Abstract
Background
Postoperative ileus is a major problem following gastrointestinal cancers surgery, several randomized controlled trials have been conducted investigating the use of probiotics or synbiotics to reduce postoperative ileus, but their findings are controversial.
Objective
We conducted a meta-analysis to determine the effect of probiotics or synbiotics on early postoperative recovery of gastrointestinal function in patients with gastrointestinal cancer.
Methods
The Embase, Cochrane Library, PubMed, and Web of Science databases were comprehensively searched for randomized controlled trials (RCTs) that evaluated the effects of probiotics or synbiotics on postoperative recovery of gastrointestinal function as of April 27, 2021. Outcomes included the time to first flatus, time to first defecation, days to first solid diet, days to first fluid diet, length of postoperative hospital stay, incidence of abdominal distension and incidence of postoperative ileus. The results were reported as the mean difference (MD) and relative risk (RR) with 95% confidence intervals (CI).
Results
A total of 21 RCTs, involving 1776 participants, were included. Compared with the control group, probiotic and synbiotic supplementation resulted in a shorter first flatus (MD, -0.53 days), first defecation (MD, -0.78 days), first solid diet (MD, -0.25 days), first fluid diet (MD, -0.29 days) and postoperative hospital stay (MD, -1.43 days). Furthermore, Probiotic and synbiotic supplementation reduced the incidence of abdominal distension (RR, 0.62) and incidence of postoperative ileus (RR, 0.47).
Conclusion
Perioperative supplementation of probiotics or synbiotics can effectively promote the recovery of gastrointestinal function after gastrointestinal cancer surgery.
Introduction
Gastrointestinal cancers account for about 25% of new cancer cases worldwide and cause more than 35% of cancer-related deaths [1]. Surgery is an essential treatment for gastrointestinal cancer. Postoperative ileus is an inevitable and most common complication of gastrointestinal surgery, with up to 30% of patients suffering from postoperative ileus [2–4]. Postoperative ileus refers to the delayed recovery of gastrointestinal function after surgery, with clinical manifestations of abdominal distension, abdominal pain, vomiting, and delayed defecation of exhaust, leading to prolonged hospital stay and increased morbidity [4–7]. Postoperative ileus is a significant financial burden for patients, adding more than 1,000,000,000 dollars in additional medical costs annually in the United States [8]. Although a number of strategies have been explored for the prevention of postoperative ileus, such as gum chewing, intravenous lidocaine, and preoperative activities, their efficacy remains controversial [5].
Probiotics are living microorganisms that are beneficial to the human body when supplemented in appropriate amounts [9]. Prebiotics are substances, such as inulin and fructooligosaccharides that promote beneficial gut microbe growth [10]. Probiotics combined with prebiotics are called synbiotics [9]. Historically, probiotics and synbiotics have been widely used in the adjuvant treatment of gastrointestinal diseases [11]. In recent years, a large number of studies have found that probiotics and synbiotics can reduce the risk of infection complications after abdominal surgery [12]. In addition, probiotics and synbiotics could also promote gastrointestinal motility [13]. Probiotics and synbiotics are inexpensive, readily available, and safe [14]. Based on these findings, probiotics and synbiotics may be potential strategies to promote recovery of gastrointestinal function after gastrointestinal cancer surgery and to reduce the incidence of postoperative ileus. However, clinical studies have shown conflicting results [15, 16]. Therefore, it is extremely important to establish strong evidence to determine whether perioperative probiotics or synbiotics can prevent postoperative ileus.
Hence, we systematically collected evidence from current randomized controlled trails (RCTs) and performed a meta-analysis to determine the effect of probiotics or synbiotics on early postoperative recovery of gastrointestinal function in patients with gastrointestinal cancer.
Materials and methods
The meta-analysis is reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [17] (see S1 Checklist, PRISMA checklist, which contains PRISMA 2009 checklist).
Search strategy
Systematic literature searches were conducted on Web of Science, Cochrane Library, Embase, and PubMed databases with no filters until April 27, 2021. The search terms were: (synbiotics OR prebiotic OR probiotics OR probiotic OR prebiotics OR synbiotic) AND (operation OR surgery) AND (cancer OR neoplasm OR carcinoma OR tumour) (S1 Table). Additionally, the reference lists of related reviews were also searched to reduce omissions.
Study selection
Studies that met the following criteria were included: (1) study design: RCTs, (2) participants: gastrointestinal cancer patients undergoing surgery, (3) intervention: intervention with probiotics or synbiotics, (4) comparison: the control group received the standard treatment or a placebo, and (5) the outcomes included any of the following: time to first flatus, time to first defecation, postoperative ileus, days to first solid diet, abdominal distension, days to first fluid diet, and length of postoperative hospital stay. Duplicate studies, reviews, abstracts, non-randomized trails, animal studies, letters, and case reports were excluded.
Data extraction
The first author, gender, year, primary disease, sample size, type of surgery, type of study, age, treated days, intervention, control group data, and outcomes were extracted from each study. If the essential data could not be obtained from the article, the corresponding author was contacted to try to obtain the missing data.
Quality assessment
Risk of bias for eligible studies was assessed by the ROB-2 tool available in the Cochrane Handbook, including the following domains: (1) Randomization process, (2) Deviations from intended interventions, (3) Missing outcome data, (4) Measurement of the outcome, (5) Selection of the reported result, and (6) Overall. Literature retrieval, selection of article, data extraction, and risk of bias assessment were performed independently by two authors (Gang Tang and Jie Tao). If there was a disagreement between the authors, it was discussed and resolved with a third author (Wang Huang).
Statistical analysis
For continuous data, the mean differences (MD) with 95% confidence intervals (CIs) were calculated. Relative risks (RRs) were calculated for dichotomous variable data [18]. The I2 statistic was used to assess the magnitude of heterogeneity between studies; The random effect model was used in all quantitative analyses, and the fixed effect model was selected only when heterogeneity was low [19]. For result robustness, the one-study exclusion test was used to investigate the influence of each study on the total effect size. Subgroup analysis was performed by intervention type (probiotics or synbiotics). Egger’s test was performed using Stata 12.0 (Stata Corp., College Station, TX, USA) to assess potential publication bias. In addition, funnel plots were used when the number of included studies > 10. All statistical analyses were performed using Review 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration 2014; Copenhagen, Denmark). P <0.05 was considered significant.
GRADE assessment
To grade the quality of evidence, a GRADE assessment was performed through GRADEpro online tools (https://gradepro.org/). GRADE assessed the evidence as four levels: very low, low, medium, and high. The two researchers (Gang Tang and Jie Tao) independently assess the certainty of the evidence, and if there was dispute, they would discuss and resolve it.
Results
Literature retrieval
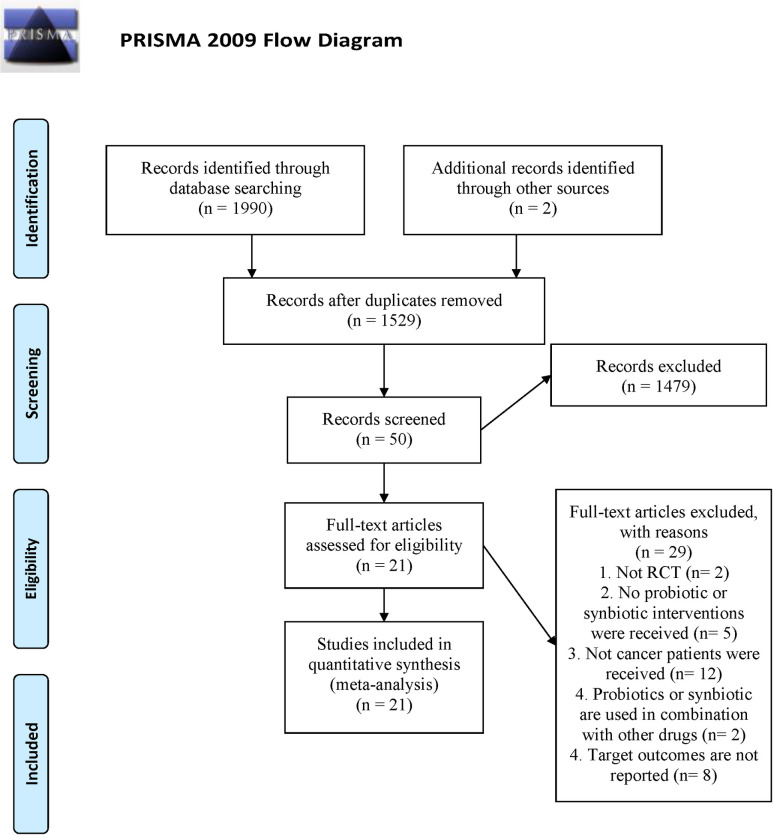
Our search strategy yielded 1,992 records and 463 duplicates were removed. 1479 of the results were excluded after reading the headings and abstracts, and the remaining 50 records were evaluated for the full text. Finally, 21 eligible studies [16, 20–39] were included. The flow chart of literature retrieval is shown in Fig 1.
Fig 1. Flow chart of literature search and screening.
Study characteristics
Between 2005 and 2020, 21 studies were published with 1776 total participants (875 in the intervention group and 901 in the control group). Twelve studies [16, 20, 22, 25, 29, 30, 32, 35–39] used only probiotics, and nine [21, 23, 24, 26–28, 31, 33, 34] used synbiotics. The indications for surgery were colorectal cancer, gastric cancer, liver cancer, gallbladder cancer, esophageal cancer and periampullary cancer. The characteristics of eligible studies are detailed in S2 Table.
Quality assessment
Ten of the studies [16, 22, 23, 27–29, 31, 32, 34, 36] conducted an appropriate randomization process. Deviations from intended interventions were evaluated as a low bias risk in six studies [16, 20, 22, 31, 34, 36]. Missing outcome data, measurement of the outcome, and selection of the reported result in all studies were assessed as a low bias risk (Fig 2). The overall risk of 10 studies [16, 22, 23, 27–29, 31, 32, 34, 36] was assessed as low risk of bias.
Fig 2. Risk of bias for each included study.
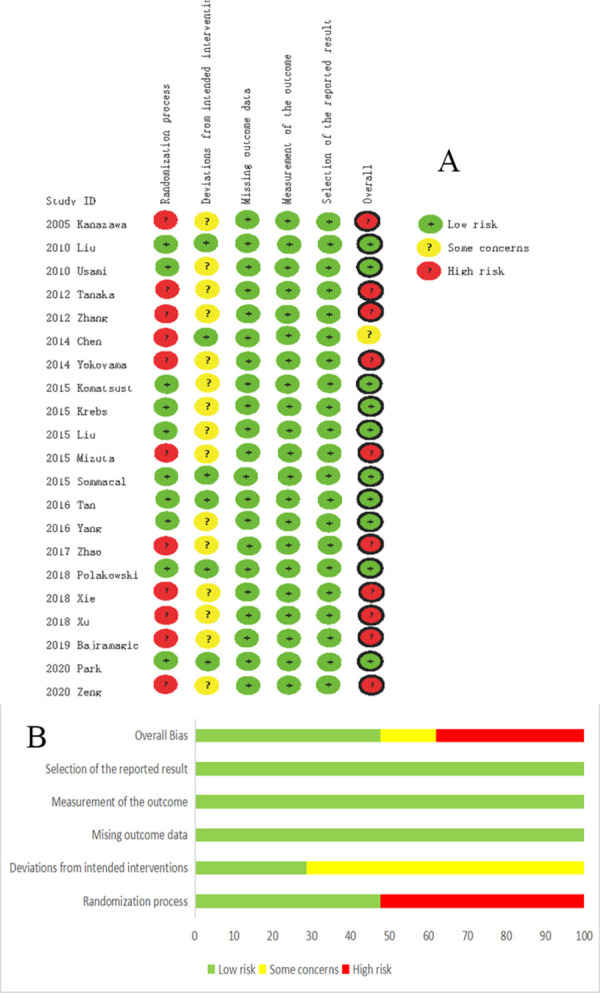
(A), risk of bias summary. (B), risk of bias graph.
Meta-analysis
Time to first flatus
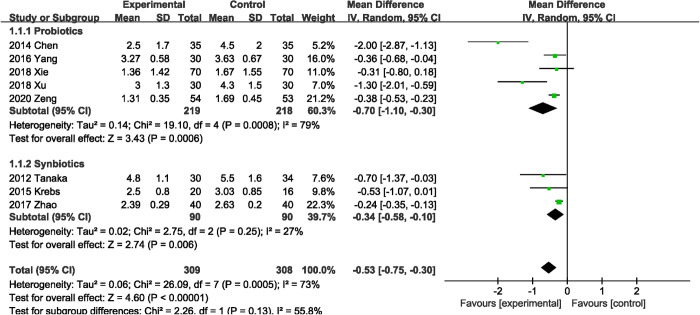
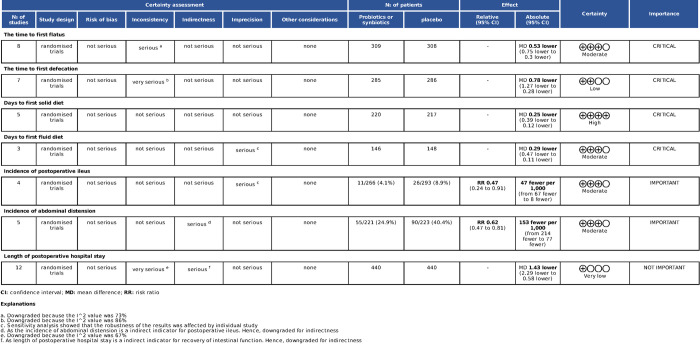
Eight RCTs [20, 24, 28, 32, 33, 37–39] (617 patients) reported on time to first flatus. Probiotics or synbiotics supplementation was associated with a significant reduction in time to first flatus (MD, -0.53 days; 95% CI, -0.75, -0.30; P < 0.00001) (Fig 3), with significant heterogeneity (I2 = 73%, P = 0.0005). The results of subgroup analysis showed that both probiotics (MD, -0.70 days; 95% CI, -1.10, -0.30; P = 0.0006) alone and synbiotics (MD, -0.34 days; 95% CI, -0.58, -0.10; P = 0.006) supplementation were associated with shorter first exhaust time.
Fig 3. Effect of probiotics or synbiotics supplementation on time to first flatus.
Time to first defecation
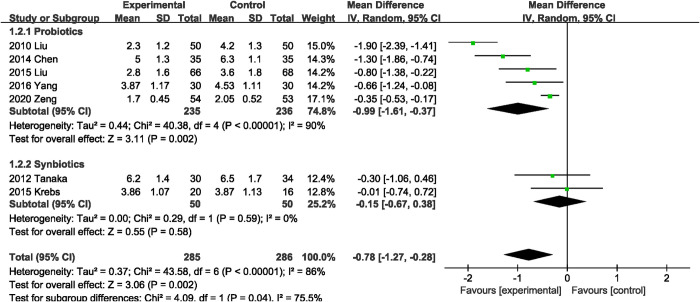
Seven studies [20, 22, 24, 28, 29, 32, 39] measured time to first defecation as an outcome. Compared with the control group, probiotics or synbiotics significantly reduced the time to first defecation, with significant heterogeneity (MD, -0.78 days; 95% CI, -1.27, -0.28; P = 0.002; I2 = 86%, P < 0.00001) (Fig 4). Subgroup analysis indicated that this benefit was observed only in the subgroup supplemented with probiotics alone (MD, -0.99 days; 95% CI, -1.61, -0.37).
Fig 4. Effect of probiotics or synbiotics supplementation on time to first defecation.
Days to first solid diet
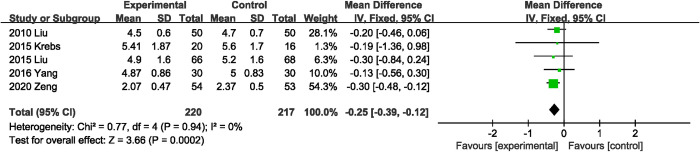
Five studies [22, 28, 29, 32, 39] reported data for days to first solid diet, pooled results showed that probiotics or synbiotics supplementation significantly shortened the days to first solid diet (MD, -0.25 days; 95% CI, -0.39, -0.12; P = 0.0002) (Fig 5). In addition, no significant heterogeneity was shown between RCTs (I2 = 0%, P = 0.94).
Fig 5. Effect of probiotics or synbiotics supplementation on days to first solid diet.
Days to first fluid diet
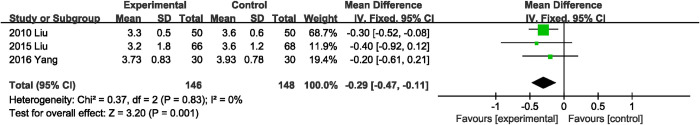
Three RCTs [22, 29, 32] mentioned days to first fluid diet. Probiotics or synbiotics significantly shortened the days to first fluid diet (MD, -0.29 days; 95% CI, -0.47, -0.11; P = 0.001) (Fig 6), and no significant heterogeneity was observed between the three studies (I2 = 0%, P = 0.83).
Fig 6. Effect of probiotics or synbiotics supplementation on days to first fluid diet.
Length of postoperative hospital stay
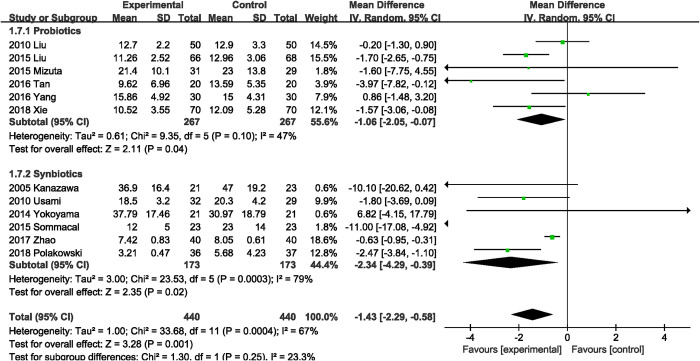
Twelve RCTs [16, 21–23, 26, 29–33, 37] with a total of 440 participants were in the probiotics or synbiotics group and 440 in the control. The combined result favored probiotics or synbiotics supplementation, with a MD of 1.43 days reduction (MD, -1.43 days; 95% CI, -2.29, -0.58; P = 0.001; I2 = 67%; Fig 7). Subgroup analysis showed that both probiotics (MD, -1.06 days; 95% CI, -2.05, -0.07; P = 0.04) and synbiotics (MD, -2.34 days; 95% CI, -4.29, -0.39; P = 0.02) supplementation reduced length of postoperative hospital stay.
Fig 7. Effect of probiotics or synbiotics supplementation on length of postoperative hospital stay.
Postoperative ileus
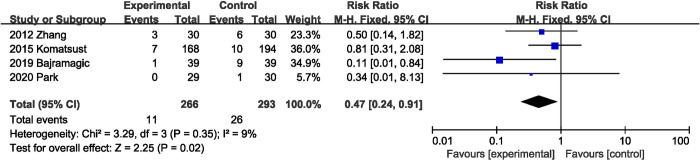
Of the 21 eligible RCTs, four studies [25, 27, 35, 36] (559 participants) reported findings on postoperative ileus, the combined total effect size showed that supplementation with probiotics or synbiotics significantly reduced the incidence of postoperative ileus (RR, 0.47; 95% CI, 0.24, 0.91, P = 0.02; I2 = 9%, P = 0.35) (Fig 8).
Fig 8. Effect of probiotics or synbiotics supplementation on the incidence of postoperative ileus.
Abdominal distension
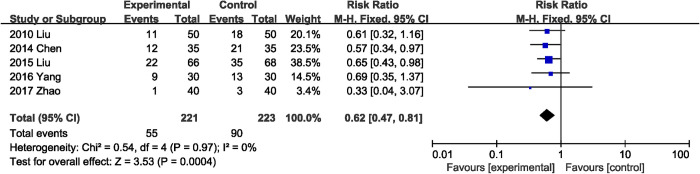
Five RCTs [20, 22, 29, 32, 33] presented data on incidence of abdominal distension. Supplementation with probiotics or synbiotics was associated with a significant reduction in the incidence of postoperative abdominal distension (RR, 0.62; 95% CI, 0.47, 0.81; P = 0.0004) (Fig 9), with low heterogeneity (I2 = 0%, P = 0.97).
Fig 9. Effect of probiotics or synbiotics supplementation on the incidence of postoperative abdominal distension.
Sensitivity analysis
The results of the sensitivity analysis indicated that excluding any one study did not affect the total effect size of the time to first flatus, time to first defecation, days to first solid diet, length of postoperative hospital stay and incidence of abdominal distension. The overall effect size for the days to first fluid diet changed when the study by Liu et al. [22] (MD, -0.28 days; 95% CI, -0.60, 0.04; P = 0.09) was excluded. The overall effect size of the incidence of postoperative ileus was influenced by the study of Bajramagic et al. [35] (RR, 0.66; 95% CI, 0.32, 1.37, P = 0.26).
Publication bias
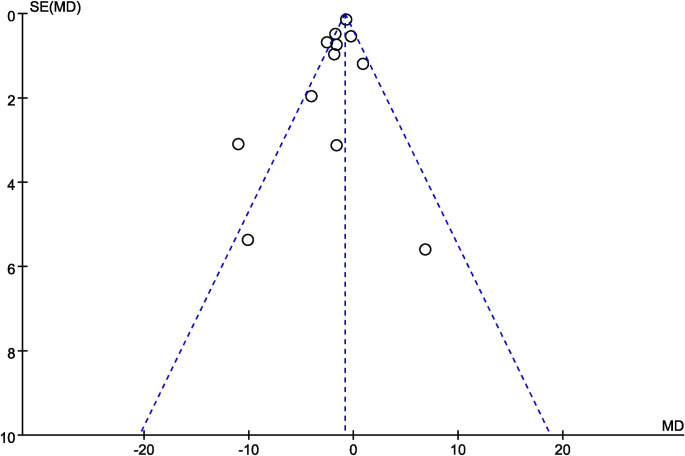
Egger’s test results did not show potential publication bias of the time to first flatus (P = 0.214), time to first defecation (P = 0.754), days to first solid diet (P = 0.609), days to first fluid diet (P = 0.991), length of postoperative hospital stay (P = 0.970), incidence of abdominal distension (P = 0.530) and incidence of postoperative ileus (P = 0.265). Visual inspection of the funnel plot (length of postoperative hospital stay) identified basically symmetric (Fig 10).
Fig 10. Funnel plot of effect of probiotics or synbiotics supplementation on the length of postoperative hospital stay.
GRADE analysis
We evaluated the quality of evidence in this study (Fig 11). A part of the evidence (the time to first flatus, days to first fluid diet, incidence of abdominal distension and incidence of postoperative ileus) was in a medium level, one (length of postoperative hospital stay) was very low, one (the time to first defecation) was low, one (days to first solid diet) was high.
Fig 11. Grade evidence synthesis and summary of findings.
Discussion
Postoperative gastrointestinal function, as the core part of the accelerated recovery of patients with gastrointestinal cancer undergoing surgery, has important clinical significance and has been paid close attention by surgeons [40]. To our knowledge, this is the first meta-analysis to evaluate the effect of probiotics or synbiotics on gastrointestinal function recovery after gastrointestinal cancer surgery. Evidence from this meta-analysis was based on 21 RCTs with 1776 participants. The results showed that peri-operative probiotics or synbiotics supplementation signifcantly reduced the time to first flatus, time to first defecation, days to first solid diet, days to first fluid diet and length of postoperative hospital stay. The time to first flatus and time to first defecation are the key to evaluate gastrointestinal dysfunction and postoperative ileus. They are generally considered to be the relief of postoperative ileus, and are also important indicators to evaluate the efficacy of intervention methods [4]. The results of subgroup analysis showed that either probiotics alone or synbiotics alone could shorten the time to first exhaust and first defecation. In addition, probiotics or prebiotics could also reduce the incidence of postoperative abdominal distension and postoperative ileus. This study has important clinical significance because our meta-analysis provides clear evidence that probiotics or synbiotics could promote gastrointestinal recovery normality after surgery for gastrointestinal cancer. Hence, probiotics or synbiotics are potential strategies that clinicians should consider in the prevention of postoperative ileus.
The mechanism of postoperative ileus is not clear and may involve the interaction of many factors [2], inhibition of gastrointestinal motility caused by surgical overstimulation of the sympathetic nerve may be the most important factor [41]. In addition, substance P and nitric oxide secreted by the enteric nervous system also prolong the duration of postoperative ileus. Furthermore, surgery stimulates the inflammatory cascade, releasing a large number of inflammatory mediators, such as interleukin-6, interleukin-1, monocytechemoattractantprotein-1 and cell adhesion molecule-1, which damage intestinal muscles and further inhibit the recovery of gastrointestinal function [2, 41]. Some drugs have also been associated with increased the risk of ileus after surgery [2]. Probiotics or synbiotics are an alternative therapy widely used in cancer patients to prevent postoperative infection, relieve symptoms and improve quality of life, with beneficial effects in a variety of gastrointestinal diseases having been demonstrated [42]. Peri-operative supplementation with probiotics or synbiotics could modulate local and systemic immune homeostasis, reduce inflammatory responses, and reduce concentrations of pro-inflammatory factors, tumor necrosis factor-α, interleukin-6, C-reactive protein, and nitric oxide which could aggravate postoperative ileus by ameliorating operationally induced intestinal flora dysregulation [42–46]. In addition, Schmitter et al. found that probiotics significantly reduced the release of interleukin-6, interleukin-8, and prostaglanin E2 from monocytes compared with placebo [47]. Studies have shown that dendritic cells in the gastrointestinal tract can interact with intestinal nerve cells and intestinal microorganisms. Probiotics or synbiotics may stimulate nerve cells to promote gastrointestinal function recovery by regulating intestinal microorganisms [42].
Several excluded clinical studies have also supported the beneficial effects of probiotics or synbiotics on postoperative ileus. A non-RCT study by Aisu et al. [48] showed that perioperative probiotics supplementation significantly reduced the time to first exhaust and first feeding. Kotzampassi et al. [49] found that a capsule containing four probiotics significantly shortened the time to first defecation in patients undergoing colorectal surgery, compared with a placebo. In addition, Xu et al. [50] demonstrated that early use of synbitin after colon cancer surgery can improve immune function, reduce inflammatory response, and promote gastrointestinal function recovery.
This study has several strengths. First, only RCTs were included in our meta-analysis in order to synthesize the strongest evidence. Second, this study conducted a comprehensive literature search to reduce bias. Furthermore, we used advanced statistical methods to find no potential publication bias. Finally, we confirmed the robustness of our results (including time to first exhaust, time to first defecate, days to first fluid diet, incidence of abdominal distension and length of hospital stay) through sensitivity analysis.
Our meta-analysis also had several limitations. First, several studies with small sample sizes were included. Second, some outcome measures (incidence of postoperative ileus and incidence of postoperative abdominal distension) were quantitatively synthesized based on a small number of studies. Third, Significant heterogeneity was observed in our study, which may be related to significant differences in type of surgery (radical colorectomy, liver resection, esophagectomy, colorectal cancer resection, gastrectomy and pancreatoduodenectomy), duration of probiotics or synbiotics supplementation (from 3 days to 28 days), species of probiotics or synbiotics and dose of probiotics or synbiotics. Future research should explore the specific species of probiotics or synbiotics with the greatest benefit for gastrointestinal function recovery, as well as the most appropriate course and dose of probiotics or synbiotics supplementation. Finally, this study only included patients with gastrointestinal cancer who underwent elective surgery, so our findings may not be generalizable to patients undergoing emergency surgery.
Conclusions
In conclusion, our study showed that perioperative supplementation of probiotics or synbiotics can effectively promote the recovery of gastrointestinal function after gastrointestinal cancer surgery, including shorting the time to first flatus, time to first defecation, days to first solid diet, days to first fluid diet and length of postoperative hospital stay, and reducing the incidence of postoperative abdominal distention and postoperative ileus. But these conclusions need to be treated with caution, given some limitations that cannot be ignored. High-quality, large-sample RCTs are necessary to confirm the benefit of probiotics or synbiotics supplementation for gastrointestinal function recovery after gastrointestinal cancer surgery.
Supporting information
(DOC)
(DOC)
CFU: colony forming units; C: Control group; DB: Double blind; I: Intervention group; GOS: galacto-oligosaccharides; PD: pancreatoduodenectomy; N: not available; RCT: randomized controlled trial; SC: standard care; TF: time to first flatus; TD: time to first defecation; LOP: Length of postoperative hospital stay; PI: Postoperative ileus; DS: Days to first solid diet; AB; abdominal distension; DF: days to first fluid die.
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol Motil. 2004;16 Suppl 2:54–60. doi: 10.1111/j.1743-3150.2004.00558.x [DOI] [PubMed] [Google Scholar]
- 3.Mochiki E, Ohno T, Yanai M, Toyomasu Y, Andoh H, Kuwano H. Effects of glutamine on gastrointestinal motor activity in patients following gastric surgery. World journal of surgery. 2011;35(4):805–10. doi: 10.1007/s00268-011-0962-5 [DOI] [PubMed] [Google Scholar]
- 4.Liu YH, Dong GT, Ye Y, Zheng JB, Zhang Y, Lin HS, et al. Effectiveness of Acupuncture for Early Recovery of Bowel Function in Cancer: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2017;2017:2504021. doi: 10.1155/2017/2504021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman SJ, Pericleous A, Downey C, Jayne DG. Postoperative ileus following major colorectal surgery. Br J Surg. 2018;105(7):797–810. doi: 10.1002/bjs.10781 [DOI] [PubMed] [Google Scholar]
- 6.Gong J, Xie Z, Zhang T, Gu L, Yao W, Guo Z, et al. Randomised clinical trial: prucalopride, a colonic pro-motility agent, reduces the duration of post-operative ileus after elective gastrointestinal surgery. Alimentary pharmacology & therapeutics. 2016;43(7):778–89. doi: 10.1111/apt.13557 [DOI] [PubMed] [Google Scholar]
- 7.Gan TJ, Robinson SB, Oderda GM, Scranton R, Pepin J, Ramamoorthy S. Impact of postsurgical opioid use and ileus on economic outcomes in gastrointestinal surgeries. Curr Med Res Opin. 2015;31(4):677–86. doi: 10.1185/03007995.2015.1005833 [DOI] [PubMed] [Google Scholar]
- 8.Viscusi ER, Goldstein S, Witkowski T, Andonakakis A, Jan R, Gabriel K, et al. Alvimopan, a peripherally acting mu-opioid receptor antagonist, compared with placebo in postoperative ileus after major abdominal surgery: results of a randomized, double-blind, controlled study. Surg Endosc. 2006;20(1):64–70. doi: 10.1007/s00464-005-0104-y [DOI] [PubMed] [Google Scholar]
- 9.Notay M, Foolad N, Vaughn AR, Sivamani RK. Probiotics, Prebiotics, and Synbiotics for the Treatment and Prevention of Adult Dermatological Diseases. American journal of clinical dermatology. 2017;18(6):721–32. doi: 10.1007/s40257-017-0300-2 [DOI] [PubMed] [Google Scholar]
- 10.Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547–61; quiz 6, 62. doi: 10.1038/ajg.2014.202 [DOI] [PubMed] [Google Scholar]
- 11.Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nature reviews Gastroenterology & hepatology. 2019;16(10):605–16. doi: 10.1038/s41575-019-0173-3 [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury AH, Adiamah A, Kushairi A, Varadhan KK, Krznaric Z, Kulkarni AD, et al. Perioperative Probiotics or Synbiotics in Adults Undergoing Elective Abdominal Surgery: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Annals of surgery. 2020;271(6):1036–47. doi: 10.1097/SLA.0000000000003581 [DOI] [PubMed] [Google Scholar]
- 13.Folwarski M, Dobosz M, Małgorzewicz S, Skonieczna-Żydecka K, Kaźmierczak-Siedlecka K. Effects of Lactobacillus rhamnosus GG on early postoperative outcome after pylorus-preserving pancreatoduodenectomy: a randomized trial. Eur Rev Med Pharmacol Sci. 2021;25(1):397–405. doi: 10.26355/eurrev_202101_24407 [DOI] [PubMed] [Google Scholar]
- 14.Szántó M, Dózsa A, Antal D, Szabó K, Kemény L, Bai P. Targeting the gut-skin axis-Probiotics as new tools for skin disorder management? Exp Dermatol. 2019;28(11):1210–8. doi: 10.1111/exd.14016 [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Padilla Á, Morales-Martín G, Pérez-Quintero R, Gómez-Salgado J, Balongo-García R, Ruiz-Frutos C. Postoperative Ileus after Stimulation with Probiotics before Ileostomy Closure. Nutrients. 2021;13(2). doi: 10.3390/nu13020626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan CK, Said S, Rajandram R, Wang Z, Roslani AC, Chin KF. Pre-surgical Administration of Microbial Cell Preparation in Colorectal Cancer Patients: A Randomized Controlled Trial. World journal of surgery. 2016;40(8):1985–92. doi: 10.1007/s00268-016-3499-9 [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. 2011. Available from www.cochrane-handbook org. Available from www.cochrane-handbook org.
- 19.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–58. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Xia Y, Shi C, Liang Y, Yang Y, Qin H. Effects of perioperative probiotics administration on patients with colorectal cancer. Chinese Journal of Clinical Nutrition. 2014;22(2):74–81. 10.3760/cma.j.issn.1674-635X.2014.02.002. [DOI] [Google Scholar]
- 21.Kanazawa H, Nagino M, Kamiya S, Komatsu S, Mayumi T, Takagi K. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbeck’s archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2005;390(2):104‐13. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01081899/full. CN-01081899 [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Qin H, Yang Z, Xia Y, Liu W, Yang J, et al. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery—a double-blind study. Alimentary pharmacology & therapeutics. 2011;33(1):50‐63. CN-00779488 doi: 10.1111/j.1365-2036.2010.04492.x [DOI] [PubMed] [Google Scholar]
- 23.Usami M, Miyoshi M, Kanbara Y, Aoyama M, Sakaki H, Shuno K, et al. Effects of perioperative synbiotic treatment on infectious complications, intestinal integrity, and fecal flora and organic acids in hepatic surgery with or without cirrhosis. Journal of Parenteral and Enteral Nutrition. 2011;35(3):317–28. doi: 10.1177/0148607110379813 [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, Yano M, Motoori M, Kishi K, Miyashiro I, Ohue M, et al. Impact of perioperative administration of synbiotics in patients with esophageal cancer undergoing esophagectomy: A prospective randomized controlled trial. Surgery (United States). 2012;152(5):832–42. doi: 10.1016/j.surg.2012.02.021 [DOI] [PubMed] [Google Scholar]
- 25.Zhang JW, Du P, Gao J, Yang BR, Fang WJ, Ying CM. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. American Journal of the Medical Sciences. 2012;343(3):199–205. doi: 10.1097/MAJ.0b013e31823aace6 [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama Y, Nishigaki E, Abe T, Fukaya M, Asahara T, Nomoto K, et al. Randomized clinical trial of the effect of perioperative synbiotics versus no synbiotics on bacterial translocation after oesophagectomy. British Journal of Surgery. 2014;101(3):189–99. WOS:000331175500007 doi: 10.1002/bjs.9385 [DOI] [PubMed] [Google Scholar]
- 27.Komatsu S, Sakamoto E, Norimizu S, Shingu Y, Asahara T, Nomoto K, et al. Efficacy of perioperative synbiotics treatment for the prevention of surgical site infection after laparoscopic colorectal surgery: a randomized controlled trial. Surg Today. 2016;46(4):479–90. WOS:000373159300014 doi: 10.1007/s00595-015-1178-3 [DOI] [PubMed] [Google Scholar]
- 28.Krebs B. Prebiotic and Synbiotic Treatment before Colorectal Surgery—Randomised Double Blind Trial. Collegium antropologicum. 2016;40(1):35–40. https://www.embase.com/search/results?subaction=viewrecord&id=L611329695&from=export. [PubMed] [Google Scholar]
- 29.Liu Z, Li C, Huang M, Tong C, Zhang X, Wang L, et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: a double-center and double-blind randomized clinical trial. BMC Gastroenterol. 2015;15:34. CN-01215318 doi: 10.1186/s12876-015-0260-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizuta M, Endo I, Yamamoto S, Inokawa H, Kubo M, Udaka T, et al. Perioperative supplementation with bifidobacteria improves postoperative nutritional recovery, inflammatory response, and fecal microbiota in patients undergoing colorectal surgery: a prospective, randomized clinical trial. Bioscience of Microbiota Food and Health. 2016;35(2):77–87. 10.12938/bmfh.2015-017. WOS:000426585800003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sommacal HM, Bersch VP, Vitola SP, Osvaldt AB. Perioperative Synbiotics Decrease Postoperative Complications in Periampullary Neoplasms: A Randomized, Double-Blind Clinical Trial. Nutrition and Cancer-an International Journal. 2015;67(3):457–62. WOS:000352316200009 doi: 10.1080/01635581.2015.1004734 [DOI] [PubMed] [Google Scholar]
- 32.Ma Y, Wei Q, Yang Y, Qin H. The effect of perioperative probiotics treatment for colorectal cancer: Shortterm outcomes of a randomized controlled trial. Cancer Res. 2016;76(14). 10.1158/1538-7445.AM2016-CT155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao R, Wang Y, Huang Y, Cui Y, Xia L, Rao Z, et al. Effects of fiber and probiotics on diarrhea associated with enteral nutrition in gastric cancer patients: A prospective randomized and controlled trial. Medicine (Baltimore). 2017;96(43):e8418. doi: 10.1097/MD.0000000000008418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polakowski CB, Kato M, Preti VB, Schieferdecker MEM, Campos ACL. Impact of the preoperative use of synbiotics in colorectal cancer patients: A prospective, randomized, double-blind, placebo-controlled study. Nutrition. 2019;58:40–6. WOS:000457068900008 doi: 10.1016/j.nut.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 35.Bajramagic S, Hodzic E, Mulabdic A, Holjan S, Smajlovic SV, Rovcanin A. Usage of Probiotics and its Clinical Significance at Surgically Treated Patients Sufferig from Colorectal Carcinoma. Medical archives (Sarajevo, Bosnia and Herzegovina). 2019;73(5):316–20. doi: 10.5455/medarh.2019.73.316-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park IJ, Lee JH, Kye BH, Oh HK, Cho YB, Kim YT, et al. Effects of probiotics on the symptoms and surgical outcomes after anterior resection of colon cancer (Postcare): a randomized, double-blind, placebo-controlled trial. Journal of clinical medicine. 2020;9(7):1‐16. CN-02142695 doi: 10.3390/jcm9072181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie H, Lu Q, Wang H, Zhu X, Guan Z. Effects of probiotics combined with enteral nutrition on immune function and inflammatory response in postoperative patients with gastric cancer. J buon. 2018;23(3):678–83. [PubMed] [Google Scholar]
- 38.Xu Q, Xu P, Cen Y, Li W. Effects of preoperative oral administration of glucose solution combined with postoperative probiotics on inflammation and intestinal barrier function in patients after colorectal cancer surgery. Oncology letters. 2019;18(1):694–8. doi: 10.3892/ol.2019.10336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng X, Yang SW, Yang HQ, Chen Y, Pan QJ. Effect of bifid triple viable combined with enteral nutrition support on gastrointestinal function and nutritional indexes in patients with gastric cancer after operation. World Chinese Journal of Digestology. 2020;28(11):410–6. 10.11569/wcjd.v28.i11.410. [DOI] [Google Scholar]
- 40.Wei S, Yu-Han Z, Wei-Wei J, Hai Y. The effects of intravenous lidocaine on wound pain and gastrointestinal function recovery after laparoscopic colorectal surgery. Int Wound J. 2020;17(2):351–62. doi: 10.1111/iwj.13279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vásquez W, Hernández AV, Garcia-Sabrido JL. Is gum chewing useful for ileus after elective colorectal surgery? A systematic review and meta-analysis of randomized clinical trials. J Gastrointest Surg. 2009;13(4):649–56. doi: 10.1007/s11605-008-0756-8 [DOI] [PubMed] [Google Scholar]
- 42.Theodoropoulos GE, Memos NA, Peitsidou K, Karantanos T, Spyropoulos BG, Zografos G. Synbiotics and gastrointestinal function-related quality of life after elective colorectal cancer resection. Ann Gastroenterol. 2016;29(1):56–62. [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng C, Chen T, Wang Y, Gao Y, Kong Y, Liu Z, et al. A randomised trial of probiotics to reduce severity of physiological and microbial disorders induced by partial gastrectomy for patients with gastric cancer. J Cancer. 2019;10(3):568–76. doi: 10.7150/jca.29072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skonieczna-Żydecka K, Kaczmarczyk M, Łoniewski I, Lara LF, Koulaouzidis A, Misera A, et al. A Systematic Review, Meta-Analysis, and Meta-Regression Evaluating the Efficacy and Mechanisms of Action of Probiotics and Synbiotics in the Prevention of Surgical Site Infections and Surgery-Related Complications. J Clin Med. 2018;7(12). doi: 10.3390/jcm7120556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn SI, Cho S, Choi NJ. Effect of dietary probiotics on colon length in an inflammatory bowel disease-induced murine model: A meta-analysis. J Dairy Sci. 2020;103(2):1807–19. doi: 10.3168/jds.2019-17356 [DOI] [PubMed] [Google Scholar]
- 46.Zaharuddin L, Mokhtar NM, Muhammad Nawawi KN, Raja Ali RA. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019;19(1):131. doi: 10.1186/s12876-019-1047-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitter T, Fiebich BL, Fischer JT, Gajfulin M, Larsson N, Rose T, et al. Ex vivo anti-inflammatory effects of probiotics for periodontal health. Journal of oral microbiology. 2018;10(1):1502027. doi: 10.1080/20002297.2018.1502027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aisu N, Tanimura S, Yamashita Y, Yamashita K, Maki K, Yoshida Y, et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Experimental and therapeutic medicine. 2015;10(3):966–72. doi: 10.3892/etm.2015.2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotzampassi K, Stavrou G, Damoraki G, Georgitsi M, Basdanis G, Tsaousi G, et al. A Four-Probiotics Regimen Reduces Postoperative Complications After Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World journal of surgery. 2015;39(11):2776–83. doi: 10.1007/s00268-015-3071-z [DOI] [PubMed] [Google Scholar]
- 50.Xu R, Ding Z, Zhao P, Tang L, Tang X, Xiao S. The Effects of Early Post-Operative Soluble Dietary Fiber Enteral Nutrition for Colon Cancer. Nutrients. 2016;8(9). doi: 10.3390/nu8090584 [DOI] [PMC free article] [PubMed] [Google Scholar]