PURPOSE
Balstilimab (antiprogrammed death-1) and zalifrelimab (anticytotoxic T-lymphocyte–associated antigen-4) are two new checkpoint inhibitors emerging as promising investigational agents for the treatment of advanced cervical cancer. This phase II trial (ClinicalTrials.gov identifier: NCT03495882) evaluated the combination of balstilimab plus zalifrelimab in patients with recurrent and/or metastatic cervical cancer who relapsed after prior platinum-based therapy.
PATIENTS AND METHODS
Patients were intravenously dosed with balstilimab 3 mg/kg once every 2 weeks and zalifrelimab 1 mg/kg once every 6 weeks, for up to 24 months. The primary end point was objective response rate (ORR, RECIST version 1.1, assessed by independent central review). Secondary end points included duration of response, safety and tolerability, and survival.
RESULTS
In total, 155 women (median age, 50 years [range, 24-76 years]) were enrolled and treated with balstilimab plus zalifrelimab; 125 patients had measurable disease at baseline and one prior line of platinum-based therapy in the advanced setting, and these patients constituted the efficacy-evaluable population. The median follow-up was 21 months. The confirmed ORR was 25.6% (95% CI, 18.8 to 33.9), including 10 complete responders and 22 partial responders, with median duration of response not reached (86.5%, 75.5%, and 64.2% at 6, 9, and 12 months, respectively). The ORRs were 32.8% and 9.1% in patients with programmed death ligand-1–positive and programmed death ligand-1–negative tumors, respectively. For patients with squamous cell carcinoma, the ORR was 32.6%. The overall disease control rate was 52% (95% CI, 43.3 to 60.6). Hypothyroidism (14.2%) and hyperthyroidism (7.1%) were the most common immune-mediated adverse events.
CONCLUSION
Promising and durable clinical activity, with favorable tolerability, was seen in this largest trial to date evaluating dual programmed death-1/cytotoxic T-lymphocyte–associated antigen-4 blockade in patients with recurrent and/or metastatic cervical cancer. Further investigation of the balstilimab and zalifrelimab combination in this setting is continuing.
INTRODUCTION
Cervical cancer remains the most common gynecologic malignancy and fourth leading cause of cancer mortality in women worldwide.1 Strides are being made to improve the outlook for patients with recurrent and/or metastatic disease, yet current treatments remain suboptimal,2-4 particularly for those who progress after first-line chemotherapy doublet plus bevacizumab treatment.5 To date, the only immunotherapeutic indicated for use in this setting is the antiprogrammed death-1 (PD-1) antibody pembrolizumab, which received accelerated approval for the treatment of patients with programmed death ligand-1 (PD-L1)–positive (defined as a combined positive score ≥ 1%) recurrent and/or metastatic cervical cancer after disease progression on or after chemotherapy. This approval established the feasibility of targeting the PD-1 and PD-L1 immune checkpoint axis as a therapeutic approach in this malignancy.6-9 More recently, cemiplimab became the first PD-1 inhibitor to show an overall survival (OS) benefit when given as monotherapy to women with previously treated, recurrent and/or metastatic cervical cancer, on the basis of the findings of the phase III EMPOWER-CERVICAL/GOG-3016/ENGOT-CX9 study (NCT03257267).10 Despite this progress, the majority of patients fail to respond to single-agent immunotherapy11 and considerable opportunity exists to improve on current outcomes.
CONTEXT
Key Objective
This large phase II study assessed the feasibility and effectiveness of dual immune checkpoint blockade using two novel agents, the antiprogrammed death-1 antibody balstilimab and the anticytotoxic T-lymphocyte–associated antigen-4 antibody zalifrelimab, in patients with recurrent and/or metastatic cervical cancer who had progressed after prior platinum-based therapy.
Knowledge Generated
Balstilimab plus zalifrelimab demonstrated encouraging and durable clinical activity in patients with previously treated, recurrent and/or metastatic cervical cancer. The high proportion of complete responders and activity irrespective of tumor programmed death ligand-1 status or histology confirm the feasibility of dual targeted immunotherapy for this disease. The safety profile was manageable and consistent with that reported for other programmed death-1/cytotoxic T-lymphocyte–associated antigen-4 inhibitor combinations.
Relevance
This novel combination regimen provides meaningful clinical benefit for a patient population with significant unmet medical need and lack of effective therapies. On the basis of these findings, further evaluation of balstilimab plus zalifrelimab as second-line treatment in this disease setting is warranted.
Dual targeted immunotherapy is a clinically validated strategy for optimizing antitumor activity over anti–PD-1 monotherapy alone,12 typically involving the addition of antibodies directed to cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4).11 Mechanistically, inhibition of PD-1 and/or PD-L1 signaling can restore the responsiveness of tumor reactive T cells that become inactivated after prolonged exposure to chronic stimulation within the tumor microenvironment.13 By contrast, CTLA-4 blockade promotes activation of effector T cells and reduces the suppressive activity of regulatory T cells, leading to enhanced antitumor immunity.14 These distinct yet complementary mechanisms of action underlie the improved outcomes seen with concomitant blockade of the PD-1 and CTLA-4 pathways.
The most widely used anti–PD-1 and CTLA-4 combination is nivolumab plus ipilimumab, which is approved across a broad array of metastatic solid tumors.15 The early experience with this combination showed that although treatment could significantly improve response rates and prolong survival, it also led to an increased incidence of immune-mediated adverse events (imAEs).16-19 Immune-related toxicity has been reduced by modifying the dose and schedule of the CTLA-4 antibodies, specifically lower and less frequent dosing of ipilimumab. The evaluation of other immunomodulatory combinations remains a highly active area of clinical research,11 with the goal of maximizing antitumor immunity and identifying agents that preserve the superior efficacy of dual PD-1 and CTLA-4 inhibition along with favorable tolerability.
Balstilimab (AGEN2034) is a fully human monoclonal antibody that binds with high affinity to PD-1, preventing the interaction between the receptor and its ligands PD-L1 and/or PD-L2.20 As monotherapy, balstilimab demonstrated clinical activity in a large phase II study in patients with recurrent and/or metastatic cervical cancer who had progressed after prior platinum-based therapy,21 and a randomized phase III trial comparing balstilimab with investigators' choice chemotherapy in this setting has recently been initiated (BRAVA, NCT04943627). Coupled with a favorable safety profile, balstilimab represents an attractive candidate for combination-based immunotherapeutic approaches. Zalifrelimab (AGEN1884) is a novel CTLA-4–targeting monoclonal antibody that antagonizes the inhibitory checkpoints of immune cell activation regulated by CTLA-4 signaling. Preclinically, zalifrelimab potentiated the activity of other immunomodulatory antibodies and combined effectively with PD-1 inhibition to elicit T-cell–associated proliferative responses in nonhuman primate models.22 Zalifrelimab is undergoing clinical evaluation as monotherapy in patients with PD-1–refractory solid tumors, where it has exhibited encouraging signals of activity and good tolerability,23 underscoring its potential as a suitable partnering agent.
Here, we present findings from a global phase II trial evaluating the safety and efficacy of balstilimab coadministered with zalifrelimab as second-line treatment for patients with recurrent and/or metastatic cervical cancer who had relapsed after prior platinum-based therapy. To our knowledge, this is the largest study report assessing dual targeting of PD-1 and CTLA-4 as a therapeutic modality in this disease setting to date.
PATIENTS AND METHODS
Study Design and Patients
This was an open-label, single-arm, global phase II clinical trial undertaken at 45 sites throughout the United States, Europe, South America, and Australia. Patients were enrolled from August 27, 2018, to May 7, 2020. The trial was conducted in accordance with the International Conference on Harmonisation guidelines for Good Clinical Practice and the Declaration of Helsinki. The study was approved by local Human Investigations Committee and institutional review board requirements at each participating center.
Eligible patients had a pathologically confirmed diagnosis of squamous cell carcinoma, adenosquamous carcinoma, or adenocarcinoma of the cervix, with recurrent and/or metastatic disease at the time of enrollment. Patients were required to have at least one lesion measurable by RECIST version 1.124 and disease that had relapsed after a first-line, platinum-based treatment regimen (patients who received chemotherapy concurrently with primary radiation or adjuvant chemotherapy after completion of radiation therapy and progressed within 6 months were permitted to enroll). Patients were included regardless of PD-L1 expression status at baseline, which was analyzed in archival tumor biopsy specimens using the validated PD-L1 immunohistochemistry 22C3 pharmDx assay25 at a central laboratory. Patients were additionally required to be age ≥ 18 years; have an Eastern Cooperative Oncology Group performance status score of 0 or 1; and have adequate hematologic, renal, and hepatic function. Key exclusion criteria included prior immune checkpoint targeted therapy, known hypersensitivity to humanized monoclonal antibodies, or > 1 systemic treatment regimen for recurrent/metastatic cervical cancer. All patients provided written informed consent in accordance with federal, local, and institutional guidelines.
Study Treatment
Patients received 3 mg/kg balstilimab once every two weeks plus 1 mg/kg zalifrelimab once every six weeks over a 6-week cycle, each of which included three doses of biweekly balstilimab and one dose of zalifrelimab. Balstilimab was delivered intravenously on days 1, 15, and 29 of each cycle; zalifrelimab was administered intravenously after completion of the balstilimab infusion on day 1 only. Patients were monitored for infusion reactions for at least 30 minutes after dosing. Treatment was continued until disease progression, development of unacceptable toxicity, or investigator and/or patient decision and permitted for up to 24 months.
End Points and Assessments
The primary efficacy end point was objective response rate (ORR), assessed by an independent end point review committee, according to RECIST version 1.1. To evaluate tumor response, computed tomography or magnetic resonance imaging was performed at baseline and every 6 weeks on-study. Secondary objectives included duration of response (DOR), disease control rate (DCR; defined as complete response [CR], partial response [PR], or stable disease [SD] ≥ 12 weeks), duration of SD, safety and tolerability, and survival. The association of PD-L1 expression with clinical response was an exploratory end point.
AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03 and monitored continuously from the time of the first study dose until at least 30 days after the last dose of study drug (safety follow-up visit) or for 90 days for adverse events of special interest. Adverse events of special interests included infusion-related reactions and imAEs.
Statistical Analysis
The data cutoff was April 29, 2021. Trial enrollment for this phase II cohort was planned for 150 patients. The primary end point of ORR was estimated as the binomial proportion of best overall response of a confirmed PR or CR and reported using a two-sided, 95% Wilson score CI. With 150 patients in the final analysis, the power to exclude the ORR of 10% by the lower limit of the 95% Wilson score interval is 94% assuming a true ORR for the combination of balstilimab with zalifrelimab of 20%. The sample size additionally provided ≥ 77% probability to observe an AE with an underlying rate of ≥ 1%. Kaplan-Meier methods were used to estimate medians and 95% CIs for DOR, DCR, progression-free survival (PFS), and OS. Descriptive statistics were used to summarize trial results, ie, statistics for continuous variables included medians and ranges and categorical variables were summarized by counts and percentages.
RESULTS
Patients
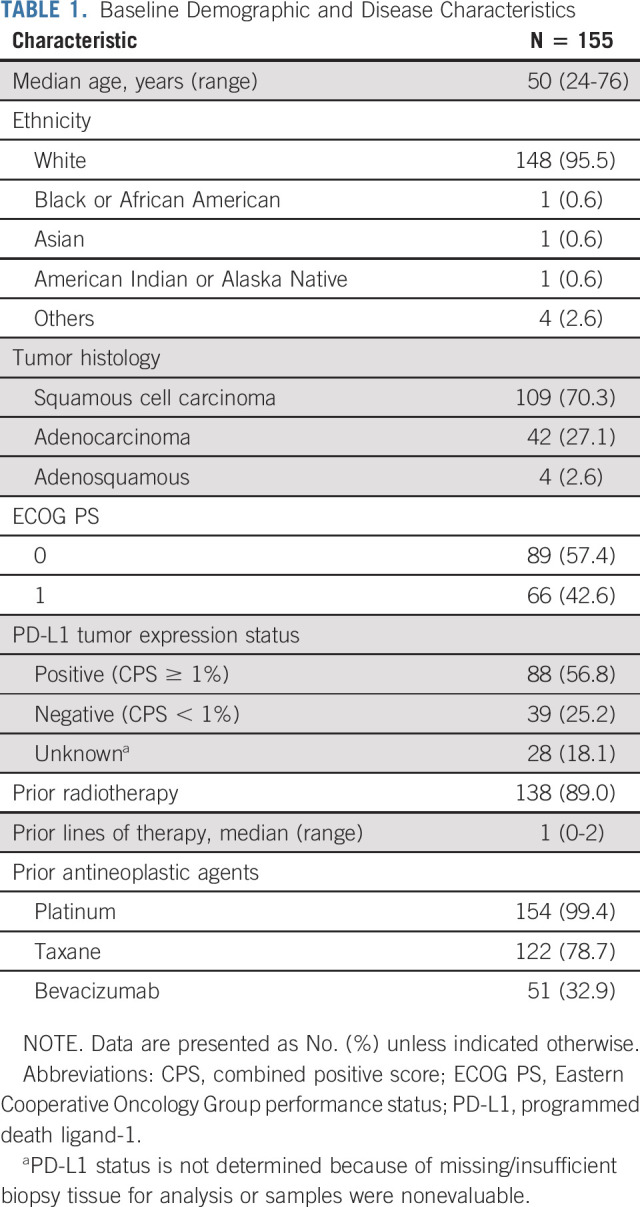
Between August 27, 2018, and May 7, 2020, 155 patients were enrolled and received combination treatment with balstilimab plus zalifrelimab. Baseline demographic and disease characteristics of this safety population are summarized in Table 1. The median age was 50 years (range, 24-76 years), and more than half of all patients had an Eastern Cooperative Oncology Group performance status of 0 (57.4%). Eighty-six patients (55.5%) had PD-L1–positive tumors (combined positive score ≥ 1%), and 38 (24.5%) were determined to be PD-L1–negative; either 20% of unknown cases were not evaluable or tissue was not available. The histologic breakdown was squamous cell carcinoma (70.3%), adenocarcinoma (27.1%), and adenosquamous carcinoma (2.6%). All but one patient had prior platinum exposure, and 51 patients (32.9%) had received bevacizumab as part of a previous therapeutic regimen. The median duration of follow-up (time on study from first dose to data cutoff) was 21.0 months (minimum, 11.8 months; maximum, 32.1+ months).
TABLE 1.
Baseline Demographic and Disease Characteristics
Efficacy
Ten patients enrolled into the study lacked measurable disease at baseline and were excluded from the efficacy analyses. Of the 145 patients with measurable disease and who had received at least one dose of combination treatment with balstilimab and zalifrelimab, 20 had received chemoradiotherapy (with or without neoadjuvant/adjuvant chemotherapy) in the front-line setting for locally advanced cervical cancer and progressed within 6 months before enrollment. Baseline characteristics and outcomes for this subset are listed in Appendix Tables A1 and A2 (online only). The remaining 125 patients had received one prior line of platinum-based therapy in the recurrent and/or metastatic disease setting. This defined group of patients who had relapsed after a platinum-based treatment regimen for metastatic disease constituted the efficacy-evaluable population, with 12 patients ongoing at time of analyses (Appendix Fig A1, online only).
The confirmed ORR in these patients, as assessed by RECIST v1.1 per independent central review, was 25.6% (95% CI, 18.8 to 33.9) and included 10 CRs (8.0%) and 22 PRs (17.6%; Table 2). Best change from baseline in target lesion size for all patients and changes in tumor burden over time for responders are shown in Figure 1. Examples of two patients who achieved a CR to combination therapy are presented in Figure 2. Responses were durable, with the median DOR not reached (NR; 95% CI, 9.7 to NR). The median time to response was 2.7 months (95% CI, 1.3 to 15.8). The DCR (CR, PR, or SD ≥ 12 weeks) was 52% (95% CI, 43.3 to 60.6).
TABLE 2.
Antitumor Activity Assessed by Independent Central Review
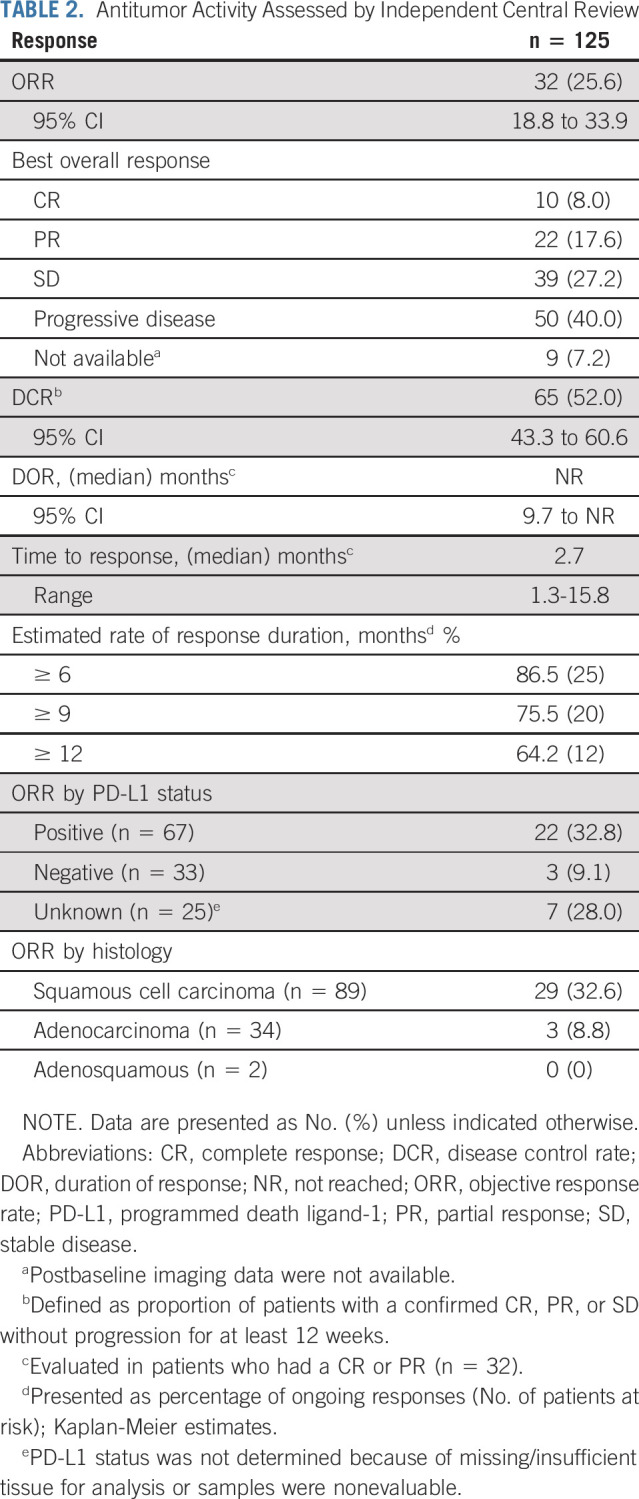
FIG 1.
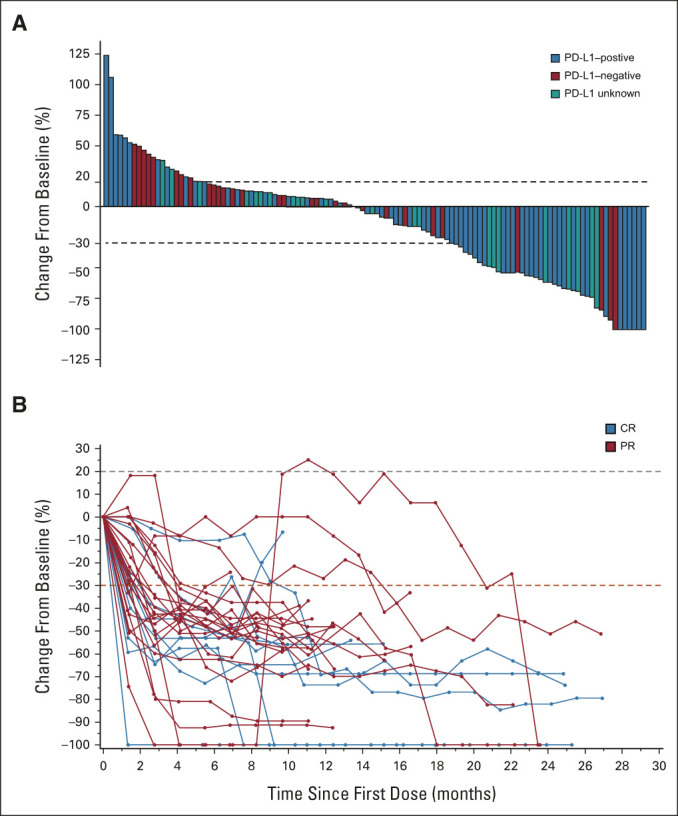
Antitumor activity of the balstilimab-zalifrelimab combination. (A) Maximum percentage change from baseline in the sum of tumor target lesion diameters according to RECIST, version 1.1. PD-L1 status is indicated by color coding of bars. (B) Kinetics of tumor burden over time presented as percentage change in RECIST sum in patients with confirmed responses to combination treatment. The dashed red line corresponds to the 30% decrease in tumor size. CR, complete response; PD-L1, programmed death ligand-1; PR, partial response.
FIG 2.
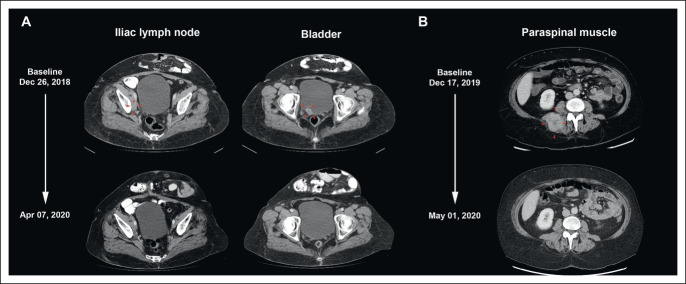
CRs to balstilimab-zalifrelimab combination therapy. (A) Computed tomography scans shown for a 59-year-old patient who received her first dose of the combination on January 22, 2019. Target lesions at baseline were an external iliac lymph node lesion (16 mm) and bladder lesion (23 mm). The first documentation of CR was on April 7, 2020, after 10 cycles of treatment, with regression of both target lesions. The patient remained cancer free and completed the study after 24 months, per protocol. (B) Scans for a 58-year-old patient, initially dosed on December 30, 2019, showing complete clearance of a large paraspinal muscle target lesion (49 mm) on May 1, 2020, after three cycles of treatment. CR, complete response.
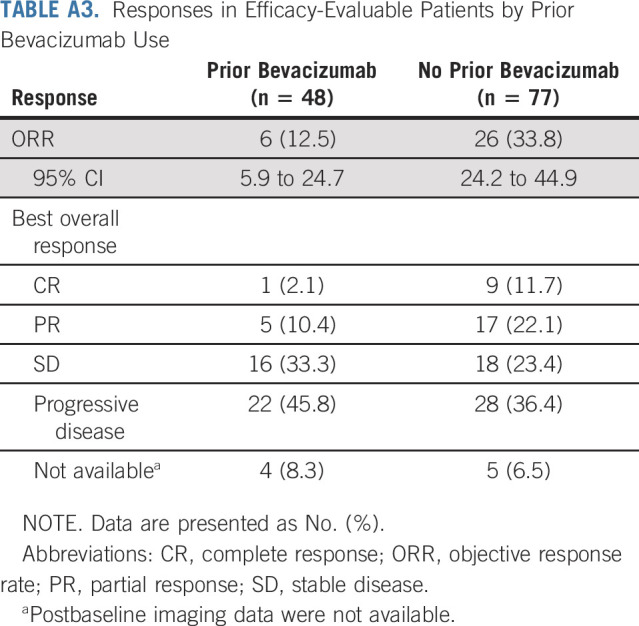
Exploratory subset analyses showed that the ORR was 32.8% (95% CI, 22.8 to 44.7) in patients with PD-L1–positive tumors (n = 67) and responses were also seen in patients who were PD-L1–negative (3 of 33, 9.1%; Table 2). Responses were observed across histologic subtypes, including in 32.6% of patients with squamous tumors (29 of 89) and 8.8% of patients with cervical adenocarcinomas (3 of 34). Six responses (one CR and five PRs) occurred in patients with prior bevacizumab treatment (6 of 48; 12.5%; Appendix Table A3, online only).
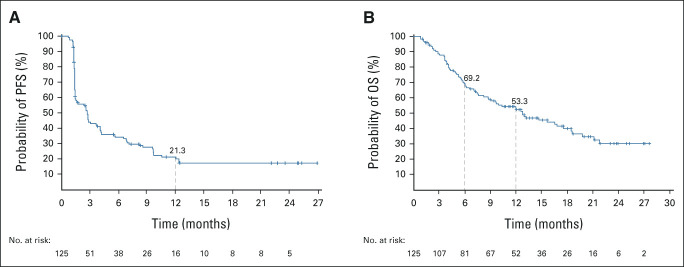
At data cutoff, the median PFS was 2.7 months (95% CI, 1.5 to 3.7; Fig 3A) and the 12-month PFS was 21.3% (95% CI, 14.1 to 29.4). The median OS was 12.8 months (95% CI, 8.8 to 17.6) with 6- and 12-month OS rates of 69.2% (95% CI, 60.1 to 76.7) and 53.3% (95% CI, 43.8 to 61.9), respectively (Fig 3B).
FIG 3.
Kaplan-Meier estimates of survival outcomes in the efficacy population (n = 125). (A) PFS and (B) OS assessed by the independent review committee per RECIST v1.1. OS, overall survival; PFS, progression-free survival.
Safety
The most common treatment-related adverse events (TRAEs) of any grade were hypothyroidism (16.8%), diarrhea (14.2%), fatigue (11.6%), and nausea (9.0%; Table 3), with the majority of events being either grade 1 or 2 in severity. The overall incidence of ≥ grade 3 TRAEs was 20.0%, with increased ALT (2.6%) and diarrhea (1.9%) most frequently reported. TRAEs leading to dose interruptions or discontinuations occurred in 19 (12.3%) and 12 (7.7%) patients, respectively. Elevations in AST (four patients) or ALT levels (three patients) were the leading cause of dose interruptions, and diarrhea was the leading cause of treatment discontinuation (three patients). Serious TRAEs were experienced by a total of 16 patients (10.3%), with immune-mediated enterocolitis (three patients) being the most common events. Three deaths (1.3%) assessed by investigators are related to treatment occurred on study. These involved individual cases attributed to pneumonitis, immune-mediated nephritis, and diabetes mellitus.
TABLE 3.
TRAEs (safety population)
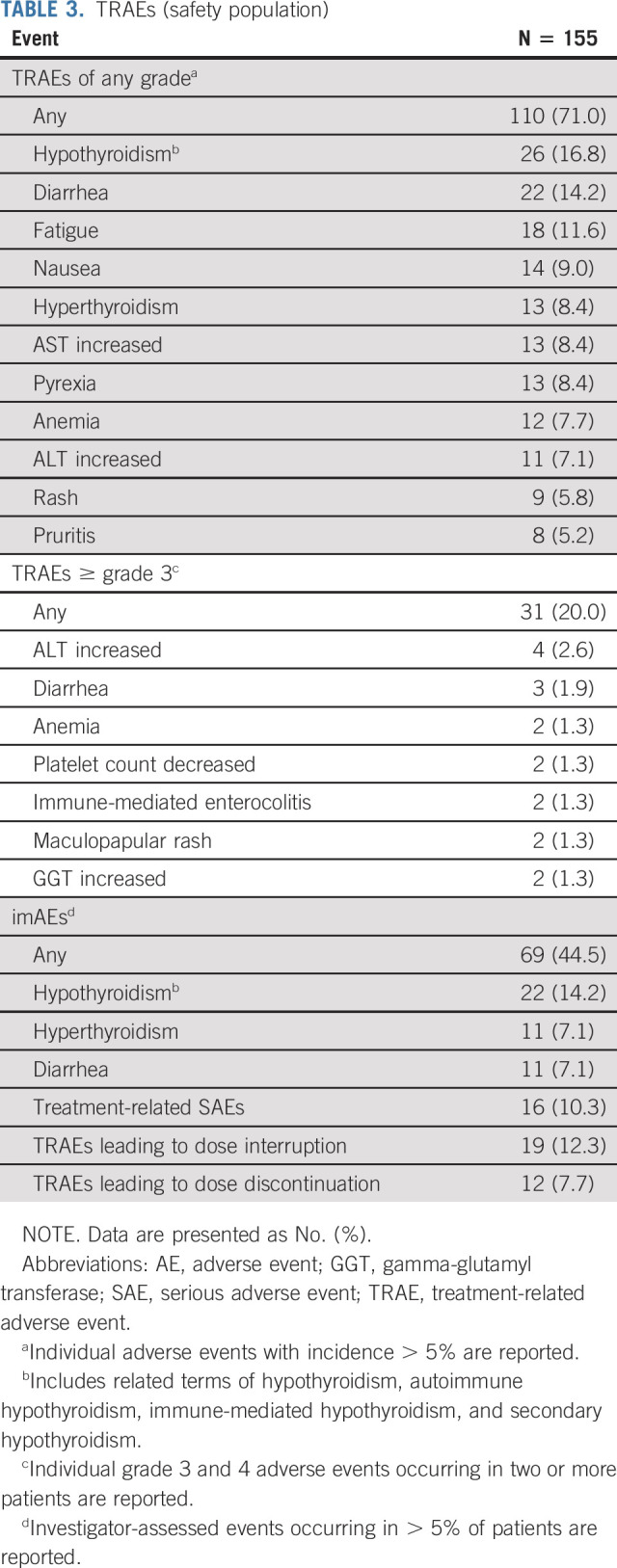
imAEs were observed in 69 patients (44.5%), with hypothyroidism (22 patients, 14.2%), hyperthyroidism, and diarrhea (each 11 patients, 7.1%) reported most frequently (Table 3). These events were generally managed with appropriate supportive care (including replacement therapy), corticosteroids, or withholding treatment. Infusion-related reactions were uncommon and reported in only five patients (3.2%).
DISCUSSION
The combination of balstilimab and zalifrelimab elicited compelling clinical activity in women with cervical cancer who had progressed after prior treatment with platinum-based chemotherapy in the recurrent and/or metastatic disease setting. The primary end point of ORR was 25.6%, including 8% of patients who experienced a CR to combination therapy. Moreover, responses were durable with a median DOR NR after a median study follow-up of 21 months. Enhanced benefit was observed in patients with quantifiable tumor cell PD-L1 expression (ORR, 32.8%); however, clinical activity was also seen in PD-L1–negative patients. Furthermore, responses were observed irrespective of tumor histology, with the greatest benefit seen in patients with squamous cell carcinoma (ORR, 32.6%). These ORRs are higher than those achieved with currently used second-line therapies, which typically range between 4% and 14%.2 The outcomes, particularly for patients with PD-L1–positive or squamous-type tumors, also compare favorably with the 24% ORR recently reported for the investigational agent tisotumab vedotin, a tissue factor–targeted antibody-drug conjugate, in a similar setting.26 Because of its cytotoxic monomethyl auristatin E payload, tisotumab vedotin has a differentiated safety profile to combination immunotherapy, characterized by ocular adverse events and other toxicities typically seen with chemotherapy (eg, alopecia and higher grade neuropathies).26
Despite the caveat of an indirect cross-trial comparison, it is informative to consider these outcomes in the context of the results of an independent phase II study of balstilimab monotherapy in a patient population with the same enrollment criteria.21 In that trial, the primary efficacy outcomes were a confirmed ORR of 15.0% (95% CI, 10.0 to 21.8) and a median DOR of 15.4 months (95% CI, 5.7 to NR), with both measures lower than the 25.6% ORR (95% CI, 18.8 to 33.9) and median DOR NR (95% CI, 9.7 to NR) achieved with combination treatment. The addition of zalifrelimab to balstilimab also resulted in numerically higher complete and PR rates (8.0 v 3.6%; 17.6% v 11.4%, respectively) compared with balstilimab monotherapy.21 Collectively, these findings support the hypothesis that combined anti–PD-1 and CTLA-4 inhibition enhances antitumor activity compared with PD-1 inhibition alone in this patient population.
The ORRs observed for the combination in the adenocarcinoma (8.8%) and PD-L1–negative subsets of patients (9.1%) were consistent with those seen with balstilimab monotherapy.21 For adenocarcinoma, additional studies on the potential benefits of the addition of zalifrelimab with respect to depth and durability of response are ongoing.27 The latter finding provides further evidence that PD-L1 expression determined by immunohistochemistry is an imperfect biomarker for predicting response to checkpoint inhibitor therapy in this and other tumor types.28 Contributing technical factors include a multiplicity of antibodies used in different companion diagnostic assays, the comparative performance characteristics of which are not established, as well as differences in defined thresholds for PD-L1 tumor positivity.29 PD-L1 expression is inherently heterogeneous, within tumors and at metastatic sites, and is dynamically regulated by a variety of tumor-intrinsic and tumor-extrinsic mechanisms,30 including modulation in response to prior antineoplastic therapies. Thus, the temporal and spatial expression of PD-L1 can limit interpretability of baseline tumor testing. The identification of more reliable predictive biomarkers will be of clinical relevance for identifying those patients most likely to receive benefit from balstilimab plus zalifrelimab therapy.
Of note, to our knowledge, this study represents the largest data set to date evaluating dual targeting of PD-1 and CTLA-4 as a therapeutic modality in patients with recurrent and/or metastatic cervical cancer. It was previously shown that ipilimumab as monotherapy had negligible activity in patients with metastatic cervical cancer who progressed after at least one line of platinum chemotherapy.31 Interim results have now been reported from the ongoing CheckMate 358 trial that is assessing two alternate dosing regimens of nivolumab plus ipilimumab in patients with recurrent and/or metastatic disease.32 Appropriate intertrial comparisons cannot be made, because of key differences in dose schedules and patient populations. For example, CheckMate 358 enrolled an exclusively squamous cell carcinoma population that was less heavily pretreated, with 42% and 52% of patients in each investigational arm having received no prior therapy for metastatic disease. However, in the small subsets of patients who had received at least one line of prior therapy and were dosed using a similar regimen (n = 26), the ORR was 23.1%, which included one complete responder (3.8%), whereas the high-dose ipilimumab regimen (n = 22) showed 36.4% ORR including three complete responders (13.6%).32 Similar to the present study, activity was seen in patients irrespective of PD-L1 tumor expression status. Taken together, it is reasonable to suggest that combined PD-1 and CTLA-4 blockade is a feasible and promising strategy for improving outcomes in this malignancy, similar to what has been demonstrated in other solid tumor types.
Beyond improvements in response rates, the addition of CTLA-4 blockade to PD-1 inhibition enhances depth and durability of response, shown to directly translate to longer-term disease control in other tumor types.33 Indeed, a key feature of the CheckMate 067 trial that led to initial approval of nivolumab plus ipilimumab for patients with advanced melanoma was a significant improvement in OS for the combination compared with nivolumab alone.34 Importantly, the plateau in the survival curve was maintained with longer-term follow-up, indicative of a continuing survival benefit conferred by the addition of a CTL4-targeting agent to PD-1 inhibition.35,36 In this regard, survival data for the current study are not yet mature, as evidenced by the high degree of censoring beyond 12 months. However, the median OS of 12.8 months and the 12-month OS rate of 53.3% are encouraging and it will be of considerable interest to determine whether the durable efficacy outcomes reported here for the balstilimab-zalifrelimab combination in cervical cancer will similarly translate to sustained survival benefit with longer follow-up.
Combination treatment was well tolerated, as evidenced by a low discontinuation rate because of TRAEs (7.7%, primarily because of elevations in hepatic enzymes), and no unexpected toxicities were observed. The overall rate of any-grade TRAEs (71%) was comparable with that reported for balstilimab monotherapy in a similar patient population21 although differences in the adverse event profile were found. These included higher relative frequencies of hypothyroidism, hyperthyroidism, and elevations in AST and ALT, most of which were grade 1 or 2. The incidence of grade 3 or higher TRAEs (20%) is similar to previous trials that led to the approval of the nivolumab-ipilimumab combination in nongynecologic malignancies.34,37-40
The imAE profile was consistent with that of other PD-1 and CTLA-4 inhibitors, which have also been associated with endocrine (hypothyroidism and hyperthyroidism), gastrointestinal (diarrhea and colitis), and hepatic side effects (increased ALT and/or AST).41-43 Immune-mediated pneumonitis and nephritis were rare imAEs seen with the combination during the study (reported in three and one patients, respectively); however, both these toxicities were associated with a treatment-related fatality. This observation underscores the clinical challenges inherent to immunotherapy in that checkpoint inhibitors are associated with distinctive and often high-risk toxicities, the optimal management of which requires early recognition and appropriate interventions.44
In conclusion, the balstilimab plus zalifrelimab doublet elicited high ORRs, durable responses, and an acceptable safety profile as second-line treatment for patients with recurrent and/or metastatic cervical cancer. The high proportion of complete responders and activity irrespective of tumor PD-L1 status or histology were particularly promising outcomes that confirm the feasibility of dual targeted immunotherapy for this disease. Given the lack of effective therapies and poor prognosis for patients in this setting, the findings suggest that this novel regimen may provide meaningful clinical benefit in this difficult-to-treat population and further evaluation of the combination is warranted. Accordingly, a randomized phase II study assessing the safety and efficacy of balstilimab, both alone and in combination with zalifrelimab, in the second-line setting for recurrent and/or metastatic cervical cancer (RaPiDS/GOG-3028; NCT03894215) is underway.
ACKNOWLEDGMENT
We would like to thank all the patients who participated and their families, as well as coinvestigators, nurses, study coordinators, and operations staff at each of the clinical sites. We also thank Richard Bates, Director of Scientific Communications at Agenus, who provided drafts and editorial assistance during the production of this article.
APPENDIX
FIG A1.
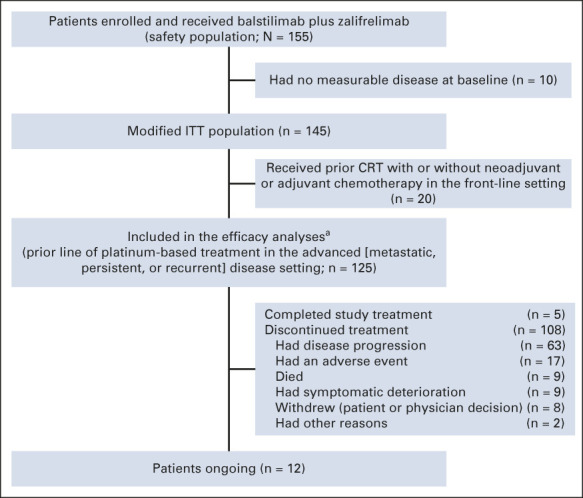
Patient enrollment and disposition. CRT, chemoradiotherapy; ITT, intent-to-treat. aPatients who received ≥ 1 dose of study treatment, with measurable disease at baseline, and had prior line of platinum-based treatment in the metastatic, persistent, or recurrent setting (per Independent Review Committee).
TABLE A1.
Baseline Demographics and Disease Characteristics (CRT subset)
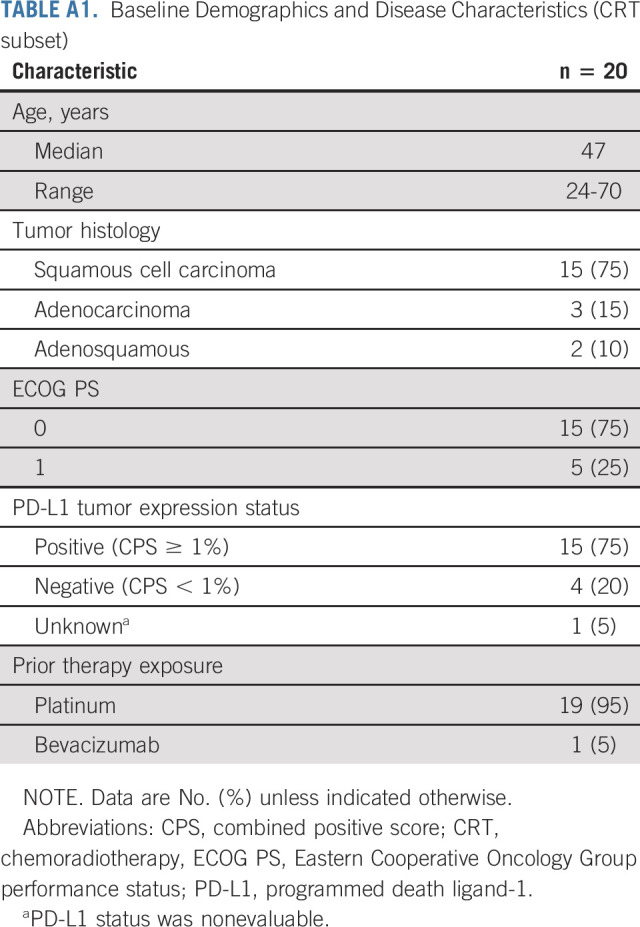
TABLE A2.
Antitumor Activity in the CRT Subset
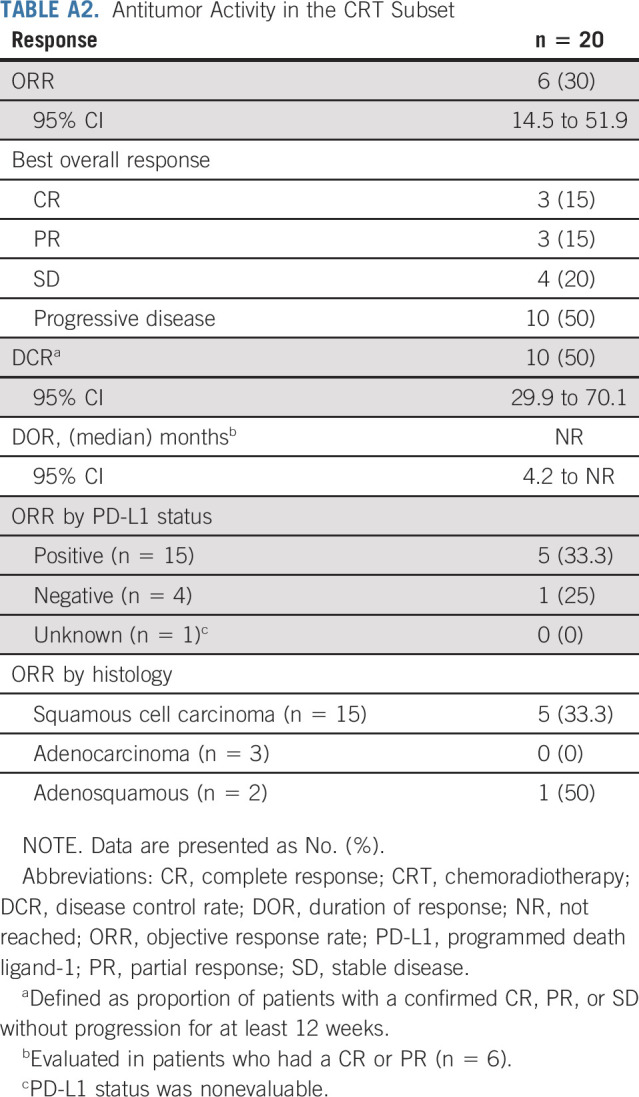
TABLE A3.
Responses in Efficacy-Evaluable Patients by Prior Bevacizumab Use
David M. O'Malley
Consulting or Advisory Role: Janssen Oncology, AstraZeneca, Clovis Oncology, Tesaro, Novocure, AbbVie, Genentech/Roche, OncoQuest, Immunogen, GOG Foundation, Translational Genomics Research Institute, Agenus, Marker Therapeutics, Eisai, Genelux, Iovance Biotherapeutics, Ambry Genetics, Tarveda Therapeutics, Leap Therapeutics, Myriad Genetics, GlaxoSmithKline, Regeneron, Sorrento Therapeutics, Rubius Therapeutics, Elevar Therapeutics¸ Novartis, Seattle Genetics, BBI Healthcare, Arquer Diagnostics¸ Toray Medical, Takeda, InxMed, Celsion, Roche Diagnostics MSA
Research Funding: Amgen (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), Regeneron (Inst), Immunogen (Inst), Janssen Research & Development (Inst), Clovis Oncology (Inst), EMD Serono (Inst), Ergomed (Inst), Ajinomoto (Inst), Immunogen (Inst), Cerulean Pharma (Inst), PharmaMar (Inst), Array BioPharma (Inst), Bristol Myers Squibb (Inst), Agenus (Inst), Tesaro (Inst), TRACON Pharma (Inst), Genmab (Inst), Seattle Genetics (Inst), Iovance Biotherapeutics (Inst), Merck (Inst), AbbVie/Stemcentrx (Inst), AbbVie (Inst), Mersana (Inst), Eisai (Inst), BBI Healthcare (Inst), Sumitomo Dainippon Pharma Oncology (Inst),
Bradley J. Monk
Leadership: US Oncology
Honoraria: AbbVie, Advaxis, Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Asymmetric Therapeutics, Boston Biomedical, ChemoID, Clovis Oncology, Deciphera Pharmaceuticals, Eisai¸ Geistlich Pharma, Genmab/Seattle Genetics, ImmunoGen, Immunomedics, Incyte, Iovance Biotherapeutics, Laekna Health Care, Mersana, Merck, Myriad Pharmaceuticals, NuCana, OncoMed, OncoQuest, OncoSec, Perthera, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, Senti Biosciences, Takeda, Tarveda Therapeutics, Tesaro/GSK, Vavotar Life Sciences, Vascular Biogenics, Vigeo Therapeutics, GOG Foundation, Starton Therapeutics, Elevar Therapeutics, Novocure, Iovance Biotherapeutics, Gradalis, Karyopharm Therapeutics
Consulting or Advisory Role: AbbVie, Advaxis, Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Asymmetric Therapeutics, Boston Biomedical, ChemoID, Clovis Oncology, Deciphera Pharmaceuticals, Eisai¸ Geistlich Pharma, Genmab/Seattle Genetics, GOG Foundation, ImmunoGen, Immunomedics, Iovance Biotherapeutics, Laekna Health Care, Mersana, Merck, Myriad Pharmaceuticals, NuCana, OncoMed, OncoQuest, OncoSec, Perthera, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, Senti Biosciences, Takeda, Tarveda Therapeutics, Tesaro/GSK, Vavotar Life Sciences, Vascular Biogenics, Vigeo Therapeutics, Gradalis, Karyopharm Therapeutics, Sorrento Therapeutics, Novocure, Chemocare
Speakers' Bureau: Roche/Genentech, AstraZeneca, Clovis Oncology, Eisai, Tesaro/GSK, Merck
Research Funding: Amgen (Inst), Genentech (Inst), Lilly (Inst), Janssen (Inst), Array BioPharma (Inst), Tesaro (Inst), Morphotek (Inst), Pfizer (Inst), Advaxis (Inst), AstraZeneca (Inst), ImmunoGen (Inst), Regeneron (Inst), NuCana (Inst)
Marilyn Huang
Consulting or Advisory Role: Tesaro, Seattle Genetics, Aptitude Health, Agenus, Cooper Surgical, touchExpert opinions
Research Funding: Merck (Inst)
Tarek M. Meniawy
Honoraria: AstraZeneca/MedImmune
Consulting or Advisory Role: AstraZeneca/MedImmune, Novartis, GlaxoSmithKline, BMS
Research Funding: Bristol Myers Squibb (Inst), BeiGene (Inst), Incyte (Inst), AstraZeneca/MedImmune (Inst), Regeneron (Inst), Bayer (Inst), Merck Serono (Inst), Roche/Genentech (Inst)
Travel, Accommodations, Expenses: BMS
Waldo Ortuzar Feliu
Employment: Agenus
Stock and Other Ownership Interests: Agenus
Travel, Accommodations, Expenses: Agenus
Marek Ancukiewicz
Employment: Agenus, LFB Biotechnologies, Boston Scientific (I)
Stock and Other Ownership Interests: Agenus, Boston Scientific (I)
Iwona Lugowska
Honoraria: Roche, Agen7s, MSD, Amgen
Research Funding: Roche (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
No other potential conflicts of interest were reported.
SUPPORT
Supported by Agenus Inc.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: David M. O'Malley, Bradley J. Monk, Waldo Ortuzar Feliu, Marek Ancukiewicz, Iwona Lugowska
Administrative support: Waldo Ortuzar Feliu
Provision of study materials or patients: David M. O'Malley, Maryna Neffa, Tamar Melkadze, Anna Kryzhanivska, Iurie Bulat, Edward W. Wang
Collection and assembly of data: David M. O'Malley, Maryna Neffa, Bradley J. Monk, Marilyn Huang, Anna Kryzhanivska, Iurie Bulat, Tarek M. Meniawy, Andrea Bagameri, Edward W. Wang, Bernard Doger de Speville Uribe, Roberto Hegg, Waldo Ortuzar Feliu, Marek Ancukiewicz
Data analysis and interpretation: David M. O'Malley, Bradley J. Monk, Tamar Melkadze, Marilyn Huang, Edward W. Wang, Waldo Ortuzar Feliu, Marek Ancukiewicz, Iwona Lugowska
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Dual PD-1 and CTLA-4 Checkpoint Blockade Using Balstilimab and Zalifrelimab Combination as Second-Line Treatment for Advanced Cervical Cancer: An Open-Label Phase II Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David M. O'Malley
Consulting or Advisory Role: Janssen Oncology, AstraZeneca, Clovis Oncology, Tesaro, Novocure, AbbVie, Genentech/Roche, OncoQuest, Immunogen, GOG Foundation, Translational Genomics Research Institute, Agenus, Marker Therapeutics, Eisai, Genelux, Iovance Biotherapeutics, Ambry Genetics, Tarveda Therapeutics, Leap Therapeutics, Myriad Genetics, GlaxoSmithKline, Regeneron, Sorrento Therapeutics, Rubius Therapeutics, Elevar Therapeutics¸ Novartis, Seattle Genetics, BBI Healthcare, Arquer Diagnostics¸ Toray Medical, Takeda, InxMed, Celsion, Roche Diagnostics MSA
Research Funding: Amgen (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), Regeneron (Inst), Immunogen (Inst), Janssen Research & Development (Inst), Clovis Oncology (Inst), EMD Serono (Inst), Ergomed (Inst), Ajinomoto (Inst), Immunogen (Inst), Cerulean Pharma (Inst), PharmaMar (Inst), Array BioPharma (Inst), Bristol Myers Squibb (Inst), Agenus (Inst), Tesaro (Inst), TRACON Pharma (Inst), Genmab (Inst), Seattle Genetics (Inst), Iovance Biotherapeutics (Inst), Merck (Inst), AbbVie/Stemcentrx (Inst), AbbVie (Inst), Mersana (Inst), Eisai (Inst), BBI Healthcare (Inst), Sumitomo Dainippon Pharma Oncology (Inst),
Bradley J. Monk
Leadership: US Oncology
Honoraria: AbbVie, Advaxis, Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Asymmetric Therapeutics, Boston Biomedical, ChemoID, Clovis Oncology, Deciphera Pharmaceuticals, Eisai¸ Geistlich Pharma, Genmab/Seattle Genetics, ImmunoGen, Immunomedics, Incyte, Iovance Biotherapeutics, Laekna Health Care, Mersana, Merck, Myriad Pharmaceuticals, NuCana, OncoMed, OncoQuest, OncoSec, Perthera, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, Senti Biosciences, Takeda, Tarveda Therapeutics, Tesaro/GSK, Vavotar Life Sciences, Vascular Biogenics, Vigeo Therapeutics, GOG Foundation, Starton Therapeutics, Elevar Therapeutics, Novocure, Iovance Biotherapeutics, Gradalis, Karyopharm Therapeutics
Consulting or Advisory Role: AbbVie, Advaxis, Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Asymmetric Therapeutics, Boston Biomedical, ChemoID, Clovis Oncology, Deciphera Pharmaceuticals, Eisai¸ Geistlich Pharma, Genmab/Seattle Genetics, GOG Foundation, ImmunoGen, Immunomedics, Iovance Biotherapeutics, Laekna Health Care, Mersana, Merck, Myriad Pharmaceuticals, NuCana, OncoMed, OncoQuest, OncoSec, Perthera, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, Senti Biosciences, Takeda, Tarveda Therapeutics, Tesaro/GSK, Vavotar Life Sciences, Vascular Biogenics, Vigeo Therapeutics, Gradalis, Karyopharm Therapeutics, Sorrento Therapeutics, Novocure, Chemocare
Speakers' Bureau: Roche/Genentech, AstraZeneca, Clovis Oncology, Eisai, Tesaro/GSK, Merck
Research Funding: Amgen (Inst), Genentech (Inst), Lilly (Inst), Janssen (Inst), Array BioPharma (Inst), Tesaro (Inst), Morphotek (Inst), Pfizer (Inst), Advaxis (Inst), AstraZeneca (Inst), ImmunoGen (Inst), Regeneron (Inst), NuCana (Inst)
Marilyn Huang
Consulting or Advisory Role: Tesaro, Seattle Genetics, Aptitude Health, Agenus, Cooper Surgical, touchExpert opinions
Research Funding: Merck (Inst)
Tarek M. Meniawy
Honoraria: AstraZeneca/MedImmune
Consulting or Advisory Role: AstraZeneca/MedImmune, Novartis, GlaxoSmithKline, BMS
Research Funding: Bristol Myers Squibb (Inst), BeiGene (Inst), Incyte (Inst), AstraZeneca/MedImmune (Inst), Regeneron (Inst), Bayer (Inst), Merck Serono (Inst), Roche/Genentech (Inst)
Travel, Accommodations, Expenses: BMS
Waldo Ortuzar Feliu
Employment: Agenus
Stock and Other Ownership Interests: Agenus
Travel, Accommodations, Expenses: Agenus
Marek Ancukiewicz
Employment: Agenus, LFB Biotechnologies, Boston Scientific (I)
Stock and Other Ownership Interests: Agenus, Boston Scientific (I)
Iwona Lugowska
Honoraria: Roche, Agen7s, MSD, Amgen
Research Funding: Roche (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
No other potential conflicts of interest were reported.
REFERENCES
- 1.Arbyn M, Weiderpass E, Bruni L, et al. : Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob Health 8:e191-e203, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boussios S, Seraj E, Zarkavelis G, et al. : Management of patients with recurrent/advanced cervical cancer beyond first line platinum regimens: Where do we stand? A literature review. Crit Rev Oncol Hematol 108:164-174, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Marth C, Landoni F, Mahner S, et al. : Cervical cancer: ESMO clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv72-iv83, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Minion LE, Tewari KS: Cervical cancer—State of the science: From angiogenesis blockade to checkpoint inhibition. Gynecol Oncol 148:609-621, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tewari KS, Sill MW, Long HJ, III, et al. : Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 370:734-743, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung HC, Ros W, Delord JP, et al. : Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol 37:1470-1478, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Saglam O, Conejo-Garcia J: PD-1/PD-L1 immune checkpoint inhibitors in advanced cervical cancer. Integr Cancer Sci Ther 5: 10.15761/ICST.1000272, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Wu L, Tong R, et al. : PD-1/PD-L1 inhibitors in cervical cancer. Front Pharmacol 10:65, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kagabu M, Nagasawa T, Fukagawa D, et al. : Immunotherapy for uterine cervical cancer. Healthcare (Basel) 7:108, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tewari KS, Monk BJ, Vergote I, et al. : VP4-2021: EMPOWER-cervical 1/GOG-3016/ENGOT-cx9: Interim analysis of phase III trial of cemiplimab vs. investigator’s choice (IC) chemotherapy (chemo) in recurrent/metastatic (R/M) cervical carcinoma. Ann Oncol 32:940-941, 2021 [Google Scholar]
- 11.Rotte A: Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res 38:255, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popovic A, Jaffee EM, Zaidi N: Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J Clin Invest 128:3209-3218, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei SC, Levine JH, Cogdill AP, et al. : Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell 170:1120-1133 e17, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchbinder EI, Desai A: CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am J Clin Oncol 39:98-106, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bristol Myers Squibb : S.-Food-and-Drug-Administration-Approves-Opdivo-nivolumab--Yervoy-ipilimumab-as-the-First-and-Only-Immunotherapy-Treatment-for-Previously-Untreated-Unresectable-Malignant-Pleural-Mesothelioma/default.aspx”>https://news.bms.com/news/details/2020/U.S.-Food-and-Drug-Administration-Approves-Opdivo-nivolumab--Yervoy-ipilimumab-as-the-First-and-Only-Immunotherapy-Treatment-for-Previously-Untreated-Unresectable-Malignant-Pleural-Mesothelioma/default.aspx, 2020
- 16.Hao C, Tian J, Liu H, et al. : Efficacy and safety of anti-PD-1 and anti-PD-1 combined with anti-CTLA-4 immunotherapy to advanced melanoma: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 96:e7325, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Li S, Yao Q, et al. : The efficacy and safety of combined immune checkpoint inhibitors (nivolumab plus ipilimumab): A systematic review and meta-analysis. World J Surg Oncol 18:150, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson DB, Balko JM, Compton ML, et al. : Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375:1749-1755, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cappelli LC, Gutierrez AK, Baer AN, et al. : Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 76:43-50, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore KN, Dresher C, Liu J, et al. : Phase 1/2 open-label, multiple ascending dose trial of AGEN2034, an anti-PD-1 monoclonal antibody, in advanced solid malignancies: Results of dose escalation. J Clin Oncol 36, 2018. (suppl; abstr 3086) [Google Scholar]
- 21.O'Malley DM, Oaknin A, Monk BJ, et al. : Phase II study of the safety and efficacy of the anti-PD-1 antibody balstilimab in patients with recurrent and/or metastatic cervical cancer. Gynecol Oncol 163:274-280, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Gombos RB, Gonzalez A, Manrique M, et al. : Toxicological and pharmacological assessment of AGEN1884, a novel human IgG1 anti-CTLA-4 antibody. PLoS One 13:e0191926, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez CA, Wesolowski R, Wilky BA, et al. : Single-agent zalifrelimab (anti-CTLA-4) shows clinical benefit in rare tumors - case reports from a phase 2 study (NCT02694822). J Immunother Cancer 8:A279, 2020. (suppl 3) [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Roach C, Zhang N, Corigliano E, et al. : Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol 24:392-397, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman RL, Lorusso D, Gennigens C, et al. : Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 22:609-619, 2021 [DOI] [PubMed] [Google Scholar]
- 27.O'Malley DM, Randall LM, Jackson CG, et al. : RaPiDS (GOG-3028): Randomized phase II study of balstilimab alone or in combination with zalifrelimab in cervical cancer. Future Oncol 17:3433-3443, 2021 [DOI] [PubMed] [Google Scholar]
- 28.Grossman JE, Vasudevan D, Joyce CE, et al. : Is PD-L1 a consistent biomarker for anti-PD-1 therapy? The model of balstilimab in a virally-driven tumor. Oncogene 40:1393-1395, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel SP, Kurzrock R: PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 14:847-856, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Dang F, Ren J, et al. : Biochemical aspects of PD-L1 regulation in cancer immunotherapy. Trends Biochem Sci 43:1014-1032, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Lheureux S, Butler MO, Clarke B, et al. : Association of ipilimumab with safety and antitumor activity in women with metastatic or recurrent human papillomavirus-related cervical carcinoma. JAMA Oncol 4:e173776, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naumann RW, Oaknin A, Meyer T, et al. : Efficacy and safety of nivolumab (Nivo) + ipilimumab (Ipi) in patients (pts) with recurrent/metastatic (R/M) cervical cancer: Results from CheckMate 358. Ann Oncol 30:v851-v934, 2019. (suppl 5) [Google Scholar]
- 33.Pires da Silva I, Ahmed T, Reijers ILM, et al. : Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: A multicentre, retrospective, cohort study. Lancet Oncol 22:836-847, 2021 [DOI] [PubMed] [Google Scholar]
- 34.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. : Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377:1345-1356, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. : Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 19:1480-1492, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535-1546, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Larkin J, Hodi FS, Wolchok JD: Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:1270-1271, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Motzer RJ, Tannir NM, McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277-1290, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overman MJ, Lonardi S, Wong KYM, et al. : Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol 36:773-779, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. : Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 381:2020-2031, 2019 [DOI] [PubMed] [Google Scholar]
- 41.Kumar V, Chaudhary N, Garg M, et al. : Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 8:49, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu C, Chen YP, Du XJ, et al. : Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ 363:k4226, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kooshkaki O, Derakhshani A, Hosseinkhani N, et al. : Combination of ipilimumab and nivolumab in cancers: From clinical practice to ongoing clinical trials. Int J Mol Sci 21:4427, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou S, Khanal S, Zhang H: Risk of immune-related adverse events associated with ipilimumab-plus-nivolumab and nivolumab therapy in cancer patients. Ther Clin Risk Manag 15:211-221, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]