Keywords: finger, fMRI, neurofeedback, plasticity
Abstract
Previous work has shown that functional magnetic resonance imaging (fMRI) activity patterns associated with individual fingers can be shifted by temporary impairment of the hand. Here, we investigated whether these neural activity patterns could be modulated endogenously and whether any behavioral changes result from this modulation. We used decoded neurofeedback in healthy individuals to encourage participants to shift the neural activity pattern in sensorimotor cortex of the middle finger toward the index finger, and the ring finger toward the little finger. We first mapped the neural activity patterns for all fingers of the right hand in an fMRI pattern localizer session. Then, in three subsequent neurofeedback sessions, participants were rewarded after middle/ring finger presses according to their activity pattern overlap during each trial. A force-sensitive keyboard was used to ensure that participants were not altering their physical finger coordination patterns. We found evidence that participants could learn to shift the activity pattern of the ring finger but not of the middle finger. Increased variability of these activity patterns during the localizer session was associated with the ability of participants to modulate them using neurofeedback. Participants also showed an increased preference for the ring finger but not for the middle finger in a postneurofeedback motor task. Our results show that neural activity and behaviors associated with the ring finger are more readily modulated than those associated with the middle finger. These results have broader implications for rehabilitation of individual finger movements, which may be limited or enhanced by individual finger plasticity after neurological injury.
NEW & NOTEWORTHY It may be possible to remobilize fingers after neurological injury by altering neural activity patterns. Toward this end, we examined whether finger-related neural activity patterns could be modified in healthy individuals without physical intervention, using fMRI neurofeedback. Our findings show that greater variability of neural patterns at baseline predicted a participant’s ability to successfully shift these patterns. Because neural variability is common in individuals poststroke, this illustrates a potential clinical benefit of this procedure.
INTRODUCTION
Movements of individual fingers have distinct neural activity patterns that can be measured using multivoxel pattern analysis (MVPA) of functional magnetic resonance imaging (fMRI) data from sensorimotor cortex (1–3). In animal models, the underlying neural activity maps in sensorimotor cortex have long been known to adapt dynamically with experience and learning (4, 5). More recently, it was found that neural finger representations in humans will adapt and shift when two of the fingers are glued together for a short period of time (6). Furthermore, this temporary adaptation of cortical maps was correlated with behavioral deficits recorded after the fingers were unglued.
Instead of physically intervening at the hand (e.g., by gluing fingers together) and measuring the associated cortical changes, our approach is to directly induce cortical remapping and observe whether any changes in motor behavior or tactile sensation of the fingers occur. Current neural stimulation techniques such as transcranial magnetic stimulation (TMS) and transcranial direct-current stimulation (tDCS) operate on the scale of centimeters and are therefore unable to target the millimeter-scale neural activity patterns associated with individual finger movements in humans (7, 8). However, a recent technique known as decoded neurofeedback is able to target activity patterns directly, for example, to modulate the fMRI patterns associated with different visual orientations in primary visual cortex (9). Critically, the modulation of these patterns correlated with changes in visual perception, establishing a link between direct modulation of neural activity patterns and their associated behavior. This technique has since been used to investigate neural representations such as fear conditioning (10, 11) and confidence judgments (12) but has yet to be used to target motor behaviors or tactile sensation.
Therefore, we wanted to investigate whether decoded neurofeedback could be applied to neural maps in sensorimotor cortex and whether this would result in behavior changes similar to those found in physical manipulations such as gluing or repeated stimulation of individual fingers. Instead of physically attaching the fingers together, we used a form of decoded neurofeedback known as associative decoded neurofeedback (13). This technique was used by Amano et al. (13) to associate a neutral visual stimulus (gray orientation grating) with another visual stimulus (colored orientation grating). In this study, we used associative decoded neurofeedback to associate presses of one finger (either the middle or ring finger) with the outwardly adjacent finger (index or little finger). This manipulation was intended to match the neural changes found by Kolasinski et al. (6), in which the representations of the middle and ring finger were shifted apart, and the representations of the ring and little finger moved closer together. We hypothesized that individual finger representations and their associated behaviors would be altered after neurofeedback training.
Although we aimed to alter neural representations and finger behaviors in healthy individuals, our approach is intended to explore possible future applications for brain-based therapy of neurological injuries that affect motor behavior, such as stroke. In such patients, decoded neurofeedback of individual finger movements may be able to improve cortical impairments and their associated behaviors. Overall, this approach offers insight into how changes in neuroplasticity can alter sensorimotor behavior, with implications for recovery following neurological injury such as stroke.
MATERIALS AND METHODS
Participants
Fourteen healthy right-handed participants were recruited for the experiment approved by the University of Texas at Austin Institutional Review Board. Informed consent was obtained from all participants. One participant became uncomfortable during the first fMRI session and did not continue in the experiment. Two participants completed the first fMRI session but elected not to continue in the experiment. One additional participant completed the familiarization session outside of the MRI scanner but did not continue the experiment due to illness. Thus, 10 participants completed the experiment and remained in the final analysis (4 female, average age 25.5 yr, SD = 5.2). This sample size is in line with previous studies of finger plasticity (6, 14). As neurofeedback is a new approach to studying finger plasticity, this study aimed to test the viability of this technique.
Apparatus
Finger presses were recorded from the index, middle, ring, and little fingers of the right hand using a custom force-sensitive keyboard at 120 Hz. For tests outside of the MRI scanner, the keyboard (force sensor: TE FS20) was placed on a desk, and visual instructions and feedback were presented on a laptop screen using Python and Pygame. For tests inside the MRI scanner, the keyboard (force sensor: Honeywell FSS015) was affixed to a wooden board, which lay on the participant’s lap, and visual instructions and feedback were provided through a back-projection screen. For vibrotactile stimuli, two vibrotactile stimulators (Soberton E-12041808) were placed on top of the force-sensitive keyboard.
Imaging Parameters
Participants were scanned in a Siemens Skyra 3 T scanner with a 32-channel head coil. For all fMRI sessions, the same EPI sequence was used (TR = 2 s; 36 slices; in-plane resolution 2.3 × 2.3 mm; 100 × 100 matrix size; 2.15-mm slice thickness; 0.15-mm slice gap; 2× multiband factor). After auto-alignment to the AC-PC plane, a manual adjustment was performed to ensure coverage of the motor cortex. The same manual adjustment parameters were applied to the subsequent neurofeedback sessions. A high-resolution anatomical scan (MEMPRAGE, 1-mm isotropic voxels) was also acquired during the first localizer session to identify the primary sensorimotor cortex (M1 + S1) using Freesurfer. Subsequent real-time fMRI scans were rigidly aligned to this template using AFNI’s 3dvolreg for motion correction (48).
General Procedure
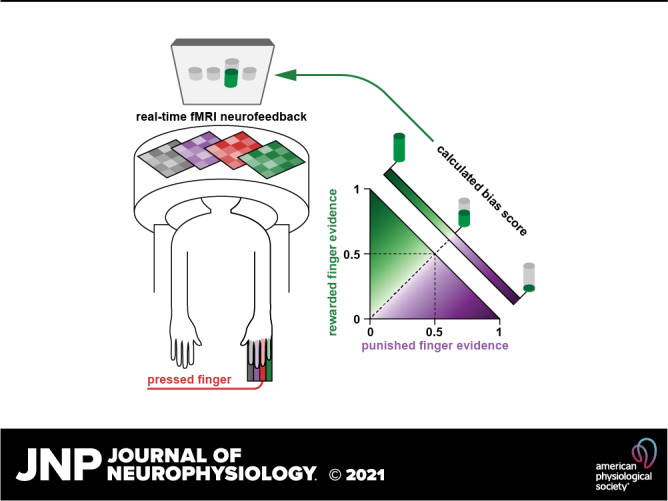
To associate presses of one finger with the outwardly adjacent finger, we first constructed a decoder to determine the relative real-time evidence of each of the four fingers of the right hand during normal button pressing. We then constructed a neurofeedback “finger bias score” calculation that would increase with evidence of the desired (outwardly adjacent) finger and decrease with evidence of the undesired (inwardly adjacent) finger, effectively “biasing” participants toward the desired finger and away from the undesired finger. In subsequent neurofeedback sessions, this neurofeedback “bias score” was presented as a feedback thermometer to participants at the end of each trial of pressing using the calculated decoder evidence values from the fMRI data of that trial. We used a within-subject control by providing two different types of neurofeedback on alternating trials: one based on index-middle finger coordination and one based on ring-little finger coordination. For example, during presses of the middle finger, the bias score would be large if the evidence for the index (desired) finger was greater than the evidence for the ring (undesired) finger, leading to a large feedback thermometer. The height of the feedback thermometer was directly tied to monetary compensation provided at the end of the experiment—therefore, we also consider the outwardly adjacent finger to be a “rewarded” finger (greater evidence leads to more reward), and the inwardly adjacent finger to be a “punished” finger (greater evidence leads to less reward).
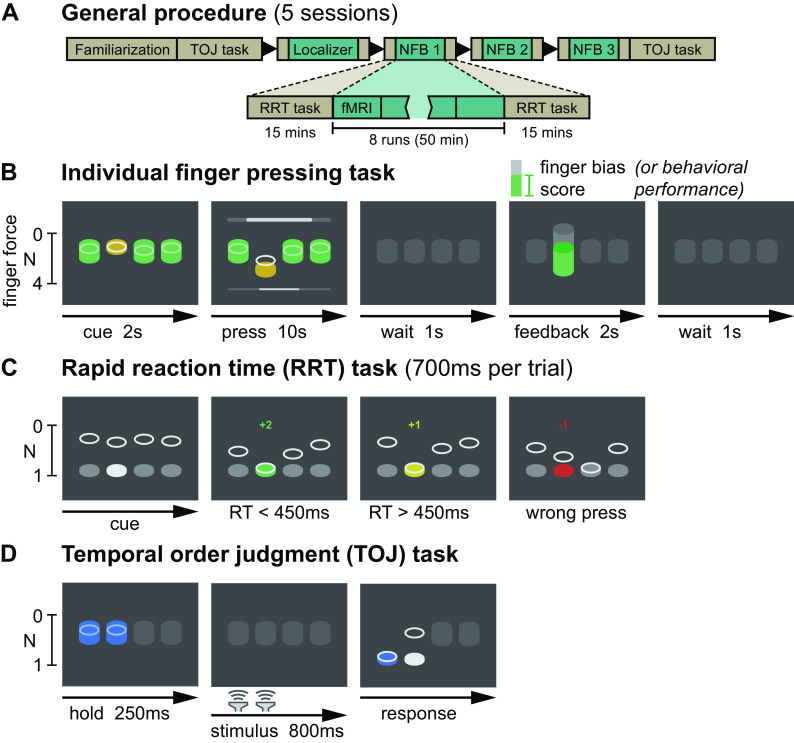
The experiment consisted of five sessions: one familiarization session outside of the MRI scanner, one finger pattern localizer session inside the MRI scanner, and three decoded neurofeedback sessions inside the MRI scanner (Fig. 1A). Neurofeedback sessions were scheduled by participant availability, with a goal of 1 session per week, with sessions separated by at least 2 days. Neurofeedback sessions had a separation of 6.1 ± 3.2 (mean ± SD) days, minimum 2 days, maximum 12 days. During the pattern localizer, participants pressed each of the fingers an equal number of times, whereas during neurofeedback participants pressed only the middle and ring fingers. Pressing behavior was controlled using a custom force-sensitive keyboard to ensure participants exerted the same amount of force and did not alter their finger coordination patterns during each trial (Fig. 1B). Motor behavior was assessed before and after each fMRI session using a rapid reaction time task (Fig. 1C). This task captured behavioral changes resulting from any short-term plasticity associated with the neurofeedback manipulation as well as any changes due to repeated presses of only the middle and ring fingers over the course of each neurofeedback session. Somatosensory perception was assessed before and after the full experiment using a tactile temporal order judgment task in which adjacent finger pairs were stimulated in quick succession (Fig. 1D). This assessment determined if any changes in tactile discrimination ability occurred due to neurofeedback.
Figure 1.
Experimental design. A: the five-session experiment consisted of behavioral familiarization, a finger pattern localizer fMRI session, and three neurofeedback (NFB) sessions. Each fMRI session included behavioral pre- and posttests. B: an individual finger pressing task was used as the basis for the localizer and neurofeedback sessions. Participants were required to make individual presses with one of four fingers (index, middle, ring, or little) while maintaining constant pressure on all other keys. At the end of each trial, feedback was presented related to their motor behavior (localizer session) or their ability to bias the fMRI patterns related to finger presses (neurofeedback sessions). C: a rapid reaction time (RRT) task was used to assess motor confusion before and after each fMRI session. Participants were encouraged to make rapid presses (reaction time below 450 ms) through a point system. D: a temporal order judgment (TOJ) task was used to assess the hand representation of participants before and after the entire neurofeedback protocol. During a 800-ms stimulus blank period, a brief vibrotactile stimulus was delivered to two adjacent fingers in rapid succession. Participants then judged which of the two stimuli happened first. fMRI, functional magnetic resonance imaging.
Individual Finger Pressing Task
An individual finger pressing task was performed during all fMRI scanning sessions (Fig. 1B). This task was also performed for 15 min during the familiarization session. Each trial of the task consisted of 10 s of individual finger pressing followed by 2 s of feedback. Each trial was preceded by a 2-s cue for the target finger, and feedback was both preceded and followed by 1-s wait periods, leading to a 16-s trial duration. In the familiarization and localizer sessions, feedback was related to maintaining normal finger coordination patterns on the individual finger pressing task, whereas in the neurofeedback sessions, feedback was related to neural activity in sensorimotor cortex. Each fMRI session consisted of eight runs of 20 trials each, lasting about an hour.
Participants were instructed to press the target finger (one of index, middle, ring, or little) while keeping a constant pressure on the three other (nontarget) fingers. During the cue and pressing period, the real-time force of each finger was displayed as a gray disk (Fig. 1B). For the target finger, a yellow target cylinder was visible indicating the target force range. Two types of targets were displayed in alternating order: low force (between 0.2 N and 1.2 N) and high force (between 2.0 N and 3.0 N). Participants were required to keep the gray finger disk in the target cylinder for 200 ms before moving on to the next target (e.g., for high force targets, maintaining a force between 2.0 N and 3.0 N for 200 ms). Participants had 10 s to hit as many targets as possible, indicated by a timer bar at the bottom of the screen. For each nontarget finger, a cylinder was visible indicating a constant force range to maintain (0.2–1.5 N). While fingers exerted a force in this range, the nontarget cylinder turned green, and the gray disk was hidden by the cylinder; if fingers exerted more or less force than this force range, the cylinder turned red and the current force of the finger was visible as a gray disk, either above or below the cylinder depending on whether the force was less or greater than the desired force range.
Points were tallied during this task based on hitting targets with the target finger and maintaining constant force on the nontarget fingers. Specifically, targets were assessed as “hits” (+1 point, green score message: “+1”), “misses” (0 points, red score message: “miss!”), or “nontarget fingers off” (0 points, red score message: “off!”). Hits were tallied if the target finger remained inside the yellow target cylinder for 200 ms and all nontargets fingers remained inside the constant force cylinders. If the target finger entered and exited the target cylinder in less than 200 ms, a miss was recorded. If the target was hit but any of the nontarget fingers exited their constant force cylinder, then a “nontarget finger off” was recorded. Participants were encouraged to gain at least 15 points each trial. At the beginning of each trial, a gray score bar appeared at top of the screen, at the same width as the timer bar. As targets were hit, the score bar decreased in size, up to a score of 15. After 15 targets were hit, the score bar turned green and increased in size. After 10 s of pressing, a feedback thermometer appeared in the position of the target finger, related to the participant’s performance during that trial. If at least 15 targets were hit, the feedback thermometer cylinder turned green; if less than 15 targets were hit, the feedback thermometer was gray. Feedback was either related to the number of targets hit (familiarization and finger pattern localizer sessions) or the participant’s neural activity during pressing (neurofeedback sessions).
Behavioral Feedback
In the familiarization and finger pattern localizer sessions, the feedback thermometer was directly related to the number of targets hit. Initially, the maximum height of the thermometer was equivalent to 15 points. For each finger, the maximum score achieved by the participant in that session was used to recalibrate the maximum height of the thermometer. The numerical score was also shown below the feedback thermometer during the feedback period (in green for scores 15 or above or in gray for scores below 15). At the end of each run, the total score over all trials was shown at the bottom of the screen.
Individual Finger Decoding
The fMRI data from the finger pattern localizer task was used to create a finger decoder to be used in the subsequent neurofeedback sessions. Standard preprocessing (rigid body motion correction, detrending, and Z-scoring) was performed. The preprocessed fMRI data from the last 6 s of pressing was averaged over time for each trial to yield 160 time-averaged trials, 40 per finger. The hand area of the primary sensorimotor cortex was identified in each participant by first training a sparse multinomial logistic regression decoder (SMLR, Ref. 15; PyMVPA, Ref. 16) on the larger primary sensorimotor cortex (Brodmann areas 4A, 4P, 3A, and 3B identified using Freesurfer’s surface reconstruction and alignment, Ref. 17, and Freesurfer’s probabilistic cytoarchitectonic map, Ref. 18), and then selecting only those voxels within a 15-mm radius of the center of the hand area. A final SMLR decoder was trained on only these voxels and exported for use in neurofeedback. In all, 214 ± 26.5 voxels were selected by the final decoders (mean ± SD across participants). An SMLR decoder was selected over other possible decoders as we have previously shown its suitability for real-time decoding of finger movements, selecting only the relevant hand region of primary motor and somatosensory cortices (19). SMLR also performs feature selection concurrently with decoding, which can select relevant voxels that may be ignored by univariate feature selection (20).
Real-Time Finger Decoding and Decoded Neurofeedback Logistics
For real-time processing, three files were exported from the offline decoder construction stage: 1) a reference functional image (RFI) which was the mean of the first fMRI run of the pattern localizer session, 2) a mask of primary sensorimotor cortex, and 3) the PyMVPA SMLR decoder trained on voxels within primary sensorimotor cortex. These files were placed on a separate real-time processing computer running custom real-time fMRI Python software (Instabrain; github.com/LewisPeacockLab/instabrain). Each neurofeedback session began with an anatomical localizer “Scout” scan to first align the functional volume to the AC-PC plane. The functional acquisition was then translated and rotated from the AC-PC plane according to parameters entered during the pattern localizer session. This ensured that the functional volume was roughly aligned with the pattern localizer acquisition (to be further corrected with rigid body alignment to the RFI). During neurofeedback, raw fMRI slice data acquired by the MRI scanner was sent to the real-time processing computer, which reconstructed these into NIFTI images. These images were then aligned to the RFI using AFNI’s 3dvolreg (48). They were finally imported to our custom Python software for further time-series processing and decoder application, described in the following section.
Our real-time pipeline, including experimental timings and data processing parameters, follows an optimized procedure developed in Oblak et al. (19). Each volume was loaded into Python, and the voxels of interest were extracted using the mask of primary sensorimotor cortex. Each run began with 20 s of baseline rest, used to estimate the baseline variance of activation in each voxel of interest. At each subsequent TR, data were linearly detrended using the current time history and Z-scored using the variance estimated from the initial baseline period. At decoding TRs (i.e., immediately before feedback presentation), the Z-scored and detrended fMRI data from the previous three TRs was averaged over time. The PyMVPA SMLR decoder was then applied to this time-averaged data, yielding a probability for each of the four fingers that was used to calculate the neurofeedback bias scores (described in the next section). Time points used for decoding were identical to the finger pattern localizer task (i.e., the time-averaged fMRI data from the last 6 s of pressing).
Neurofeedback Bias Score Calculation
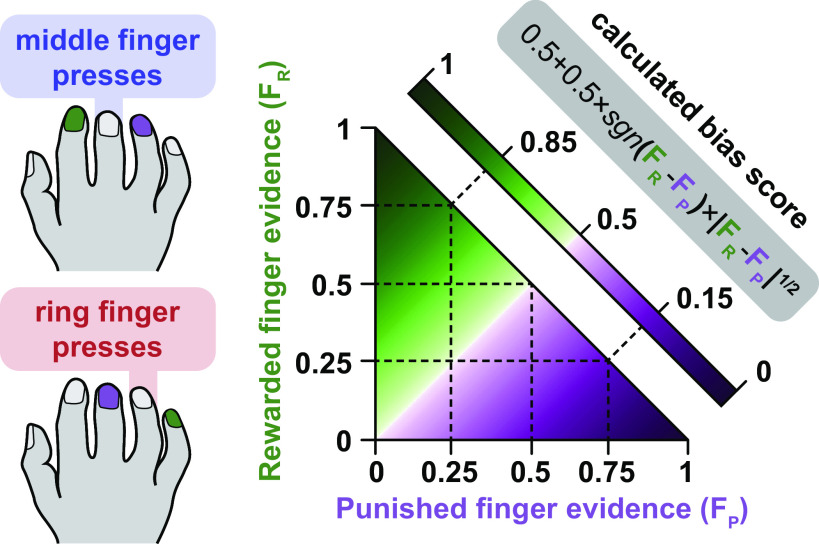
In neurofeedback sessions, participants were rewarded up to $30 per session for shifting the neural activity pattern of the middle finger toward that of the index, and for shifting the neural pattern of the ring finger toward the little finger. Specifically, the neurofeedback score is summarized in Eq. 1, where fingerreward is the decoder output for the finger adjacent to the target finger that is being rewarded (middle: index; ring: little) and fingerpunish is the decoder output for the finger adjacent to the target finger that is being punished (middle: ring; ring: middle). The score calculation is also shown in Fig. 2. We sought to develop a score between 0 and 1 (that could be mapped to a thermometer height) that would increase with evidence of the desired (rewarded) finger, decrease with evidence of the nondesired (punished) finger, and be neutral (0.5) if there was equal evidence of each finger. Finally, we aimed for the score to have sufficient variance for changes to be perceived by participants (i.e., was not always close to 0.5). To spread out the distribution of scores, we calculated sample bias scores using control data from Oblak et al. (19), in which participants performed individual finger presses without neurofeedback. We found that using an exponent of 1/2 (square root) on the difference between rewarded and punished fingers was sufficient to spread the scores without becoming completely bimodal (i.e., scores being calculated almost always as 0 or 1).
| (1) |
Figure 2.
Neurofeedback bias score calculation. Bias scores were calculated based on the real-time decoder evidence for the two fingers adjacent to the pressed finger. Rewarded fingers are shown in green, punished fingers are shown in purple (middle finger presses: index rewarded, ring punished; ring finger presses: little rewarded, middle punished). If rewarded and punished finger evidence were equal, the bias score was 0.5. The square root of the difference between finger evidence was used as the basis of the bias score calculation. For example, with evidence of 0.75 for the rewarded finger (FR) and 0.25 for the punished finger (FP), the calculated bias score would be approximately 0.85. If the evidence for the punished finger was greater than the evidence for the rewarded finger, the bias score was less than 0.5 [i.e., if the sign (sgn) of the difference between rewarded and punished fingers was negative]. The heatmap of bias scores only includes possible decoder output combinations: the four-finger SMLR decoder could output four possible values, which must sum to 1. Therefore, the sum of the evidence of two fingers could never exceed 1. SMLR, sparse multinomial logistic regression.
Participants were not told the explicit purpose of the experiment or the neurofeedback manipulation (i.e., to bias fingers toward one another). Participants were simply instructed to regulate their brain activity during pressing to maximize the height of the feedback thermometer (scoreadjusted), and that the neural activity signature would be different for each finger. If they achieved at least 15 points on the individual pressing task, they saw their neural activity feedback score as a green thermometer, indicating reward proportional to the height of the thermometer; if they did not achieve this minimum score, they saw their neural activity as a gray feedback thermometer and did not receive reward. Trials were blocked in groups of five repetitions of the same finger (i.e., five trials of middle finger presses followed by five trials of ring finger presses). The amount of money earned was displayed at the bottom of the screen at the end of each run.
Post Hoc Calculation of Bias Scores
For all statistical analyses and plots, bias scores were recalculated offline to ensure that they were not skewed by real-time processing. Variance for Z-scoring was estimated using the baseline period at the beginning of each run, as in the neurofeedback sessions. Offline detrending was performed using GLM correction that accounted for the explicit finger presses during the neurofeedback sessions (21). The same SMLR decoder and feedback calculation equation as in the neurofeedback sessions were then applied.
Pattern Visualizations
Changes in fMRI patterns for the ring finger were visualized using PyCortex (22). The four participants with the highest bias scores for the ring finger during neurofeedback were selected to highlight successful pattern shifts. Four patterns were computed for each selected participant: a template importance map showing important regions for each of the rewarded (little) and punished (middle) fingers, a pattern for the pressed (ring) finger relative to the decoder weights for the rewarded and punished fingers during the localizer, a pattern for the pressed finger relative to rewarded and punished decoder weights during neurofeedback, and a pattern showing the change from localizer to neurofeedback.
Template patterns were computed by subtracting the importance map (23) for the punished finger from the importance map of the rewarded finger during the localizer. Localizer and neurofeedback session patterns were computed by taking the mean pattern for the pressed (ring) finger during each session and multiplying it by the difference between the rewarded (little) finger decoder weights and the punished (middle) finger decoder weights. Changes were computed by subtracting the localizer session patterns from the neurofeedback session patterns.
Calculation of Univariate Activation
Univariate activations were calculated as percent signal change (PSC) for each finger in each run of each session. Because we were concerned about the impact of univariate activation on bias scores, the univariate region of interest (ROI) for each participant included only voxels that had a nonzero decoder weight for at least one finger. The mean activation from this ROI was extracted from the motion corrected data, without any Z-scoring or detrending. A design matrix was constructed with a regressor for each finger as well as a constant and linear term for detrending estimation. Finger regressors were constructed as a boxcar during each 10 s of pressing, convolved with a double gamma canonical hemodynamic response function. A least-squares fit between the model and univariate activation was then calculated with Python and Numpy (numpy.linalg.lstsq). PSC was estimated for each finger in each run as the model estimate for each finger divided by the model’s constant term estimate. PSC was calculated for each session by averaging the eight run estimates.
Behavioral Assessment: Rapid Reaction Time Task
We assessed short-term plasticity associated with each neurofeedback session using a motor confusion task in which participants performed rapid button presses (700 ms between presses) of pseudorandomly cued fingers (Fig. 1C), adapted from Kolasinski et al. (6). “Motor confusion” within a finger pair refers to presses cued for one finger in the pair but pressed by the other finger. For example, for the index-middle finger pair, presses cued for the index finger but performed by the middle finger, or presses cued for the middle finger but performed by the index finger, are counted as “confused” presses within that pair. This behavior was found to be altered in Kolasinski et al. (6) along with changes in fMRI patterns in the “confused” finger pair. Therefore, we aimed to determine if similar motor confusion would arise from our neurofeedback manipulation.
Participants viewed the real-time force of each finger as a gray disk that moved up and down with each finger. Under each finger’s disk was a corresponding target gray disk. Every 700 ms, one of the target disks would light up white, indicating the finger to be pressed. A selected finger was detected as the first finger to exceed a force of 1 N. Participants were encouraged to make rapid presses of the correct finger through a point system. If an incorrect finger was selected, the target disk lit up red and a score of −1 point was tallied. If the correct finger was pressed with a reaction time greater than 450 ms, the target disk lit up yellow and a score of +1 point was tallied. If the correct finger was pressed with a reaction time faster than 450 ms, the target disk lit up green and a score of +2 points was tallied. At the end of each run (217 presses), the participant’s score was displayed. The press order in each run was randomized to ensure an equal number of each possible finger transition (18 transitions for each of the 12 possible finger transitions). Participants performed six runs of this task (∼15 min) immediately before and after each fMRI session in a room adjacent to the scanner.
Behavioral Assessment: Temporal Order Judgment Task
Long-term changes in hand representation were assessed using a temporal order judgment task in which participants had to judge which of two adjacent fingers received a vibrotactile stimulus first (Fig. 1D), adapted from Kolasinski et al. (6). Although our neurofeedback manipulation used a motor task without an explicit somatosensory component, we nevertheless included this assessment to mirror the behavioral assessments in Kolasinski et al. (6). As our neurofeedback region of interest included both motor and somatosensory areas, it is possible that the neurofeedback manipulation could influence both motor and somatosensory behaviors.
This task was conducted during the familiarization session, as well as after the final neurofeedback session. Each run of the task targeted two adjacent fingers (index-middle, middle-ring, or ring-little). Participants rested their fingers on top of vibrotactile stimulators (Soberton E-12041808) which lay on the force-sensitive keyboard. The real-time applied force of the two targets fingers were displayed as gray disks. To begin each trial, participants were required to apply a constant force on the stimulators (between 0.2 N and 0.5 N, indicated by a blue cylinder) for 250 ms. After this, the display went blank for 800 ms. During this blank period, the two fingers were stimulated one after the other (20-ms duration, 200-Hz sinusoidal pulse), with possible interstimulus intervals (ISIs) of +400, −400, +250, −250, +180, −180, +120, −120, +70, −70, +30, or −30 ms where, by convention, a positive ISI indicates the rightward digit was stimulated first. The onset of the first pulse was randomly jittered, starting between 150 ms and 350 ms after the beginning of the blank period. After the blank period, a target disk appeared in white for each finger, and the real-time force of each finger also reappeared as a gray disk. Participants were instructed to select the finger which was first stimulated. Selections were detected as the first finger to exceed a force of 1 N. The selected target lit up blue, and a blue asterisk appeared above the selected finger; however, no feedback was given as to whether the selection was correct. At this point, the two blue cylinders appeared again, and the next trial began once the participant kept the constant (between 0.2 N and 0.5 N) force on the stimulators for 250 ms. At the end of each run (120 trials, 10 per ISI), summary accuracy over the run was displayed to participants. In each session of the temporal order judgment task, participants completed nine runs total (3 for each finger pair) in pseudorandomized order, lasting ∼45 min. Participants listened to pink noise over headphones to mask any auditory cues from the vibrotactile stimulators.
Finger Coordination Assessment
Coordination between the target finger and the adjacent (rewarded and punished) fingers during neurofeedback was assessed on a trial-by-trial basis. Each trial was subdivided into a number of presses equal to the number of high force targets passed. For the target finger, the force of each press was evaluated as the maximum force within ±200 ms of the high force target being passed, minus the minimum force within ±200 ms of the previous low force target being passed. Because participants were required to stay in each target for 200 ms, this window ensured that the peak and trough forces of each press were typically captured. The timing of the maximum and minimum force in the target finger were then used to evaluate the corresponding pressing force of the adjacent fingers. Coordination was assessed as the mean pressing force of the adjacent finger (over all presses within the trial) divided by the mean pressing force of the target finger. Note that this coordination metric (evaluated as a ratio of forces) can be either positive or negative because the press timing is determined only by the peaks and troughs of the target finger presses, which may be out of phase with the peaks and troughs of the adjacent fingers (e.g., if participants are slightly lifting the adjacent finger during pressing of the target finger). Because participants were required to maintain some constant force on the keyboard and could therefore have relatively negative force application during presses, these sorts of lifting behaviors were accounted for in our analysis. For each participant, a heatmap of the coordination of the rewarded and punished fingers was generated using a bivariate kernel density estimate (Fig. 6, bottom, with Python and Seaborn’s kdeplot).
Statistical Analyses
Paired t tests were used to assess participants’ ability to bias the neural activity pattern associated with each finger relative to baseline, as well as to directly compare between fingers. A linear mixed effects model with participant as a random effect was used to assess the interaction between neurofeedback training and finger (ring or middle). For all bias score analyses, the mean bias score for each finger over all three neurofeedback sessions was compared with the mean values calculated for the pattern localizer session. Bias scores were recalculated offline, taking into account the explicit finger pressing task during neurofeedback, to ensure that they were not skewed.
Linear mixed effects models with participant as a random effect were used to determine the relationship between univariate activations and finger bias scores. A separate model was constructed for each finger (middle or ring). A general linear hypothesis test was used to calculate P values for the slope of each model.
The relationship between baseline variability and participants’ ability to bias patterns was calculated using Spearman’s rho. Significance was established using 10,000 bootstrap correlations selecting either the ring or middle finger randomly for each participant, with the P value calculated as the proportion of iterations with rho < 0.
Paired t tests were used to assess within-finger changes in finger preference at each neurofeedback session. Significance was assessed using Bonferroni adjusted alpha levels of 0.0125 per finger (0.05/4). Changes in preference were also compared directly between fingers with a linear mixed effects model (with participant as random effect) of the change in finger preference for each session, using a general linear hypothesis test of adjacent finger contrasts (index-middle, middle-ring, and ring-little). Changes in motor confusion between finger pairs at each neurofeedback session was assessed by creating a linear mixed effects model for each session with participant as a random effect. Tukey’s post hoc test (alpha < 0.05) was used to determine if there were statistically significant differences between finger pairs. A similar linear mixed effects model was created for assessing changes in tactile discrimination behavior due to all three neurofeedback sessions.
A linear model using coordination of each of the adjacent fingers was used to assess the effect of average coordination patterns on mean neurofeedback scores. A linear mixed effects model with participant as a random effect was used to assess the effect of trial-by-trial coordination patterns on trial-by-trial neurofeedback bias scores, again using the coordination of the two adjacent fingers as fixed effects. Finally, a general linear hypothesis test was used to calculate P values for linear and linear mixed effects models.
Statistical software.
For all analyses, linear mixed effects models were constructed with lme from nlme in R (24). Linear models without mixed effects were constructed with lm from base R. Interaction effects were assessed with anova from base R. Post hoc tests for main effects were conducted using glht from multcomp in R (25). Basic statistics (paired t tests, Spearman’s rho) were calculated with Python’s scipy.stats.
Subjective Assessments and Questionnaires
A neurofeedback strategy questionnaire was administered after each neurofeedback session to determine the strategies used by participants for each finger. At the end of the last neurofeedback session, participants were asked what they thought the feedback signal for each finger was related to, and were finally given a forced-choice test to guess which nontarget finger they thought the feedback thermometer was related to (for each target finger).
RESULTS
Differential Modulation of Neural Patterns Related to Individual Fingers
Presses of each of the ring and middle fingers were associated with a “bias score” intended to shift their representations based on real-time decoded patterns of fMRI activity. After each 10-s trial of pressing, this score was displayed to participants as a feedback thermometer (Fig. 3B). For the middle finger, the score was higher when the real-time decoded pattern appeared more similar to the index finger, and lower when the decoded pattern was more similar to the ring finger. For the ring finger, the score increased with little finger pattern similarity and decreased with middle finger pattern similarity. Participants received monetary reward related to their ability to increase the bias scores for each finger, up to a total of $30 persession.
Figure 3.
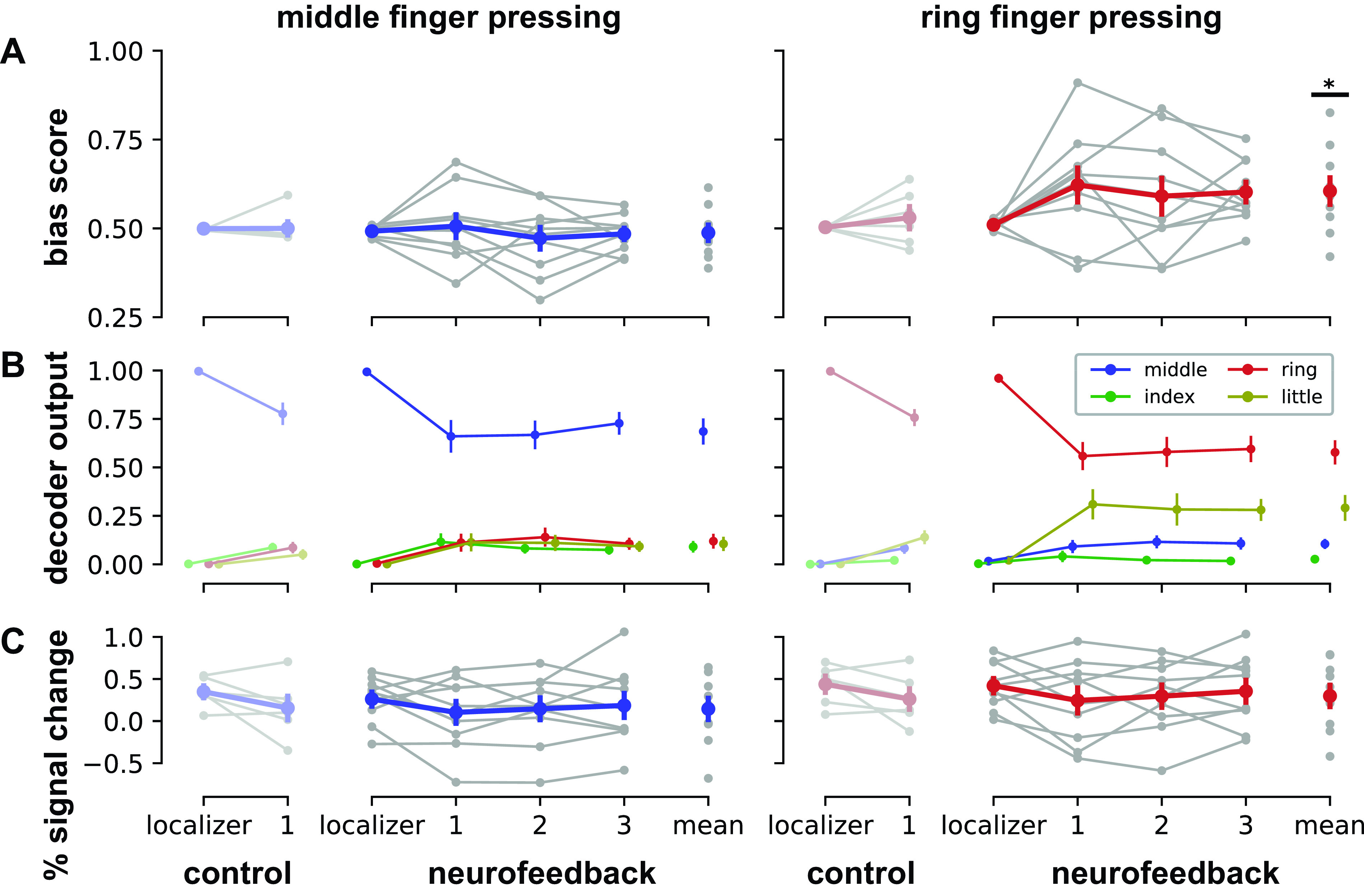
Individual finger pattern bias modulation. A: mean bias scores by session for presses of middle finger (blue, left column) and ring finger (red, right column). See Eq. 1 in methods for bias score calculation details. B: mean decoder outputs by session. During middle finger presses, bias scores encouraged participants to increase the index finger decoder output (green) relative to the ring finger output (red). During ring finger presses, bias scores encouraged participants to increase the little finger output (yellow) relative to the middle finger output (blue). C: mean univariate activations by session, calculated as the mean percent signal change within the voxels selected by each decoder. Univariate activations were found to be unrelated to changes in bias score. Control data were acquired from participants (N = 6) who performed two sessions of finger pressing but did not receive neurofeedback in the second session (19). Neurofeedback data (N = 10) are shown by session (localizer: pattern localizer; 1–3: neurofeedback sessions; mean: average over all neurofeedback sessions). All data (control and neurofeedback) are shown relative to a decoder trained on each participant’s localizer session. Gray dots indicate means for each participant; colored dots indicate means across the entire group. Errors bars indicate SE. Statistical differences relative to the pattern localizer sessions are indicated at *P < 0.05.
Participants were unable to reliably increase the middle finger bias score above the baseline level from the pattern localizer [t(9) = −0.22, P = 0.83, Cohen’s dz = −0.073; Fig. 3A, left column]. However, participants were able to increase the ring finger bias score above baseline: mean bias scores for the ring finger were significantly greater during neurofeedback compared with the pattern localizer session [t(9) = 2.63, P = 0.027, dz = 0.88; Fig. 3A, right column]. A linear mixed effects model of bias score with participant as a random effect confirmed a significant interaction between session (localizer or neurofeedback) and finger [middle or ring; F(1,27) = 5.15, P = 0.031]. A direct comparison between bias scores also showed significantly higher scores for the ring finger compared with the middle finger during neurofeedback [t(9) = 2.47, P = 0.035, dz = 0.82].
A visualization of ring finger pattern shifts in the most successful participants demonstrates how participants were able to increase the ring finger bias score (Fig. 4). In all participants, a template map for the rewarded (little) and punished (middle) finger aligns with the expected somatotopy, with the little finger pattern biased medially and the middle finger pattern biased laterally. Some participants were able to increase pattern strength in both rewarded and punished areas, while others primarily exhibited changes in either rewarded or punished areas.
Figure 4.
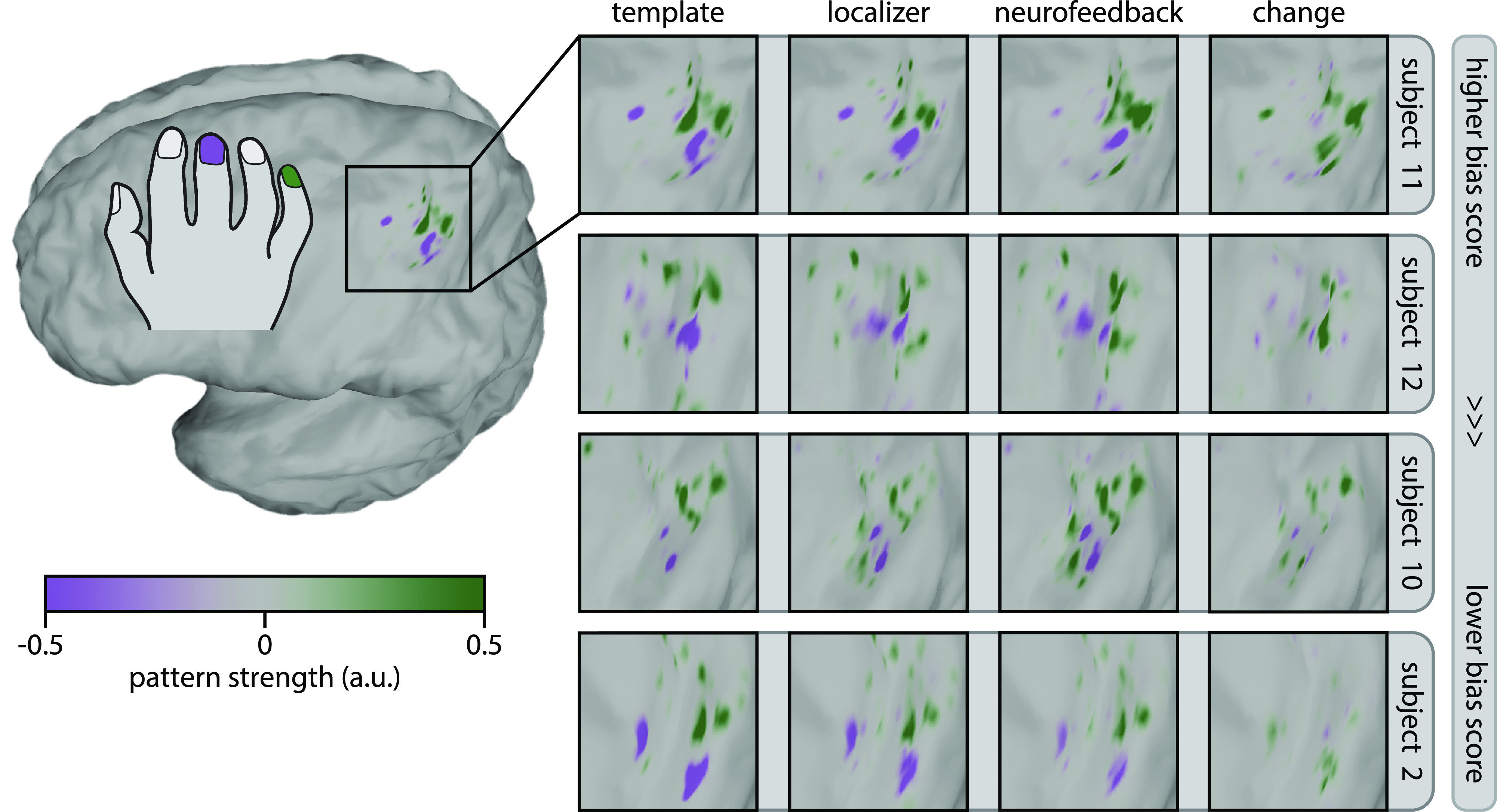
Visualization of ring finger pattern changes with neurofeedback. Pattern shifts are shown for the four participants with the highest bias scores for the ring finger during neurofeedback, in descending order. Template patterns were computed using the decoder weights and mean fMRI patterns during pressing of the rewarded (little) finger and punished (middle) finger from the localizer session. For template patterns, green indicates regions related to the little finger, whereas purple indicates regions related to the middle finger. Localizer and neurofeedback patterns were computed by taking the mean fMRI patterns during localizer and neurofeedback sessions for the pressed (ring) finger and multiplying them by the decoder weights for the rewarded and punished fingers. Change patterns were simply the difference between neurofeedback and localizer patterns. For localizer, neurofeedback, and change patterns, green indicates regions with pattern strength that increases bias scores, whereas purple indicates regions with pattern strength that decreases bias scores. See methods for full pattern visualization details. a.u., arbitrary units; fMRI, functional magnetic resonance imaging.
Changes in Univariate Activations Did Not Inflate Bias Scores
Extensive practice of a motor task can result in increases or decreases in univariate neural activation in primary sensorimotor cortex (26–28). Changes in univariate activations could potentially drive bias scores, for example, if the decoder weights are biased toward one finger or if changes in signal to noise ratio (SNR) alter the decoder’s confidence. We therefore investigated whether the univariate signal (percent signal change; PSC) within the voxels selected by the decoder in each participant were related to their calculated bias scores. We analyzed this relationship in neurofeedback participants as well as in a control data set where participants performed individual finger pressing in two subsequent sessions but did not receive neurofeedback (19).
We found no significant relationship between mean univariate activation and finger bias score in the control data set for either the middle finger (linear mixed effects model with participant as random effect; general linear hypothesis test; P = 0.705) or ring finger (P = 0.664). In the neurofeedback group, this relationship was trending for the middle finger (P = 0.072) but not for the middle finger (P = 0.334). For the middle finger, the model estimate for the relationship between PSC and bias score was negative for both the control (β = −0.022) and neurofeedback (β = −0.068) datasets, implying that decreased univariate activity during middle finger presses could increase middle finger bias scores. For the ring finger, this model estimate was positive for both control (β = 0.041) and neurofeedback (β = 0.073) datasets, implying that increased univariate activity for the ring finger could increase ring finger bias scores. However, the mean univariate activation of the ring finger during neurofeedback was not increased relative to the pattern localizer session [t(9) = −1.45, P = 0.18], and the negative estimate of PSC (β = −0.12) was in the opposite direction of the potential relationship between PSC and bias score. Therefore, we are confident that epiphenomenal changes in univariate activation did not contribute to apparent neuromodulation success during ring finger presses.
Baseline Variability Relates with Neuromodulation Ability
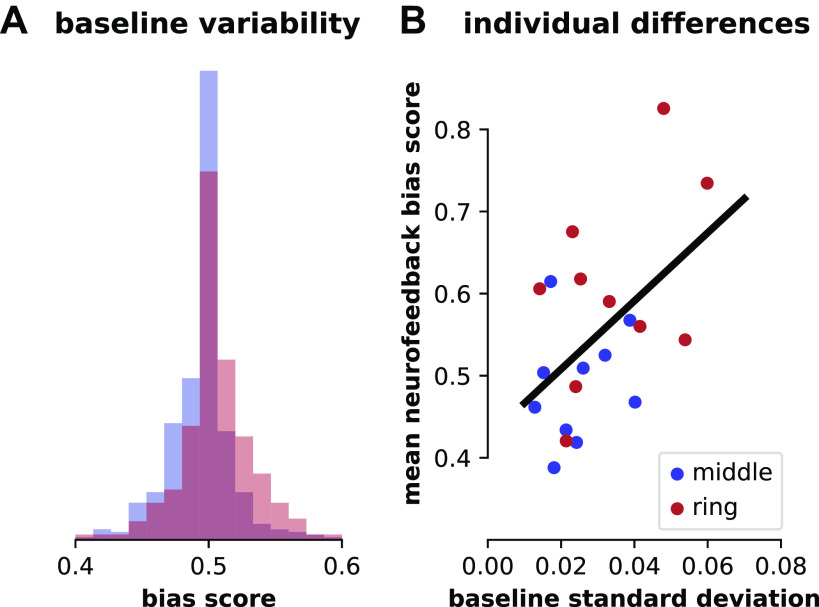
When bias scores were taken from the pattern localizer session for all participants, mean bias scores were similar for the middle (0.494) and ring (0.507) fingers, but variability (standard deviation) was on average larger for the ring (SD = 0.038) than the middle (SD = 0.027; Fig. 5A). This variability was not reliably different for each finger when compared within subject [t(9) = 1.67, P = 0.13] but did correlate with individual participants’ abilities to increase each finger’s bias score (Spearman’s rho = 0.39, P = 0.036; 10,000 bootstrapped correlations performed using only one randomly selected finger per participant per correlation; see methods for further bootstrapping details; Fig. 5B).
Figure 5.
Baseline variability of individual finger patterns. A: distributions of bias scores for middle (blue) and ring (red) fingers for all participants during the baseline pattern localizer session. Each distribution includes 400 bias scores (40 trials of pressing from each of the 10 participants for each finger). B: relationship between individual participant bias pattern variability during the pattern localizer session (standard deviation) and mean bias score achieved during neurofeedback sessions for each finger. Best-fit line shown for reference; see methods for statistics.
Neuromodulation Was Not Driven by Finger Coordination
We used a custom force-sensitive keyboard to ensure that no overt movements were performed with the nontarget fingers. It was nevertheless possible that participants could learn to subtly shift their finger coordination to increase the bias scores. We therefore examined whether bias scores were related to finger coordination. We hypothesized that increased movement of the rewarded finger (press middle/reward index; press ring/reward little) would increase bias scores, and increased movement of the punished finger (press middle/punish ring; press ring/punish middle) would decrease bias scores. No such relationship was found for each participant on average (Fig. 6, top). Mean coordination patterns with the adjacent fingers in each participant did not explain each participant’s mean neurofeedback scores for either the middle finger (linear model without random effects; general linear hypothesis test; index fixed effect: P = 0.838; ring fixed effect: P = 0.428) or the ring finger (middle: P = 0.986; little: P = 0.446).
Figure 6.
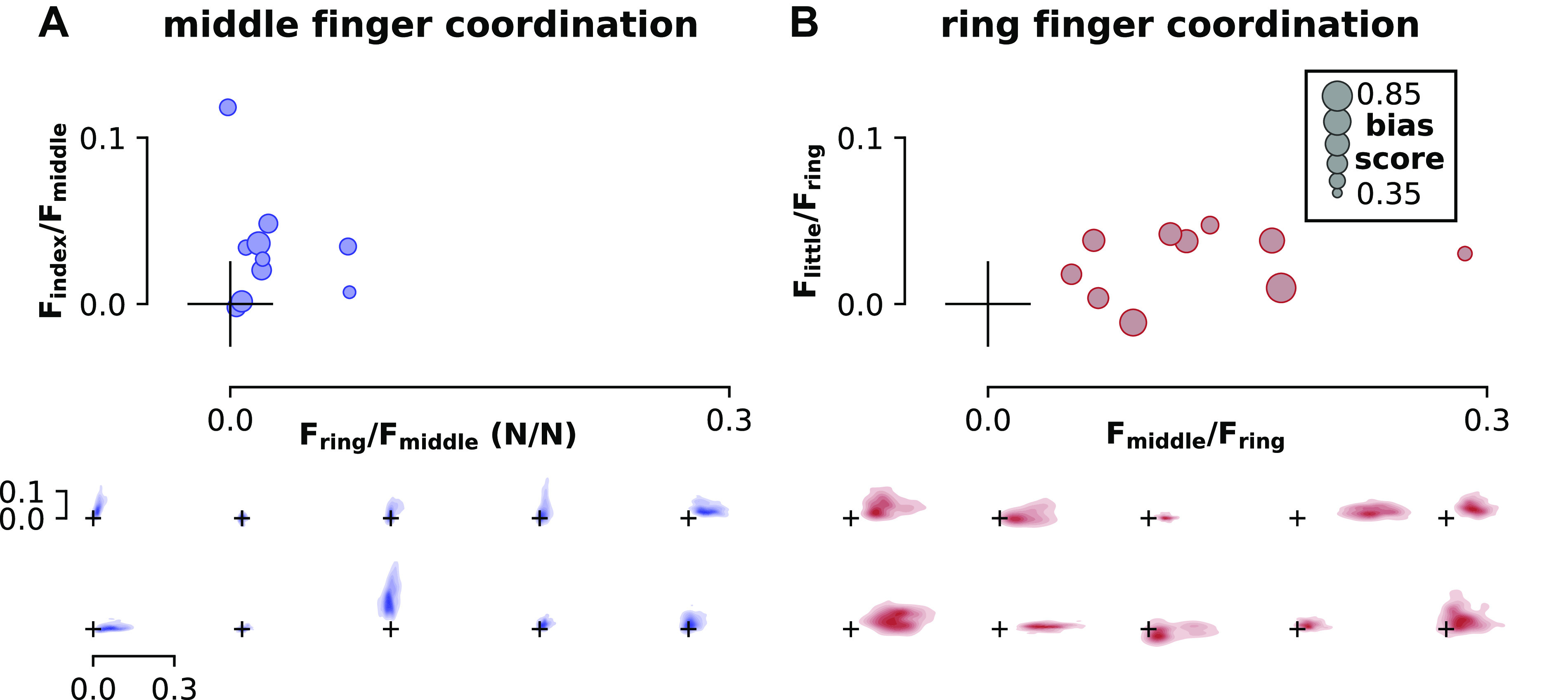
Finger coordination patterns. A: coordination of middle finger presses with the rewarded finger (index, y-axis) and punished finger (ring, x-axis). B: coordination of ring finger presses with the rewarded finger (little, y-axis) and punished finger (middle, x-axis). Mean coordination values for each participant (top, N = 10) are shown as a ratio of the force of the rewarded or punished finger to the pressed finger (N/N). The size of each marker indicates the mean bias score achieved by each participant, with larger circles indicating a greater bias score. These plots illustrate that coupling between adjacent fingers did not correlate with bias scores; if this were true, we would expect larger circles toward the top of each plot (rewarded finger coupling increasing on the y-axis) and smaller circles toward the right of each plot (punished finger coupling increasing on the x-axis). Individual coordination heatmaps for each participant are also shown in the bottom. Each heatmap in the bottom corresponds to one circular marker in the top, with the size and position of the origin marker cross serving as a scale reference. See methods for details of coordination pattern calculation and heatmap generation. F, force exerted by each finger; N/N, ratio of the force of the rewarded or punished finger to the pressed finger.
Although average coordination patterns for each participant did not predict their mean bias scores, it is possible that within-subject coordination pattern variability could explain bias scores on a trial-by-trial basis. Individuals demonstrated coordination patterns with unique distributions (Fig. 6, bottom). However, we found no relationship between trial-by-trial coordination patterns and bias scores in either the middle finger (linear mixed effects model with participant as random effect; general linear hypothesis test; index fixed effect: P = 0.11; ring fixed effect: P = 1.00) or ring finger (middle: P = 0.924; little: P = 0.663).
We also investigated whether finger coordination could explain variability in bias scores during the localizer session. As in the neurofeedback sessions, we found no such effect in either the middle finger (linear mixed effects model with participant as random effect; general linear hypothesis test; index fixed effect: P = 0.936; ring fixed effect: P = 0.489) or ring finger (middle: P = 0.918; little: P = 0.390).
Participants Were Unaware of the Neurofeedback Manipulation
Participants reported their subjective strategies for each finger at the end of each neurofeedback session. Only three of 10 participants reported motor-related strategies, including “pressing slowly and accurately,” “focusing only on the target finger,” and “trying not to push both middle and ring fingers.” The remaining seven participants reported various strategies such as “thinking about math problems and counting,” “relaxing and looking at the top of the thermometer,” and “thinking positive thoughts.”
After the final neurofeedback session, participants also reported what they thought the height of the feedback thermometer was related to. Four of 10 reported “I don't know” or “no idea.” Only two participants reported different strategies for each finger (first participant: “eye focus” for the middle finger, “ring finger pressure” for the ring finger; second participant: “heightened emotions” for the middle finger, “confidence and focus” for the ring finger). The remaining four participants reported various guesses for the thermometer’s height, with the same guess being reported for both fingers: 1) “positive thoughts,” 2) “creative thoughts while accurately performing motor task,” 3) “ability to isolate finger movements,” and 4) “maintaining focus while engaging in higher order logical thinking.”
Finally, it was revealed to participants that increases in the height of the thermometer for each finger was related to one of the nontarget fingers, and they were asked to guess which of the three that was. For the middle finger target, three participants guessed the index finger, three guessed the ring finger, and four guessed the little finger. For the ring finger, six participants guessed the index finger, three guessed the middle finger, and one guessed the little finger.
Overall, these subjective results suggest that participants were unaware of the purpose of the neurofeedback manipulation. A minority of participants reported motor-related strategies, which were mostly related to performing the ongoing task accurately (i.e., pressing the target finger independently). Although one participant did report “trying not to push both middle and ring fingers” after one neurofeedback session, this was not reiterated during the final questionnaire regarding the height of the thermometer. Even when it was finally revealed that the neurofeedback reward was related to nontarget fingers, participants did not show a bias toward the rewarded fingers when asked to choose between nontarget fingers.
It may be possible that participants who report certain explicit strategies such as using motor-related strategies or using different strategies for each finger are able to achieve greater neurofeedback success despite ultimately being unaware of the purpose of the manipulation. The three participants with motor-related strategies had a mean bias score of 0.47 for the middle finger and 0.64 for the ring finger. The two participants reporting different optimal strategies for each finger had a mean bias score of 0.54 for the middle finger and 0.51 for the ring finger. As the overall mean score for the middle finger was 0.49, and the overall mean for the ring finger was 0.62, neither subgroup performed better than the mean with both fingers, showing no clear benefit for either strategy subgroup.
Differential Modulation of Finger Preference
Motor behavior was assessed before and after each fMRI session in a rapid reaction time task. The pressing order in this task was randomized to have an equal number of presses of each finger and an equal number of transitions between each possible two-finger combination. We considered two factors that would influence participants’ pressing behavior: the neurofeedback manipulation as well as the fact that participants would only be pressing the middle and ring fingers (and not the index or little fingers) throughout each fMRI neurofeedback session.
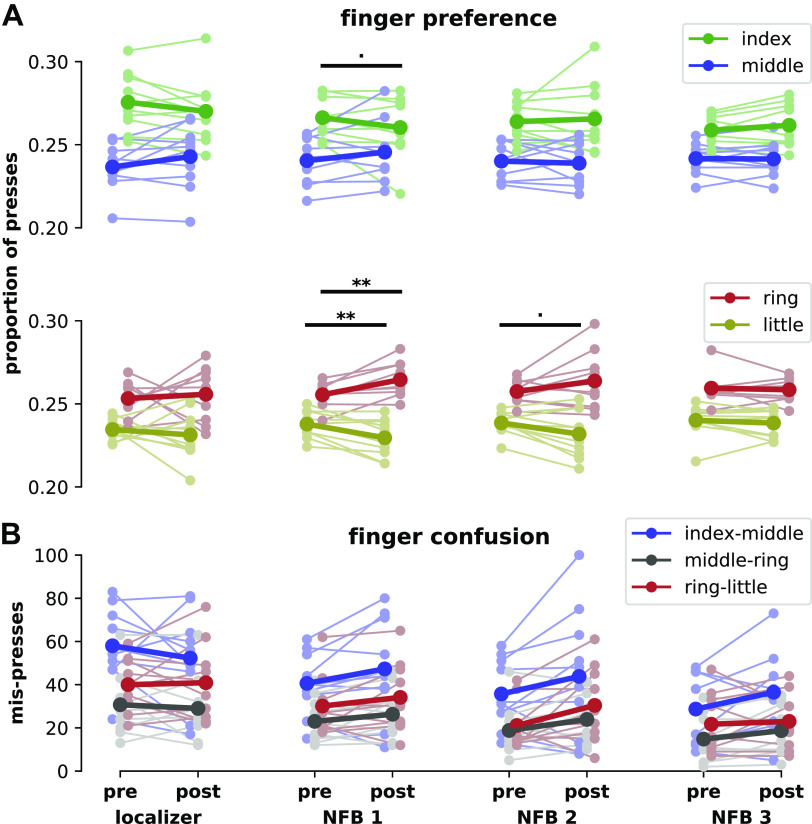
To assess the effect of motor repetition, we measured finger preference as the proportion of total presses by each finger during each testing session (Fig. 7A). Each session’s assessment was analyzed separately due to potential practice effects with the rapid reaction time task leading to ceiling performance in later sessions. We hypothesized that participants would have increased preference for the middle and ring fingers after each neurofeedback session due to motor repetition. Consistent with this hypothesis, we found that after the first neurofeedback session the preference for the ring finger significantly increased [t(9) = 3.82, P = 0.004] and preference for the little finger significantly decreased [t(9) = −3.50, P = 0.007]. Preference for the index finger was trending toward a decrease [t(9) = −1.87, P = 0.094], but contrary to our hypothesis, preference for the middle finger did not significantly increase [t(9) = 1.56, P = 0.15]. After the second neurofeedback session, preference for the little finger was trending toward a decrease [t(9) = −2.19, P = 0.056] but no other near-significant results were found (all other P > 0.1). After the third neurofeedback session, preferences were unchanged (all P > 0.1). For all changes in finger preference, significance was assessed using Bonferroni-adjusted alpha levels of 0.0125 per finger (baseline alpha level of 0.05 divided by 4 fingers).
Figure 7.
Finger pressing behavior before and after each fMRI scan, including each neurofeedback (NFB) session. A: finger preference in pre- and post-fMRI rapid reaction time tests, as a proportion of the total number of presses. Faded colors indicate each participant’s performance, solid colors indicate means across the group. Within-finger statistical differences from pre- to posttest are shown at ■P < 0.1 and **P < 0.01. B: confusion between fingers in pre- and post-fMRI rapid reaction time tests. Mis-presses are assigned to a finger pair when the target finger was one of the fingers of the pair but the other finger in the pair was pressed instead. fMRI, functional magnetic resonance imaging.
We also assessed the relative change in finger preference by contrasting the change in preference of adjacent fingers, on a session-by-session basis. For the localizer session and the third neurofeedback session, there were no significant differences in the change in preference between any adjacent fingers (general linear hypothesis test, all adjacent finger contrasts P > 0.1). After the first neurofeedback session, the increase in ring finger preference was significant in contrast to the decrease of the little finger (P < 0.001). The increase in middle finger preference was also significant in contrast to the index finger (P = 0.016). There was no significant difference between the change in ring and middle finger preferences (P = 0.63). After the second neurofeedback session, the increase in ring finger preference was still significant relative to the little finger (P = 0.049), but there was no significant difference between index and middle (P = 0.91) or middle and ring (P = 0.38) preference changes.
Both the absolute and relative changes in finger preference were most noticeable after the first neurofeedback session, barely present after the second, and completely gone after the third. By the end of the third neurofeedback session posttest, participants had ∼120 min of exposure to the rapid reaction time task, and their mean pressing accuracy had increased from 81.4% (localizer session, pretest) to 92.6% (neurofeedback session, posttest). Therefore, it is likely that overtraining on the rapid reaction time task led to decreased sensitivity to finger preference changes during later neurofeedback sessions.
To assess the neurofeedback manipulation, we measured motor confusion between finger pairs using the number of mis-presses within each finger pair. A mis-press was defined as a press that was supposed to be with one finger of the pair but was instead performed with the other finger of the pair. We hypothesized that the neurofeedback manipulation would result in increased confusion (mis-presses) within index-middle and ring-little finger combinations, and decreased confusion within the middle-ring finger pair, as was found by Kolasinski et al. (6). Despite the changes in finger preference found earlier, there were no significant differences between the change in mis-presses in any finger pairs [Tukey’s honestly significant difference (HSD), all sessions, all finger pair contrasts P > 0.1; Fig. 7B].
Tactile Discrimination Did Not Change Differentially between Finger Pairs
We also assessed somatosensory ability before and after the entire experiment using the just noticeable difference (JND) in tactile discrimination within each finger pair. There were no significant differences between the tactile performance changes in any finger pairs (Tukey’s HSD, all finger pair contrasts P > 0.1). These results show that although motor repetition may have caused changes in motor finger behavior, there is no evidence that increasing pattern overlap through neurofeedback was able to increase confusion between fingers in the sensory domains.
DISCUSSION
In this study, both neural activity and behavior associated with the ring finger were manipulated, whereas the same was not possible for the middle finger. Participants implicitly learned to shift the neural representation of the ring finger toward that of the little finger, but on average were unable to shift the representation of the middle finger toward the index finger. Moreover, the baseline variability of participants’ individual finger representations was predictive of their ability to bias each finger’s representation. After the first neurofeedback session, participants also showed increased preference for the ring finger and decreased preference for the little finger, although no such preference shifts occurred with the middle or index fingers. These results imply greater plasticity for the ring finger than the middle finger, which is supported by the natural variability of these fingers’ neural representations.
Our results showing differential neural plasticity are complementary to those found by Kolasinski et al. (6). Although they physically glued the index and middle fingers together and hypothesized plasticity changes within those fingers, they only found evidence of shifts in the representation of the ring finger, with the strongest behavioral changes also found within the ring and little fingers. These results also agree with animal experiments in which electrophysiologically recorded signals were easier to control when they aligned with more naturally varying neural activity patterns, rather than along an unnatural manifold (29). In decoded neurofeedback, there is also within-subject evidence that certain patterns are easier to regulate than others. In a crossover design, Cortese et al. (30) found that although participants could learn to both up- and downregulate patterns related to confidence judgments, upregulation of these patterns was easier and had a stronger effect on subsequent learning of downregulation compared with the reversed condition. The relationship discovered between neural pattern variability and learning ability supports our previous findings in which pattern localizer data was able to predict participants’ abilities in a subsequent decoded neurofeedback task (19). By examining baseline variability of patterns, future studies may be able to determine the feasibility of controlling specific neural patterns.
Although decoded neurofeedback has targeted behaviors such as visual perception, confidence ratings, and fear responses, this work represents the first decoded neurofeedback study to target motor behaviors (for comprehensive overview, see Ref. 31). Furthermore, it is the first fMRI neurofeedback study to target individual fingers, rather than modulating overall sensorimotor cortex activity (32, 33). These results also have implications for rehabilitation of individual finger movements after stroke. The strength and coordination of individual finger movements are impaired after stroke, recovering significantly through the first year poststroke but often leaving chronic deficits (34). Recent work has suggested that this recovery and subsequent chronic impairment can be tracked through MVPA of individual finger representations (35), although the to-date published results of the fMRI data from this study report only average region-of-interest analyses (36). Although advanced robotic techniques exist for improving finger individuation (37, 38), neurofeedback enables targeted neuroplasticity, and with it, the potential ability to avoid maladaptive neuroplasticity. This approach has a long history of use in altering spinal reflexes in rats (39), nonhuman primates (40), and humans (41). More recently, it been used with success to improve gait in spinal cord injury patients without requiring conscious effort (42), showing promise for rehabilitation. As our technique shows the ability for participants to gain control over the neural activity patterns related to individual fingers, we suggest that we may be able to improve individual finger coordination after stroke using decoded neurofeedback.
The choice of control groups in fMRI neurofeedback is controversial, with no clear consensus on the optimal control condition (43). For example, if a control group receives neurofeedback from a brain region that is unrelated to the task, the level of reward can be heavily skewed between groups. Alternatively, if control participants are given “yoked sham” feedback corresponding to the exact feedback signals viewed by experimental participants, participants lose agency over the feedback signal and can become disengaged in the task. Given these difficulties, instead of using a between-subject control, we chose to use a within-subject control to maximize statistical power (44, 45). Previous decoded neurofeedback experiments have successfully used such within-subject control, for example, comparing multiple visual orientations (9) or colors (13) within each participant. Similarly, our within-subject control, in this case comparing the middle and ring finger, allows us to achieve greater statistical power with a limited number of participants relative to multi-group neurofeedback studies. One limitation of our within-subject control was that only the middle and ring fingers were pressed during neurofeedback sessions, instead of all four fingers as was done in the localizer session. This meant that our post hoc analysis was limited to applying the pre-existing decoder to the neurofeedback session, rather than calculating pattern distances between fingers, for example, using representational similarity analysis (RSA; Ref. 46). Although RSA could be performed by using data across sessions, the results would be skewed as within-session data would always appear more similar than across-session data. Future studies along these lines should aim to collect data from all possible fingers in at least one run of the neurofeedback session to examine these effects.
A major limitation of this study is that there was no observable change in the targeted finger behavior that could be conclusively linked to the neurofeedback manipulation. This is not entirely unexpected. For example, in the study of spinal reflexes, healthy participants can learn to self-regulate these reflexes using operant conditioning, which is similar to neurofeedback. Despite learning to alter their spinal reflexes, these healthy participants do not show any change in behavior such as gait patterns (41). However, patient groups undergoing the same spinal reflex training demonstrate significant gait improvements (42). This paradoxical difference between healthy participants and patients is explained through a negotiated equilibrium model: when plasticity that produces a new behavior also improves a previous behavior, overall plasticity is increased, leading to greater changes in patient populations (47). Therefore, it may be necessary to perform decoded neurofeedback training on patients with significant deficits to observe any changes in finger behavior.
Another limitation in our behavioral motor assessment was that participants became overtrained on the rapid reaction time task, reaching ceiling performance by the final neurofeedback session. Therefore, any effects of neurofeedback on finger preference or motor confusion in later sessions are likely overpowered by the practice effect on the rapid reaction time task. We chose to perform frequent assessments to catch any potential transient effects of neurofeedback training, especially considering the rapid plasticity potential of the motor cortex. However, this had the downside of participants becoming well-practiced at the assessment task. Future studies should aim to minimize this practice effect, for example, by performing fewer assessments or staircasing the difficulty of the task to force more errors as participants improve at the task. The rapid plasticity of sensorimotor cortex may also have led to a performance ceiling in the neurofeedback task in the first session, as we did not see differences between neurofeedback performance across sessions. This limitation, namely, that we did not see across-session learning of neurofeedback performance, has also been observed in other studies (e.g., Ref. 13) and may be expected in decoded neurofeedback.
Furthermore, a major behavioral confound in our study (pressing of only two of the fingers during neurofeedback training) may have masked any small effects of decoded neurofeedback. An ideal experiment would involve training of all four fingers; however, this would involve learning twice as many patterns as the present experiment. Not only would this double the duration of the experiment, but it is also unknown whether learning four different neurofeedback targets is feasible within a single experimental group. As this is the first study of its kind, we compromised between too little variation (e.g., only training one finger and losing the within-subject control of two different fingers) and presenting participants with too difficult of a task (the confusion associated with training all four fingers). This allowed us to have a within-subject control (training of 2 different fingers) without presenting a potentially impossible task to participants. As this study shows promise toward controlling multiple patterns, future work should attempt to include all possible fingers. This could be achieved either through careful consideration of the training schedule to ensure participants can learn to control the multitude of patterns, or by including multiple groups, with different finger pairs selected for each group. We also found no changes in sensory perception, which may be due to the neurofeedback task being primarily a motor task. Future studies could include vibrotactile stimulation during the neurofeedback phase to enhance changes in sensory behavior.
Overall, the first attempt at decoded neurofeedback in sensorimotor cortex demonstrated both promise and limitations for the technique. Our results show that certain patterns and behaviors may be difficult to manipulate in certain fingers or in individuals with decreased sensorimotor cortex plasticity. However, our results were able to support existing literature and provide a unique window into the plasticity and representations of individual finger movements in humans. Furthermore, we present a unique technique that may be able to enhance rehabilitation of individual fingers without requiring physical intervention at the limb or knowledge of the manipulation from patients.
GRANTS
This work was support by a Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation Medical Research Grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.O., J.L-P., and J.S. conceived and designed research; E.O. performed experiments; E.O. analyzed data; E.O., J.L-P., and J.S. interpreted results of experiments; E.O. prepared figures; E.O. drafted manuscript; E.O., J.L-P., and J.S. edited and revised manuscript; E.O., J.L-P., and J.S. approved final version of manuscript.
REFERENCES
- 1.Diedrichsen J, Wiestler T, Krakauer JW. Two distinct ipsilateral cortical representations for individuated finger movements. Cereb Cortex 23: 1362–1377, 2013. doi: 10.1093/cercor/bhs120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ejaz N, Hamada M, Diedrichsen J. Hand use predicts the structure of representations in sensorimotor cortex. Nat Neurosci 18: 1034–1040, 2015. doi: 10.1038/nn.4038. [DOI] [PubMed] [Google Scholar]
- 3.Kolasinski J, Makin TR, Jbabdi S, Clare S, Stagg CJ, Johansen-Berg H. Investigating the stability of fine-grain digit somatotopy in individual human participants. J Neurosci 36: 1113–1127, 2016. doi: 10.1523/JNEUROSCI.1742-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci 21: 149–186, 1998. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 5.Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science 310: 810–815, 2005. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- 6.Kolasinski J, Makin TR, Logan JP, Jbabdi S, Clare S, Stagg CJ, Johansen-Berg H. Perceptually relevant remapping of human somatotopy in 24 hours. eLife 5: e17280, 2016. doi: 10.7554/eLife.17280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul 5: 175–195, 2012. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh V, Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Nat Rev Neurosci 1: 73–80, 2000. doi: 10.1038/35036239. [DOI] [PubMed] [Google Scholar]
- 9.Shibata K, Watanabe T, Sasaki Y, Kawato M. Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science 334: 1413–1415, 2011. doi: 10.1126/science.1212003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koizumi A, Amano K, Cortese A, Shibata K, Yoshida W, Seymour B, Kawato M, Lau H. Fear reduction without fear through reinforcement of neural activity that bypasses conscious exposure. Nat Hum Behav 1: 0006, 2017. doi: 10.1038/s41562-016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taschereau-Dumouchel V, Cortese A, Chiba T, Knotts J, Kawato M, Lau H. Towards an unconscious neural reinforcement intervention for common fears. Proc Natl Acad Sci USA 115: 3470–3475, 2018. doi: 10.1073/pnas.1721572115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortese A, Amano K, Koizumi A, Kawato M, Lau H. Multivoxel neurofeedback selectively modulates confidence without changing perceptual performance. Nat Commun 7: 13669, 2016. doi: 10.1038/ncomms13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amano K, Shibata K, Kawato M, Sasaki Y, Watanabe T. Learning to associate orientation with color in early visual areas by associative decoded fMRI neurofeedback. Curr Biol 26: 1861–1866, 2016. doi: 10.1016/j.cub.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pleger B, Foerster AF, Ragert P, Dinse HR, Schwenkreis P, Malin JP, Nicolas V, Tegenthoff M. Functional imaging of perceptual learning in human primary and secondary somatosensory cortex. Neuron 40: 643–653, 2003. doi: 10.1016/s0896-6273(03)00677-9. [DOI] [PubMed] [Google Scholar]
- 15.Krishnapuram B, Carin L, Figueiredo MA, Hartemink AJ. Sparse multinomial logistic regression: Fast algorithms and generalization bounds. IEEE Trans Pattern Anal Mach Intell 27: 957–968, 2005. doi: 10.1109/TPAMI.2005.127. [DOI] [PubMed] [Google Scholar]
- 16.Hanke M, Halchenko YO, Sederberg PB, Hanson SJ, Haxby JV, Pollmann S. PyMVPA: a python toolbox for multivariate pattern analysis of fMRI data. Neuroinformatics 7: 37–53, 2009. doi: 10.1007/s12021-008-9041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale A, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9: 179–194, 1999. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 18.Fischl B, Rajendran N, Busa E, Augustinack J, Hinds O, Yeo BT, Mohlberg H, Amunts K, Zilles K. Cortical folding patterns and predicting cytoarchitecture. Cereb Cortex 18: 1973–1980, 2008. doi: 10.1093/cercor/bhm225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oblak EF, Sulzer JS, Lewis-Peacock JA. A simulation-based approach to improve decoded neurofeedback performance. Neuroimage 195: 300–310, 2019. doi: 10.1016/j.neuroimage.2019.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita O, Sato M, Yoshioka T, Tong F, Kamitani Y. Sparse estimation automatically selects voxels relevant for the decoding of fMRI activity patterns. Neuroimage 42: 1414–1429, 2008. doi: 10.1016/j.neuroimage.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopel R, Sladky R, Laub P, Koush Y, Robineau F, Hutton C, Weiskopf N, Vuilleumier P, Van De Ville D, Scharnowski F. No time for drifting: comparing performance and applicability of signal detrending algorithms for real-time fMRI. Neuroimage 191: 421–429, 2019. doi: 10.1016/j.neuroimage.2019.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao JS, Huth AG, Lescroart MD, Gallant JL. Pycortex: an interactive surface visualizer for fMRI. Front Neuroinform 9: 23, 2015. doi: 10.3389/fninf.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDuff SGR, Frankel HC, Norman KA. Multivoxel pattern analysis reveals increased memory targeting and reduced use of retrieved details during single-agenda source monitoring. J Neurosci 29: 508–516, 2009. doi: 10.1523/JNEUROSCI.3587-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinheiro J, Bates D, DebRoy S, Sarkar D; R Core Team. nlme: linear and nonlinear mixed effects models (Online). https://CRAN.R-project.org/package=nlme [2021 Feb 4].
- 25.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J 50: 346–363, 2008. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 26.Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron 72: 443–454, 2011. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage 67: 283–297, 2013. doi: 10.1016/j.neuroimage.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steele CJ, Penhune VB. Specific increases within global decreases: a functional magnetic resonance imaging investigation of five days of motor sequence learning. J Neurosci 30: 8332–8341, 2010. doi: 10.1523/JNEUROSCI.5569-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadtler PT, Quick KM, Golub MD, Chase SM, Ryu SI, Tyler-Kabara EC, Byron MY, Batista AP. Neural constraints on learning. Nature 512: 423–426, 2014. doi: 10.1038/nature13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortese A, Amano K, Koizumi A, Lau H, Kawato M. Decoded fMRI neurofeedback can induce bidirectional confidence changes within single participants. Neuroimage 149: 323–337, 2017. doi: 10.1016/j.neuroimage.2017.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31..Watanabe T, Sasaki Y, Shibata K, Kawato M. Advances in fMRI real-time neurofeedback. Trends Cogn Sci 21: 997–1010, 2017. doi: 10.1016/j.tics.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blefari ML, Sulzer J, Hepp-Reymond M-C, Kollias S, Gassert R. Improvement in precision grip force control with self-modulation of primary motor cortex during motor imagery. Front Behav Neurosci 9: 18, 2015. doi: 10.3389/fnbeh.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bray S, Shimojo S, O’Doherty JP. Direct instrumental conditioning of neural activity using functional magnetic resonance imaging-derived reward feedback. J Neurosci 27: 7498–7507, 2007. doi: 10.1523/JNEUROSCI.2118-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Ejaz N, Hertler B, Branscheidt M, Widmer M, Faria AV, Harran MD, Cortes JC, Kim N, Celnik PA, Kitago T, Luft AR, Krakauer JW, Diedrichsen J. Separable systems for recovery of finger strength and control after stroke. J Neurophysiol 118: 1151–1163, 2017. doi: 10.1152/jn.00123.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Ejaz N, Hertler B, Branscheidt M, Widmer M, Kim N, Harran M, Cortes JC, Faria AV, Celnik PA, Kitago T, Luft A, Krakauer JW, Diedrichsen J. Changes in neural activity patterns during recovery of fine finger control after stroke . 45th Annual Meeting of Society for Neuroscience (SfN), Chicago, IL, Oct 17–21, 2015. [Google Scholar]
- 36.Ejaz N, Xu J, Branscheidt M, Hertler B, Schambra H, Widmer M, Faria AV, Harran MD, Cortes JC, Kim N, Celnik PA, Kitago T, Luft AR, Krakauer JW, Diedrichsen J. Evidence for a subcortical origin of mirror movements after stroke: a longitudinal study. Brain 141: 837–847, 2018. doi: 10.1093/brain/awx384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taheri H, Rowe JB, Gardner D, Chan V, Gray K, Bower C, Reinkensmeyer DJ, Wolbrecht ET. Design and preliminary evaluation of the FINGER rehabilitation robot: controlling challenge and quantifying finger individuation during musical computer game play. J Neuroeng Rehabil 11: 10, 2014. doi: 10.1186/1743-0003-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thielbar KO, Lord TJ, Fischer HC, Lazzaro EC, Barth KC, Stoykov ME, Triandafilou KM, Kamper DG. Training finger individuation with a mechatronic-virtual reality system leads to improved fine motor control post-stroke. J Neuroeng Rehabil 11: 171, 2014. doi: 10.1186/1743-0003-11-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen XY, Wolpaw JR. Operant conditioning of H-reflex in freely moving rats. J Neurophysiol 73: 411–415, 1995. doi: 10.1152/jn.1995.73.1.411. [DOI] [PubMed] [Google Scholar]
- 40.Wolpaw JR. Operant conditioning of primate spinal reflexes: the H-reflex. J Neurophysiol 57: 443–459, 1987. doi: 10.1152/jn.1987.57.2.443. [DOI] [PubMed] [Google Scholar]
- 41..Thompson AK, Chen XY, Wolpaw JR. Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci 29: 5784–5792, 2009. doi: 10.1523/JNEUROSCI.4326-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson AK, Pomerantz FR, Wolpaw JR. Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci 33: 2365–2375, 2013. doi: 10.1523/JNEUROSCI.3968-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorger B, Scharnowski F, Linden DE, Hampson M, Young KD. Control freaks: towards optimal selection of control conditions for fMRI neurofeedback studies. Neuroimage 186: 256–265, 2019. doi: 10.1016/j.neuroimage.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cremers HR, Wager TD, Yarkoni T. The relation between statistical power and inference in fMRI. PLoS One 12: e0184923, 2017. doi: 10.1371/journal.pone.0184923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, Nichols TE, Poline J-B, Vul E, Yarkoni T. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 18: 115–126, 2017. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kriegeskorte N, Mur M, Bandettini PA. Representational similarity analysis—connecting the branches of systems neuroscience. Front Syst Neurosci 2: 4, 2008. doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolpaw JR. The negotiated equilibrium model of spinal cord function. J Physiol 596: 3469–3491, 2018. doi: 10.1113/JP275532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173, 1996. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]