Abstract
Timely reaction to perturbation is important in activities of daily living. Modulation of reaction time to and early recovery from perturbation via vibrotactile noise was investigated. It was hypothesized that subthreshold vibrotactile noise applied to the upper extremity can accelerate a person’s reaction to and recovery from handle perturbation. This intervention was developed based on previous studies in which the earliest cue available for people to detect handle perturbation was somatosensation detecting changes in pressure on the hand whose sensitivity can improve with subthreshold vibrotactile noise. To induce a handle perturbation, a sudden upward load was applied to the handle that subjects were lightly grasping. Eighteen healthy subjects were instructed to stop the handle from moving up when they detected the perturbation. The muscle reaction time and handle stabilization time with and without vibrotactile noise were determined. The results showed that the muscle reaction time and handle stabilization time significantly decreased by 3 ms (p=0.018) and 6 ms (p=0.023), respectively, when vibrotactile noise was applied to the upper extremity, regardless of where the noise was applied among four different locations within the upper extremity (p>0.05). In conclusion, the application of subthreshold vibrotactile noise enhanced persons’ muscle reaction time to handle perturbation and led to early recovery from the perturbation. Use of the vibrotactile noise may increase a person’s ability to rapidly respond to perturbation of a grasped object in potentially dangerous situations such as holding onto ladder rungs from elevation or manipulating knives.
Keywords: Handle Perturbation, Muscle Reaction Time, Stochastic Resonance
I. Introduction
OBJECT manipulation with a stable hand grip is one of the most frequent activities of daily living. Once the stability of hand grip is threatened by perturbation of the grasped objects, timely response to tighten the grip against the perturbation is critical. This is especially true when a person’s safety is at risk such as grasping a ladder rung to climb in workplace or manipulating a knife in the kitchen. Therefore, investigating factors that modulate grip reaction time to object perturbation may have potentials to enhance a person’s safety and efficiency during handle grasping or object manipulation.
Reaction time to object perturbation can be modulated by several factors. The reaction time for children to increase pinch grip force in response to a sudden increase in the grasped object’s weight gradually becomes shorter with increasing age, reaching adult values at 6-10 years [1]. People can react faster to object perturbation during pinch grip when the object’s weight suddenly increases compared to when the object’s weight suddenly decreases [2]. Pinch grip response to object perturbation is faster when the arm is moving the object up, compared to when the arm is stationary [2]. Anticipation of object perturbation also shortens reaction time to object perturbation [1], [3].
Another potential candidate to shorten reaction time to object perturbation is enhancement of somatosensation via subthreshold vibrotactile noise. Somatosensation detecting pressure on the hand has been shown to play an important role in people’s ability to react to handle perturbation [4]. A body of literature in neuroscience shows that somatosensation, specifically tactile sensation, can be improved by using subthreshold vibrotactile noise [5]–[7]. Enhancement of somatosensation via vibrotactile noise may be accomplished by a phenomenon called stochastic resonance where nonzero subthreshold noise maximizes a response of a nonlinear system to a weak input signal [5]–[7]. In general, stochastic resonance indicates that the flow of information through a system (i.e., the coherence between the input signal and the system response) is maximized when the input signal is accompanied with a noise whose intensity is set to an optimal value. Stochastic resonance [8] specifically involves three components: a threshold, a signal, and noise. A signal that does not reach the sensory threshold is not detectable (Fig. 1a). When small random noise is added to the system, the noise-added signal may become detectable (Fig. 1b), increasing tactile acuity.
Fig. 1.
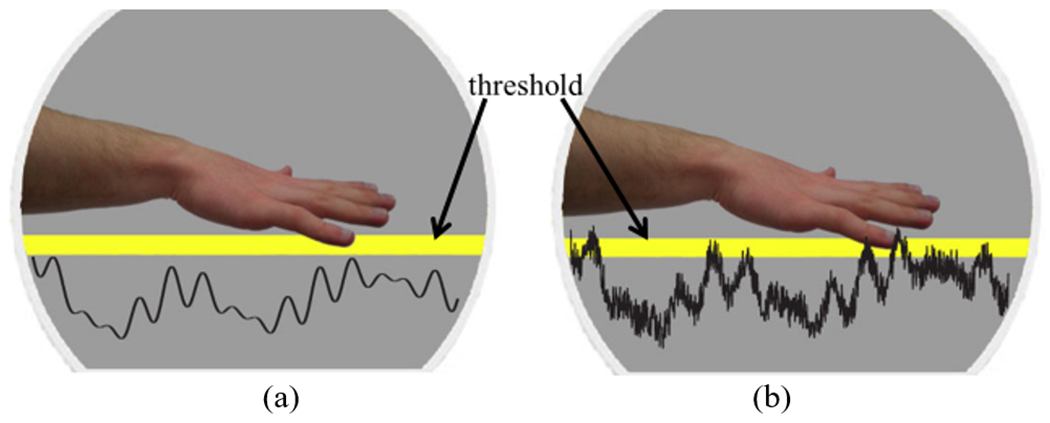
Illustration of threshold stochastic resonance. In threshold stochastic resonance [8], there are three necessary components: a threshold, a signal, and noise. (a): A weak signal below the sensory threshold is not detectable. (b): If an optimal level of random noise is added to the signal, the noise-added signal may be detectable, increasing a person’s tactile acuity.
The concept of stochastic resonance has been applied to enhance somatosensation in various populations. Subthreshold vibrotactile noise applied to the feet has been shown to enhance vibrotactile sensitivity of the feet in both young and old adults [9]. In addition, the ability of detecting vibration in the hand for older adults, stroke patients, and diabetic patients significantly improved with the application of subthreshold noise to the hand [6]. Furthermore, detection of monofilament touch at the fingertip improved when subthreshold vibrotactile noise was applied at the wrist and dorsal hand among stroke patients [10]. It has been postulated that such enhancement of somatosensation is achieved via the low-level noise raising the resting potential of mechanoreceptors or sensory neurons within the somatosensory pathways, thus making the mechanoreceptors or sensory neurons more readily depolarized with a weak input signal [6], [10]–[12]. However, it is not known whether the application of vibrotactile noise to the hand to enhance somatosensation of the hand can also elicit faster muscular reaction time to and thus faster recovery from perturbation of a grasped object.
The objective of this study was to investigate the effect of vibrotactile noise on grip reaction time and stabilization time in response to handle perturbation. It was hypothesized that people react faster to handle perturbation and stabilize the handle faster with vibrotactile noise applied to the upper extremity than without vibrotactile noise. The functional significance of this study is that understanding of the role of vibrotactile noise on early biomechanical reaction to perturbation may be used to help enhance people’s safety and efficiency during object manipulation.
II. Methods
A. Subjects
Eighteen right-handed healthy young adults (14 males and 4 females, mean ± standard deviation of age = 25 ± 6 years) with no history of neuromuscular or orthopedic conditions participated in the study. All subjects signed a consent form and followed a protocol approved by the Institutional Review Board.
B. Procedure
An experiment was conducted to determine subjects’ muscle reaction time and handle stabilization time in response to perturbation of a grasped handle with vs. without subthreshold vibrotactile noise. Our previous study simulating perturbation of a grasped ladder rung showed that somatosensation detecting pressure on the hand was the sensory cue available for people to react to the perturbation [4], providing a suitable testing paradigm for the present study. Thus, the same experimental setup was used: Initially, subjects were instructed to grasp an overhead circular handle (radius 24.7 mm) lightly with the hand without extra effort (Fig. 2a). While the subject was lightly grasping the handle, a latch holding a weight was opened at a random time, and the weight pulled the handle suddenly upward (Fig. 2b). Subjects were instructed to stop the handle from moving upward as soon as they noticed the handle perturbation.
Fig. 2.
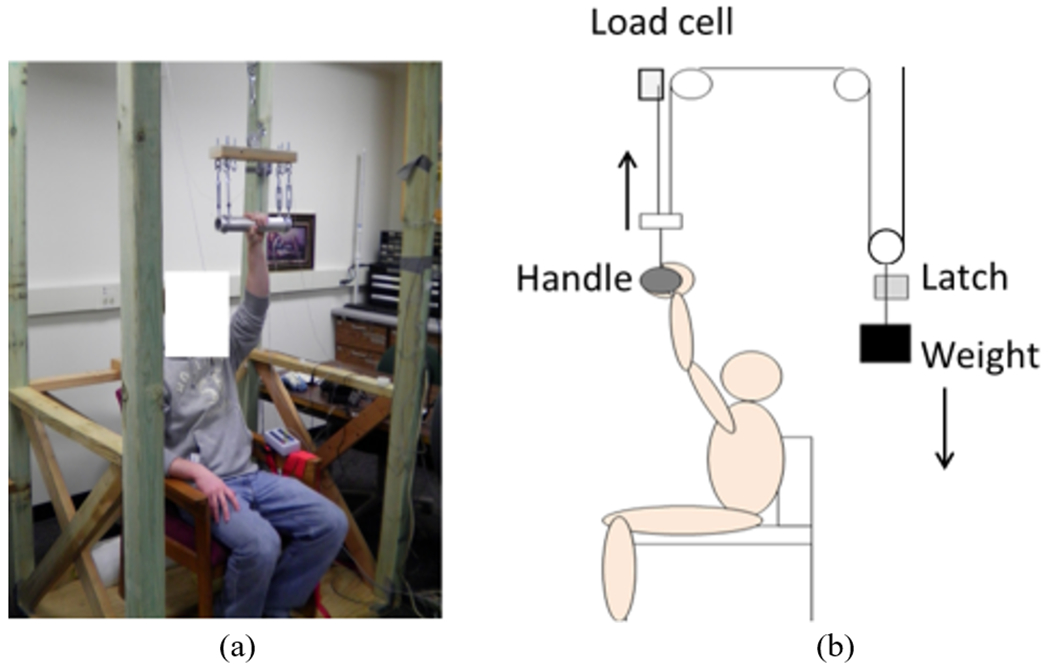
Experimental setup. (a): Subjects were instructed to sit in a chair with the hand resting on the handle. (b): At a random time, the latch was released, dropping the weight (20% of each subject’s hand strength), which applied sudden upward force to the handle. The subjects were instructed to stabilize the handle as soon as they noticed the perturbation.
The latch and weights were located behind the subjects and the subjects wore headphones during the experiment to ensure that they did not receive any visual or auditory cues from the latch or weight movement related to perturbation. The subjects were also instructed to look forward at the eye level instead of toward the overhead handle. Six practice trials were given prior to testing for each subject so that subjects became familiar with the protocol. The weight was equivalent to 20% of the subject’s hand strength to hold onto the handle [13] as in the previous study [4]. The nondominant hand was used because people usually hold onto the ladder rung with the nondominant hand while performing another task (e.g., painting, spraying water, nailing, hanging decorations) with the dominant hand, and injuries due to falls from ladders are critical issues in workplaces and home environment [14]. Also, the nondominant hand is typically used to stabilize a food object that is being cut by the dominant hand using a knife [15].
Vibrotactile noise was applied to upper extremity skin using EAI C-3 Tactor (Engineering Acoustics, Inc. Casselberry, FL) (Fig. 3). The vibrotactile device generated white random noise, low-pass filtered at 500 Hz because this range covers the sensitive frequencies for all mechanoreceptors [16]. The noise intensity was adjusted to be 50% of the sensory threshold of each noise application location, since noise at 50% of the sensory threshold was shown to enhance tactile acuity the most in healthy adults [9]. The sensory threshold was determined as the lowest noise intensity at which subjects were able to feel the vibration. During the noise off trials, the vibrotactile device was still attached to the skin and was turned off. Since subthreshold vibrotactile noise was used, subjects could not tell when the vibration was on.
Fig. 3.

The vibrotactile device (C-3 Tactor). The contactor is the vibrating part in contact with the skin.
To examine the effect of the location to which the vibrotactile noise is applied in the upper extremity, the vibrotactile device was applied to four different upper extremity sites in separate trials. The four sites were: the tip of the middle finger; the thenar eminence; the volar forearm, approximately a third of the forearm length from the wrist; and the dorsal forearm, approximately a third of the forearm length from the wrist (Fig. 4). For the fingertip location, the perturbation signal (i.e., increase of force/pressure at the fingertip) added with vibrotactile noise is to be sensed by the middle fingertip, whereas for the other three sites the perturbation signal and noise are received by different body parts and may be integrated in the central nervous system [10]. The former condition (application of noise directly to the perturbation signal) has been typically used to demonstrate the benefit of stochastic resonance in the past [6], [9], [11], [12]. The latter conditions (application of noise remotely from the signal) were tested because, if effective, they may be more applicable as an assistive device to benefit hand somatosensation during activities of daily living without having the noise-generating device physically interfering with finger-object manipulation.
Fig. 4.
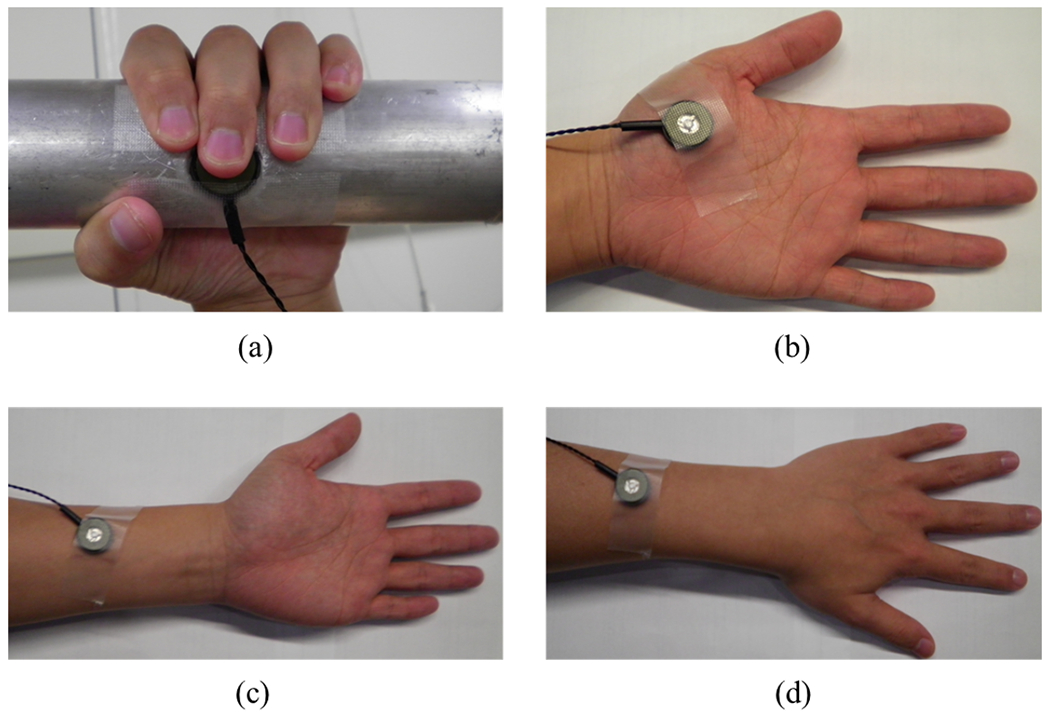
The four skin sites for noise were the middle fingertip (a), thenar eminence (b), volar forearm (c), and dorsal forearm (d). For the fingertip location, the vibrotactile device was embedded into the handle (a).
To quantify muscle reaction time, muscle activity was recorded using surface electromyography (EMG) (Bortec Biomedical Ltd., Calgary, Alberta, Canada). Muscle activities for the following muscles were recorded: flexor digitorum superficialis (FDS), flexor carpi ulnaris (FCU), extensor digitorum communis (EDC), biceps, triceps, deltoid, pectoralis major (Pmajor) and latissimus dorsi (Ldorsi) muscles (Fig. 5). These muscles were examined, since they contribute to moving and stabilizing the upper limb [17], [18]. Bipolar surface electrodes were placed on the skin overlying the muscle belly. The muscles were located using the anatomical landmarks following literature [19] and confirmed through palpation and visual observation of the EMG signals using an oscilloscope while subjects performed muscle-specific movements [20]. The EMG data were sampled at 1 kHz.
Fig. 5.
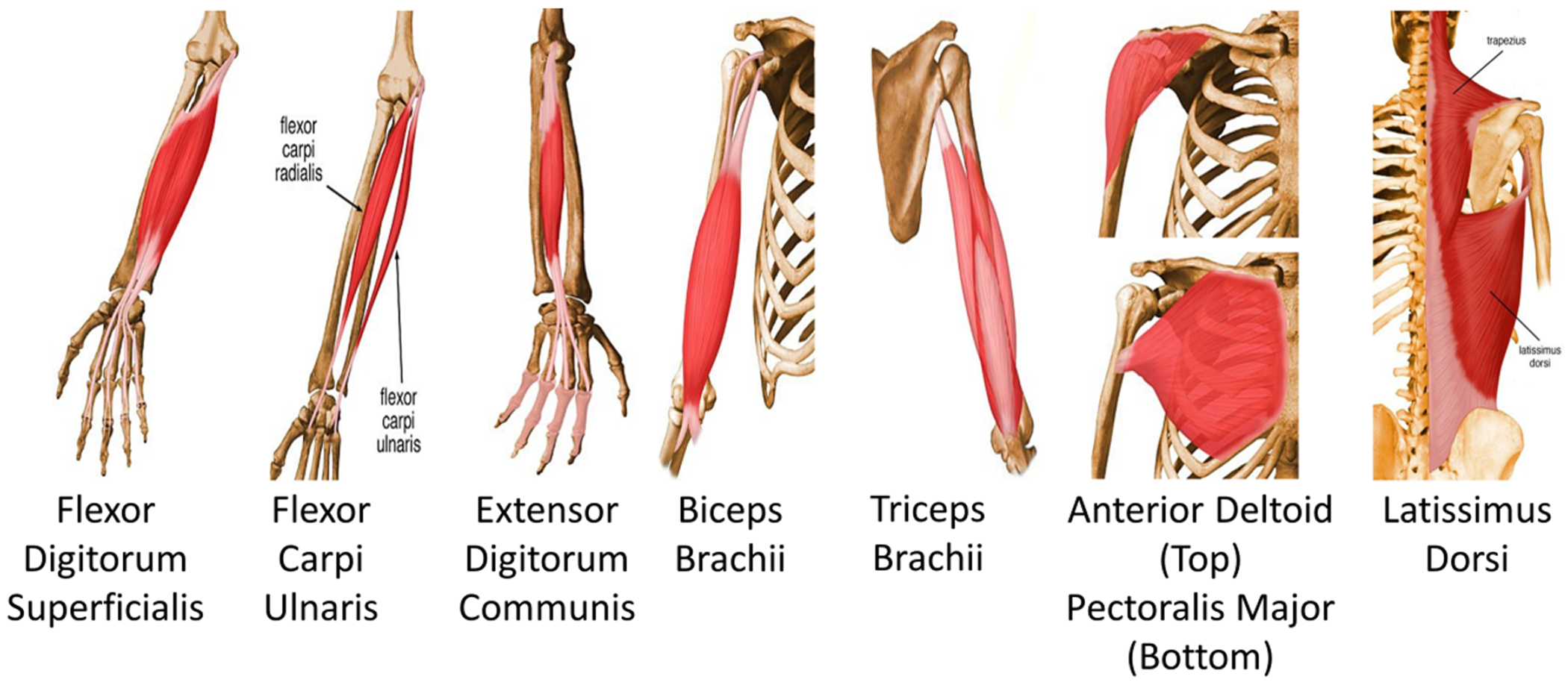
To determine muscular responses to the handle perturbation, EMG for eight upper limb muscles were recorded. These muscles contribute to moving and stabilizing the upper limb [37].
To quantify handle stabilization time, the position of the handle was recorded by using a marker-based motion capture system (Optotrak 3D Investigator Motion Capture System, NDI, Waterloo, ON, Canada). The marker data were sampled at 100 Hz. To mark the onset of perturbation, force on the handle was recorded by using a load cell (SM-1000, Interface Inc., Scottsdale, AZ). The force data were sampled at 1 kHz. The handle force data were applied with the low pass Butterworth filter at the cutoff frequency of 30 Hz to remove line noise and noises from the load cell and amplifier. Testing of the four noise locations was randomized. Within each location, six repetitions (3 with noise and 3 without noise) were randomized. Data for three repetitions for each noise condition were averaged for statistical analysis. Breaks of approximately 2 min were given between consecutive trials to prevent muscle fatigue.
C. Analysis
The EMG data were used to determine the muscle reaction time. For each of the 8 muscles, an envelope of the EMG signal was obtained by using 10 ms moving root mean square (RMS) technique. Each muscle’s reaction time was determined as the time interval between the onset of handle perturbation and when the RMS EMG exceeded mean + 3 standard deviations (SD) of the baseline RMS EMG (Fig. 6a). The baseline was defined as the 100 ms time period immediately before the onset of handle perturbation (Fig. 6a). The onset of handle perturbation was defined as when the handle force started increasing at more than 50 N/s for at least 20 ms, as in the previous study (Fig. 6a) [4]. The earliest reaction time among the 8 muscles was used for analysis.
Fig. 6.
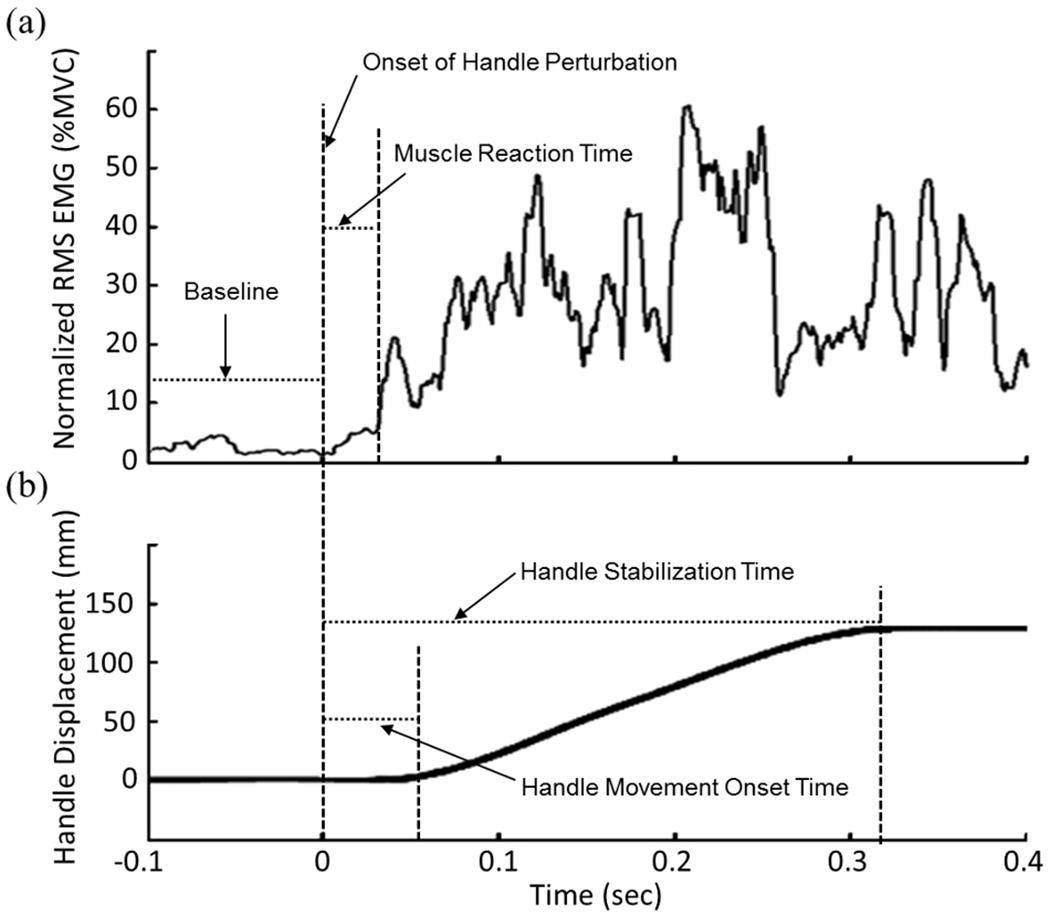
(a) Time course of normalized RMS EMG from the FDS muscle. (b) Time course of handle displacement. Muscle reaction time, handle movement onset time, handle stabilization time, onset of handle perturbation and baseline are shown from a sample data.
Handle movement onset time was defined as when the slope of displacement-time curve exceeded 100 mm/s for more than 50 ms (Fig. 6b) [4]. Handle stabilization time was defined as when the slope of handle displacement-time curve became less than 50 mm/s for more than 100 ms after the handle began moving (Fig. 6b) [4].
Statistical analyses were performed to determine whether muscle reaction time, handle stabilization time and handle movement onset time were significantly affected by the within-subject variables of noise application (on vs. off), noise location (fingertip, thenar area, volar forearm, and dorsal forearm) and their interaction (SPSS Inc, Chicago, IL; v17). Two-way repeated-measures ANOVA was used for each response. In addition, to examine time sequence of the events (i.e., muscle reaction time, handle movement onset time and handle stabilization time) after handle perturbation, one-way repeated-measures ANOVA was performed. A significance level of 0.05 was used.
III. Results
The application of vibrotactile noise significantly shortened the muscle reaction time (p=0.018) and shortened the handle stabilization time (p=0.023), regardless of where the noise was applied (Fig. 7, 8). The muscle reaction time following the handle perturbation decreased from 36 ± 2 ms (mean ± standard error) without vibrotactile noise to 33 ± 2 ms with vibrotactile noise. The muscle reaction times decreased with noise when noise was applied to the fingertip (from 36 ± 2 ms without noise to 31 ± 2 ms with noise), thenar area (from 37 ± 3 ms to 33 ± 3 ms), dorsal forearm (from 40 ± 2 ms to 36 ± 3 ms), and volar forearm (from 33 ± 3 ms to 32 ± 3 ms with noise) (Fig. 7). Repeated measures ANOVA showed that the effect of noise on the muscle reaction time was statistically significant (p=0.018), while there was neither significant effect of noise location nor interaction between noise application and noise location on the muscle reaction time (p>0.05).
Fig. 7.
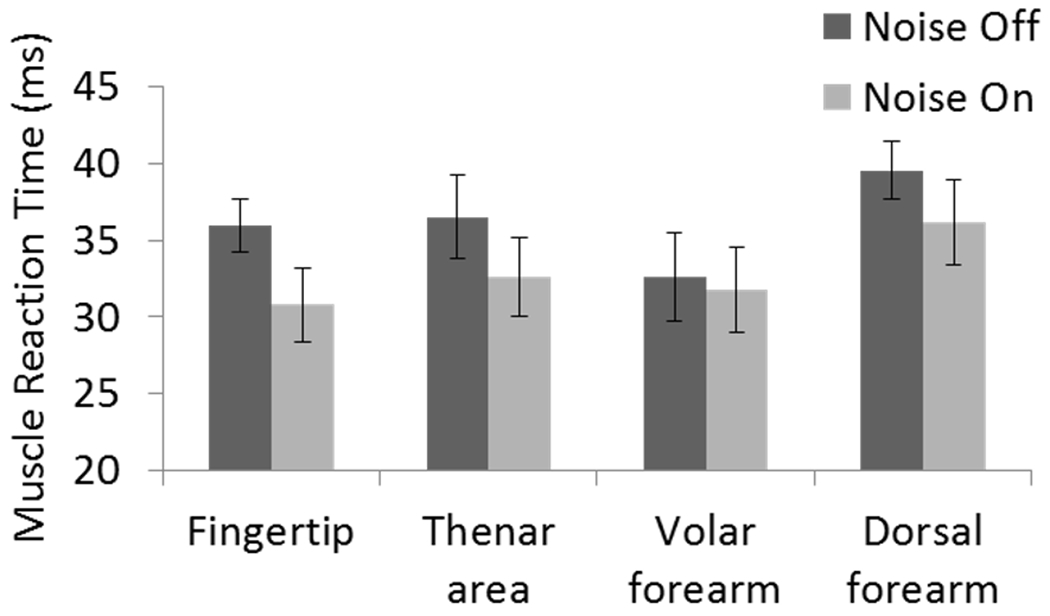
The muscle reaction time with vs. without subthreshold vibrotactile noise. Vibrotactile noise shortened muscle reaction time (p=0.018). No significant effect of noise location and no significant interaction of noise×location were found. Error bar represents ± one standard error.
Fig. 8.
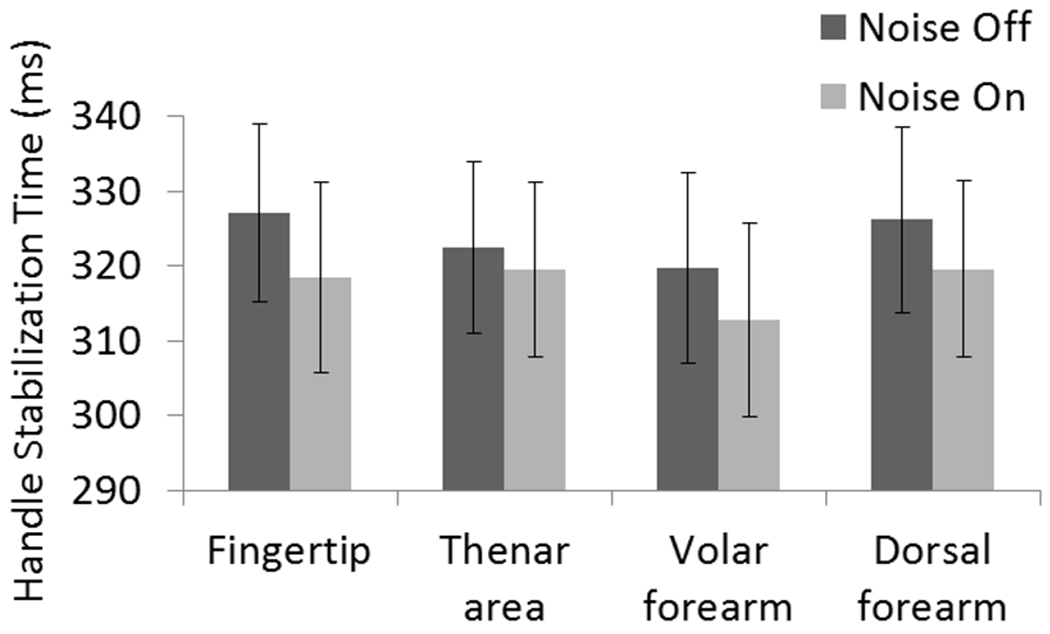
The handle stabilization time with vs. without subthreshold vibrotactile noise. Vibrotactile noise shortened handle stabilization time (p=0.023). No significant effect of noise location and no significant interaction of noise×location were found. Error bar represents ± one standard error.
Handle stabilization time decreased from 324 ± 12 ms without vibrotactile noise to 318 ± 12 ms with vibrotactile noise. The handle stabilization times decreased with noise when noise was applied to the fingertip (from 327 ± 12 ms without noise to 318 ± 13 ms with noise), thenar area (from 322 ± 11 ms to 319 ± 12 ms with noise), dorsal forearm (from 326 ± 12 ms to 320 ± 12 ms), and volar forearm (from 320 ± 13 ms to 313 ± 13 ms), (Fig. 8). Repeated measures ANOVA showed that the effect of noise on the handle stabilization time was statistically significant (p=0.023), while there was neither significant effect of noise location nor interaction between noise application and noise location on handle stabilization time (p>0.05). Handle movement onset time was not affected by noise application (52 ± 2 ms without noise vs. 52 ± 1 ms with noise), noise location or their interaction (p > 0.05).
The time course of the perturbation event was such that muscle reaction occurred, on average, at 35 ± 2 ms after the handle force increase (perturbation), followed by the handle movement onset at 52 ± 2 ms and by the handle stabilization at 321 ± 11 ms after the handle perturbation (pooled for noise application and noise location conditions). In agreement with the finding from the previous study [4], the three events occurred at different times in the above mentioned order (p<0.001 for event in ANOVA). This result verifies that somatosensation detecting force/pressure increase on the hand was the cue for subjects to react to the perturbation, not the sensation related to movement and muscle length changes, as in the previous study [4].
IV. Discussion
Subthreshold vibrotactile noise applied to the upper extremity significantly shortened the muscle reaction time to handle perturbation as well as the handle stabilization time. The muscle reaction time and handle stabilization time decreased regardless of where the noise was applied among the middle fingertip, thenar area, volar forearm, and dorsal forearm although the most reduction occurred when the noise was applied to the fingertip. To our best knowledge, this study is the first study to show that a mechanical subthreshold vibrotactile noise applied at the upper extremity shortened muscle reaction time to handle perturbation and subsequently accelerated recovery from the perturbation. This study is also the first to show that not only noise applied directly to the body part sensing the perturbation (finger in this study) but also noise applied to other body parts not involved in sensing the perturbation (the thenar area and forearm) had an effect facilitating motor behavior in response to perturbation. The finding in this study suggests a potential for development of a device generating low-level subthreshold vibrotactile noise to accelerate people’s upper limb reaction to and recovery from perturbation as a means to enhance a person’s safety and efficiency during handle grasping and object manipulation.
Noise shortened the muscle reaction time, probably by accelerating sensory detection of the perturbation. As in Hur et al. [4], we believe that the sensory cue available to detect the perturbation in the present study was somatosensation detecting force/pressure change on the hand. Specifically, proprioception related to limb movement or muscle length change does not appear to have resulted in the muscular reaction to the perturbation, because handle movement was not observed before the onset of muscle activation for both noise on and off conditions. Instead, a reflex mechanism involving somatosensory detection of the force/pressure increase at the hand is more likely to have caused muscle reaction, since the muscle reaction time of 35 ms after the handle force increase is similar with the latency of upper extremity cutaneous reflex of 33-53 ms [21–24] as well as the latency of proprioceptive reflex for upper extremity soft tissue stimulation of approximately 40 ms [25].
Subthreshold vibrotactile noise may have improved detection of the force/pressure increase on the hand via stochastic resonance. Noise applied directly to a tactile signal has been shown to improve persons’ detection of the signal [6], [7]. These previous studies have postulated that noise may increase the resting potential and thus excitability of mechanoreceptors at the periphery [6], [7].
Besides the direct addition of noise to a signal, recent studies show that persons’ detection of an external sensory signal can also improve with noise applied remotely from the signal, through integration of noise and signal within the central nervous system [26]. For instance, Enders et al. [10] reported that touch sensation of the fingertips for stroke survivors enhanced when subthreshold vibrotactile noise was applied at the wrist or dorsum of the hand. Manjarrez et al. [27] showed that remote noise applied to the third hindpaw pad of cats increased sensory evoked potentials at both the spinal cord and somatosensory cortex in response to tactile stimuli at the central pad of the hindpaw, suggesting increased excitability of sensory neurons at the spinal as well as supraspinal levels through interneuronal connections between different parts of the body [28]. It is unlikely that the remote vibrotactile noise at the thenar area or the forearm influenced mechanoreceptors at the fingertip, since the vibration substantially decays as it travels through the skins [29], [30]. In summary, the present study showed that application of noise at the fingertip as well as other parts of the upper extremity (thenar area, volar forearm, dorsal forearm) resulted in faster muscular reaction to handle perturbation and early recovery from handle perturbation, possibly via increased excitability of mechanoreceptors and/or sensory neurons resulting in the input signal (the force/pressure change on the hand) crossing over the sensory threshold earlier, thereby accelerating muscular response. However, the exact mechanisms will have to be investigated in the future.
The finding in this study is limited to the specific subthreshold vibrotactile noise used in this study. For instance, tendon vibrations which are typically suprathreshold at a fixed frequency between 50 and 250 Hz are known to suppress sensation or sensation-induced muscle activity [31–33]. Transcutaneous electrical nerve stimulation which are also typically suprathreshold at a fixed frequency between 10 and 250 Hz has been shown to reduce excitability for the agonist muscle, increase excitability for the antagonist muscles [34], [35], inhibit primary somatosensory cortex activity [36], and decrease sensory threshold [35] depending on stimulation frequency. It should also be noted that this study investigated only the immediate effect of subthreshold vibrotactile noise on reaction to handle perturbation. Future studies may examine the effects of long-term exposure to subthreshold vibrotactile noise on persons’ response to handle perturbation. Further, the benefit of subthreshold vibrotactile noise for populations with reduced sensation such as elderly population or stroke survivors may be worth investigating.
V. Conclusion
This study demonstrated that use of subthreshold vibrotactile noise at the upper extremity shortened the muscle reaction time to handle perturbation and contributed to early recovery from handle perturbation, possibly via enhanced somatosensation of the hand. Shortened muscle reaction time to perturbation and early recovery was achieved for all four noise locations on the upper extremity. This study demonstrates a potential for use of vibrotactile noise to increase a person’s ability to respond to object perturbation in a timely manner, increasing the likelihood that the person may recover from perturbation in dangerous activities of daily living or occupation including manipulating knives and holding onto scaffolds from elevation.
Acknowledgment
This study was funded by the NIOSH UIC ERC T42 OH008672 and American Heart Association Midwest Affiliate Postdoctoral Fellowship 12POST12090039.
References
- [1].Eliasson A, Forssberg H, Ikuta K, Apel I, Westling G, and Johansson R, “Development of human precision grip. V. anticipatory and triggered grip actions during sudden loading,” Exp Brain Res, vol. 106, no. 3, pp. 425–433, 1995. [DOI] [PubMed] [Google Scholar]
- [2].Mrotek L, Hart B, Schot P, and Fennigkoh L, “Grip responses to object load perturbations are stimulus and phase sensitive,” Exp Brain Res, vol. 155, no. 4, pp. 413–420, 2004. [DOI] [PubMed] [Google Scholar]
- [3].Kourtis D, Kwok H, Roach N, Wing A, and Praamstra P, “Maintaining Grip: Anticipatory and Reactive EEG Responses to Load Perturbations,” J Neurophysiol2, vol. 99, no. 2, pp. 545–553, 2008. [DOI] [PubMed] [Google Scholar]
- [4].Hur P, Motawar B, and Seo NJ, “Muscular responses to handle perturbation with different glove condition,” J EMG Kines, p. doi: 10.1016/j.jelekin.2013.10.004, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wiesenfeld K and Moss F, “Stochastic resonance and the benefits of noise: from ice ages to crayfish and SQUIDs,” Nature, vol. 373, no. 6509, pp. 33–36, 1995. [DOI] [PubMed] [Google Scholar]
- [6].Liu W, Lipsitz LA, Montero-Odasso M, Bean J, Kerrigan DC, and Collins JJ, “Noise-enhanced vibrotactile sensitivity in older adults, patients with stroke, and patients with diabetic neuropathy,” Arch. Phys. Med. Rehabil, vol. 83, no. 2, pp. 171–176, 2002. [DOI] [PubMed] [Google Scholar]
- [7].Collins J, Imhoff T, and Grigg P, “Noise-mediated enhancements and decrements in human tactile sensation,” Phys. Rev. E, 1997. [Google Scholar]
- [8].Gingl Z, Kiss LB, and Moss F, “Non-dynamical stochastic resonance: Theory and experiments with white and arbitrarily coloured noise,” EPL (Europhysics Lett., vol. 29, p. 191, 1995. [Google Scholar]
- [9].Wells C, Ward LM, Chua R, and Inglis JT, “Touch noise increases vibrotactile sensitivity in old and young,” Psychol. Sci, vol. 16, no. 4, pp. 313–320, 2005. [DOI] [PubMed] [Google Scholar]
- [10].Enders L, Hur P, Johnson M, and Seo NJ, “Remote vibrotactile noise improves light touch sensation in stroke survivors’ fingertips via stochastic resonance,” J NeuroEng Rehab, pp. doi: 10.1186/1743-0003-10-105, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Richardson KA, Imhoff TT, Grigg P, and Collins JJ, “Using electrical noise to enhance the ability of humans to detect subthreshold mechanical cutaneous stimuli,” Chaos, vol. 8, no. 3, pp. 599–603, 1998. [DOI] [PubMed] [Google Scholar]
- [12].Dhruv NT, Niemi JB, Harry JD, Lipsitz LA, and Collins JJ, “Enhancing tactile sensation in older adults with electrical noise stimulation,” Neuroreport, vol. 13, no. 5, p. 597, 2002. [DOI] [PubMed] [Google Scholar]
- [13].Hur P, Motawar B, and Seo NJ, “Hand breakaway strength model—Effects of glove use and handle shapes on a person’s hand strength to hold onto handles to prevent fall from elevation,” J. Biomech, vol. 45, no. 6, pp. 958–964, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].CPSC, “Ladder Safety 101, Retrieved Jul, 2013. (http://www.cpsc.gov/onsafety/2011/12/ladder-safety-101/).” 2011.
- [15].Sainburg R and Duff S, “Does motor lateralization have implications for stroke rehabilitation?,” J. Rehabil. Res. Dev, vol. 43, no. 3, pp. 311–322, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Johansson RS and Flanagan JR, “Coding and use of tactile signals from the fingertips in object manipulation tasks,” Nat. Rev. Neurosci, vol. 10, no. 5, pp. 345–359, 2009. [DOI] [PubMed] [Google Scholar]
- [17].Di Domizio J and Keir PJ, “Forearm posture and grip effects during push and pull tasks,” Ergonomics, vol. 53, no. 3, pp. 336–343, 2010. [DOI] [PubMed] [Google Scholar]
- [18].Richardson ML, Muscle Atlas of the Extremities. Bare Bones Books, 2011. [Google Scholar]
- [19].V Basmajian J, Biofeedback: Principles and practice for clinicians, 3rd ed. Williams & Wilkins, 1989. [Google Scholar]
- [20].Smith L, Weiss E, and Lehmkuhl D, Brunnstrom’s Clinical Kinesiology, 5th ed. Philadelphia, PA: F.A. Davis Company, 1996. [Google Scholar]
- [21].Garnett R and Stephens JA, “The reflex responses of single motor units in human first dorsal interosseous muscle following cutaneous afferent stimulation,” J. Physiol, vol. 303, pp. 351–364, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jenner JR and Stephens JA, “Cutaneous reflex responses and their central nervous pathways studied in man,” J. Physiol, vol. 333, no. 1, pp. 405–419, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Corden D, Lippold O, Buchanan K, and Norrington C, “Long-latency component of the stretch reflex in human muscle is not mediated by intramuscular stretch receptors,” J. Neurophysiol, vol. 84, no. 1, pp. 184–188, 2000. [DOI] [PubMed] [Google Scholar]
- [24].Zehr EP, Collins DF, and Chua R, “Human interlimb reflexes evoked by electrical stimulation of cutaneous nerves innervating the hand and foot.,” Exp. brain Res, vol. 140, no. 4, pp. 495–504, Oct. 2001. [DOI] [PubMed] [Google Scholar]
- [25].Hagert E, Persson JKE, Werner M, and Ljung B-O, “Evidence of wrist proprioceptive reflexes elicited after stimulation of the scapholunate interosseous ligament.,” J. Hand Surg. Am, vol. 34, no. 4, pp. 642–51, Apr. 2009. [DOI] [PubMed] [Google Scholar]
- [26].Hidaka I, Nozaki D, and Yamamoto Y, “Functional stochastic resonance in the human brain: noise induced sensitization of baroreflex system,” Phys. Rev. Lett, vol. 85, no. 17, pp. 3740–3743, 2000. [DOI] [PubMed] [Google Scholar]
- [27].Manjarrez E, Rojas-Piloni G, and Mendez I, “Stochastic resonance within the somatosensory system: effects of noise on evoked field potentials elicited by tactile stimuli,” J Neurosci, vol. 23, no. 6, pp. 1997–2001, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Merzenich M, Kaas J, Wall J, and Sur M, “Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys,” Neuroscience, 1983. [DOI] [PubMed] [Google Scholar]
- [29].Manfredi LR, Baker AT, Elias DO, Dammann JF, Zielinski MC, Polashock VS, and Bensmaia SJ, “The effect of surface wave propagation on neural responses to vibration in primate glabrous skin.,” PLoS One, vol. 7, no. 2, p. e31203, Jan. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kurita Y, Shinohara M, and Ueda J, “Wearable Sensorimotor Enhancer for Fingertip Based on Stochastic Resonance Effect,” IEEE Trans. Human-Machine Syst, vol. 43, no. 3, pp. 333–337, May 2013. [Google Scholar]
- [31].Conrad MO, Scheidt RA, and Schmit BD, “Effects of wrist tendon vibration on arm tracking in people poststroke.,” J. Neurophysiol, vol. 106, no. 3, pp. 1480–8, Sep. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Conrad MO, Scheidt RA, and Schmit BD, “Effects of wrist tendon vibration on targeted upper-arm movements in poststroke hemiparesis,” Neurorehabil. Neural Repair, vol. 25, no. 1, p. 61, 2011. [DOI] [PubMed] [Google Scholar]
- [33].Smith AC, Mummidisetty CK, Rymer WZ, and Knikou M, “Effects of mechanical vibration of the foot sole and ankle tendons on cutaneomuscular responses in man.,” Neurosci. Lett., vol. 545, pp. 123–6, Jun. 2013. [DOI] [PubMed] [Google Scholar]
- [34].Tinazzi M, Zarattini S, Valeriani M, Romito S, Farina S, Moretto G, Smania N, Fiaschi A, and Abbruzzese G, “Long-lasting modulation of human motor cortex following prolonged transcutaneous electrical nerve stimulation (TENS) of forearm muscles: evidence of reciprocal inhibition and facilitation.,” Exp. Brain Res, vol. 161, no. 4, pp. 457–64, Mar. 2005. [DOI] [PubMed] [Google Scholar]
- [35].Mima T, Oga T, Rothwell J, Satow T, Yamamoto J, Toma K, Fukuyama H, Shibasaki H, and Nagamine T, “Short-term high-frequency transcutaneous electrical nerve stimulation decreases human motor cortex excitability.,” Neurosci. Lett, vol. 355, no. 1–2, pp. 85–8, Jan. 2004. [DOI] [PubMed] [Google Scholar]
- [36].Macefield G and Burke D, “Long-lasting depression of central synaptic transmission following prolonged high-frequency stimulation of cutaneous afferents: a mechanism for post-vibratory hypaesthesia.,” Electroencephalogr. Clin. Neurophysiol, vol. 78, no. 2, pp. 150–8, Feb. 1991. [DOI] [PubMed] [Google Scholar]
- [37].Richardson M, Muslce atlas of the extremities., 1st ed. Seattle: Bare Bone Books, 1997, p. Chapter 1. [Google Scholar]


