Abstract
Preeclampsia (PE) is a life-threatening human gestational syndrome with incompletely understood etiopathogenesis. The disorder has a spectrum of clinical features, likely due to a complex interaction between maternal predisposing factors and abnormalities at the maternal-fetal interface. Poor trophoblast cell invasion, inadequate uterine vascular remodeling, and placental hypoperfusion are considered as key placental events leading to PE. Kisspeptins, a family of small peptides derived from the KISS1 gene, have been implicated in the development of this syndrome. Most studies of kisspeptin expression in PE have reported an upregulation of kisspeptins and/or their cognate receptor in preeclamptic placentas. Conversely, maternal peripheral blood concentration of kisspeptins is reportedly lower in PE than in uncomplicated pregnancies. This apparent paradox remains to be further elucidated. Although kisspeptins were initially known for inhibiting cellular migration and invasion, other biological activities attributed to these peptides include neuroendocrine regulation of reproduction, metabolism regulation, inhibition of angiogenesis, and induction of apoptosis. This review summarizes the current knowledge on expression and biological activity of kisspeptins at the maternal-fetal interface in the context of PE.
Keywords: KISS1, placenta, preeclampsia, pregnancy, trophoblast
INTRODUCTION
Preeclampsia (PE) is a leading cause of adverse pregnancy outcomes, greatly contributing to maternal and fetal mortality worldwide (1, 2). This pregnancy-specific multiorgan disorder is characterized by hypertension after 20 wk of gestation, often associated with glomerular endotheliosis and proteinuria (1). PE occurs as a new-onset syndrome in previously normotensive women or superimposed in patients with preexisting chronic hypertension (1). In severe cases, the clinical presentation may include thrombocytopenia, renal insufficiency, hepatocellular necrosis, pulmonary edema, and neurological disturbances (1). Women who develop PE may be predisposed to cardiovascular disease later in life, and the affected fetuses have a higher predisposition for premature delivery, intrauterine growth restriction, prenatal death, and postnatal metabolic, neurological, and cardiovascular disorders (3, 4). Maternal therapeutic options for PE are limited, and the delivery of the fetus and the placenta is often required to alleviate the clinical signs (1, 3).
The etiopathogenesis of PE is not completely understood. Because of the range of clinical presentations, subclassification based on disease severity and gestational age has been proposed (1, 5). Accordingly, early- and late-onset PE refer to the development of clinical signs before or after 34 to 35 wk of gestation, respectively, and each category has been associated with distinct etiologies and outcomes (1, 5). Based on integrated transcriptomics and proteomics studies, the heterogeneity of clinical features of PE may result from a complex interaction between maternal predisposing factors and abnormalities at the maternal-fetal interface (6). Correspondingly, an event more extensively associated with the pathogenesis of early-onset PE is the inadequate invasion of the maternal uterus by conceptus-derived trophoblast cells, leading to poor uterine vascular remodeling, placental hypoperfusion, and, ultimately, local and systemic endothelial dysfunction (6–9). Contrastingly, maternal pregestational disorders, including hypertension, obesity, and diabetes mellitus, may lead to a preexisting proinflammatory milieu, often implicated with the late-onset syndrome (10, 11).
Kisspeptins are a family of small peptides containing 54, 14, 13, and 10 amino acids (KP-54, KP-14, KP-13, and KP-10) derived from the posttranslational cleavage of a 145-amino-acid peptide (KP-145). The prepropeptide KP-145 is encoded by the KISS1 gene, located in the long arm of chromosome 1. KP-54 was the first peptide of this family to be identified and initially named “metastin” because of its ability to regulate cellular migration and inhibit metastatic invasion of neoplastic cells (12). The role of kisspeptins in the reproductive system began to be elucidated in 2003 with the discovery of their function as neuroendocrine regulators of the hypothalamic-hypophyseal-gonadal axis (13, 14). Along with neurokinin B and dynorphin A, kisspeptins have been proposed to mediate the interaction between energy balance and reproduction in the central nervous system (15, 16). Interestingly, kisspeptins may also exert important metabolic functions in peripheral tissues, and associations between kisspeptins/kisspeptin receptor dysregulations and disorders such as obesity and diabetes have been reviewed elsewhere (15, 16).
The expression of KISS1 mRNA in the normal human placenta was first reported in the pioneer study describing the gene (12). Further investigations have suggested important roles of kisspeptins as regulators of embryonic implantation, decidualization, and placentation (17–19). Comparable with the biological activity exerted by kisspeptins in tumoral cells, these peptides have been proposed to inhibit migration and invasion of trophoblast cells in humans, bovids, and rodents (20–23). In uncomplicated human pregnancies, kisspeptin/kisspeptin receptor mRNA and protein expressions are higher in the first-trimester than term placentas, corroborating with the proposed physiological regulatory activity during early placentation (20, 21). Intriguingly, peripheral blood concentration of kisspeptins is inversely associated with placental expression in normal pregnancies (24). A series of studies have reported an upregulation of kisspeptins and/or their cognate receptor in preeclamptic placentas (Table 1). Therefore, it is speculated that kisspeptin upregulation at the maternal-fetal interface during early gestation may contribute to the pathological inhibition of trophoblast cell invasion seen in PE. The potential use of kisspeptins as biomarkers for PE has also been investigated (Table 2). Contrary to placental expression, peripheral blood kisspeptins are lower in women with PE than in those with uncomplicated pregnancies, an apparent paradox that remains to be further elucidated. In addition to their role in cellular invasion, kisspeptins may also exert proapoptotic and antiangiogenic activities in neoplasms and placentas (24, 40). Considering the multiple biological activities attributed to those peptides, the aim of this review is to highlight the potential roles of kisspeptins at the maternal-fetal interface in the context of PE.
Table 1.
Kisspeptin/kisspeptin receptor mRNA and protein expression in preeclamptic and normal placental tissue and trophoblast cells
| Experimental Groups | Model | Kisspeptins |
Kisspeptin Receptor |
Study | ||
|---|---|---|---|---|---|---|
| mRNA | Protein | mRNA | Protein | |||
| NP and PE | Trophoblast cells, C-section | PE > NP (RT-PCR) |
PE > NP (WB, KP-54) |
Qiao et al. (25) | ||
| NP (first, second, and third trimesters) and term PE | Placenta, elective pregnancy termination and C-section | NP > PE (RT-PCR) |
Qiao et al. (26) | |||
| NP and PE | Placenta | PE > NP (RT-PCR) |
PE > NP (WB, KP-?) |
Zhang et al. (27) | ||
| NP and PE (GA > 35) | Placenta, C-section | PE > NP (RT-PCR) |
PE > NP (WB, KP-?) |
Zhang et al. (28) | ||
| NP (GA 39.4 ± 1.1) and PE (GA 37.6 ± 2.6) | Placenta, C-section | NP > PE (RT-PCR) |
NP > PE (WB, IHC, KP-10) |
PE > NP (RT-PCR) |
PE > NP (WB, IHC) |
Cartwright and Williams (29) |
| LRI and HRI (GA 8.8 ± 0.9) | Placenta, surgical termination of pregnancy | LRI >HRI (RT-PCR) |
LRI >HR I(WB, IHC, KP-10) |
HRI >LRI (RT-PCR) |
HRI >LR I(WB, IHC) |
Cartwright and Williams (29) |
| EPE (GA 28.8 ± 3.1), LPE (GA 37.1 ± 2.2), and NP (GA-matched) | Placenta | EPE > LPE = NP (RT-PCR) |
EPE > LPE = NP (WB, IHC, KP-54) |
NP = EPE = LPE (RT-PCR) |
NP = EPE = LPE (WB, IHC) |
Qiao et al. (30) |
| NP, EPE, and LPE | Placenta | EPE > NP | EPE > NP > LPE | Vodneva et al. (31) | ||
| NP (GA 38.03 ± 0.06) and EPE (GA 32.95 ± 0.53) | Placenta, elective C-section | EPE > NP* (RT-PCR) |
EPE > NP (IHC, KP-10) |
NP = EPE (RT-PCR) |
Matjila et al. (32) | |
C-section, cesarean section; EPE, early-onset preeclampsia; GA, gestational age at delivery in weeks (means ± standard deviation); IHC, immunohistochemistry; KP, kisspeptin; KP-?, unknown KP peptide; LPE, late-onset preeclampsia; LRI and HRI, low- and high-resistance index of maternal uterine artery, respectively, assessed via Doppler velocimetry; NP, normal pregnancy; PE, preeclampsia; RT-PCR, real-time polymerase chain reaction; WB, Western blotting. *mRNA expression was reportedly higher but not statistically significant.
Table 2.
Blood concentration of kisspeptins in women with normal and preeclamptic pregnancies
| Experimental Groups | Sample | Kisspeptin Concentration | Method | Study |
|---|---|---|---|---|
| AGA and SGA (GA 8–14)* | Plasma | SGA < AGA (KP-10) |
RIA | Smets et al. (33) |
| NP, IUGR, and PE (GA 16–20)* | Serum | PE < NP (KP-54) |
ELISA | Armstrong et al. (34) |
| NP (GA 31.6 ± 0.5), PE (GA 35.4 ± 1.1), PIH (GA 34.1 ± 0.9) | Plasma | NP = PE = PIH (KP-54, KP-14, KP-10) |
RIA | Nijher et al. (35) |
| NP (GA 37.66 ± 0.39), mPE (GA 35.42 ± 0.83), and sPE (GA 33.09 ± 0.75) | Plasma | sPE < mPE < NP (KP-?) |
ELISA | Adali et al. (36) |
| NP and PE (GA 21–25 and 32–36) | Plasma | PE < NP (KP-?) |
RIA | Ćetković et al. (37) |
| lNP, oNP, and oPE (GA 16) | Plasma | oPE < oNP < lNP | ELISA | Logie et al. (38) |
| NP (GA 38.03 ± 0.06) and PE (GA 32.95 ± 0.53) | Serum | PE < NP (KP-10) |
ELISA | Matjila et al. (32) |
| NP, mPE, and sPE (GA 20–27 and 28–40) | Plasma | sPE < mPE < NP (KP-10) |
ELISA | Ziyaraa et al. (39) |
AGA, appropriate for gestational age neonates; ELISA, enzyme-linked immunosorbent assay; GA, gestational age at delivery in weeks (means ± standard deviation); IUGR, intrauterine growth restriction; KP, kisspeptin; lNP, lean women with normal pregnancy; mPE, mild preeclampsia; NP, normal pregnancy; oNP, obese women with normal pregnancy; oPE, obese women who later developed preeclampsia; PE, preeclampsia; PIH, pregnancy-induced hypertension (hypertension after 20 wk of gestation without proteinuria); RIA, radioimmunoassay; SGA, small for gestational age neonates (birthweight below the 10th percentile); sPE, severe preeclampsia. *Retrospective case-control study with samples collected at 16–20 wk of gestation.
KISSPEPTINS ACTIVATE CELL TYPE-SPECIFIC SIGNALING PATHWAYS
Kisspeptins are highly conserved among species and exert their action by binding to a G protein (Gq/11)-coupled receptor encoded by KISS1R (41). The kisspeptin receptor has seven transmembrane domains and is known as KISS1R, GPR54, AXOR12, CPPBI, hOT7175, or HH8 (26). KP-145 is biologically inactive until cleavage. Based on in vitro studies, the cleaved products KP-54, KP-14, KP-13, and KP-10 have similar receptor binding affinities, which is attributed to the shared 10-amino-acid sequence (41, 42). Nonetheless, KP-54 and KP-10 have specific pharmacokinetics, likely associated with the distinct in vivo responses reported after exogenous administration (43).
The diverging biological functions of kisspeptins have been associated with the activation of cell type-specific sets of signaling pathways (18, 44). Generally, the kisspeptin receptor activation triggers a typical G protein (Gq/11)-coupled receptor cascade, including activation of phospholipase C (PLC), hydrolysis of phosphatidylinositol bisphosphate (PIP2), 1,4,5-trisphosphate (IP3)-mediated intracellular calcium release, and activation of diacylglycerol and protein kinase C (PKC) (18, 44). Intriguingly, however, when first-trimester human trophoblast cells were stimulated in vitro with the different KPs, only KP-10 led to increased levels of intracellular calcium (20). The kisspeptin-activated PLC-PKC cascade is connected to distinct pathways in specific cell types, including phosphorylation of mitogen-activated protein kinases (MAPK) ERK1/2 and p38, activation/inhibition of PI3k/Akt, and interaction with the prometastatic chemokine receptor CXCR4 (18, 44). Downstream, ERK1/2 phosphorylation and PI3k/Akt phosphorylation are linked to nuclear factor (NF) κB-mediated inhibition of matrix metalloproteinases (MMP)-2 and MMP-9, important enzymes for the degradation of the extracellular matrix (ECM) as the cells migrate into tissues (7, 45).
KISSPEPTINS AND TROPHOBLAST CELL INVASION
Trophoblast cell migration and invasion are controlled by molecular pathways mediated by cytokines, growth factors, and hormones, as extensively reviewed elsewhere (7, 46). The extravillous cytotrophoblast (evCT) is a motile and highly invasive subtype of trophoblast cells of the human placenta (7). In normal pregnancies, evCT migrates through the maternal decidua and myometrial interstitium, infiltrates the walls of the spiral arteries, and acquires an endothelial-like phenotype. The evCT vascular infiltration leads to remodeling of the spiral arteries from low-flow, high-resistance to high-flow, low-resistance vessels (46). In PE, insufficient interstitial and endovascular invasion by evCT may lead to poor vascular remodeling, placental hypoperfusion, and systemic release of proinflammatory mediators (9, 46).
A holistic understanding of the mechanisms leading to inadequate trophoblast cell invasion in PE is lacking (47). The evCT cells share interesting similarities with neoplastic cells, as they proliferate, invade the uterine wall, promote angiogenesis, and modulate the maternal immune response (7). However, differently than tumoral cells, evCT invasion is spatially and temporarily regulated (7). The molecular pathways associated with the invasive phenotype of evCT cells include the secretion of leukemia inhibitory factor (LIF) by the maternal decidua, stimulating trophoblast cell adhesion to the ECM, and secretion of MMP-2 and MMP-9 by trophoblast cells, leading to ECM degradation (7). The trophoblast cell invasion appears to be limited by multiple factors, including tumor necrosis factor-α (TNF-α), secreted by macrophages in the maternal decidua, and transforming growth factor-β1 (TGF-β1), secreted by decidual cells and known to upregulate tissue inhibitors of metalloproteinases (TIMPs) (7, 45).
It is suggested that kisspeptins are additional physiological modulators of trophoblast invasion, inhibiting evCT penetration of deeper layers of the myometrium via downregulation of MMP-2 and MMP-9 (20, 48). Kisspeptin-mediated activation of ERK1/2 and PI3k/Akt pathways may regulate the nuclear translocation and DNA-binding activity of NF-κB, ultimately reducing the NF-κB binding and activation of the MMP-9 gene promoter region (18). Furthermore, kisspeptins have been shown to suppress MMP-9 via activation of p38 MAPK in tumoral cells and to upregulate TIMP-1 and TIMP-3 in first-trimester trophoblast cells (45, 49). Additional compelling evidence of the aforementioned role of these family of peptides is the low kisspeptin expression in the highly invasive trophoblast cells of choriocarcinomas and the high expression reported in PE and the noninvasive cells of benign molar pregnancies (21). Based on studies with KISS1 knockout (−/−) mice, kisspeptins have also been suggested to act as positive regulators of LIF expression in response to estrogen stimulation (18).
ANTIANGIOGENESIS
The pathogenesis of PE has been associated with abnormal expression of pro- and antiangiogenic factors. Vascular endothelial growth factor (VEGF), placental growth factor (PlGF), and the shared endothelial receptor fms-like tyrosine kinase-1 (Flt-1) are proangiogenic mediators highly expressed by evCT, stimulating placental vasculogenesis and stabilization of endothelial cells locally and systemically (50). In response to hypoxia, the preeclamptic placenta may secrete high concentrations of the antiangiogenic factors soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng) weeks before the development of clinical signs (1, 50). High concentrations of sFlt-1 and sEng are hypothesized to ultimately inactivate VEGF, TGF-β1, and PlGF, leading to systemic endothelial dysfunction, glomerular endotheliosis, and hypertension (50).
Inhibition of VEGF-A has been reported via the kisspeptin activation of the ERK1/2 pathway in neoplasms, ovarian cells, human umbilical vein endothelial cells (HUVECs), and, importantly, human first-trimester primary trophoblast cells (45, 51). Interestingly, maternal serum concentrations of kisspeptins, sFlt-1, and sEng appear to share a similar profile in normal pregnancies, with blood concentration increasing in peripheral circulation from the first to the third trimesters and normalizing after parturition (50). In PE, the reduction in sFlt-1 blood concentration postdelivery is usually concomitant with the improvement of clinical signs (50). It remains to be determined whether a common upstream pathway is associated with kisspeptin, sFlt-1, and sEng expressions in pregnancy. Further studies are also needed to investigate whether kisspeptin-associated downregulation of proangiogenic factors could be either an inciting factor of poor placentation or a downstream effect leading to systemic disorders.
PROAPOPTOSIS
Increased rate of trophoblast cell apoptosis has been demonstrated in pregnancies complicated by PE and fetal intrauterine growth restriction (52). In an elegant study using high-resolution immunofluorescence, increased caspase-associated apoptosis was limited to cytotrophoblasts, whereas increased apoptosis rate was not detected in syncytiotrophoblasts (52). Interestingly, apoptotic cytotrophoblasts and derived vesicles were noted to migrate within the syncytium (52). In normal pregnancies, cytotrophoblasts continuously fuse with the multinucleated syncytiotrophoblasts to maintain the syncytiotrophoblast structure and function. Therefore, it is hypothesized that increased rate of cytotrophoblast apoptosis may be associated with placental underdevelopment and reduced syncytium repair capacity in preeclamptic pregnancies (52).
Flow cytometry studies have shown that the kisspeptin receptor activation leads to cell cycle arrest between interphase subphase G2 and mitosis, a common first step for the induction of programmed cell death (53). Multiple proapoptotic genes and pathways are upregulated by the activation of the kisspeptin receptor, and critical pathways for cell survival and directional motility are blocked (40, 53). Kisspeptin receptor activation in mammary carcinoma cell lines (MDA-MB-435S) resulted in increased intracellular calcium concentrations and activation of NF-κB pathway. Downstream, PLC-PKC-dependent upregulation of the proapoptotic/cell cycle regulator genes GADD45α, GADD45β, p21CIP-1/WAF-1, and MIHC was reported (53). Accordingly, proapoptotic activity has been reported in human placenta explants collected from elective caesarean section at term and postterm, 38–40 wk and >41 wk of gestation, respectively (40).
KISSPEPTIN/KISSPEPTIN RECEPTOR EXPRESSION IN NORMAL AND PREECLAMPTIC MATERNAL-FETAL INTERFACES
In the nonpregnant reproductive tract, kisspeptin/kisspeptin receptor mRNA and protein have been detected in endometrial samples from reproductive-age and postmenopausal women, with immunostaining noted in both luminal and glandular epithelial cells (17). Kisspeptin/kisspeptin receptor were also detected in the epithelial lining of the uterine tubes but not detected in the nonpregnant myometrium during secretory, proliferative, or menstrual phases of the menstrual cycle (19). When different stages of the menstrual cycle were compared, kisspeptin expression and kisspeptin receptor expression appear to vary according to the steroidal hormone milieu, with higher expression found in endometrial epithelium and decidualized stromal cells during the late secretory phase (17).
Based on DNA microarray profiling, KISS1 gene was highly expressed in first-trimester normal human placentas when compared with term placentas from uncomplicated vaginal deliveries (20). This finding was corroborated by independent studies reporting higher kisspeptin/kisspeptin receptor mRNA and protein expression in the normal maternal-fetal interface during the peak of trophoblast cell invasion and a decrease in expression throughout placental maturation (20, 21, 29). It has been suggested that autocrine and paracrine kisspeptin cellular signaling exist between subpopulations of first-trimester human trophoblast cells and, potentially, the endometrium (20, 29). Pioneer studies reported a high expression of kisspeptins in noninvasive syncytiotrophoblasts, whereas the kisspeptin receptor was equally expressed by syncytiotrophoblasts and villous and invasive extravillous cytotrophoblasts (20). However, other studies have described similar expression of peptides and receptor in both syncytiotrophoblasts and cytotrophoblasts (54). A circadian rhythm of kisspeptin expression in term placentas has also been described, comparable with the expression profiles of the placental proinflammatory cytokines TNF-α, IL-1β, and IL-6 (55).
Studies investigating kisspeptin/kisspeptin receptor expression in preeclamptic placentas have yielded conflicting results (Table 1). The discrepancies have been attributed to different gestational ages, methods of fetoplacental delivery, peptide studied (i.e., KP-54 vs. KP-10), and, importantly, lack of distinction of subcategories of PE (32). Kisspeptin expression appears to be consistently higher in placentas of women with early-onset PE when compared with those with uncomplicated pregnancies (30–32). Conversely, a coherent pattern of kisspeptin/kisspeptin receptor expression in late-onset PE when compared with nonaffected pregnancies has not been elucidated. When samples of human placenta, placental bed, and decidua parietalis were collected after elective caesarean section, kisspeptin upregulation was limited to preeclamptic placental tissues, with no difference in kisspeptin/kisspeptin receptor expression levels in the placental bed or decidua of normal and PE pregnancies (32).
The investigation of uteroplacental kisspeptin/kisspeptin receptor expression during early gestation in preeclamptic women has not been performed. The obvious ethical challenges of sample collection and the lack of reliable methods for early diagnosis of PE may hinder confirmatory studies of the role of kisspeptins in the pathogenesis of PE. Even though important particularities exist between the placental trophoblast cells of humans and animal models, translational studies may provide additional information about uninvestigated time points of human gestation. In mice carrying normal pregnancies, there is evidence of kisspeptin signaling in the fetoplacental unit. KISS1/KISS1R mRNA levels were generally low in the murine uterus from embryonic days e1 to e5 but increased during uterine decidualization, from e6 to e8, a period comparable with the uterine decidualization during the first trimester of human gestation (56, 57). Immunohistochemistry studies have been performed to investigate the spatiotemporal expression of kisspeptin/kisspeptin receptor protein from e1 to e8. Interestingly, kisspeptins/kisspeptin receptor protein immunostainings were initially located in the luminal and glandular epithelium from e1 to e4. From e5 to e8, however, there was increased kisspeptins/kisspeptin receptor protein expression in the decidual stroma (57). A similar expression pattern was seen in murine artificial decidualization reactions (i.e., in the absence of conceptuses), reiterating a potential role of kisspeptins in the preparation of the maternal endometrium for placentation (57).
Translational studies in mice are also suggestive of a role of sex steroidal hormones as upstream regulators of uterine kisspeptin/kisspeptin receptor expression (57). In ovariectomized females, KISS1 mRNA was low to undetectable in uterine tissues, with a significant increase after the treatment with progesterone or progesterone and estrogen in combination, whereas the effect of treatment was blocked by the coadministration of estrogen or progesterone antagonists (57). In the hypothalamus, kisspeptin neurons have been shown to coexpress estrogen receptor-α (ER-α) and exert an important role in the secretion of gonadotropin-releasing hormone (58). Moreover, high estrogen levels have been shown to suppress KISS1R expression in mouse pituitary cells via an estrogen response element in the promoter region of the gene (59). A potential role of sex steroid hormones as upstream regulators of fetoplacental kisspeptin/kisspeptin receptor expression has not been further investigated. In PE, disturbances in the steroidal hormone profile have been reported, with reduced serum estrogen levels, reduced ER-α, and increased ER-β expression levels recently found in term preeclamptic placenta (8). Further studies are warranted to investigate a potential association between sex steroidal hormones and kisspeptins/kisspeptin receptor in the human preeclamptic placentas.
CIRCULATING KISSPEPTIN LEVELS: A POTENTIAL BIOMARKER OF PREECLAMPSIA?
High levels of kisspeptins are detected in peripheral circulation of women carrying normal pregnancies. Contrary to the decrease in placental expression from first to third trimester, plasma KP-54 concentrations increase significantly throughout gestation (24). The low kisspeptin levels in men and nonpregnant women and the abrupt return to near basal levels at approximately 5 days after delivery corroborate the placental origin of the peptide (24, 60). Likewise, kisspeptin levels were 200-fold higher in the urine of third-trimester pregnant women compared with samples from nonpregnant women (61). It is proposed that the gradual increase in placental mass and, therefore, number of trophoblast cells throughout gestation may explain the inverse relationship between the increasing kisspeptin circulating levels despite the decreasing placental expression as the pregnancy progresses (18).
PE is often diagnosed late in the course of the syndrome because of the lack of alternatives for early diagnosis (52). Therefore, a myriad of biochemical markers have been studied for their potential application in early diagnosis (1). To date, there is no single biomarker shown to be clinically reliable for PE diagnosis and monitoring (52). Unlike sFlt-1 and sEng, case-control and prospective studies have shown that kisspeptin levels at 6–10 wk and 8–14 wk of gestation were lower in women who experienced miscarriage when compared with those with normal pregnancies (60, 62). In the aforementioned studies, kisspeptins presented a comparable or higher diagnostic value than human chorionic gonadotropin (60, 62). Multiple studies have reported reduced circulating kisspeptin levels throughout gestation in preeclamptic versus normal pregnancies (32, 52, 63). At 16 wk of gestation, women suffering from obesity, a well-recognized risk factor for PE, presented lower plasma kisspeptin concentration when compared with lean women, and even lower kisspeptin concentrations were described in women with obesity who later developed PE (38). In addition, a potential association with the severity of PE has been investigated, and blood kisspeptin concentrations were inversely associated with maternal proteinuria and mean arterial blood pressure (36). A prospective cohort study is warranted to further investigate the clinical applicability of using kisspeptins as biomarkers for PE.
PERSPECTIVES AND SIGNIFICANCE
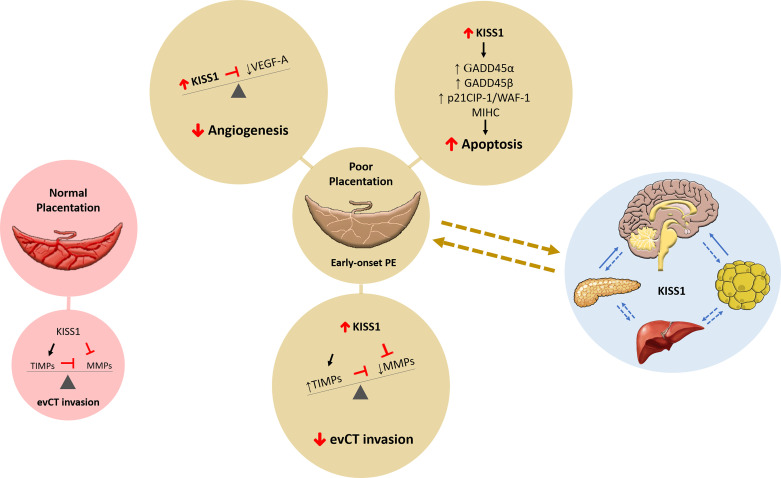
There is compelling evidence of kisspeptin signaling at the maternal-fetal interface during decidualization and placentation. The data compiled herein are suggestive of kisspeptins mRNA and protein upregulation at the maternal-fetal interface in early-onset PE. In the past 2 decades, the downstream molecular pathways and cellular responses associated with kisspeptin receptor activation began to be elucidated. Based on those findings, fetoplacental kisspeptin-mediated inhibition of evCT invasion and angiogenesis and induction of placental apoptosis in early-onset PE are speculated (Fig. 1). Hitherto, the upstream pathways leading to kisspeptin upregulation in PE remain largely unexplored. Confirmatory studies of the role of kisspeptins in the etiopathogenesis of PE in women are lacking, which may be explained by the inherent challenges of investigating early gestation in humans.
Figure 1.
Role of kisspeptins in pregnancy and preeclampsia. In a healthy pregnancy, kisspeptin (KISS1) influences normal placentation by promoting tissue inhibitors of metalloproteinases (TIMPs) while inhibiting matrix metalloproteinases (MMPs) for a balanced and adequate extravillous cytotrophoblast (evCT) invasion. In pregnancies with poor placentation, such as early-onset preeclampsia (PE), KISS1 may influence several physiological events essential for the success of pregnancy and placental development. Elevated KISS1 can decrease evCT invasion and decrease vascular endothelial growth factor (VEGF)-A and angiogenesis and increase apoptosis by increasing growth arrest and DNA damage-inducible protein (GADD) 45α and GADD45β, p21 cyclin-dependent kinase inhibitor (CIP)-1/WAF-1, and mammalian inhibitor of apoptosis protein-1 homolog C (MIHC). Our working hypothesis includes a complex role for KISS1 signaling in the brain, pancreas, liver, and adipose tissue along with the placenta to influence pregnancy outcomes.
GRANTS
This paper was supported by the Veterinary Clinical Sciences Competitive Research Program (to V. C. L. Gomes) and Theriogenology Foundation (to V. C. L. Gomes).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.C.L.G. prepared figures; V.C.L.G. drafted manuscript; V.C.L.G. and J.L.S. edited and revised manuscript; V.C.L.G. and J.L.S. approved final version of manuscript.
REFERENCES
- 1.Rana S, Lemoine E, Granger JP, Karumanchi AS. Preeclampsia: pathophysiology, challenges and perspectives. Circ Res 124: 1094–1112, 2019. doi: 10.1161/CIRCRESAHA.118.313276. [DOI] [PubMed] [Google Scholar]
- 2.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, Gülmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2: e323–e333, 2014. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 3.Lu HQ, Hu R. Lasting effects of intrauterine exposure to preeclampsia on offspring and the underlying mechanism. AJP Rep 9: e275–e291, 2019. doi: 10.1055/s-0039-1695004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, Mamas MA. Preeclampsia and future cardiovascular health: A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 10: 1–9, 2017. doi: 10.1161/CIRCOUTCOMES.116.003497. [DOI] [PubMed] [Google Scholar]
- 5.Wójtowicz A, Zembala-Szczerba M, Babczyk D, Kołodziejczyk-Pietruszka M, Lewaczyńska O, Huras H. Early- and late-onset preeclampsia: a comprehensive cohort study of laboratory and clinical findings according to the new ISHHP criteria. Int J Hypertens 2019: 4108271, 2019. doi: 10.1155/2019/4108271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Than NG, Romero R, Tarca AL, Kekesi KA, Xu Y, Xu Z, et al. Integrated systems biology approach identifies novel maternal and placental pathways of preeclampsia. Front Immunol 9: 1661, 2018. doi: 10.3389/fimmu.2018.01661.eCollection2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischof P, Meisser A, Campana A. Paracrine and autocrine regulators of trophoblast invasion - a review. Placenta 21: S55–S60, 2000. doi: 10.1053/plac.2000.0521. [DOI] [PubMed] [Google Scholar]
- 8.Lan KC, Lai YJ, Cheng HH, Tsai NC, Su YT, Tsai CC, Hsu TY. Levels of sex steroid hormones and their receptors in women with preeclampsia. Reprod Biol Endocrinol 18: 12, 2020. doi: 10.1186/s12958-020-0569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naicker T, Khedun SM, Moodley J, Pijnenborg R. Quantitative analysis of trophoblast invasion in preeclampsia. Acta Obstet Gynecol Scand 82: 722–729, 2003. doi: 10.1034/j.1600-0412.2003.00220.x. [DOI] [PubMed] [Google Scholar]
- 10.Fox R, Kitt J, Leeson P, Aye CYL, Lewandowski AJ. Preeclampsia: risk factors, diagnosis, management, and the cardiovascular impact on the offspring. J Clin Med 8: 1625, 2019. doi: 10.3390/jcm8101625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep 15: 9, 2015. doi: 10.1007/s11892-015-0579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J-H, Miele ME, Hicks DJ, Phillips KK, Trent J, Weissman B, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst 88: 1731–1737, 1996. [Erratum in J Natl Cancer Inst 89: 1549, 1997].doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 13.de Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100: 10972–10976, 2003. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MBL, Crowley WF Jr, Aparicio SAJR, Colledge WH. The GPR54 gene as a regulator of puberty from the reproductive endocrine unit. N Engl J Med 349: 1614–1627, 2003. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 15.Dudek M, Ziarniak K, Sliwowska JH. Kisspeptin and metabolism: the brain and beyond. Front Endocrinol (Lausanne) 9: 1–45., 2018. doi: 10.3389/fendo.2018.00145.eCollection2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harter CJL, Kavanagh GS, Smith JT. The role of kisspeptin neurons in reproduction and metabolism. J Endocrinol 238: R173–R183, 2018. doi: 10.1530/JOE-18-0108. [DOI] [PubMed] [Google Scholar]
- 17.Baba T, Kang HS, Hosoe Y, Kharma B, Abiko K, Matsumura N, Hamanishi J, Yamaguchi K, Yoshioka Y, Koshiyama M, Mandai M, Murphy SK, Konishi I. Menstrual cyclic change of metastin/GPR54 in endometrium. Med Mol Morphol 48: 76–84, 2015. doi: 10.1007/s00795-014-0081-0. [DOI] [PubMed] [Google Scholar]
- 18.Hu KL, Chang HM, Zhao HC, Yu Y, Li R, Qiao J. Potential roles for the kisspeptin/kisspeptin receptor system in implantation and placentation. Hum Reprod Update 25: 326–343, 2019. doi: 10.1093/humupd/dmy046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roman AC, Pinto FM, Dorta I, Almeida TA, Hernández M, Illanes M, Tena-Sempere M, Candenas L. Analysis of the expression of neurokinin B, kisspeptin, and their cognate receptors NK3R and KISS1R in the human female genital tract. Fertil Steril 97: 1213–1219, 2012. doi: 10.1016/j.fertnstert.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, Malli R, Sharabi A, Hiden U, Graier W, Knöfler M, Andreae F, Wagner O, Quaranta V, Desoye G. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci 117: 1319–1328, 2004. doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]
- 21.Janneau JL, Maldonado-Estrada J, Tachdjian G, Miran I, Motté N, Saulnier P, Sabourin JC, Coté JF, Simon B, Frydman R, Chaouat G, Bellet D. . Transcriptional expression of genes involved in cell invasion and migration by normal and tumoral trophoblast cells. J Clin Endocrinol Metab 87: 5336–5339, 2002. doi: 10.1210/jc.2002-021093. [DOI] [PubMed] [Google Scholar]
- 22.Martino NA, Rizzo A, Pizzi F, Dell’Aquila ME, Sciorsci RL. Effects of kisspeptin-10 on invitro proliferation and kisspeptin receptor expression in primary epithelial cell cultures isolated from bovine placental cotyledons of fetuses at the first trimester of pregnancy. Theriogenology 83: 978–987, 2015. doi: 10.1016/j.theriogenology.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 23.Terao Y, Kumano S, Takatsu Y, Hattori M, Nishimura A, Ohtaki T, Shintani Y. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim Biophys Acta 1678: 102–110, 2004. doi: 10.1016/j.bbaexp.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Horikoshi Y, Matsumoto H, Takatsu Y, Ohtaki T, Kitada C, Usuki S, Fujino M. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab 88: 914–919, 2003. doi: 10.1210/jc.2002-021235. [DOI] [PubMed] [Google Scholar]
- 25.Qiao C, Wang CH, Shang T, Lin Q. Clinical significance of KiSS-1 and matrix metalloproteinase-9 expression in trophoblasts of women with preeclampsia and their relation to perinatal outcome of neonates. Zhonghua Fu Chan Ke Za Zhi 40: 585–590, 2005. [PubMed] [Google Scholar]
- 26.Qiao C, Cheng DL, Zhang SL, Wang CH, Lin Q. The role of KiSS-1 and matrix metalloproteinase-9 in regulation of invasion of trophoblasts. Zhonghua Yi Xue Za Zhi 85: 839–842, 2005. [PubMed] [Google Scholar]
- 27.Zhang H, Lin Q, Qiao C. Expression of trophoblast invasion related genes mRNA and protein in human placenta in preeclampsia. Zhonghua Fu Chan Za Zhi 41: 509–513, 2006. [PubMed] [Google Scholar]
- 28.Zhang H, Long Q, Ling L, Gao A, Li H, Lin Q. Elevated expression of KiSS-1 in placenta of preeclampsia and its effect on trophoblast. Reprod Biol 11: 99–115, 2011. doi: 10.1016/S1642-431X(12)60048-5. [DOI] [PubMed] [Google Scholar]
- 29.Cartwright JE, Williams PJ. Altered placental expression of kisspeptin and its receptor in pre-eclampsia. J Endocrinol 214: 79–85, 2012. doi: 10.1530/JOE-12-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao C, Wang C, Zhao J, Liu C, Shang T. Elevated expression of KiSS-1 in placenta of Chinese women with early-onset preeclampsia. PLoS One 7: e48937, 2012. doi: 10.1371/journal.pone.0048937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vodneva DN, Dubova EA, Pavlov KA, Shmakov RG, Shchegolev AI. Role of kisspeptins in the development of early- and late-onset preeclampsia. Obstet Gyncol 8: 65–70, 2014. [Google Scholar]
- 32.Matjila M, Millar R, Van Der Spuy Z, Katz A. Elevated placental expression at the maternal-fetal interface but diminished maternal circulatory kisspeptin in preeclamptic pregnancies. Pregnancy Hypertens 6: 79–87, 2016. doi: 10.1016/j.preghy.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Smets EML, Deurloo KL, Go ATJI, van Vugt JMG, Blankenstein MA, Oudejans CBM. Decreased plasma levels of metastin in early pregnancy are associated with small for gestational age neonates. Prenat Diagn 28: 299–303, 2008. doi: 10.1002/pd.1969. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong RA, Reynolds RM, Leask R, Shearing CH, Calder AA, Riley SC. Decreased serum levels of kisspeptin in early pregnancy are associated with intra-uterine growth restriction and pre-eclampsia. Prenat Diagn 29: 982–985, 2009. doi: 10.1002/pd.2328. [DOI] [PubMed] [Google Scholar]
- 35.Nijher GMK, Chaudhri OB, Ramachandran R, Murphy KG, Zac-Varghese SEK, Fowler A, Chinthapalli K, Patterson M, Thompson EL, Williamson C, Kumar S, Ghatei MA, Bloom SR, Dhillo W. The effects of kisspeptin-54 on blood pressure in humans and plasma kisspeptin concentrations in hypertensive diseases of pregnancy. Br J Clin Pharmacol 70: 674–681, 2010. doi: 10.1111/j.1365-2125.2010.03746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adali E, Kurdoglu Z, Kurdoglu M, Kamaci M, Kolusari A, Yildizhan R. Metastin levels in pregnancies complicated by pre-eclampsia and their relation with disease severity. J Matern Neonatal Med 25: 2671–2675, 2012. doi: 10.3109/14767058.2012.708369. [DOI] [PubMed] [Google Scholar]
- 37.Ćetković A, Miljic D, Ljubić A, Patterson M, Ghatei M, Stamenković J, Nikolic-Djurovic M, Pekic S, Doknic M, Glišić A, Bloom S, Popovic V. Plasma kisspeptin levels in pregnancies with diabetes and hypertensive disease as a potential marker of placental dysfunction and adverse perinatal outcome. Endocr Res 37: 78–88, 2012. doi: 10.3109/07435800.2011.639319. [DOI] [PubMed] [Google Scholar]
- 38.Logie JJ, Denison FC, Riley SC, Ramaesh T, Forbes S, Norman JE, Reynolds RM. Evaluation of kisspeptin levels in obese pregnancy as a biomarker for pre-eclampsia. Clin Endocrinol (Oxf) 76: 887–893, 2012. doi: 10.1111/j.1365-2265.2011.04317.x. [DOI] [PubMed] [Google Scholar]
- 39.Ziyaraa MA, Hamdan FB, Mousa LR. Correlation of kisspeptin-10 and fetal well-being in preeclamptic patients. Taiwan J Obstet Gynecol 55: 840–846, 2016. doi: 10.1016/j.tjog.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 40.Torricelli M, Novembri R, Conti N, De Falco G, De Bonis M, Petraglia F. Correlation with placental kisspeptin in postterm pregnancy and apoptosis. Reprod Sci 19: 1133–1137, 2012. doi: 10.1177/1933719112443878. [DOI] [PubMed] [Google Scholar]
- 41.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KISS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411: 613–616, 2001. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 42.West A, Vojta PJ, Welch DR, Weissman BE. Chromosome localization and genomic structure of the KiSS-1 metastasis suppressor gene (KISS1). Genomics 54: 145–148, 1998. doi: 10.1006/geno.1998.5566. [DOI] [PubMed] [Google Scholar]
- 43.De Tassigny XDA, Jayasena C, Murphy KG, Dhillo WS, Colledge WH. Mechanistic insights into the more potent effect of KP-54 compared to KP-10 in vivo. PLoS One 12: e0176821, 2017. doi: 10.1371/journal.pone.0176821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castaño JP, Martínez-Fuentes AJ, Gutiérrez-Pascual E, Vaudry H, Tena-Sempere M, Malagón MM. Intracellular signaling pathways activated by kisspeptins through GPR54: do multiple signals underlie function diversity? Peptides 30: 10–15, 2009. doi: 10.1016/j.peptides.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 45.Francis VA, Abera AB, Matjila M, Millar RP, Katz AA. Kisspeptin regulation of genes involved in cell invasion and angiogenesis in first trimester human trophoblast cells. PLoS One 9: e99680, 2014. doi: 10.1371/journal.pone.0099680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod 69: 1–7, 2003. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 47.Huppertz B. Biology of preeclampsia: combined actions of angiogenic factors, their receptors and placental proteins. Biochem Biophys Acta 1866: 165349, 2018. doi: 10.1016/j.bbadis.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 48.Matjila M, Millar R, van der Spuy Z, Katz A. The differential expression of kiss1, mmp9 and angiogenic regulators across the feto-maternal interface of healthy human pregnancies: implications for trophoblast invasion and vessel development. PLoS One 8: e63574, 2013.doi: 10.1371/journal.pone.0063574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu C, Takasu C, Morine Y, Bando Y, Ikemoto T, Saito Y, Yamada S, Imura S, Arakawa Y, Shimada M. KISS1 associates with better outcome via inhibiting matrix metalloproteinase-9 in colorectal liver metastasis. Ann Surg Oncol 22: 1516–1523, 2015. doi: 10.1245/s10434-015-4891-7. [DOI] [PubMed] [Google Scholar]
- 50.Maynard SE, Ananth Karumanchi S. Angiogenic factors and preeclampsia. Semin Nephrol 31: 33–46, 2011. doi: 10.1016/j.semnephrol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhai J, Liu J, Zhao S, Zhao H, Chen ZJ, Du Y, Li W. Kisspeptin-10 inhibits OHSS by suppressing VEGF secretion. Reproduction 154: 355–362, 2017. doi: 10.1530/REP-17-0268. [DOI] [PubMed] [Google Scholar]
- 52.Longtine MS, Chen B, Odibo AO, Zhong Y, Nelson DM. Villous trophoblast apoptosis is elevated and restricted to cytotrophoblasts in pregnancies complicated by preeclampsia, IUGR, or preeclampsia with IUGR. Placenta 33: 352–359, 2012. doi: 10.1016/j.placenta.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Becker JAJ, Mirjolet JF, Bernard J, Burgeon E, Simons MJ, Vassart G, Parmentier M, Libert F. Activation of GPR54 promotes cell cycle arrest and apoptosis of human tumor cells through a specific transcriptional program not shared by other G q-coupled receptors. Biochem Biophys Res Commun 326: 677–686, 2005. doi: 10.1016/j.bbrc.2004.11.094. [DOI] [PubMed] [Google Scholar]
- 54.Wu S, Zhang H, Tian J, Liu L, Dong Y, Mao T. Expression of kisspeptin/GPR54 and PIBF/PR in the first trimester trophoblast and decidua of women with recurrent spontaneous abortion. Pathol Res Pract 210: 47–54, 2014. doi: 10.1016/j.prp.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 55.De Pedro MA, Morán J, Díaz I, Murias L, Fernández- Plaza C, González C, Díaz E. Circadian Kisspeptin expression in human term placenta. Placenta 36: 1337–1339, 2015. doi: 10.1016/j.placenta.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Fayazi M, Calder M, Bhattacharya M, Vilos GA, Power S, Babwah AV. The pregnant mouse uterus exhibits a functional kisspeptin/KISS1R signaling system on the day of embryo implantation. Reprod Biol Endocrinol 13: 105, 2015. doi: 10.1186/s12958-015-0105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang P, Tang M, Zhong T, Lin Y, Zong T, Zhong C, Zhang B, Ren M, Kuang H. Expression and function of kisspeptin during mouse decidualization. PLoS One 9: e976471, 2014. doi: 10.1371/journal.pone.0097647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L, Burger LL, Greenwald-Yarnell ML, Myers MG, Moenter SM. . Glutamatergic transmission to hypothalamic kisspeptin neurons is differentially regulated by estradiol through estrogen receptor α in adult female mice. J Neurosci 38: 1061–1072, 2018. doi: 10.1523/JNEUROSCI.2428-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeFino MC, Wacker JL, Lyssand JS, Wang EH, Hague C. Differential regulation of GPR54 transcription by specificity protein-1 and partial estrogen response element in mouse pituitary cells. Biochem Biophys Res Commun 393: 603–608, 2010. doi: 10.1016/j.bbrc.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sullivan-Pyke C, Haisenleder DJ, Senapati S, Nicolais O, Eisenberg E, Sammel MD, Barnhart KT. Kisspeptin as a new serum biomarker to discriminate miscarriage from viable intrauterine pregnancy. Fertil Steril 109: 137–141.e2, 2018. doi: 10.1016/j.fertnstert.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jayasena CN, Comninos AN, Narayanaswamy S, Abbara A, Nijher GMK, Cheema M, Malik Z, Ghatei MA, Bloom SR, Dhillo WS. The identification of elevated urinary kisspeptin-immunoreactivity during pregnancy. Ann Clin Biochem 52: 395–398, 2015. doi: 10.1177/0004563214551612. [DOI] [PubMed] [Google Scholar]
- 62.Jayasena CN, Abbara A, Izzi-Engbeaya C, Comninos AN, Harvey RA, Gonzalez Maffe JG, Sarang Z, Ganiyu-Dada Z, Padilha AI, Dhanjal M, Williamson C, Regan L, Ghatei MA, Bloom SR, Dhillo WS. Reduced levels of plasma kisspeptin during the antenatal booking visit are associated with increased risk of miscarriage. J Clin Endocrinol Metab 99: E2652–E2660, 2014. doi: 10.1210/jc.2014-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu KL, Zhao H, Yu Y, Li R. Kisspeptin as a potential biomarker throughout pregnancy. Eur J Obstet Gynecol Reprod Biol 240: 261–266, 2019. doi: 10.1016/j.ejogrb.2019.07.016. [DOI] [PubMed] [Google Scholar]