PURPOSE
Pembrolizumab demonstrated durable antitumor activity in patients with previously treated, advanced microsatellite instability–high or mismatch repair–deficient (MSI-H/dMMR) tumors, including endometrial cancer, in the nonrandomized, open-label, multicohort, phase II KEYNOTE-158 study (NCT02628067). We report efficacy and safety outcomes for patients with MSI-H/dMMR endometrial cancer enrolled in KEYNOTE-158.
METHODS
Eligible patients from cohorts D (endometrial cancer, regardless of MSI-H/dMMR status) and K (any MSI-H/dMMR solid tumor, except colorectal) with previously treated, advanced MSI-H/dMMR endometrial cancer received pembrolizumab 200 mg once every 3 weeks for 35 cycles. The primary end point was objective response rate per RECIST version 1.1 by independent central radiologic review. Secondary end points included duration of response, progression-free survival, overall survival, and safety.
RESULTS
As of October 5, 2020, 18 of 90 treated patients (20%) had completed 35 cycles of pembrolizumab and 52 (58%) had discontinued treatment. In the efficacy population (patients who received ≥ 1 dose of pembrolizumab and had ≥ 26 weeks of follow-up; N = 79), the median time from first dose to data cutoff was 42.6 (range, 6.4-56.1) months. The objective response rate was 48% (95% CI, 37 to 60), and median duration of response was not reached (2.9-49.7+ months). Median progression-free survival was 13.1 (95% CI, 4.3 to 34.4) months, and median overall survival was not reached (95% CI, 27.2 months to not reached). Among all treated patients, 76% had ≥ 1 treatment-related adverse event (grades 3-4, 12%). There were no fatal treatment-related events. Immune-mediated adverse events or infusion reactions occurred in 28% of patients (grades 3-4, 7%; no fatal events).
CONCLUSION
Pembrolizumab demonstrated robust and durable antitumor activity and encouraging survival outcomes with manageable toxicity in patients with previously treated, advanced MSI-H/dMMR endometrial cancer.
INTRODUCTION
Endometrial cancer is the second most prevalent gynecologic cancer in women worldwide, and its incidence has been increasing.1,2 Standard-of-care first-line systemic therapy for patients with advanced or recurrent endometrial cancer commonly comprises a platinum-based chemotherapy regimen such as carboplatin plus paclitaxel.3,4 However, treatment options after failure of first-line therapy are limited.3,5 For patients with advanced or recurrent endometrial cancer, 5-year survival rates of approximately 17% have been reported.6
CONTEXT
Key Objective
Endometrial cancer is the second most prevalent gynecologic cancer in women worldwide; however, treatment options after failure of first-line therapy are limited. We evaluated the efficacy and safety of pembrolizumab, an antiprogrammed death-1 antibody, in patients with previously treated advanced endometrial cancer with tumors that had high levels of microsatellite instability/mismatch repair deficiency.
Knowledge Generated
Among patients who received pembrolizumab monotherapy, 48% had an objective response. Responses were durable, and the median duration of response was not reached after a median follow-up of 42.6 months. No new safety signals were identified.
Relevance
Pembrolizumab demonstrated durable antitumor activity with manageable toxicity in patients with advanced microsatellite instability–high or mismatch repair–deficient endometrial cancer. These findings support the use of pembrolizumab as a treatment option in this setting.
Approximately 25%-31% of patients with endometrial cancer have tumors with high levels of microsatellite instability (MSI-H) and mismatch repair deficiency (dMMR).7,8 dMMR occurs as an inherited mutation (known as Lynch syndrome) in one of the mismatch repair (MMR) genes MLH1, PMS2, MSH2, and MSH6 or as sporadic methylation of the MLH1 promoter.9,10 Defects in the MMR genes result in a failure to correct DNA replication errors, leading to an accumulation of point mutations within microsatellite regions that result in microsatellite instability.10 This DNA-repair defect also leads to accumulation of point mutations in other areas of the genome,10 and MSI-H/dMMR tumors have a marked increase in somatic mutations compared with non–MSI-H/dMMR tumors.11 MSI-H/dMMR endometrial cancer is associated with a higher neoantigen load and increased CD3-positive, CD8-positive, and programmed death-1 (PD-1)–expressing tumor-infiltrating lymphocytes and programmed death ligand-1 (PD-L1)–expressing intraepithelial and peritumoral immune cells compared with microsatellite stable endometrial cancers.12
The humanized monoclonal anti–PD-1 antibody pembrolizumab has demonstrated antitumor activity in patients with MSI-H/dMMR tumors and in patients with endometrial cancer.13-15 Pembrolizumab first demonstrated antitumor activity in patients with endometrial cancer in the phase Ib KEYNOTE-028 study, in which an objective response rate (ORR) of 13% was observed in a population of patients with PD-L1–positive disease.13 In a prospective analysis from the KEYNOTE-016 study, pembrolizumab resulted in an objective response in eight of 15 (53%) patients with dMMR endometrial cancer,14 providing early evidence of antitumor activity in this setting. In the nonrandomized, open-label, multicohort, phase II KEYNOTE-158 (ClinicalTrials.gov identifier: NCT02628067) study of pembrolizumab in multiple types of advanced (unresectable and/or metastatic) rare cancers, pembrolizumab demonstrated an ORR of 34.3% among 233 patients with previously treated unresectable or metastatic MSI-H/dMMR noncolorectal cancers.15
In an initial analysis of outcomes among the first 49 patients with MSI-H/dMMR endometrial cancer enrolled in KEYNOTE-158, the ORR was 57%.16 Here, we report efficacy and safety data in a larger number of patients with longer follow-up in patients with previously treated advanced MSI-H/dMMR endometrial cancer from KEYNOTE-158.
METHODS
Study Design and Patients
As previously described,15,17 KEYNOTE-158 is an open-label, multicohort, phase II study in patients with multiple types of advanced solid tumors. Cohort D enrolled patients with endometrial carcinoma (irrespective of MSI status and excluding sarcomas and mesenchymal tumors). Cohort K enrolled patients with any advanced solid tumor (with the exception of colorectal cancer) that was MSI-H/dMMR including endometrial carcinoma. In this analysis, we report outcomes for patients from cohorts D and K who had MSI-H/dMMR endometrial cancer.
Eligible patients were age ≥ 18 years, with histologically or cytologically documented, advanced (metastatic and/or unresectable) disease that was incurable and had disease progression on or intolerance to standard therapies; provided an evaluable tissue sample for biomarker analysis from a tumor lesion not previously irradiated; had radiologically measurable disease per RECIST version 1.1 as assessed by independent central radiologic review; had an Eastern Cooperative Oncology Group performance status of 0 or 1; and had adequate organ function. Exclusion criteria included a diagnosis of immunodeficiency or receipt of systemic steroid therapy or immunosuppressive therapy within 7 days of first dose; active autoimmune disease requiring systemic therapy within 2 years; treatment with a monoclonal antibody or an investigational agent within 4 weeks; use of chemotherapy, targeted small molecule therapy, or radiation therapy within 2 weeks; known active central nervous system metastases and/or carcinomatous meningitis (patients with previously treated stable brain metastases with no evidence of new or enlarging brain metastases and no use of steroids within 7 days were eligible); current pneumonitis or history of pneumonitis (noninfectious) that required steroids; and an active infection requiring systemic therapy.
The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines and with local and/or national regulations as outlined in the Declaration of Helsinki. The Protocol (online only) and all amendments were approved by an institutional review board or independent ethics committee at each site. Patients provided written informed consent.
Study Treatments
Patients received pembrolizumab 200 mg intravenously on day 1 of each 3-week cycle for 35 cycles (approximately 2 years). Treatment continued until documented disease progression, unacceptable adverse event (AE), intercurrent illness preventing further treatment administration, investigator decision, or patient withdrawal of consent. Patients who discontinued pembrolizumab with complete response (CR), partial response (PR), or stable disease were eligible for up to 17 cycles (approximately 1 year) of retreatment (second course) with pembrolizumab after disease progression if safety criteria were met.
Assessments
Tumor imaging by computed tomography (preferred) or magnetic resonance imaging occurred at baseline, every 9 weeks for the first year, and every 12 weeks thereafter. Survival status was assessed every 12 weeks after documented disease progression or the start of a new anticancer treatment.
In cohort D, MSI/MMR status was determined retrospectively by polymerase chain reaction (PCR)–based assays at a central laboratory. In cohort K (patients with tumors that were MSI-H/dMMR), MSI/MMR status was assessed prospectively by PCR and/or immunohistochemistry (IHC) at a local laboratory.16 MSI/MMR status was determined by examining either loss of protein expression by IHC of four MMR enzymes (MLH1, MSH2, MSH6, and PMS2) or analysis of five tumor microsatellite loci using PCR-based assays (either the five mononucleotide loci [BAT25, BAT26, NR21, NR24, and Mono27] or the five mixed mononucleotide and dinucleotide loci [BAT25, BAT26, Di 5S346, Di 2S123, and Di 17S250]).15 MSI-H/dMMR was defined as ≥ 1 of four MMR enzymes absent by IHC or ≥ 2 allelic loci size shifts among five microsatellite markers as detected by PCR.
PD-L1 expression was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies, Carpinteria, CA). PD-L1 was measured using the combined positive score, defined as the number of PD-L1–staining cells (tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumor cells, multiplied by 100.
AEs were assessed throughout the study and for 30 days after the end of treatment (90 days for serious AEs) and were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
End Points
A primary end point of the KEYNOTE-158 study was ORR, defined as the proportion of patients with CR or PR per RECIST version 1.1, as assessed by independent central radiologic review, in patients with any one of the tumor types enrolled and with a positive tumor sample for one of the prespecified biomarkers. MSI-H/dMMR was included among the prespecified biomarkers for evaluation of this end point. Secondary end points included duration of response (time from the first documented evidence of CR or PR until the sign of first documented disease progression or death, whichever occurred first) and progression-free survival (PFS; time from first dose to the first documented disease progression or death due to any cause, whichever occurred first) per RECIST version 1.1 as assessed by independent central radiologic review, overall survival (OS; time from first dose to death due to any cause), and safety.
Statistical Analyses
Efficacy was assessed in patients who received ≥ 1 dose of pembrolizumab and had been enrolled ≥ 26 weeks before data cutoff (to allow sufficient time for responses to occur and be assessed). Safety was assessed in all patients who received ≥ 1 dose of pembrolizumab. The point estimate and exact Clopper-Pearson 95% CIs were provided for ORR. The Kaplan-Meier method was used to estimate duration of response, PFS, and OS.
RESULTS
Patients
A total of 90 patients with MSI-H/dMMR endometrial cancer were enrolled in cohorts D (11 patients) or K (79 patients) of KEYNOTE-158 between February 1, 2016, and September 23, 2020, from 38 sites in 15 countries. As of the data cutoff date (October 5, 2020), 79 patients had received ≥ 1 dose of pembrolizumab, were enrolled ≥ 26 weeks before data cutoff, and were therefore included in the efficacy analysis population. All 90 patients were included in the safety analysis population. The median age was 64 years (range, 42-86 years), and 61% of patients had an Eastern Cooperative Oncology Group performance status of 1; 48% had received ≥ 2 lines of prior therapy, and the majority of patients (68%) had received prior radiation therapy (Table 1). The median duration of treatment was 8.3 months (range, 1 day to 26.9 months). At the time of data cutoff, 52 patients (58%) had discontinued treatment, 18 (20%) had completed 35 cycles of pembrolizumab, and 20 (22%) were continuing study treatment (Fig 1). Two patients received second-course pembrolizumab. In the efficacy population, the median time from first dose to data cutoff was 42.6 months (range, 6.4-56.1 months).
TABLE 1.
Patient Demographics and Baseline Characteristics
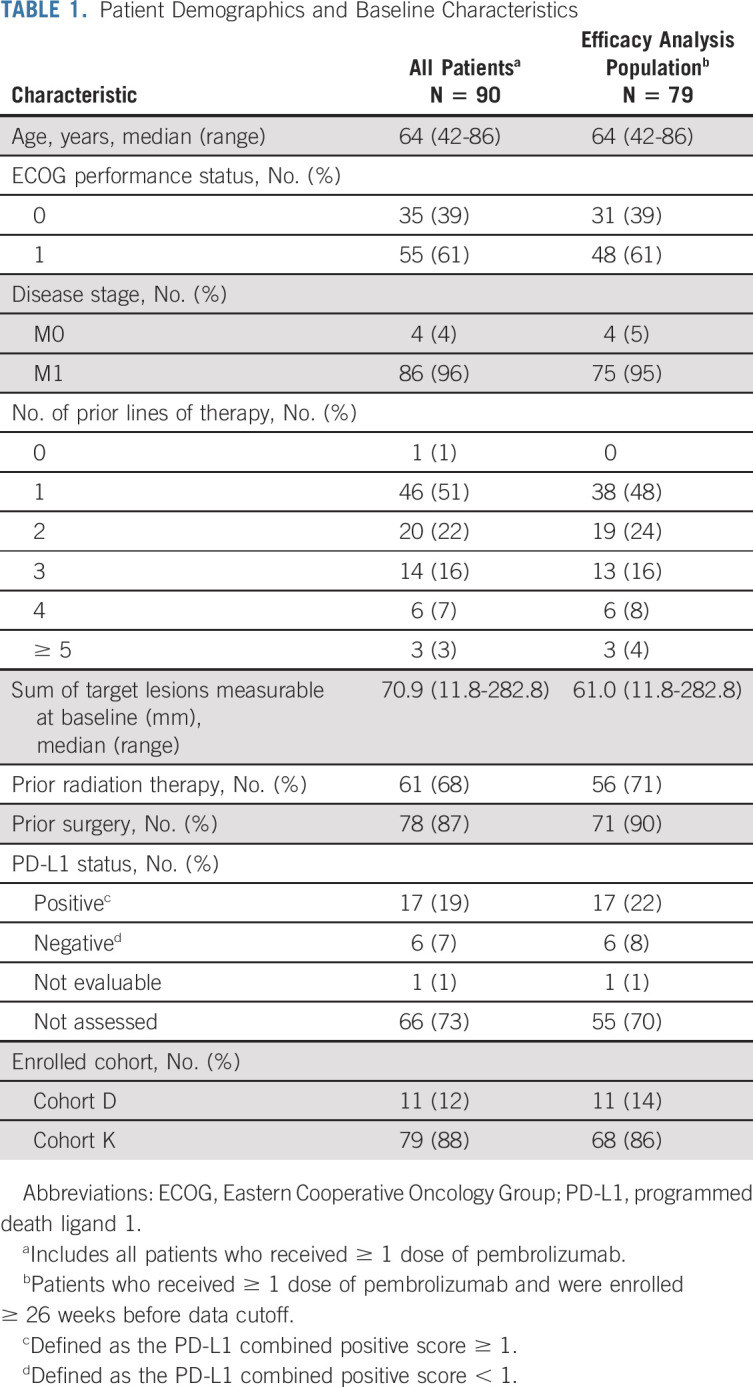
FIG 1.
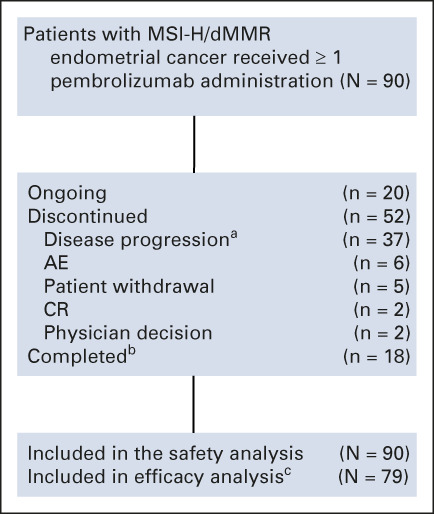
Disposition of patients' study medication status in the safety population (ie, all patients who received ≥ 1 dose of pembrolizumab). aIncludes patients with clinical and radiographic progression. bTwo patients received a second course of pembrolizumab, one of whom had second course up to cycle 3 and the other had second course up to cycle 12. cPatients who received ≥ 1 dose of pembrolizumab and had been enrolled ≥ 26 weeks before data cutoff. AE, adverse event; CR, complete response; dMMR; mismatch repair deficiency; MSI-H, high levels of microsatellite instability.
Efficacy Outcomes
Among 79 patients in the efficacy analysis population, 48% (95% CI, 37 to 60) had an objective response as determined by independent central radiologic review, including 11 patients (14%) with CR and 27 (34%) with PR (Table 2). An additional 14 patients (18%) had stable disease, 13 of whom had a reduction in tumor size from baseline. Among 75 evaluable patients in the efficacy population with ≥ 1 postbaseline tumor assessment, 56 (75%) had a reduction from baseline in target lesion size (four patients were not evaluable or had no assessment for response evaluation; Fig 2A). At the time of data cutoff, 21 of 38 patients with a confirmed response had an ongoing response, including eight of 11 patients with a confirmed CR (Fig 2B). Median duration of response was not reached (range, 2.9-49.7+ months). The Kaplan-Meier estimate of the proportion of patients with response duration ≥ 1 year was 88% and ≥ 2 years was 73%, with a plateau from ≥ 3 years at 68% (Fig 2C).
TABLE 2.
Objective Response in the Efficacy Analysis Populationa
FIG 2.

(A) Best percentage change from baseline in target lesion size. (B) Time to response and duration of response for patients with a response. (C) Kaplan-Meier analysis of duration of response per RECIST version 1.1. Light blue lines indicate 95% confidence bands. For each figure, all assessments are per RECIST version 1.1 by independent central review. aCR was the last overall response before data cutoff and was not confirmed as of the data cutoff date. CR, complete response; NR, not reached; PD, progressive disease; PR, partial response.
The ORR was 53% (95% CI, 36 to 69) among the 38 patients who had received < 2 lines of prior therapy and 44% (95% CI, 28 to 60) in the 41 patients who had received ≥ 2 lines of prior therapy (Table 2). The ORR was 52% (95% CI, 38 to 65) in the 56 patients with prior radiation therapy and 39% (95% CI, 20 to 61) in the 23 patients with no prior radiation therapy.
At data cutoff, 45 patients (57%) had experienced disease progression or died. Median PFS was 13.1 months (95% CI, 4.3 to 34.4 months). The Kaplan-Meier estimate of the PFS rate was 51% at 1 year, 41% at 2 years, and 37% at 3 and 4 years (Fig 3A). At the time of data cutoff, 29 (37%) had died. Median OS was not reached (95% CI, 27.2 months to not reached). The Kaplan-Meier estimate of the OS rate was 69% at 1 year and 64% at 2 years, with a plateau at 60% at 3 and 4 years (Fig 3B).
FIG 3.
Kaplan-Meier analysis of (A) PFS per RECIST version 1.1 by independent central radiologic review in the efficacy analysis population and (B) OS in the efficacy analysis population. Light blue lines indicate 95% confidence bands. NR, not reached; OS, overall survival; PFS, progression-free survival.
Safety
Among 90 patients included in the safety analysis population, AEs considered by the investigator to be related to study treatment occurred in 68 patients (76%), with grade 3 or 4 treatment-related AEs occurring in 11 patients (12%). There were no fatal treatment-related AEs. The most frequently occurring treatment-related AEs of any grade were pruritus (24%), fatigue (21%), and diarrhea (16%). The only grade ≥ 3 treatment-related AEs to occur in two or more patients were hyperglycemia, decreased lymphocyte count, and increased transaminases (n = 2 [2%] each; all were grade 3). There were two grade 4 treatment-related events—enterocolitis and decreased neutrophil count—both of which occurred in the same patient and subsequently resolved; no other patient experienced a grade 4 treatment-related AE. Six patients (7%) discontinued treatment because of a treatment-related AE: increased transaminases (n = 2), arthritis, drug-induced liver injury, enterocolitis, and rash (all n = 1 each; Table 3).
TABLE 3.
AEs in the Safety Population
Immune-mediated AEs and infusion reactions, regardless of attribution to study treatment or immune relatedness by the investigator, occurred in 25 patients (28%). The most frequently occurring of these events were hypothyroidism (14%), hyperthyroidism (8%), and infusion reactions (4%). Most immune-mediated AEs and infusion reactions were of grade 1 or 2 severity. Six patients (7%) had grade 3 or 4 immune-mediated AEs: severe skin reactions (n = 2), adrenal insufficiency, colitis, hepatitis, and type 1 diabetes mellitus (all, n = 1 each). There were no grade 4 or 5 infusion reactions or grade 5 immune-mediated AEs. Two patients (2%) discontinued because of an immune-mediated adverse event, including one each of colitis (resolved) and hepatitis (resolving), both of which were considered related to treatment.
DISCUSSION
In this analysis of outcomes among patients with previously treated MSI-H/dMMR advanced endometrial cancer in the KEYNOTE-158 study, pembrolizumab monotherapy demonstrated robust and clinically meaningful antitumor activity, with 48% of patients experiencing an objective response. Responses were durable: the median duration of response was not reached after a median follow-up of 42.6 months and approximately two thirds of patients were estimated to have a response duration of ≥ 3 years. As of data cutoff, responses were ongoing in 21 of 38 patients who had a confirmed response, including eight of 11 patients with a confirmed CR. In addition, PFS and OS outcomes were promising, with estimated 3-year rates of 37% and 60%, respectively. Median PFS was 13.1 months, and median OS was not reached. These findings are particularly encouraging in light of the low long-term survival rates typically observed in advanced endometrial cancer.6 The toxicity profile was manageable, and no new safety signals were identified. The current results confirm and extend initial findings from patients with MSI-H/dMMR advanced endometrial cancer enrolled in the KEYNOTE-158 study.15 In 2017, supported by findings from KEYNOTE-158, pembrolizumab received approval by the US Food and Drug Administration (FDA) for the treatment of patients with unresectable or metastatic MSI-H/dMMR solid tumors that have progressed after prior treatment and with no alternative treatment options.18
Among patients enrolled in KEYNOTE-158, 43 of 90 (48%) had received ≥ 2 lines of prior therapy. In efficacy analyses, pembrolizumab monotherapy was associated with high response rates in patients who received < 2 lines of prior therapy (n = 38) as well as in those who received ≥ 2 lines of prior therapy (n = 41). These results are particularly promising given the limited available treatment options for patients with endometrial cancer who experience disease progression after first-line therapy.3,5 The higher ORR among patients who had received < 2 lines of prior therapy (53% v 44%) may provide support for earlier use of pembrolizumab in this setting.
Pembrolizumab had manageable toxicity, and no new safety signals were identified. The majority of treatment-related AEs were mild to moderate in severity, and few patients discontinued owing to treatment-related AEs (n = 6) or immune-mediated AEs (n = 2). The overall safety profile of pembrolizumab was consistent with that observed in patients with a variety of MSI-H/dMMR advanced solid tumors enrolled in KEYNOTE-158.15 In addition, treatment-related toxicity and incidence of immune-mediated AEs and infusion reactions were consistent with previous experience with pembrolizumab monotherapy across a range of solid tumor types.13,17,19-21
A limitation of this study was its single-arm design, which precludes a definitive comparison with outcomes with standard-of-care therapies. Although there is no standard-of-care second-line therapy in this setting, the ORR in this study was particularly robust when compared with treatment with cytotoxic agents as second-line therapy, which have been associated with response rates ranging up to 27% in patients with recurrent endometrial cancer.5,22 Furthermore, too few patients were assessed for PD-L1 status to allow for a meaningful evaluation of outcomes by PD-L1 expression.
Results from other studies evaluating anti–PD-1 and anti–PD-L1 agents in MSI-H/dMMR endometrial cancer have also provided evidence of activity for these agents in this setting. A recent update from the phase I GARNET study evaluating the anti‒PD-1 antibody dostarlimab reported an ORR of 45.5% in patients with dMMR endometrial cancer (primary efficacy analysis population; n = 110).23 In an earlier analysis from the GARNET study, median OS was not reached and the median PFS was 8.1 months with a median study follow-up of 11.2 months.24 In April 2021, the FDA granted accelerated approval of dostarlimab for patients with previously treated dMMR recurrent or advanced endometrial cancer on the basis of results from the GARNET study.25 The FDA subsequently granted accelerated approval of dostarlimab-gxly for patients with dMMR recurrent or advanced solid tumors that have progressed on or after prior treatment with no satisfactory alternative treatment options in August 2021. In the phase II PHAEDRA study, the anti–PD-L1 antibody durvalumab demonstrated an ORR of 40% in patients (n = 35) with dMMR advanced endometrial cancer.26 In a phase II study evaluating the anti–PD-L1 antibody avelumab in patients with dMMR endometrial cancer (n = 15), the ORR was 27%, median OS was not reached, and median PFS was 4.4 months.27 Pembrolizumab demonstrated efficacy in patients with previously treated advanced endometrial cancer when combined with the multireceptor tyrosine kinase inhibitor lenvatinib irrespective of tumor MSI status in the nonrandomized KEYNOTE-146 study.28 On the basis of these results from KEYNOTE-146, pembrolizumab plus lenvatinib has been approved by the FDA for advanced endometrial cancer that is not MSI-H/dMMR with disease progression after prior systemic therapy and is not a candidate for curative surgery or radiation.18 This combination was also shown to improve PFS, OS, and ORR compared with doxorubicin or paclitaxel in the phase III KEYNOTE-775 study both in the overall study population (N = 827, including 130 patients with dMMR disease) and in patients with MMR-proficient disease (N = 697).29
In the current analysis, MSI-H/dMMR was assessed by IHC and PCR. In cohort D, MSI-H/dMMR expression was evaluated by PCR at a central laboratory, and in cohort K, MSI-H/dMMR expression was assessed either by IHC or PCR at a local laboratory. Although IHC and PCR analyses demonstrate high concordance in endometrial cancer and are widely recommended methods for assessing MSI-H/dMMR for immunotherapy in endometrial cancer,30 a validated testing method is yet to be established. Our results demonstrate that commonly used methods of IHC (with four MMR proteins) and PCR (using tumor microsatellite loci) can identify patients with endometrial cancer, for whom pembrolizumab may be an appropriate treatment.
In summary, pembrolizumab demonstrated robust and durable antitumor activity with manageable toxicity in patients with advanced MSI-H/dMMR endometrial cancer. These findings support the use of pembrolizumab as a treatment option for patients with advanced MSI-H/dMMR endometrial cancer with treatment failure on prior therapy.
ACKNOWLEDGMENT
We thank the patients and their families and caregivers for participating in this study, along with all investigators and site personnel. Medical writing assistance was provided by Christabel Wilson, MSc, of ICON plc (North Wales, PA).
David M. O'Malley
Consulting or Advisory Role: Janssen Oncology, AstraZeneca, Clovis Oncology, Tesaro, Novocure, AbbVie, Genentech/Roche, OncoQuest, Immunogen, GOG Foundation, Translational Genomics Research Institute, Agenus, Marker Therapeutics, Eisai, Genelux, Iovance Biotherapeutics, Ambry Genetics, Tarveda Therapeutics, Leap Therapeutics, Myriad Genetics, GlaxoSmithKline, Regeneron, Sorrento Therapeutics, Rubius Therapeutics, Elevar Therapeutics, Novartis, Seattle Genetics, BBI Healthcare, Arquer Diagnostics, Toray Medical, Takeda, InxMed, Celsion, Roche Diagnostics MSA
Research Funding: Amgen (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), Regeneron (Inst), Immunogen (Inst), Janssen Research & Development (Inst), Clovis Oncology (Inst), EMD Serono (Inst), Ergomed (Inst), Ajinomoto (Inst), Immunogen (Inst), Cerulean Pharma (Inst), PharmaMar (Inst), Array BioPharma (Inst), Bristol Myers Squibb (Inst), Agenus (Inst), Tesaro (Inst), TRACON Pharma (Inst), Genmab (Inst), Seattle Genetics (Inst), Iovance Biotherapeutics (Inst), Leap Therapeutics (Inst), Merck (Inst), AbbVie/Stemcentrx (Inst), AbbVie (Inst), Mersana (Inst), Eisai (Inst), BBI Healthcare (Inst), Sumitomo Dainippon Pharma Oncology (Inst)
Giovanni Mendonca Bariani
Consulting or Advisory Role: Libbs
Research Funding: mAbxience, Merck Sharp & Dohme, Bristol Myers Squibb
Travel, Accommodations, Expenses: Lilly
Philippe A. Cassier
Honoraria: Novartis, Roche/Genentech, Amgen, AstraZeneca, Merck Serono
Research Funding: Novartis (Inst), Roche/Genentech (Inst), Lilly (Inst), Blueprint Medicines (Inst), Bayer (Inst), AstraZeneca (Inst), Celgene (Inst), Plexxikon (Inst), AbbVie (Inst), Bristol Myers Squibb (Inst), Merck Serono (Inst), Merck Sharp & Dohme (Inst), Taiho Pharmaceutical (Inst), Toray Industries (Inst), Transgene (Inst), Loxo (Inst), GlaxoSmithKline (Inst), Innate Pharma (Inst), Janssen (Inst)
Travel, Accommodations, Expenses: Roche, Amgen, Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Netris Pharma, Bayer, Merck Serono
Aurelien Marabelle
Stock and Other Ownership Interests: PEGASCY (Inst), HiFiBiO Therapeutics, Shattuck Labs, Centessa Pharmaceuticals (Inst)
Honoraria: Bristol Myers Squibb, AstraZeneca/MedImmune, Oncovir
Consulting or Advisory Role: Lytix Biopharma, EISAI, Pierre Fabre, AstraZeneca, Servier, Roche, Redx Pharma, Sotio, Innate Pharma, ImCheck therapeutics, MSD, OSE Immunotherapeutics, HiFiBiO Therapeutics, MedinCell, Centessa Pharmaceuticals
Speakers' Bureau: BMS
Research Funding: Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Transgene (Inst), MSD (Inst)
Patents, Royalties, Other Intellectual Property: Monoclonal antibodies against CD81 (Stanford University)
Travel, Accommodations, Expenses: MSD, AstraZeneca
Aaron R. Hansen
Consulting or Advisory Role: Merck, GlaxoSmithKline, Bristol Myers Squibb, Eisai, Novartis, AstraZeneca
Research Funding: Karyopharm Therapeutics (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim, GlaxoSmithKline (Inst), Roche/Genentech (Inst), Janssen (Inst), AstraZeneca/MedImmune (Inst), Astellas Pharma (Inst), BioNTech (Inst), Pfizer/EMD Serono (Inst), Neoleukin Therapeutics (Inst)
Ana De Jesus Acosta
Consulting or Advisory Role: Merck
Research Funding: Merck (Inst), AstraZeneca (Inst)
Wilson H. Miller Jr
Honoraria: Bristol Myers Squibb Foundation, Merck, Roche, Novartis, GlaxoSmithKline, Mylan, EMD Serono, Amgen
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Roche, Novartis, Amgen, GlaxoSmithKline, Mylan, EMD Serono
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Roche (Inst), AstraZeneca (Inst), Merck (Inst), MethylGene (Inst), Bayer (Inst), Amgen (Inst), MedImmune (Inst), Pfizer (Inst), Esperas Pharma (Inst), Astellas Pharma (Inst), Sanofi (Inst), Incyte (Inst), Array BioPharma (Inst), Exelixis (Inst), Mimic Technologies (Inst), Ocellaris Pharma (Inst), Canadian Institutes of Health Research (CIHR) (Inst), Canadian Research Society (Inst), Terry Fox Research Institute (Inst), Samuel Waxman Cancer Research Foundation (Inst), Canadian Cancer Society Research Institute (CCSRI) (Inst)
Antoine Italiano
Honoraria: Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, IPSEN
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly
Research Funding: Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono
Patents, Royalties, Other Intellectual Property: BMS
Lei Xu
Employment: Merck
Stock and Other Ownership Interests: Merck
Fan Jin
Employment: Merck
Stock and Other Ownership Interests: Merck
Travel, Accommodations, Expenses: Merck
Kevin Norwood
Employment: Merck
Michele Maio
Stock and Other Ownership Interests: Theravance, Epigen Therapeutics
Honoraria: Bristol Myers Squibb, AstraZeneca, Roche, MSD, Merck, Amgen, Pierre Fabre, Alfasigma, Sanofi, Lilly
Consulting or Advisory Role: Bristol Myers Squibb, Roche, AstraZeneca, MSD, Merck, Pierre Fabre, Alfasigma
Patents, Royalties, Other Intellectual Property: DNA Hypomethylating agents for cancer therapy
Travel, Accommodations, Expenses: Bristol Myers Squibb, AstraZeneca, Roche, MSD, Merck, Amgen, Pierre Fabre, Alfasigma
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the European Society for Medical Oncology Annual Virtual Meeting 2021, September 16-21, 2021.
SUPPORT
Supported by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, USA.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
AUTHOR CONTRIBUTIONS
Conception and design: David M. O'Malley, Kevin Norwood
Provision of study materials or patients: Giovanni Mendonca Bariani, Philippe A. Cassier, Aurelien Marabelle, Ana De Jesus Acosta, Wilson H. Miller Jr, Tamar Safra, Antoine Italiano, Linda Mileshkin, Michele Maio
Collection and assembly of data: David M. O'Malley, Giovanni Mendonca Bariani, Philippe A. Cassier, Aurelien Marabelle, Aaron R. Hansen, Ana De Jesus Acosta, Wilson H. Miller Jr, Tamar Safra, Antoine Italiano, Linda Mileshkin, Fan Jin, Kevin Norwood, Michele Maio
Data analysis and interpretation: David M. O'Malley, Giovanni Mendonca Bariani, Philippe A. Cassier, Aurelien Marabelle, Ana De Jesus Acosta, Wilson H. Miller Jr, Antoine Italiano, Linda Mileshkin, Lei Xu, Fan Jin, Kevin Norwood, Michele Maio
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pembrolizumab in Patients With Microsatellite Instability–High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David M. O'Malley
Consulting or Advisory Role: Janssen Oncology, AstraZeneca, Clovis Oncology, Tesaro, Novocure, AbbVie, Genentech/Roche, OncoQuest, Immunogen, GOG Foundation, Translational Genomics Research Institute, Agenus, Marker Therapeutics, Eisai, Genelux, Iovance Biotherapeutics, Ambry Genetics, Tarveda Therapeutics, Leap Therapeutics, Myriad Genetics, GlaxoSmithKline, Regeneron, Sorrento Therapeutics, Rubius Therapeutics, Elevar Therapeutics, Novartis, Seattle Genetics, BBI Healthcare, Arquer Diagnostics, Toray Medical, Takeda, InxMed, Celsion, Roche Diagnostics MSA
Research Funding: Amgen (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), Regeneron (Inst), Immunogen (Inst), Janssen Research & Development (Inst), Clovis Oncology (Inst), EMD Serono (Inst), Ergomed (Inst), Ajinomoto (Inst), Immunogen (Inst), Cerulean Pharma (Inst), PharmaMar (Inst), Array BioPharma (Inst), Bristol Myers Squibb (Inst), Agenus (Inst), Tesaro (Inst), TRACON Pharma (Inst), Genmab (Inst), Seattle Genetics (Inst), Iovance Biotherapeutics (Inst), Leap Therapeutics (Inst), Merck (Inst), AbbVie/Stemcentrx (Inst), AbbVie (Inst), Mersana (Inst), Eisai (Inst), BBI Healthcare (Inst), Sumitomo Dainippon Pharma Oncology (Inst)
Giovanni Mendonca Bariani
Consulting or Advisory Role: Libbs
Research Funding: mAbxience, Merck Sharp & Dohme, Bristol Myers Squibb
Travel, Accommodations, Expenses: Lilly
Philippe A. Cassier
Honoraria: Novartis, Roche/Genentech, Amgen, AstraZeneca, Merck Serono
Research Funding: Novartis (Inst), Roche/Genentech (Inst), Lilly (Inst), Blueprint Medicines (Inst), Bayer (Inst), AstraZeneca (Inst), Celgene (Inst), Plexxikon (Inst), AbbVie (Inst), Bristol Myers Squibb (Inst), Merck Serono (Inst), Merck Sharp & Dohme (Inst), Taiho Pharmaceutical (Inst), Toray Industries (Inst), Transgene (Inst), Loxo (Inst), GlaxoSmithKline (Inst), Innate Pharma (Inst), Janssen (Inst)
Travel, Accommodations, Expenses: Roche, Amgen, Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Netris Pharma, Bayer, Merck Serono
Aurelien Marabelle
Stock and Other Ownership Interests: PEGASCY (Inst), HiFiBiO Therapeutics, Shattuck Labs, Centessa Pharmaceuticals (Inst)
Honoraria: Bristol Myers Squibb, AstraZeneca/MedImmune, Oncovir
Consulting or Advisory Role: Lytix Biopharma, EISAI, Pierre Fabre, AstraZeneca, Servier, Roche, Redx Pharma, Sotio, Innate Pharma, ImCheck therapeutics, MSD, OSE Immunotherapeutics, HiFiBiO Therapeutics, MedinCell, Centessa Pharmaceuticals
Speakers' Bureau: BMS
Research Funding: Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Transgene (Inst), MSD (Inst)
Patents, Royalties, Other Intellectual Property: Monoclonal antibodies against CD81 (Stanford University)
Travel, Accommodations, Expenses: MSD, AstraZeneca
Aaron R. Hansen
Consulting or Advisory Role: Merck, GlaxoSmithKline, Bristol Myers Squibb, Eisai, Novartis, AstraZeneca
Research Funding: Karyopharm Therapeutics (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim, GlaxoSmithKline (Inst), Roche/Genentech (Inst), Janssen (Inst), AstraZeneca/MedImmune (Inst), Astellas Pharma (Inst), BioNTech (Inst), Pfizer/EMD Serono (Inst), Neoleukin Therapeutics (Inst)
Ana De Jesus Acosta
Consulting or Advisory Role: Merck
Research Funding: Merck (Inst), AstraZeneca (Inst)
Wilson H. Miller Jr
Honoraria: Bristol Myers Squibb Foundation, Merck, Roche, Novartis, GlaxoSmithKline, Mylan, EMD Serono, Amgen
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Roche, Novartis, Amgen, GlaxoSmithKline, Mylan, EMD Serono
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Roche (Inst), AstraZeneca (Inst), Merck (Inst), MethylGene (Inst), Bayer (Inst), Amgen (Inst), MedImmune (Inst), Pfizer (Inst), Esperas Pharma (Inst), Astellas Pharma (Inst), Sanofi (Inst), Incyte (Inst), Array BioPharma (Inst), Exelixis (Inst), Mimic Technologies (Inst), Ocellaris Pharma (Inst), Canadian Institutes of Health Research (CIHR) (Inst), Canadian Research Society (Inst), Terry Fox Research Institute (Inst), Samuel Waxman Cancer Research Foundation (Inst), Canadian Cancer Society Research Institute (CCSRI) (Inst)
Antoine Italiano
Honoraria: Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, IPSEN
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly
Research Funding: Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono
Patents, Royalties, Other Intellectual Property: BMS
Lei Xu
Employment: Merck
Stock and Other Ownership Interests: Merck
Fan Jin
Employment: Merck
Stock and Other Ownership Interests: Merck
Travel, Accommodations, Expenses: Merck
Kevin Norwood
Employment: Merck
Michele Maio
Stock and Other Ownership Interests: Theravance, Epigen Therapeutics
Honoraria: Bristol Myers Squibb, AstraZeneca, Roche, MSD, Merck, Amgen, Pierre Fabre, Alfasigma, Sanofi, Lilly
Consulting or Advisory Role: Bristol Myers Squibb, Roche, AstraZeneca, MSD, Merck, Pierre Fabre, Alfasigma
Patents, Royalties, Other Intellectual Property: DNA Hypomethylating agents for cancer therapy
Travel, Accommodations, Expenses: Bristol Myers Squibb, AstraZeneca, Roche, MSD, Merck, Amgen, Pierre Fabre, Alfasigma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Gong TT, Liu FH, et al. : Global, regional, and national burden of endometrial cancer, 1990-2017: Results from the Global Burden of Disease study, 2017. Front Oncol 9:1440, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN) : NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Uterine Neoplasms. Version 2.2021. https://www.nccn.org/home [Google Scholar]
- 4.Miller DS, Filiaci VL, Mannel RS, et al. : Carboplatin and paclitaxel for advanced endometrial cancer: Final overall survival and adverse event analysis of a phase III trial (NRG Oncology/GOG0209). J Clin Oncol 38:3841-3850, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming GF: Second-line therapy for endometrial cancer: The need for better options. J Clin Oncol 33:3535-3540, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2021. CA Cancer J Clin 71:7-33, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Ryan NAJ, Glaire MA, Blake D, et al. : The proportion of endometrial cancers associated with Lynch syndrome: A systematic review of the literature and meta-analysis. Genet Med 21:2167-2180, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonneville R, Krook MA, Kautto EA, et al. : Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol 10.1200/PO.17.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pakish JB, Zhang Q, Chen Z, et al. : Immune microenvironment in microsatellite-instable endometrial cancers: Hereditary or sporadic origin matters. Clin Cancer Res 23:4473-4481, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer LA, Broaddus RR, Lu KH: Endometrial cancer and Lynch syndrome: Clinical and pathologic considerations. Cancer Control 16:14-22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le DT, Uram JN, Wang H, et al. : PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509-2520, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howitt BE, Shukla SA, Sholl LM, et al. : Association of polymerase E-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol 1:1319-1323, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Ott PA, Bang YJ, Berton-Rigaud D, et al. : Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: Results from the KEYNOTE-028 study. J Clin Oncol 35:2535-2541, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Le DT, Durham JN, Smith KN, et al. : Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409-413, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marabelle A, Le DT, Ascierto PA, et al. : Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol 38:1-10, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Malley D, Marabelle A, De Jesus-Acosta A, et al. : Pembrolizumab in patients with MSI-H advanced endometrial cancer from the KEYNOTE-158 study. Ann Oncol 30:v425-v426, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung HC, Ros W, Delord JP, et al. : Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol 37:1470-1478, 2019 [DOI] [PubMed] [Google Scholar]
- 18.KEYTRUDA® (Pembrolizumab). Full Prescribing Information. Merck Sharp & Dohme Corp, Whitehouse Station, NJ, 2021 [Google Scholar]
- 19.Mok TSK, Wu YL, Kudaba I, et al. : Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 393:1819-1830, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Herbst RS, Baas P, Kim DW, et al. : Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non–small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387:1540-1550, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Ott PA, Bang YJ, Piha-Paul SA, et al. : T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol 37:318-327, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Miller DS, Scambia G, Bondarenko I, et al. : ZoptEC: Phase III randomized controlled study comparing zoptarelin with doxorubicin as second line therapy for locally advanced, recurrent, or metastatic endometrial cancer (NCT01767155). Presented at American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 1-5, 2018
- 23.Oaknin A, Gilbert L, Tinker AV, et al. : Interim analysis of the immune-related endpoints of the mismatch repair deficient (dMMR) and proficient (MMRp) endometrial cancer cohorts from the GARNET study. Presented at Society of Gynecologic Oncology (SGO) Annual Meeting on Women's Cancer, March 9-25, 2021; Virtual
- 24.Oaknin A, Tinker AV, Gilbert L, et al. : Clinical activity and safety of the anti-programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: A nonrandomized phase 1 clinical trial. JAMA Oncol 6:1-7, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Food and Drug Administration : FDA Grants Accelerated Approval to Dostarlimab-Gxly for dMMR Endometrial Cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-dostarlimab-gxly-dmmr-endometrial-cancer [Google Scholar]
- 26.Antill YC, Kok PS, Robledo K, et al. : Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: The phase II PHAEDRA trial (ANZGOG1601). J Clin Oncol 37, 2019. (suppl; abstr 5501) [Google Scholar]
- 27.Konstantinopoulos PA, Luo W, Liu JF, et al. : Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J Clin Oncol 37:2786-2794, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makker V, Taylor MH, Aghajanian C, et al. : Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol 38:2981-2992, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makker V, Colombo N, Casado Herraez A, et al. : A multicenter, open-label, randomized, phase III study to compare the efficacy and safety of lenvatinib in combination with pembrolizumab versus treatment of physician's choice in patients with advanced endometrial cancer. Presented at Society of Gynecologic Oncology (SGO) Annual Meeting on Women's Cancer, March 9-25, 2021; Virtual
- 30.Luchini C, Bibeau F, Ligtenberg MJL, et al. : ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann Oncol 30:1232-1243, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.





