PURPOSE
Insertion mutations in Erb-b2 receptor tyrosine kinase 2 gene (ERBB2 or HER2) exon 20 occur in 2%-5% of non–small-cell lung cancers (NSCLCs) and function as an oncogenic driver. Poziotinib, a tyrosine kinase inhibitor, was evaluated in previously treated patients with NSCLC with HER2 exon 20 insertions.
METHODS
ZENITH20, a multicenter, multicohort, open-label phase II study, evaluated poziotinib in patients with advanced or metastatic NSCLC. In cohort 2, patients received poziotinib (16 mg) once daily. The primary end point was objective response rate evaluated by independent review committee (RECIST v1.1); secondary outcome measures were disease control rate, duration of response, progression-free survival, and safety and tolerability. Quality of life was assessed.
RESULTS
Between October 2017 and March 2021, 90 patients with a median of two prior lines of therapy (range, 1-6) were treated. With a median follow-up of 9.0 months, objective response rate was 27.8% (95% CI, 18.9 to 38.2); 25 of 90 patients achieved a partial response. Disease control rate was 70.0% (95% CI, 59.4 to 79.2). Most patients (74%) had tumor reduction (median reduction 22%). Median progression-free survival was 5.5 months (95% CI, 3.9 to 5.8); median duration of response was 5.1 months (95% CI, 4.2 to 5.5). Clinical benefit was seen regardless of lines and types of prior therapy, presence of central nervous system metastasis, and types of HER2 mutations. Grade 3 or higher treatment-related adverse events included rash (48.9%), diarrhea (25.6%), and stomatitis (24.4%). Most patients had poziotinib dose reductions (76.7%), with median relative dose intensity of 71.5%. Permanent treatment discontinuation because of treatment-related adverse events occurred in 13.3% of patients.
CONCLUSION
Poziotinib demonstrates antitumor activity in previously treated patients with HER2 exon 20 insertion NSCLC.
INTRODUCTION
Genetic alterations in the Erb-b2 receptor tyrosine kinase 2 gene (ERBB2), also known as human epidermal growth factor receptor 2 gene (HER2), occur in many cancer types and function as oncogene drivers.1,2 Insertion mutations in exon 20 of HER2 are detected in 2%-5% of non–small-cell lung cancers (NSCLCs) and are associated with never-smoker status, female sex, and adenocarcinoma histology.3-5 To date, there are no approved targeted therapies for this patient population; an unmet clinical need remains.5
CONTEXT
Key Objective
There is an unmet need for developing targeted therapy for lung cancers with HER2 exon 20 insertions. ZENITH20 cohort 2 assessed the efficacy and safety of poziotinib, a small-molecule inhibitor, in patients who had prior treatments.
Knowledge Generated
In the 90 patients who received poziotinib for their previously treated HER2 exon 20 lung cancers, the response rate was 27.8% (95% CI, 18.9 to 38.2) and disease control rate was 70.0% (95% CI, 59.4 to 79.2), with clinical benefit seen regardless of lines and types of prior therapy, presence of central nervous system metastasis, and type of HER2 insertions. Treatment-related adverse events were generally consistent with class effect, with rash, diarrhea, and stomatitis being the most common.
Relevance
These findings demonstrated that poziotinib is a potentially meaningful therapeutic option for patients with metastatic lung cancer harboring HER2 exon 20 insertion mutations.
Poziotinib is an irreversible pan-ErbB inhibitor with activity against mutations or insertions of HER1 (ErbB1; epidermal growth factor receptor [EGFR]), HER2 (ErbB2), and HER4 (ErbB4).6,7 Preclinical studies of EGFR and HER2 exon 20 insertion mutation cancer cells have demonstrated sensitivity to poziotinib, differentiating it from other EGFR tyrosine kinase inhibitors (TKIs) that have limited activity against exon 20 insertions.8-13 Given that poziotinib may represent a viable treatment option for patients with EGFR or HER2 exon 20–mutated tumors, the ZENITH20 study was initiated to evaluate poziotinib in patients with NSCLC with exon 20 insertion mutations. The 16 mg/d dose was chosen to improve tolerability as it was the highest daily dose without dose-limiting toxicity in a phase I study.8 Furthermore, 16 mg daily was also used in a single-site investigator-initiated lung cancer trial (NCT03066206) that demonstrated tolerable toxicity and antitumor efficacy.14,15
Here, we report the effects of poziotinib in a cohort of previously treated patients with HER2 exon 20 insertion-positive NSCLC in the ZENITH20 trial.
METHODS
Study Design and Patients
The ZENITH20 trial is a phase II, multicenter, multi-cohort, open-label study. In cohort 2, patients age ≥ 18 years were eligible if they were previously treated for locally advanced or metastatic NSCLC with documented HER2 exon 20 insertion mutations. Patients must have measurable NSCLC disease (per RECIST Guidelines, v1.1). Patients with known brain metastases were eligible if the patient's condition was stable, defined as asymptomatic, with no requirement for high dose or increasing doses of systemic corticosteroids, and no need for any anticonvulsant therapy for metastatic brain disease. For the patient who has had radiation therapy, sequential post-treatment magnetic resonance imaging (MRI) tests, at least 4-6 weeks apart, should show no increase in brain lesion size or volume within 4 weeks before the study. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki and in discussion with the US Food and Drug Administration. All patients provided written informed consent.
Study Procedures
Patients received 16 mg poziotinib orally once daily in an outpatient setting during each 28-day treatment cycle for up to 24 months. The dose could be reduced in 2-mg increments if necessary in the presence of toxicity. Dose interruption up to 28 days was allowed. Tumor assessments (using computed tomography, positron emission tomography-computed tomography, or MRI) were performed. Response evaluation was conducted by a blinded independent committee review using RECIST v1.1 at baseline, after 4 and 8 weeks of treatment, and every 8 weeks thereafter for up to 24 months. Patients with known brain lesions underwent MRI for tumor assessment at baseline and during the trial. MRI was not required for patients who did not have known brain metastasis.
Adverse events (AEs) were monitored throughout the study and for 35 days after poziotinib discontinuation and, along with laboratory abnormalities, were graded by investigators according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Study End Points
The primary end point was objective response rate (ORR). Secondary outcome measures were disease control rate (DCR), duration of response (DoR), progression-free survival (PFS), and safety and tolerability. Quality of life was also assessed (Data Supplement, online only). The primary analysis population included all patients who received ≥ 1 dose of poziotinib. Analyses were also conducted on the evaluable population, which included only patients who had a target lesion at baseline and were evaluable for tumor response.
Statistical Analysis
This study was designed to have 85% power to reject a nondesired ORR of 17% versus a clinically meaningful ORR of 30% in a two-sided test with a significance level of 5%. On the basis of the above test of hypotheses and per discussion with the US Food and Drug Administration, the criterion for the primary efficacy was the lower bound of 95% CI of the ORR to be above 17%. All efficacy and safety outcomes were analyzed using descriptive statistics. The ORR and DCR were also evaluated with 95% CI, and DoR and PFS were evaluated with Kaplan-Meier estimates and plots. Statistical analyses were conducted using SAS 9.4 software (SAS Institute, Cary, NC).
RESULTS
Patients
Between October 2017 and March 2021, 90 patients were enrolled and treated in ZENITH20 cohort 2 (Table 1). Median age was 60 years, 64.4% were female, and 65.6% were never-smokers. At study entry, 96.7% of patients had adenocarcinoma histopathology, 3.3% had squamous cell carcinomas, and 15.6% had stable CNS metastasis. All patients had received one to six prior lines of treatment (median: two lines; 38.9% had received ≥ 3 lines of prior therapy); 96.7% had prior platinum chemotherapy, 67.8% had received an immune checkpoint inhibitor (CPI), 27.8% received prior anti-HER2 therapy, and 65.6% of patients had chemotherapy in combination or sequentially with a CPI (Table 1, Data Supplement). Of the 25 patients who received prior anti-HER2 therapy, all had taken at least one antibody (trastuzumab, n = 22) or antibody-drug conjugate (ADC, ado-trastuzumab emtansine, n = 6; Data Supplement). At data cutoff (March 5, 2021), the median follow-up was 9.0 months (range, 0-17.6 months), treatment was ongoing in one (1.1%) patient, and 89 patients (98.9%) had discontinued treatment. Primary reasons for discontinuation were progressive disease (53 [58.9%] patients) and AEs (13 [14.4%] patients: 10 related AEs and three unrelated AEs; Data Supplement).
TABLE 1.
Demographic and Baseline Characteristics in Patients With Advanced NSCLC and HER2 Exon 20 Insertion
Efficacy
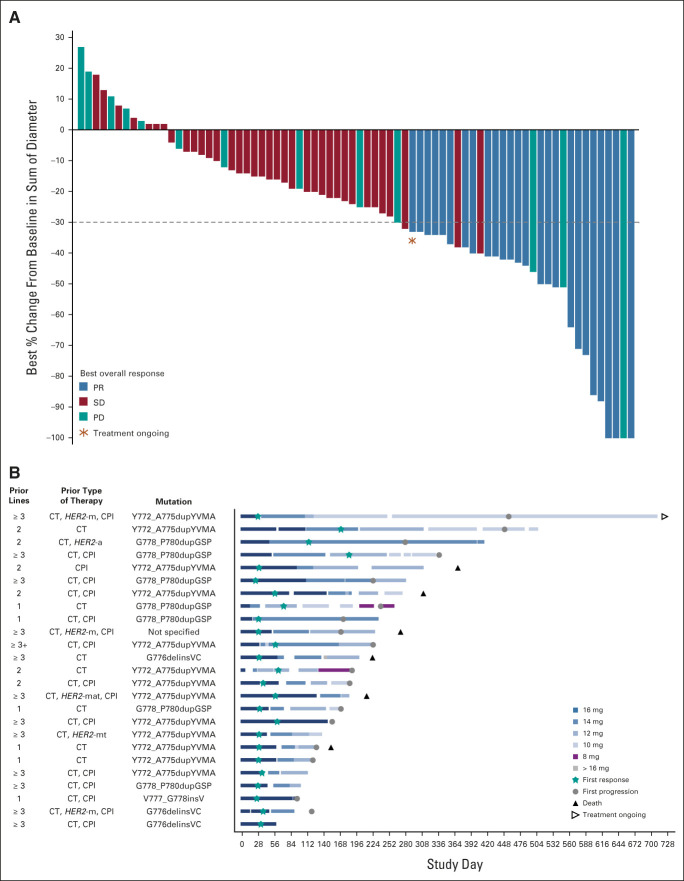
In the primary analysis population, ORR was 27.8% (95% CI, 18.9 to 38.2), with 25 of 90 treated patients achieving partial response (PR) and none achieving complete response. The DCR was 70.0% (95% CI, 59.4 to 79.2; Table 2). The evaluable population (n = 74) demonstrated an ORR and DCR of 35.1% (95% CI, 24.4 to 47.1) and 82.4% (95% CI, 71.8 to 90.3), respectively (Table 2). Most patients had tumor shrinkage (as-treated population: 74.4% [67 of 90], evaluable population: 90.5% [67 of 74]) (Fig 1A). Sixteen patients were excluded from the evaluable population due to absence of baseline target lesions (n = 4), lack of follow-up scans (n = 6), or lack of adequate follow-up for evaluable tumor response per RECIST v1.1 (n = 6) (Data Supplement).
TABLE 2.
Clinical Response (RECIST Guidelines, v1.1 by BICR) in Patients With Advanced NSCLC and HER2 Exon 20 Insertion
FIG 1.
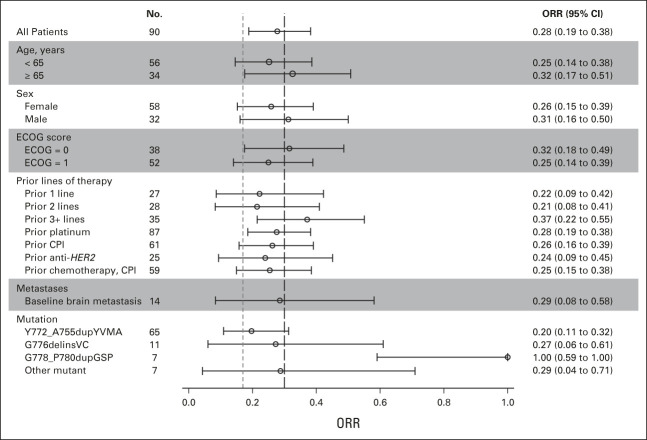
(A) Best percentage change from baseline in target lesion size (RECIST Guidelines, v1.1 by BICR) in patients with advanced NSCLC and HER2 exon 20 insertions (evaluable population, n = 74). (B). Swimmer plot for individual responders showing the number of lines of prior therapy, prior type of therapy, and mutation. The prior therapy column indicates prior exposure (not necessarily in the sequence listed). An accidental dosing error led to one patient taking 18 mg of poziotinib for 1 day. BICR, blinded independent committee review; CPI, checkpoint inhibitor; CT, chemotherapy; HER2, human epidermal growth factor receptor 2; HER2-a, HER2 antibody-drug conjugate; HER2-m, HER2-monoclonal antibody; HER2-mat, HER2-monoclonal antibody, antibody-drug conjugate, and tyrosine kinase inhibitor; HER2-mt, HER2-monoclonal antibody or tyrosine kinase inhibitor; NSCLC, non–small-cell lung cancer; PD, progressive disease; PR, partial response; SD, stable disease.
Among the 25 responders, median time to response was 32 days (range, 23-183 days), median DoR was 5.1 months (95% CI, 4.2 to 5.5), and 24% had DoR > 6 months (Table 2; Data Supplement). Median PFS for all 90 treated patients was 5.5 months (95% CI, 3.9 to 5.8), and 37.8% (95% CI, 25.5 to 50.0) were progression-free at 6 months (Table 2; Data Supplement).
Efficacy in Subgroups
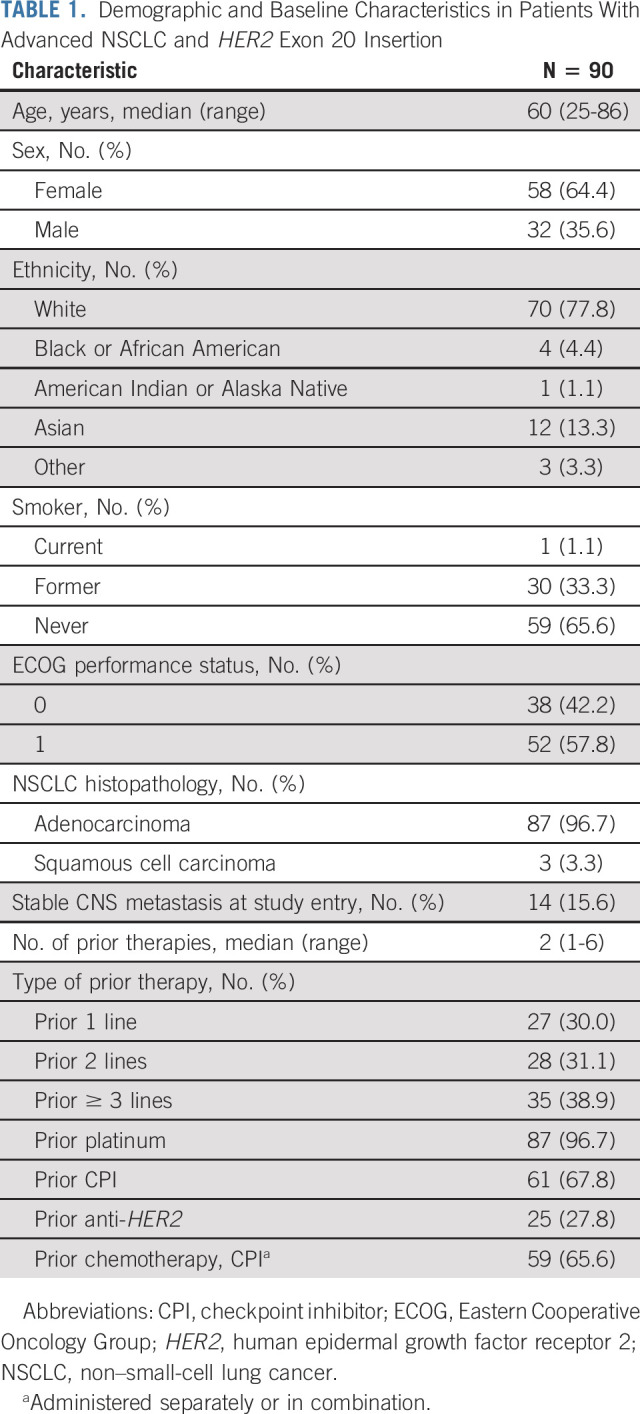
Patients derived benefit from poziotinib across demographic subgroups (Data Supplement). Efficacy was preserved in patients who had received multiple prior lines of therapy: ORR was 37.1% (95% CI, 21.5 to 55.1; n = 35) in those who had ≥ 3 prior lines of therapy, 21.4% (95% CI, 8.3 to 41; n = 28) in those who had two lines, and 22.2% (95% CI, 8.6 to 42.3; n = 27) in those with one prior systemic therapy (Fig 2).
FIG 2.
Forest plot presenting ORR by subgroups. CPI, checkpoint inhibitor; ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; ORR, objective response rate.
Of the 61 patients who had received prior CPI therapy, 16 (26.2%) were responders. Twenty-five patients had received prior anti-HER2 therapy with ≥ 1 antibody or ADC, and all had prior chemotherapy (Data Supplement). Three patients were treated previously with trastuzumab and afatinib, and one received trastuzumab and neratinib therapies. Among the 25 patients, six achieved PR (24.0%), including two who received prior trastuzumab and afatinib. The patient with prior neratinib therapy had a reported tumor reduction, but response status was not confirmed (Data Supplement).
Fourteen patients had known stable CNS metastases upon enrollment. ORR for these patients was 28.6%, with a median PFS of 7.4 months (Data Supplement). One patient with two brain lesions at baseline had an absence of both brain lesions on ≥ 2 MRI scans. Nine additional patients achieved at least CNS stable disease (SD), and the remaining four did not have adequate follow-up scans. None of the 14 patients had isolated CNS progression. Among the 14 patients, 13 received prior CNS radiation, including three who had whole-brain radiation therapy, five who underwent localized treatments such as stereotactic radiosurgery, and five who lacked detailed radiation information. Nine had CNS radiation within 12 weeks of study entry (Data Supplement).
The distribution pattern of the HER2 insertion mutations observed in our cohort was consistent with prior literature.11 The HER2 Y772_A775dupYVMA mutation was the most frequent and occurred in 65 (72.2%) patients, of whom the ORR was 20.0% (95% CI: 11.1%-31.8%), median DoR was 5.2 months, and median PFS was 5.4 months. In patients whose tumors had G778_P780dupGSP or G776delinsVC mutations, ORRs were 100% (n = 7) and 27.3% (n = 11); median PFS was 7.6 and 3.9 months; and median DoR was 5.3 and 4.6 months, respectively (Data Supplement).
Safety
Treatment-emergent AEs occurred in all patients. Treatment-related AEs (TRAEs) were reported in 88 (97.8%) patients, with 71 (78.9%) having grade 3 and four patients (4.4%) having grade 4 TRAEs (Data Supplement).
The most common TRAEs were rash (91.1%), diarrhea (82.2%), and stomatitis (68.9%), and grade ≥ 3 incidences were rash (48.9%), diarrhea (25.6%), and stomatitis (24.4%) (Table 3). Serious TRAEs occurring in ≥ 1 patient were rash (n = 3; 3.3%), asthenia, diarrhea, dehydration, and stomatitis (n = 2 each; 2.2%). Median time to onset of treatment-related rash, diarrhea, and stomatitis was 8, 6, and 7 days, respectively, with grade 3 events occurring later, at 52.5, 13, and 10 days, respectively. Treatment-emergent AEs occurring in 10% or more of patients are shown in the Data Supplement. Among the 61 patients with prior CPI treatment, the percentage of patients with grade ≥ 3 TRAEs (77.1%; rash, diarrhea, stomatitis, elevated liver function tests, and pneumonitis) was numerically lower than that of the other 29 (89.7%), suggesting that toxicity was not increased in patients receiving sequential CPI and poziotinib.
TABLE 3.
TRAEs (≥ 10% any grade and all grade 4)
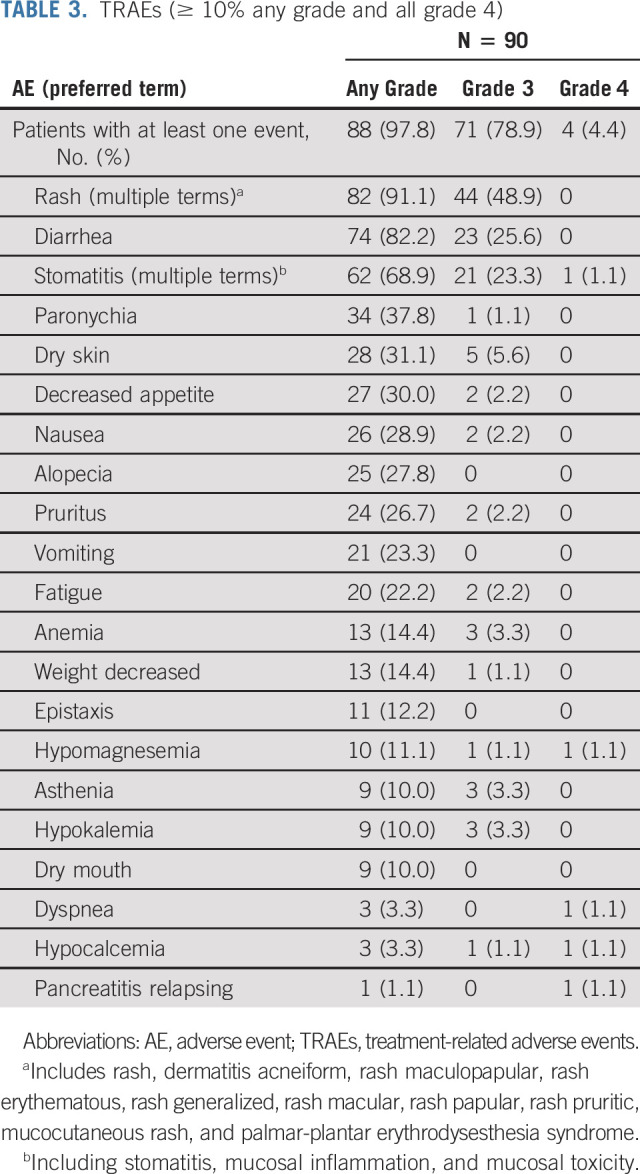
Four patients (4.4%) had five incidences of grade 4 TRAEs (stomatitis, dyspnea, hypomagnesemia, hypocalcemia, and pancreatitis relapsing), and 1 (1.1%) had a grade 5 TRAE (pneumonia). Grade 1 pneumonitis occurred in one patient. Although diarrhea was the second most common TRAE, dehydration (n = 4; 4.4%), hyponatremia (n = 4; 4.4%), and increased creatinine (n = 3; 3.3%) were uncommon. Increased alanine aminotransferase (n = 4; 4.4%) and aspartate aminotransferase (n = 3; 3.3%) were infrequent. Serious TRAEs occurred in 14.4% of patients. Twelve (13.3%) patients permanently discontinued treatment because of TRAEs (Data Supplement).
Health-related quality of life was measured using European Organisation for Research and Treatment of Cancer Quality of Life Core30 and the Quality of Life Lung Cancer 13 questionnaire, scored from 0 to 100 (≥ 10 point change from baseline [cycle 1 day 1] considered clinically meaningful; Data Supplement).16,17 Quality of Life Core30 functional and symptom scores were stable-to-improved, although without statistical significance. Quality of Life Lung Cancer 13 questionnaire mean scores indicated clinically meaningful improvement in cough (–16.5 to –13.9) from cycle 2 to cycle 7, and improvements in dyspnea (between –8.7 and –2.4) and chest pain (–6.9 to –5.5) were maintained to cycle 7 (Data Supplement).
Twenty-one patients (23.3%) remained on 16 mg poziotinib throughout the study; the remainder had ≥ 1 dose reductions such that their final dose was 14 mg (22.2%), 12 mg (30%), 10 mg (22.2%), or 8 mg (2.2%). Median relative dose intensity (percentage of total actual dose administered divided by planned dose for duration of treatment) was 71.5%. Duration of treatment ranged from 1 to 708 days (median, 112.5 days), with treatment administered for 1-675 days (median, 86.5 days). Seven patients (7.8%) were on treatment ≥ 12 months, and another four patients (4.4%) were treated ≥ 9 months. One patient who responded to the study drug from week 4 and progressed at week 64 is still receiving treatment for almost 2 years because of SD for clinical benefit as assessed by the investigator.
All 25 patients who achieved PR had a dose interruption, and 22 had a dose reduction. Eighteen responses were reported while on the 16-mg daily dose. Even with dose interruptions and reductions, disease control was maintained in the majority of patients (Fig 1B; Table 2).
DISCUSSION
An effective targeted treatment approach is lacking for patients with NSCLC harboring HER2 exon 20 insertions. The results from cohort 2 of the ZENITH20 trial demonstrate poziotinib's antitumor activity in these patients who had prior systemic treatments. In this cohort, the ORR was 27.8%, demonstrating a clinically meaningful benefit and exceeding prespecified efficacy criteria of this study conducted for registration purposes. Twenty-five patients achieved PR and an additional 38 patients had SD. Overall, 74% of patients had tumor shrinkage.
Poziotinib activity compares favorably to other irreversible TKIs targeting HER2.18 Among patients with HER2 exon 20 mutant NSCLC, one of 13 treated with afatinib achieved PR, with median PFS < 4 months.19 Three of 26 treated with dacomitinib achieved PR.20 Neratinib induced 1 PR in 26 lung cancer HER2 patients.21 Recently, pyrotinib was able to induce a partial response in 30% of patients.22
Other than small-molecule inhibitors, targeted antibodies and ADCs have demonstrated clinical efficacy for HER2-altered lung cancers. Ado-trastuzumab emtansine induced a response in 44% of patients, with approximately half carrying exon 20 insertions.23 Trastuzumab deruxtecan (T-DXd) rendered an ORR of 61.9% in 42 patients.24 Because small molecule inhibitors used sequentially with other treatments hold potential, we evaluated the efficacy of poziotinib in patients who received prior HER2 antibodies or ADCs; benefits observed were similar to the total cohort. Our data support poziotinib as a valuable additional therapeutic option in an era when various anti-HER2 therapeutics such as antibody or ADCs and TKIs are available.
HER2 exon 20 alterations are heterogeneous25,26; the most frequent insertions are Y772_A775dupYVMA, G776delinsVC, and G778_P780dupGSP.4,11,25,27,28 Evidence from prior studies indicates that HER2 exon 20 point mutations could be more responsive to TKIs, in contrast to insertions; the most common Y772_A775dupYVMA was the least responsive to therapy.10,29-32 In cohort 2, 72.2% of patients had tumors with Y772_A775dupYVMA and demonstrated an ORR of 20.0%. G776delinsVC and G778_P780dupGSP subgroups had numerically better clinical outcomes with poziotinib, although patient numbers were too small to draw definitive conclusions. Because different distributions of point mutations (none allowed in this cohort) and insertions are reported in different trials, the results cannot be compared directly; more drug-sensitive point mutations (V777L, L755P, and L755S) were allowed in both the pyrotinib and neratinib trials.21,22 In the neratinib trial, the lone responder had an L755S mutation, and none of the Y772_A775dupYVMA cases responded.21
In cohort 2, subgroups of interest benefitted, including patients with ≥ 3 prior lines of therapy or CNS metastasis. For heavily pretreated patients who had received ≥ 3 lines of therapy, ORR was 37.1% with a median DoR of 5.2 months. Pyrotinib has also shown benefit in heavily pretreated patients.22 These results suggest that the efficacy of therapy with small-molecule inhibitors is derived from target engagement, especially in difficult-to-treat mutations like exon 20.
CNS metastasis represents a clinical challenge for NSCLC: these patients have median overall survival of 6 months and 1-, 2-, and 3-year survival rates of 29.9%, 14.3%, and 8.4%, respectively.33,34 For HER2-mutant NSCLC, baseline CNS metastasis occurs in 19% of patients; during treatment, an additional 28% develop CNS metastasis.35 In cohort 2 of the ZENITH20 trial, 14 patients had CNS metastasis at enrollment; four had a response (ORR 28.6%) with median PFS of 7.4 months. One patient had complete resolution of CNS lesions, and no patients had CNS progression. Interpretation of the data was limited by small sample size (n = 14), prior radiation therapy to the brain (13 patients; three had whole-brain radiation therapy), and no requirement for baseline brain MRI; therefore, future studies are warranted to guide poziotinib use in patients with CNS metastasis.
The side-effect profile of poziotinib reported here is consistent with prior reports: rash, stomatitis, and diarrhea were the most common class-effect toxicities. Events generally manifested within the first 2 weeks of treatment. Permanent treatment discontinuation because of TRAEs occurred in 13.3% of patients. Discontinuations because of TRAEs occurred in 9% of patients receiving dacomitinib in the ARCHER1050 trial, with similar rates in trials of neratinib (7.7%) and afatinib (6%).21,36,37
In the ZENITH20 trial, standard tools were used to evaluate patient overall quality of life. Lung cancer–specific symptoms (especially cough), dyspnea, and chest pain significantly improved with poziotinib treatment. General function was also preserved throughout treatment cycles. Early recognition and intervention for treatment-related side effects is essential to optimize outcomes.
Among the 25 responders, responses were generally observed early (week 4) during 16-mg daily dosing and 24% of patients maintained their response for ≥ 6 months despite dose interruptions or reductions (Fig 1B). Given the observed side-effect profile, development of a more tolerable dosing schedule together with a proactive supportive treatment plan is ongoing to decrease toxicity and improve total dose intensity. ZENITH20 trial cohort 5 was expanded to evaluate twice-a-day dosing (b.i.d.) of poziotinib. Preliminary analysis of poziotinib b.i.d. has been shown to significantly reduce side effects while preserving efficacy.38 Specifically, the rate of grade ≥ 3 TRAEs was 19% with 8 mg b.i.d. (n = 31) versus 35% with 16 mg daily (n = 26) in cycle 1. In the 8 mg b.i.d. dosing arm, six out of 19 patients (both EGFR and HER2 exon 20) achieved a response for an ORR of 31.6%. This dosing schedule offers promise for decreasing AE rates and enhancing efficacy. Moreover, a recent ZENITH20 Protocol (online only) amendment provided detailed instructions on rash and diarrhea management, encouraging proactive use of antidiarrheal agents and oral steroids. Together, those measures will potentially decrease and mitigate toxicity, allowing patients to remain on poziotinib for maximum clinical benefit.
In summary, data from cohort 2 of the ZENITH20 trial indicate that poziotinib demonstrates clinical benefit in heavily pretreated patients with NSCLC and HER2 exon 20 insertion mutations. To date, this is the largest HER2 exon 20 insertion NSCLC study that used blinded central imaging for response analysis. The relatively large sample size for this molecularly defined patient population allowed for meaningful subgroup analyses. These findings validate HER2 exon 20 insertions as an actionable therapeutic target in lung cancer, including in patients with CNS metastasis, and present poziotinib as a potentially meaningful therapeutic option.
ACKNOWLEDGMENT
The authors thank Nicole Day, PhD, of MedVal Scientific Information Services, LLC, for medical writing and editorial assistance, which were funded by Spectrum Pharmaceuticals. The authors thank Dr Jacqulyne Robichaux for the helpful discussion about ERBB2 biology. This manuscript was prepared according to the International Society for Medical Publication Professionals' Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP3.
Xiuning Le
Consulting or Advisory Role: AstraZeneca, Lilly, EMD Serono, Spectrum Pharmaceuticals, Daiichi Sankyo/Lilly
Research Funding: Lilly (Inst), Boehringer Ingelheim (Inst)
Robin Cornelissen
Honoraria: Roche, Boehringer Ingelheim, Pfizer, Spectrum Pharmaceuticals
Consulting or Advisory Role: MSD
Marina Garassino
Honoraria: MSD Oncology, AstraZeneca/MedImmune, GlaxoSmithKline, Takeda, Roche, Bristol Myers Squibb
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Novartis, Takeda, Roche, Tiziana Life Sciences, Sanofi, Celgene, Daiiki Sankyo, Inivata, Incyte, Pfizer, Seattle Genetics, Lilly, GlaxoSmithKline, Bayer, Blueprint Medicines, Janssen, Regeneron
Speakers' Bureau: AstraZeneca, Takeda, MSD Oncology, Celgene, Incyte, Roche, Bristol Myers Squibb, Otsuka, Lilly,
Research Funding: Bristol Myers Squibb (Inst), MSD (Inst), Roche/Genentech (Inst), AstraZeneca/MedImmune (Inst), AstraZeneca (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Merck (Inst), Incyte (Inst), Takeda (Inst), Spectrum Pharmaceuticals (Inst), Blueprint Medicines (Inst), Lilly (Inst), AstraZeneca (Inst), Ipsen (Inst), Janssen (Inst), Exelixis (Inst), MedImmune (Inst), Sanofi (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca
Jeffrey M. Clarke
Honoraria: AstraZeneca, Spectrum Pharmaceuticals, Merck, Pfizer, NGM Biopharmaceuticals, Genentech
Consulting or Advisory Role: Guardant Health, AstraZeneca, Merck, Pfizer, NGM Biopharmaceuticals, Spectrum Pharmaceuticals, Genentech
Speakers' Bureau: Merck, AstraZeneca
Research Funding: Bristol Myers Squibb, Adaptimmune, Spectrum Pharmaceuticals, AbbVie, Moderna Therapeutics, GlaxoSmithKline, Array BioPharma, AstraZeneca, Grid Therapeutics, Achilles Therapeutics
Travel, Accommodations, Expenses: Merck, AstraZeneca, NGM Biopharmaceuticals, Pfizer
Uncompensated Relationships: Lung Cancer Initiative of North Carolina
Nishan Tchekmedyian
Employment: Pacific Shores Medical Group
Leadership: Pacific Shores Medical Group
Stock and Other Ownership Interests: Portola Pharmaceuticals, Halozyme (I), Infinity Pharmaceuticals (I), Global therapeutics (I), Biomarin (I), Exelixis (I), Trillium Therapeutics (I)
Consulting or Advisory Role: Seattle Genetics, IntrinsiQ, Foundation Medicine, Amgen
Research Funding: Bristol Myers Squibb (Inst), Spectrum Pharmaceuticals (Inst), Turning Point Therapeutics (Inst), Cullinan Oncology (Inst), Rain Therapeutics (Inst), Takeda (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: IntrinsiQ
Jonathan W. Goldman
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Lilly, Amgen, Pfizer
Research Funding: Lilly (Inst), Genentech/Roche (Inst), Bristol-Myers Squibb (Inst), AstraZeneca/MedImmune (Inst), Array BioPharma (Inst), AbbVie, Corvus Pharmaceuticals (Inst), Spectrum Pharmaceuticals (Inst), Advaxis (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Szu-Yun Leu
Employment: Spectrum Pharmaceuticals
Gajanan Bhat
Employment: Spectrum Pharmaceuticals
Stock and Other Ownership Interests: Spectrum Pharmaceuticals
Francois Lebel
Employment: Spectrum Pharmaceuticals
Leadership: Spectrum Pharmaceuticals
Stock and Other Ownership Interests: Spectrum Pharmaceuticals
John V. Heymach
Stock and Other Ownership Interests: Cardinal Spine, Bio-Tree
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Spectrum Pharmaceuticals, Guardant Health, Hengrui Pharmaceutical, GlaxoSmithKline, EMD Serono, Lilly, Takeda, Sanofi/Aventis, Genentech/Roche, Boehringer Ingelheim, Catalyst Biotech, Foundation medicine, Novartis, Mirati Therapeutics, BrightPath Biotheraputics, Janssen, Nexus Health Systems, Pneuma Respiratory, Kairos Ventures, Roche, Leads Biolabs
Research Funding: AstraZeneca (Inst), Spectrum Pharmaceuticals, GlaxoSmithKline
Patents, Royalties, Other Intellectual Property: Licensing agreement between Spectrum and MD Anderson (including myself) regarding intellectual property for treatment of EGFR and HER2 exon 20 mutations
Mark A. Socinski
Honoraria: Genentech, Bristol Myers Squibb, Celgene, AstraZeneca, Guardant Health, Bayer, Merck, Roche/Genentech, Lilly, Genentech, AstraZeneca/MedImmune, Lilly, Janssen, Novartis
Speakers' Bureau: Genentech, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Bayer, Merck, Amgen, Blueprint Medicines, G1 Therapeutics, Guardant Health, Lilly, Regeneron/Sanofi, Jazz Pharmaceuticals, Janssen Oncology
Research Funding: Genentech (Inst), Spectrum Pharmaceuticals (Inst), AstraZeneca/MedImmune (Inst)
No other potential conflicts of interest were reported.
See accompanying editorial on page 693
PRIOR PRESENTATION
Presented Previously at the European Society for Medical Oncology Virtual Congress, September 19-21, 2020.
SUPPORT
Spectrum Pharmaceuticals sponsored the study design and conduct, data collection, and data analysis and interpretation.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
The authors certify that this manuscript reports original clinical trial data. Data reported in this manuscript are available within the article or are posted publicly at www.clinicaltrials.gov, according to required timelines. Additional study data are available upon reasonable request.
AUTHOR CONTRIBUTIONS
Conception and design: Xiuning Le, Nishan Tchekmedyian, Jonathan W. Goldman, Szu-Yun Leu, Gajanan Bhat, Francois Lebel, John V. Heymach, Mark A. Socinski
Financial support: Francois Lebel
Administrative support: Francois Lebel
Provision of study materials or patients: Robin Cornelissen, Jeffrey M. Clarke, Nishan Tchekmedyian, Jonathan W. Goldman, Francois Lebel, Mark A. Socinski
Collection and assembly of data: Xiuning Le, Robin Cornelissen, Jeffrey M. Clarke, Nishan Tchekmedyian, Jonathan W. Goldman, Szu-Yun Leu, Francois Lebel, Mark A. Socinski
Data analysis and interpretation: Xiuning Le, Robin Cornelissen, Marina Garassino, Jeffrey M. Clarke, Nishan Tchekmedyian, Szu-Yun Leu, Gajanan Bhat, Francois Lebel, John V. Heymach, Mark A. Socinski
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Poziotinib in Non–Small-Cell Lung Cancer Harboring HER2 Exon 20 Insertion Mutations After Prior Therapies: ZENITH20-2 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Xiuning Le
Consulting or Advisory Role: AstraZeneca, Lilly, EMD Serono, Spectrum Pharmaceuticals, Daiichi Sankyo/Lilly
Research Funding: Lilly (Inst), Boehringer Ingelheim (Inst)
Robin Cornelissen
Honoraria: Roche, Boehringer Ingelheim, Pfizer, Spectrum Pharmaceuticals
Consulting or Advisory Role: MSD
Marina Garassino
Honoraria: MSD Oncology, AstraZeneca/MedImmune, GlaxoSmithKline, Takeda, Roche, Bristol Myers Squibb
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Novartis, Takeda, Roche, Tiziana Life Sciences, Sanofi, Celgene, Daiiki Sankyo, Inivata, Incyte, Pfizer, Seattle Genetics, Lilly, GlaxoSmithKline, Bayer, Blueprint Medicines, Janssen, Regeneron
Speakers' Bureau: AstraZeneca, Takeda, MSD Oncology, Celgene, Incyte, Roche, Bristol Myers Squibb, Otsuka, Lilly,
Research Funding: Bristol Myers Squibb (Inst), MSD (Inst), Roche/Genentech (Inst), AstraZeneca/MedImmune (Inst), AstraZeneca (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Merck (Inst), Incyte (Inst), Takeda (Inst), Spectrum Pharmaceuticals (Inst), Blueprint Medicines (Inst), Lilly (Inst), AstraZeneca (Inst), Ipsen (Inst), Janssen (Inst), Exelixis (Inst), MedImmune (Inst), Sanofi (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca
Jeffrey M. Clarke
Honoraria: AstraZeneca, Spectrum Pharmaceuticals, Merck, Pfizer, NGM Biopharmaceuticals, Genentech
Consulting or Advisory Role: Guardant Health, AstraZeneca, Merck, Pfizer, NGM Biopharmaceuticals, Spectrum Pharmaceuticals, Genentech
Speakers' Bureau: Merck, AstraZeneca
Research Funding: Bristol Myers Squibb, Adaptimmune, Spectrum Pharmaceuticals, AbbVie, Moderna Therapeutics, GlaxoSmithKline, Array BioPharma, AstraZeneca, Grid Therapeutics, Achilles Therapeutics
Travel, Accommodations, Expenses: Merck, AstraZeneca, NGM Biopharmaceuticals, Pfizer
Uncompensated Relationships: Lung Cancer Initiative of North Carolina
Nishan Tchekmedyian
Employment: Pacific Shores Medical Group
Leadership: Pacific Shores Medical Group
Stock and Other Ownership Interests: Portola Pharmaceuticals, Halozyme (I), Infinity Pharmaceuticals (I), Global therapeutics (I), Biomarin (I), Exelixis (I), Trillium Therapeutics (I)
Consulting or Advisory Role: Seattle Genetics, IntrinsiQ, Foundation Medicine, Amgen
Research Funding: Bristol Myers Squibb (Inst), Spectrum Pharmaceuticals (Inst), Turning Point Therapeutics (Inst), Cullinan Oncology (Inst), Rain Therapeutics (Inst), Takeda (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: IntrinsiQ
Jonathan W. Goldman
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Lilly, Amgen, Pfizer
Research Funding: Lilly (Inst), Genentech/Roche (Inst), Bristol-Myers Squibb (Inst), AstraZeneca/MedImmune (Inst), Array BioPharma (Inst), AbbVie, Corvus Pharmaceuticals (Inst), Spectrum Pharmaceuticals (Inst), Advaxis (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Szu-Yun Leu
Employment: Spectrum Pharmaceuticals
Gajanan Bhat
Employment: Spectrum Pharmaceuticals
Stock and Other Ownership Interests: Spectrum Pharmaceuticals
Francois Lebel
Employment: Spectrum Pharmaceuticals
Leadership: Spectrum Pharmaceuticals
Stock and Other Ownership Interests: Spectrum Pharmaceuticals
John V. Heymach
Stock and Other Ownership Interests: Cardinal Spine, Bio-Tree
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Spectrum Pharmaceuticals, Guardant Health, Hengrui Pharmaceutical, GlaxoSmithKline, EMD Serono, Lilly, Takeda, Sanofi/Aventis, Genentech/Roche, Boehringer Ingelheim, Catalyst Biotech, Foundation medicine, Novartis, Mirati Therapeutics, BrightPath Biotheraputics, Janssen, Nexus Health Systems, Pneuma Respiratory, Kairos Ventures, Roche, Leads Biolabs
Research Funding: AstraZeneca (Inst), Spectrum Pharmaceuticals, GlaxoSmithKline
Patents, Royalties, Other Intellectual Property: Licensing agreement between Spectrum and MD Anderson (including myself) regarding intellectual property for treatment of EGFR and HER2 exon 20 mutations
Mark A. Socinski
Honoraria: Genentech, Bristol Myers Squibb, Celgene, AstraZeneca, Guardant Health, Bayer, Merck, Roche/Genentech, Lilly, Genentech, AstraZeneca/MedImmune, Lilly, Janssen, Novartis
Speakers' Bureau: Genentech, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Bayer, Merck, Amgen, Blueprint Medicines, G1 Therapeutics, Guardant Health, Lilly, Regeneron/Sanofi, Jazz Pharmaceuticals, Janssen Oncology
Research Funding: Genentech (Inst), Spectrum Pharmaceuticals (Inst), AstraZeneca/MedImmune (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Subramanian J, Katta A, Masood A, et al. : Emergence of ERBB2 mutation as a biomarker and an actionable target in solid cancers. Oncologist 24:e1303-e1314, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JW, Soung YH, Seo SH, et al. : Somatic mutations of ERBB2 kinase domain in gastric, colorectal, and breast carcinomas. Clin Cancer Res 12:57-61, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Takeda M, Sakai K, Hayashi H, et al. : Clinical characteristics of non-small cell lung cancer harboring mutations in exon 20 of EGFR or HER2. Oncotarget 9:21132-21140, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazières J, Peters S, Lepage B, et al. : Lung cancer that harbors an HER2 mutation: Epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 31:1997-2003, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Mazières J, Barlesi F, Filleron T, et al. : Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: Results from the European EUHER2 cohort. Ann Oncol 27:281-286, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Cha MY, Lee KO, Kim M, et al. : Antitumor activity of HM781-36B, a highly effective pan-HER inhibitor in erlotinib-resistant NSCLC and other EGFR-dependent cancer models. Int J Cancer 130:2445-2454, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Kang MH, Moon SU, Sung JH, et al. : Antitumor activity of HM781-36B, alone or in combination with chemotherapeutic agents, in colorectal cancer cells. Cancer Res Treat 48:355-364, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HJ, Kim HP, Yoon YK, et al. : Antitumor activity of HM781-36B, a pan-HER tyrosine kinase inhibitor, in HER2-amplified breast cancer cells. Anticancer Drugs 23:288-297, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Nam HJ, Kim HP, Yoon YK, et al. : Antitumor activity of HM781-36B, an irreversible pan-HER inhibitor, alone or in combination with cytotoxic chemotherapeutic agents in gastric cancer. Cancer Lett 302:155-165, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Robichaux JP, Elamin YY, Tan Z, et al. : Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 24:638-646, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robichaux JP, Elamin YY, Vijayan RSK, et al. : Pan-cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-DM1 activity. Cancer Cell 36:444-457.e7, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasuda H, Kobayashi S, Costa DB: EGFR exon 20 insertion mutations in non-small-cell lung cancer: Preclinical data and clinical implications. Lancet Oncol 13:e23-31, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Koga T, Kobayashi Y, Tomizawa K, et al. : Activity of a novel HER2 inhibitor, poziotinib, for HER2 exon 20 mutations in lung cancer and mechanism of acquired resistance: An in vitro study. Lung Cancer 126:72-79, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Kim TM, Lee KW, Oh DY, et al. : Phase 1 studies of poziotinib, an irreversible pan-HER tyrosine kinase inhibitor in patients with advanced solid tumors. Cancer Res Treat 50:835-842, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heymach J, Negrao M, Robichaux J, et al. : A phase II trial of poziotinib in EGFR and HER2 exon 20 mutant non-small cell lung cancer (NSCLC). J Thorac Oncol 13:S323-S324, 2018. (abstr OA02.06) [Google Scholar]
- 16.Aaronson NK, Ahmedzai S, Bergman B, et al. : The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365-376, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Bergman B, Aaronson NK, Ahmedzai S, et al. : The EORTC QLQ-LC13: A modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer 30A:635-642, 1994 [DOI] [PubMed] [Google Scholar]
- 18.De Grève J, Teugels E, Geers C, et al. : Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer 76:123-127, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Dziadziuszko R, Smit EF, Dafni U, et al. : Afatinib in NSCLC with HER2 mutations: Results of the prospective, open-label phase II NICHE trial of European Thoracic Oncology Platform (ETOP). J Thorac Oncol 14:1086-1094, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Kris MG, Camidge DR, Giaccone G, et al. : Targeting HER2 aberrations as actionable drivers in lung cancers: Phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol 26:1421-1427, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyman DM, Piha-Paul SA, Won H, et al. : HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554:189-194, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou C, Li X, Wang Q, et al. : Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: A multicenter, open-label, single-arm, phase II study. J Clin Oncol 38:2753-2761, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Li BT, Shen R, Buonocore D, et al. : Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: Results from a phase II basket trial. J Clin Oncol 36:2532-2537, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smit EF, Nakagawa K, Nagasaka M, et al. : Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-mutated metastatic non-small cell lung cancer (NSCLC): Interim results of DESTINY-Lung01. J Clin Oncol 38, 2020. (suppl 15; abstr 9504) [Google Scholar]
- 25.Connell CM, Doherty GJ: Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open 2:e000279, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Jiang T, Qin Z, et al. : HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Ann Oncol 30:447-455, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang SE, Narasanna A, Perez-Torres M, et al. : HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 10:25-38, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Shigematsu H, Takahashi T, Nomura M, et al. : Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res 65:1642-1646, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Kosaka T, Tanizaki J, Paranal RM, et al. : Response heterogeneity of EGFR and HER2 exon 20 insertions to covalent EGFR and HER2 inhibitors. Cancer Res 77:2712-2721, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao S, Fang W, Pan H, et al. : Conformational landscapes of HER2 exon 20 insertions explain their sensitivity to kinase inhibitors in lung adenocarcinoma. J Thorac Oncol 15:962-972, 2020 [DOI] [PubMed] [Google Scholar]
- 31.Fang W, Zhao S, Liang Y, et al. : Mutation variants and co-mutations as genomic modifiers of response to afatinib in HER2-mutant lung adenocarcinoma. Oncologist 25:e545-e554, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Lv J, Wu Y, et al. : HER2 exon 20 insertion mutations in lung adenocarcinoma: Case series and response to pyrotinib. Front Oncol 10:1162, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nayak L, Lee EQ, Wen PY: Epidemiology of brain metastases. Curr Oncol Rep 14:48-54, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Waqar SN, Samson PP, Robinson CG, et al. : Non-small-cell lung cancer with brain metastasis at presentation. Clin Lung Cancer 19:e373-e379, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Offin M, Feldman D, Ni A, et al. : Frequency and outcomes of brain metastases in patients with HER2-mutant lung cancers. Cancer 125:4380-4387, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu YL, Cheng Y, Zhou X, et al. : Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol 18:1454-1466, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Park K, Tan EH, O'Byrne K, et al. : Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol 17:577-589, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Le X, Shum E, Suga JM, et al. : Poziotinib administered twice daily improves safety and tolerability in patients with EGFR or HER2 exon 20 mutant NSCLC(ZENITH20-5) [poster]. Presented at the 112th Annual Meeting of the American Association for Cancer Research, Philadelphia, PA, April 10-15, 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors certify that this manuscript reports original clinical trial data. Data reported in this manuscript are available within the article or are posted publicly at www.clinicaltrials.gov, according to required timelines. Additional study data are available upon reasonable request.