Abstract
We report a case of a patient with mixed dementia successfully treated with a personalized multimodal therapy. Monotherapeutics are inadequate for the treatment of Alzheimer’s disease (AD) and mixed dementia; therefore, we approach treatment through an adaptive personalized multimodal program. Many multimodal programs are pre-determined, and thus may not address the underlying contributors to cognitive decline in each particular individual. The combination of a targeted, personalized, precision medicine approach using a multimodal program promises advantages over monotherapies and untargeted multimodal therapies for multifactorial dementia. In this case study, we describe successful treatment for a patient diagnosed with AD, using a multimodal, programmatic, precision medicine intervention encompassing therapies targeting multiple dementia diastheses. We describe specific interventions used in this case that are derived from a comprehensive protocol for AD precision medicine. After treatment, our patient demonstrated improvements in quantitative neuropsychological testing, volumetric neuroimaging, PET scans, and serum chemistries, accompanied by symptomatic improvement over a 3.5-year period. This case outcome supports the need for rigorous trials of comprehensive, targeted combination therapies to stabilize, restore, and prevent cognitive decline in individuals with potentially many underlying causes of such decline and dementia. Our multimodal therapy included personalized treatments to address each potential perturbation to neuroplasticity. In particular, neuroinflammation and metabolic subsystems influence cognitive function and hippocampal volume. In this patient with a primary biliary cholangitis (PBC) multimorbidity component, we introduced a personalized diet that helped reduce liver inflammation. Together, all these components of multimodal therapy showed a sustained functional and cognitive benefit. Multimodal therapies may have systemwide benefits on all dementias, particularly in the context of multimorbidity. Furthermore, these therapies provide generalized health benefits, as many of the factors – such as inflammation – that impact cognitive function also impact other systems.
Keywords: multimodal, multimorbidity, Alzheimer, dementia, lifestyle, neuroplasticity, primary biliary cholangitis, dementia-multifactorial
INTRODUCTION
Recently, there has been increased interest in multimodal interventions for reducing the risk of Alzheimer’s Disease (AD). For example, the World Health Organization [1] published guidelines to reduce risk for cognitive decline and dementia that emphasize multimodal interventions. Reported lifestyle interventions include increased cognitive, physical, and social activity for reducing risk of cognitive decline [2–4]. Combination therapies and multimodal approaches have produced therapeutic success for chronic illnesses such as cancers, HIV, and cardiovascular disease. These initial successes demonstrate that targeted, multimodal approaches to AD deserve further and more detailed study.
In this paper, we present a single case study with full details of the clinical course and outcome. We describe a successful treatment response for a patient with previously diagnosed AD using a precison medicine, multimodal intervention with specific focus on treating potential contributors such as steroid hormone deficiency, thyroid deficiency, kidney function, liver function, vascular health, tick-borne infections, mercury toxicity, and mycotoxins. Each of these may independently contribute in this individual as an underlying driver of cognitive decline and the AD disease process. In presenting this case, we take a step towards filling a paucity in the literature for methods to evaluate and treat cognitive symptoms secondary to biotoxicity. Treating multimodal disease with potentially synergistic targeted interventions towards each disease modality should be a paradigm for the future of personalized medical treatment of many chronic diseases, particularly those that affect aging individuals.
CASE PRESENTATION
Background Information
A 78-year-old, left-handed retired female physician presented with a one-year history of severe, progressive memory loss, such that her significant other described her memory for recent events as “disastrous.” She described a lifelong mild dyslexia, and amnesia for many events of her early childhood and adolescence. She noted an awareness of mild cognitive problems for approximately 20 years, but these worsened in the year prior to evaluation. She also noted poor recall for movies that she had watched and books that she had read. She noted problems with name recall, and often called her pets by the wrong name. She noticed increased susceptibility to stress and fatigue which seemed to worsen when her son died a tragic death in 2001. At presentation, she reported leading an active life, enjoying golfing, hiking, birding, and gardening. She worked in private practice as a psychiatrist until she retired at the age of 75. She has always been actively involved in many civic associations, and remains active in political circles. She has a family history of dementia and memory issues: her mother developed dementia secondary to hydrocephalus, and her sister developed memory problems but died from an aneurysm at the age of 80. She reported exposure to mold in her partner’s home. She also stated that she has several dogs that she sleeps with in her bed. She lives in New York, does not use tick spray on the dogs, and reports an average of 10 tick bites per year. She had a remote history of Bell’s palsy on the left side of unknown etiology. Her past medical history is also significant for Raynaud’s syndrome, elevated gamma-glutamyl transpeptidase secondary to primary biliary cholangitis (PBC), and elevated mercury. She had elevated inflammatory markers suggesting exposure to biotoxins. She was diagnosed with early AD by her neurologist in 2016. In particular, a fluorodeoxyglucose-positron emission tomography (FDG-PET) scan revealed decreased glucose utilization in the anterior superior precuneus bilaterally and the anterolateral left temporal lobe which is consistent with the earliest manifestations of AD. Her neurologist recommended treatment with donepezil and memantine. She refused both because she had read about their minimal effects on decline and because she had read about anecdotal successes with programmatic treatment.
Mulitmodal Interventions for Alzheimer’s Disease
Recently, there has been increased interest in multimodal interventions for reducing the risk of Alzheimer’s Disease (AD). The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) was the first randomized controlled trial (RCT) demonstrating a beneficial effect of a 2-year multidomain intervention (using nutrition, physical activity, and cognitive training) on cognitive performance in older adults who were at risk for dementia based on vascular parameters [2]. World Health Organization guidelines include multimodal interventions [1]. Many others have reported success with lifestyle intervention (increased cognitive, physical, and social activity) in reducing risk of cognitive decline among individuals with mild cognitive impairment (MCI), and with aerobic exercise in improving executive function in those with early stage-cognitive impairment [5,6].
A recent publication by Isaacson et al. [7] provided further support for the application of multimodal lifestyle interventions to improve cognition and reduce AD and cardiovascular risk scores in patients at risk for AD. The authors used a personalized medicine approach when considering treatment options for patients. They found that multidomain interventions reduced AD and cardiovascular risk scores. In addition, in those seeking prevention, both high and low levels of compliance were associated with improved cognition with individualized multidomain interventions. Patients already exhibiting early cognitive impairment (MCI) showed improved cognition only with high levels of compliance with the individualized multidomain interventions, and cognition declined for those in the low compliance group. Anecdotal sustained improvements have been reported in over 100 patients with AD or MCI using a personalized, precision medicine approach that addresses presumptive contributors to cognitive decline, such as insulin resistance, systemic inflammation, and pathogens [8], although these are often not reported with detailed descriptions of the interventions and clinical course for each patient. Combination therapies and multimodal approaches have produced therapeutic success for chronic illnesses such as cancer, HIV, and cardiovascular disease [3]. Because of the complex nature of AD, with many potential and variable contributors, the initial success in prevention trials, and the anecdotal success with cognitive improvement in MCI and AD, a targeted, multimodal approach deserves further detailed study and documentation.
Individuals may vary due to genetics, environment, and lifestyle. Our precision medicine approach considers individual variability by utilizing an extensive evaluation including detailed medical history, lifestyle variables, blood biomarkers, neuroimaging with volumetrics, and quantitative neurocognitive assessments. Practitioners utilizing this approach can further examine whether patients with cognitive issues harbor a significant burden of toxicity contributing to their cognitive decline. Possible multimodal lifestyle interventions for cognitive decline in AD include diet, nutrition, exercise, mindfulness, and stress management. In addition to the effects of lifestyle, epidemiological and pathological studies suggest that there are numerous other underlying drivers such as environmental toxins and chronic infections. Environmental toxins may include mercury from seafood and amalgam fillings or biotoxins such as mycotoxins from mold exposure. Chronic infections could include herpes, gingival disease, COVID-19, or tick born disease such as Lyme disease [9–11]. The recent COVID-19 pandemic has raised the possibility of long-term cognitive effects given the neuroinvasive potential of this novel coronavirus [12]. Furthermore, pandemic stressors may worsen cognitive issues [13].
One study showed an 8% increase in hippocampal volume over a 12-week period with a multifaceted lifestyle program [14]. Additional literature has noted the influence of lifestyle-related factors such as obesity on brain atrophy, and the beneficial effects of physical activity and diet on gray matter volume [15,16]. Physical activity itself has also been linked with reduced burden of amyloid on PET scans [17]. A systematic review of multiple lifestyle factors including alcohol use and smoking suggested that fMRI also reflects both positive and negative influences of these factors on the physiologic changes reflected by this modality [18]. A randomized clinical trial of physical activity in 24 elderly women (75–83 years old) assigned either to 3 months of biweekly 90-min sessions focused on aerobic exercise, strength training, and physical therapy versus rest showed that the intervention group had improved glucose metabolism [19]. This parallels a larger randomized study showing improved hippocampal volumes in a physical activity group versus a passive stretching group on volumetric MR quantification [20]. A recent Canadian study suggested that a screening imaging program for dementia based in part by modifiable risk factors was financially manageable [21].
We recommended multimodal interventions for the treatment of this patient. These were personalized, and in addition to diet and exercise interventions, included hormone replacement, DMSA therapy for posisble heavy metal toxicity, Lyme therapy for posisble Borrelia infection, and cognitive training.
DMSA Therapy.
DMSA has a half life of 4 hours and it is important to keep the drug at a steady state so that the bound metals do not become reabsorbed and deposit in other tissues of the body. It is important to note that the order in which treatment progressed was very slow and chelation of heavy metals was actually one of the last therapies. The patient was treated in a progressive order that the chelation of heavy metals was one of the last things she was treated for in an effort to ensure that her gut health was good and that she was stronger and able to handle the chelation of mercury. With a history of PBC there was concern about her ability to sustain good liver detoxification. She was very sensitive to DMSA and noted a decrease in her cognition when she took a dose for provocation. While on chelation she was given herbs that enhance liver detoxification along with a rigorous nutrient regimen. The chelation protocol is: (1) Low Dose DMSA 10 mg/kg is the dose to be taken every 4 hours around the clock for 4 days drinking at least 8 ounces of water with each dose; (2) 10 days off between each 4 day treatment cycle, and (3) during the holiday between oral chelation we recommended patients replenish minerals and nutrients that have been removed with chelation.
Lyme Therapy.
Given dementia symptoms and the patients serologies, medications used for treatment of Lyme are: (1) Cefdinir 300 mg bid; (2) Azithromycin 500 mg qd; and (3) the Byron White herbal regimen.
Cognitive Training.
This patient engages in cognitive training using BrainHQ by Posit Science [22]. When she first embarked on this program she was unable to do BrainHQ and found it very frusterating. She initially was able to do a brain training program Elevate. She has since graduated to the BrainHQ and is now in the 94th percentile. She states that she uses her BrainHQ score to help let her know how her cognition is going and it alarms her to any new problems. She is currently using BrainHQ 45 minutes per day.
Baseline Neurological and Cognitive Assessments
The patient underwent memory testing at the age of 65 while attending Canyon Ranch Health Resort. Testing consisted of the Wechsler Memory Scale-III (WMS-III). Results indicated that her Working Memory Index (WMI) was in the average range of ability (WMS-III WMI=108, 70th percentile); however, her Immediate Memory Index (89, 23rd percentile), Delayed Memory Index (88, 21st percentile), and her General Memory Index (88, 21st percentile) were all in the low average range of ability (see Table 1). Interpretation by the health resort clinical psychologist was that her memory was poorer than expected based on her vocation of medical doctor. It was therefore recommended that she undergo more extensive neuropsychological testing.
Table 1.
Hippocampal Volume Measurements. From 2016 to 2019 overall hippocampal volume increased 3%. Timepoints: 1 = 2016; 2 = 2017; 3 = 2019.
| Timepoint | Left Hippocampus Volume (ml) | Left Hippocampus Vol/mTIV ratio | Left Hippocampus NR Index | Left Hippocampus Z-score | Left Hippocampus % |
|---|---|---|---|---|---|
| 1 | 3.86 | 0.199 | 0.804 | 0.054 | 52.17 |
| 2 | 3.76 | 0.191 | −1.28 | −0.087 | 46.55 |
| 3 | 4.06 | 0.211 | 4.91 | 0.33 | 63.03 |
| Timepoint | Right Hippocampus Volume (ml) | Right Hippocampus Vol/mTIV ratio | Right Hippocampus NR Index | Right Hippocampus Z-score | Right Hippocampus % |
|---|---|---|---|---|---|
| 1 | 4.05 | 0.208 | 2.76 | 0.187 | 57.41 |
| 2 | 3.99 | 0.203 | 1.41 | 0.095 | 53.79 |
| 3 | 4.09 | 0.213 | 4.52 | 0.306 | 62.03 |
| Timepoint | Hippocampus Volume (ml) | Hippocampus Vol/mTIV ratio |
|---|---|---|
| 1 | 7.91 | 0.41 |
| 2 | 7.75 | 0.39 |
| 3 | 8.15 | 0.42 |
However, the patient did not seek further assistance for her memory complaints for several years due to actually forgetting. Her cognitive problems continued to progress insidiously, with an acceleration in cognitive decline starting at approximately age 74. She noted that she was starting to mix up the names of people and pets and that she was starting to have difficulty with navigating spaces, such as having difficulty finding her way back to her table at a restaurant after using the bathroom. An acquaintance encouraged her to look into integrative approaches to brain health, and she began a multimodal program under the supervision of Dr. Ross at what is now the BHRI clinic. She initiated the multimodal program in January 2017 at age 75.
Prior to starting the program, she saw a neurologist in order to obtain confirmatory diagnostic testing. She scored a 28/30 on the MMSE at the initial neurology evaluation in 9/2016. Her neurological exam was normal. She underwent an MRI of the brain in 9/2016 that revealed mild biparietal atrophy with hippocampal cysts on the right side. MRI also revealed a few scattered small foci of hyperintensity in the bilateral hemispheric white matter and paramedian pons, and evidence of a partially empty sella with flattening of the pituitary gland. Volumetric analysis revealed decreased hippocampal volume bilaterally. FDG-PET conducted in 9/2016 indicated mildly decreased FDG activity in the anterior superior precuneus bilaterally as well as in the anterolateral left temporal lobe. The findings were thought to represent early AD pathology and she was referred for neuropsychological testing.
A full neuropsychological evaluation was conducted in 11/2016. She reported to the evaluating neuropsychologist that her overall energy and cognitive problems had improved somewhat over the past few months with the lifestyle changes. Even so, her neuropsychological performances revealed impairments in reaction time, visual organization/constructional skills, and learning/recognition of unstructured information, within the context of estimated high average baseline intellectual capacity. She struggled on the testing with the intial learning of a word list and did not appear to benefit from repetition of the list. In fact, she reported that the words were “dropping out” following the learning trials. Her learning slope was in the borderline-impaired range (7th percentile). Recognition performance was also notable for a remarkably high number of false positive errors. Learning improved when the verbal material was presented in a contextual format of a story. Reaction time on a computerized measure was slowed, and her approach to drawing a complex geometric figure was disorganized and poorly planned, resulting in inaccuracies in the placement and spacing of the figure details. She performed in the borderline-impaired range on this task (2nd-5th percentile). This constellation of findings was felt to be most compatible with early AD.
Clinical Course
A multimodal program was prepared for this patient tailoring the appropriate neutraceuticals and medications to fit this patient’s specific needs. Labs and biomarkers were evaluataed and targeted neutraceuticals were used to optimize levels of hormones, vitamins, glucose, and insulin. Her history of tick bites and Bell’s palsy prompted a workup that revealed a positive Western blot IgM for Lyme disease. She was placed on oral antibiotics and an herbal regimen for 3 months. Her thyroid and sex hormones were suboptimal, so she was placed on bioidentical hormone therapy as well as natural thyroid replacement. She was tested for heavy metals using a pre- and post-provocation test (Doctors Data) that revealed elevated mercury. She initially went to an integrative practice in New York for IV glutathione while she underwent oral chelation [23]. She was placed on an oral chelation protocol using DMSA. The patient was hospitalized with ehrilichiosis and received IV antibiotics; she states that she noticed an increase in her energy following the antibiotics. She had a home sleep study in 2020 and was found to have moderate sleep apnea. She began CPAP, and now reports even better energy and subjective cognitive function. She also trains her cognitive function with BrainHQ. She also uses a Vielight Gamma, Oura Ring, and MUSE Headband. She meets with a neuro physical therapist via Zoom twice weekly for excise sessions that are coupled with dual tasking. She feels that exercising with dual tasking has contributed greatly to her cognitive rehabilitation. We measured system-wide clinical markers and followed their longitudinal trajectory (Figure 1). The average date of the inflection point towards a positive trend in these markers was in May 2017 five months after start of therapy, suggesting a synergistic and cumulative impact of multimodal interventions within months of commencing therapy.
Figure 1.
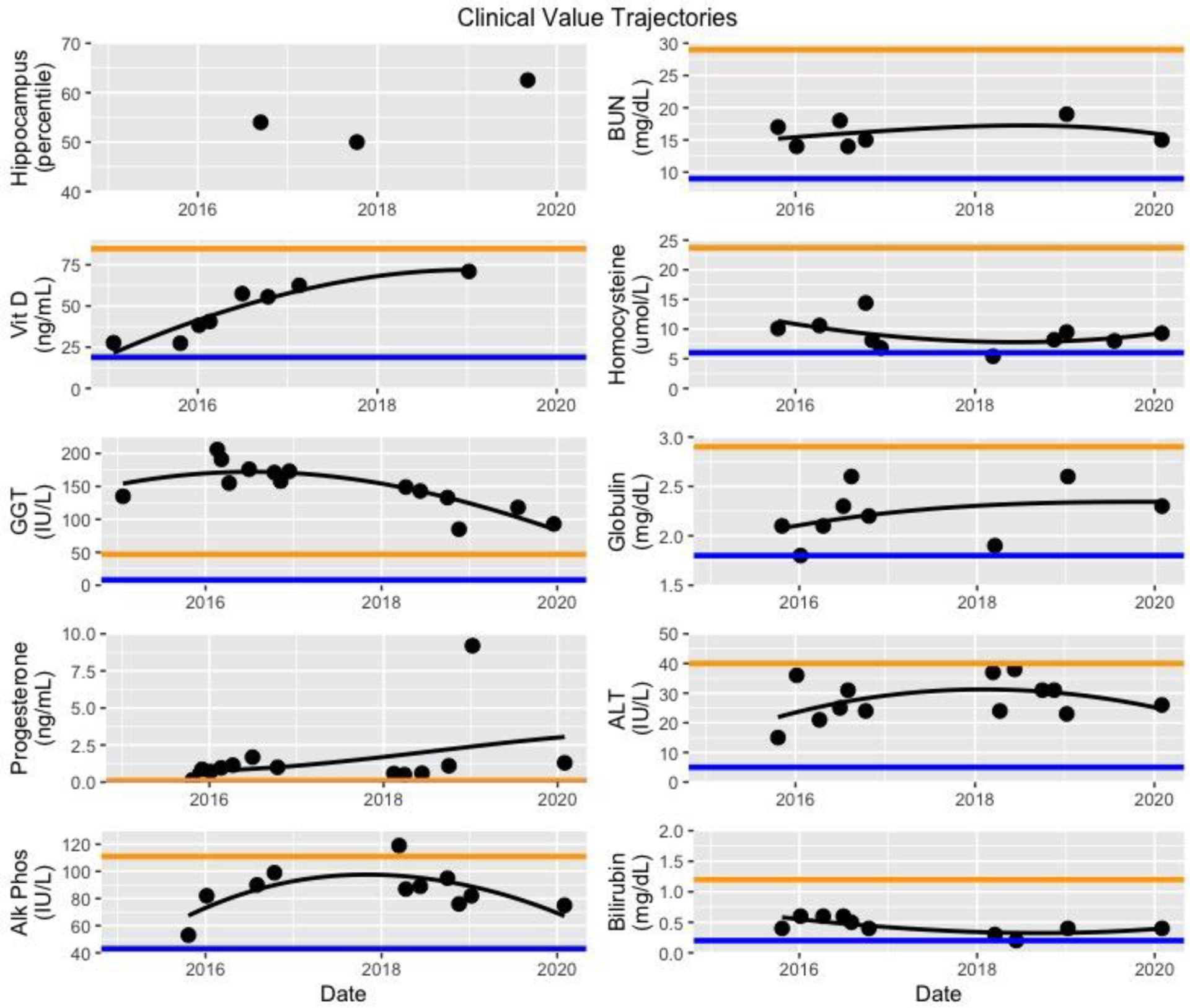
Trajectories of select clinical chemistries. Hippocampal volume measurements are shown for comparison. Orange line; upper bound of reference. Blue line; lower bound of reference. Multimodal treatment of the entire individual results in subtle changes that impact many physiological subsystems. Although these impacts often change trajectories only within traditional reference ranges, the cumulative effect on well being may be pronounced.
Neuroimaging
The patient’s MRI of the brain on 9/2016 showed a quantified hippocampal volume of 16th percentile on NeuroQuant. Her FDG-PET scan was qualitatively interpreted as having low metabolism in the anterior precuneus and anterolateral temporal lobes. This report also noted a qualitative description of “mild biparietal atrophy.” Neuroquant, the software program that measures these volumes initially has been shown to underestimate hippocampal volume [24] compared to another FDA-cleared program called Neuroreader, which is also utilized for MRI brain structural quantification [25]. While each software program compares brain volumes to a normal database, they utilize different databases. It has been published with Neuroreader that its normal database comes from the Alzheimer’s Disease Neuroimaging Initiative Database (ADNI), a well validated and well published research cohort [25]. It should be noted therefore that while the intial Neuroquant analysis suggested a hippocampal volume at 16th percentile, the Neuroreader analysis for that same scan showed a more normal value of 54th percentile. It should also be noted that while the intial FDG PET scan suggested AD based on the parietal hypometabolism, there was no medial temporal lobe hypometabolism reported, although as noted above, there was hypometabolism noted in the precuneus.
The visually interpreted mild biparietal atrophy on the MRI from 2016 was not supported by the quantitative result from Neuroreader, showing a normal parietal lobe volume of 49th percentile. This discrepancy highlights the need for quantitative evaluations of atrophy, which are usually more accurate than visual evaluations [26]. Longitudinal evalutions are also key in determining suggestive evidence of AD, since progressive atrophy is noted in conjunction with neurodegenerative disease [27].
With respect to these longitudinal evaluations, the 2017 brain MRI scan showed a reduction in hippocampal volumes compared to 2016, and this was observed with Neuroquant, as well, although, again, with a lower percentile from that scan at 11th percentile compared to the still normal hippocampal volume percentile on Neuroreader of 50th percentile. Additionally, as with the 2016 scan, none of the patient’s brain volumes were found to be abnormally low. It should also be noted that while the Neuroquant percentiles of 11th or 16th percentile may be considered abnormally low, the Neuroquant software program uses a 5th percentile cutoff to determine abnormally low volume,s while Neuroreader uses a 25th percentile threshold. Overall, the patient’s hippocampal volume from 2016 to 2017 declined on Neuroreader by 3%, which is an abnormal rate of atrophy, as it should normally be declining by only 0.5% per year. Such enhanced rate of decline is a poor prognostic feature in Alzheimer’s patients and in the the risk for conversion from MCI to AD [27]. However, from 2017 to 2019 the patient’s hippocampal volume to mTIV ratio showed an increase by 8% to the 62nd percentile – up considerably from the 50th percentile in 2017 (Table 1). Previous publications have shown that the hippocampus is neuroplastic enough to increase in volume when exposed to multimodal personalized treatment programs and lifestyle changes. The volumes for other brain regions, as with 2017 and 2016 studies, were found to be in the normal range. The summary of changes in hippocampal volumes are shown in Figure 2.
Figure 2.
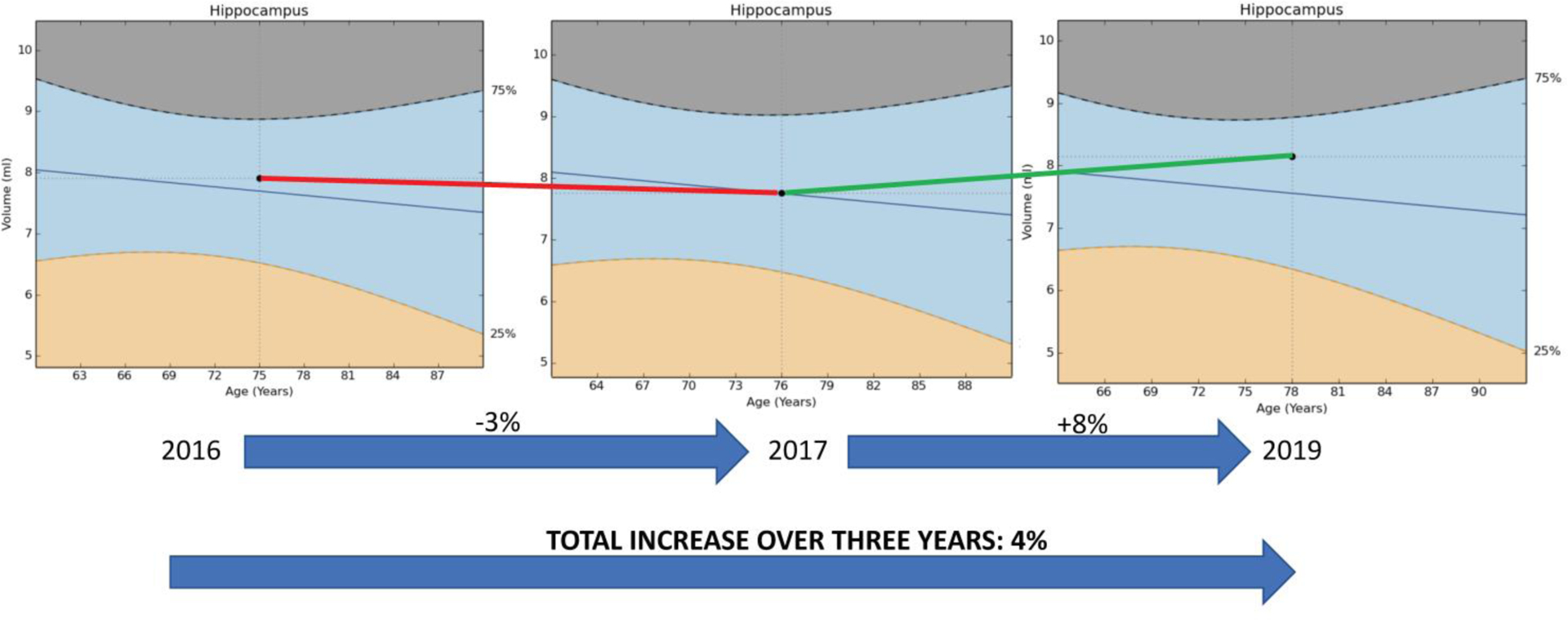
Hippocampal volume changes in the patient from 2016 to 2019. The hippocampal volume decreased from 2016 to 2017 (red line) and then increased between 2017 and 2019 (green line). Volumes were determined with Neuroreader software.
Longitudinal Biomarker Analyses
For each biomarker, we fit a quadratic regression to all longitudinal values. To compute the inflection point (typically the nadir) for each trajectory, we computed the vertex of the parabola resulting from the quadratic regression. In some cases, such as monotonically increasing values (e.g., Vitamin D) we did not compute an inflection point. For the following analytes dates of inflection are: GGT 11/4/16, BUN 7/3/17, alkaline phosphate 12/9/16, homocysteine 9/12/17, ALT 5/15/17, bilirubin 12/21/17. The average inflection date is May 23, 2017. These inflection points group together at approximately the same time (late 2016 through 2017) which is consistent with the timing of full impact of the multimodal intervention.
Improvements in Cognition and Function
As noted above, the patient’s presentation, pattern of cognitive decline, chronicity and progression, neuropsychological testing, MRI volumetrics, and FDG-PET scan results were compatible with a diagnosis of early AD. She has undergone treatment from 2016 to the present. From 2016 until 2020, the patient noted marked subjective improvements in memory. She noted that she was able to remember her golf strokes and those of her friends once again. She no longer failed to feed the parking meters and did not leave her car in the road while still running. Her significant other noted that her memory improved from “disastrous” to “just plain lousy” and ultimately to “normal.”
In the later part of 2019 this patient was able to fly from New York to Seattle unaccompanied and navigated the entire trip on her own with no problem; a patient dignosed with AD in 2016 would most likely not be traveling across the country alone 3 years later. These marked subjective changes were accompanied by objective changes. She consistently used BrainHQ cognitive training; her position in the reference range provided by BrainHQ improved from 9th to 97th percentile. Her Montreal Cognitive Assessment (MoCA) was 23 in 10/2017, was stable at 23 in 12/18 and improved to 26 in 11/2019. Her hippocampal volume increased from 50th to 62nd percentile, a volume increase of 8%; a positive change this large over this period of time was seen in only 3% of reference AD participants in ADNI [28,25]. Her FDG-PET scan also showed improvement. Her initial FDG-PET revealed mildly decreased FDG activity in the anterior superior precuneus bilaterally as well as in the anterolateral left temporal lobe. The remainder of the brain parenchyma had normal ativity. An interpretation could be that the mild biparietal and hippocampal volume loss with concordant subtle FDG hypometabolism represented the earliest imaging manifestations of underlying AD pathology in her case [14]. The repeat FDG-PET, which was conducted on the same machine, revealed mild patchy hypometabolism of the superior parietal and anterior temporal lobes along with the superior parietal volume loss which was unchanged from the previous measurement. These findings suggested that age-related changes could account for mild cognitive impairment.
The patient underwent follow-up neuropsychological testing in 11/2019 at the age of 78, after following multimodal treatment recommendations for over three years. Results were compared with her memory testing conducted in 12/2006 at the age of 65 and full neuropsychological testing conducted on 11/2016 at the age of 75 (Table 2).
Table 2.
Neuropsychological Test Performances.
| Test | 2006 | 2019 | Change from 2016–2019 |
|
|---|---|---|---|---|
| Memory Test Battery | ||||
| Immediate Memory (IM) Stories (scaled score) | 9 | 13 | 12 | −1 |
| Recall IM (percent) | 41 | 57 | 68 | 11% |
| Delayed Memory (DM) Stories (scaled score) | 9 | 13 | 11 | −2 |
| Recall DM (percent) | 30 | 46 | 51 | 5% |
| Delayed Retention (percent) | ND | 67.7 | 83 | 15% |
| Recognition (percent) | ND | 83 | 86 | 3% |
| CVLT-II LDFR (scaled score) | ND | 7 | 9 | 2 |
| CVLT-II LDCR (scaled score) | ND | 7 | 9 | 2 |
| Visual Immediate (IM) (scaled score) | 7 | 6 | 13 | 7 |
| Visual Delayed Memory DM (scaled score) | 13 | 10 | 14 | 4 |
| Other Psychometric Tests | ||||
| DigSpan (scaled score) | ND | 11 | 12 | 1 |
| Coding (scaled score) | ND | 9 | 11 | 2 |
| Trails A (scaled score) | ND | 8 | 9 | 1 |
| Rey Copy (scaled score) | ND | 4 | 8 | 4 |
| Phonemic Fluency (scaled score) | ND | 10 | 9 | −1 |
| Semantic Fluency (scaled score) | ND | 11 | 9 | −2 |
| Trails B (scaled score) | ND | 11 | 10 | −1 |
| WCST (scaled score) | ND | 8 | 8 | 0 |
Her estimated intellectual function was stable over time. In 11/2019, she obtained an estimated Full-Scale IQ of 122 which corresponds with the 93rd percentile and the superior range of functioning. This was considered commensurate with her performance of an estimated Full-Scale IQ of 117 (87th percentile, high average range) at the 2016 neuropsychological evaluation.
In 11/2019, she scored a 26/30 on the MoCA and a perfect score of 58/58 on the WMS-IV Brief Cognitive Status Evaluation. Her performance on the MoCA showed improvement at this 2019 timepoint in comparison with scores of 23/30 in 2/2017, 23/30 on 10/2017 and 23/30 in 12/2018. Her performance on tests of basic attention, working memory, and processing speed were in the average range (WAIS-IV Digit Span, 75th percentile; WAIS-IV Coding, 63rd percentile; Trailmaking Test – Part A, 58th percentile). These performances reflected slight improvements in comparison to her 2016 evaluation. Performances on measures of both phonemic and semantic verbal fluency were slightly declined in comparison to her 2016 evaluation. She performed at the 32nd percentile on a test of semantic verbal fluency at the 2019 evaluation, in comparison to the 63rd percentile although both performances were in the average range of ability. She performed in the low average range on a test of phonemic verbal fluency (FAS, 21st percentile) at the 2019 evaluation, which was slightly lower than her 2016 performance (39th percentile).
In terms of visuospatial skills, she had significant difficulty with copying the cube on the MoCA at past evaluations but was able to complete the task with full credit given at the most recent evaluation in 2019. She performed in the average range when copying a complex figure (Rey Complex Figure Test – Copy, 32/36 which is >16th percentile), reflecting marked improvement in her performance in comparison to the 2016 evaluation (Rey Complex Figure Test – Copy, 24/36 which is 2nd-5th percentile) (Figure 3). Her performance on the WAIS-IV Block Design subtest was in the average range (37th percentile) and consistent with her 2016 performance (50th percentile).
Figure 3.
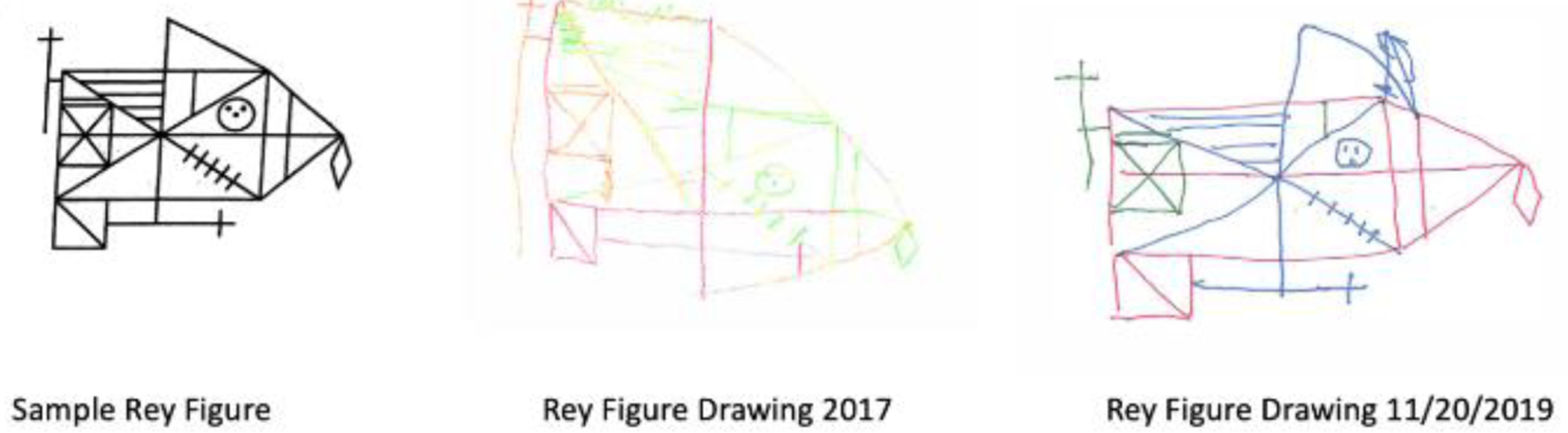
Organization and detail of Rey figure drawing from 2017 to 2019.
Executive functions were stable over time. She performed in the upper end of the average range on a task of mental flexibility (Trailmaking B Test, 68th percentile) in 2019 and this was consistent with her 2016 performance (70th percentile). She easily completed a problem-solving task, achieving all 6 of 6 categories on the Wisconsin Card Sorting Test, with error responses all in the average range. This was also consistent with her 2016 performances. Her ability to learn and immediately recall a complex line drawing was in the high average range for both immediate (86th percentile) and delayed (92nd percentile) memory. Although the recall portion of this test was not given at her 2016 evaluation, her performance was in the average range on a similar visual memory task.
Her performance on a verbal list learning task was generally consistent with her performance at the 2016 evaluation. She did improve to learn and recall one additional word, moving her performance to the average range (30th percentile) at the 2019 evaluation in comparison to her low average (16th percentile) performance at the 2016 evaluation for short delayed cued recall and long delayed cued recall. Her performance was stable on a story memory task. At the 2019 evaluation, she performed in the average range for learning and memory of auditory information and she was able to retain 83 percent of what she initially learned after a 30-minute delay.
Her most recent cognitive testing indicated generally intact cognitive function across most neurocognitive skills. Her neuropsychological profile indicates general stability over time, with some possible restoration of cognitive function in visuospatial skills, auditory learning and memory, nonverbal memory, and processing speed. Given her high level of intellectual capacity, it is likely that performances in the average range may actually indicate a change in cognition relative to her true abilities. Minor weaknesses continued to be observed in the learning, free recall, and recognition of unstructured information (e.g. word list) and also in phonemic verbal fluency.
Overall, from 2017 to 2019 she manifested measured improvement of cognitive function in visuospatial skills, auditory learning and memory, nonverbal memory, and processing speed, with stability in executive functioning and in other areas of cognition. Stability in cognitive function over a period of 2–3 years for a person diagnosed with early AD is a clinical success. Most patients experience cognitive decline, especially in learning and memory. This patient demonostrated improvements across a variety of cognitive skills.
DISCUSSION
Given the need for effective AD treatments, it is instructive to identify patients who show improvement or long-term stability, and then evaluate connections between treatments and outcomes. Since AD is a complex multimodal chronic illness, it is essential to evaluate numerous potential contributing factors. Here, we present a case study of multimodal therapy for multimorbid dementia. The patient demonstrated improvements in symptoms, neuropsychological assessments, and brain imaging. An advantage of multimodal intervention is that the health and homeostasis of diverse physiological subsystems can be addressed concurrently. Since many of these subsystems contribute, possibly incrementally, possibly synergistically, or possibly substantially to cognitive function, they all need to be considered. Where appropriate, those that are disease-perturbed should be addressed. In particular, interventions in this individual addressed two key subsystems that influence cognition: neuroinflammation and metabolism. Neuroinflammation appeared to be induced by Borrelia burgdorferi, Ehrlichiosis and heavy metal toxicity – as well as suboptimal nutrient and hormone levels. There is evidence that chronic infection with Borrelia burdorferi such as neuroborreliosis can play a role in the development of AD [29–31]. Our multimodal therapy included personalized treatments to address each of these potentially underlying neuroinflammatory causes. Liver and other metabolic subsystems also influence cognitive function and hippocampal volume [32]. We introduced a personalized diet that helped reduce liver inflammation due to PBC evidenced by a sustained drop in GGT (Figure 1). Together, all these components of multimodal therapy showed a sustained functional and cognitive benefit. Furthermore, multimodal therpaies may have systemwide benefits as many of the factors – such as inflammation – that impact cognitive function also impact other systems [33]. Therefore multimodal therapies are particularly appropriate for individuals with multimorbidities.
To some extent, multimodal intervention can be likened to pulling an airplane out of a dive. There may not be an immediate shift from decreasing function to increasing function. Because many factors are changing, one expects a time delay between when one starts to intervene and the lowest point of the curve. Furthermore, one does not necessarily expect all the inflections of all possible assays at exactly the same time, as each physiologic subsystem has distinct dynamics. However, with a comprehensive multimodal program, we do expect them roughly the same time, as we see in these data (Figure 1).
It is essential to take a detailed history and listen to the patient and family members recount the events leading up to the patient’s illness. It is imperative when faced with a patient like this that we consider their entire “exposozome” including their daily living environment. We should consider factors such as mold, in-home toxins, biotoxin exposure, heavy metals, diet, stress management, hormone balance and lifestyle. We believe that an approach of first identifying the many potential contributors to cognitive decline and then applying a personalized, precision medicine approach is essential to designing effective treatments for AD. Such an approach is quite distinct from the typical single drug-centric approach that pre-determines a treatment that is unrelated to the potential etiologic contributors. Chronic illness as it relates to AD is a continuum that starts decades earlier. Cases such as this one suggest that many of these factors can be systematically addressed and reversed. As this is expanded and tested in clinical trials, this represents an exciting approach to 21st-century medicine and the treatment of chronic disease with a new lens. The COCOA and PREVENTION trials are examples of trials using this approach [34,35].
This case also highlights differences between multiple defintions of “Alzheimer’s disease” in use spanning research and clinical contexts. The Alzheimer’s Association and National Institute of Aging provide a fairly clear framework for the definition of AD to be used in a research context [36]. This framework requires the documentation of molecular (e.g., amyloid) pathology. We do not know if the patient in this case study has such molecular pathology and cannot definitely classify this patient using the NIA-AA framework. In clinical practice, AD is often diagnosed presumptively, without assays for molecular markers [37]. The absence of testing for such markers is not always due to diagnostic malpractice; in some cases these tests are not available, are too expensive, are too invasive, would not impace care, or are otherwise contraindicated. Compared to some other diseases, such as infectious diseases, the clinical utility of a confident and precise diagnosis of AD is less. These other diseases have more clearly understood causal pathologies that clearly point to particular monotherapies. One advantage of a personalized multimodal approach for dementia is that it is robust to imprecision in diagnosis and nascent understandings of causalities. Treating multiple possible causes of dementia, personalized based on clinical and molecular evidence, and responding dynamically based on patient response can lead to clinical benefit even in complex cases of mixed dementia.
We have presented a single case example. In our experience, this patient is representative of many whose manifestations and pathophysiology are complex. Each patient has a slightly different presentation and combination of drivers of dementia, but treatments and outcomes via this precison medicine approach share more similarities than differences. The improvement observed in this patient underlines the need for clinical trials to test treatment protocols such as the one described here. The treatment program for this person may provide a guidepost for preventing or reversing the cognitive impairment of others with dementia.
Acknowledgement
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at adni.loni.usc.edu.
Funding Statement
Providence St. Joseph Health provided generous funding for the Alzheimer’s Translational Pillar (ATP) at ISB. Analysis was supported by NIH U01AG046139, RF1AG057443, U01AG061359, & R01AG062514.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.World Health Organization (2019) Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. Geneva: World Health Organization. [PubMed] [Google Scholar]
- 2.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, et al. (2015) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385(9984):2255–63. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 3.Ornish D, Scherwitz LW, Doody RS, Kesten D, McLanahan SM, et al. (1983) Effects of stress management training and dietary changes in treating ischemic heart disease. JAMA 249(1):54–9. [PubMed] [Google Scholar]
- 4.Rosenberg A, Ngandu T, Rusanen M, Antikainen R, Bäckman L, et al. (2018) Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The FINGER trial. Alzheimers Dement 14(3):263–270. doi: 10.1016/j.jalz.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Rovner BW, Casten RJ, Hegel MT, Leiby B (2018) Preventing Cognitive Decline in Black Individuals With Mild Cognitive Impairment: A Randomized Clinical Trial. JAMA Neurol 75(12):1487–1493. doi: 10.1001/jamaneurol.2018.2513.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenthal JA, Smith PJ, Mabe S, Hinderliter A, Lin PH, et al. (2019) Lifestyle and neurocognition in older adults with cognitive impairments: A randomized trial. Neurology 92(3):e212–e223. doi: 10.1212/WNL.0000000000006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isaacson RS, Hristov H, Saif N, Hackett K, Hendrix S, et al. (2019) Individualized clinical management of patients at risk for Alzheimer’s dementia. Alzheimers Dement 15(12):1588–1602. doi: 10.1016/j.jalz.2019.08.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bredesen DE, Sharlin K, Jenkins D, Okuno M, Youngberg W, et al. (2018) Reversal of cognitive decline: 100 patients. Journal of Alzheimer’s Disease and Parkinsonism 8(5):1000450. doi: 1 10.4172/2161–0460.1000450. [Google Scholar]
- 9.Carbone I, Lazzarotto T, Ianni M, Porcellini E, Forti P, et al. (2014) Herpes virus in Alzheimer’s disease: relation to progression of the disease. Neurobiol Aging 35(1):122–9. doi: 10.1016/j.neurobiolaging.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Carter CJ (2017) Genetic, Transcriptome, Proteomic, and Epidemiological Evidence for Blood-Brain Barrier Disruption and Polymicrobial Brain Invasion as Determinant Factors in Alzheimer’s Disease. J Alzheimers Dis Rep 1(1):125–157. doi: 10.3233/ADR-170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itzhaki RF (2017) Herpes simplex virus type 1 and Alzheimer’s disease: possible mechanisms and signposts. FASEB J 31(8):3216–3226. doi: 10.1096/fj.201700360. [DOI] [PubMed] [Google Scholar]
- 12.Fotuhi M, Mian A, Meysami S, Raji CA (2020) Neurobiology of COVID-19. J Alzheimers Dis 76(1):3–19. doi: 10.3233/JAD-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb L (2021) COVID-19 lockdown: A perfect storm for older people’s mental health. J Psychiatr Ment Health Nurs 28(2):300. doi: 10.1111/jpm.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fotuhi M, Lubinski B, Trullinger M, Hausterman N, Riloff T, et al. (2016) A Personalized 12-week “Brain Fitness Program” for Improving Cognitive Function and Increasing the Volume of Hippocampus in Elderly with Mild Cognitive Impairment. J Prev Alzheimers Dis 3(3):133–137. doi: 10.14283/jpad.2016.92. [DOI] [PubMed] [Google Scholar]
- 15.Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, et al. (2010) Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology 75(16):1415–22. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raji CA, Erickson KI, Lopez OL, Kuller LH, Gach HM, et al. (2014) Regular fish consumption and age-related brain gray matter loss. Am J Prev Med 47(4):444–51. doi: 10.1016/j.amepre.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, et al. (2010) Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol 68(3):311–8. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topiwala H, Terrera GM, Stirland L, Saunderson K, Russ TC, et al. (2018) Lifestyle and neurodegeneration in midlife as expressed on functional magnetic resonance imaging: A systematic review. Alzheimers Dement (NY) 4:182–194. doi: 10.1016/j.trci.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimada H, Ishii K, Makizako H, Ishiwata K, Oda K, et al. (2017) Effects of exercise on brain activity during walking in older adults: a randomized controlled trial. J Neuroeng Rehabil 14(1):50. doi: 10.1186/s12984-017-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, et al. (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 108(7):3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prato FS, Pavlosky WF, Foster SC, Thiessen JD, Beaujot RP (2019) Screening for Dementia Caused by Modifiable Lifestyle Choices Using Hybrid PET/MRI. J Alzheimers Dis Rep 3(1):31–45. doi: 10.3233/ADR-180098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes DE, Yaffe K, Belfor N, Jagust WJ, DeCarli C, et al. (2009) Computer-based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial. Alzheimer Dis Assoc Disord 23(3):205–10. doi: 10.1097/WAD.0b013e31819c6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjørklund G, Mutter J, Aaseth J (2017) Metal chelators and neurotoxicity: lead, mercury, and arsenic. Arch Toxicol 91(12):3787–3797. doi: 10.1007/s00204-017-2100-0. [DOI] [PubMed] [Google Scholar]
- 24.Tanpitukpongse TP, Mazurowski MA, Ikhena J, Petrella JR; Alzheimer’s Disease Neuroimaging Initiative (2017) Predictive Utility of Marketed Volumetric Software Tools in Subjects at Risk for Alzheimer Disease: Do Regions Outside the Hippocampus Matter? AJNR Am J Neuroradiol 38(3):546–552. doi: 10.3174/ajnr.A5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahdidan J, Raji CA, DeYoe EA, Mathis J, Noe KØ, et al. (2016) Quantitative Neuroimaging Software for Clinical Assessment of Hippocampal Volumes on MR Imaging. J Alzheimers Dis 49(3):723–32. doi: 10.3233/JAD-150559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross DE, Ochs AL, DeSmit ME, Seabaugh JM, Havranek MD, et al. (2015) Man Versus Machine Part 2: Comparison of Radiologists’ Interpretations and NeuroQuant Measures of Brain Asymmetry and Progressive Atrophy in Patients With Traumatic Brain Injury. J Neuropsychiatry Clin Neurosci 7(2):147–52. doi: 10.1176/appi.neuropsych.13040088. [DOI] [PubMed] [Google Scholar]
- 27.Jack CR Jr, Shiung MM, Gunter JL, O’Brien PC, Weigand SD, et al. (2004) Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 62(4):591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyman BT, Harvey DJ, Crawford K, Bernstein MA, Carmichael O, et al. (2013) Standardization of analysis sets for reporting results from ADNI MRI data. Alzheimers Dement 9(3):332–7. doi: 10.1016/j.jalz.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding Z, Sun L, Bi Y, Zhang Y, Yue P, et al. (2020) Integrative Transcriptome and Proteome Analyses Provide New Insights Into the Interaction Between Live Borrelia burgdorferi and Frontal Cortex Explants of the Rhesus Brain. J Neuropathol Exp Neurol 79(5):518–529. doi: 10.1093/jnen/nlaa015. [DOI] [PubMed] [Google Scholar]
- 30.Miklossy J, Khalili K, Gern L, Ericson RL, Darekar P, et al. (2004) Borrelia burgdorferi persists in the brain in chronic lyme neuroborreliosis and may be associated with Alzheimer disease. J Alzheimers Dis 6(6):639–49; discussion 673–81. doi: 10.3233/jad-2004-6608. [DOI] [PubMed] [Google Scholar]
- 31.Cuellar J, Pietikäinen A, Glader O, Liljenbäck H, Söderström M, et al. (2019) Borrelia burgdorferi Infection in Biglycan Knockout Mice. J Infect Dis 220(1):116–126. doi: 10.1093/infdis/jiz050. [DOI] [PubMed] [Google Scholar]
- 32.Mosher VAL, Swain MG, Pang JXQ, Kaplan GG, Sharkey KA, et al. (2018) Magnetic resonance imaging evidence of hippocampal structural changes in patients with primary biliary cholangitis. Clin Transl Gastroenterol;9(7):169. doi: 10.1038/s41424-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juran BD, Lazaridis KN (2014) Environmental factors in primary biliary cirrhosis. Semin Liver Dis. 34(3):265–72. doi: 10.1055/s-0034-1383726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McEwen SC, Merrill DA, Bramen J, Porter V, Panos S, et al. (2021) A systems-biology clinical trial of a personalized multimodal lifestyle intervention for early Alzheimer’s disease. Alzheimers Dement (NY) 7(1):e12191. doi: 10.1002/trc2.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roach JC, Hara J, Lovejoy J, Fridman D, Heim L, et al. (2019) Dense Longitudinal Molecular Data for Turbocharging Clinical Trials. Clinical Trials on Alzheimer’s Disease Annual Conference (CTAD), San Diego, CA. [Google Scholar]
- 36.Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, et al. (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larson EB (2021) Evaluation of cognitive impairment and dementia. In: UpToDate, DeKosky ST, Schmader KE, Wilterdink JL (Eds), UpToDate, Waltham, MA. [Google Scholar]


