Abstract
Background:
We evaluated the association of inflammation and dysbosis on cervicovaginal fluid (CVF) tenofovir (TFV) concentrations in women taking oral tenofovir disoproxil fumarate/emtricitable (TDF/FTC) for HIV pre-exposure prophylaxis in the United States.
Setting:
Thirty-five women in a HIV PrEP implementation study attended their week 24 visit at a San Diego research clinic and provided CVF specimens.
Methods:
Women in the Adherence Enhancement Guided by Individualized Texting and Drug Levels (AEGiS) study had their CVF specimens evaluated for (i) sexually transmitted bacterial (Neisseria gonorrhoeae, Chlamydia trachomatis, Gardnerella and Trichomonas vaginalis), viral (HPV, CMV and HSV-1/2) and fungal (Candida) infections; (ii) microbiome composition by 16S sequencing (V3-V4 region); and (iii) cytokine profiles by ELISA (IL-8, MIP-1a, MIP-1b and IP-10). Univariate statistical analysis was used to determine factors associated with CVF TFV concentrations. CVF TFV of 100–1000 ng/mL benchmarked typical genital concentrations and tenofovir diphosphate (TFV-DP) in dried blood spots (DBS) of 700 fmol/punch was considered adequate adherence.
Results:
Thirty-five women had CVF specimens collected. No factor was associated with CVF TFV concentrations or discordance of blood and vaginal concentrations. Among 27 participants assessed for vaginosis (Candida, Gardnerella or Trichomonas), women with Gardnerella (n=11) were more likely to have high (>1000 ng/mL) CVF TFV concentrations (82% versus 33%, p=0.02).
Conclusions:
Presence of genital viruses, cytokines or vaginal community state types were not associated with low CVF TFV concentrations in cisgender women taking oral TDF/FTC for pre-exposure prophylaxis. The surprising association observed between presence of Gardnerella and higher vaginal TFV concentrations needs further evaluation.
Keywords: HIV preexposure prophylaxis, women, Gardnerella, vaginal microbiome, tenofovir
INTRODUCTION
Daily oral pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) is safe and effective for reducing the risk of HIV infection in clinical trials of heterosexual men and men who have sex with men (MSM).1–3 Clinical trials of oral PrEP in cisgender women have had mixed results with some studies demonstrating efficacy 2,3 and others finding no efficacy.4,5 Nevertheless, TDF/FTC is considered effective in women to lower HIV acquisition but drug concentrations at the vaginal mucosa are lower than at the rectal mucosa.6 What biologic factors [e.g., inflammation from sexually transmitted infections (STIs) or alterations in the vaginal microbiota, etc.] at the vaginal mucosa may alter tenofovir (TFV) concentrations have not been fully explored.
Genital inflammation as evidenced by higher genital concentrations of cytokines MIP-1α, MIP-1β, IL-8 and IP-10 was associated with increased risk of HIV acquisition in women in the Centre for the AIDS Programme of Research in South Africa (CAPRISA) tenofovir gel trial.7 In this study, there was no identifiable cause of elevated genital cytokine concentrations despite extensive investigation of many potential factors, including STIs. Other studies suggest that inflammation may impact intracellular TFV concentrations.8
One cause of inflammation could be related to changes in the microbial environments of the vagina. A Lactobacillus-dominant microbiome is characterized as having Lactobacillus-species as the main bacteria with overall lower bacterial diversity and consequent low pH.9 Higher diversity, as observed clinically in bacterial vaginosis (BV), represents an unhealthy microbiome and is associated with increased HIV risk.10,11 Vaginal dysbiosis with non-Lactobacillus dominant bacteria may decrease TFV concentration when it is given as a topical gel, and could undermine the efficacy of TFV gel to protect women from HIV.12,13 With tenofovir gels, Gardnerella-dominated vaginal communities were associated with lower TFV concentration.12 Oral TDF/FTC was not different among women with abnormal versus normal vaginal microbiota;14 however, tenofovir concentration were not measured so it is unknown if there was any impact of microbiota on mucosal drug concentration, even if there was no effect on HIV incidence. Thus, the aim of this study was to determine whether certain biologic factors including genital inflammation, infections and changes in the vaginal microbiota could lower cervicovaginal fluid (CVF) TFV concentration.
METHODS
Study Cohort:
Adherence Enhancement Guided by Individualized Texting and Drug Levels (AEGiS) was a 48-week PrEP demonstration project in cisgender women 18 years old at-risk for HIV conducted at five Southern California sites. Adherence was supported using two-way text messaging (Individualized Texting for Adherence Behavior; iTAB) and titrated adherence counseling based on rapid-turnaround tenofovir diphosphate (TFV-DP) concentrations. Study visits occurred at baseline, week 4, week 12 and then quarterly through week 48. Demographics were collected with computer surveys. Adherence was assessed by quantifying TFV-DP concentrations in dried blood spots (DBS). This sub-study included women from the AEGiS study who agreed to the collection of additional vaginal swabs and CVF specimens located at the University of California, San Diego (UCSD) study site. Specimens were collected by research staff from women who reported taking their oral TDF/FTC at their week 24 study visit. IRB approval for the study was obtained from the UCSD Human Research Protection Program.
Measures
Sexually transmitted bacterial infections (Gonorrhea and Chlamydia) were assessed by nucleic acid amplifications using Hologic Aptima Combo2 performed at the Long Beach Public Health Lab. A vaginitis panel (Candida, Gardnerella and Trichomonas) using the BD Affirm VPIII was performed at the UCSD CALM lab. HPV subtypes 16 and 18 were assessed using the Roche HPV High Risk Genotypes Nucleic Acid Test (UCSD CALM lab). Herpesviruses 1, 2 and 5 (Herpes simplex virus 1 and 2, cytomegalovirus) DNA levels were measured by RT-PCR as previously described.15
For microbiome and cytokine analysis, a Catchall and Weckel swab were collected, respectively. To measure cytokines from cervicovaginal swabs, swabs were first extracted in 1 mL of PBS. Next, total protein was measured by bicinchoninic acid assay (Sigma Aldrich, St. Louis, MO); then levels for MIP1α and MIP1β [R&D Systems, Minneapolis, MN], and IL-8 and IP10 [Biolegend, San Diego, CA] were measured by ELISA following manufacturer instructions. Microbiome profiles were determined by soaking vaginal swabs in Qiagen lysis buffer using the blood and tissue DNA isolation kits. Bacteria were lysed in this buffer with bead beating for 5 minutes. The recovered genomic DNAs were used to amplify the V3-V4 region of 16S rRNA and sequenced using the Mi-Seq instrument as described.16 Vaginal microbiota types were divided in community state types (CST) 1–5 based on predominance of particular Lactobacillus species representing dominant taxa.17
CVF was collected by a standardized protocol via aspirator from the posterior fornix. TFV was quantified in CVF using validated HPLC-MS/MS methods.18 Samples were diluted in 0.9% sodium chloride and TFV was extracted by protein precipitation with stable isotopically-labeled internal standard (13C5-tenofovir). Calibration standards and QCs were prepared in human CVF diluted in a 1:4 ratio with 0.9% sodium chloride and met 15% precision and accuracy acceptance criteria. Concentrations were normalized to the volume of specimen extracted by calculating the mass of CVF collected based on pre- and post-weights of the specimen collection tubes, and assuming the density of CVF was 1g/mL. The calibrated linear range of the assay was 2 – 5000 ng/mL. Levels of serum estradiol at week 24 were measured using the Estradiol ELISA kit (Ref EIA-2693, DRG International, Inc.) following manufacturers’ protocols.
Statistical Analysis:
Descriptive baseline analyses were performed to describe the study participants that consented for the collection of vaginal swabs and cervical vaginal fluid collection. Univariate statistical analysis was used to determine factors associated with low and high CVF TFV. CVF TFV concentrations >1000 ng/mL were considered to be “high” (expected target in CVF of 100ng/mL).19 Discordant CVF and DBS concentrations was defined as CVF TFV <1000ng/mL and TFV-DP≥700 fmol/punch. Serum estradiol was considered high above the rounded upper quartile of 100 pg/mL. Continuous variables were compared by Student t-test unless the distribution was not normal; in this case, the Wilcoxon test was used. Pearson correlation coefficient was used for two continuous variables. Dichotomous outcomes were compared with Fisher exact test. Statistically significant associations for the outcome of high CVF TFV concentration were placed in logistic regression models to adjust for any confounding factors associated with DBS TFV-DP concentrations. Analyses were performed in SAS v9 (Cary NC).
RESULTS
Thirty-five women attended their week 24 visit and provided cervicovaginal specimens. The women had a median age of 43 years (IQR 35–47) with 5 non-Hispanic Black, 17 non-Hispanic White and 10 Latina (Table 1). Adherence to TDF/FTC was high among these women with a median DBS TFV-DP level of 1007 fmol/punch (IQR 726–1581). Twenty-eight (80%) of the women had a TFV-DP concentrations of 700 fmol/punch or greater consistent with dosing four days per week, and 14 (40%) had concentrations of 1250 fmol/punch consistent with dosing seven days per week. Among all women, the median TFV CVF concentration was 854 (IQR 125–2350) ng/mL. Almost half of the women (47%) had TFV CVF concentrations of ≥1000 ng/mL. DBS TFV-DP poorly correlated with CVF TFV (r=0.009, p=0.96) suggesting that many other factors were possibly influencing CVF concentrations.
Table 1:
Demographic and Clinical Characteristics of Participants at Baseline
| Characteristic | (n = 35) |
|---|---|
| Age years, median (IQR) | 43 (35–47) |
| Race or Ethnic Group | |
| Black or African American | 5 (14%) |
| White | 17 (49%) |
| Other | 3 (9%) |
| Hispanic or Latina | 10 (28%) |
| DBS TFV-DP level fmol/punch, median (IQR) | 1007 (726–1581) |
| CVF TFV level ng/mL, median (IQR) (n=34) | 854 (125–2350) |
| Sexually transmitted infections (n=34) | |
| Chlamydia | 0 (0%) |
| Gonorrhea | 0 (0%) |
| Vaginitis | |
| Gardnerella (n=27) | 11 (41%) |
| Candida (n=27) | 2 (7%) |
| Trichomonas (n=27) | 0 (0%) |
| Herpes Simplex Virus 1/2 | 4 (11%) |
| Cytomegalovirus | 2 (6%) |
| HPV 16 or 18 (n=28) | 6 (21%) |
| Vaginal Microbiota community group (n=34) | |
| I | 8 (24%) |
| II | 1 (3%) |
| III | 11 (32%) |
| IV | 12 (35%) |
| V | 2 (6%) |
| Cytokine detected | |
| MIP 1alpha | 7 (20%) |
| MIP 1 beta | 7 (20%) |
| IP-10 | 8 (22.9%) |
| IL-8 (>20) | 21 (60%) |
While no women had genital Chlamydia or Gonorrhea detected, of those that had swabs, there were high rates of positivity for Gardnerella (11 out of 27, 41%) and Candida (2 out of 27, 7%). HSV-1 DNA was detected in 4 women (11%) and two of these women were also positive for CMV DNA. HPV 16/18 was detected in 6 out of the 28 (21%) women tested. Vaginal community state types (CST) were assigned to 34 women with 8 (24%) CST I (L. crispatus dominated), 1 (3%) CST II (L. gasseri dominated), 11 (32%) CST III (L. iners dominated), 12 (35%) CST IV (non-Lactobacillus dominant) and 2 (6%) CST V (L. jensenii dominated). Vaginal cytokines MIP-1a, MIP-1b and IP10 were detectable in less than a third of participants while IL-8 was more common.
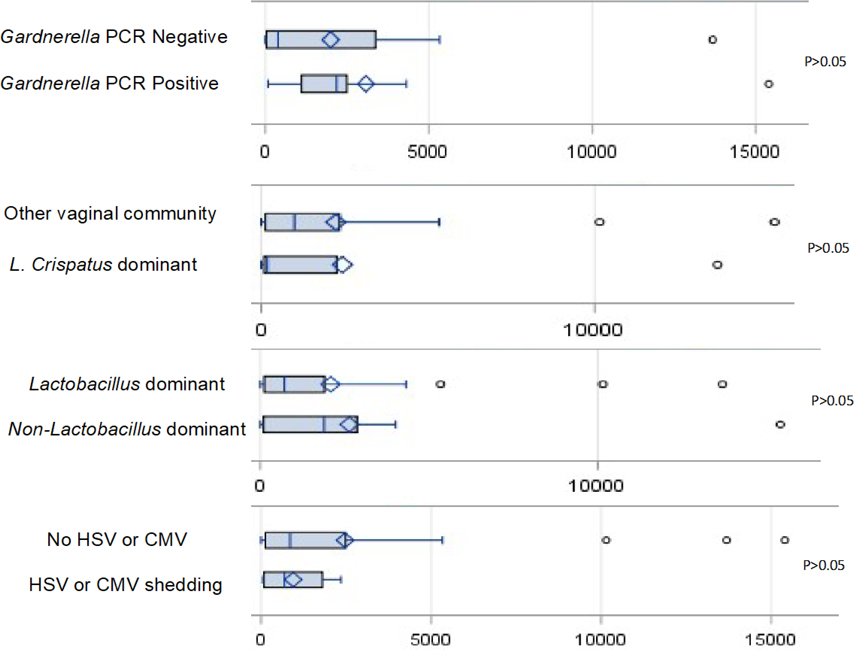
There was no association of the considered vaginal factors with low or high CVF TFV concentrations (Table 2, Figure 1) except in those assessed for vaginitis organisms (Candida, Gardnerella and Trichomonas). Positivity for Gardnerella was associated with higher than the median TFV CVF concentrations (Figure 1) in 9 out of 11 versus 6 out of of 15 individuals without Gardnerella (p=0.05). Similarly, those with Gardnerella (n=9) were more likely to have a vaginal TFV of 1000 ng/mL or greater compared to those without Gardnerella (n=5) (82% versus 33%, p=0.02). This finding remained even when adjusting for adherence by absolute TFV-DP DBS concentration by logistic regression with odds ratio of 10.0 (95% CI 1.5–67.3) for association of Gardnerella with vaginal TFV ≥1000 ng/mL (p=0.02). Elevation of cytokines in the presence of Gardnerella compared to its absence did not reach significance (88% versus 64%, p=0.19). No difference was seen in TFV CVF concentrations by vaginal community type. CST IV had 7 of 12 (58%) with CVF TFV ≥1000 ng/mL compared to 5 of 18 (28%) of Lactobacillus dominant vaginal community types (p=0.3). Figure 2 shows the vaginal microbiota composition in each participant ordered by CVF concentration.
Table 2:
Predictors of High Vaginal TFV Levels
| Vaginal TFV level ≥1000 ng/mL | Vaginal TFV level <1000 ng/mL | P-value | |
|---|---|---|---|
|
| |||
| DBS TFV-DP ≥700 fmol/punch | 13 | 14 | 1.00 |
| DBS TFV-DP <700 fmol/punch | 3 | 4 | |
|
| |||
| DBS TFV-DP ≥1250 fmol/punch | 4 | 9 | 0.17 |
| DBS TFV-DP <1250 fmol/punch | 12 | 9 | |
|
| |||
| Plasma estrogen ≥100 pg/mL | 13 | 13 | 0.70 |
| Plasma estrogen <100 pg/mL | 3 | 5 | |
|
| |||
| Gardnerella positive | 9 | 2 | 0.02 |
| Gardnerella negative | 5 | 10 | |
|
| |||
| Candida positive | 1 | 1 | 1.00 |
| Candida negative | 13 | 11 | |
|
| |||
| HSV/CMV positive | 2 | 2 | 1.00 |
| HSV/CMV negative | 15 | 15 | |
|
| |||
| HPV16/18 positive | 3 | 3 | 1.00 |
| HPV16/18 negative | 11 | 10 | |
|
| |||
| L. crispatus dominant | 2 | 5 | 0.41 |
| Other vaginal type | 13 | 13 | |
|
| |||
| Lactobacillus dominant | 5 | 13 | 0.30 |
| Non-Lactobacillus dominant | 7 | 5 | |
|
| |||
| Elevated cytokines | 12 | 15 | 0.68 |
| No elevated cytokines | 3 | 4 | |
|
| |||
Figure 1:
Vaginal Tenofovir Levels by Viruses and Bacteria Detected
FIGURE 2:
Vaginal Microbiome Composition ordered by CVF (ng/mL) Concentration
The X-axis shows the IDs of the 34 participants who provided CVF samples and had a CVF tenofovir concentration measured. CVF concentration is ordered from high (64b = 15400 ng/mL) to low (6hb = below limit of quantification). The arrow represents where 1000ng/mL falls. The Y-axis represents % abundance. The remainder is comprised of several other bacteria outside the group listed.
Less than 20% of women (5/27) with DBS TFV-DP >700 fmol/punch had low vaginal TFV concentrations (<100 ng/mL). No factor was associated with a difference in vaginal TFV levels or discordant CVF and DBS concentrations. Women with non-Lactobacillus dominant vaginal community types had 25% discordance of CVF and DBS concentrations as compared to 32% in other women (Fisher Exact p=0.33). The three participants with discordant CVF and DBS concentrations with non-Lactobacillus dominant vaginal microbiomes did not have Gardnerella or Candida, but had microbiota that were dominated by other organisms [Enterobacter cloacae (2), Escherichia fergusonii (1)].
DISCUSSION
In this study of cisgender women taking oral TDF/FTC for HIV prevention, we found high concentrations of TFV in the CVF in nearly all participants. Our study did not find differences in CVF TFV levels with presence of HSV, CMV, HPV, inflammatory cytokines (MIP-1a, MIP-1b, IP10 and IL-8) or vaginal community group. Non-Lactobacillus-dominated vaginal community types and the presence of Gardnerella were not associated with lower TFV concentrations with oral TDF/FTC.
Interestingly, presence of Gardnerella by DNA probe was associated with being 2.5 times more likely of having TFV in CVF of ≥1000 ng/mL, compared to those without Gardnerella. Higher concentrations of TFV could be produced through a unique vaginal environment created by Gardnerella. This is in contrast to the lower concentrations of TFV found in the CVF of women with Gardnerella who were using topical TFV gel.12 The local cytokine and cellular milieu with Gardnerella is surprisingly not well defined.20 Bacterial vaginosis does not usually appear to be related to extensive inflammation, and may actually have fewer granulocytes but greater numbers of CD4+ lymphocytes.21 Thus, CD4+ lymphocytes recruited to the vagina in bacterial vaginosis could be a source of TFV elevations through dephosphorylation of TFV-DP.22 Further investigation is needed to determine the influential sources of TFV in cervicovaginal fluid.
After two PrEP clinical trials did not show adequate HIV protection in cisgender women4,5, there were concerns that, in addition to low adherence and pharmacokinetic data favoring near perfect dosing23, biological mechanisms such as STIs, BV and inflammation could be diminishing PrEP efficacy. However, studies have demonstrated that these biological effects depend on product delivery, with vaginal dysbiosis influencing topical12,13 but not oral14 PrEP efficacy. We found high concentrations of TFV in the CVF, consistent with modeling studies showing protection against HIV acquisition with oral TDF/FTC23, despite the presence of various biological phenomena. These data serve to support the effect of oral PrEP with TDF/FTC is not undermined by abnormal vaginal microbiota or inflammatory changes.
There were a number of limitations to our observational study. The sample size of only 35 women participating was small, and was likely due to the cost, complexity of the study and limited number of women taking oral PrEP to recruit from. In addition, not all factors were assessed in the 35 women, limiting the sample size further. We also could not assess the presence of Chlamydia, Gonorrhea or Trichomonas with TFV concentrations because none of the women in this study tested positive for these organisms. The influence of genital tract STIs warrants further research.
Overall, our findings should be reassuring to providers that prescribe PrEP to women in the form of oral TDF/FTC. There does not appear to be changes in TFV penetration from common bacterial and viral flora. Women who take tenofovir alfenamide fumarate (TAF) were not part of this study as it has not yet been approved for PrEP through receptive vaginal intercourse. As the prevention efforts moves increasingly towards TAF/FTC, further studies are needed in women to determine the penetration of TAF/FTC into cervicovaginal fluid.
Acknowledgements:
The authors would like to thank and acknowledge the study participants for their time and contributions to this research. This work was supported by the following grants California HIV/AIDS Research Program (CHRP) Grant EI11-SD-005B and NIH Grants KL2TR001444, AI036214, AI147821, DA051915, P30 AI050410.
Funding Sources:
This work was supported by the following grants California HIV/AIDS Research Program (CHRP) Grant EI11-SD-005B and NIH Grants KL2TR001444, AI036214, AI147821, DA051915, AI050410.
Conflict of Interest:
MC, SP, SR, DP, AK, AC, and SG have no conflicts. MK has research funding paid to the institution from Gilead Sciences and ViiV Healthcare. JB has research funding paid to the institution from Gilead Sciences. PLA has received personal fees and research funding paid to his institution from Gilead Sciences. SRM has research funding paid to the institution from Gilead Sciences and Merck. SRM has financial interests in Bristol Myers Sqibb, Geron, Pfizer and Aspera Biomedicines and has acted as consultant to Primmune Therapeutics.
REFERENCES
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010;363(27):2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine. 2012;367(5):423–434. [DOI] [PubMed] [Google Scholar]
- 4.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendrix CW, Andrade A, Bumpus NN, et al. Dose Frequency Ranging Pharmacokinetic Study of Tenofovir-Emtricitabine After Directly Observed Dosing in Healthy Volunteers to Establish Adherence Benchmarks (HPTN 066). AIDS research and human retroviruses. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masson L, Passmore JA, Liebenberg LJ, et al. Genital inflammation and the risk of HIV acquisition in women. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;61(2):260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumond JB, Bay CP, Nelson JAE, et al. Intracellular Tenofovir and Emtricitabine Concentrations in Younger and Older Women with HIV Receiving Tenofovir Disoproxil Fumarate/Emtricitabine. Antimicrob Agents Chemother. 2020;64(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopinath S, Iwasaki A. Cervicovaginal microbiota: simple is better. Immunity. 2015;42(5):790–791. [DOI] [PubMed] [Google Scholar]
- 10.Sewankambo N, Gray RH, Wawer MJ, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350(9077):546–550. [DOI] [PubMed] [Google Scholar]
- 11.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS (London, England). 2008;22(12):1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klatt NR, Cheu R, Birse K, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science. 2017;356(6341):938–945. [DOI] [PubMed] [Google Scholar]
- 13.Hillier SL, Meyn LA, Bunge K, et al. Impact of Vaginal Microbiota on Genital Tissue and Plasma Concentrations of Tenofovir. CROI; 2017; Seattle, Washington. [Google Scholar]
- 14.Heffron R, McClelland RS, Balkus JE, et al. Efficacy of oral pre-exposure prophylaxis (PrEP) for HIV among women with abnormal vaginal microbiota: a post-hoc analysis of the randomised, placebo-controlled Partners PrEP Study. The lancet HIV. 2017;4(10):e449–e456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianella S, Smith DM, Vargas MV, et al. Shedding of HIV and human herpesviruses in the semen of effectively treated HIV-1-infected men who have sex with men. Clin Infect Dis. 2013;57(3):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson CT, Sharma V, Uchitel S, et al. Prebiotic Potential of Herbal Medicines Used in Digestive Health and Disease. J Altern Complement Med. 2018;24(7):656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences of the United States of America. 2011;108 Suppl 1:4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cottrell ML, Prince HM, Allmon A, et al. Cervicovaginal and Rectal Fluid as a Surrogate Marker of Antiretroviral Tissue Concentration: Implications for Clinical Trial Design. J Acquir Immune Defic Syndr. 2016;72(5):498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashuba AD, Gengiah TN, Werner L, et al. Genital Tenofovir Concentrations Correlate With Protection Against HIV Infection in the CAPRISA 004 Trial: Importance of Adherence for Microbicide Effectiveness. J Acquir Immune Defic Syndr. 2015;69(3):264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosca AS, Castro J, Sousa LGV, Cerca N. Gardnerella and vaginal health: the truth is out there. FEMS Microbiol Rev. 2020;44(1):73–105. [DOI] [PubMed] [Google Scholar]
- 21.Giraldo PC, de Carvalho JB, do Amaral RL, da Silveira Gonçalves AK, Eleutério J Jr., Guimarães F. Identification of immune cells by flow cytometry in vaginal lavages from women with vulvovaginitis and normal microflora. Am J Reprod Immunol. 2012;67(3):198–205. [DOI] [PubMed] [Google Scholar]
- 22.Shen Z, Rodriguez-Garcia M, Patel MV, Bodwell J, Wira CR. Epithelial Cells and Fibroblasts from the Human Female Reproductive Tract Accumulate and Release TFV and TAF to Sustain Inhibition of HIV Infection of CD4+ T cells. Sci Rep. 2019;9(1):1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cottrell ML, Yang KH, Prince HM, et al. A Translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis Against HIV in Men and Women Using Tenofovir Disoproxil Fumarate With or Without Emtricitabine. J Infect Dis. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This work was supported by the following grants California HIV/AIDS Research Program (CHRP) Grant EI11-SD-005B and NIH Grants KL2TR001444, AI036214, AI147821, DA051915, AI050410.