between subjects who underwent a surgical vs. transcatheter approach to IP. Assignment to transcatheter or surgical groups was based on the first palliative procedure (intention to treat), understanding that some crossover from transcatheter to surgical palliation was likely. The rate of crossover was measured.
All analyses were performed by the CCRC DCC using SAS software version 9.4 (SAS Institute, Cary, NC, United States of America) and R version 4.0.2 (R Development Core Team, Vienna, Austria).
RESULTS:
Study population:
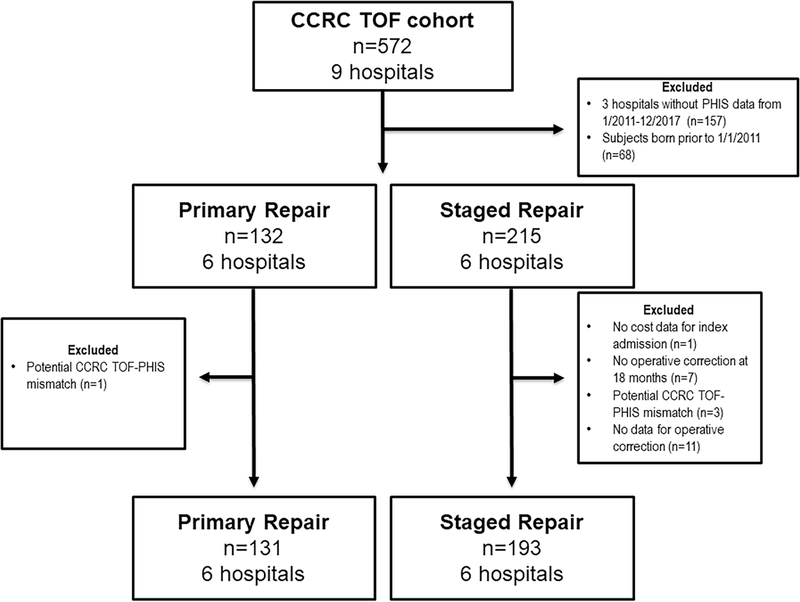
The study cohort (Figure 1) was comprised of 324 individuals (40% PR) from 6 centers, which represented 57% of the initial CCRC TOF cohort. The use of PR varied significantly across study centers with a range between 16 and 64% (p<0.001). The proportion of subjects receiving pre-procedural invasive ventilation was higher amongst SR subjects (27%) than those who underwent PR (15%, p=0.006). The proportion of SR subjects (25%) born <37 weeks gestation was also higher than that in PR subjects (17%), but this was not statistically significant (p=0.08). There were no other significant differences in the characteristics of PR and SR subjects (Table 1).
Figure 1: Study Population.
This cohort diagram depicts which subjects from the original CCRC TOF cohort were included in the current study. In total, 57% of the original CCRC TOF cohort received care at a hospital contributing data to PHIS during a single contemporary period (birth date between 1/2011–12/2017).
Table 1:
Characteristics of the study population
| Primary Repair (n=131) | Staged Repair (n=193) | p | |
|---|---|---|---|
| Center * | <0.001 | ||
| 1 | 65% (11) | 35% (6) | |
| 2 | 64% (68) | 36% (37) | |
| 3 | 33% (16) | 67% (33) | |
| 4 | 23% (5) | 77% (17) | |
| 5 | 29% (22) | 71% (52) | |
| 6 | 16% (9) | 84% (48) | |
| Prematurity (<37 weeks gestation) | 17% (22) | 25% (48) | 0.08 |
| Gestational age | 38.5 (IQR: 37.0–39.4) | 38.3 (36.7–39.1) | 0.11 |
| Birth weight | 2.9 (IQR: 2.4–3.3) | 2.9 (IQR: 2.4–3.3) | 0.23 |
| Anatomic diagnosis | 0.35 | ||
| Tetralogy of Fallot with pulmonary stenosis | 48% (63) | 53% (103) | |
| Tetralogy of Fallot with pulmonary atresia | 52% (68) | 47% (90) | |
| 22q11.2 microdeletion (DiGeorge) syndrome | 11% (36) | 8% (10) | 0.10 |
| Non-cardiac co-morbidities | 10% (13) | 12% (23) | 0.58 |
| Invasive ventilation prior to index procedure | 15%(19) | 27% (53) | 0.006 |
For Center, percentages are percentage of subjects with primary repair vs. staged repair (row percentages). For the rest of the table percentages refer to the percentage within primary or staged repair subjects (column percentages).
Clinical outcomes:
Mortality at 18 months was not significantly different between PR and SR subjects (7 vs. 4%, p=0.19, Table 2). The 18-month risks of procedural complications and reintervention were not significantly different between PR and SR (p=0.10 and 0.20, respectively). PR was associated with a higher likelihood of extracorporeal membrane oxygenation (6%) than SR (1%, p=0.004). PR was associated with both lower 18-month hospital and ICU LOS (p<0.001 for both). Comparisons of outcomes for index procedure (PR vs. IP) and operative correction (PR vs. CR) are summarized in Supplementary Table 2.
Table 2:
Clinical outcomes
| Primary Repair (n=131) | Staged Repair (n=193) | p | |
|---|---|---|---|
| Mortality | |||
| Through 3 months | 5% (6) | 1% (1) | 0.02 |
| Through 18 months | 7% (9) | 4% (7) | 0.19 |
| Procedural complications | 47% (61) | 37% (72) | 0.10 |
| Reintervention | 11% (15) | 17% (33) | 0.20 |
| Extracorporeal membrane oxygenation | 6% (8) | 1% (1) | 0.004 |
| Ventilation (days) | 3 (1–8) | 4 (2–8) | 0.14 |
| Hospital length of stay (days) | 21 (14–35) | 32.5 (22–49) | <0.001 |
| Intensive care unit length of stay (days) | 8 (5–18) | 12 (8–19.5) | <0.001 |
Data presented as % (n) for categorical data and median (interquartile range) for continuous data.
Cost data:
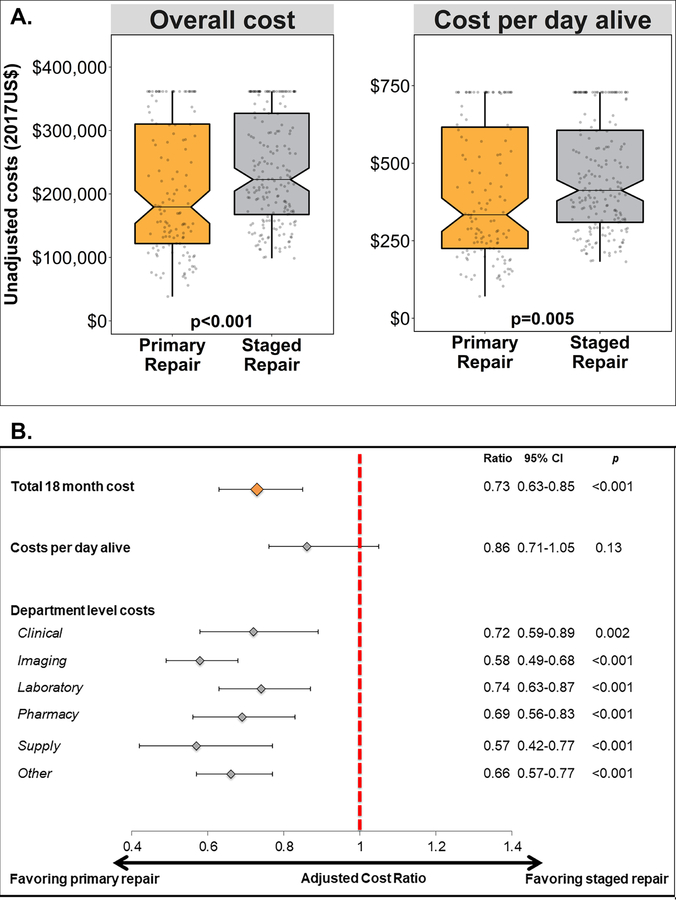
Total 18-month median costs for PR ($179,494, IQR: 121,760–310,721) were lower than for SR ($222,799, IQR: 167,581–327,113, p<0.001, Table 3, Central Figure A). Similarly, median cost-per-day-alive for PR ($334 and IQR: 225–623) was also lower than for SR ($412 and IQR: 310–606, p=0.005). With regards to department-level costs, the median clinical cost for PR was lower than for SR, but this difference was not statistically significant (p=0.06). All other department-level costs were significantly lower for PR than for SR (Table 3).
Table 3:
Observed costs through 18 months of life
| Primary Repair (n=131) | Staged Repair (n=193) | p | |
|---|---|---|---|
| Total costs | $179,494 (121,760–310,721) | $222,799 (167,581–327,113) | <0.001 |
| Cost per day alive | $334 (225–623) | $412 (310–606) | 0.005 |
| Department level costs | |||
| Clinical | $19,870 (9,766–39,385) | $21,802 (14,703–40,161) | 0.06 |
| Imaging | $7,463 (4,824–13,287) | $14,265 (8,522–22,744) | <0.001 |
| Laboratory | $16,423 (9,952–29,732) | $21,552 (15,206–33,904) | <0.001 |
| Pharmacy | $7,328 (3,668–14,080) | $12,706 (7,843–20,543) | <0.001 |
| Supply | $15,051 (8,638–29,198) | $19,816 (11,398–31,130) | 0.048 |
| Other | $76,980 (46,939–135,510) | $111,515 (80,723–167,386) | <0.001 |
All costs expressed in 2017 United States Dollars ($) and measured over first 18 months.
Values are presented as median (interquartile range).
Central Figure: Comparisons of costs associated with Primary and Staged Repair Strategies.
A. This notched box plots depicts the unadjusted observed outcomes: total 18-month cost (left) and cost-per-day-alive (right). The central line depicts the median value, notches the 95% confidence interval of the median, the top and bottom of the box depict the 25th and 75th percentiles, whiskers depict 1.5 × the interquartile range, and gray circles depict individual observations. Data are truncated at the 80th percentile.
B. This Forest plot depicts the results of propensity score adjusted analyses. Depicted are the point estimate (diamond) and 95% CI (brackets). The cost ratio between primary repair and staged repair is depicted on the x-axis. The red dashed line marks a cost ratio of 1. The specific cost ratios, confidence intervals, and p-values are reproduced to the right of the graph.
After propensity score adjustment, PR was associated with lower total 18-month costs (cost ratio: 0.73, 95% CI: 0.63–0.85, p<0.001). Utilizing bootstrapping techniques to estimate the costs over the first 18 months of life for a hypothetical patient with equal likelihood of receiving PR and SR, undergoing PR would be expected to cost $154,047 (95% CI: $150,025–158,178) compared to SR, which would be expected to cost $253,316 (95% CI: $229,747–241,019). The point estimate for cost-per-day-alive suggested a cost benefit for PR (cost ratio: 0.86) but the association was not statistically significant (p=0.13). All department-level costs also favored PR (Central Figure B).
In a pre-planned secondary analysis, the costs of the index hospitalization for PR were compared to the cumulative costs of the IP and CR hospitalizations for SR subjects. As in the primary analysis, observed costs were lower for PR (p<0.001), as were all department level costs (Table 4A). In propensity-score adjusted analyses, PR was associated with lower total cost (cost ratio: 0.65, 95% CI: 0.55–0.78, p<0.0001) and all department-level costs (p<0.0001 for all) (Table 4B).
Table 4:
Analysis of costs for primary repair hospitalization versus summed costs for staged repair hospitalizations
| A. OBSERVED | |||
| Primary Repair (n=131) | Staged Repair (n=193) | p | |
| Total cost | $147,427 (107,458 249,752) | $191,110 (145,251–273,467) | <0.001 |
| Department level costs | |||
| Clinical | $13,699 (7,259–30,218) | $17,523 (11,162–30,890) | 0.01 |
| Imaging | $5,963 (4,055–9,602) | $11,128 (6,989–18,025) | <0.001 |
| Laboratory | $14,978 (9,025–20,423) | $19,015 (14,188–30,364) | <0.001 |
| Pharmacy | $6,169 (3,052–12,353) | $11,253 (6,848–21,138) | <0.001 |
| Supply | $10,793 (5,049–21,282) | $14,718 (9,314–25,173) | 0.002 |
| Other | $61,075 (39,719–100,563) | $98,442 (66,733–151,501) | <0.001 |
| B. PROPENSITY SCORE ADJUSTED | |||
| Cost ratio | 95% CI | p | |
| Total cost | 0.65 | 0.55–0.78 | <0.001 |
| Department level costs | |||
| Clinical | 0.53 | 0.37–0.77 | <0.001 |
| Imaging | 0.55 | 0.47–0.65 | <0.001 |
| Laboratory | 0.62 | 0.49–0.79 | <0.001 |
| Pharmacy | 0.52 | 0.39–0.68 | <0.001 |
| Supply | 0.38 | 0.24–0.60 | <0.001 |
| Other | 0.55 | 0.44–0.70 | <0.001 |
All costs expressed in 2017 United States Dollars ($) and measured over first 18 months.
Values are presented as median (interquartile range).
Cost ratios are the adjusted difference in costs between primary repair and staged repair strategies. Cost ratios <1 reflect cost savings favoring primary repair.
Comparison of transcatheter and surgical palliation:
Among SR subjects, 70% underwent IP with a surgical aortopulmonary shunt. The remaining patients underwent a transcatheter approach to IP, including balloon pulmonary valvuloplasty (48%), RVOT stent (14%), and PDA stent (38%). Of these transcatheter IP patients, 13% underwent a subsequent surgical aortopulmonary shunt prior to CR (i.e. crossover from transcatheter to surgical palliation).
Comparing the observed costs for surgical and transcatheter palliation, there was no significant difference in the total 18 month cost (p=0.17) or cost-per-day-alive (p=0.24, Table 5A). Observed laboratory costs were higher in surgical palliation patients than in recipients of transcatheter palliation (p=0.01), but there were no other significant differences in cost. These findings were similar to those obtained with propensity-score adjustment (Table 5B). As a sensitivity analysis, cases in which transcatheter palliation was followed by conversion to a surgical shunt were excluded. In this sensitivity analysis, exclusion of the 7 crossover subjects had no effect on the observed associations (data not shown).
Table 5:
Comparison of costs for transcatheter and surgical palliation
| A. OBSERVED | |||
| Transcatheter Palliation (n=56) | Surgical Palliation (n=137) | p | |
| Total cost | $213,127 (147,506–294,145) | $225,265 (172,236–342,378) | 0.17 |
| Cost per day alive | $403 (273–547) | $416 (318–633) | 0.24 |
| Department level costs | |||
| Clinical | $19,959 (15,062–37,810) | $23,623 (14,219–41,152) | 0.74 |
| Imaging | $16,581 (8,367–25023) | $13,231 (8,584–21,451) | 0.18 |
| Laboratory | $18,937 (11,968–29,105) | $21,880 (16,462–37,825) | 0.01 |
| Pharmacy | $10,821 (6,855–18,756) | $12,899 (8,179–22,139) | 0.12 |
| Supply | $20,809 (11,855–31,715) | $19,603 (10,681–31,130) | 0.46 |
| Other | $103,346 (64,730–152,673) | $119,223 (83,080–175,617) | 0.06 |
| B. ADJUSTED | |||
| 95% CI | p | ||
| Total cost | 1.09 | 0.89–1.33 | 0.41 |
| Cost per day alive | 1.09 | 0.88–1.35 | 0.41 |
| Department level costs | |||
| Clinical | 0.98 | 0.74–1.30 | 0.91 |
| Imaging | 0.91 | 0.73–1.12 | 0.37 |
| Laboratory | 1.34 | 1.07–1.67 | 0.01 |
| Pharmacy | 1.25 | 0.96–1.64 | 0.10 |
| Supply | 0.81 | 0.61–1.07 | 0.14 |
| Other | 1.18 | 0.96–1.46 | 0.12 |
All costs expressed in 2017 United States Dollars ($) and measured over first 18 months.
Values are presented as median (interquartile range).
Cost ratios are the adjusted difference in costs between transcatheter and surgical palliation. Cost ratios >1 reflect cost savings favoring transcatheter palliation.
Discussion:
In this multicenter cohort study comparing costs associated with PR and SR in neonates with sTOF, PR was associated with lower total costs through 18 months, even after addressing confounding by indication. Cost-per-day-alive, a metric that mitigates potential bias introduced by early mortality was also lower in PR in unadjusted analyses. In adjusted analyses the point estimates for this metric also demonstrated a cost advantage for PR, but the difference was no longer statistically significant. It is not possible to determine if this was due to inadequate statistical power (type II error) or the effect of the modeling strategy. Secondary analyses demonstrated that all department level costs favored PR. Although prior investigations have explored the cost of care in this population(19), to our knowledge, this is the first that combines administrative data with directly reviewed patient-level data.
The optimal therapy for neonates with sTOF has been a source of considerable and ongoing debate. The hypothesis that delaying operative correction and delivering a larger, healthier patient to the operating room would reduce the risk of both mortality and morbidity is intuitively compelling. However, as demonstrated recently in a large multicenter retrospective cohort study, the early survival benefit of SR dissipates if patients are followed through their eventual CR(8). In that study, the risks of hospital and procedural complications, and reintervention post-repair, also did not differ between management strategies(8). If there is no significant difference in the likelihood of meaningful, measurable clinical outcomes, deciding on the optimal approach to sTOF is more complicated.
The findings in the current study complement the findings of the previously published study, specifically that PR was associated with shorter LOS and less frequent reintervention(9), underscoring the impact of these observed differences. First, where the early survival advantage of SR dissipated by the time of definitive operative correction and no significant advantage is evident for SR,, lower costs associated with PR may represent better value. Second, analyzing costs provides a means of evaluating the impact of a range of disparate clinical outcomes, integrating the time spent receiving care and the intensity of that care and providing a surrogate for morbidity that may complement traditional outcome measures. Together, these findings support consideration of a PR strategy for neonates with sTOF where feasible.
TOF is one of the most common sources of cyanotic heart disease but only occurs in 3–5 per 10,000 live births(20). However, the public health impact is magnified by its cost. As stratified by annual in-hospital spending, TOF is the 3rd most costly congenital cardiac diagnosis and 14th most costly overall pediatric diagnosis(21). Though they represent a minority of patients with TOF, symptomatic neonates represent an especially vulnerable subgroup with higher risk of mortality and morbidity(3), and who likely incur greater burden of resource utilization and healthcare costs. The optimal strategy for repair potentially sets a course for better long-term health and well-being, underscoring the importance of establishing the ideal strategy.
How to translate the present findings into clinical practice and achieve the best outcomes for these vulnerable patients remains an important question. Encouraging and disseminating high value practices (in this case, PR) from high-performing centers has the potential to improve outcomes across a broad range of hospitals. If it is not possible to reproduce these outcomes broadly, our data support directing care of this vulnerable subgroup to centers with excellent results. Potential benefits of regionalization(22, 23) are likely to be magnified in high-risk populations. Whether regionalization would achieve this aim and the potential knock-on effects on families (travel time and lost productivity) are not addressable in this study
Realistically, the impact of this study is to inform decisions made by heart teams at individual centers. An important question for each center is how applicable the results are to them. CCRC participating centers have a range of volumes and practice patterns for PR and SR. Propensity score adjustment was used to mitigate confounding by indication from measurable covariates (including center). If local results differ for either PR or SR, this should inform program-wide choices for how to treat these patients. The reported results can, however, provide a benchmark that can guide program development and quality improvement in a variety of settings(24).
We performed a series of pre-specified secondary and sensitivity analyses, seeking to clarify the factors associated with cost differences between PR and SR. Modifying outcomes in these areas (through improvement efforts) could potentially mitigate or overcome the demonstrated cost difference. First, we evaluated cost differences by department. In previous analyses comparing the relative costs of transcatheter and surgical interventions, device costs result in higher supply costs for transcatheter interventions, while operative interventions incur longer LOS (and accompanying costs)(6, 9, 10, 25), with the cost advantage determined by the balance of these components. In this case, all of the component costs favor PR. Overcoming the broad cost advantage of PR, would require similarly broad improvements in SR (i.e. shorter total hospital LOS). Second, we studied whether the cost advantage of PR was due to the higher frequency of interstage reinterventions in the SR group(8). However, in comparisons of the costs of primary procedural hospitalizations in each group (PR vs. IP + CR) excluding reinterventions occur outside of these hospitalizations, PR’s cost advantage persisted, suggesting that It can be deduced, then, that the cost savings in PR cases is not primarily derived from fewer reinterventions. Whatever benefits accrue by delaying CR in the SR management pathway do not (at least in terms of measurable costs) overcome the costs of a second hospitalization.
Finally, we demonstrated that no significant differences in cost between transcatheter and surgical palliations. This sub-analysis was limited by a relatively small sample size and crossover between the groups, both of which might obscure a difference in costs between transcatheter and operative palliation. While these findings suggest that the cost advantage of PR would not be overcome by increased utilization of transcatheter palliation, it is important to note that this analysis reflects the practice and outcomes of the study period. In the intervening years, there has been an accumulation of experience and technical facility (both in the catheterization laboratory and in subsequent operations) with both ductal (4, 5, 7) and right ventricular outflow tract stent palliations(26, 27). Increased facility and experience with a technique may be associated with improved outcomes and/or reduced cost, while the cost of necessary equipment (e.g. drug eluting coronary stents) and potential for more interstage reinterventions may offset potential savings. Additional studies are needed as experience with these palliation techniques continues to accrue. The Pediatric Heart Network-funded COMPASS trial, a randomized clinical trial evaluating ductal stent vs. surgical shunts for ductal-dependent pulmonary blood flow in neonates, is an example of a prospective clinical trial better equipped to answer these questions.
It is important to acknowledge that mortality, major adverse events, reintervention and cost are not the only relevant clinical outcomes, nor are they a comprehensive assessment of functional status. For instance, neurodevelopment(28, 29), cardiopulmonary exercise performance(30–32), and health-related quality of life (33) are all diminished in cohorts of repaired TOF patients. The impact of PR or SR on these important outcomes in this vulnerable cohort is not incorporated into this analysis. The additional cost of SR may be warranted if there are significant benefits in these long-term clinical and functional outcomes. Incorporating these measures into ongoing research is critical to determining the optimal care of the next generation of neonates with sTOF.
Limitations:
We acknowledge several additional limitations. Outpatient costs (e.g., outpatient echocardiograms and emergency room visits) are not recorded in PHIS, but these generate a minority of costs in this population(34). Unmeasured confounding is always a concern, but a strength of the current study is that it combines detailed medical record data using standardized definitions and methods with cost data from an administrative registry that has a track record of comparing costs across member institutions. Next, though we sought to identify the strategy that produced the best value on average, there may factor(s) in which SR is preferable. With a fixed study sample, a limited number of factors were included in the propensity score and it may be that there are other factors that favor one strategy over the other (e.g, size <2.5 kg at repair (35)). No significant difference in clinical outcomes was seen (for example in 18-month mortality), but the study was limited in its statistical power by a fixed sample size and the risk of type 2 error. Finally, SR subjects were more likely to be excluded because of late operative correction after 18 months or complete repair at another hospital, which reduces statistical power. However, this would alter our conclusions only if the sum of follow-up and operative correction costs in this minority (<10%) overcame the savings seen in the rest of the cohort, which is unlikely.
Conclusions:
While acknowledging these limitations, we conclude that PR was associated with superior value, lower cost with similar mortality risk, through 18 months of age. Where feasible and appropriate at the center-level, PR should be considered as the primary strategy for management of neonates with sTOF. Future data from a broader range of centers, greater experience with transcatheter palliation, and inclusion of longer-term clinical and functional health outcomes, could influence these conclusions.
Supplementary Material
Clinical Perspectives:
Competency in Patient Care and Procedural Skills:
In infants with tetralogy of Fallot, primary surgical repair is associated with lower costs than staged intervention without a significant increase in mortality.
Translational Outlook:
Additional analyses of larger datasets would expand the generalizability of these cost and value assessments and inform the selection of neonates with tetralogy of Fallot for early intervention.
Funding sources:
Financial support for this research was derived in part from the Kennedy Hamill Pediatric Cardiac Research Fund, the Liam Sexton Foundation, and a Heart Like Ava. Dr. O’Byrne received research support from the National Institute of Health/National Heart Lung and Blood Institute (K23 HL130420-01). The funding agencies had no role in the planning or execution of the study, nor did they edit the manuscript as presented. The manuscript represents the opinions of the authors alone.
Abbreviations:
- CI
Confidence intervals
- CHD
Congenital heart disease
- CCRC
Congenital Cardiac Research Collaborative
- CR
Cost ratios
- IP
Initial palliation
- LOS
Length of stay
- PHIS
Pediatric Health Information Systems
- PR
Primary repair
- SR
Staged repair
- sTOF
Symptomatic tetralogy of Fallot
- TOF
Tetralogy of Fallot
Footnotes
Disclosures: Dr. Glatz has a consulting relationship with Ampio Pharmaceuticals. Dr. Goldstein has consulting relationships with Medtronic, W.L. Gore & Associates, and Mezzion Pharma. He is also a consultant and advisory board member for PECA Labs. Dr. Qureshi has consulting relationships with Medtronic, W.L. Gore & Associates, Edwards Lifesciences, and Abiomed. Dr. Shahanavaz has consulting relationship with Medtronic, Edwards Lifesciences, W.L Gore & Associates, and Abbott Inc. The other authors have no relevant financial conflicts to disclose.
Tweet: New in @jaccjournals: 6-hospital cohort study combining data from @CCRCresearch and PHIS database demonstrate that primary repair of symptomatic neonates with tetralogy of Fallot incurs less costs through 18-months than staged repair, suggesting superior value @ACCACPC #chd
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Smith CA, McCracken C, Thomas AS, et al. Long-term Outcomes of Tetralogy of Fallot: A Study From the Pediatric Cardiac Care Consortium. JAMA Cardiol 2019;4:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Brien SM, Clarke DR, Jacobs JP, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg 2009;138:1139–1153. [DOI] [PubMed] [Google Scholar]
- 3.Kirsch RE, Glatz AC, Gaynor JW, et al. Results of elective repair at 6 months or younger in 277 patients with tetralogy of Fallot: a 14-year experience at a single center. J Thorac Cardiovasc Surg 2014;147:713–717. [DOI] [PubMed] [Google Scholar]
- 4.Bentham JR, Zava NK, Harrison WJ, et al. Duct Stenting Versus Modified Blalock-Taussig Shunt in Neonates With Duct-Dependent Pulmonary Blood Flow: Associations With Clinical Outcomes in a Multicenter National Study. Circulation 2018;137:581–588. [DOI] [PubMed] [Google Scholar]
- 5.Glatz AC, Petit CJ, Goldstein BH, et al. Comparison Between Patent Ductus Arteriosus Stent and Modified Blalock-Taussig Shunt as Palliation for Infants With Ductal-Dependent Pulmonary Blood Flow: Insights From the Congenital Catheterization Research Collaborative. Circulation 2018;137:589–601. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein BH, O’Byrne ML, Petit CJ, et al. Differences in Cost of Care by Palliation Strategy for Infants With Ductal-Dependent Pulmonary Blood Flow. Circ Cardiovasc Interv 2019;12:e007232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratnayaka K, Nageotte SJ, Moore JW, et al. Patent Ductus Arteriosus Stenting for All Ductal-Dependent Cyanotic Infants: Waning Use of Blalock-Taussig Shunts. Circ Cardiovasc Interv 2021;14:e009520. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein BH, Petit CJ, Qureshi AM, et al. Comparison of Management Strategies for Neonates With Symptomatic Tetralogy of Fallot. J Am Coll Cardiol 2021;77:1093–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of transcatheter and operative closures of ostium secundum atrial septal defects. Am Heart J 2015;169:727–735.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of Transcatheter and Operative Pulmonary Valve Replacement (from the Pediatric Health Information Systems Database). Am J Cardiol 2016;117:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Byrne ML, Glatz AC, Faerber JA, et al. Interhospital Variation in the Costs of Pediatric/Congenital Cardiac Catheterization Laboratory Procedures: Analysis of Data From the Pediatric Health Information Systems Database. J Amer Heart Assoc 2019;8:e011543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasquali SK, Sun JL, d’Almada P, et al. Center Variation in Hospital Costs for Patients Undergoing Congenital Heart Surgery. Circ Cardiovasc Qual Outcomes 2011;4:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petit CJ, Qureshi AM, Glatz AC, et al. Comprehensive comparative outcomes in children with congenital heart disease: The rationale for the Congenital Catheterization Research Collaborative. Congenit Heart Dis 2019;14:341–349. [DOI] [PubMed] [Google Scholar]
- 14.Pettus JA, Pajk AL, Glatz AC, et al. Research Collaborative. Data Quality Methods Through Remote Source Data Verification Auditing:. Cardiol Young 2021;e-published ahead of print:1–6. [DOI] [PubMed] [Google Scholar]
- 15.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Statist. Med. 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Byrne ML, Shinohara RT, Mi L, et al. Inter-hospital Variation in Costs of Pediatric Cardiac Catheterization: An Analysis of the PHIS Database. Circ Cardiovasc Qual Outcomes 2018;11:A227. [Google Scholar]
- 17.O’Byrne ML, Glatz AC, Song L, et al. Association Between Variation in Preoperative Care Before Arterial Switch Operation and Outcomes in Patients With Transposition of the Great Arteries. Circulation 2018;138:2119–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ 2005;24:465–488. [DOI] [PubMed] [Google Scholar]
- 19.Ramakrishnan KV, Zurakowski D, Pastor W, Jonas RA, Sinha P. Symptomatic Tetralogy of Fallot in Young Infants: Primary Repair or Shunt-Pediatric Health Information System Database Analysis. World J Pediatr Cong Heart Surg 2018;9:539–545. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman JIE, Kaplan S. The Incidence of Congenital Heart Disease. J Am Coll Cardiol 2002;39:1890–1900. [DOI] [PubMed] [Google Scholar]
- 21.Keren R, Luan X, Localio R, et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med 2012;166:1155–1164. [DOI] [PubMed] [Google Scholar]
- 22.Welke KF, Pasquali SK, Lin P, et al. Regionalization of Congenital Heart Surgery in the United States. Sem Thorac Cardiovasc Surg 2020;32:128–137. [DOI] [PubMed] [Google Scholar]
- 23.Welke KF, Pasquali SK, Lin P, et al. Theoretical Model for Delivery of Congenital Heart Surgery in the United States. Ann Thoracic Surg 2021;111:1628–1635. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein BH, Petit CJ, Qureshi AM, McCracken CE, Glatz AC. Reply: Neonates With Symptomatic TOF: The Shorter Path Need Not Always Be the Desirable Path. J Am Coll Cardiol 2021;77:2984–2985. [DOI] [PubMed] [Google Scholar]
- 25.Ooi YK, Kelleman M, Ehrlich A, et al. Transcatheter Versus Surgical Closure of Atrial Septal Defects in Children: A Value Comparison. JACC Cardiovasc Interv 2016;9:79–86. [DOI] [PubMed] [Google Scholar]
- 26.Quandt D, Ramchandani B, Stickley J, et al. Stenting of the Right Ventricular Outflow Tract Promotes Better Pulmonary Arterial Growth Compared With Modified Blalock-Taussig Shunt Palliation in Tetralogy of Fallot-Type Lesions. JACC Cardiovasc Interv 2017;10:1774–1784. [DOI] [PubMed] [Google Scholar]
- 27.Dohlen G, Chaturvedi RR, Benson LN, et al. Stenting of the right ventricular outflow tract in the symptomatic infant with tetralogy of Fallot. Heart 2009;95:142–147. [DOI] [PubMed] [Google Scholar]
- 28.Bellinger DC, Rivkin MJ, DeMaso D, et al. Adolescents with tetralogy of Fallot: neuropsychological assessment and structural brain imaging. Cardiol Young 2015;25:338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Favilla E, Faerber JA, Hampton LE, et al. Early Evaluation and the Effect of Socioeconomic Factors on Neurodevelopment in Infants with Tetralogy of Fallot. Pediatr Cardiol 2021;42:643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kipps AK, Graham DA, Harrild DM, Lewis E, Powell AJ, Rhodes J. Longitudinal exercise capacity of patients with repaired tetralogy of fallot. Am J Cardiol 2011;108:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Byrne ML, Mercer-Rosa L, Ingall E, McBride MG, Paridon S, Goldmuntz E. Habitual Exercise Correlates With Exercise Performance in Patients With Conotruncal Abnormalities. Pediatr Cardiol 2013;34:853–860. [DOI] [PubMed] [Google Scholar]
- 32.Duppen N, Kapusta L, de Rijke YB, et al. The effect of exercise training on cardiac remodelling in children and young adults with corrected tetralogy of Fallot or Fontan circulation: a randomized controlled trial. Int J Cardiol 2015;179:97–104. [DOI] [PubMed] [Google Scholar]
- 33.Goldmuntz E, Cassedy A, Mercer-Rosa L, Fogel MA, Paridon SM, Marino BS. Exercise Performance and 22q11.2 Deletion Status Affect Quality of Life in Tetralogy of Fallot. J Pediatr 2017;189:162–168. [DOI] [PubMed] [Google Scholar]
- 34.O’Byrne ML, DeCost G, Katcoff H, et al. Resource Utilization in the First 2 Years Following Operative Correction for Tetralogy of Fallot: Study Using Data From the Optum’s De- Identified Clinformatics Data Mart Insurance Claims Database. J Amer Heart Assoc 2020;169:e2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qureshi AM, Caldarone CA, Romano JC, et al. Comparison of management strategies for neonates with symptomatic tetralogy of Fallot and weight <2.5 kg. J Thorac Cardiovasc Surg 2021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.