Fifteen percent of injured workers were not able to return to work or sustain work. Mood was not related to work ability, whereas vestibular, cognitive, sleep, visual and hearing symptoms, which did not lessen or abate, were related. Many exposed workers suffered persistent ill effects warranting long term specialized care.
Keywords: cluster; cognitive symptoms; directed pulsed radio frequency energy; mild traumatic brain injury; neurological symptoms; return to work; US diplomat; work accommodations; work-related injury, Havana, Cuba; Havana Syndrome
Objective:
To determine factors associated with return to work in US diplomats injured during a work assignment in Cuba.
Methods:
In this case series work ability was determined at each visit. Questionnaires used included the Symptom Score Questionnaire, Beck Anxiety Inventory, Beck Depression Inventory, Quality-of-Life Inventory, and Patient Health Questionnaire.
Results:
Of the 45 employees referred to Occupational Medicine, the mean age was 42.5 years, 60% were men, 68% were never out of work, 22% were out of work for some period, and 15% remain out of work. Vestibular, cognitive, hearing, sleep, and visual symptoms, and a higher initial symptom score were significantly associated with work inability while psychiatric symptoms were not.
Conclusions:
This exposure resulted in prolonged illness with cognitive impairment and other clinical manifestations associated with work inability.
From July 2016 to 2018, US diplomats serving in Havana, Cuba reported exposures described as high-pitched, loud noises, buzzing and cicada like sounds, rumbling and experiences of head and/or ear pressure.1 The resulting symptoms were cognitive (difficulty remembering, mental fogginess, difficulty concentrating, slowed processing speed), behavioral/emotional (irritability, feeling more emotional), vestibular (balance, dizziness), visual (visual disturbance, light sensitivity, difficulty reading), and auditory (sound sensitivity, tinnitus, hearing reduction). Also reported were sleep impairment (drowsiness, decreased sleep duration, trouble falling asleep, increased fatigue), nausea, and headaches. Detailing of their symptom constellations and treatment rendered were previously described.1 They were referred to University of Pennsylvania Medical Center Occupational and Environmental Medicine (OEM) Division for evaluation and treatment. Specialty consultation with physical medicine and rehabilitation, neuro-optometry, neurology, neurosurgery, psychology, neuropsychology, audiology, and sleep medicine and referral to occupational, speech language and pathology, vision and vestibular therapies, cognitive rehabilitation, and psychological treatment, were managed.2
Comprehensive multidisciplinary evaluation described previously1 ranged from within normal limits to impairments in executive functioning and processing speed (demonstrated by neuropsychological testing) and impairments in static postural stability and dynamic balance, convergence insufficiency, abnormal smooth pursuits, and saccadic dysfunctions revealed through neuro-optometry evaluations.1,2 Illness incurred as a result of these exposures has resulted in various degrees of work disability. Whereas some returned to work full duty immediately after evaluation, others returned to modified work or were never able to return to work (RTW) in any capacity.
Return to work after an injury can be complex and challenging involving multiple parties: the injured worker, the OEM physician and other treating health care personnel, the employer/supervisor, and the claims adjuster.3 A main determinant of successful RTW is the ability of the worker to perform the essential functions of the position—fitting the job to the worker and not vice versa.4,5 If work accommodations are necessary, and the employer/supervisor willing to implement them, the worker may RTW to modified duty until full duty is attained. If modifications remain necessary long term, the employer/supervisor may allow permanent job modifications, or the worker will have to leave the position. Expectations of recovery and successful return-to-work have been found to be related to physical, psychological, and psychosocial factors,6 as well as the worker's education and age.7,8 Methods to predict future work success are not well described in the literature.9
US diplomats are generally motivated, skilled, and culturally adaptable professionals assigned to challenging positions requiring sound cognitive and physical abilities.10 The source of injury in this cluster of employees, previously described as an uncharacterized environmental exposure,1 is now determined by the National Academy of Medicine as due to directed pulsed radio frequency energy.11 It has often been referred to as “Havana Syndrome”. This study was designed to determine the factors associated with RTW among this cluster of US government personnel.
METHODS
Design
In this dynamic case series, US Diplomats referred for clinical evaluation, treatment, coordination of care and RTW determination, were followed prospectively from time of initial visit of the first patient in August 2017 to time of RTW full/modified duty on December 2019. A medical and work history, physical assessment, and evaluation of work status were completed at each visit by an OEM physician. Based on this assessment in collaboration with the multidisciplinary team,2 work status assessments of full duty, modified duty or out of work designations were determined. Various questionnaires were administered at each OEM visit. The outcomes of clinical evaluations, neuropsychological testing, cognitive rehabilitation, and occupational, vestibular and/or vision therapy, and various assessment measures were used to determine factors potentially related RTW.
Study Instruments
Given that the symptom complex with which these diplomats presented suggested potential mild traumatic brain injury,1 a 23-item Symptom Score Questionnaire (SSQ), derived from the symptom evaluation section of the 22 item Sports Concussion Assessment Tool (SCAT),12 was administered at each visit (Table 1). Five additional questions were added to the SSQ: 1) hearing difficulty in the form of tinnitus, 2) sleeping less than usual, 3) sleeping more than usual, 4) anxiety, and 5) numbness or tingling, the latter added given that a toxicologic source13,14 was being considered in the differential diagnosis at the time. Four questions were removed: 1) confusion as it was evaluated elsewhere, 2) neck pain, 3) pressure in head because there was no evidence of physical head injury, and 4) “don’t feel right.”
TABLE 1.
Symptom Score Questionnaire
| Symptoms Filled Out by Patients | Symptom Subset∗ |
| Balance problems | Vestibular |
| Dizziness | |
| Nausea | |
| Feeling slowed down | Cognitive |
| Feeling mentally foggy | |
| Difficulty remembering | |
| Difficulty concentrating | |
| Sensitivity to noise | Hearing |
| Hearing problems (Tinnitus)† | |
| Trouble falling asleep | Sleep |
| Sleeping more than usual† | |
| Drowsiness | |
| Fatigue Sleeping less than usual | |
| Sensitivity to light | Vision |
| Visual problems (eg, blurred, double vision) | |
| Irritability | Mood |
| Sadness | |
| Nervousness | |
| Feeling more emotional | |
| Anxiety† | |
| Headache | |
| Numbness or tingling† |
Subset scores were created to determine if some of the symptoms were localized to a specific area.
Symptoms added that were not included in the SCAT 3.
Respondents were asked to circle the number best representing how they felt, reflecting the past 24 hours, utilizing a Likert scale, where 0 = no symptoms to 6 = most severe symptoms. The combined score average for the symptoms reported by each individual was calculated to constitute an overall symptom score for each visit. Symptom sub-scores were created for vestibular symptoms (vestibular), cognitive function (cognitive), hearing problems (hearing), sleep challenges (sleep), visual challenges (vision), and mood changes (mood), to further examine specific aspects of the total score. A score ranging from 0 to 0.5 was considered no symptoms, 0.6 to 2.5 mild symptoms, 2.6 to 4.5 moderate symptoms, and 4.6 to 6 severe symptoms.
To better understand, the psychological factors associated with RTW for this group, standardized assessment measures were administered: The Beck Anxiety Inventory (BAI),15 the Beck Depression Inventory (BDI),16 the Quality-of-Life Inventory (QOLI),17 the Patient Health Questionnaire (PHQ-9),18 and the Patient-Reported Outcomes Measurement Information (PROMIS) Sleep Disturbance item bank.19 These measures were administered at clinical visits starting in June 2018. Patients were seen every 1 to 12 months depending on the severity of their symptoms.
Return to Work Modifications
Employees in the group never out of work (NOOW) were returned to work full duty with either no or a minimal work modification allowing scheduled breaks. The OOW/RTW group consisted of employees initially out of work initially who later returned full duty or modified duty. Modified duty assignments ranged from scheduled breaks only to breaks plus limited number worked days each week, reduced workload, extra time for tasks, reducing multi-tasking, quiet work environments, reduced workplace lighting, use of blue light filtering apps, use of tinted glasses, and arrangement of workstations to reduce visual scanning. Employees were returned to work in a supervised, measured, graduated way with the goal of sustainable full duty. The third group of employees were out of work (OOW).
Statistical Analysis
Summary statistics such as frequencies and percentages or means and range, were used to describe the demographics and symptom assessment. To assess differences in initial symptom severity between the 3 RTW groups, 1-way analysis of variance (ANOVA) was performed. To assess changes in overall symptom score and sub-scores between initial and last visit, a linear mixed model was performed where work status was a fixed factor and time a random factor. To adjust for multiple comparisons, for both the ANOVA and linear mixed models, post hoc pairwise Tukey–Kramer tests were performed. Lastly, Kaplan–Meier survival estimates were calculated to assess median time to RTW in the group that was initially out of work but then returned either full duty or modified duty (OOW/RTW). All analyses were performed using SAS statistical software (version 9.4, SAS Institute, Cary NC). This study was approved by the University of Pennsylvania's Perelman School of Medicine institutional review board with a waiver of HIPAA authorization.
RESULTS
Participant Characteristics
Of 45 employees referred to the OEM clinic between July 2016 and July 2018, the mean age was 42.5 years (range: 24–66) and 27 (60%) were men. Twenty-eight (62%) were NOOW, 10 (22%) were OOW/RTW), and 7 (16%) in the OOW group. There were no differences in RTW status by age or gender.
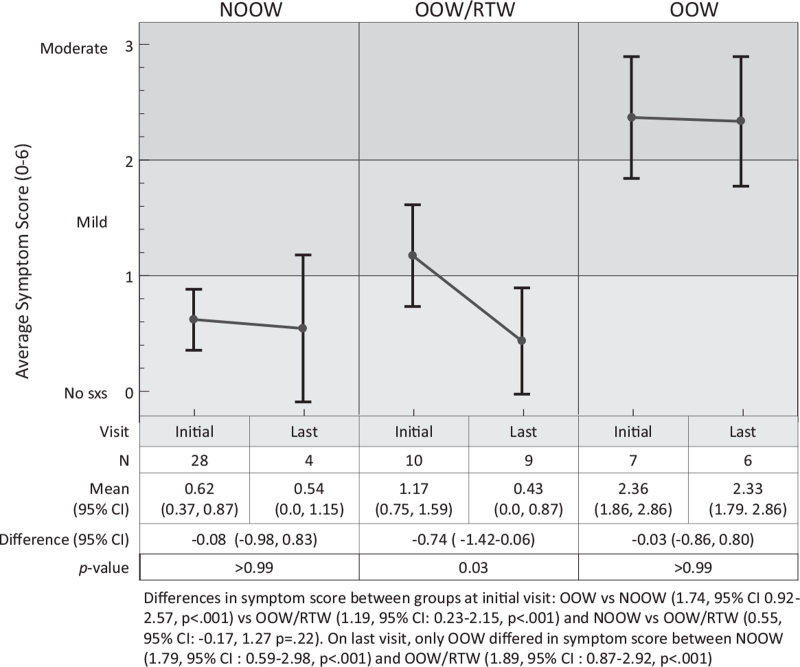
Initial overall mean symptom score differed significantly between the OOW group and both the OOW/RTW and NOOW groups (Fig. 1). The NOOW reported the lowest score on average on initial visit, which ranged from none to minimal severity (score = 0.62, 95%CI: 0.37, 0.87), followed by OOW/RTW group with mild severity (score = 1.17, 95% CI: 0.75, 1.59). The OOW group reported moderate symptom severity (score = 2.36 [95% CI: 1.86, 2.86]). Between the first and last visit, the only group in which symptoms significantly improved was the OOW/RTW group (mean difference = −0.74, 95% CI: −1.42 to 0.06, 63% decrease), P = .03).
FIGURE 1.
Changes in Symptom Score over time.
Initial vestibular, cognitive, hearing, sleep, and vision symptoms ranged from 1.57 to 3.57 (mild to moderate) with an average of 3.00 ± 0.61, for the OOW group, compared to 0.2 to 2.3 (none to mild) with an average of 1.29 ± 0.57 for the OOW/RTW group and 0.18 to 1.04 (none to mild) with an average of 0.62 ± 0.31 for the NOOW group. The OOW group reported more severe vestibular symptoms, that is, balance problems, and dizziness ((3.75, 3.43, moderate for both) compared to both the NOOW (score = 0.68, 0.21, none to minimal) and the OOW/RTW (score = 0.60, 1.0, minimal to mild) groups (differences ranged 2.43–3.21, P < .001, for all comparisons, Table 2). The initial dizziness symptom score reported for the OOW/RTW group was significantly different than for the NOOW group (difference = 0.79, 95% CI: 0.05–1.52, P = 0.03). The OOW and OOW/RTW groups reported mild nausea, on average (1.57, 0.6), the NOOW group reported none (0.18), and the OOW and NOOW groups differed significantly (difference: 1.39, 95% CI: 0.55, 2.23, P < 0.001, Table 2). Between initial and last visit, there was no significant change for any of the groups (Table 3), however, the change in score for the OOW/RTW group was 48% and 88% larger for the OOW and NOOW groups, respectively. This was consistent with the overall symptom score reported (Fig. 1).
TABLE 2.
Initial Symptom Scores Reported by Return-to-Work Status
| OOW n = 7 | OOW/RTW n = 10 | NOOW N = 28 | OOW vs. OOW/RTW | OOW vs. NOOW | OOW/RTW vs. NOOW | ||||
| Symptom | Mean ± SD | Mean ± SD | Mean ± SD | Δ (95% CI) | P | Δ (95% CI) | P | Δ (95% CI) | P |
| Vestibular sub-score | 2.86 ± 1.15 | 0.73 ± 0.84 | 0.36 ± 0.48 | 2.12 (1.29, 2.96) | <0.001 | 2.50 (1.78, 3.22) | <0.001 | 0.38 (−0.25, 1.00) | 0.32 |
| Balance problems | 3.57 ± 0.98 | 0.60 ± 1.07 | 0.68 ± 1.02 | 2.97 (1.74, 4.20) | <0.001 | 2.89 (1.84, 3.95) | <0.001 | −0.08 (−1.00, 0.84) | 0.98 |
| Dizziness | 3.43 ± 1.40 | 1.00 ± 1.05 | 0.21 ± 0.50 | 2.43 (1.44, 3.41) | <0.001 | 3.21 (2.37, 4.06) | <0.001 | 0.79 (0.05, 1.52) | 0.03 |
| Nausea | 1.57 ± 1.40 | 0.60 ± 1.07 | 0.18 ± 0.48 | 0.97 (−0.01, 1.95) | 0.053 | 1.39 (0.55, 2.23) | 0.0007 | 0.42 (−0.31, 1.16) | 0.35 |
| Cognitive sub-score | 3.32 ± 1.23 | 1.63 ± 1.44 | 0.93 ± 1.36 | 1.7 (0.07, 3.32) | 0.04 | 2.39 (1.00, 3.79) | <0.001 | 0.7 (−0.52, 1.91) | 0.35 |
| Feeling slowed down | 3.29 ± 0.95 | 1.20 ± 0.92 | 0.82 ± 1.44 | 2.09 (0.55, 3.62) | 0.005 | 2.46 (1.15, 3.78) | <0.001 | 0.38 (−0.77, 1.53) | 0.70 |
| Feeling mentally foggy | 3.29 ± 1.25 | 1.50 ± 1.72 | 0.96 ± 1.53 | 1.79 (−0.05, 3.62) | 0.06 | 2.32 (0.75, 3.90) | 0.003 | 0.54 (−0.84, 1.91) | 0.61 |
| Difficulty remembering | 3.29 ± 1.50 | 2.10 ± 2.08 | 1.04 ± 1.40 | 1.19 (−0.71, 3.08) | 0.29 | 2.25 (0.62, 3.88) | 0.005 | 1.06 (−0.35, 2.48) | 0.17 |
| Difficulty concentrating | 3.43 ± 1.81 | 1.70 ± 1.95 | 0.89 ± 1.34 | 1.73 (−0.14, 3.60) | 0.08 | 2.54 (0.93, 4.14) | 0.001 | 0.81 (−0.59, 2.21) | 0.35 |
| Hearing sub-score | 3.07 ± 1.37 | 1.15 ± 1.43 | 0.96 ± 1.39 | 1.92 (0.25, 3.59) | 0.02 | 2.11 (0.68, 3.54) | 0.003 | 0.19 (−1.06, 1.43) | 0.93 |
| Noise sensitivity | 2.86 ± 1.86 | 1.20 ± 1.75 | 0.93 ± 1.65 | 1.66 (−0.39, 3.70) | 0.13 | 1.93 (0.18, 3.68) | 0.03 | 0.27 (−1.26, 1.80) | 0.90 |
| Tinnitus | 3.29 ± 1.80 | 1.10 ± 1.85 | 1.00 ± 1.59 | 2.19 (0.18, 4.20) | 0.03 | 2.29 (0.56, 4.01) | 0.007 | 0.10 (−1.40, 1.60) | 0.99 |
| Sleep sub-score | 2.26 ± 1.51 | 1.26 ± 0.97 | 0.54 ± 0.64 | 1.00 (−0.07, 2.06) | 0.07 | 1.72 (0.81, 2.64) | <0.001 | 0.72 (−0.07, 1.52) | 0.08 |
| Trouble falling asleep | 2.71 ± 1.98 | 2.00 ± 1.83 | 0.39 ± 0.79 | 0.71 (−0.83, 2.26) | 0.51 | 2.32 (1.00, 3.65) | <0.001 | 1.61 (0.45, 2.76) | 0.004 |
| Fatigue | 3.29 ± 1.80 | 1.50 ± 1.78 | 0.82 ± 0.98 | 1.79 (0.20, 3.38) | 0.02 | 2.46 (1.10, 3.83) | <0.001 | 0.68 (−0.51, 1.87) | 0.36 |
| Sleeping more | 1.71 ± 2.36 | 0.20 ± 0.63 | 0.25 ± 0.59 | 1.51 (0.26, 2.77) | 0.02 | 1.46 (0.39, 2.54) | 0.006 | −0.05 (−0.99, 0.89) | 0.99 |
| Sleeping less | 1.14 ± 1.68 | 1.70 ± 1.83 | 0.93 ± 1.49 | −0.56 (−2.47, 1.35) | 0.76 | 0.21 (−1.42, 1.85) | 0.95 | 0.77 (−0.66, 2.20) | 0.40 |
| Drowsiness | 2.43 ± 1.90 | 0.90 ± 1.20 | 0.29 ± 0.66 | 1.53 (0.27, 2.79) | 0.01 | 2.14 (1.06, 3.22) | <0 001 | 0.61 (−0.33, 1.55) | 0.26 |
| Vision sub-score | 3.21 ± 1.70 | 1.40 ± 1.71 | 0.46 ± 0.79 | 1.81 (0.37, 3.25) | 0.01 | 2.75 (1.51, 3.99) | <0.001 | 0.94 (−0.14, 2.01) | 0.10 |
| Light sensitivity | 3.00 ± 1.83 | 1.30 ± 1.77 | 0.50 ± 0.96 | 1.70 (0.12, 3.28) | 0.03 | 2.50 (1.15, 3.85) | <0.001 | 0.80 (−0.38, 1.98) | 0.24 |
| Visual problems | 3.43 ± 2.57 | 1.50 ± 2.22 | 0.43 ± 0.92 | 1.93 (0.02, 3.84) | 0.05 | 3.00 (1.36, 4.64) | <0.001 | 1.07 (−0.36, 2.50) | 0.18 |
| Mood sub-score | 0.91 ± 0.60 | 0.92 ± 0.72 | 0.66 ± 0.73 | −0.01 (−0.86, 0.84) | >.99 | 0.26 (−0.47, 0.99) | 0.67 | 0.26 (−0.37, 0.90) | 0.58 |
| Irritability | 2.43 ± 1.72 | 1.30 ± 1.34 | 1.18 ± 1.54 | 1.13 (−0.70, 2.96) | 0.30 | 1.25 (−0.32, 2.82) | 0.14 | 0.12 (−1.25, 1.49) | 0.97 |
| Sadness | 0.29 ± 0.76 | 0.70 ± 0.82 | 0.43 ± 0.96 | −0.41 (−1.50, 0.67) | 0.63 | −0.14 (−1.07, 0.79) | 0.93 | 0.27 (−0.54, 1.08) | 0.70 |
| Nervousness | 1.14 ± 1.07 | 0.90 ± 0.99 | 0.79 ± 1.03 | 0.24 (−0.99, 1.47) | 0.88 | 0.36 (−0.70, 1.41) | 0.69 | 0.11 (−0.81, 1.04) | 0.95 |
| Feeling more emotional | 0.57 ± 1.13 | 1.40 ± 2.07 | 0.64 ± 1.22 | −0.83 (−2.55, 0.89) | 0.48 | −0.07 (−1.55, 1.40) | 0.99 | 0.76 (−0.53, 2.04) | 0.33 |
| Anxiety | 0.14 ± 0.38 | 0.30 ± 0.48 | 0.25 ± 0.44 | −0.16 (−0.69, 0.37) | 0.75 | −0.11 (−0.56, 0.35) | 0.84 | 0.05 (−0.35, 0.45) | 0.95 |
| Headache | 3.43 ± 0.98 | 2.30 ± 1.70 | 0.54 ± 0.88 | 1.13 (−0.21, 2.47) | 0.11 | 2.89 (1.74, 4.04) | <0.001 | 1.76 (0.76, 2.77) | <0.001 |
| Numbness/tingling | 0.71 ± 1.25 | 0.10 ± 0.32 | 0.21 ± 0.69 | 0.61 (−0.27, 1.50) | 0.22 | 0.50 (−0.26, 1.26) | 0.26 | −0.11 (−0.78, 0.55) | 0.91 |
Scoring: 0–0.5 = no symptoms; 0.6–2.5 = mild symptoms; 2.6–4.5 = moderate symptoms; 4.6–6 = severe symptoms.
TABLE 3.
Differences Between Initial and Last Visit by RTW Status∗
| Symptom Sub-score† | OOW (n = 7, 6) | OOW/RTW (n = 10, 9) | NOOW (n = 28, 4) | ||||||
| Initial | Last | Δ (95% CI) | Initial | Last | Δ (95% CI) | Initial | Last | Δ (95% CI) | |
| Vestibular | 2.86 | 2.52 | 0.34 (−0.57, 1.25) | 0.73 | 0.09 | 0.65 (−0.11, 1.4) | 0.36 | 0.28 | 0.08 (−0.86, 1.01) |
| Cognitive | 3.32 | 3.51 | −0.19 (−1.74, 1.35) | 1.63 | 0.72 | 0.91 (−0.36, 2.17) | 0.93 | 0.98 | −0.05 (−1.84, 1.74) |
| Hearing | 3.07 | 3.36 | −0.29 (−1.03, 0.45) | 1.15 | 0.93 | 0.22 (−0.39, 0.83) | 0.96 | 1.36 | −0.39 (−1.26, 0.48) |
| Sleep | 2.54 | 1.55 | 0.98 (−0.29, 2.25) | 1.15 | 0.5 | 0.65 (−0.4, 1.69) | 0.44 | 0.34 | 0.10 (−1.18, 1.38) |
| Vision | 3.21 | 3.5 | −0.29 (−2.02, 1.44) | 1.40 | 0.77 | 0.63 (−0.81, 2.06) | 0.46 | 0.26 | 0.21 (−1.42, 1.83) |
| Mood | 0.91 | 1.17 | −0.25 (−1.05, 0.55) | 0.92 | 0.13 | 0.79‡ (0.12, 1.45) | 0.66 | 0.16 | 0.5 (−0.18, 1.19) |
Means and confidence intervals derived from linear mixed models.
Symptom scales: 0 = no symptoms, 1–2 = mild symptoms, 3–4 = moderate symptoms, 5–6 = severe symptoms.
Differences are statistically significant using post hoc pairwise Tukey Kramer tests.
The OOW group reported moderate cognitive symptom scores initially (range 3.29–3.43), compared to the NOOW group (range 0.82–1.04, none to minimal, difference range 2.25–2.54, P < 0.005) or the OOW/RTW group (range 1.2–2.1, mild, difference 2.09, 95% CI:0.55, 3.62, P = 0.005 but only for feeling slowed down, Table 2). Both the NOOW and OOW/RTW groups reported mild symptoms of difficulty remembering, on average (range 1.04–2.10), whereas the OOW group reported moderate (3.29) symptoms. There was no significant difference in the initial score for difficulty remembering for the NOOW and OOW/RTW groups, similar to nausea. The OOW/RTW group improved between initial and last visit—mild (1.63) to minimal (0.72) symptom sub-score (difference = .91, 95% CI: −0.36 to 2.17, Table 3).
The NOOW group's headache symptom score was minimal (0.54) and significantly different from the OOW/RTW (2.30, mild) and OOW groups (3.43, moderate, P < 0.001 for both, Table 2). As with dizziness and difficulty remembering, no significant difference between the OOW/RTW and OOW groups was found.
The OOW group had moderate symptom scores for both noise sensitivity and tinnitus (2.86, 3.29), differing significantly from the NOOW group (0.93, 1.0, none to minimal, difference 1.93 and 2.29, P < 0.005 for all). The OOW/RTW and OOW group symptom scores were mild (1.1–1.2) and not significantly different from the OOW and NOOW groups. There was no significant difference in the hearing sub-score, between the initial and last visits for any group (Table 3). Two diplomats suffered hearing loss, and were in the OOW/RTW.
All sleep symptoms queried were ranked mild to moderate in the OOW group (range 1.71–3.29), compared to the NOOW with none to minimal symptoms (range 0.29–0.82, overall score difference = 1.0, 95% CI: −0.37 to 2.06), and the OOW/RTW groups (range 0.2–1.5, none to mild; difference = 1.72, 95% CI: 0.81–2.64). The overall sleep sub-score and question response regarding sleep differed significantly between the NOOW and the OOW group. There was no significant difference between the OOW and the OOW/RTW groups for sleeping less or more than usual and for drowsiness (Table 2). As with the hearing sub-score, there was no significant difference between the initial and last visits for any group (Table 3). Based on the Sleep Disturbance PROMIS item bank, those in the OOW group continued to report sleep disturbances.
The OOW group, which reported moderate symptom scores for light sensitivity and visual problems (range 3–3.34), was significantly different from the NOOW (range 0.43–0.5, none to minimal (difference = 2.75, 95% CI: 1.51–3.99) and OOW/RTW groups (range 1.3–1.5, mild (difference = 1.81, 95% CI: −0.37 to 2.75, P < 0.05 for both). The latter two groups did not differ significantly. As with the hearing sub-score, there was no significant difference between the initial and last visits for any group (Table 3).
There was no statistically significant difference on initial visit for psychiatric symptom scores related to mood—irritability, sadness, nervousness, anxiety, or feeling more emotional (Table 2). Eleven individuals responded to the BAI reporting minimal to moderate anxiety. Twelve individuals responded to the BDI reporting minimal to no depression. No responses were consistent with severe anxiety or severe depression. The NOOW group (N = 2) had significantly lower PHQ-9 scores than the OOW/RTW (N = 7) and OOW groups (N = 4); P < 0.01. Responses from all groups were consistent with mild depression on the PHQ-9, none with severe depression. The NOOW group reported scores reflecting an average QOLI (mean score = 1.8; N = 4), the OOW/RTW group with low QOLI (mean score = 1.1; N = 5) the OOW group with very low QOLI (mean score = 0.8; N = 6). None reported a high quality of life (QOLI). The groups were not statistically significantly different as regards psychiatric symptoms.
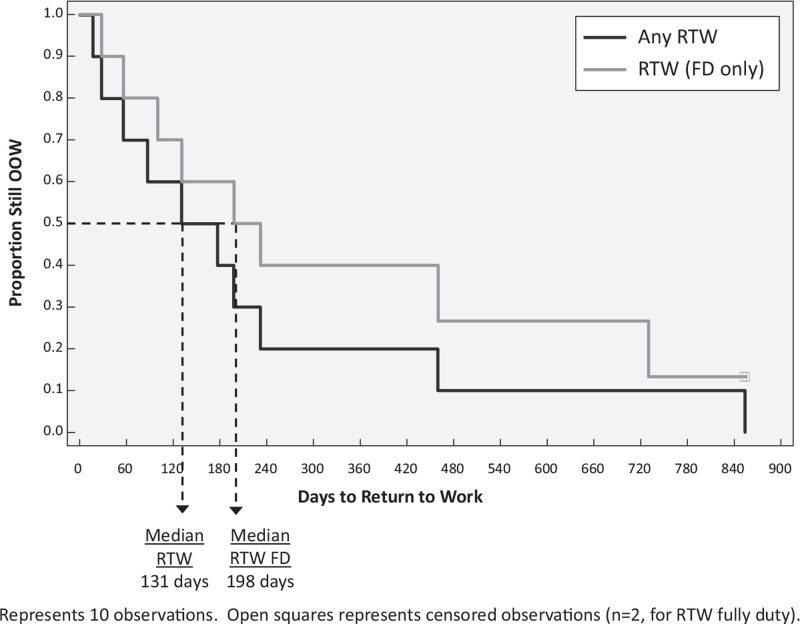
All 7 (15%) in the OOW group, remain out of work currently. Two were sufficiently injured that they were never able to RTW. Five were assigned modified duty but were unable to sustain the work tasks so they were taken out of work. All within the OOW/RTW group are currently working. Two are on modified duty and 8 full duty, albeit not at their premorbid level. The median time for the OOW/RTW group to return modified or full duty was 131 days. For those who returned full duty it was 198 days (Fig. 2).
FIGURE 2.
Time to Return to Work for employees in OOW/RTW Group (n = 10).
DISCUSSION
This longitudinal epidemiological assessment of factors associated with RTW in this cluster of American diplomats injured in Havana, Cuba allows insight into the more consequential symptoms likely to prevent workers from returning to full time employment. The work-related injury and illness suffered “arose out of or in the course of employment.”6 While 84% of workers have returned to work, most have not done so at their premorbid level of functioning. Workers rendered unable to return to their livelihood, now suffer a permanent disability.
Employees in the OOW group reported the highest initial SSQ score, which did not change significantly by the last visit, suggesting that a high initial symptom score is associated with work inability. Conversely, symptom scores for the NOOW group were the lowest, none to minimal, did not change significantly and most had RTW and not given a follow-up visit. Only the OOW/RTW group, initially removed from work until they were well enough to be reintegrated into the workforce, reported significant reductions on their SSQ, which decreased from mild, to none or minimal symptoms by the last visit.
The OOW group reported significantly more severe vestibular, cognitive, hearing, sleep, and visual symptoms than the OOW/RTW or NOOW groups. Cognitive symptoms improved significantly, on average, in the individuals in the OOW/RTW group. Interestingly, the OOW group reported no improvement of cognition. They remain out of work with limited prospects of returning to employment, some medically retired. This suggests that cognitive impairment is associated with work inability, similar to findings found with mild traumatic brain injury.20
The OOW/RTW and the NOOW groups had similar symptom scores on initial visit except for dizziness, trouble falling asleep, and headache, which were significantly more pronounced in the OOW/RTW group, implying that dizziness, trouble falling asleep, and headache differentiated the OOW/RTW from the others. The dizziness and difficulty sleeping may have affected the OOW/RTW group such that they were unable to RTW without some rehabilitation, suggesting that although the OOW/RTW and NOOW groups differed significantly from the OOW group, they were also quite dissimilar from each other. The OOW/RTW and OOW groups reported similar nausea, difficulty remembering, noise sensitivity, and trouble falling asleep. Physical symptoms such as cognitive, vestibular and sleep, that did not improve over time likely prevented these workers from returning to their livelihood. Psychiatric symptoms (mood) was not related to ability to work. All had none to minimal symptoms initially. The OOW group improved over time. No severe depression or severe anxiety were evident. Two had a prior history of PTSD.
Overall, the OOW group suffered more severely with vestibular, cognitive, hearing, sleep, vision, and headache challenges, which did not lessen or abate over time rendering them unable to RTW. Employees in the OOW/RTW group had mild to moderate initial symptoms, which improved with therapy over time. Employees in the NOOW group had none to mild symptoms.
The objective for returning injured employees to work is not for return to a state completely without pain or impairment, but functional restoration.7,21 Both the OOW and OOW/RTW groups underwent similar intensive rehabilitation,1 implying that the OOW group sustained injuries such that RTW has not been possible. For most of these diplomats, however, RTW was achievable.
One of the limitations of this study is that the SSQ score is reflective of a moment in time and may not be fully representative of fluctuating symptomatology. However, the SSQ was assessed at each visit longitudinally by the same OEM provider allowing recording consistency. Symptoms were self-reported, and respondents may have over or under emphasized symptoms.22 However, the symptoms were relatively stable in this group. Questions have been grouped thematically and though not formally validated, it was consistently applied to all individuals. The PHQ-9, QOLI, BAI, and BDI were not completed by employees in the NOOW group as SSQ administration started after they had been RTW. They were completed at clinic visits, to minimize intrusiveness or disruption to these employees who had experienced trauma. The magnitude of the exposure was not captured, as often the employees unaware of an exposure only realizing later that they had unexplained symptoms, at which point they sought help. This also sometimes precluded an exact exposure date. RTW can be affected by factors not accounted for in this analysis such as supervisor relationships.6 Finally, the medical and neuropsychological assessments were conducted in a retrospective fashion based on diplomats’ historical self-report, making the results potentially influenced by participation, recall, and confirmation bias.
Another potential limitation is that co-morbidities were not included in the analysis. However, this was a relatively young and healthy cohort and except for hypertension in three individuals, prior PTSD in two, and hyperlipidemia in two, diabetes in one, PTSD and history of prior TBI in two, no other significant chronic diseases were noted in the OOW or OOW/RTW groups.
The factors most closely associated with RTW were vestibular, cognitive, and sleep and intensity of initial symptoms. This constellation of symptoms remained relatively stable. Psychiatric symptoms were not prominent and did not affect work ability. Significant injury was incurred. Work modifications were integral to the successful RTW process.
Acknowledgments
The authors would like to thank Kourtney Pony and Jamie Curran for their assistance with data entry, and Drs. Randell Swanson, Sajjad Savul, Stephen Hampton, Sean Grady, Michael Gallaway, Alyssa Gallaway-Beckett, Ramon Arrastia-Diaz, Douglas H. Smith, Diana Duda and Via Strong as well as Patricia Fletcher and Jennifer Ryder, who were part of the care team. We are also grateful to Cheryl Boberick, Rachel Greenlee, Timothy McInnes, and Tara Byrd for their assistance.
Footnotes
Research was supported in part by training grants from the National Institute of Occupational Safety and Health—grant number: 5-TO1–0H008628 and the Health Resources and Services Administration—grant number: D33HP25770–01–00. The opinions expressed in this article are the authors own and do not necessarily reflect the view of the granting agencies. They provided salary support to Dr Matthei, an Occupational Medicine resident, Both Drs Green-McKenzie and Shofer had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
IRB Approval: The University of Pennsylvania Institutional Review Board approved the study prior to data collection.
Dr Marla Deibler has the following conflicts of interest: 1. TLC Foundation for Body-Focused Repetitive Behaviors (Consultation and Speaker Fees); 2. NJ Center for Tourette Syndrome (Speaker Fees); 3. The Center for Emotional Health of Greater Philadelphia (private practice); 4. State of NJ (Psychological Testing and Expert Testimony); 5. Voorhees School District (Speaker Fees, Consultation).
The authors report no conflicts of interest.
REFERENCES
- 1.Swanson RL, Hampton S, Green-McKenzie J, et al. Neurological manifestations among US Government Personnel Reporting Directional Audible and Sensory Phenomena in Havana, Cuba. JAMA 2018; 319:1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffer M, Levin B, Snapp H, Buskirk J, Balaban C. Acute findings in an acquired neurosensory dysfunction. Laryngoscope Investig Otolaryngol 2019; 4:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wendt C, Emmett E. The Art of Directing a workers’ compensation claim: personal observations on the role of the workers compensation claim adjuster. Clin Occup Environ Med Green-McKenzie J (eds) 2004; 4:237–248. [DOI] [PubMed] [Google Scholar]
- 4. OSHA REF United States Department of labor. Occupational Safety and Health Administration. By Standard Number 1904.5 – Determination of work-relatedness. Available at: https://www.osha.gov/laws-regs/regulations/standardnumber/1904/1904.5 Accessed January 15, 2021. [Google Scholar]
- 5.Rinker J, Dinenberg R, Zappaterra M, Pransky G. Disability management & prevention. In: Current Diagnosis and Treatment. Occupational and Environmental Medicine. 5th ed. LaDou J, Harrison R, eds. Lange McGraw Hills; 2014. [Google Scholar]
- 6.Cancelliere C, Donovan J, Stochkendahl S, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropractic Manual Ther 2016; 24:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volberding PA, Aranda MP, Dennerlein JT, et al. The National Academies of Sciences, Engineering, and Medicine. The National Academies Press. May 2019 Notes: Selected to Sub-Committee that briefed The House ways and Means Committee (Congress) as well as the Social Security Administration on the study findings. Requested to sit on Committee convened by the Social Security Board (SSAB) to discuss Functional Disability in Adults. PMCID: doi: 10.17226/25376. [DOI] [Google Scholar]
- 8. Office of Disability Employment Policy. Accommodations. Available at: https://www.dol.gov/agencies/odep/program-areas/employers/accommodations. Accessed March 3, 2021. [Google Scholar]
- 9.Kuijer W, Groothoff JW, Brouwer S, Geertzen JH, Dijkstra PU. Prediction of sickness absence in patients with chronic low back pain: a systematic review. J Occup Rehabil 2006; 16:439–467. [DOI] [PubMed] [Google Scholar]
- 10. National Academies of Sciences, Engineering, and Medicine 2020. An Assessment of Illness in U.S. Government Employees and Their Families at Overseas Embassies. Washington, DC: The National Academies Press. Available at: 10.17226/25889. [DOI] [PubMed] [Google Scholar]
- 11. Becoming a Foreign Service Officer. I am diplomacy. I am America. Available at: https://careers.state.gov/wp-content/uploads/2019/05/Becoming-a-Foreign-Service-Officer-Specialist.pdf) careers.state.gov. Accessed September 1, 2017. [Google Scholar]
- 12. Sports Concussion Assessment Tool SCAT. 2013. Concussion in Sports Group. Br J Sports Med 2013; 47:259–. [Google Scholar]
- 13.Naughton SX, Terry AV. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology 2018; 408:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman A, Calkin C, Adams A, et al. Havana syndrome among Canadian diplomats: brain imaging reveals acquired neurotoxicity. medRxiv 2019; doi: 10.1101/19007096. [Google Scholar]
- 15.Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 16.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571. [DOI] [PubMed] [Google Scholar]
- 17.Frisch MB. Quality of Life Inventory (QOLI). Minneapolis, MN: National Computer Systems; 1994. [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patient-Reported Outcomes Measurement Information System: Sleep Related Impairment. Available at: http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Sleep-Related_Impairment_Scoring_Manual.pdf. Accessed September 1, 2017. [Google Scholar]
- 20.Benedictus M, Spikman J, van der Naalt J. Cognitive and behavioral impairment in traumatic brain injury related to outcome and return to work. Arch Phys Med Rehabil 2010; 91:1436–1441. [DOI] [PubMed] [Google Scholar]
- 21.Green-McKenzie J.Bender J, Rothstein M, Leone F, et al. Workers’ compensation. Occupational Health Services. Occupational Health Services: A Practical Approach. New York, NY: Routledge; 2012. [Google Scholar]
- 22.Short ME, Goetzel RZ, Pei X, et al. How accurate are self-reports? Analysis of self-reported health care utilization and absence when compared with administrative data. J Occup Environ Med 2009; 51:786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]