Abstract
Background:
Chronic inflammation contributes to the pathogenesis of depression in persons with HIV (PWH). Neopterin, a biomarker of HIV-related immune activation that partially normalizes with antiretroviral therapy (ART), correlates with major depressive disorder (MDD) and sub-clinical depressive symptoms in persons without HIV and acutely-infected, young PWH. The sensitivity of neopterin, however, to both lifetime and current depression is poorly understood in older PWH on suppressive ART.
Methods:
Participants were 70 PWH and 35 persons without HIV (HIV-) who were at least 50 years of age and completed standardized neurobehavioral and neuromedical assessments. Depressive symptoms in the past two weeks, measured with the Beck Depression Inventory-II (BDI-II), and lifetime MDD diagnoses, defined as meeting DSM-IV criteria for a depressive episode at any point in one’s lifetime, were separately modeled as a function of plasma neopterin levels in the full sample and by HIV serostatus.
Results:
Compared to HIV- adults, PWH had higher neopterin levels (p<.001) and BDI-II scores (p<.01) and were more likely to have lifetime MDD (p<.01). Higher neopterin related to lifetime MDD but only in PWH, even after controlling for clinically-relevant comorbidities and treatment factors in logistic regression (OR=3.11, p=.002). Higher neopterin correlated with higher BDI-II scores in the full sample (rs=0.25; p=0.010), but not within either group (PWH: rs=0.03, p=0.819; HIV-: rs=0.09, p=0.588).
Conclusion:
Neopterin was associated with lifetime MDD but not current depressive symptoms in older PWH on suppressive ART. This may reflect a legacy of inflammation-related disruptions to amino acid metabolism and neurotransmitter synthesis, similar to prior observations. Identification of biopsychosocial and resilience factors underlying the null association between neopterin and current depression in older PWH is warranted.
Keywords: aging, HIV, depression, inflammation, neopterin, antiretroviral therapy
Introduction
Persons with HIV (PWH) have greater odds of meeting criteria for major depressive disorder (MDD) as compared to the general population1. Among older PWH, 52% reported depressive symptoms within the previous year2, which is markedly higher than the general population (9.1%)3. In the general population, prevalence of depression tends to decline with age; however, PWH have plateauing, rather than declining, levels of depression with age4. Depression in aging PWH is of particular importance given that over half (51%) of PWH in the U.S. and dependent areas are aged 50 years old and older5. Underlying causes of the higher prevalence of depression among older PWH are not fully understood, but chronic inflammation plays a role.
Numerous studies link depression to inflammation among persons with chronic diseases, including HIV. For example, central and peripheral inflammation due to immunometabolic dysregulation contributes to MDD risk in patients with metabolic syndrome6,7. Similarly, risk of depressive symptoms is elevated among PWH with high levels of the acute phase reactant, C-reactive protein8. Increased peripheral blood concentrations of pro-inflammatory cytokines, particularly interleukin-6 (IL-6) and tumor necrosis factor (TNF), are also frequently observed in depressed patients with9 and without HIV10. Elevated cerebrospinal fluid (CSF) concentrations of cytokines and tryptophan catabolites may also reflect a transdiagnostic neuroimmune feature of major psychiatric disorders, including schizophrenia, bipolar disorder, and MDD11. Understanding the influence of inflammation is clinically relevant since it is associated with treatment-resistant depression12. Meta-analysis of randomized controlled trials show anti-inflammatory treatments may have significant antidepressant effects when compared to placebo13.
Neopterin is a product of guanosine triphosphate (GTP) metabolism that is produced by macrophages. Its concentrations in blood are reduced by antiretroviral therapy (ART)14 but can remain elevated years after initiation15. Neopterin is synthesized in response to interferon-gamma (IFN-γ), which also induces tryptophan degradation via activation of indoleamine 2,3-dioxygenase (IDO)16. Because tryptophan is a precursor of serotonin, increased neopterin may signal a concurrent reduction in serotonin and vulnerability for depression16. Neopterin is increased in individuals experiencing two or more episodes of depression compared to others17. Consistent with the influence of neopterin on IDO, people with MDD have lower tryptophan levels and higher IFN-γ18. In the limited literature on the association between neopterin and depression among PWH, similar trends have been found19–21.
The existing research linking neopterin with depression in PWH has typically evaluated relatively young cohorts with recent HIV diagnosis. It is well established that typical biological aging involves increased inflammation22, including elevations in neopterin23,24, and the combination of HIV disease and older age may enhance the likelihood of a chronic inflammatory state25. As PWH grow older, studying depression and its association with immune function becomes increasingly important. The study reported here investigates the relationship between neopterin in blood plasma and depression, both lifetime MDD and current depressive symptoms, in a cohort of older PWH on suppressive ART. We hypothesized that: 1) older PWH would have higher rates of lifetime MDD and more current depressive symptoms than their HIV-counterparts; 2) older PWH would also exhibit higher plasma neopterin levels; and 3) higher neopterin would relate to higher odds of lifetime MDD and more current depressive symptoms irrespective of HIV serostatus, but these associations would be stronger among PWH.
Methods
Participants and Procedure
This study examined 70 PWH and 35 persons without HIV (HIV-) adults from the UCSD HIV Neurobehavioral Research Program’s Successfully Aging Seniors with HIV (SASH) cohort. All participants provided written informed consent and the study protocol was approved by the Institutional Review Board. In order to be included in this analysis, participants were at least 50 years of age and had neopterin measured in blood plasma. All PWH were taking ART and had a plasma HIV RNA ≤ 50 copies/mL. All participants underwent standardized neuromedical and psychiatric assessments.
Psychiatric Assessments
Participants were evaluated for lifetime (any point in one’s lifetime) and current (last 30 days) MDD and substance use disorder (dependence or abuse) diagnoses using the Composite International Diagnostic Interview (CIDI)26, a computerized psychodiagnostic clinical interview based on the DSM-IV, as study methodology was developed prior to the release of the DSM-5. In addition to the diagnostic evaluation, participants were administered the Beck Depression Inventory II (BDI-II)27 to provide a continuous measure of depressive symptoms (possible range: 0 to 63) experienced in the past two weeks. In order to preserve statistical power in primary analyses, we chose to model current depression with the continuous total BDI-II score instead of a current MDD diagnosis, as only 10 total study participants had a current MDD diagnosis. Domain-specific BDI-II scores reflecting cognitive (possible range: 0 to 27), affective (possible range: 0 to 12), and somatic (possible range: 0 to 24) symptoms of depression were computed based on a previous factor analysis of the BDI-II in 1,583 PWH28.
Neuromedical Assessment
Medical comorbidities and medications were determined by interview. For the group of PWH, additional HIV disease-related variables were collected. These included history of Acquired Immunodeficiency Syndrome (AIDS), estimated duration of HIV disease (years), current CD4+ T cell count, nadir CD4+ T cell count, and duration of ART use (years). Current use of efavirenz is reported for the present study given its known associations with neurobehavioral disturbance29. HIV RNA level was measured in plasma by RT-PCR (Abbott Diagnostics; lower limit of quantitation 50 copies/mL).
Neopterin Assay
Blood was collected by venipuncture and aliquots were stored at −80C until assayed. Neopterin levels were quantified from the blood by enzyme-linked immunosorbent assay (ALPCO, Salem, NH USA) and are expressed as nmol/L. Assays were performed in duplicate, and assays for the samples with coefficients of variation greater than 20% or outliers that were more than 4 standard deviations from the mean were repeated. Assays for 10% of the samples were also repeated to ensure operator and batch consistency.
Statistical Analyses
Univariable HIV serostatus comparisons on demographic, medical, biomarker, and psychiatric data were performed with two-tailed t-test, Wilcoxon rank-sum, or likelihood ratio χ2 tests, as appropriate. Neopterin was log10 transformed to produce a more normal distribution. Hedge’s g (g) statistic for binary predictors and Pearson’s coefficient (r) /Spearman’s rho (rs) for continuous predictors were used to generate effect sizes for predictors of neopterin.
To examine conditions associated with neopterin in our mixed sample of PWH and persons without HIV, multivariable linear regression modeled neopterin levels as a function of HIV serostatus and clinical factors of interest, which included lifetime MDD. Clinical factors were considered based on which variables in Table 1 demonstrated univariable associations with HIV serostatus or neopterin at a critical α=0.10. Stepwise regression models based on Akaike Information Criterion (AIC) were conducted to select the optimal model. The same AIC-based regression approach was employed to identify the optimal set of predictors of neopterin among PWH only. Clinical factors were considered for inclusion based on which variables in Table 1 demonstrated univariable associations with neopterin, among PWH only, at a critical α=0.10.
Table 1:
Demographic, medical, and psychiatric characteristics of sample (N=105)
| HIV- (n=35) | PWH (n=70) | p-value | |
|---|---|---|---|
| Descriptive demographics | |||
| Age, median [IQR] | 59 [54 – 64] | 57 [51 – 61] | .19 |
| Education, median [IQR] | 14 [12 – 16] | 14 [12 – 16] | .96 |
| Male, n (%) | 23 (65.7%) | 60 (85.7%) | .02 |
| Non-Hispanic White, n (%) | 22 (62.9%) | 56 (80.0%) | .06 |
| Medical comorbidities | |||
| Hyperlipidemia, n (%)a | 11 (31.4%) | 38 (56.7%) | .01 |
| Hypertension, n (%)a | 14 (40.0%) | 28 (41.8%) | .86 |
| Ever smoker, n (%)a | 14 (40.0%) | 27 (40.3%) | .98 |
| Current smoker, n (%)a | 9 (25.7%) | 24 (35.8%) | .30 |
| Diabetes mellitus, n (%)a | 7 (20.0%) | 17 (25.4%) | .54 |
| Hepatitis C Virus, n (%)a | 6 (17.1%) | 14 (20.9%) | .65 |
| BMI median [IQR] | 28 [23 – 29] | 26 [23 – 29] | .24 |
| Current medications | |||
| NSAID, n (%) | 8 (22.9%) | 21 (30.0%) | .44 |
| Antihypertensive drug, n (%) | 9 (25.7%) | 22 (31.4%) | .54 |
| Lipid-lowering drug, n (%) | 8 (22.9%) | 28 (40.0%) | .08 |
| Psychiatric characteristics/diagnoses | |||
| BDI-II, median [IQR] | 2 [0 – 6] | 8 [2 – 16] | .0001 |
| Cognitive, median [IQR] | 0 [0 – 1] | 2 [0 – 5] | .0001 |
| Affective, median [IQR] | 0 [0 – 1] | 2 [0 – 3] | .0005 |
| Somatic, median [IQR] | 1 [0 – 4] | 5 [1 – 8] | .0003 |
| Current MDD, n (%)b | 1 (2.9%) | 9 (13.0%) | .16† |
| LT MDD, n (%) | 10 (28.6%) | 39 (55.7%) | .008 |
| LT GAD, n (%) | 0 (0%) | 7 (6.7%) | .09† |
| LT alcohol use disorder, n (%)b | 17 (50.0%) | 33 (47.1%) | .78 |
| LT cannabis use disorder, n (%)b | 7 (20.6%) | 21 (30.0%) | .30 |
| LT meth use disorder, n (%)b | 8 (23.5%) | 21 (30.0%) | .49 |
| LT cocaine use disorder, n (%)b | 6 (17.7%) | 16 (22.9%) | .54 |
| Psychotropic medications | |||
| Antidepressant drug, n (%) | 4 (11.4%) | 30 (42.9%) | .0006 |
| On SSRI, n (%) | 2 (5.7%) | 14 (20.0%) | .04 |
| On SNRI, n (%) | 0 (0%) | 5 (7.1%) | .17 |
| On Atypicals, n (%) | 3 (8.6%) | 15 (21.4%) | .08 |
| On Tricyclics, n (%) | 0 (0%) | 5 (7.1%) | .17 |
| HIV disease characteristics | |||
| AIDS, n (%) | 43 (61.4%) | ||
| Duration of HIV disease (years), median [IQR]c | 20 [11 – 25] | ||
| Current CD4+ T cell count, median [IQR]d | 649 [478 – 835] | ||
| Nadir CD4+ T cell count, median [IQR]c | 180 [43 – 300] | ||
| Duration of exposure to ARVs (years), median [IQR] | 11 [6 – 17] | ||
| On efavirenz, n (%) | 17 (16%) | ||
Note: AIDS=acquired immunodeficiency syndrome; ARV=antiretroviral; BDI-II=Beck Depression Inventory-II; CD4=cluster of differentiation 4; CESD=Center for Epidemiological Studies Depression; LT=lifetime; MDD=Major Depressive Disorder; meth=methamphetamine; NSAID=nonsteroidal anti-inflammatory drug
n=102
n=104
n=69
n=67
Group comparisons: Wilcoxon for continuous variables; likelihood ratio test for dichotomous variables
p-value associated with Fisher’s exact test
After establishing the clinical factors independently associated with neopterin, primary analyses specifically focused on neopterin as a predictor of depression characteristics. In order to capture both lifetime and current depression, analyses modeled lifetime MDD diagnoses and BDI-II scores (total and domain scores) as separate outcomes. Based on the pattern of univariable associations between neopterin and lifetime MDD, a confirmatory AIC-based logistic regression analysis examined lifetime MDD status as a function of neopterin, HIV status, and their interaction, with a follow-up analysis examining the neopterin and lifetime MDD association in PWH only. The pattern of univariable associations between neopterin and BDI-II scores did not indicate a differential association by HIV serostatus and therefore an AIC-based linear regression analysis examined BDI-II scores as a function of neopterin only in the total sample. For each regression analysis, covariates included clinical correlates of neopterin identified in prior analysis and additional psychosocial and treatment covariates univariably associated with lifetime MDD or total BDI-II scores at a critical α=0.10. All analyses were performed using JMP Pro version 14.0.0 (SAS Institute Inc., Cary, NC).
Results
Sample Characteristics
Demographic, medical and psychiatric characteristics of the sample are summarized in Table 1. The full sample consisted predominantly of middle-aged [median 58 (IQR 52–62) years], non-Hispanic white (74.5%) men (79.2%) with some college education [median 14 (IQR 12–16) years]. PWH and HIV- groups were comparable (i.e., p values for group differences>.05) across many demographic and medical characteristics, except PWH were more likely to be male (85.7% vs. 65.7%, p=.02) and have hyperlipidemia (56.7% vs. 31.4%, p=.01). With respect to psychiatric characteristics, PWH had more evidence of historical and current affective distress than the HIV- group. Specifically, the PWH group had higher rates of lifetime MDD (55.7% vs 28.6%, p<.01), higher scores on BDI-II [median 8 (IQR 2 – 16) vs. median 2 (IQR 0 – 6), p<.01], and a higher proportion of individuals on any antidepressant medication (42.9% vs 11.4%, p<.01) as well as selective serotonin reuptake inhibitors (20.0% vs 5.7%, p=.04). The prevalence of lifetime substance use disorders did not significantly differ by HIV serostatus (ps>0.29). Among PWH, the median estimated duration of HIV disease was 20.0 years, the median CD4+ T cell count was 649 cells/mm3, and the median nadir CD4+ T cell count was 180 cells/mm3.
Clinical Predictors of Neopterin
Univariably, PWH had higher levels of plasma neopterin [median=9.9 nmol/L (IQR 7.6 – 11.9)] than the HIV- group [median=6.3 nmol/L (IQR 5.0 – 7.6); g=1.25; p<0.001]. With respect to other univariable relationships between neopterin and clinical factors across the entire sample, lifetime MDD (g=0.56; p=0.004), diabetes (g=0.50; p=0.049), male sex (g=0.68; p=0.005), hepatitis C seropositivity (g=0.43; p=0.082), and lifetime methamphetamine use disorder (g=0.37; p=0.087) were associated with higher levels of neopterin. In an AIC-based multiple linear regression model (R2=0.33; F[5, 96]=10.94; p<0.001, see Table 2), higher neopterin was associated with HIV seropositivity (β=0.14; p<0.001), male sex (β=0.07; p=0.028), lifetime MDD (β=0.05, p=0.052), diabetes (β=0.06; p=0.077) and hepatitis C (β=0.06; p=0.082). In addition to these covariates, a model that was restricted to PWH (R2=0.38; F[6, 60]=6.14; p<0.001) also included lifetime cannabis use disorder (β=−0.09; p=0.006) and efavirenz use (β=−0.07; p=0.040). Each of these additional covariates were associated with lower neopterin levels.
Table 2:
Multivariable linear regression model selected based on AIC to model log-transformed neopterin as a function of clinical predictors of interest in the full sample and PWH only
| Full Sample | PWH Only | |||||
|---|---|---|---|---|---|---|
| Predictor | β (SE) | 95% CI | p | β (SE) | 95% CI | p |
| PWH [ref: HIV-] | 0.14 (0.03) | 0.08, 0.20 | <0.001 | -- | -- | -- |
| Male [ref: female] | 0.07 (0.03) | 0.01, 0.14 | 0.028 | 0.08 (0.04) | −0.16, 0.01 | 0.063 |
| LT MDD [ref: no dx] | 0.05 (0.03) | 0.00, 0.11 | 0.052 | 0.07 (0.03) | 0.01, 0.14 | 0.024 |
| Diabetes [ref: no dx] | 0.06 (0.03) | −0.01, 0.12 | 0.077 | 0.07 (0.03) | 0.00, 0.14 | 0.041 |
| Hepatitis C virus [ref: no dx] | 0.06 (0.03) | −0.01, 0.12 | 0.082 | 0.08 (0.04) | 0.00, 0.15 | 0.041 |
| LT cannabis use disorder [ref: no dx] | -- | -- | -- | −0.08 (0.03) | −0.14, −0.01 | 0.018 |
| Efavirenz [ref: no efavirenz] | -- | -- | -- | −0.07 (0.04) | −0.15, −0.00 | 0.040 |
Note: dx=diagnosis; LT=lifetime; MDD=Major Depressive Disorder;
Neopterin and Lifetime MDD in PWH
As previously noted, higher neopterin was associated with lifetime MDD in PWH compared to those without a history of MDD (g=0.59; p=0.028), whereas neopterin levels did not significantly differ by lifetime MDD status in the HIV- group (g=−0.19; p=0.361; see Figure 1). A logistic regression testing the interaction between neopterin and HIV serostatus confirmed a significant interaction effect between neopterin and HIV on lifetime MDD (OR=4.62, p=.015). Thus, a follow-up logistic regression analysis within the PWH group only was conducted to determine if neopterin uniquely explained lifetime MDD status in PWH after accounting for clinical correlates of neopterin identified in prior analysis (i.e., sex, diabetes, hepatitis C, lifetime cannabis use disorder, efavirenz use) as well as additional psychosocial and treatment factors univariably associated with lifetime MDD: non-Hispanic White race/ethnicity (OR=2.78; p=.09), current antidepressant use (OR=2.85; p=.04), lifetime alcohol use disorder (OR=3.02, p=.03), lifetime methamphetamine use disorder (OR=2.60; p=.08). The optimal model was overall significant (pseudo R2=0.22; χ2[4, 65]=21.39; p<0.001) and higher neopterin levels remained significantly associated with lifetime MDD (OR=3.11 per 1 standard deviation increase in neopterin levels; p=.002) in PWH. With respect to covariates, the optimal model also contained antidepressant use, lifetime alcohol use disorder, and lifetime cannabis use disorder as predictors of higher odds of lifetime MDD in PWH (see Table 3).
Figure 1. Plasma neopterin levels are elevated in persons with HIV (PWH) and a history of Major Depressive Disorder (MDD).
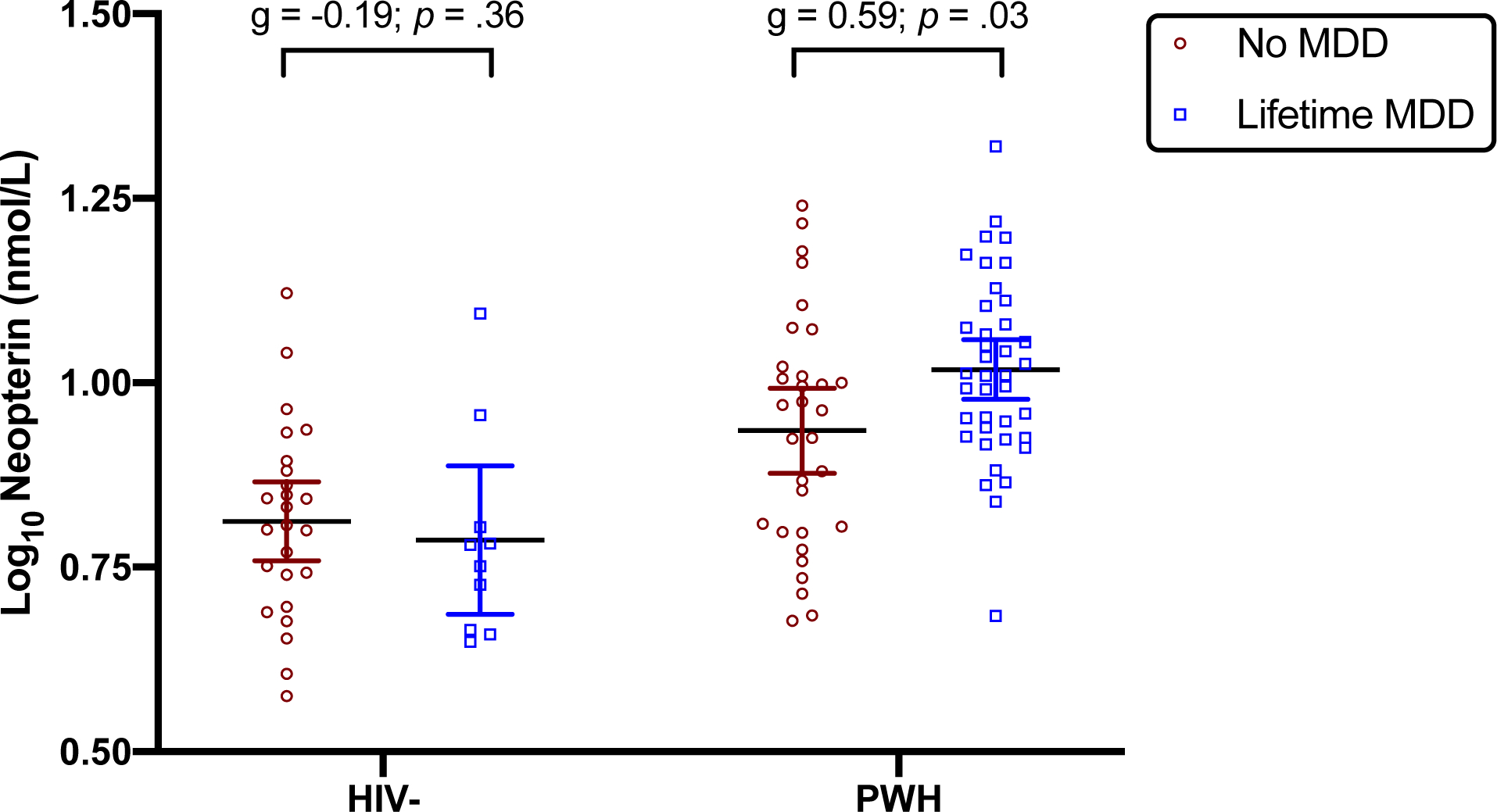
PWH [median=9.9 nmol/L (IQR 7.6 – 11.9)] had higher levels of plasma neopterin than the HIV-negative group [median=6.3 nmol/L (IQR 5.0 – 7.6); g=1.25; p<0.001]. Lifetime MDD related to significantly higher neopterin levels within PWH (g=0.59, p=.028) but not within HIV- (g=−0.19, p=.361). Statistical analyses conducted on log10 neopterin levels, which are expressed as mean ± 95% confidence interval.
Table 3:
Multivariable logistic regression model selected based on AIC to model lifetime Major Depressive Disorder as a function of log-transformed neopterin and clinical covariates in PWH
| Predictor | beta (SE) | p | Odds Ratio | 95% CI |
|---|---|---|---|---|
| Log10 neopterin [per 1SD increase] | 1.13 (0.36) | 0.002 | 3.11 | 1.52 – 6.34 |
| Antidepressant use [ref: no use] | 1.41 (0.61) | 0.020 | 4.08 | 1.24 – 13.38 |
| LT alcohol use disorder [ref: no dx] | 1.07 (0.64) | 0.095 | 2.93 | 0.83 – 10.31 |
| LT cannabis use disorder [ref: no dx] | 1.16 (0.75) | 0.121 | 3.19 | 0.74 – 13.79 |
Note: dx=diagnosis; LT=lifetime
Neopterin and BDI-II
To determine the univariable associations between neopterin and current depressive symptoms, a series of Spearman’s correlations examined relationships between neopterin and BDI-II scores across the full sample and by HIV serostatus (see Figure 2). In the full sample, higher neopterin significantly correlated with higher BDI-II scores (rs=0.25; p=0.010). Despite the significant correlations between neopterin and BDI-II scores in the full sample, the correlation between neopterin and total BDI-II scores was substantially weaker and did not reach significance within either group (HIV-: rs=0.09; p=0.588; PWH: rs=0.03; p=0.819). Thus, a linear regression analysis was conducted in the entire sample only to determine if neopterin uniquely explained BDI-II scores across the cohort after accounting for clinical correlates of neopterin in the full sample (i.e., sex, diabetes, hepatitis C) as well as additional psychosocial and treatment factors univariably associated with BDI-II scores: non-Hispanic White race/ethnicity (d=0.42; p=.06), current antidepressant use (d=0.76; p<.001), lifetime alcohol use disorder (d=0.59, p<.01), lifetime cannabis use disorder (d=0.67, p<.01), lifetime methamphetamine use disorder (d=0.47; p=.03). The optimal model was overall significant (R2=0.20; F[4, 99]=7.42; p<0.001) and higher neopterin levels remained significantly associated with total BDI-II scores (beta=1.67 per 1 standard deviation increase in neopterin levels; p=.04). Similar to the lifetime MDD analysis, the optimal model also retained antidepressant use, lifetime alcohol use disorder, and lifetime cannabis use disorder as covariates of higher BDI-II scores (ps<.07). With respect to BDI-II subdomains, higher neopterin was significantly associated with higher cognitive (rs=0.27; p=0.006) and somatic subscale scores (rs=0.22; p=0.022), and approached statistical significance with higher affective subscale scores (rs=0.17; p=0.091). Similar to the total BDI-II results, correlations between neopterin and BDI-II domains did not reach significance when stratified by HIV serostatus (HIV-: rs<0.13; ps>0.178; PWH: rs<0.08; ps>0.550).
Figure 2. Plasma neopterin levels and current depressive symptoms.
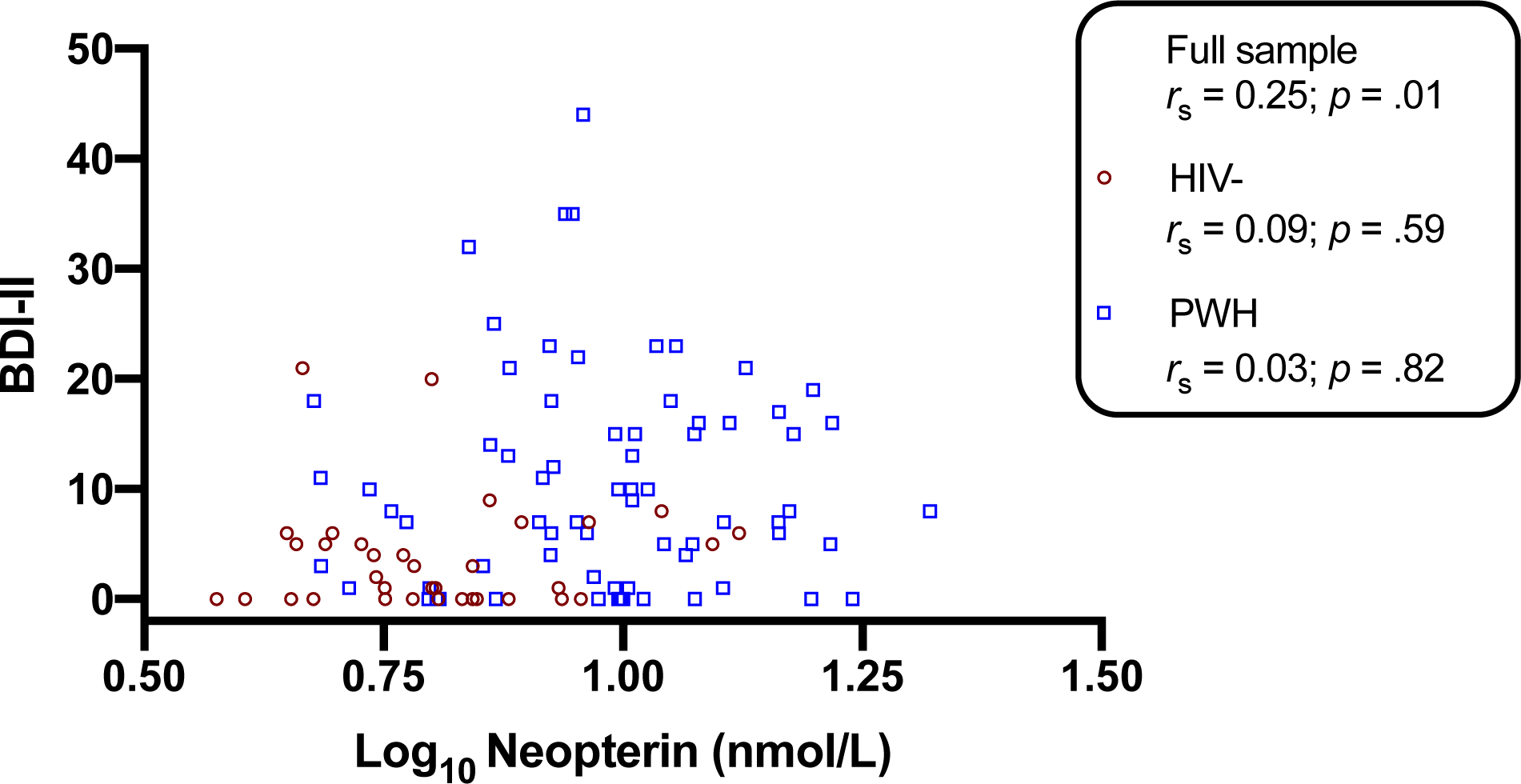
Higher plasma neopterin correlated with higher Beck Depression Inventory-II (BDI-II) scores in the full sample (rs=0.25; p=0.010), but not within either group (PWH: rs=0.03, p=0.819; HIV-: rs=0.09, p=0.588). Correlations conducted with Spearman’s rho.
Discussion
In a cohort of well-characterized older PWH on suppressive ART and age-matched HIV-adults, HIV disease was related to elevated plasma neopterin levels. In turn, higher plasma neopterin levels related to higher odds of a clinically-significant depression history, but only within PWH. Plasma neopterin was also elevated in individuals with comorbid chronic illnesses (i.e., diabetes, HCV) and was notably lower in PWH with a history of cannabis use disorder and currently taking efavirenz. Neopterin remained robustly sensitive to lifetime MDD after statistical adjustment for these clinical factors and additional depression-related psychosocial and treatment factors. Plasma neopterin levels were also modestly correlated with current depressive symptoms in the full sample, but this relationship was not replicated in analyses stratified by HIV serostatus.
Our findings align with several previous studies that have investigated the relationship between neopterin and depression in PWH. Hellmuth et. al reported that higher plasma neopterin correlated with worse initial depression scores in acutely-infected PWH19, while a similar study in ART-naïve PWH found the same relationship only among patients taking antidepressants21. Another study reported that higher plasma viral load and lower CD4 counts related to higher urinary neopterin concentrations, and urinary neopterin outperformed these HIV disease indicators as a predictor of BDI scores in multivariable analysis20. In contrast, Gold et. al, did not detect a relationship between BDI scores and neopterin levels in a male cohort of PWH during primary HIV infection (<1-year duration)30. These null findings may relate to the observation that the majority of participants self-reported minimal (52%) to mild (17%) depression whereas fewer participants reported moderate (17%) and severe (14%) depression.
Our findings extend the literature on neopterin as a marker of HIV-related immune activation with sensitivity to depression characteristics by characterizing these relationships in ART-treated and virally-suppressed older PWH. The mentioned studies focused on younger adults (average/median ages: 2813, 35.614, 3616, 37.615) as compared to this study (median age: 58). Additionally, these existing studies investigated a more acutely HIV infected population (average/median duration of infection: 19.8 days13, 103.5 days16) as compared to our study (median duration of infection: 20 years). In our study, plasma neopterin levels related to presence of lifetime MDD and higher current depressive symptoms, as measured by the BDI-II. However, the association between neopterin and current depressive symptoms was only significant in the total sample and not observed within either of the HIV serostatus groups. Although the severity of self-reported depression symptoms was higher in the PWH group, the majority of participants had subthreshold depression (i.e., about 22% of the PWH group had BDI-II total scores >17). Therefore, we have limited power to detect associations between current clinically-significant depression and immune activation in this sample.
Based on previous studies, levels of neopterin decline with effective ART31,32. The elevated levels of neopterin observed in our sample of older PWH, relative to the HIV- sample, may reflect legacy effects of prior immune compromise [e.g., 61.4% of the PWH group were diagnosed with AIDS and median nadir CD4+ T cell count was 180 (IQR: 43 – 300)]33,34. PWH who have more advanced disease may also have greater translocation of gut microbes into the blood. Neopterin in blood correlated with an indicator of microbial translocation, (1→3)-β-D-glucan35 in a cohort of virally suppressed PWH36. The association between neopterin and (1→3)-β-D-glucan may be relevant to our findings, as gut dysbiosis has been implicated in depressive disorders37. In addition to neopterin, other immune biomarkers such as C-reactive protein and a host of pro-inflammatory cytokines (e.g., IFN-γ, IL-6, IL-15, IP-10, TNF-α) may have utility for capturing acute inflammatory changes in PWH that track with neurobehavioral status9,38–40. More research is needed to identify biomarkers of inflammation that are sensitive to current depression symptoms versus prior immune responses associated with previous MDD. Such research should use gold-standard measures of depression, such as a structured clinical interview and validated self-report measure like the BDI-II41,42. Of note, the diagnostic value of the BDI-II does not appear to be weakened in the presence of somatic or affective symptom overlap with both depression and HIV28. Our observation that neopterin most strongly correlated with the cognitive subscale of the BDI-II, which includes items reflecting negative thought patterns and cognitive distortions28 (e.g., rumination, pessimism), also suggests a link between immune dysfunction and psychological features (versus physical ailments) of depression.
The neurobiological underpinnings of depression with inflammation have yet to be fully elucidated, yet disruptions in amino acid metabolism43 and neurotransmitter synthesis44 likely occur. Elevated neopterin is consistently correlated with conversion of tryptophan to kynurenine, resulting in less serotonin synthesis. Plasma and CSF levels of serotonin are reduced in PWH45–47 and greater tryptophan degradation (indexed by kynurenine/tryptophan ratios) has been linked to depression20,48 and impulsivity/risk-taking49 in PWH. In addition to serotonergic deficiencies, neopterin also correlates with decelerated conversion of phenylalanine to tyrosine, resulting in less dopamine synthesis43. This concurrent increase in neopterin and decreased dopamine production may reflect oxidative stress-induced reductions in tetrahydrobiopterin (BH4), a critical cofactor in phenylalanine metabolism43. This pathway converges with the robust observation that pro-inflammatory cytokine signaling compromises the synthesis and release of dopamine in cortico-striatal reward pathways44,51,52. Consistent with this notion, dopaminergic abnormalities have been widely reported in PWH53–55 and shown to correlate with depressive symptoms and neurocognitive deficits54,56.
HIV disease is hypothesized to accelerate the aging processes in part due to persistent chronic inflammation, even in the presence of suppressive ART1. As a result, PWH are at increased risk of developing comorbid diseases that reciprocally enhance inflammation and further accelerate aging in this population. Limiting and managing the accumulation of comorbidities is a central tenant of HIV clinical care. In the present study, PWH with comorbid HCV or diabetes (or both) exhibited significantly increased neopterin levels as compared to PWH without these comorbidities. Interestingly, a history of lifetime cannabis use disorder was linked to lower neopterin levels. This mirrors prior observations that cannabis possesses anti-inflammatory properties that may mitigate HIV-related gut and blood-brain-barrier permeability, oxidative stress, and possibly protect against neurocognitive impairment57–61. In contrast, we observed that lifetime cannabis use disorder related to increased odds of lifetime MDD within PWH. This underscores the complexity of a disorder-based classification of cannabis use that not only reflects historical exposure levels, but also use-related psychosocial difficulties (e.g., interference with occupational functioning) that may correlate with mood. A more surprising clinical factor that also related to lower neopterin levels was use of efavirenz (n=17), a non-nucleoside reverse transcriptase inhibitor (NNRTI) that carries a high CNS toxicity risk profile and should be used with caution in older PWH with neuropsychiatric histories62,63. Of note, a prospective study observed that switching from efavirenz to the less neurotoxic NNRTI dolutegravir related to increased plasma kynurenine concentrations, but not neopterin or kynurenine/tryptophan ratios64. Although efavirenz has been linked to neuropsychiatric disturbances and related to lower neopterin in our sample, it did not influence the unique relationship between neopterin and lifetime MDD.
Our study findings should be considered in light of its limitations. First, our small sample size (N=105) prohibits more complex analyses requiring greater power to detect small-to-medium effects. Second, our cross-sectional data precludes discussion of temporality among our variables of interest. Therefore, the extent to which levels of neopterin have changed over time and whether elevated levels of neopterin preceded or succeeded episodes of MDD is unknown. Third, the present studied focused on neopterin given it operates within a well-defined biological pathway with putative relevance to depression and HIV. Other immune biomarkers, however, may also be associated with depression in older PWH. Fourth, although blood-based biomarker assays are more feasible and scalable in both research and clinical settings, the absence of CSF neopterin data limits our ability to draw inferences about CNS-specific immune activation. Fifth, male sex was associated with neopterin despite our study being underpowered to study sex-specific mechanisms of immune response and depression. Sex may moderate immune and neurobehavioral associations65 and therefore should be considered in future research. Last, the PWH group consisted of mostly non-Hispanic white men, which is not representative of the demographics of PWH in the United States. Race/ethnicity is important to consider as prior research indicates markers of systemic inflammation are associated with depressive symptoms differentially across race/ethnicity groups66.
Taken together, our findings provide important context to the literature on neopterin and depression in HIV disease. As expected, PWH exhibited higher neopterin levels and greater lifetime and current depression characteristics than their HIV- counterparts. Given that plasma neopterin was a more sensitive indicator of a lifetime history of clinical depression rather than current depressive symptoms in the PWH group, these individuals may possess unmeasured biopsychosocial factors that confer resilience against the adverse effects of neopterin on current depression that have been documented in younger and less treated cohorts. Continued monitoring and management of comorbidity burden, exploration of novel anti-inflammatory therapeutics, and identification of positive behaviors and psychological factors may further facilitate successful neurobehavioral aging in this population as well as inform care for those with more recently acquired HIV and comorbid depression.
Conflicts of Interest and Source of Funding:
This study was supported by California HIV/AIDS Research Program IDEA Award ID10-SD-057 (PI: David J. Moore); the HIV Neurobehavioral Research Center (HNRC) Award P30MH062512 (PI: Robert Heaton); the NIA T35 AG26757 Award (PI: Dilip V. Jeste); the NIMH R25MH108389 (Sustained Training on Aging and HIV Research; STAHR) Award; and the UC San Diego Sam and Rose Stein Institute for Research on Aging at the University of California San Diego. Mr. Saloner is supported by NIA award F31AG064989.
References
- 1.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158(5):725–730. [DOI] [PubMed] [Google Scholar]
- 2.Havlik RJ, Brennan M, Karpiak SE. Comorbidities and depression in older adults with HIV. Sex Health. 2011;8(4):551–559. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Current depression among adults---United States, 2006 and 2008. Morbidity and Mortality Weekly Report. 2010;59(38):1229–1235. [PubMed] [Google Scholar]
- 4.Rabkin JG, McElhiney M. Depression and distress in older HIV+ adults. GMHC Treat Issues. 2007;21(2):3–5. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. HIV and Older Americans. 2020; https://www.cdc.gov/hiv/pdf/group/age/olderamericans/cdc-hiv-older-americans.pdf. Accessed 02/04/2021.
- 6.Schachter J, Martel J, Lin CS, et al. Effects of obesity on depression: A role for inflammation and the gut microbiota. Brain Behav Immun. 2018;69:1–8. [DOI] [PubMed] [Google Scholar]
- 7.Chan KL, Cathomas F, Russo SJ. Central and Peripheral Inflammation Link Metabolic Syndrome and Major Depressive Disorder. Physiology (Bethesda). 2019;34(2):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poudel-Tandukar K, Bertone-Johnson ER, Palmer PH, Poudel KC. C-reactive protein and depression in persons with Human Immunodeficiency Virus infection: the Positive Living with HIV (POLH) Study. Brain Behav Immun. 2014;42:89–95. [DOI] [PubMed] [Google Scholar]
- 9.Norcini Pala A, Steca P, Bagrodia R, et al. Subtypes of depressive symptoms and inflammatory biomarkers: An exploratory study on a sample of HIV-positive patients. Brain, behavior, and immunity. 2016;56:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller AH, Haroon E, Felger JC. Therapeutic Implications of Brain-Immune Interactions: Treatment in Translation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017;42(1):334–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang AK, Miller BJ. Meta-analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophrenia Bulletin. 2018;44(1):75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamberlain SR, Cavanagh J, de Boer P, et al. Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry. 2019;214(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2018;23(2):335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalak L, Bulska M, Strzabala K, Szczesniak P. Neopterin as a marker of cellular immunological response. Postepy Hig Med Dosw (Online). 2017;71(1):727–736. [DOI] [PubMed] [Google Scholar]
- 15.Brew BJ, Letendre SL. Biomarkers of HIV related central nervous system disease. Int Rev Psychiatry. 2008;20(1):73–88. [DOI] [PubMed] [Google Scholar]
- 16.Wirleitner B, Schroecksnadel K, Winkler C, Fuchs D. Neopterin in HIV-1 infection. Mol Immunol. 2005;42(2):183–194. [DOI] [PubMed] [Google Scholar]
- 17.Celik C, Erdem M, Cayci T, et al. The association between serum levels of neopterin and number of depressive episodes of major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(2):372–375. [DOI] [PubMed] [Google Scholar]
- 18.Maes M, Scharpe S, Meltzer HY, et al. Increased neopterin and interferon-gamma secretion and lower availability of L-tryptophan in major depression: further evidence for an immune response. Psychiatry Res. 1994;54(2):143–160. [DOI] [PubMed] [Google Scholar]
- 19.Hellmuth J, Colby D, Valcour V, et al. Depression and Anxiety are Common in Acute HIV Infection and Associate with Plasma Immune Activation. AIDS Behav. 2017;21(11):3238–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroecksnadel K, Sarcletti M, Winkler C, et al. Quality of life and immune activation in patients with HIV-infection. Brain Behav Immun. 2008;22(6):881–889. [DOI] [PubMed] [Google Scholar]
- 21.Warriner EM, Rourke SB, Rourke BP, et al. Immune activation and neuropsychiatric symptoms in HIV infection. J Neuropsychiatry Clin Neurosci. 2010;22(3):321–328. [DOI] [PubMed] [Google Scholar]
- 22.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nature Reviews Endocrinology. 2018;14(10):576–590. [DOI] [PubMed] [Google Scholar]
- 23.Capuron L, Geisler S, Kurz K, Leblhuber F, Sperner-Unterweger B, Fuchs D. Activated immune system and inflammation in healthy ageing: relevance for tryptophan and neopterin metabolism. Current Pharmaceutical Design. 2014;20(38):6048–6057. [DOI] [PubMed] [Google Scholar]
- 24.Spencer ME, Jain A, Matteini A, et al. Serum levels of the immune activation marker neopterin change with age and gender and are modified by race, BMI, and percentage of body fat. J Gerontol A Biol Sci Med Sci. 2010;65(8):858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thurman M, Johnson S, Acharya A, Pallikkuth S, Mahesh M, Byrareddy SN. Biomarkers of Activation and Inflammation to Track Disparity in Chronological and Physiological Age of People Living With HIV on Combination Antiretroviral Therapy. Frontiers in Immunology. 2020;11:2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Composite Diagnositic International Interview (CIDI, version 2.1). Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 27.Beck A, Steer R, Brown G. Manual for Beck Depression Inventory II (BDI-II). San Antonio, TX, Psychology Corporation. 1996. [Google Scholar]
- 28.Hobkirk AL, Starosta AJ, De Leo JA, Marra CM, Heaton RK, Earleywine M. Psychometric validation of the BDI-II among HIV-positive CHARTER study participants. Psychological assessment. 2015;27(2):457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apostolova N, Funes HA, Blas-Garcia A, Galindo MJ, Alvarez A, Esplugues JV. Efavirenz and the CNS: what we already know and questions that need to be answered. Journal of Antimicrobial Chemotherapy. 2015;70(10):2693–2708. [DOI] [PubMed] [Google Scholar]
- 30.Gold JA, Grill M, Peterson J, et al. Longitudinal characterization of depression and mood states beginning in primary HIV infection. AIDS Behav. 2014;18(6):1124–1132. [DOI] [PubMed] [Google Scholar]
- 31.Zangerle R, Widner B, Quirchmair G, Neurauter G, Sarcletti M, Fuchs D. Effective antiretroviral therapy reduces degradation of tryptophan in patients with HIV-1 infection. Clin Immunol. 2002;104(3):242–247. [DOI] [PubMed] [Google Scholar]
- 32.Eisenhut M Neopterin in Diagnosis and Monitoring of Infectious Diseases. J Biomark. 2013;2013:196432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. [DOI] [PubMed] [Google Scholar]
- 34.Dinh DM, Volpe GE, Duffalo C, et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis. 2015;211(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris A, Hillenbrand M, Finkelman M, et al. Serum (1-->3)-beta-D-glucan levels in HIV-infected individuals are associated with immunosuppression, inflammation, and cardiopulmonary function. J Acquir Immune Defic Syndr. 2012;61(4):462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoenigl M, de Oliveira MF, Perez-Santiago J, et al. Correlation of (1-->3)-beta-D-glucan with other inflammation markers in chronically HIV infected persons on suppressive antiretroviral therapy. GMS Infect Dis. 2015;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zalar B, Haslberger A, Peterlin B. The Role of Microbiota in Depression - a brief review. Psychiatr Danub. 2018;30(2):136–141. [DOI] [PubMed] [Google Scholar]
- 38.Saloner R, Paolillo EW, Heaton RK, et al. Chronically elevated depressive symptoms interact with acute increases in inflammation to predict worse neurocognition among people with HIV. J Neurovirol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivera-Rivera Y, García Y, Toro V, et al. Depression Correlates with Increased Plasma Levels of Inflammatory Cytokines and a Dysregulated Oxidant/Antioxidant Balance in HIV-1-Infected Subjects Undergoing Antiretroviral Therapy. Journal of clinical & cellular immunology. 2014;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Derry HM, Johnston CD, Burchett CO, et al. Links Between Inflammation, Mood, and Physical Function Among Older Adults With HIV. The Journals of Gerontology: Series B. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aalto AM, Elovainio M, Kivimaki M, Uutela A, Pirkola S. The Beck Depression Inventory and General Health Questionnaire as measures of depression in the general population: a validation study using the Composite International Diagnostic Interview as the gold standard. Psychiatry Res. 2012;197(1–2):163–171. [DOI] [PubMed] [Google Scholar]
- 42.Hobkirk AL, Starosta AJ, De Leo JA, et al. Psychometric validation of the BDI-II among HIV-positive CHARTER study participants. Psychol Assess. 2015;27(2):457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gostner JM, Becker K, Kurz K, Fuchs D. Disturbed Amino Acid Metabolism in HIV: Association with Neuropsychiatric Symptoms. Frontiers in Psychiatry. 2015;6(97). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Felger JC. The Role of Dopamine in Inflammation-Associated Depression: Mechanisms and Therapeutic Implications. Current topics in behavioral neurosciences. 2017;31:199–219. [DOI] [PubMed] [Google Scholar]
- 45.Kumar AM, Berger JR, Eisdorfer C, Fernandez JB, Goodkin K, Kumar M. Cerebrospinal Fluid 5-Hydroxytryptamine and 5-Hydroxyindoleacetic Acid in HIV-1 Infection. Neuropsychobiology. 2001;44(1):13–18. [DOI] [PubMed] [Google Scholar]
- 46.Pierluigi L, Alessandro P, Stefano C, et al. A Study of Tryptophan Metabolism via Serotonin in Ventricular Cerebrospinal Fluid in HIV-1 Infection Using a Neuroendoscopic Technique. Current HIV research. 2007;5(2):267–272. [DOI] [PubMed] [Google Scholar]
- 47.Launay J-M, Copel L, Callebert J, et al. Decreased Whole Blood 5-Hydroxytryptamine (Serotonin) in AIDS Patients. JAIDS Journal of Acquired Immune Deficiency Syndromes. 1988;1(4). [PubMed] [Google Scholar]
- 48.Martinez P, Tsai AC, Muzoora C, et al. Reversal of the Kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. Journal of acquired immune deficiency syndromes (1999). 2014;65(4):456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J, Lee J-Y, Meade CS, et al. Tryptophan degradation is associated with risk-taking propensity in methamphetamine users with treated HIV infection. Journal of neurovirology. 2020;26(5):779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keegan MR, Chittiprol S, Letendre SL, et al. Tryptophan Metabolism and Its Relationship with Depression and Cognitive Impairment Among HIV-infected Individuals. Int J Tryptophan Res. 2016;9:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Felger JC, Li L, Marvar PJ, et al. Tyrosine metabolism during interferon-alpha administration: Association with fatigue and CSF dopamine concentrations. Brain, behavior, and immunity. 2013;31:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol. 2012;33(3):315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar AM, Fernandez JB, Singer EJ, et al. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. Journal of neurovirology. 2009;15(3):257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. Journal of neurovirology. 2011;17(1):26–40. [DOI] [PubMed] [Google Scholar]
- 55.Larsson M, Hagberg L, Forsman A, Norkrans G. Cerebrospinal fluid catecholamine metabolites in HIV-infected patients. Journal of neuroscience research. 1991;28(3):406–409. [DOI] [PubMed] [Google Scholar]
- 56.Saloner R, Cherner M, Grelotti DJ, et al. Lower CSF homovanillic acid relates to higher burden of neuroinflammation and depression in people with HIV disease. Brain, behavior, and immunity. 2020;90:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saloner R, Campbell LM, Serrano V, et al. Neurocognitive SuperAging in Older Adults Living With HIV: Demographic, Neuromedical and Everyday Functioning Correlates. Journal of the International Neuropsychological Society : JINS. 2019;25(5):507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ellis RJ, Peterson S, Cherner M, et al. Beneficial Effects of Cannabis on Blood Brain Barrier Function in HIV. Clinical Infectious Diseases. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swinton MK, Carson A, Telese F, et al. Mitochondrial biogenesis is altered in HIV+ brains exposed to ART: Implications for therapeutic targeting of astroglia. Neurobiology of disease. 2019;130:104502–104502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saloner R, Fields JA, Marcondes MCG, et al. Methamphetamine and Cannabis: A Tale of Two Drugs and their Effects on HIV, Brain, and Behavior. Journal of Neuroimmune Pharmacology. 2020;15(4):743–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watson CW-M, Paolillo EW, Morgan EE, et al. Cannabis Exposure is Associated With a Lower Likelihood of Neurocognitive Impairment in People Living With HIV. Journal of acquired immune deficiency syndromes (1999). 2020;83(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burgess MJ, Zeuli JD, Kasten MJ. Management of HIV/AIDS in older patients-drug/drug interactions and adherence to antiretroviral therapy. HIV AIDS (Auckl). 2015;7:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abers MS, Shandera WX, Kass JS. Neurological and Psychiatric Adverse Effects of Antiretroviral Drugs. CNS Drugs. 2014;28(2):131–145. [DOI] [PubMed] [Google Scholar]
- 64.Keegan MR, Winston A, Higgs C, Fuchs D, Boasso A, Nelson M. Tryptophan metabolism and its relationship with central nervous system toxicity in people living with HIV switching from efavirenz to dolutegravir. Journal of neurovirology. 2019;25(1):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubin LH, Neigh GN, Sundermann EE, Xu Y, Scully EP, Maki PM. Sex Differences in Neurocognitive Function in Adults with HIV: Patterns, Predictors, and Mechanisms. Curr Psychiatry Rep. 2019;21(10):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beydoun MA, Obhi HK, Weiss J, et al. Systemic inflammation is associated with depressive symptoms differentially by sex and race: a longitudinal study of urban adults. Mol Psychiatry. 2020;25(6):1286–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]


