Abstract
Tick borne relapsing fever (TBRF) is a zoonosis caused by various Borrelia species transmitted to humans by both soft-bodied and (more recently recognized) hard-bodied ticks. In recent years, molecular diagnostic techniques have allowed to extend our knowledge on the global epidemiological picture of this neglected disease. Nevertheless, due to the patchy occurrence of the disease and the lack of large clinical studies, the knowledge on several clinical aspects of the disease remains limited. In order to shed light on some of these aspects, we have systematically reviewed the literature on TBRF and summarized the existing data on epidemiology and clinical aspects of the disease. Publications were identified by using a predefined search strategy on electronic databases and a subsequent review of the reference lists of the obtained publications. All publications reporting patients with a confirmed diagnosis of TBRF published in English, French, Italian, German, and Hungarian were included. Maps showing the epidemiogeographic mosaic of the different TBRF Borrelia species were compiled and data on clinical aspects of TBRF were analysed.
The epidemiogeographic mosaic of TBRF is complex and still continues to evolve. Ticks harbouring TBRF Borrelia have been reported worldwide, with the exception of Antarctica and Australia. Although only molecular diagnostic methods allow for species identification, microscopy remains the diagnostic gold standard in most clinical settings. The most suggestive symptom in TBRF is the eponymous relapsing fever (present in 100% of the cases). Thrombocytopenia is the most suggestive laboratory finding in TBRF. Neurological complications are frequent in TBRF. Treatment is with beta-lactams, tetracyclines or macrolids. The risk of Jarisch-Herxheimer reaction (JHR) appears to be lower in TBRF (19.3%) compared to louse-borne relapsing fever (LBRF) (55.8%). The overall case fatality rate of TBRF (6.5%) and LBRF (4–10.2%) appears to not differ. Unlike LBRF, where perinatal fatalities are primarily attributable to abortion, TBRF-related perinatal fatalities appear to primarily affect newborns.
Author summary
Tick-borne relapsing fever (TBRF) is a bacterial disease characterized by eponymous recurrent fever episodes. The disease is common on all continents except Australia and Antarctica and is caused by several species of Borrelia bacteria. The Borrelia bacteria causing relapsing fever circulate naturally between ticks and various animal hosts (usually small rodents). Humans become infected when they are accidentally bitten by an infected tick.
Although the disease has been known since 1904, many aspects of the disease have never been investigated in larger studies and are therefore still not conclusively understood. To shed light on some of these aspects, we reviewed the published literature on TBRF and analysed all reported data on the geographic distribution of the different TBRF-causing Borrelia spp. as well as on the clinical presentations of the disease, its complications, its diagnosis and treatment and its outcome, and compiled them in this review.
Introduction
Two febrile illnesses related to human pathogenic spirochetes belonging to the genera Borrelia present as “relapsing fevers”, louse-borne relapsing fever (LBRF) and tick-borne relapsing fever (TBRF). While LBRF is an anthroponotic disease exclusively caused by Borrelia recurrentis, TBRF is a zoonotic disease caused by various Borrelia species. In different regions of the world different Borrelia spp. have been identified to be endemic. They differ in their natural enzootic cycles (involving different tick species and their hosts, mostly small rodents) and they are capable of infecting humans as accidental dead-end hosts [1]. An exemption is B. duttonii, for which humans may be the reservoir [2]. Since LBRF and TBRF present clinically identical and LBRF- and TBRF-Borrelia are microscopically indistinguishable, differentiation between the two diseases was historically limited to the epidemiological circumstances (LBRF: outbreaks, epidemics, occurrence in vulnerable populations exposed to body lice; TBRF: sporadic cases in persons exposed to ticks). Finally, the advent of molecular diagnostic techniques not only enabled to distinguish TBRF from LBRF, but also significantly changed the understanding of the diversity and epidemiology of TBRF. To summarize the current knowledge on epidemiology and clinical relevant aspects of TBRF we reviewed and analysed the existing literature, analogously to our recently published review on LBRF [3,4].
Epidemiology
Historically, in most regions of the world TBRF has always been overshadowed by LBRF which was more prominent and epidemiologically relevant because of its epidemic occurrence. With the decline of lice-infested populations in most regions of the world, LBRF became a rare disease, while TBRF received increasing attention, especially in recent years.
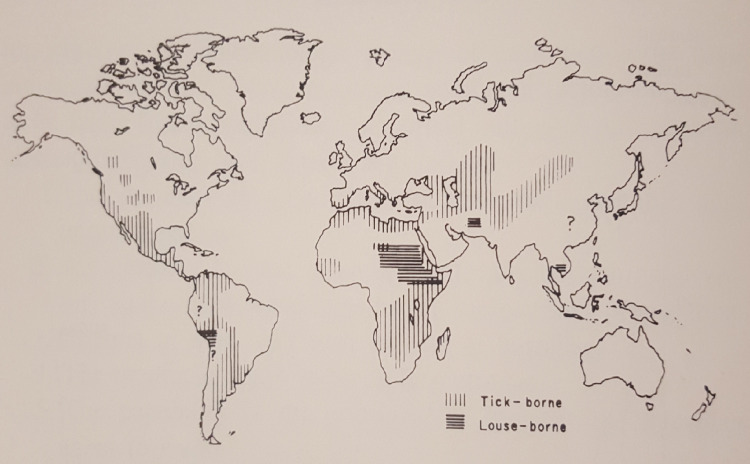
TBRF has been recognized in Africa since 1904, owing to the researches of Ross, Dutton and others [5]. In the early 1920s, TBRF was also recognized as an endemic disease in the United States of America (USA), although a tick vector was not recognized until 1930 [6]. In the following years, case reports of TBRF showed the extend of endemic areas in the USA and various tick species were identified as vectors [7]. Fig 1 shows a map with the assumed global distribution of TBRF and LBRF published in 1971 [8].
Fig 1. Assumed global distribution of TBRF and LBRF, 1950–1969 (Felsenfeld O. Borrelia; Strains, Vectors, Human and Animal Borreliosis. St. Louis: Warren H. Green; 1971[8]).
Today, TBRF is reported from all continents except Australia and Antarctica [9] and constitutes an important public health problem in some parts of the world. In Western Africa, TBRF accounts for about 13% of febrile illnesses [10] and in endemic regions of East Africa, TBRF is one of the diseases with the highest lethality among children [11].
Tick vectors
Historically, TBRF was considered to be exclusively transmitted by soft ticks (Ornithodoros spp.) [8]. In 2011, this paradigm changed when Borrelia miyamotoi, a Borrelia species discovered in Japan in 1995 [12], was reported to cause TBRF transmitted by hard ixodid ticks in Russia [4], a finding later confirmed in Europe, Japan and the USA [13–15]. Nevertheless, since most TBRF Borrelia are transmitted by soft ticks, several distinct and epidemiological relevant differences between soft and hard ticks deserve to be highlighted. Soft ticks differ from hard ticks not only by the eponymous lack of a hard shell around the mouthparts, but also by the fact that they do not wait on leaves or blades of grass for their prey to walk by. Instead, they live in close proximity to their small mammal hosts (e.g. mice, rats, squirrels, rabbits) and rarely leave the confines of their hosts’ nest or burrow. Humans may be targeted by these night active ticks when sleeping close to their habitats. Because soft ticks feed rapidly (15–90 minutes) and then return to the place from which they came, their attack is rarely noticed. Persistent infection of the ticks’ salivary glands [16] allows quick transmission of TBRF Borrelia during the short feeding period, possibly after only 30 seconds of attachment [17]. Soft tick females lay clutches of eggs after each blood meal. This reproductive pattern is strikingly different from that of hard ticks, where adult females reproduce only once in their lifetime [18]. The life cycle stages of soft ticks include egg, larva, several successive nymphs and the adult. After hatching, all stages are obligate blood feeders and capable of transmitting Borrelia [18]. Once infected, ticks remain infectious for the duration of their life. Since soft ticks may live for more than 10 years and survive up to 5 years without feeding [19], they can outlive their rodent hosts and infect several cohorts of rodents over the course of their lifespan [20].
Clinical picture
The incubation period of TBRF is 4–18 days. Thereafter, up to 12 recurrent febrile episodes occur. These fever episodes last 2–7 days and are separated by afebrile periods of up to 10 days [18,21,22]. A broad range of accompanying unspecific symptoms (e.g. headache, myalgia, chills, nausea, vomiting, arthralgia) as well as neurologic complications (e.g. meningitis, encephalitis, hemiplegia, facial palsy, radiculopathy, occasionally subarachnoid hemorrhage) may occur. They generally become more prominent after the second febrile episode [1,23,24]. The characteristic disease pattern of recurrent febrile episodes, interspersed with afebrile episodes, is attributable to the antigenic variation of different, sequentially expressed versions of the bacterium’s outer-membrane lipoprotein (vmp), allowing the bacterium to temporarily evade the host’s humoral immune response. Once the host’s immune system mounts antibodies against a specific vmp variant, a new vmp variant is expressed by the Borrelia, camouflaging itself, until antibodies are also generated against the new vmp variant [25]. The clinical presentation of LBRF is very similar to TBRF and the pathophysiological mechanism of the recurrent fever episodes is identical. However, the number of recurrent fever episodes is overall lower in LBRF (mostly less than 2) compared to TBRF (≥2), while the paroxysms last longer in LBRF (up to 10 days) compared to TBRF (≤7 days). The frequently observed neurological complications in TBRF are rare in LBRF, and TBRF is usually milder and lethality reportedly lower compared to LBRF [26].
Diagnostics
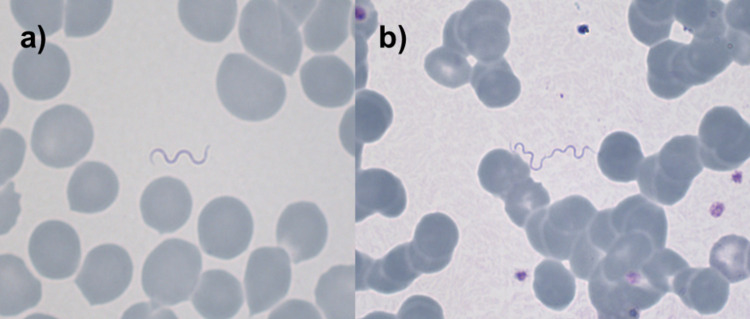
Relapsing fever Borrelia cause massive, microscopically visible bacteremia during febrile episodes. Therefore, the microscopic examination of blood smears (Fig 2) has been the diagnostic method of choice since relapsing fever Borrelia were first microscopically detected in the blood of patients by Obermeier in 1873 [3]. The optimum time to obtain blood is during the presence of fever, as Borrelia are usually not detectable once the temperature is decreasing or back to normal. Thick and thin blood films are taken and stained with e.g. Giemsa, May-Grünwald Giemsa, Wright, Wright-Giemsa, Field’s or Diff-Quick stain. Various techniques, including centrifugation of the blood samples before microscopy [27], quantitative buffy coat (QBC) preparation [28], dark-field microscopy and direct or indirect immunofluorescence [29] have been used to improve the sensitivity of microscopic detection.
Fig 2. Microscopical detection of TBRF Borrelia in blood films.
Microscopic images of Giemsa-stained thin blood films (original magnifications ×1’000) showing TBRF Borrelia in a patient suffering from TBRF fever due to Borrelia persica (courtesy of Dr. Veronika Muigg).
No commercial serological assays have been developed to diagnose TBRF. This is due to cross-reactivity among Borrelia spp., including LBRF, Lyme disease and other spirochetes (e.g. Treponema pallidum) [30] as well as the fact that serology is not helpful to diagnose acute infection due to the time to seroconversion. Culture of Borrelia spp. is difficult and time-consuming and thus largely remains restricted to research institutions. Animal inoculation was considered as a putative adjunct diagnostic tool in the late 1940s [31] but, being cumbersome, was never routinely used for diagnostic reasons alone.
With the introduction of polymerase chain reaction (PCR) and sequencing techniques in the 1980s, highly sensitive and specific diagnostic tools became available. However, the availability of these molecular diagnostic tools still remains largely restricted to research institutions and microscopy remains the diagnostic gold standard for TBRF even in affluent countries [32,33]. Table 1 summarizes the advantages and disadvantages of the different diagnostic methods.
Table 1. Overview of laboratory methods applied in TBRF and their advantages, disadvantages and use.
| Method | Advantage | Disadvantage | Use |
|---|---|---|---|
| PCR | Species specific; high sensitivity allows to differentiate TBRF- from LBRF-Borrelia and among TBRF-Borrelia | Currently no standardized protocol available; availability in resource-poor countries limited |
Largely restricted to research institutions |
| Microscopy | Fast; widely available | Variable sensitivity (spirochete density, inter-observer variability, methodological differences); does not allow species differentiation | Diagnostic gold standard |
| Culture | Isolation and growth of Borrelia spp. | Time and resource demanding; overall challenging | Largely restricted to research institutions |
| Animal inoculation | Enhanced sensitivity in cases with negative microscopy; allows differentation between TBRF and LBRF* | Time and resource demanding | Historical research method; formerly also used to "transport" Borrelia |
| Serology | Allows retrospective evaluation of infection | Not useful as acute diagnostic method due to delayed seroconversion; cross-reactivity with other non-RF Borrelia |
Restricted to epidemiological studies |
LBRF, louse borne relapsing fever; PCR, polymerase chain reaction; TBRF, tick borne relapsing fever; RF: relapsing fever.
* Note: rodents are susceptible to TBRF Borrelia spp. but not susceptible to B. recurrentis infection.
(Table adapted from [3])
Molecular epidemiology
Historically, TBRF Borrelia species are geographically grouped into Old World species (e.g. Borrelia duttonii, B. persica, B. hispanica, B. crocidurae a.o.) and New World species (e.g. B. hermsii, B. turicatae, B. parkeri a.o.). For some Borrelia species (identified in vectors and animal hosts only) their humanpathogenic potential still remains to be determined (e.g. B. cachapoal, B. osphepa a.o. [34,35]).
With the advent of PCR and sequencing techniques, it became not only possible to differentiate TBRF from LBRF, but these techniques also allowed genetic characterization of the different TBRF Borrelia species. Currently, 12 different Borrelia spp. and an additional 4 proposed "Candidatus" spp. have been reported to cause TBRF. The Candidatus status is used for newly discovered species for which more than a mere nucleic acid sequence is available but for which characteristics required for description according to the International Code of Nomenclature of Bacteria are lacking [36,37]. In general, to confirm the novelty of a bacterial species, 16S rRNA gene sequencing is performed and the sequence is compared to archived reference sequences. A threshold of 98.7% of 16S rRNA gene sequence similarity with the phylogenetically closest species with standing in the nomenclature was suggested by Stackebrandt and Ebers to classify a new bacterial species [38]. With the increasing availability of molecular diagnostic techniques, the number of reported species is likely to continue expanding in the future.
Treatment, Jarisch-Herxheimer reaction (JHR) and outcome
In the first half of the 20th century, arsenicals and emetine bismuth iodide were the only available drugs for the treatment of relapsing fever [39]. After the discovery of penicillin, treatment shifted towards this antimicrobial agent in the second half of the century with alternative therapeutic agents becoming available over time (i.e. tetracyclines, macrolides). Today, the preferred antibiotics to treat TBRF are tetracyclines, β-lactams and macrolids [40] to which Borrelia are invariably susceptible [41].
Treatment may be complicated by Jarisch-Herxheimer reaction (JHR), which mostly occurs after administering the first dose of the antibiotic. JHR is characterized by intense chills and a rise in temperature about 1–2 hours after initiating antibiotic treatment and may be complicated by hypotension. JHR shares pathophysiological features of a classic endotoxin reaction mediated by proinflammatory cytokines (tumor necrosis factor α [TNF-α], interleukin 6 (IL-6), IL-8) [42]. JHR is not restricted to relapsing fever, but may also occur when treating other spirochete infections like syphilis, leptospirosis, and Lyme disease. In TBRF, JHR is reported to occur in up to 54.1% of cases [43]. Symptoms usually resolve within a few hours. Although JHR is rarely fatal, it is a clinically relevant complication which may require appropriate clinical therapeutic measures [4].
The lethality of untreated TBRF is reported to be 2–10% [44]. With antibiotic treatment the lethality is reported to be <2% [45]. Of note, TBRF is more serious in expatriates and visitors to an endemic area compared to indigenous people, who have usually been exposed to the pathogen previously [26].
TBRF infection during pregnancy is associated with an increased risk of death in pregnant women [46,47]. Infections during pregnancy are claimed to cause up to 10–15% of neonatal deaths worldwide and a perinatal lethality of up to 43.6% has been reported [1,23,44,48–50].
The aim of this study is to review and analyse the existing literature on TBRF and to summarize the epidemiological, clinical, diagnostic and treatment aspects of the disease, including its transmission through ticks, its vector reservoir and its clinical outcome.
Methods
We performed a systematic literature search of the databases Biosis Citation Index, Biosis Previews, CINAHL, Cochrane, Current Contents Connect, Data Citation Index, Derwent Innovations Index, EMBASE Elsevier, EMBASE Ovid, Inspec, Medline, PMC, PubMed, SciELO Citation Index, Scopus, Web of Science, and Zoological Record on 04/Dec/2020, using the search term ("tick" OR "ticks" OR "tick borne" OR "Ornithodoros" OR "Borrelia" OR "Borrelia miyamotoi" OR "Borrelia turicatae" OR "Borrelia hermsii" OR "Borrelia parkeri" OR "Borrelia persica" OR "Borrelia hispanica" OR "Borrelia crocidurae" OR "Borrelia duttonii" OR "Borrelia caucasica" OR "Borrelia microti" OR "Borrelia brasiliensis" OR "Borrelia mazzottii" OR "Borrelia venezuelensis" OR "Borrelia graingeri" OR "Borrelia latyschewii" OR "Borrelia dugesii" OR "Borrelia infections" OR "Borrelia") AND ("relapsing fever" OR "recurrent fever" OR "relapsing fever disease") adapted to the search format of the different databases.
A detailed description of the literature search is available in S2 Text. After removing duplicates by EndNote (Version X9.2, Clarivate Analytics) and manually, the publications were pre-screened by title and abstract, removing those not concerning TBRF or not including the objectives of this study (epidemiology, transmission, vector, clinic, diagnostic, treatment, outcome). A full-text review of the remaining publications was then performed excluding those not meeting the inclusion criteria, according to the systematic review protocol (concerning TBRF and the objectives of the study, published in English, German, French, Italian or Hungarian), as shown in S1 Text. Publications that could neither be retrieved through the respective journals, nor by contacting libraries, or after contacting the authors, were classified as ‘not retrievable’ and excluded. During the full-text review, the reference lists of the articles were screened for additional relevant publications not identified previously («snowball-search» strategy). From the finally identified eligible studies, the following data were extracted: author, title, year of publication, type of study, study location, study period, location of acquisition/infection of Borrelia, Borrelia species, tick species, vector, percentage of ticks or vectors infected with Borrelia, hospital location for diagnosis, diagnostic method (microscopy, serology, molecular diagnostic, animal inoculation), grade of diagnostic certainty, number of patients, age of patient(s) (median and range), gender, symptoms, number of fever relapses, pregnancies, complications, used drug(s) and treatment regimen(s), number of treated or untreated patients, lethality of treated or untreated patients, frequency of JHR. To minimize bias, the same reviewer conducted a second full data extraction one month after the first extraction. Discrepancies and unclear cases were resolved by consulting a second reviewer. The probability of a correctly diagnosed TBRF was graded according to the diagnostic method used in the different studies, with PCR having the highest (grade A) and serology the lowest (grade C) evidence for a correct diagnosis (Table 2).
Table 2. Diagnostic grading system to judge the certainty of the correct diagnosis of TBRF.
| Diagnostic method | Grade of diagnostic certainty | Case classification | Comment |
|---|---|---|---|
| PCR | A | Confirmed diagnosis | Highest level of evidence, detection even at low level of spirochetemia |
| Microscopy | B | Microscopic diagnosis | High level of evidence, easy to carry out, examiner-dependent, likelihood of detection depends on level of spirochetemia |
| Culture | B | Microscopic diagnosis | High level of evidence, difficult to carry out, time demanding |
| Animal inoculation | B | Microscopic diagnosis | High level of evidence, difficult to carry out, time demanding |
| Serology | C | Indirect evidence | Intermediate level of evidence, not standardized, cross-reaction with other Borrelia (e.g. Lyme disease) possible |
PCR, polymerase chain reaction.
The data extraction sheet is available in S1 Table.
To visualize the worldwide distribution of TBRF cases, the causative TBRF Borrelia spp. and the transmitting tick species, we used the free online geographic application Mapchart (www.mapchart.net).
Results
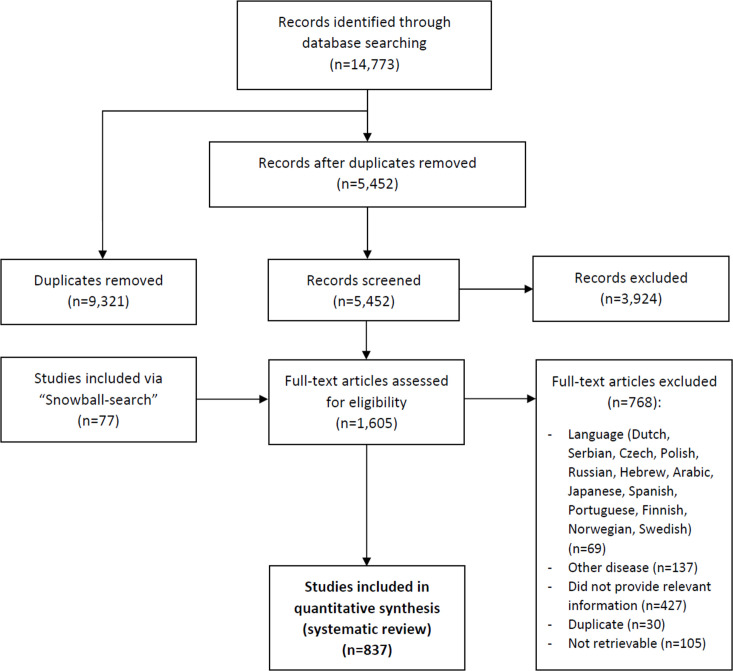
Our search identified 14,773 publications, of which 837 proved to be eligible for inclusion in the review (Fig 3). The reference list of the included and excluded publications and the PRISMA Checklist for systematic reviews are available in S3 Text and S1 PRISMA Checklist.
Fig 3. Flow diagram of search and selection of eligible publications.
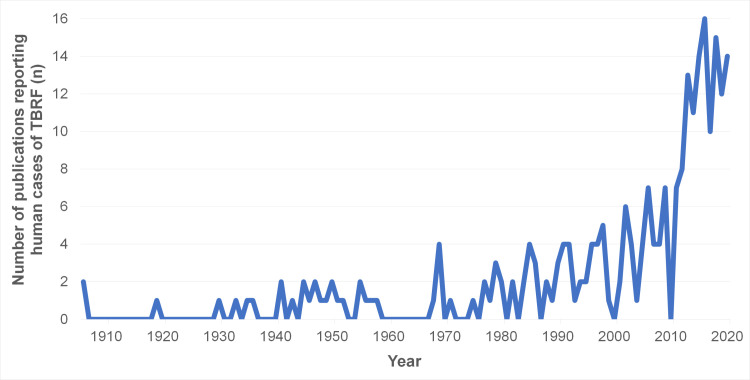
Fig 4 shows the number of TBRF case studies published from 1906 to 2020.
Fig 4. Number of TBRF case studies published from 1906 to 2020.
TBRF, tick borne relapsing fever.
Geographic distribution of human TBRF cases and worldwide prevalence of TBRF-transmitting ticks and Borrelia species
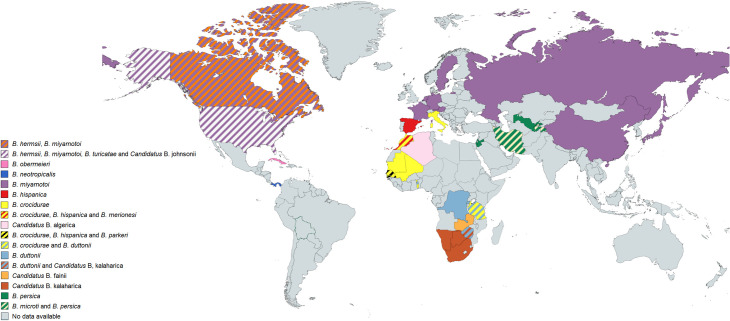
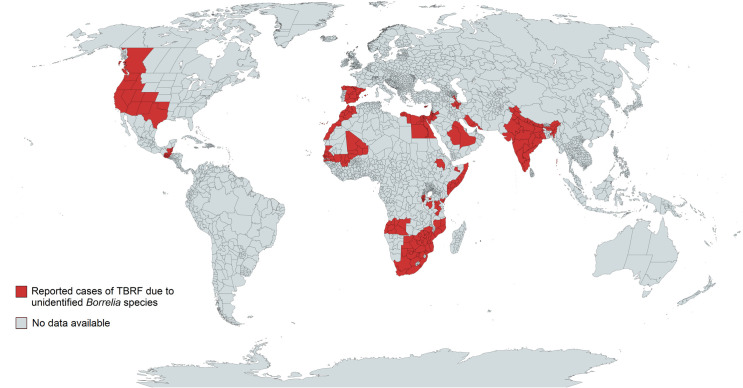
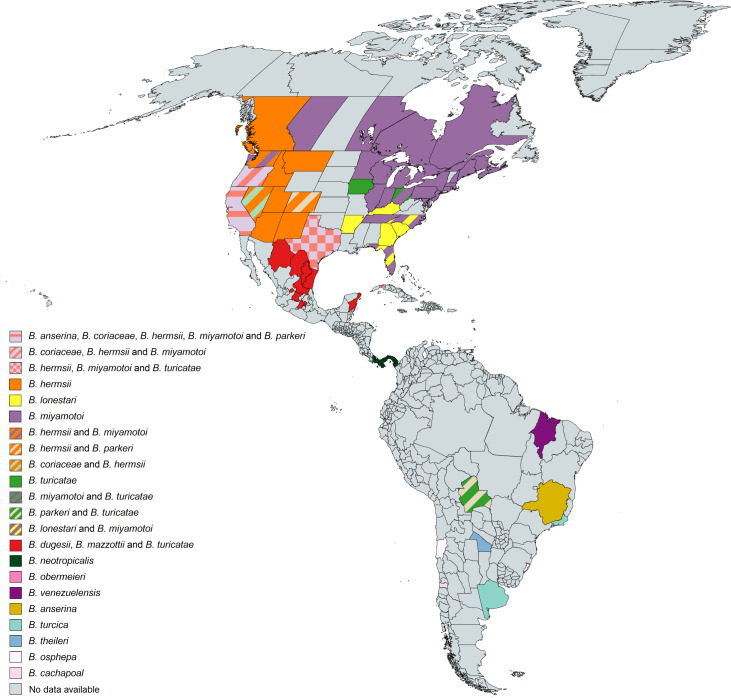
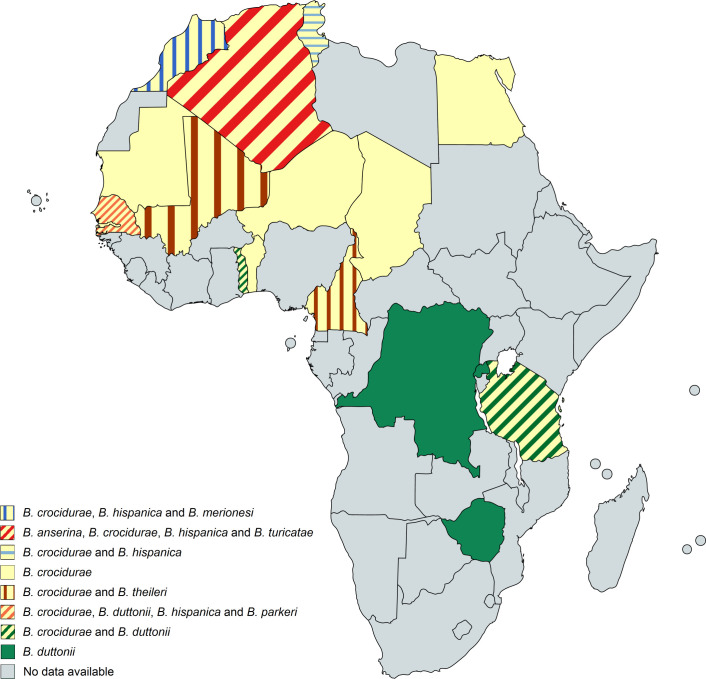
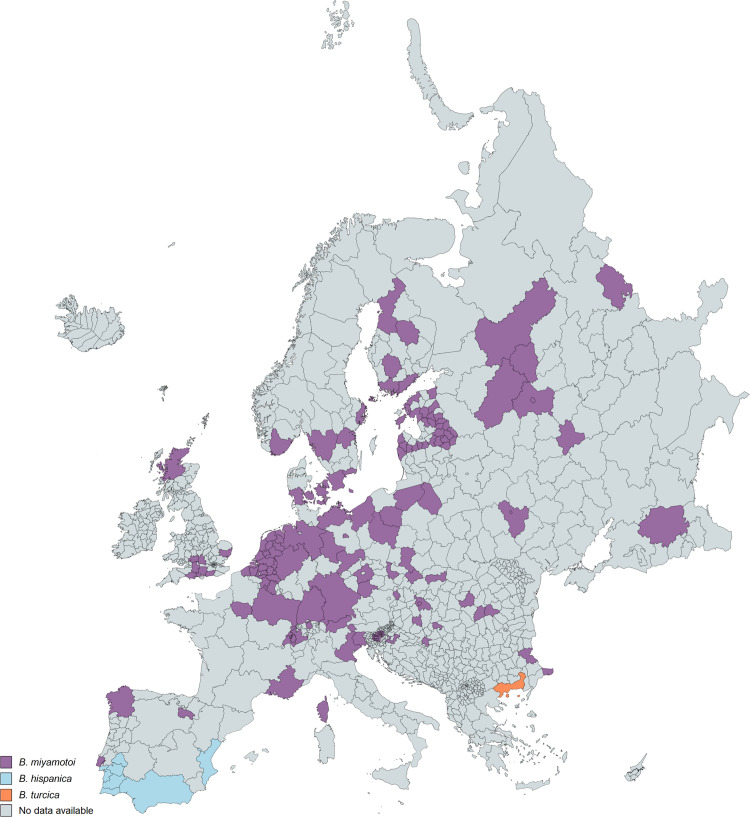
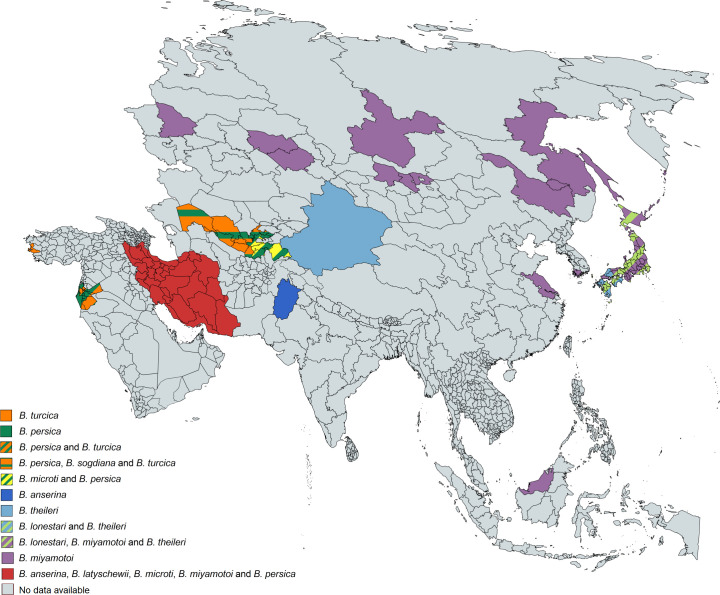
385 of the 837 analysed studies reported the distribution of either ticks, Borrelia spp. or both. Some of the reported Borrelia are not yet acknowledged as official species and are currently considered “Candidatus” species. In North America, four Borrelia species (B. miyamotoi, B. hermsii, B. turicatae, Candidatus B. johnsonii) causing TBRF in humans are reported [15,43,51–104], in Central and South America four species (B. obermeieri, B. neotropicalis, B. turicatae, B. parkeri) [105–107], in Africa eight species (B. crocidurae, B. hispanica, B. merionesi, B. parkeri, B. duttonii, Candidatus B. algerica, Candidatus B. fainii, Candidatus B. kalaharica) [10,50,108–141], in Europe three species (B. miyamotoi, B. hispanica, B. crocidurae) [13,142–154] and in Asia three species (B. miyamotoi, B. persica, B. microti) [14,155–176]. No cases of TBRF or ticks known to transmit TBRF Borrelia are reported in Australia. The detailed list of Borrelia and ticks reported in the different continents and regions can be found in S1 Data. The worldwide distribution of reported TBRF cases by country and the causative Borrelia species are shown in Fig 5. The worldwide distribution of reported TBRF cases caused by unidentified Borrelia species is shown in Fig 6. The prevalence of Borrelia species causing TBRF in America, Africa, Europe, and Asia (based on detection in animal blood samples and/or ticks) is shown in Figs 7, 8, 9 and 10, respectively. The prevalence of competent vector ticks for TBRF Borrelia in America, Africa, Europe, and Asia can be found in S1 Fig.
Fig 5. Reported TBRF cases by country and causative Borrelia species.
B., Borrelia. Map created on www.mapchart.net.
Fig 6. Reported TBRF cases caused by unidentified Borrelia species.
TBRF, Tick borne relapsing fever. Map created on www.mapchart.net.
Fig 7. Reported presence of TBRF Borrelia species in ticks and animal hosts in America.
B., Borrelia. Map created on www.mapchart.net.
Fig 8. Reported presence of TBRF Borrelia species in ticks and animal hosts in Africa.
B., Borrelia. Map created on www.mapchart.net.
Fig 9. Reported presence of TBRF Borrelia species in ticks and animal hosts in Europe.
B., Borrelia. Map created on www.mapchart.net.
Fig 10. Reported presence of TBRF Borrelia species in ticks and animal hosts in Asia.
B., Borrelia. Map created on www.mapchart.net.
Known and putative TBRF spp. and their animal host(s) and transmitting tick species
124 studies reported data on Borrelia spp. and their associated animal hosts and transmitting ticks. Table 3 lists the known humanpathogenic TBRF Borrelia spp. as well as Borrelia spp. with yet unknown humanpathogenic potential, their known animal hosts and transmitting tick species.
Table 3. Known and putative TBRF Borrelia spp. and their animal host(s) and transmitting tick species.
| Borrelia spp. causing TBRF (number of reported human cases with unequivocal species identification*) | Animal host(s) | Transmitting tick species |
| B. crocidurae (425) | Rodents, shrews | O. erraticus, O. sonrai |
| B. duttonii (141) | Chicken, pigs | O. moubata, O. porcinus |
| B. hermsii (616) | Chipmunks, deer, dogs, owls, rodents, squirrels | O. hermsii |
| B. hispanica (128) | Cats, cattle, dogs, hedgehogs, pigs, rodents, sheep, warblers | O. erraticus, O. marocanus, O. occidentalis |
| B. merionesi | ? | ? |
| B. microti (1) | Hedgehogs, rodents, toads | O. erraticus |
| B. miyamotoi (639) | Birds, cats, cattle, deer, dogs, hedgehogs, ponies, rodents, sheep, squirrels, wild boar | Am. americanum, D. reticulatus, D. variabilis, Ha. concinna, Ha. inermis, Ha. longicornis, Ha. punctata, I. dentatus, I. hexagonus, I. nipponensis, I. pacificus, I. pavlovskyi, I. persulcatus, I. ricinus, I. scapularis |
| B. neotropicalis (106) | ? | ? |
| B. obermeieri (1) | ? | ? |
| B. parkeri | Horses | O. parkeri |
| B. persica (415) | Camel, cat, cattle, dog, hyrax | O. tholozani |
| B. turicatae (4) | Birds, coyotes, dogs, foxes, rats, tortoises | C. capensis, C. kelleyi, O. turicata |
| Candidatus B. algerica (1) | ? | ? |
| Candidatus B. fainii (1) | Rodents | ? |
| Candidatus B. johnsonii (1) | Bats | C. kelleyi |
| Candidatus B. kalaharica (2) | ? | O. savigni |
| Borrelia spp. with yet unknown human-pathogenic potential | Animal host(s) | Transmitting tick species |
| B. anserina | Birds | Ar. minatus, Ar. persicus |
| B. baltazardii | ? | ? |
| B. brasiliensis | ? | O. brasiliensis |
| B. caucasica | ? | ? |
| B. coriaceae | Deer | O. coriaceus |
| B. dugesii | Rats | ? |
| B. graingeri | ? | O. graingeri |
| B. latyschewii | Birds | O. tartakovskyi |
| B. lonestari | Birds, deer, dogs | Am. americanum, C. capensis |
| B. lonestari-like | Deer | Ha. spp. |
| B. mazzottii | Rats | O. talaje |
| B. osphepa | ? | O. spheniscus |
| B. sogdiana | Rodents | O. papillipes |
| B. theileri | Bats, deer | Rh. geigyi |
| B. turcica | Birds, camels, cattle, tortoises | Am. aureolatum, Am. longirostre, Hy. aegyptium |
| B. venezuelensis | ? | O. rudis |
| Candidatus B. mvumi | ? | O. porcinus |
| Candidatus B. texasensis | Coyotes | D. variabilis |
| Unidentified Borrelia spp. | Bats, buffalos, cats, cattle, chipmunks, deer, dogs, lizards, penguins, rabbits, rodents, sheep, shrews, snakes, tortoises, turtles, wild boar | Multiple tick species |
Am., Amblyomma; Ar., Argas; B., Borrelia; C., Carios; D., Dermacentor; Ha, Haemaphysialis; Hy., Hyalomma; I., Ixodes; O., Ornithodoros; Rh., Rhipicephalus; spp., species (plural);?, unknown.
*In total, we found 9,372 reported cases of TBRF in the literature. The table contains only the unequivocally attributable (PCR confirmed) number of cases caused by the respective Borrelia species.
TBRF case studies
228 (27.2%) of the 837 analysed publications reported a total of 9,372 human TBRF cases. For 5,755 cases, the patients’ gender was reported: 3,164 (55.0%) were male, 2,591 (45.0%) were female. For 2,775 cases, the patient’s age was reported: the median age of male patients was 32.7 years (range <1–90), the median age of female patients was 34.6 years (range <1–90). Table 4 lists the countries where the infections were acquired.
Table 4. Number of publications on TBRF cases by country where the infections were most likely acquired (n = 240 studies).
| Number of publications | Country where the TBRF cases reported in the publication acquired their infection (number of cases) |
|---|---|
| 84 | USA (1,341; 182*) |
| 20 | Senegal (229; 238*) |
| 14 | Iran (2,538), Israel (753) |
| 13 | Spain (267; 3*) |
| 12 | Tanzania (930) |
| 7 | Canada (55; 182*) |
| 6 | Morocco (131; 3*), Russia (317) |
| 5 | India (158; 1*) |
| 4 | Japan (5), Mali (3; 238*), South Africa (23; 3*), Tajikistan (2; 2*) |
| 3 | Botswana (3*), Cyprus (111), France (58), Mauritania (3; 238*), Namibia (1; 2*), Netherland (3), Rwanda (109), Uzbekistan (1; 2*), Jordan (237), Zimbabwe (14; 1*) |
| 2 | Egypt (1; 9*), Libya (4; 9*), Mexico (2), Saudi Arabia (3), Somalia (1,147) |
| 1 | Algeria (1), Angola (4), Austria (1), Belize (1*), Burundi (1), China (14), Cuba (1), Democratic Republic of the Congo (13), Ethiopia (262), Germany (1), Guatemala (1*), Italy (1), Kenya (49), Mozambique (1*), Nepal (1*), Palestine (4), Panama (106), Sweden (2), Togo (21), Zambia (1) |
TBRF, tick borne relapsing fever; USA, United States of America.
* Number of additional cases which may have contracted TBRF in the respective country, but since the ill person visited additional countries within the presumed incubation period, the infection may have also been acquired elsewhere.
Information about travel-related TBRF cases are shown in Table 5.
Table 5. Case analysis on TBRF in travelers.
| Year | No. cases | Infection acquired in | Imported to | Borrelia spp. | Complications | Ref. |
|---|---|---|---|---|---|---|
| 1982 | 1 | Namibia | South Africa | ? | None reported | [177] |
| 1985 | 1 | Cyprus | England | ? | None reported | [178] |
| 1988 | 1 | Israel | USA | ? | JHR (n = 1) | [179] |
| 1991 | 2 | Senegal | Belgium | ? | Meningoencephalitis (n = 1), JHR (n = 1) | [180] |
| 1993 | 3 | USA | Canada | B. hermsii | JHR (n = 1) | [67] |
| 1995 | 1 | Saudi Arabia | USA | ? | None reported | [181] |
| 1996 | 1 | Nepal or India | Denmark | ? | None reported | [182] |
| 1999 | 2 | Gambia or Senegal | Netherlands | B. crocidurae | Meningitis (n = 1) | [116] |
| 1999 | 1 | Senegal | Italy | ? | None reported | [183] |
| 2005 | 3 | Spain or Morocco | France | B. crocidurae, B. hispanica | None reported | [140] |
| 2006 | 1 | Guatemala or Belize | Netherlands | ? | None reported | [184] |
| 2006 | 1 | Senegal | Italy | B. crocidurae | None reported | [125] |
| 2007 | 1 | Mali | France | ? | None reported | [185] |
| 2008 | 4 | Senegal | France | ? | Meningoencephalitis (n = 1), JHR (n = 1) | [186] |
| 2009 | 1 | Senegal | France | B. crocidurae | None reported | [120] |
| 2010 | 1 | Senegal | Belgium | B. crocidurae | Meningoencephalitis (n = 1), JHR (n = 1) | [112] |
| 2010 | 1 | Uzbekistan | Japan | B. persica | None reported | [164] |
| 2011 | 1 | Uzbekistan or Tajikistan | France | B. persica | None reported | [175] |
| 2009–2011 | 4 | Senegal | France | B. crocidurae | Encephalitis (n = 2), meningitis (n = 3) | [119] |
| 2015 | 1 | Southern Africa | Germany | Candidatus B. kalaharica | None reported | [109] |
| 2016 | 1 | Southern Africa | Germany | Candidatus B. kalaharica | JHR (n = 1) | [110] |
| 2017 | 1 | Morocco | Belgium | B. hispanica | None reported | [137] |
| 2017 | 1 | USA | Japan | B. miyamotoi | None reported | [95] |
| 2018 | 1 | Senegal | France | B. crocidurae | None reported | [118] |
| 2019 | 1 | Botswana or South Africa | Netherlands | ? | None reported | [187] |
| 2019 | 1 | Tajikistan | Switzerland | B. persica | JHR (n = 1) | [174] |
| 2020 | 1 | Jordan | USA | B. persica | None reported | [170] |
| 2020 | 2 | Mali | France | B. crocidurae | None reported | [117] |
| 2020 | 1 | Tajikistan | Italy | B. microti | None reported | [155] |
B., Borrelia; JHR, Jarisch-Herxheimer reaction; Ref., reference; spp., species (plural); USA, United States of America; ?, unknown.
Symptoms related to TBRF
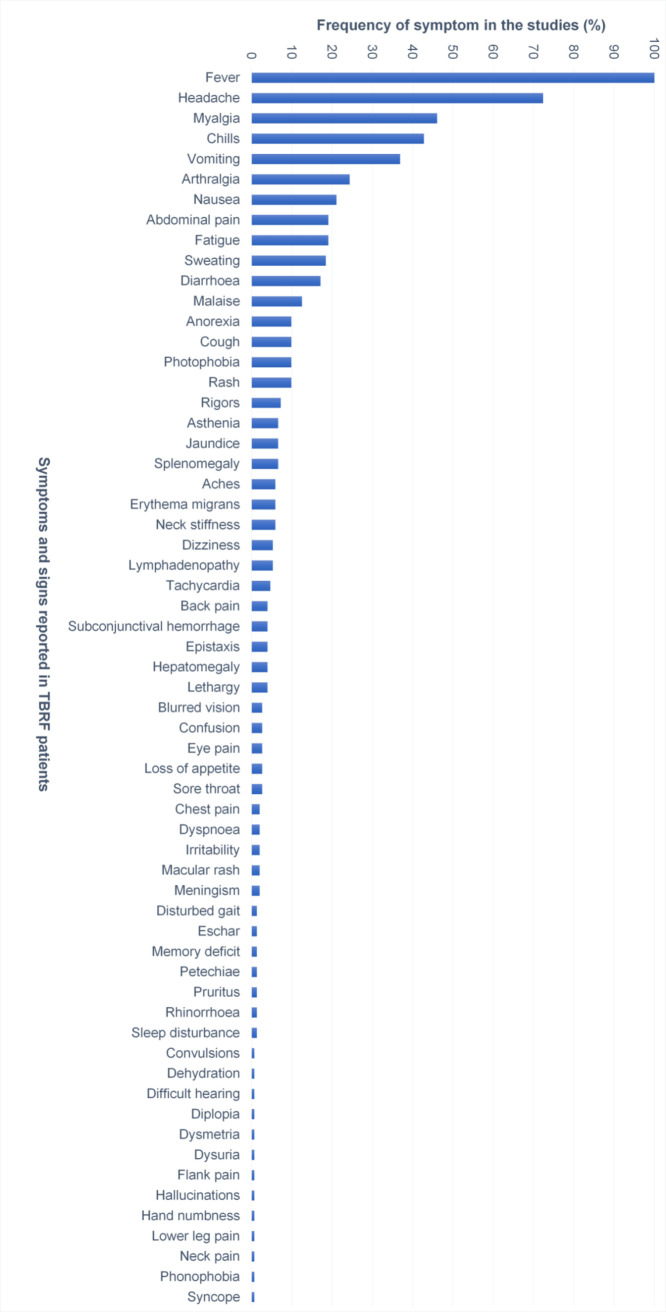
A total of 152 publications reported specific symptoms related to TBRF. Fig 11 shows the relative frequency of these symptoms.
Fig 11. Relative frequency of signs and symptoms (in %) related to TBRF (n = 152 studies).
TBRF, tick borne relapsing fever.
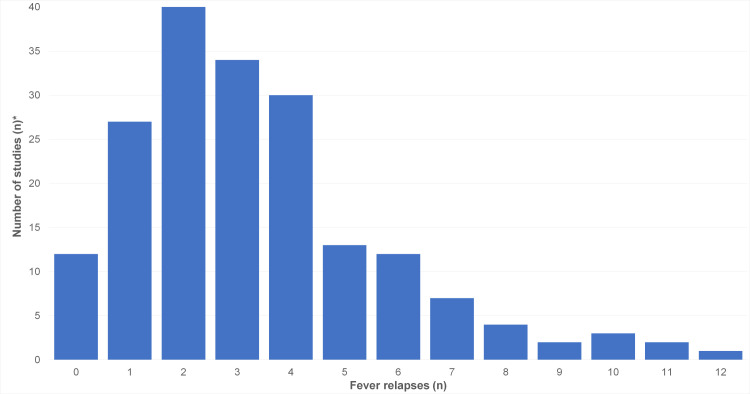
The number of relapsing fever episodes was reported in 67 publications (Fig 12).
Fig 12. Number of relapsing fever episodes in studies on TBRF (n = 67 studies).
* Note: Since the number of relapsing fever episodes within single studies was mostly reported as median, an evaluation per case was not possible.
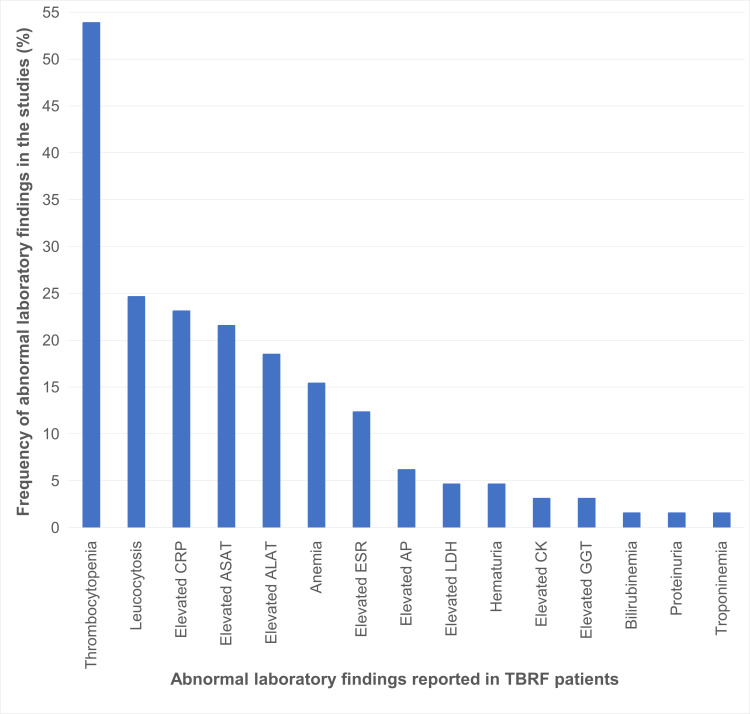
Abnormal laboratory findings, were described in 65 studies (Fig 13).
Fig 13. Abnormal laboratory findings related to TBRF (n = 65 studies).
ALAT, alanine aminotransferase; AP, alkaline phosphatase; ASAT, aspartate transaminase; CK, creatine kinase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GGT, gamma-glutamyltransferase; LDH, lactate dehydrogenase; TBRF, tick borne relapsing fever.
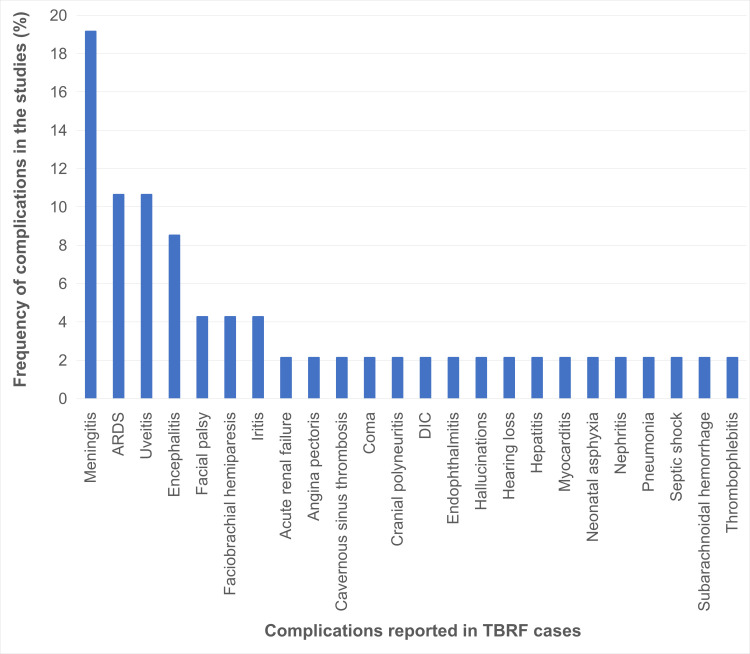
Information on complications other than preterm delivery (61 cases), was available in 47 studies for 433 of the analysed 9,372 TBRF cases (Fig 14).
Fig 14. Complications of TBRF (n = 47 studies).
ARDS, acute respiratory distress syndrome; DIC, disseminated intravascular coagulation; TBRF, tick borne relapsing fever.
Diagnostic
Details on the diagnostic methods used to diagnose TBRF was available for 7,612 (81.2%) of the analysed 9,372 cases (Table 6).
Table 6. Diagnostic methods used to diagnose TBRF in 7,612 cases (n = 240 studies).
| Diagnostic method | Grade of diagnostic certainty | Number of cases in which this diagnostic method was applied | Number of cases diagnosed only by this method | Number of cases diagnosed by a combination of diagnostic methods | Number of cases where this method was the method with the highest grade of diagnostic certainty |
|---|---|---|---|---|---|
| PCR | A | 3,443 | 2,051 | 1,392 | 3,443 |
| Microscopy | B | 5,159 | 2,732 | 2,427 | 3,792 |
| Culture | B | 129 | 0 | 129 | 0 |
| Animal inoculation | B | 756 | 0 | 756 | 0 |
| Serology | C | 1,139 | 377 | 762 | 377 |
PCR, polymerase chain reaction.
Note: in 2,452 (32%) of the 7,612 cases a combination of diagnostic tests was used to establish the diagnosis. Thus, the number of tests exceeds the number of cases.
Treatment
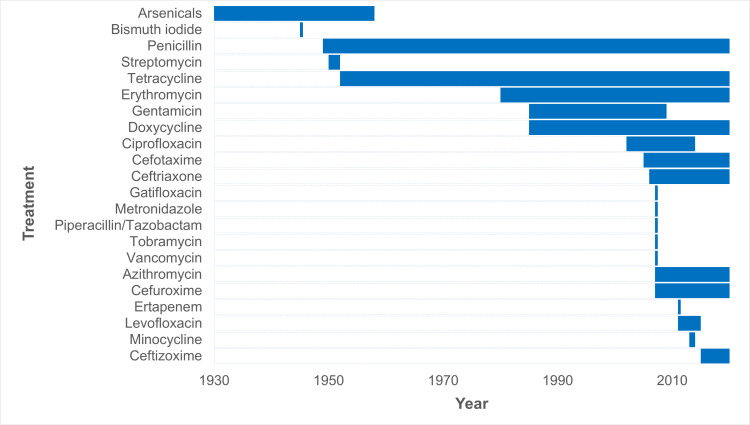
Information on antimicrobial treatment was available for 1,274 (13.6%) of the analysed 9,372 TBRF cases. 1,238 patients received antimicrobial treatment, 36 patients received no antimicrobial treatment. Fig 15 shows the use of the different antimicrobial compounds/drugs, as reported in the studies, from 1930 until today. Detailed data on the used treatment regimens (frequency, dosage, length of treatment) can be found in S2 Table. Because of the large heterogeneity and the lack of precise data, a detailed analysis of the used treatment regimens was omitted.
Fig 15. Use of different antimicrobial compounds/drugs to treat TBRF as reported from 1930 until today (n = 172 studies).
JHR and outcome
Information on the occurrence of JHR was available for 1,189 (12.7%) of the 9,372 analysed TBRF cases. JHR occurred in 230 (19.3%) of antimicrobially treated patients. Data on antibiotic treatment and the occurrence/absence of JHR was reported in 65 studies (Table 7). JHR was fatal in 15 (6.5%) cases [46,133,188].
Table 7. Treatment specific frequency of JHR in TBRF (n = 65 studies).
| Antimicrobial treatment regimen | Number of patients with reported treatment regimen and reported occurrence/absence of JHR (N) | Number of reported JHR (N) | Frequency of JHR (%) |
|---|---|---|---|
| Tetracyclines | 116 | 28 | 24.1 |
| Doxycycline | 83 | 18 | 21.7 |
| Tetracycline | 33 | 10 | 30.3 |
| β-lactams | 26 | 4 | 15.4 |
| Penicilline | 12 | 1 | 8.3 |
| Ceftriaxon | 12 | 2 | 16.7 |
| Cefuroxim | 2 | 1 | 50.0 |
| Erythromycin | 13 | 4 | 30.8 |
JHR, Jarisch-Herxheimer-reaction.
Information on the clinical outcome was available for 1,454 (15.5%) of the analysed 9,372 TBRF cases. 95 (6.5%) of the 1,454 cases were fatal [39,46,115,133–135,188–198]. 88 fatal cases were reported from Africa (Tanzania 72, Ethiopia 12, Democratic Republic of Congo 1, Egypt 1, Rwanda 1, Senegal 1), 5 from the USA, and 2 from Israel. Table 8 lists the outcome of TBRF in different patient groups.
Table 8. Case fatality analysis of TBRF (n = 17 studies).
| Patient group | Number of fatal cases (%) | Number of documented fatal cases among cases with documented antimicrobial treatment (N = 629) | Number of documented fatal cases among cases with unknown antimicrobial treatment status (N = 816) | Number of documented fatal cases among documented untreated cases (N = 9) |
|---|---|---|---|---|
| All cases of TBRF not infected during pregnancy and not infected in the fetal or peripartal period (N = 992) | 43 (4.3%) | 38 | 5 | 0 |
| Pregnant women (N = 231) | 11 (4.8%) | 0 | 11 | 0 |
| Fetuses and neonates (N = 231) | 0 | |||
| Intrauterine death | 11 (4.8%) | 2 | 9 | 0 |
| Postpartal death | 30 (13.0%) | 12 | 18 | 0 |
| Total | 95 (6.5%) | 52 | 43 | 0 |
CFR, case fatality rate.
Discussion
Publications on TBRF
Over the last 30 years, the number of published case studies on TBRF has increased significantly (Fig 4). The increasing number of publications over time might be attributable to the advent of molecular diagnostic tools as well as the increasing awareness and recognition of the disease, which previously was very likely underdiagnosed.
Epidemiology
With the advent of molecular diagnostic techniques and the resulting identification of multiple new TBRF causing Borrelia spp., as well as of previously unknown epidemic areas, the historic classification of TBRF Borrelia into Old World and New World TBRF Borrelia spp. has been replaced by a more complex picture. This is particularly evident when comparing the map outlining the TBRF endemic areas as assumed by Felsenfeld five decades ago (Fig 1) with maps compiled from confirmed data (Figs 5 and 6). Interestingly, some regions of the world, like e.g. Europe or Japan, emerged as endemic regions. Contrastingly, other regions like South America or central Africa were apparently considered more endemic in the past than they actually are (Figs 5 and 6). However, rating the relevance of TBRF in a particular region of the world demands not only to integrate the mere presence of TBRF Borrelia and the occurrence of human cases but also needs to take into account differences in available diagnostic capacities as well as a reporting bias accruing from differences in academic publishing traditions (Table 4). It is to be expected that in the future additional endemic areas, additional Candidatus- as well as proven TBRF-Borrelia spp., additional animal host reservoirs, and additional transmitting tick species will emerge (Table 3) and further expand the granularity of our picture of TBRF.
The rather recent discovery of B. miyamotoi’s wide geographic distribution (Figs 5, 7, 9 and 10), as well the surprising finding that this Borrelia sp. is not only transmitted by various soft tick species [156,199–202] but also by hard ticks (Ixodes spp. [152,203]), which were previously considered non-vector-competent for TBRF Borrelia, serve as good example. The demand for an adequate ecological niche serving not only a specific animal host but also the transmitting tick species may be the main reason why TBRF is restricted to certain geographic areas. There are two ways for TBRF Borrelia spp. to spread geographically: either the infected host animals move into new areas with locally prevalent vector competent tick species or infected ticks, attached to overland migrating host animals or to migratory birds, translocate into new areas with suitable animal hosts and a suitable habitat allowing the ticks survival. Its potential to infect a broad range of host animals, and especially migratory birds, may be the reason why B. miyamotoi shows a wide geographic distribution all around the Northern hemisphere (Figs 5, 7, 9 and 10). Since migration by birds demands prolonged attachment of the vector tick species, the unusual vector competence of hard ticks may also play a pivotal role in the extend geographic distribution of B. miyamotoi. Unlike the rather short attachment and feeding time of soft ticks, the prolonged attachment and feeding of hard ticks would be favourable for migration over large distances [1,204]. In contrast, TBRF Borrelia spp. with a narrow and spatially limited host range are rather unlikely to extend their geographic range, as for instance in the case of B. coriaceae. With deer being the only associated animal host, this Borrelia species is to date only reported in the Western part of the USA (California, Nevada, Oregon). Whether and to what extend climatic changes will influence the epidemiology and possible spread of TBRF in the future remains to be seen [205,206]. Reports of TBRF in international travelers are rare. To date, only 42 cases have been published (Table 5). Although underdiagnosing and underreporting is likely, existing surveillance data on infectious diseases in travelers confirm the apparently overall low exposure risk and the rare occurrence of TBRF in travelers [207].
Signs, symptoms and complications
Presenting similar to a multitude of other febrile infectious diseases without specific signs and symptoms (Fig 11), TBRF may easily be missed or misdiagnosed. Furthermore, the under- or misdiagnosis of TBRF may also be caused by its mostly benign and, even without antimicrobial treatment, self-limiting course. The sensitivity of TBRF Borrelia to standard antibiotics widely available and used for empirically treatment may also contribute to underdiagnosing.
Even in endemic regions, cases presenting without the diagnosis-suggestive relapsing fever episodes or without complications leading to a thorough diagnostic work-up, are likely to be missed. The most helpful symptom to differentiate TBRF from other febrile illnesses are the recurrent fever episodes, which are found in the majority of cases (Fig 12). When looking at Fig 12 it needs to be emphasized that the presented data do not reflect the natural course of TBRF as almost all reported and analysed cases received antimicrobial treatment. Thus, the number of fever relapses in untreated TBRF is very likely higher. In addition, it must be kept in mind that the number of fever relapses indirectly reflects the time elapsing before a patient seeks medical help or has access to medical care. This may differ widely across countries and health care systems. Likewise, it is very likely that in endemic areas physicians familiar with the disease will suspect, diagnose and treat TBRF much earlier compared to their colleagues in non-endemic areas.
To date, there is largely insufficient data to compare different TBRF Borrelia species with regard to possible differences in their clinical manifestations. We performed a subgroup analysis regarding the signs and symptoms, complications and number of fever relapses reported in TBRF caused by B. hermsii and B. crocidurae as for these the most data were available (S2 Fig). However, due to the low number of cases as well as the inhomogeneity of the available data, conclusions regarding possible differences in clinical manifestations of different TBRF Borrelia species remain difficult.
The only exception may be the reported rates of neurologic complications: in contrast to LBRF, neurological complications are common in TBRF [26], reported to occur in 10–40% of the cases [33,208]. Our analysis confirms the prominence of central nervous system (CNS) involvement among the reported complications of TBRF (Fig 14). A comprehensive review on neurologic and ophthalmologic involvement and complications in TBRF has been published by Cadavid and Barbour in 1998 [33]. Generalized neurologic symptoms like dizziness, apathy or delirium are considered attributable to spirochetemia and high fever rather than to direct invasion of the CNS by Borrelia and were reported in LBRF as well as TBRF. Neuropsychiatric abnormalities not solely attributable to high fevers have been reported for both TBRF and LBRF and cases of encephalitis or encephalopathy are occasionally observed in TBRF and LBRF [33]. Neurologic complications differ in their frequency and pathogenesis among the two diseases. While neurologic complications in LBRF are rare and primarily attributed to CNS hemorrhage and not to direct invasion of the CNS by the pathogen, neurologic complications in TBRF are frequently observed and attributed to direct invasion of the CNS invasion by Borrelia [33]. Neurologic manifestations are more likely to present during subsequent, rather than the initial febrile period [33]. A frequently reported complication in TBRF (and not reported in LBRF) is cranial neuritis, most often presenting in the form of facial palsy. Its frequency varies with different TBRF Borrelia species between 3% (7/230) of B. hispanica-related cases to 38% (8/21) of B. turicatae-related cases [33]. However, as mentioned above, data are limited, inhomogeneous, and come from times when molecular species identification was not possible. Nevertheless, data from animal studies also suggest that the different TBRF Borrelia species are neuroinvasive to varying degrees [33].
Reports of B. miyamotoi associated meningoencephalitis in immunocompromised patients suggests that the pathogens may behave like an opportunistic pathogen in this population [13,15,153]. Whether and to what extend B. miyamotoi may also cause neurological complications in non-immunocompromised patients is currently unknown.
Limited data suggest possible differences regarding the clinical presentation of soft tick-borne RF and hard tick-borne/B. miyamotoi RF ("Cases with the characteristic recurring febrile episodes interspersed with non-febrile intervals that typify classical RF have only been described sporadically [in B. miyamotoi RF]. … Furthermore, unlike RF spirochetes, epistaxis, abortion, jaundice and major organ failure have not appeared as features of B. miyamotoi infection" [209]), but the currently available data is limited and has yet to prove itself. Pooled clinical data, like presented in Figs 11–14, may thus not necessarily reflect the true picture on species level, but as mentioned above, the overall low number of reported cases and the inhomogeneity of the available data do not allow for robust subgroup analyses. Ocular involvement in TBRF includes iritis, cyclitis, choroiditis, and optic neuritis (Figs 11 and 14). When eye involvement is reported in TBRF, it is bilateral in one-third of the cases and almost always occurs after the third or fourth febrile episode. Involvement of the eyes during LBRF has not been reported [33].
The historical statement that the occurrence of vomiting in TBRF is exclusively related to meningitis [26] cannot be confirmed, as overall, gastrointestinal symptoms are quite common in TBRF (Fig 11).
Interestingly, Erythema migrans, a symptom highly specific for Lyme disease, has been reported in some cases of TBRF (Fig 11). Thus, it may be speculated that coinfections with other locally endemic tick-borne pathogens could lead to overlapping presentations making it difficult to attribute signs and symptoms to a specific pathogen. This speculation is strongly supported by the fact that all reported TBRF cases presenting Erythema migrans were reported from Russia, the Netherlands, and Japan and caused by B. miyamotoi, the only TBRF Borrelia transmitted by hard ticks and thus, by ticks plausibly capable of co-transmitting Lyme Borrelia. In analogy, the report of an eschar (Fig 11), a symptom primarily associated with rickettsial infections, suggests coinfection.
The frequency and relevance of such coinfections remains unclear. An outbreak of a febrile illness in West Texas was initially wrongly attributed to Lyme disease, based on a combination of facial palsy and a positive B. burgdorferi serology in some of the cases. However, in the end the disease was identified as TBRF due to B. turicatae and the serological results recognized as cross-reactivity [210].
Bleeding signs like petechiae, epistaxis, subconjunctival hemorrhage or hemorrhagic complications like subarachnoidal hemorrhage or disseminated intravascular coagulation (DIC, Fig 14) are only rarely reported in TBRF (Fig 11) when compared to LBRF, where subconjunctival hemorrhages and epistaxis are common (25%) and severe hemorrhage (hemoptysis, gastrointestinal bleeding, retinal hemorrhages) and DIC (leading to intracranial, massive gastrointestinal, pulmonary or peripartum hemorrhage) are feared complications [211].
Laboratory findings
Like the signs and symptoms, the laboratory findings are rather unspecific. Only thrombocytopenia is a feature present with a rather high frequency, possibly helping to support the tentative diagnosis (Fig 13). However, fever and thrombocytopenia occur in a variety of infections, including malaria, a broad range of common viral infections and notably also in many other tick-borne diseases (e.g., rickettsioses, ehrlichiosis, anaplasmosis, tularemia, Q fever, babesiosis, arboviral infections).
Diagnostic
To date, microscopy of thin and thick blood smears remains the most frequently reported diagnostic method for diagnosing TBRF (Table 6). Regarding the sensitivity of microscopy, it is important to remember that the positivity thresholds of thin and thick smear preparations are estimated at 105 and 104 spirochetes per mL of blood, respectively [28] and that the number of Borrelia in the peripheral blood is considered to be lower in TBRF compared to LBRF (an observation repeatedly quoted, but for which clear evidence is missing) [3]. To improve the sensitivity of microscopy using equipment that is easily available in small health centers, a method based on enrichment of bacteria by centrifugation followed by Giemsa staining was developed. This methods reduces the detection level to fewer than 10 spirochetes per mL of blood [27]. RF Borrelia are not infrequently detected in blood smears ordered because of the clinical suspicion of malaria. With the trend to progressively replace microscopy with rapid diagnostic tests (RDTs) to diagnose malaria, the incidental finding of RF Borrelia in malaria smears will become less, potentially further increasing the underdiagnosing of this disease.
PCR has grown in importance and is now the second most frequently reported diagnostic method. PCR is the most sensitive and specific diagnostic method available and the only diagnostic method to definitively differentiate between TBRF and LBRF (although, in most cases of microscopically detected RF Borrelia the epidemiological circumstances will allow to conclude whether TBRF or LBRF is the more likely diagnosis [3]) and to differentiate the different TBRF Borrelia species. However, because Borrelia are highly related at the molecular level (16S rRNA gene sequence variability ≤1%), the development of discrimination PCR assays is challenging and they will not always be able to provide species identification [128,182,212,213]. For instance, for B. duttonii and B. recurrentis, which are genetically and genomically very closely related, even PCR assays fail to provide species discrimination [214]. Nevertheless, several studies have been conducted using multiplex real-time PCR assays allowing the detection and speciation of several RF Borrelia (B. crocidurae, B. duttonii/B. recurrentis, B. hispanica) found in Africa [215]. Although the successful introduction of PCR as point-of-care routine diagnostic in rural Senegal has been reported [114], the availability of PCR still remains largely restricted in resource poor settings.
Serology plays no relevant role in diagnosing TBRF for several reasons. Within endemic areas, seroprevalence is high, which may not necessarily reflect acute infection but previous infection (seroscars). Furthermore, the time to seroconversion is too long to influence treatment decisions in acutely ill patients. Additionally, as mentioned above, cross-reactivity of assays may confuse TBRF borrelioses and Lyme borreliosis [216]. The latter issue can be circumvented by using an assay detecting antibodies to the GlpQ protein, which is produced by RF Borrelia species, but not by Lyme Borrelia species [217].
Culture of TBRF Borrelia is restricted to very few laboratories in the world, has primarily been used in the context of research and has no role in routine diagnostic.
Treatment
Over time, many antibiotic compounds have been evaluated for the treatment of TBRF (Fig 15). However, as with LBRF [218], neither well-designed studies to determine the best treatment regimens nor comparative studies of the efficacy of different antimicrobial agents are available. Data evaluating putative differences between different TBRF Borrelia species regarding antimicrobial susceptibility or treatment response are scarce or non-existing.
Due to their successful use in patients with syphilis, the arsenic compounds arsphenamine (salvarsan; the first marketed antibiotic which cured a bacterial infection [219]) and its less toxic derivative neoarsphenamine (neosalvarsan) developed by Ehrlich and Hata [220] were the first antimicrobial compounds used to treat relapsing fever Borrelia infections.
In the 1940s, the considerably less toxic and more effective penicillin became available and replaced the arsenical compounds for the treatment of the spirochetal infections syphilis and RF. It quickly became apparent that unlike LBRF, for which a single administration of intramuscular procaine penicillin proved highly effective [218], TBRF required prolonged and sufficiently high-dose penicillin treatments to prevent relapse and achieve cure [221–224]. In this regard, and from the pathogen’s neurotropic persistence demonstrated in animal models, an early analogy between TBRF and syphilis was drawn [225]. This analogy, as well as the marked differences in the treatment response of LBRF and TBRF, strengthened the suggestion that sufficient antibiotic target levels in the CNS are critical to successfully treat TBRF. Due to the lack of emerging resistance in spirochetal infections, penicillin remains an option for these infections up until today. However, the often restricted availability of procaine penicillin for intramuscular injection and the need to dose intravenously administered penicillin several times per day to achieve sufficient blood and tissue levels restricts the drug’s use in clinical practice. Today, the use of β-lactams is mostly restricted to the treatment of TBRF patients with CNS involvement, similar to early CNS involvement in Lyme disease or neurosyphilis, and ceftriaxone (2g once daily for 10–14 days) is preferred over penicillin in these cases [226]. Of note, in vitro data suggesting resistance of B. miyamotoi to amoxicillin but susceptibility to ceftriaxone have been reported [227]. However, the validity and generalizability of these findings is called into question by the successful treatment of a case of B. miyamotoi TBRF with amoxicillin (and sultamicillin) [158].
In the 1950s, tetracycline was introduced and became the drug of choice for oral treatment of uncomplicated TBRF. Similar to β-lactams, a correlation between administered dose and length of treatment and relapse rate/treatment success exists [102,228]. In the absence of CNS involvement, oral or parenteral treatment with a tetracycline (tetracycline 500mg every 6 hours for 10 days [226] or doxycycline 100mg every 12 hours for 7–10 days [229]) is the recommended treatment for adults. While tetracycline remains contraindicated in children due to the risk of irreversible dental staining, the administration of doxycycline is considered safe for up to 21 days regardless of age [229–231]. The recommended pediatric dose of doxycycline is 4.4mg/kg body weight/day divided in 2 doses (max. 200mg/day) [229]. Several studies have evaluated antibiotic postexposure prophylaxis/preemptive therapy with doxycycline to prevent TBRF following tick bites within endemic areas. Studies on preemptive therapy with a short course of doxycycline (day 1: 200mg/d, day 2–5: 100mg/d) were found to be highly effective in this regard [40,232,233]. A more recent study suggests, that even a single dose of doxycycline is sufficient and as effective [234].
For patients unable to take a β-lactam or a tetracycline, erythromycin (500mg or 12.5 mg/kg body weight every 6 hours for 7–10 days) is the most widely recommended alternative [226,229]. It is likely that the better tolerated azithromycin is equally effective, but dosing data are lacking [227].
Given the lack of comparative studies on antimicrobial treatment regimens of TBRF, well-designed studies evaluating different therapeutic regimens in the different TBRF species would be desirable.
JHR, outcome
The pathogenesis and frequency of JHR in spirochete infections has repeatedly been reviewed by several authors [42,235,236]. The reported frequency of JHR in spirochete infections varies widely (Lyme disease: 5–30%, syphilis: 8–75%, leptospirosis: 9–83%, LBRF: 0–100%, TBRF: 1–39%) [42]. However, due to the lack of a uniform definition and a standardised assessment of JHR, reliable data on the true incidence and possible differences in incidence of JHR in spirochete infections remain missing [4]. Thus, a critical appraisal of the incidence of JHR in TBRF is difficult. Nevertheless, compared to LBRF, where we found a JHR incidence rate of 55.8% [4], we found a considerably lower JHR incidence rate of 19.3% in TBRF. Considering the proposed underlying pathomechanisms, the lower incidence of JHR in TBRF may primarily be attributable to the overall lower number of Borrelia in the peripheral blood compared to LBRF [3,236]. Experimental animal data suggest that TBRF Borrelia species can differ in their degree of spirochaemia. This suggests that there may also be a species-specific risk of JHR. Unfortunately, the existing data are not sufficient to confirm or refute such an assumption.
The choice of antibiotics used for the treatment of spirochetal infections is considered to affect the incidence and severity of JHR, although studies in this regard provide conflicting views [237–239]. In their systematic review and meta-analysis comparing different antibiotic regimens in LBRF, Guerrier and Doherty found a benefit in favour of penicillin when comparing the rate of JHR (in 3/5 eligible studies) and concluded that treatment with a tetracycline appears to be associated with a higher rate of JHR [218]. Our analysis suggests that this may also be true in TBRF, with penicillin showing a considerably lower rate of reported JHR when compared to tetracyclines or erythromycin (Table 7). Overall, existing data suggest that in RF tetracycline treatment is associated with a higher rate of JHR but a lower relapse rate and penicillin treatment is associated with low rate of JHR but a higher relapse rate [211,218]. Overall, we found a TBRF-related CFR of 6.5%. This is in line with the generally reported TBRF-related CFR range of 2–10% [44]. The frequently encountered postulation that TBRF is less fatal than LBRF [18,240,241] may simply reflect the fact that the CFR of LBRF estimated in the literature has been too high [4]. This assumption is supported by our review on LBRF, were we found a CFR of 4% (treated)– 10.2% (untreated) [4]. Therefore, we speculate that under similar medical conditions the overall CFR of TBRF and LBRF is not significantly different. Mortality in TBRF appears to be primarily due to neurologic complications and ARDS, although reported data on attributable causes of death are largely lacking. It appears that JHR contributes little to the overall death rate, as only 6.5% of cases with JHR (from the 19.3% of antimicrobial-treated patients) die from it. In a clinical trial setting involving 184 patients with LBRF in Ethiopia, the CFR attributed to JHR was 3.3% [242]. Considering the probably above average quality of care in such clinical trial settings, CFR due to JHR in TBRF and LBRF may, overall, not be significantly different.
Regarding adverse pregnancy outcomes in TBRF, rates between 30% and 44% have been reported [192,243–246]. This is in analogy with LBRF, where adverse pregnancy outcomes, primarily in the form of abortions, are reported to occur in at least 70.9% of the cases [4]. Interestingly, our analysis suggests that the CFR for unborn children and for pregnant women does not differ from the CFR of other patients. Only the CFR of newborns appears to be considerably higher compared to non-neonatal cases (Table 8).
Table 9 comparatively summarizes the disease specific characteristics of TBRF and LBRF.
Table 9. Summary of characteristics of TBRF compared to LBRF.
| TBRF | LBRF [3,4] | |
|---|---|---|
| Causative Borrelia spp. | Various Borrelia spp. | B. recurrentis (only) |
| Epidemiology | Occurrence of sporadic cases (affecting persons exposed to ticks) |
Occurrence of outbreaks/epidemics (affecting vulnerable populations exposed to body lice) |
| Number of relapsing fever episodes | Mostly ≥2 | Mostly <2 |
| Duration of febrile episodes | Mostly ≤7 days | Up to 10 days |
| Treatment | Prolonged antibiotic treatment demanded (7–10 days; in the case of CNS involvement 10–14 days) |
Single dose antibiotic treatment sufficient |
| Complications | Neurological complications are common (attributable to direct CNS invasion by Borrelia) | Neurologic complications are rare (attributable to hemorrhagic diathesis/bleeding complications rather than direct CNS invasion by Borrelia) |
| Ocular involvement may occur | No ocular involvement reported | |
| Hemorrhagic diathesis/bleeding complications are rare | Subconjunctival hemorrhages and epistaxis are common. | |
| Risk of JHR | 19.3% | 55.8% |
| Overall CFR | 6.5% | 4–10.2% |
| Perinatal fatalities | Primarily postpartal complications/affecting newborns | Primarly prepartal complications/affecting fetuses |
TBRF, tick-borne relapsing fever; LBRF, louse-borne relapsing fever; CNS, central nervous system; JHR, Jarisch-Herxheimer reaction; CFR, case fatality rate.
Our analysis has several limitations. First, data and results of studies and case series were often reported as overall numbers, medians or percentages and thus attributing data to individual cases was not possible. Second, the heterogeneity of the reviewed studies from very different geographic regions and clinical settings leads to the inherent problem of incomplete and not always compatible data, limiting the overall validity of the analysis. Third, the overall small number in subgroup analyses limits their validity and results may not reflect the true picture.
Key learning points
TBRF is widespread worldwide, with transmission occurring by soft as well as hard ticks
although only PCR-based methods allow for species identification, microscopy remains the diagnostic gold standard in most clinical settings
the risk of JHR is apparently lower in TBRF compared to LBRF
the overall case fatality rate of TBRF and LBRF appears not to differ
unlike LBRF, where perinatal fatalities are primarily attributable to abortion, TBRF-related perinatal fatalities appear to primarily affect newborns
Supporting information
Established to conduct this systematic review.
(PDF)
Terms used for the study research in the different databases.
(PDF)
Reference list of included and excluded publications.
(PDF)
Used for screening and selecting eligible publications.
(PDF)
Treatment details: antimicrobial treatment regimen, dosage and duration.
(PDF)
Twenty-seven-item checklist for systematic reviews. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
(PDF)
List of Borrelia spp. and ticks associated to TBRF reported worldwide.
(XLSX)
Excel sheet containing the underlying numerical data.
(XLSX)
Distribution of competent vector ticks for TBRF Borrelia spp.
(PDF)
Subgroup analysis of B. crocidurae and B. hermsii.
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Rodino KG, Theel ES, Pritt BS. Tick-Borne Diseases in the United States. Clinical chemistry. 2020;66(4):537–48. doi: 10.1093/clinchem/hvaa040 [DOI] [PubMed] [Google Scholar]
- 2.Talagrand-Reboul E, Boyer PH, Bergström S, Vial L, Boulanger N. Relapsing fevers: Neglected tick-borne diseases. Frontiers in Cellular and Infection Microbiology. 2018;8(APR). doi: 10.3389/fcimb.2018.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahlig P, Paris DH, Neumayr A. Louse-borne relapsing fever—A systematic review and analysis of the literature: Part 1—Epidemiology and diagnostic aspects. PLOS Neglected Tropical Diseases. 2021;15(3):e0008564. doi: 10.1371/journal.pntd.0008564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahlig P, Neumayr A, Paris DH. Louse-borne relapsing fever—A systematic review and analysis of the literature: Part 2—Mortality, Jarisch–Herxheimer reaction, impact on pregnancy. PLOS Neglected Tropical Diseases. 2021;15(3):e0008656. doi: 10.1371/journal.pntd.0008656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson FD. TICK FEVER IN PALESTINE. Br Med J. 1919;2(3077):811. doi: 10.1136/bmj.2.3077.811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weller B, Graham GM. Relapsing fever in central Texas. Journal of the American Medical Association. 1930;95(24):1834. [Google Scholar]
- 7.Davis GE. TICKS AND RELAPSING FEVER IN THE UNITED STATES. Public Health Reports. 1940;55(51):2347–51. [Google Scholar]
- 8.Felsenfeld O. Borrelia: Strains, Vectors, Human and Animal Borreliosis. St Louis: Warren H Green. 1971. [Google Scholar]
- 9.Burrascano JJ. Relapsing fever. Clinical Infectious Disease 2010. p. 1135–8. [Google Scholar]
- 10.Parola P, Diatta G, Socolovschi C, Mediannikov O, Tall A, Bassene H, et al. Tick-borne relapsing fever borreliosis, rural senegal. Emerging Infectious Diseases. 2011;17(5):883–5. doi: 10.3201/eid1705.100573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talbert A, Nyange A, Molteni F. Spraying tick-infested houses with lambda-cyhalothrin reduces the incidence of tick-borne relapsing fever in children under five years old. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1998;92(3):251–3. doi: 10.1016/s0035-9203(98)90998-1 [DOI] [PubMed] [Google Scholar]
- 12.Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, et al. Genetic and Phenotypic Analysis of Borrelia miyamotoi sp. nov., Isolated from the Ixodid Tick Ixodes persulcatus, the Vector for Lyme Disease in Japan. International Journal of Systematic Bacteriology. 1995;45(4):804–10. doi: 10.1099/00207713-45-4-804 [DOI] [PubMed] [Google Scholar]
- 13.Hovius JWR, De Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. The Lancet. 2013;382(9892):658. doi: 10.1016/S0140-6736(13)61644-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato K, Takano A, Konnai S, Nakao M, Ito T, Koyama K, et al. Human infections with Borrelia miyamotoi, Japan. Emerging Infectious Diseases. 2014;20(8):1391–3. doi: 10.3201/eid2008.131761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gugliotta JL, Goethert HK, Berardi VP, Telford SR. Meningoencephalitis from Borrelia miyamotoi in an Immunocompromised Patient. New England Journal of Medicine. 2013;368(3):240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwan TG, Hinnebusch BJ. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science. 1998;280(5371):1938–40. doi: 10.1126/science.280.5371.1938 [DOI] [PubMed] [Google Scholar]
- 17.Davis G. The endemic relapsing fevers. Springfield (IL): Charles C Thomas; 1955. 552–65 p. [Google Scholar]
- 18.Dworkin MS, Schwan TG, Anderson DE Jr, Borchardt SM. Tick-Borne Relapsing Fever. Infectious Disease Clinics of North America. 2008;22(3):449–68. doi: 10.1016/j.idc.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francıs E. Longevity of the Tick Ornithodoros turicata and of Spirochaeta recurrentis within This Tick. Public Health Reports (1896–1970). 1938;53(51):2220. [Google Scholar]
- 20.Johnson TL, Landguth EL, Stone EF. Modeling Relapsing Disease Dynamics in a Host-Vector Community. PLoS Neglected Tropical Diseases. 2016;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez JE, Krishnavahjala A, Garcia MN, Bermudez S. Tick-Borne Relapsing Fever Spirochetes in the Americas. Vet Sci. 2016;3(3). doi: 10.3390/vetsci3030016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briggs LH. Relapsing Fever*. Cal West Med. 1935;42(5):350–4. [PMC free article] [PubMed] [Google Scholar]
- 23.Bryant K. Tickborne Infections. Principles and Practice of Pediatric Infectious Diseases 2018. p. 542-6.e2. [Google Scholar]
- 24.Roscoe C, Epperly T. Tick-borne relapsing fever. American Family Physician. 2005;72(10):2039–44. [PubMed] [Google Scholar]
- 25.Barbour AG. Antigenic Variation in Borrelia. Relapsing Fever and Lyme Borreliosis. Antigenic Variation 2003. p. 319–56. [Google Scholar]
- 26.Cook GC, Zumla A. Manson’s Tropical Diseases. 21 ed: Saunders; 2003. 2003. p. 1153–61 p. [Google Scholar]
- 27.Larsson C, Bergström S. A novel and simple method for laboratory diagnosis of relapsing Fever borreliosis. Open Microbiol J. 2008;2:10–2. doi: 10.2174/1874285800802010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hovette P, Aubron C, Perrier-Gros-Claude JD, Schieman R, N’Dir MC, Camara P. [Value of Quantitative Buffy Coat (QBC) in borreliasis-malaria co-infection]. Med Trop (Mars). 2001;61(2):196–7. [PubMed] [Google Scholar]
- 29.Fotso AF, Mediannikov O, Nappez C, Azza S, Raoult D, Drancourt M. Monoclonal antibodies for the diagnosis of borrelia crocidurae. American Journal of Tropical Medicine and Hygiene. 2016;94(1):61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talagrand-Reboul E, Raffetin A, Zachary P, Jaulhac B, Eldin C. Immunoserological Diagnosis of Human Borrelioses: Current Knowledge and Perspectives. Frontiers in Cellular and Infection Microbiology. 2020;10. doi: 10.3389/fcimb.2020.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coghill NF, Gambles RM. Discussion of methods for differentiating tick- from louse-borne relapsing fever spirochaetes. Ann Trop Med Parasitol. 1948;42(1):113–7. doi: 10.1080/00034983.1948.11685354 [DOI] [PubMed] [Google Scholar]
- 32.Cutler SJ, Rudenko N, Golovchenko M, Cramaro WJ, Kirpach J, Savic S, et al. Diagnosing Borreliosis. Vector-Borne and Zoonotic Diseases. 2017;17(1):2–11. doi: 10.1089/vbz.2016.1962 [DOI] [PubMed] [Google Scholar]
- 33.Cadavid D, Barbour AG. Neuroborreliosis during relapsing fever: Review of the clinical manifestations, pathology, and treatment of infections in humans and experimental animals. Clinical Infectious Diseases. 1998;26(1):151–64. doi: 10.1086/516276 [DOI] [PubMed] [Google Scholar]
- 34.Muñoz-Leal S, Marcili A, Fuentes-Castillo D, Ayala M, Labruna MB. A relapsing fever Borrelia and spotted fever Rickettsia in ticks from an Andean valley, central Chile. Exp Appl Acarol. 2019;78(3):403–20. doi: 10.1007/s10493-019-00389-x [DOI] [PubMed] [Google Scholar]
- 35.Muñoz-Leal S, Lopes MG, Marcili A, Martins TF, González-Acuña D, Labruna MB. Anaplasmataceae, Borrelia and Hepatozoon agents in ticks (Acari: Argasidae, Ixodidae) from Chile. Acta Tropica. 2019;192:91–103. doi: 10.1016/j.actatropica.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 36.Murray RG, Stackebrandt E. Taxonomic note: implementation of the provisional status Candidatus for incompletely described procaryotes. Int J Syst Bacteriol. 1995;45(1):186–7. doi: 10.1099/00207713-45-1-186 [DOI] [PubMed] [Google Scholar]
- 37.Parker CT, Garrity GM, Tindall BJ. International Code of Nomenclature of Prokaryotes. International Journal of Systematic and Evolutionary Microbiology. 2019;69(1A):S1–S111. doi: 10.1099/ijsem.0.000778 [DOI] [PubMed] [Google Scholar]
- 38.Stackebrandt E, Ebers J. Taxonomic parameters revisited: tarnished gold standards. MICROBIOLOGY TODAY. 2006;33(4):152–5. [Google Scholar]
- 39.Dewar HA, Walmsley R. Relapsing fever with nephritis and subarachnoid haemorrhage. Lancet (London, England). 1945;2(6379):630. doi: 10.1016/s0140-6736(45)90763-x [DOI] [PubMed] [Google Scholar]
- 40.Hasin T, Davidovitch N, Cohen R, Dagan T, Romem A, Orr N, et al. Postexposure treatment with doxycycline for the prevention of tick-borne relapsing fever. New England Journal of Medicine. 2006;355(2):148–55. doi: 10.1056/NEJMoa053884 [DOI] [PubMed] [Google Scholar]
- 41.Aguero-Rosenfeld Marie E, Stanek G. Borrelia. 12th ed 2019. [Google Scholar]
- 42.Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: A review of recent cases and our understanding of pathogenesis. American Journal of Tropical Medicine and Hygiene. 2017;96(1):46–52. doi: 10.4269/ajtmh.16-0434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dworkin MS, Anderson DE Jr., Schwan TG, Shoemaker PC, Banerjee SN, Kassen BO, et al. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin Infect Dis. 1998;26(1):122–31. doi: 10.1086/516273 [DOI] [PubMed] [Google Scholar]
- 44.Goddard J, Goddard J. Tick-Borne Diseases 2018. 91–147 p. [Google Scholar]
- 45.Barbour AG. Relapsing fever. Goodman JL, Dennis DT, Sonenshine DE, editors 2005. 268–91 p. [Google Scholar]
- 46.Jongen VHWM, Van Roosmalen J, Tiems J, Van Holten J, Wetsteyn JCFM. Tick-borne relapsing fever and pregnancy outcome in rural Tanzania. Acta Obstetricia et Gynecologica Scandinavica. 1997;76(9):834–8. doi: 10.3109/00016349709024361 [DOI] [PubMed] [Google Scholar]
- 47.Dotters-Katz SK, Kuller J, Heine RP. Arthropod-borne bacterial diseases in pregnancy. Obstetrical and Gynecological Survey. 2013;68(9):635–49. doi: 10.1097/OGX.0b013e3182a5ed46 [DOI] [PubMed] [Google Scholar]
- 48.Paris DH, Neumayr A. Ticks and tick-borne infections in Asia: Implications for travellers. Travel Medicine and Infectious Disease. 2018;26:3–4. doi: 10.1016/j.tmaid.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 49.Lambert JS. An Overview of Tickborne Infections in Pregnancy and Outcomes in the Newborn: The Need for Prospective Studies. Front Med (Lausanne). 2020;7. doi: 10.3389/fmed.2020.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Holten J, Tiems J, Jongen VHWM. Neonatal Borrelia duttoni infection: A report of three cases. Tropical Doctor. 1997;27(2):115–6. doi: 10.1177/004947559702700229 [DOI] [PubMed] [Google Scholar]
- 51.Jones JM, Hranac CR, Schumacher M, Horn K, Lee DM, Terriquez J, et al. Tick-borne relapsing fever outbreak among a high school football team at an outdoor education camping trip, Arizona, 2014. American Journal of Tropical Medicine and Hygiene. 2016;95(3):546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mafi N, Yaglom HD, Levy C, Taylor A, O’Grady C, Venkat H, et al. Tick-Borne Relapsing Fever in the White Mountains, Arizona, USA, 2013–2018. Emerg Infect Dis. 2019;25(4):649–53. doi: 10.3201/eid2504.181369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyer KM, Munford RS, Maupin GO, Pattison CP, Fox MD, Barnes AM, et al. Tick borne relapsing fever: an interstate outbreak originating at Grand Canyon National Park. American Journal of Epidemiology. 1977;105(5):469–79. doi: 10.1093/oxfordjournals.aje.a112406 [DOI] [PubMed] [Google Scholar]
- 54.Banerjee SN, Banerjee M, Fernando K, Burgdorfer W, Schwan TG. Tick-borne relapsing fever in British Columbia, Canada: First isolation of Borrelia hermsii. Journal of Clinical Microbiology. 1998;36(12):3505–8. doi: 10.1128/JCM.36.12.3505-3508.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hussein H, Showler A, Tan DHS. Tick-borne relapsing fever in pregnancy. CMAJ. 2014;186(2):131–4. doi: 10.1503/cmaj.122053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spiller GW. Tick-borne relapsing fever due to Borrelia hermsii in British Columbia. Canadian Medical Association Journal. 1986;134(1):46–7. [PMC free article] [PubMed] [Google Scholar]
- 57.Morshed MG, Drews SJ, Lee MK, Fernando K, Man S, Mak S, et al. Tick-borne relapsing fever in british Columbia: A 10-year review (2006–2015). British Columbia Medical Journal. 2017;59(8):412–7. [Google Scholar]
- 58.Gholkar N, Lehman D. Borrelia hermsii (relapsing fever). New England Journal of Medicine. 2013;368(3):266. [DOI] [PubMed] [Google Scholar]
- 59.Fritz CL, Bronson LR, Smith CR, Schriefer ME, Tucker Jr., Schwan TG. Isolation and characterization of Borrelia hermsii associated with two foci of tick-borne relapsing fever in California. Journal of Clinical Microbiology. 2004;42(3):1123–8. doi: 10.1128/JCM.42.3.1123-1128.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy FK, Parker S, Stokich D, Murray M, Fogelman V, Todd R, et al. Acute respiratory distress syndrome in persons with tickborne relapsing fever—Three states, 2004–2005. Morbidity and Mortality Weekly Report. 2007;56(41):1073–6. [PubMed] [Google Scholar]
- 61.Feldman KA, Gage K, Maupin G, Riddle D, Klouse P, Schriefer M, et al. Tick-borne relapsing fever in Clark County, Nevada, October 2000. Clinical Infectious Diseases. 2001;33(7):1244-. [Google Scholar]
- 62.Gaither M, Schumacher M, Nieto N, Corrigan J, Murray H, Maurer M. Where Are the Ticks? Solving the Mystery of a Tickborne Relapsing Fever Outbreak at a Youth Camp. Journal of environmental health. 2016;78(8):8–11. [PubMed] [Google Scholar]
- 63.Jones JM, Schumacher M, Peoples M, Souders N, Horn K, Fox L, et al. Tickborne Relapsing Fever Outbreak at an Outdoor Education Camp—Arizona, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(23):651–2. [PMC free article] [PubMed] [Google Scholar]
- 64.Trevejo RT, Schriefer ME, Gage KL, Safranek TJ, Orloski KA, John Pape W, et al. An interstate outbreak of tick-borne relapsing fever among vacationers at a Rocky Mountain cabin. American Journal of Tropical Medicine and Hygiene. 1998;58(6):743–7. [DOI] [PubMed] [Google Scholar]
- 65.Centers for Disease C, Prevention. Tickborne relapsing fever in a mother and newborn child—Colorado, 2011. MMWR Morbidity and mortality weekly report. 2012;61(10):174–6. [PubMed] [Google Scholar]
- 66.Skar G, Snowden J. Recurrent fever and thrombocytopenia in a 4-year-old girl. Pediatrics in Review. 2015;36(3):130–1. doi: 10.1542/pir.36-3-130 [DOI] [PubMed] [Google Scholar]
- 67.Tilley PA, Azar R, Banerjee S, Bell A. Three cases of relapsing fever associated with lakeside cabins in Idaho. Canada communicable disease report = Relevé des maladies transmissibles au Canada. 1994;20(4):29–31. [PubMed] [Google Scholar]
- 68.Uhlmann EJ, Seed PC, Schwan TG, Storch GA. Polymerase chain reaction of tick-borne relapsing fever caused by Borrelia hermsii. Pediatric Infectious Disease Journal. 2007;26(3):267–9. doi: 10.1097/01.inf.0000254392.99545.69 [DOI] [PubMed] [Google Scholar]
- 69.Christensen J, Fischer RJ, McCoy BN, Raffel SJ, Schwan TG. Tickborne relapsing fever, bitterroot valley, Montana, USA. Emerging Infectious Diseases. 2015;21(2):217–23. doi: 10.3201/eid2102.141276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Centers for Disease C, Prevention. Tickborne relapsing fever outbreak after a family gathering: New Mexico, August 2002. Morbidity and Mortality Weekly Report. 2003;52(34):809–12. [PubMed] [Google Scholar]
- 71.Paul WS, Maupin G, Scott-Wright AO, Craven RB, Dennis DT. Outbreak of tick-borne relapsing fever at the North Rim of the Grand Canyon: Evidence for effectiveness of preventive measures. American Journal of Tropical Medicine and Hygiene. 2002;66(1):71–5. doi: 10.4269/ajtmh.2002.66.71 [DOI] [PubMed] [Google Scholar]
- 72.Aviles ES, Oakes M, Algranati M, Mansoor AM. Tick-borne relapsing fever. BMJ case reports. 2020;13(7). doi: 10.1136/bcr-2020-237296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lim LL, Rosenbaum JT. Borrelia Hermsii Causing Relapsing Fever and Uveitis. American Journal of Ophthalmology. 2006;142(2):348–9. doi: 10.1016/j.ajo.2006.03.030 [DOI] [PubMed] [Google Scholar]
- 74.Schwan TG, Raffel SJ, Schrumpf ME, Webster LS, Marques AR, Spano R, et al. Tick-borne relapsing fever and Borrelia hermsii, Los Angeles County, California, USA. Emerging Infectious Diseases. 2009;15(7):1026–31. doi: 10.3201/eid1507.090223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thompson RS, Burgdorfer W, Russell R, Francis BJ. Outbreak of tick-borne relapsing fever in Spokane County, Washington. JAMA: the journal of the American Medical Association. 1969;210(6):1045–50. [PubMed] [Google Scholar]
- 76.Badger MS. Tick talk: unusually severe case of tick-borne relapsing fever with acute respiratory distress syndrome—case report and review of the literature. Wilderness Environ Med. 2008;19(4):280–6. doi: 10.1580/07-WEME-CR-140.1 [DOI] [PubMed] [Google Scholar]
- 77.Flanigan TP, Schwan TG, Armstrong C, Van Voris LP, Salata RA. Relapsing fever in the US Virgin Islands: a previously unrecognized focus of infection. J Infect Dis. 1991;163(6):1391–2. doi: 10.1093/infdis/163.6.1391 [DOI] [PubMed] [Google Scholar]
- 78.Shehab KW, Banaei N. Unexplained fever after a camping trip in the american Southwest. Journal of the Pediatric Infectious Diseases Society. 2012;1(3):254–5. doi: 10.1093/jpids/pis067 [DOI] [PubMed] [Google Scholar]
- 79.Felder H, Hoekstra KA. Borrelia hermsii relapsing fever. Blood. 2014;123(2):160. doi: 10.1182/blood-2013-09-523373 [DOI] [PubMed] [Google Scholar]
- 80.Hoekstra K, Kelly M. Elevated troponin and Jarish-Herxheimer reaction in tick borne relapsing fever. Clinical Chemistry. 2011;57(10):A165–A6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwan TG, Policastro PF, Miller Z, Thompson RL, Damrow T, Keirans JE. Tick-borne relapsing fever caused by Borrelia hermsii, Montana. Emerging Infectious Diseases. 2003;9(9):1151–4. doi: 10.3201/eid0909.030280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kingry LC, Anacker M, Pritt B, Bjork J, Respicio-Kingry L, Liu GP, et al. Surveillance for and Discovery of Borrelia Species in US Patients Suspected of Tickborne Illness. Clinical Infectious Diseases. 2018;66(12):1864–71. doi: 10.1093/cid/cix1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campbell SB, Klioueva A, Taylor J, Nelson C, Tomasi S, Replogle A, et al. Evaluating the risk of tick-borne relapsing fever among occupational cavers-Austin, TX, 2017. Zoonoses Public Health. 2019;66(6):579–86. doi: 10.1111/zph.12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jobe DA, Lovrich SD, Oldenburg DG, Kowalski TJ, Callister SM. Borrelia miyamotoi Infection in Patients from Upper Midwestern United States, 2014–2015. Emerging Infectious Diseases. 2016;22(8):1471–3. doi: 10.3201/eid2208.151878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marcos LA, Smith K, Reardon K, Weinbaum F, Spitzer ED. Presence of Borrelia miyamotoi infection in a highly endemic area of Lyme disease. Annals of Clinical Microbiology and Antimicrobials. 2020;19(1). doi: 10.1186/s12941-020-00360-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fiorito T, Godding M, Reece R, Flanigan T, Silverblatt F. Utility of borrelia miyamotoi polymerase chain reaction in Rhode island: A case series. Open Forum Infectious Diseases. 2016;3. [Google Scholar]
- 87.Marcos L, Smith K, Weinbaum F, Spitzer E. An emerging tick-borne disease in Long Island, New York: Relapsing fever caused by Borrelia miyamotoi. Open Forum Infectious Diseases. 2018;5:S241. [Google Scholar]
- 88.Smith RP, Elias SP, Cavanaugh CE, Lubelczyk CB, Lacombe EH, Brancato J, et al. Seroprevalence of Borrelia burgdorferi, B. miyamotoi, and Powassan Virus in Residents Bitten by Ixodes Ticks, Maine, USA. Emerg Infect Dis. 2019;25(4):804–7. doi: 10.3201/eid2504.180202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kadkhoda K, Dumouchel C, Brancato J, Gretchen A, Krause PJ. Human seroprevalence of Borrelia miyamotoi in Manitoba, Canada, in 2011–2014: a cross-sectional study. CMAJ Open. 2017;5(3):E690–e3. doi: 10.9778/cmajo.20170070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krause PJ, Schwab J, Narasimhan S, Brancato J, Xu G, Rich SM. Hard tick relapsing fever caused by Borrelia miyamotoi in a Child. Pediatric Infectious Disease Journal. 2016;35(12):1352–4. doi: 10.1097/INF.0000000000001330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu LT, Tsibris AM, Branda JA. Case 24–2015: A 28-Year-Old Pregnant Woman with Fever, Chills, Headache, and Fatigue. New England Journal of Medicine. 2015;373(5):468–75. [DOI] [PubMed] [Google Scholar]
- 92.Chowdri HR, Gugliotta JL, Berardi VP, Goethert HK, Molloy PJ, Sterling SL, et al. Borrelia miyamotoiInfection Presenting as Human Granulocytic Anaplasmosis. Annals of Internal Medicine. 2013;159(1):21. doi: 10.7326/0003-4819-159-1-201307020-00005 [DOI] [PubMed] [Google Scholar]
- 93.Molloy PJ, Telford SR, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, et al. Borrelia miyamotoi Disease in the Northeastern United States A Case Series. Annals of Internal Medicine. 2015;163(2):91-+. doi: 10.7326/M15-0333 [DOI] [PubMed] [Google Scholar]
- 94.Krause PJ, Carroll M, Fedorova N, Brancato J, Dumouchel C, Akosa F, et al. Human Borrelia miyamotoi infection in California: Serodiagnosis is complicated by multiple endemic Borrelia species. PLoS ONE. 2018;13(2). doi: 10.1371/journal.pone.0191725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oda R, Kutsuna S, Sekikawa Y, Hongo I, Sato K, Ohnishi M, et al. The first case of imported Borrelia miyamotoi disease concurrent with Lyme disease. Journal of Infection and Chemotherapy. 2017;23(5–6):333–5. doi: 10.1016/j.jiac.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 96.Sudhindra P, Wang G, Schriefer ME, McKenna D, Jian Z, Krause PJ, et al. Insights into Borrelia miyamotoi infection from an untreated case demonstrating relapsing fever, monocytosis and a positive C6 Lyme serology. Diagnostic Microbiology and Infectious Disease. 2016;86(1):93–6. doi: 10.1016/j.diagmicrobio.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fiorito TM, Reece R, Flanigan TP, Silverblatt FJ. Borrelia miyamotoi Polymerase Chain Reaction Positivity on a Tick-Borne Disease Panel in an Endemic Region of Rhode Island: A Case Series. Infectious Diseases in Clinical Practice. 2017;25(5):250–4. [Google Scholar]
- 98.Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T, et al. Human Borrelia miyamotoi Infection in the United States. N Engl J Med. 2013;368(3):291–3. doi: 10.1056/NEJMc1215469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Delaney SL, Murray LA, Aasen CE, Bennett CE, Brown E, Fallon BA. Borrelia miyamotoi Serology in a Clinical Population With Persistent Symptoms and Suspected Tick-Borne Illness. Frontiers in Medicine. 2020;7. doi: 10.3389/fmed.2020.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dykstra EA, Oltean HN, Kangiser D, Marsden-Haug N, Rich SM, Guang X, et al. Ecology and Epidemiology of Tickborne Pathogens, Washington, USA, 2011–2016. Emerging Infectious Diseases. 2020;26(4):648–832. doi: 10.3201/eid2604.191382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bissett JD, Ledet S, Krishnavajhala A, Armstrong BA, Klioueva A, Sexton C, et al. Detection of tickborne relapsing fever Spirochete, Austin, Texas, USA. Emerging Infectious Diseases. 2018;24(11):2003–9. doi: 10.3201/eid2411.172033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Linnemann CC Jr, Barber LC, Dine MS, Body AE. Tick-borne relapsing fever in the Eastern United States. American journal of diseases of children (1960). 1978;132(1):40–2. [DOI] [PubMed] [Google Scholar]
- 103.Christensen AM, Pietralczyk E, Lopez JE, Brooks C, Schriefer ME, Wozniak E, et al. Diagnosis and Management of Borrelia turicatae Infection in Febrile Soldier, Texas, USA. Emerg Infect Dis. 2017;23(5):883–4. doi: 10.3201/eid2305.162069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davis H, Vincent JM, Lynch J. Tick-Borne relapsing fever caused by Borrelia turicatae. Pediatric Infectious Disease Journal. 2002;21(7):703–5. doi: 10.1097/00006454-200207000-00020 [DOI] [PubMed] [Google Scholar]
- 105.Calero C. Relapsing fever on the Isthmus of Panama; report of 106 cases. The American journal of tropical medicine and hygiene. 1946;26(6):761–9. doi: 10.4269/ajtmh.1946.s1-26.761 [DOI] [PubMed] [Google Scholar]
- 106.Lebredo MG. A Case of Recurrent Fever Observed in Havana. Public Health Pap Rep. 1906;32(Pt 1):238–47. [PMC free article] [PubMed] [Google Scholar]
- 107.Ciceroni L, Bartoloni A, Guglielmetti P, Paradisi F, Barahona HG, Roselli M, et al. Prevalence of antibodies to Borrelia burgdorferi, Borrelia parkeri and Borrelia turicatae in human settlements of the Cordillera Province, Bolivia. J Trop Med Hyg. 1994;97(1):13–7. [PubMed] [Google Scholar]
- 108.Qiu Y, Nakao R, Hang’ombe BM, Sato K, Kajihara M, Kanchela S, et al. Human Borreliosis Caused by a New World Relapsing Fever Borrelia–like Organism in the Old World. Clinical Infectious Diseases. 2019;69(1):107–12. doi: 10.1093/cid/ciy850 [DOI] [PubMed] [Google Scholar]
- 109.Stete K, Rieg S, Margos G, Häcker G, Wagner D, Kern WV, et al. Case report and genetic sequence analysis of candidatus Borrelia Kalaharica, Southern Africa. Emerging Infectious Diseases. 2018;24(9):1659–64. doi: 10.3201/eid2409.171381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fingerle V, Pritsch M, Wächtler M, Margos G, Ruske S, Jung J, et al. "Candidatus Borrelia kalaharica" Detected from a Febrile Traveller Returning to Germany from Vacation in Southern Africa. PLoS Neglected Tropical Diseases. 2016;10(3). doi: 10.1371/journal.pntd.0004559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fotso Fotso A, Angelakis E, Mouffok N, Drancourt M, Raoult D. Blood-Borne Candidatus Borrelia algerica in a Patient with Prolonged Fever in Oran, Algeria. Am J Trop Med Hyg. 2015;93(5):1070–3. doi: 10.4269/ajtmh.15-0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bottieau E, Verbruggen E, Aubry C, Socolovschi C, Vlieghe E. Meningoencephalitis complicating relapsing fever in traveler returning from Senegal. Emerging Infectious Diseases. 2012;18(4). doi: 10.3201/eid1804.111771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vial L, Diatta G, Tall A, Hadj Ba E, Bouganali H, Durand P, et al. Incidence of tick-borne relapsing fever in west Africa: longitudinal study. Lancet. 2006;368(9529):37–43. doi: 10.1016/S0140-6736(06)68968-X [DOI] [PubMed] [Google Scholar]
- 114.Sokhna C, Mediannikov O, Fenollar F, Bassene H, Diatta G, Tall A, et al. Point-of-Care Laboratory of Pathogen Diagnosis in Rural Senegal. PLoS Negl Trop Dis. 2013;7(1). doi: 10.1371/journal.pntd.0001999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fall NS, Diagne N, Mediannikov O, Fenollar F, Parola P, Sokhna C, et al. Detection of Borrelia crocidurae in a vaginal swab after miscarriage, rural Senegal, Western Africa. International Journal of Infectious Diseases. 2019;91:261–3. doi: 10.1016/j.ijid.2019.12.020 [DOI] [PubMed] [Google Scholar]
- 116.Van Dam AP, Van Gool T, Wetsteyn JCFM, Dankert J. Tick-borne relapsing fever imported from West Africa: Diagnosis by quantitative buffy coat analysis and in vitro culture of Borrelia crocidurae. Journal of Clinical Microbiology. 1999;37(6):2027–30. doi: 10.1128/JCM.37.6.2027-2030.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yahia SA, Faibis F, Benmoussa M, Lantohasina N, Dupont A, Abdesselam TA. Tick-borne relapsing fever: An unrecognized cause of fever in travellers. Revue De Medecine Interne. 2020;41(6):418–20. [DOI] [PubMed] [Google Scholar]
- 118.Guiheneuf E, Desjardins N, Guiheneuf R. It is not always malaria: diagnosis of Borrelia recurrent fever on blood smear. Annales de biologie clinique. 2018;76(1):118–9. doi: 10.1684/abc.2017.1320 [DOI] [PubMed] [Google Scholar]
- 119.Goutier S, Ferquel E, Pinel C, Bosseray A, Hoen B, Couetdic G, et al. Borrelia crocidurae Meningoencephalitis, West Africa. Emerging Infectious Diseases. 2013;19(2):301–4. doi: 10.3201/eid1902.121325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Million M, Cazorla C, Doudier B, Scola BL, Parola P, Drancourt M, et al. Molecular identification of Borrelia crocidurae in a patient returning from Senegal. BMJ Case Reports. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Diallo MA, Kane BS, Ndiaye M, Dieng M, Diongue K, Badiane AS, et al. Plasmodium falciparum malaria co-infection with tick-borne relapsing fever in Dakar. Malaria Journal. 2017;16(1):1–3. doi: 10.1186/s12936-016-1650-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gras E, Bailly E, Le Brun C, Lemaignen A, Lanotte P. Borrelia crocidurae tick-borne relapsing fever upon return from Senegal. Medecine et Maladies Infectieuses. 2019;49(8):624–5. doi: 10.1016/j.medmal.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 123.Mediannikov O, Socolovschi C, Bassene H, Diatta G, Ratmanov P, Fenollar F, et al. High incidence of Borrelia crocidurae in acute febrile patients in Senegal. International Journal of Infectious Diseases. 2014;21:218. doi: 10.3201/eid2008.130550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mediannikov O, Socolovschi C, Bassene H, Diatta G, Ratmanov P, Fenollar F, et al. Borrelia crocidurae Infection in Acutely Febrile Patients, Senegal. Emerging Infectious Diseases. 2014;20(8):1335–8. doi: 10.3201/eid2008.130550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tordini G, Giaccherini R, Corbisiero R, Zanelli G. Relapsing fever in a traveller from Senegal: determination of Borrelia species using molecular methods. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100(10):992–4. doi: 10.1016/j.trstmh.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 126.Trape JF, Godeluck B, Diatta G, Rogier C, Legros F, Albergel J, et al. The spread of tick-borne borreliosis in West Africa and its relationship to sub-Saharan drought. American Journal of Tropical Medicine and Hygiene. 1996;54(3):289–93. doi: 10.4269/ajtmh.1996.54.289 [DOI] [PubMed] [Google Scholar]
- 127.Reller ME, Clemens EG, Schachterle SE, Mtove GA, Sullivan DJ, Dumler JS. Multiplex 5′ nuclease-quantitative PCR for diagnosis of relapsing fever in a large Tanzanian cohort. Journal of Clinical Microbiology. 2011;49(9):3245–9. doi: 10.1128/JCM.00940-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nordstrand A, Bunikis I, Larsson C, Tsogbe K, Schwan TG, Nilsson M, et al. Tickborne relapsing fever diagnosis obscured by Malaria, Togo. Emerging Infectious Diseases. 2007;13(1):117–23. doi: 10.3201/eid1301.060670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Malatre I, Giocanti R, Macaigne F, Ripert C. A study of the Borrelia fever focus of Gisenyi (Rwanda). Medecine Tropicale. 1991;51(1):49–52. [PubMed] [Google Scholar]
- 130.Anderson IG. A note on relapsing fever occurring in two Europeans. The Central African journal of medicine. 1958;4(10):444–5. [PubMed] [Google Scholar]
- 131.Gear JHS. Tropical thrombophlebitis. The role of relapsing fever in its causation. South African Medical Journal. 1975;49(49):2057–8. [PubMed] [Google Scholar]
- 132.Melkert P, Kahema L, van der Velden J, van Roosmalen J. Relapsing fever, a disappearing cause of fever and maternal death in Sengerema, East Africa. East African medical journal. 2013;90(4):137–41. [PubMed] [Google Scholar]
- 133.Rustenhoven-Spaan I, Melkert P, Nelissen E, van Roosmalen J, Stekelenburg J. Maternal mortality in a rural tanzanian hospital: Fatal Jarisch-Herxheimer reaction in a case of relapsing fever in pregnancy. Tropical Doctor. 2013;43(4):138–41. doi: 10.1177/0049475513497477 [DOI] [PubMed] [Google Scholar]
- 134.Brasseur D. Tick-borne relapsing fever in a premature infant. Annals of Tropical Paediatrics. 1985;5(3):161–2. doi: 10.1080/02724936.1985.11748384 [DOI] [PubMed] [Google Scholar]
- 135.Dupont HT, La Scola B, Williams R, Raoult D. A focus of tick-borne relapsing fever in southern Zaire. Clinical Infectious Diseases. 1997;25(1):139–44. doi: 10.1086/514496 [DOI] [PubMed] [Google Scholar]
- 136.Kisinza WN, McCall PJ, Mitani H, Talbert A, Fukunaga M, Kisinza WN, et al. A newly identified tick-borne Borrelia species and relapsing fever in Tanzania. Lancet. 2003;362 North American Edition(9392):1283–4. doi: 10.1016/s0140-6736(03)14609-0 [DOI] [PubMed] [Google Scholar]
- 137.Leen I, Bruynseels P, Mukadi BK, Van Oort M, Van Den Akker M. A 13-year old girl with pancytopenia at the presentation of a Borrelia hispanica infection: A case report and review of the literature. Journal of Medical Case Reports. 2017;11(1). doi: 10.1186/s13256-017-1225-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Heida J, van Arkel A, Verweij JJ, Tijssen CC. Meningitis due to infection with Borrelia hispanica. Ned Tijdschr Geneeskd. 2019;163. [PubMed] [Google Scholar]
- 139.Sarih M, Garnier M, Boudebouch N, Bouattour A, Rihani A, Hassar M, et al. Borrelia hispanica relapsing fever, Morocco. Emerging Infectious Diseases. 2009;15(10):1626–9. doi: 10.3201/eid1510.090403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wyplosz B, Mihaila-Amrouche L, Baixench M-T, Bigel M-L, Berardi-Grassias L, Fontaine C, et al. Imported tickborne relapsing fever, France. Emerging Infectious Diseases. 2005;11(11):1801–3. doi: 10.3201/eid1111.050616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Diatta G, Souidi Y, Granjon L, Arnathau C, Durand P, Chauvancy G, et al. Epidemiology of Tick-Borne Borreliosis in Morocco. PLoS Neglected Tropical Diseases. 2012;6(9). doi: 10.1371/journal.pntd.0001810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Malincarne L, Schiaroli E, Ciervo A, Scaglione V, Paciaroni M, Mancini F, et al. Meningitis with cranial polyneuritis and cavernous sinus thrombosis by Borrelia crocidurae: First autochthonous case in Europe. International Journal of Infectious Diseases. 2019;82:30–2. doi: 10.1016/j.ijid.2019.02.028 [DOI] [PubMed] [Google Scholar]
- 143.Garcia-Soler P, Nunez-Cuadros E, Milano-Manso G, Ruiz Sanchez P. Severe Jarisch-Herxheimer reaction in tick-borne relapsing fever. [Spanish]. Enfermedades Infecciosas y Microbiologia Clinica. 2011;29(9):710–1. doi: 10.1016/j.eimc.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 144.Domínguez MC, Vergara S, Gómez MC, Roldán ME. Epidemiology of tick-borne relapsing fever in endemic area, Spain. Emerging Infectious Diseases. 2020;26(5):849–56. doi: 10.3201/eid2605.190745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cerdan M, Martínez IS, Cabanes BP, Guarnizo EC, Fernandez PA, Nieto RE, et al. Borrelia hispanica: An emerging infectious agent causing neuroborreliosis. Neurology. 2015;84. doi: 10.1186/s12883-015-0340-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boyer PH, Koetsveld J, Zilliox L, Sprong H, Talagrand-Reboul É, Hansmann Y, et al. Assessment of Borrelia miyamotoi in febrile patients and ticks in Alsace, an endemic area for Lyme borreliosis in France. Parasites and Vectors. 2020;13(1). doi: 10.1186/s13071-020-04071-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tobudic S, Burgmann H, Stanek G, Winkler S, Schotta A-M, Obermuller M, et al. Human Borrelia miyamotoi Infection, Austria. Emerging infectious diseases. 2020;26(9):2201–4. doi: 10.3201/eid2609.191501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Franck M, Ghozzi R, Pajaud J, Lawson-Hogban NE, Mas M, Lacout A, et al. Borrelia miyamotoi: 43 Cases Diagnosed in France by Real-Time PCR in Patients With Persistent Polymorphic Signs and Symptoms. Frontiers in Medicine. 2020;7. doi: 10.3389/fmed.2020.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Aubry C, Socolovschi C, Raoult D, Parola P. Bacterial agents in 248 ticks removed from people from 2002 to 2013. Ticks and Tick-borne Diseases. 2016;7(3):475–81. doi: 10.1016/j.ttbdis.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 150.Hoornstra D, Koetsveld J, Sprong H, Platonov AE, Hovius JW. Borrelia miyamotoi disease in an immunocompetent patient, Western Europe. Emerging Infectious Diseases. 2018;24(9):1770–2. doi: 10.3201/eid2409.180806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jahfari S, Hofhuis A, Fonville M, van der Giessen J, van Pelt W, Sprong H. Molecular Detection of Tick-Borne Pathogens in Humans with Tick Bites and Erythema Migrans, in the Netherlands. PLoS Negl Trop Dis. 2016;10(10). doi: 10.1371/journal.pntd.0005042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, et al. Humans infected with relapsing fever spirochete borrelia miyamotoi, Russia. Emerging Infectious Diseases. 2011;17(10):1816–23. doi: 10.3201/eid1710.101474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Boden K, Lobenstein S, Hermann B, Margos G, Fingerle V. Borrelia miyamotoi–Associated Neuroborreliosis in Immunocompromised Person. Emerging Infectious Diseases. 2016;22(9):1617–20. doi: 10.3201/eid2209.152034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Henningsson AJ, Asgeirsson H, Hammas B, Karlsson E, Parke Å, Hoornstra D, et al. Two Cases of Borrelia miyamotoi Meningitis, Sweden, 2018. Emerg Infect Dis. 2019;25(10):1965–8. doi: 10.3201/eid2510.190416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mancini F, Innocenti P, Baumgartner M, Binazzi R, Troi C, Pagani E, et al. Borrelia microti infection in an Italian woman returning from Kyrgyzstan and Tajikistan. Travel Medicine and Infectious Disease. 2020;35. [DOI] [PubMed] [Google Scholar]
- 156.Jiang BG, Jia N, Jiang JF, Zheng YC, Chu YL, Jiang RR, et al. Borrelia miyamotoi Infections in Humans and Ticks, Northeastern China. Emerg Infect Dis. 2018;24(2):236–41. doi: 10.3201/eid2402.160378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sato K, Sakakibara K, Masuzawa T, Ohnishi M, Kawabata H. Case control study: Serological evidence that Borrelia miyamotoi disease occurs nationwide in Japan. Journal of Infection and Chemotherapy. 2018;24(10):828–33. doi: 10.1016/j.jiac.2018.06.017 [DOI] [PubMed] [Google Scholar]
- 158.Yamano K, Ito T, Kiyanagi K, Yamazaki H, Sugawara M, Saito T, et al. Case report: Clinical features of a case of suspected borrelia miyamotoi disease in Hokkaido, Japan. American Journal of Tropical Medicine and Hygiene. 2017;97(1):84–7. doi: 10.4269/ajtmh.16-0699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sarksyan DS, Platonov AE, Karan LS, Shipulin GA, Sprong H, Hovlus JWR. Probability of Spirochete Borrelia miyamotoi Transmission from Ticks to Humans. Emerging Infectious Diseases. 2015;21(12):2273–4. doi: 10.3201/eid2112.151097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sarksyan DS, Maleev VV, Platonov AE, Platonova OV, Karan LS. Relapsing (recurrent) disease caused by Borrelia miyamotoi. Terapevticheskiĭ arkhiv. 2015;87(11):18–25. doi: 10.17116/terarkh2015871118-25 [DOI] [PubMed] [Google Scholar]
- 161.Savel’eva MV, Krasnova EI, Khokhlova NI, Provorova VV, Filimonova ES, Rar VA, et al. Clinical and laboratory characteristics of diseases caused by Borrelia spp. In the inhabitants of the Novosibirsk region in 2015–2017. Jurnal Infektologii. 2018;10(2):68–75. [Google Scholar]
- 162.Karan L, Makenov M, Kolyasnikova N, Stukolova O, Toporkova M, Olenkova O. Dynamics of Spirochetemia and Early PCR Detection of Borrelia miyamotoi. Emerg Infect Dis. 2018;24(5):860–7. doi: 10.3201/eid2405.170829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Platonov AE, Toporkova MG, Kolyasnikova NM, Stukolova OA, Dolgova AS, Brodovikova AV, et al. Clinical presentation of Ixodes tick-borne borreliosis caused by Borrelia miyamotoi in the context of an immune response to the pathogen. Ter Arkh. 2017;89(11):35–43. doi: 10.17116/terarkh2017891135-43 [DOI] [PubMed] [Google Scholar]
- 164.Kutsuna S, Kawabata H, Kasahara K, Takano A, Mikasa K. Case report: The first case of imported relapsing fever in Japan. American Journal of Tropical Medicine and Hygiene. 2013;89(3):460–1. doi: 10.4269/ajtmh.13-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yossepowitch O, Gottesman T, Schwartz-Harari O, Soroksky A, Dan M. Aseptic meningitis and adult respiratory distress syndrome caused by Borrelia persica. Infection. 2012;40(6):695–7. doi: 10.1007/s15010-012-0296-8 [DOI] [PubMed] [Google Scholar]
- 166.Halperin T, Orr N, Cohen R, Hasin T, Davidovitch N, Klement E, et al. Detection of relapsing fever in human blood samples from Israel using PCR targeting the glycerophosphodiester phosphodiesterase (GlpQ) gene. Acta Tropica. 2006;98(2):189–95. doi: 10.1016/j.actatropica.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 167.Hashavya S, Gross I, Gross M, Hurvitz N, Weiser G, Temper V, et al. Tickborne Relapsing Fever, Jerusalem, Israel, 2004–2018. Emerg Infect Dis. 2020;26(10):2420–3. doi: 10.3201/eid2610.181988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shaked Y, Maier MK, Samra Y. Relapsing fever and salmonella bacteraemia simultaneously affecting a healthy young man. Journal of Infection. 1986;13(3):308–9. doi: 10.1016/s0163-4453(86)91718-4 [DOI] [PubMed] [Google Scholar]
- 169.Eisenberg S, Gunders AE, Cohen AM. Tick-borne relapsing fever in the Judean hills, including a case with massive haematuria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1968;62(5):679–81. doi: 10.1016/0035-9203(68)90119-3 [DOI] [PubMed] [Google Scholar]
- 170.Snavely E, Hymas W, Couturier MR, Couturier MR. The brief case: Tick-borne relapsing fever in a returned traveler. Journal of Clinical Microbiology. 2020;58(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shayeghi M, Piazak N, Gollampuor A, Nasirian H, Abolhassani M. Tick-borne relapsing fever in Sabzevar (Khorasan Razavy Province), North-Eastern Iran. Bangladesh Journal of Medical Science. 2016;15(4):551–5. [Google Scholar]
- 172.Kassiri H, Kasiri A, Karimi M, Kasiri E, Lotfi M. The seven-year longitudinal study on relapsing fever borreliosis in Western Iran. Asian Pacific Journal of Tropical Disease. 2014;4(S2):S679–S83. [Google Scholar]
- 173.Moemenbellah-Fard MD, Benafshi O, Rafinejad J, Ashraf H. Tick-borne relapsing fever in a new highland endemic focus of western Iran. Annals of Tropical Medicine and Parasitology. 2009;103(6):529–37. doi: 10.1179/136485909X451852 [DOI] [PubMed] [Google Scholar]
- 174.Muigg V, Seth-Smith HMB, Goldenberger D, Egli A, Nickel B, Dürig R, et al. Tick-Borne Relapsing Fever Caused by Borrelia persica in Traveler to Central Asia, 2019. Emerging Infectious Diseases. 2020;26(4):424–6. doi: 10.3201/2604.191771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.de Verdiére NC, Hamane S, Assous MV, Sertour N, Ferquel E, Cornet M. Tickborne relapsing fever caused by Borrelia persica, Uzbekistan and Tajikistan. Emerging Infectious Diseases. 2011;17(7):1325–7. doi: 10.3201/eid1707.101894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Masoumi Asl H, Goya MM, Vatandoost H, Zahraei SM, Mafi M, Asmar M, et al. The epidemiology of tick-borne relapsing fever in Iran during 1997–2006. Travel Medicine and Infectious Disease. 2009;7(3):160–4. doi: 10.1016/j.tmaid.2009.01.009 [DOI] [PubMed] [Google Scholar]
- 177.Rosenthal E. Relapsing fever in Cape Town. A case report. South African Medical Journal. 1982;61(21):801–2. [PubMed] [Google Scholar]
- 178.Simon JW. Tick borne relapsing fever imported into the United Kingdom. Journal of the Royal Army Medical Corps. 1985;131(2):65–7. doi: 10.1136/jramc-131-02-02 [DOI] [PubMed] [Google Scholar]
- 179.McNamara JJ, Kay HH. Relapsing fever (Borrelia) in an adolescent tourist in Israel. Journal of Adolescent Health Care. 1988;9(5):421–3. doi: 10.1016/0197-0070(88)90042-3 [DOI] [PubMed] [Google Scholar]
- 180.Colebunders R, Serrano PD, Gompel AV, Wynants H, Blot K, Van Den Enden E, et al. Imported relapsing fever in european tourists. Scandinavian Journal of Infectious Diseases. 1993;25(4):533–6. doi: 10.3109/00365549309008539 [DOI] [PubMed] [Google Scholar]
- 181.Keung YK, Cobos E, Kimbrough RC, Carver RC. Borreliosis as a Cause of fever in a woman who recently returned from Saudi Arabia. Clinical Infectious Diseases. 1995;21(2):447–8. doi: 10.1093/clinids/21.2.447 [DOI] [PubMed] [Google Scholar]
- 182.Poulsen LW, Iversen G. Relapsing fever: A differential diagnosis to malaria. Scandinavian Journal of Infectious Diseases. 1996;28(4):419–20. doi: 10.3109/00365549609037932 [DOI] [PubMed] [Google Scholar]
- 183.Chatel G, Gulletta M, Matteelli A, Marangoni A, Signorini L, Oladeji O, et al. Diagnosis of tick-borne relapsing fever by the quantitative buffy coat fluorescence method. American Journal of Tropical Medicine and Hygiene. 1999;60(5):738–9. doi: 10.4269/ajtmh.1999.60.738 [DOI] [PubMed] [Google Scholar]
- 184.Heerdink G, Petit PLC, Hofwegen H, Van Genderen PJJ. A patient with fever following a visit to the tropics: Tick-borne relapsing fever discovered in a thick blood smear preparation. Nederlands Tijdschrift voor Geneeskunde. 2006;150(43):2386–9. [PubMed] [Google Scholar]
- 185.Gallien S, Sarfati C, Haas L, Lagrange-Xelot M, Molina JM. Borreliosis: A rare and alternative diagnosis in travellers’ febrile illness. Travel Medicine and Infectious Disease. 2007;5(4):247–50. doi: 10.1016/j.tmaid.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 186.Patrat-Delon S, Drogoul AS, Le Ho H, Biziraguzenyuka J, Rabier V, Arvieux C, et al. Recurrent tick-borne fever: A possible diagnosis in patients returning from Senegal. Medecine et Maladies Infectieuses. 2008;38(7):396–9. doi: 10.1016/j.medmal.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 187.Lambregts MMC, Bentvelsen RG, Makiello PE, De Wever B, Kuijper EJ, Visser LG. Relapsing fever after traveling in the tropics: A story with a twist. Nederlands Tijdschrift voor Geneeskunde. 2019;163(25). [PubMed] [Google Scholar]
- 188.Mitiku K, Mengistu G. Relapsing fever in Gondar, Ethiopia. East African medical journal. 2002;79(2):85–7. doi: 10.4314/eamj.v79i2.8908 [DOI] [PubMed] [Google Scholar]
- 189.Melkert P, Melkert D, Kahema L, Van Der Velden K, Van Roosmalen J. Estimation of changes in maternal mortality in a rural district of northern Tanzania during the last 50 years. Acta Obstetricia et Gynecologica Scandinavica. 2015;94(4):419–24. doi: 10.1111/aogs.12589 [DOI] [PubMed] [Google Scholar]
- 190.Mayegga E, Ljøstad U, Mygland Å, Monstad P. Absence of focal neurological involvement in tick-borne relapsing fever in northern Tanzania. European Journal of Neurology. 2005;12(6):449–52. doi: 10.1111/j.1468-1331.2005.01003.x [DOI] [PubMed] [Google Scholar]
- 191.Barclay AJG, Coulter JBS. Tick-borne relapsing fever in central Tanzania. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1990;84(6):852–6. doi: 10.1016/0035-9203(90)90106-o [DOI] [PubMed] [Google Scholar]
- 192.Melkert PWJ. Relapsing fever in pregnancy: analysis of high-risk factors. BJOG: An International Journal of Obstetrics & Gynaecology. 1988;95(10):1070–2. doi: 10.1111/j.1471-0528.1988.tb06516.x [DOI] [PubMed] [Google Scholar]
- 193.Yagupsky P, Moses S. Neonatal Borrelia species infection (relapsing fever). American Journal of Diseases of Children. 1985;139(1):74–6. doi: 10.1001/archpedi.1985.02140030076034 [DOI] [PubMed] [Google Scholar]
- 194.Makwabe CM. Tick borne relapsing fever in Tanzanian children. The Central African journal of medicine. 1984;30(8):148, 50. [PubMed] [Google Scholar]
- 195.Fihn S, Larson EB. Tick-borne relapsing fever in the Pacific Northwest: An underdiagnosed illness? Western Journal of Medicine. 1980;133(3):203–9. [PMC free article] [PubMed] [Google Scholar]
- 196.Malison MD. Relapsing fever. Journal of the American Medical Association. 1979;241(26):2819–20. [PubMed] [Google Scholar]
- 197.Horton JM, Blaser MJ. The spectrum of relapsing fever in the Rocky Mountains. Archives of Internal Medicine. 1985;145(5):871–5. [PubMed] [Google Scholar]
- 198.Fuchs PC, Oyama AA. Neonatal relapsing fever due to transplacental transmission of Borrelia. JAMA: the journal of the American Medical Association. 1969;208(4):690–2. [PubMed] [Google Scholar]
- 199.Scott MC, Rosen ME, Hamer SA, Baker E, Edwards E, Crowder C, et al. High-Prevalence Borrelia miyamotoi scapin Wild Turkeys (Meleagris gallopavo) in Tennessee. Journal of Medical Entomology. 2010;47(6):1238–42. doi: 10.1603/me10075 [DOI] [PubMed] [Google Scholar]
- 200.Yang Y, Yang Z, Kelly P, Li J, Ren Y, Wang C. Borrelia miyamotoi sensu lato in Père David Deer and Haemaphysalis longicornis Ticks. Emerg Infect Dis. 2018;24(5):928–31. doi: 10.3201/eid2405.171355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Heglasová I, Rudenko N, Golovchenko M, Zubriková D, Miklisová D, Stanko M. Ticks, fleas and rodent-hosts analyzed for the presence of Borrelia miyamotoi in Slovakia: the first record of Borrelia miyamotoi in a Haemaphysalis inermis tick. Ticks and Tick-borne Diseases. 2020;11(5). [DOI] [PubMed] [Google Scholar]
- 202.Grech-Angelini S, Stachurski F, Vayssier-Taussat M, Devillers E, Casabianca F, Lancelot R, et al. Tick-borne pathogens in ticks (Acari: Ixodidae) collected from various domestic and wild hosts in Corsica (France), a Mediterranean island environment. Transboundary and Emerging Diseases. 2020;67(2):745–57. doi: 10.1111/tbed.13393 [DOI] [PubMed] [Google Scholar]
- 203.Bernard Q, Helezen E, Boulanger N. Tick-Borne Bacteria and Host Skin Interface. Skin and Arthropod Vectors 2018. p. 293–324. [Google Scholar]
- 204.Guberman D, Vardy DA, Klapholz L, Klaus SN. Vector-borne infections: a hazard for adventure visitors to Israel. Journal of Wilderness Medicine. 1994;5(3):254–62. [Google Scholar]
- 205.Donaldson TG, de Leon AAP, Li AI, Castro-Arellano I, Wozniak E, Boyle WK, et al. Assessment of the Geographic Distribution of Ornithodoros turicata (Argasidae): Climate Variation and Host Diversity. PloS Neglected Tropical Diseases. 2016;10(2):e0004383. doi: 10.1371/journal.pntd.0004383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Souidi Y, Boudebouch N, Ezikouri S, Belghyti D, Jean-François T, Sarih M. Borrelia crocidurae in Ornithodoros ticks from northwestern Morocco: A range extension in relation to climatic change? Journal of Vector Ecology. 2014;39(2):316–20. doi: 10.1111/jvec.12106 [DOI] [PubMed] [Google Scholar]
- 207.Jensenius M, Schlagenhauf P, Loutan L, Parola P, Schwartz E, Leder K, et al. Acute and Potentially Life-Threatening Tropical Diseases in Western Travelers—A GeoSentinel Multicenter Study, 1996–2011. The American Journal of Tropical Medicine and Hygiene. 2013;88(2):397–404. doi: 10.4269/ajtmh.12-0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Southern PMJ, Sanford JP. RELAPSING FEVER: A Clinical and Microbiological Review. Medicine. 1969;48(2). doi: 10.1097/00005792-196903000-00003 [DOI] [PubMed] [Google Scholar]
- 209.Cutler S, Vayssier-Taussat M, Estrada-Peña A, Potkonjak A, Mihalca AD, Zeller H. A new Borrelia on the block: Borrelia miyamotoi–a human health risk? Euro Surveill. 2019;24(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rawlings JA. An overview of tick-borne relapsing fever with emphasis on outbreaks in Texas. Texas medicine. 1995;91(5):56–9. [PubMed] [Google Scholar]
- 211.Warrell DA. Louse-borne relapsing fever (Borrelia recurrentisinfection). Epidemiology and Infection. 2019;147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.De Zulueta J, Nasrallah S, Karam JS, Anani AR, Weatman GKS, Muir DA. Finding of tick-borne relapsing fever in jordan by the malaria eradication service. Annals of Tropical Medicine and Parasitology. 1971;65(4):491–5. doi: 10.1080/00034983.1971.11686782 [DOI] [PubMed] [Google Scholar]
- 213.Fotso AF, Drancourt M. Laboratory Diagnosis of Tick-Borne African Relapsing Fevers: Latest Developments. Frontiers in Public Health. 2015;3. doi: 10.3389/fpubh.2015.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lescot M, Audic S, Robert C, Nguyen TT, Blanc G, Cutler SJ, et al. The genome of Borrelia recurrentis, the agent of deadly louse-borne relapsing fever, is a degraded subset of tick-borne Borrelia duttonii. PLoS Genetics. 2008;4(9). doi: 10.1371/journal.pgen.1000185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Elbir H, Henry M, Diatta G, Mediannikov O, Sokhna C, Tall A, et al. Multiplex Real-Time PCR Diagnostic of Relapsing Fevers in Africa. PLoS Neglected Tropical Diseases. 2013;7(1). doi: 10.1371/journal.pntd.0002042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Magnarelli LA, Anderson JF, Johnson RC. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. Journal of Infectious Diseases. 1987;156(1):183–8. doi: 10.1093/infdis/156.1.183 [DOI] [PubMed] [Google Scholar]
- 217.Schwan TG, Schrumpf ME, Hinnebusch BJ, Anderson DE Jr., Konkel ME. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J Clin Microbiol. 1996;34(10):2483–92. doi: 10.1128/jcm.34.10.2483-2492.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Guerrier G, Doherty T. Comparison of antibiotic regimens for treating louse-borne relapsing fever: A meta-analysis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2011;105(9):483–90. doi: 10.1016/j.trstmh.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 219.Schwartz RS. Paul Ehrlich’s Magic Bullets. New England Journal of Medicine. 2004;350(11):1079–80. doi: 10.1056/NEJMp048021 [DOI] [PubMed] [Google Scholar]
- 220.Ehrlich P, Hafa S. Die experimentelle Chemotherapie der Spirillosen. Springer-Verlag Berlin, Heidelberg. 1910;VIII:178. [Google Scholar]
- 221.Taft WC, Pike JB. Relapsing fever; report of a sporadic outbreak, including treatment with penicillin. Journal of the American Medical Association. 1945;129:1002–5. doi: 10.1001/jama.1945.02860490014004 [DOI] [PubMed] [Google Scholar]
- 222.Tucker WAL. A report on the treatment of tick relapsing fever with sodium penicillin. East African medical journal. 1946;23:13–8. [PubMed] [Google Scholar]
- 223.Muwazi EM. Penicillin in treatment of relapsing fever. East African medical journal. 1946;23:55–64. [PubMed] [Google Scholar]
- 224.Quin CE, Perkins ES. Tick-borne relapsing fever in East Africa. The Journal of tropical medicine and hygiene. 1946;49:30–2. [PubMed] [Google Scholar]
- 225.Charters AD. Tick-borne relapsing fever in Somaliland with special reference to the blood sedimentation rate. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1950;43(4):427–34. doi: 10.1016/0035-9203(50)90038-1 [DOI] [PubMed] [Google Scholar]
- 226.CDC. Tick borne relapsing fever treatment Atlanta, GA: US Centers for Disease Control and Prevention; 2018 [updated Nov 26. Available from: https://www.cdc.gov/relapsing-fever/clinicians/index.html.
- 227.Koetsveld J, Draga ROP, Wagemakers A, Manger A, Oei A, Visser CE, et al. In vitro susceptibility of the relapsing-fever spirochete borrelia miyamotoi to antimicrobial agents. Antimicrobial Agents and Chemotherapy. 2017;61(9). doi: 10.1128/AAC.00535-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.De Vera Andrey R, Maldonado Sampedro M. Study on the use of terramycin in the treatment of Spanish recurrent fever. Revista de sanidad e higiene pública. 1956;30(9–10):598–647. [PubMed] [Google Scholar]
- 229.The Sanford guide to antimicrobial therapy 2021. In: Gilbert DN, Chambers HF, Saag MS, Pavia AT, Boucher HW, Black D, et al., editors. Sperryville, VA, USA:: Antimicrobial Therapy, Inc.; 2021. [Google Scholar]
- 230.Todd SR, Dahlgren FS, Traeger MS, Beltrán-Aguilar ED, Marianos DW, Hamilton C, et al. No Visible Dental Staining in Children Treated with Doxycycline for Suspected Rocky Mountain Spotted Fever. The Journal of Pediatrics. 2015;166(5):1246–51. doi: 10.1016/j.jpeds.2015.02.015 [DOI] [PubMed] [Google Scholar]
- 231.Cross R, Ling C, Day NPJ, McGready R, Paris DH. Revisiting doxycycline in pregnancy and early childhood–time to rebuild its reputation? Expert Opinion on Drug Safety. 2016;15(3):367–82. doi: 10.1517/14740338.2016.1133584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Balicer RD, Mimouni D, Bar-Zeev Y, Levine H, Davidovitch N, Ankol OH, et al. Post exposure prophylaxis of tick-borne relapsing fever. European Journal of Clinical Microbiology and Infectious Diseases. 2010;29(3):253–8. doi: 10.1007/s10096-009-0846-x [DOI] [PubMed] [Google Scholar]
- 233.Moran-Gilad J, Levine H, Schwartz E, Bartal C, Huerta-Hartal M, Schwaber MJ, et al. Postexposure prophylaxis of tick-borne relapsing fever: Lessons learned from recent outbreaks in Israel. Vector-Borne and Zoonotic Diseases. 2013;13(11):791–7. doi: 10.1089/vbz.2013.1347 [DOI] [PubMed] [Google Scholar]
- 234.Binenbaum Y, Ben-Ami R, Baneth G, Langford B, Negev Y, Friedlander E, et al. Single dose of doxycycline for the prevention of tick-borne relapsing fever. Clinical Infectious Diseases. 2020;71(7):1768–71. doi: 10.1093/cid/ciaa034 [DOI] [PubMed] [Google Scholar]
- 235.Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BSN. The Jarisch-Herxheimer reaction: Revisited. Travel Medicine and Infectious Disease. 2013;11(4):231–7. doi: 10.1016/j.tmaid.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 236.Guerrier G, D’Ortenzio E. The Jarisch-Herxheimer Reaction in Leptospirosis: A Systematic Review. PLoS One. 2013;8(3). doi: 10.1371/journal.pone.0059266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gebrehiwot T, Fiseha A. Tetracycline versus penicillin in the treatment of louse-borne relapsing fever. Ethiopian Medical Journal. 1992;30(3):175–81. [PubMed] [Google Scholar]
- 238.Butler T, Jones PK, Wallace CK. Borrelia recurrentis infection: single-dose antibiotic regimens and management of the Jarisch-Herxheimer reaction. J Infect Dis. 1978;137(5):573–7. doi: 10.1093/infdis/137.5.573 [DOI] [PubMed] [Google Scholar]
- 239.Nadelman RB, Luger SW, Frank E, Wisniewski M, Collins JJ, Wormser GP. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117(4):273–80. doi: 10.7326/0003-4819-117-4-273 [DOI] [PubMed] [Google Scholar]
- 240.El-Bahnsawy MM, Labib NA, Abdel-Fattah MAH, Ibrahim AM, Morsy TA. Louse and tick borne relapsing fevers. Journal of the Egyptian Society of Parasitology. 2012;42(3):625–38. [PubMed] [Google Scholar]
- 241.Wang G. Borrelia burgdorferi and Other Borrelia Species. Molecular Medical Microbiology: Second Edition. 32014. p. 1867–909. [Google Scholar]
- 242.Seboxa T, Rahlenbeck SI. Treatment of louse-borne relapsing fever with low dose penicillin or tetracycline: A clinical trial. Scandinavian Journal of Infectious Diseases. 1995;27(1):29–31. doi: 10.3109/00365549509018969 [DOI] [PubMed] [Google Scholar]
- 243.Goubau PF. Relapsing fevers. A review. Annales de la Société belge de médecine tropicale. 1984;64(4):335–64. [PubMed] [Google Scholar]
- 244.Melkert PW. Mortality in high risk patients with tick-borne relapsing fever analysed by the Borrelia-index. East African medical journal. 1991;68(11):875–9. [PubMed] [Google Scholar]
- 245.Larsson C, Andersson M, Guo BP, Nordstrand A, Hägerstrand I, Carlsson S, et al. Complications of pregnancy and transplacental transmission of relapsing-fever borreliosis. Journal of Infectious Diseases. 2006;194(10):1367–74. doi: 10.1086/508425 [DOI] [PubMed] [Google Scholar]
- 246.McConnell J. Tick-borne relapsing fever under-reported. The Lancet infectious diseases. 2003;3(10):604. doi: 10.1016/s1473-3099(03)00787-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Established to conduct this systematic review.
(PDF)
Terms used for the study research in the different databases.
(PDF)
Reference list of included and excluded publications.
(PDF)
Used for screening and selecting eligible publications.
(PDF)
Treatment details: antimicrobial treatment regimen, dosage and duration.
(PDF)
Twenty-seven-item checklist for systematic reviews. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
(PDF)
List of Borrelia spp. and ticks associated to TBRF reported worldwide.
(XLSX)
Excel sheet containing the underlying numerical data.
(XLSX)
Distribution of competent vector ticks for TBRF Borrelia spp.
(PDF)
Subgroup analysis of B. crocidurae and B. hermsii.
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.