Abstract
Study objective
This study sought to assess the predictive value of H2FPEF score in patients with COVID-19.
Design
Retrospective study.
Setting
Rush University Medical Center.
Participants
A total of 1682 patients had an echocardiogram in the year preceding their COVID-19 admission with a preserved ejection fraction (≥50%). A total of 156 patients met inclusion criteria.
Interventions
Patients were divided into H2FPEF into low (0–2), intermediate (3–5), and high (6–9) score H2FPEF groups and outcomes were compared.
Main outcome measures
Adjusted multivariable logistic regression models evaluated the association between H2FPEF score group and a composite outcome for severe COVID-19 infection consisting of (1) 60-day mortality or illness requiring (2) intensive care unit, (3) intubation, or (4) non-invasive positive pressure ventilation.
Results
High H2FPEF scores were at increased risk for severe COVID-19 infection when compared intermediate to H2FPEF score groups (OR 2.18 [CI: 1.01–4.80]; p = 0.049) and low H2FPEF score groups (OR 2.99 [CI: 1.22–7.61]; p < 0.05). There was no difference in outcome between intermediate H2FPEF scores (OR 1.34 [CI: 0.59–3.16]; p = 0.489) and low H2FPEF score.
Conclusions
Patients with a high H2FPEF score were at increased risk for severe COVID-19 infection when compared to patients with an intermediate or low H2FPEF score regardless of regardless of coronary artery disease and chronic kidney disease.
Keywords: COVID-19, SARS-CoV-2, Heart Failure with Preserved Ejection Fraction
1. Introduction
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly grown into a global pandemic with a surge in hospitalizations and deaths. COVID-19 can present with a wide spectrum of symptoms ranging from mild symptoms to acute respiratory distress syndrome (ARDS), septic shock, and multiorgan failure. In addition to respiratory complications, recent reports suggest that 20–25% of patients will suffer from cardiac complications [1] and that patients with pre-existing cardiovascular disease are at even higher risk of severe COVID-19 infection and death [2], [3], [4], [5], [6], [7], [8].
Given the rising proportion of HFpEF hospitalizations in the United States [9] and the scarcity of current information [10], [11], it would be helpful to explore the relationship between COVID-19 and HFpEF. It has been suggested that COVID-19 may cause exacerbation of HFpEF through direct mechanisms such as viral infiltration, inflammation, or cardiac fibrosis but also unmask subclinical HFpEF or promote the development of new HFpEF [12]. This paucity of studies examining the relationship between HFpEF and COVID-19 could partly be due to the challenges in diagnosing HFpEF. The H2FPEF score was developed as a tool to address the diagnostic difficulty in discriminating exertional dyspnea due to HFpEF from non-cardiac causes. The weighted score, as seen in Table 1, is based on 6 variables – “heavy” (obesity), treatment with ≥2 antihypertensives, atrial fibrillation, pulmonary hypertension (pulmonary artery systolic pressure > 35 mmHg), age > 60 years, and elevated filling pressures (E/e′ ratio > 9). The score ranges from 0 to 9, with higher scores associated with an increased likelihood of HFpEF. Patients with a score > 5 have a >90% probability of having HFpEF [13].
Table 1.
Variables with associated point values for calculating the H2FPEF score.
| Variable | Criteria | Points |
|---|---|---|
| Heavy | BMI > 30 kg/m2 | 2 |
| Hypertensive | On 2 or more anti-hypertensives | 1 |
| Atrial fibrillation | Paroxysmal or persistent | 3 |
| Pulmonary hypertension | Pulmonary artery systolic pressure > 35 mmHg | 1 |
| Elder | Age > 60 years old | 1 |
| Filling pressure | E/e′ > 9 on echocardiography | 1 |
This study of hospitalized COVID-19 patients sought to compare 60-day mortality and additional secondary outcomes in patients with an H2FPEF score of 0–2, 3–5, and 6–9. This study will assess whether patients with a higher H2FPEF score and therefore an increased likelihood of HFpEF diagnosis are associated with an increased risk of severe COVID-19 infection.
2. Materials and methods
2.1. Study population and design
A retrospective study was performed of patients ≥18 years old who were admitted with COVID-19 between March to November 2020 to the Rush University System for Health (RUSH) in Chicago, Illinois, USA. The patients' medical course was followed for a minimum of 60 days from their admission. This study was approved by the Institutional Review Board (IRB) of Rush University Medical Center.
2.2. Data collection and outcomes
Data was obtained through both manual and automatic data collection. Demographics, laboratory measurements, diagnoses, comorbidities, electrocardiogram (ECG) results, transthoracic echocardiogram (TTE) results, and outcomes (60-day mortality, need for intensive care unit [ICU], intubation, and mechanical ventilation) were collected from electronic health records. Comorbidities were extracted using the International Classification of Disease-10th (ICD10) Revision codes. Additionally, the electronic medical record was reviewed up to 60 days after the initial admission for various complications, and major adverse cardiovascular events (MACE) defined as non-fatal myocardial injury (defined as troponin greater than the upper limit of normal, >0.09 ng/mL at our hospital system), non-fatal stroke, and cardiac death. During this subsequent chart review, the electronic medical record was also assessed for patient readmission and mortality.
To be included in our cohort, patients must have (1) a TTE in the year before their COVID-19 admission and (2) had a normal left ventricular ejection fraction (EF) of greater than 50% on that TTE. H2FPEF scores were calculated using the previously published scoring system by Reddy et al., as summarized in Table 1. Obesity was defined as BMI >30 kg/m2. Atrial fibrillation was determined from clinical history and ECG. The number of antihypertensives was determined using the list of the patient's ambulatory medications on the day of admission. The E/e′ measurement is the ratio of early diastolic mitral inflow velocity to septal mitral annulus tissue relaxation velocity. The E/e′ ratio and pulmonary artery systolic pressure were manually extracted from the most recent TTE completed within 1 year before the COVID admission date.
Patients were divided into three groups, a low, intermediate, and high score group consisting of H2FPEF scores from 0 to 2, 3–5, and 6–9, respectively. The primary outcome of the study was a composite for severe COVID-19 infection consisting of (1) 60-day mortality or illness requiring (2) intensive care unit, (3) intubation, or (4) non-invasive positive pressure ventilation.
Due to the potential confounder that the cohort of patients that required a prior TTE may represent an overall sicker population at baseline, an additional analysis was performed by propensity score matching each H2FPEF score group (low, intermediate, and high) to a similar group lacking a prior TTE. Covariables for the propensity score matching were pre-defined and chosen using clinical experience and comorbidities known to have a worse prognosis in patients with COVID-19. For this additional analysis only, body mass index (1.4% of patients were missing BMI data) was imputed with linear regression modeling prior to matching.
A logistic regression model was used to generate propensity scores, and matching was performed using a 2:1 nearest neighbor method with a caliper width of 0.20. The incidence of both 60-day mortality and the composite outcome was then compared between each matched group.
2.3. Statistical analysis
Both data and statistical analyses were performed with RStudio version 1.3 (Boston, Massachusetts). Propensity score matching was performed using the MatchIt package, and Kaplan-Meier survival estimates were created and plotted using the survival and survminer packages.
Normally distributed continuous variables are reported with mean and standard deviation, while non-normally distributed variables are described with median and interquartile range. Categorical variables are reported as counts and proportions. Continuous and categorical variables were compared with t-tests and Pearson chi-square tests, respectively. Logistic regression was performed between the H2FPEF score groups as a predictor for severe COVID-19 infection and is reported with odds ratios (OR) with 95% confidence intervals (CI). A multivariable logistic regression model was created for the primary composite outcome for severe COVID-19 infection, adjusted for coronary artery disease and chronic kidney disease. The 60-day survival outcome is univariable due to the fewer number of event counts. The threshold for statistical significance was set to a p-value < 0.05.
3. Results
3.1. Baseline characteristics
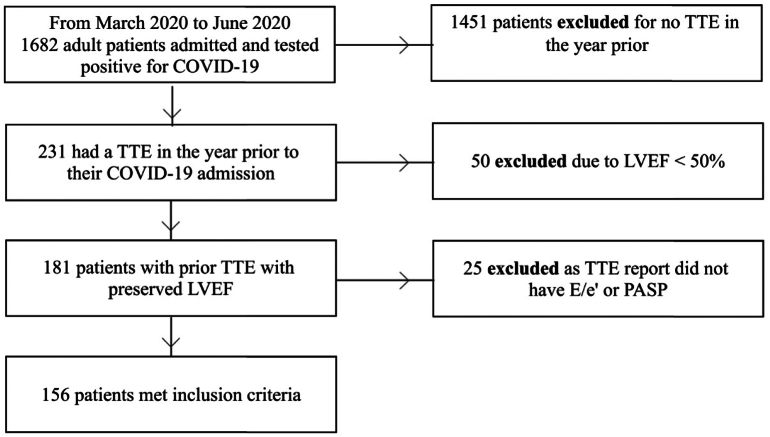
There were 1682 patients admitted with COVID-19 during the period of data collection. In this cohort, 231 patients (13.7%) had a TTE within the year preceding their admission. Of these patients, 50 were excluded for having a reduced left ventricular ejection fraction of less than 50%. An additional 25 were excluded for not having the necessary variables for calculating the H2FPEF scores in the TTE report (Fig. 1).
Fig. 1.
Flow diagram of patients meeting study including criteria
Abbreviations: COVID-19 = coronavirus disease 2019; TTE = transthoracic echocardiogram; LVEF = left ventricular ejection fraction; PASP = pulmonary artery systolic pressure.
Our total cohort that met inclusion criteria for this study consisted of 156 patients who had a TTE with preserved ejection fraction in the year leading up to their COVID-19 admission (Fig. 1). When H2FPEF scores were calculated, 40 patients (25.6%) had low scores (0–2), 72 (46.2%) had intermediate scores (3–5), and 44 (28.2%) had high scores (6–9).
The median age of our entire cohort was 59 years (interquartile range 46–71). The higher score groups tended to be older with a prior history of atrial fibrillation, both of which are individual components of the H2FPEF score calculation (Table 2). Body mass index, another component of the H2FPEF score, was highest in the intermediate group and lowest in the low score group. The proportion of patients with a history of ventricular arrhythmias was greatest in the high score group. The remainder of the comorbidities tested, including coronary artery disease, hypertension, and diabetes mellitus, were similar across the groups.
Table 2.
Baseline characteristics stratified by H2FPEF score category.
| Low score⁎ | Intermediate score⁎ | High score⁎ | p-Value | |
|---|---|---|---|---|
| n | 40 | 72 | 44 | |
| Age | 60.00 [50.75, 74.00] | 67.00 [56.75, 78.00] | 74.00 [66.00, 81.00] | 0.014 |
| Male (%) | 23 (57.5) | 34 (47.2) | 17 (38.6) | 0.224 |
| BMI | 26.95 [22.25, 29.40] | 33.20 [25.55, 39.24] | 31.20 [27.95, 36.05] | <0.001 |
| Race (%) | 0.325 | |||
| White | 9 (22.5) | 22 (30.6) | 17 (39.5) | |
| Other | 12 (30.0) | 14 (19.4) | 11 (25.6) | |
| Black | 19 (47.5) | 36 (50.0) | 15 (34.9) | |
| Comorbidities | ||||
| Current smoker (%) | 2 (5.1) | 0 (0.0) | 0 (0.0) | 0.052 |
| Atrial fibrillation (%) | 2 (5.0) | 11 (15.3) | 33 (75.0) | <0.001 |
| Coronary artery disease (%) | 19 (47.5) | 39 (54.2) | 28 (63.6) | 0.324 |
| Hypertension (%) | 35 (87.5) | 67 (93.1) | 42 (95.5) | 0.373 |
| Chronic kidney disease (%) | 17 (42.5) | 40 (55.6) | 20 (45.5) | 0.345 |
| COPD (%) | 5 (12.5) | 7 (9.7) | 10 (22.7) | 0.140 |
| Diabetes mellitus (%) | 21 (52.5) | 45 (62.5) | 27 (61.4) | 0.564 |
| Asthma (%) | 7 (17.5) | 10 (13.9) | 12 (27.3) | 0.194 |
| Cancer (%) | 11 (27.5) | 17 (23.6) | 12 (27.3) | 0.865 |
| Ventricular arrhythmia (%) | 2 (5.0) | 5 (6.9) | 10 (22.7) | 0.011 |
| Stroke (%) | 16 (40.0) | 20 (27.8) | 12 (27.3) | 0.340 |
| Acute myocardial infarction (%) | 8 (20.0) | 18 (25.0) | 10 (22.7) | 0.833 |
| DVT or pulmonary embolism (%) | 8 (20.0) | 19 (26.4) | 17 (38.6) | 0.149 |
| Labs | ||||
| Troponin | 0.02 [0.01, 0.06] | 0.05 [0.02, 0.10] | 0.04 [0.02, 0.09] | 0.231 |
| White blood cell count | 6.42 [4.57, 7.68] | 5.58 [4.18, 7.08] | 7.65 [5.39, 10.15] | 0.003 |
| Lymphocyte number | 0.99 [0.70, 1.45] | 0.90 [0.63, 1.35] | 1.09 [0.70, 1.35] | 0.468 |
| Hemoglobin | 11.85 [9.88, 12.50] | 10.85 [9.17, 12.93] | 12.65 [10.57, 13.75] | 0.024 |
| Platelet Count | 156.50 [128.25, 225.25] | 188.00 [142.50, 244.75] | 240.50 [168.75, 300.50] | 0.002 |
| Creatinine | 1.19 [0.83, 1.96] | 1.60 [0.93, 2.86] | 1.36 [0.99, 2.47] | 0.291 |
| CRP | 82.70 [19.50, 138.10] | 81.10 [37.08, 145.25] | 67.00 [31.02, 122.67] | 0.778 |
| Ferritin | 567.00 [251.18, 1381.13] | 832.50 [276.53, 1993.75] | 453.00 [187.43, 872.60] | 0.088 |
| LDH | 265.00 [226.50, 387.50] | 326.50 [234.50, 380.75] | 296.50 [246.25, 404.50] | 0.672 |
| Vital signs | ||||
| Systolic BP | 131.50 [112.75, 151.25] | 131.50 [118.50, 149.00] | 130.00 [112.75, 149.00] | 0.732 |
| Diastolic BP | 73.50 [61.75, 82.25] | 71.00 [61.00, 79.25] | 76.00 [58.75, 94.00] | 0.403 |
| Heart Rate | 90.50 [87.00, 105.00] | 90.50 [77.50, 99.00] | 87.00 [73.75, 98.25] | 0.357 |
| Respiratory Rate | 18.00 [18.00, 20.00] | 20.00 [18.00, 22.00] | 21.00 [18.00, 24.00] | 0.006 |
| Pulse Oximetry | 97.00 [93.00, 98.00] | 97.00 [93.00, 98.25] | 96.00 [92.75, 97.00] | 0.397 |
| Temperature | 98.95 [98.12, 99.80] | 99.25 [98.38, 100.03] | 98.45 [97.65, 99.32] | 0.024 |
ULN = upper limit of normal; BMI = body mass index; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disorder; DVT = deep venous thrombosis; CRP = c-reactive protein; LDH = lactate dehydrogenase; BP = blood pressure.
Low, intermediate, and high score groups defined as H2FPEF scores from 0 to 2, 3–5, and 6–9, respectively.
The intermediate score group had the lowest median values of initial white blood cell count and hemoglobin (5.6 and 10.9, respectively). The high score group had the highest values of each (7.65 and 12.65, respectively). Additionally, the median platelet count was lowest in the low score group (157) and highest in the high score group (240.5). The median values for cardiac troponin, c-reactive protein, and lactate dehydrogenase were similar across the groups.
Of the 156 patients included in the study, 71 (45.5%) had dyspnea as a presenting symptom for their hospitalization, while 80 patients did not. The presence or absence of dyspnea was not documented in 5 patients in the study cohort.
3.2. Comparing patients with versus patients without a prior echocardiogram
In the propensity score matching analysis to see how the 156 patients with a prior TTE who met our inclusion criteria compared to the 1451 patients without one within each H2FpEF group, a total of 436 patients were matched (Supplemental Table 1). After matching, each of the covariates had a standardized mean difference (SMD) < 0.10, indicating well-balanced groups were generated. Once balanced with matching, there was no difference in the incidence of either the composite outcome or 60-day mortality in the low, intermediate, or high score group between those with versus without an echocardiogram.
3.3. Primary and secondary outcomes
The primary outcome of severe COVID-19 infection occurred among 64 patients in our cohort with an incidence of 30.0%, 37.5%, and 56.8% in the low, intermediate, and high score categories, respectively. In multivariable logistic regression adjusted for coronary artery disease and chronic kidney disease, those with high H2FPEF scores (OR 2.99 [CI: 1.22–7.61]; p < 0.05) were at significantly higher risk of severe COVID-19 infection compared to those with low H2FPEF scores (Table 3). Those with high H2FPEF scores were also at an increased risk (OR 2.18 [CI: 1.01–4.80]; p = 0.049) for severe infection when compared to those with intermediate scores.
Table 3.
H2FPEF score level as a predictor for severe COVID-19 infection, adjusted for age and body mass index.
| Low score⁎, |
Intermediate score⁎, |
High score⁎, |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
n = 40 |
n = 72 |
n = 44 |
|||||||
| Incidence | aOR (95% CI) | p-Value | Incidence | aOR (95% CI) | p-Value | Incidence | aOR (95% CI) | p-Value | |
| Severe COVID-19 infection† | 30.0% | Ref | – | 37.5% | 1.38 (0.57–3.40) | 0.480 | 56.8% | 3.10 (1.18–8.50) | 0.024 |
Abbreviations: aOR = adjusted odds ratio; CI = confidence interval; COVID-19 = coronavirus disease 2019; Ref = reference.
Low, intermediate, and high score groups defined as H2FPEF scores from 0 to 2, 3–5, and 6–9, respectively.
Severe COVID-19 infection defined as a composite of (1) 60-day mortality, (2) needing the intensive care unit, or requiring (3) intubation or (4) non-invasive positive pressure ventilation.
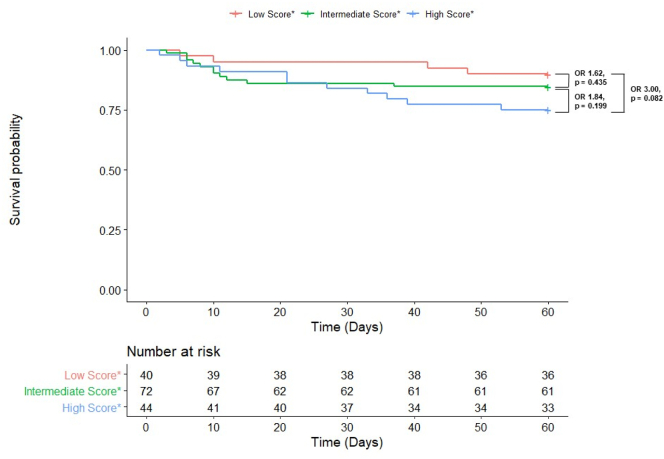
In our cohort, 26 patients suffered 60-day mortality. In a univariable model, both the high score (OR 3.00 [CI: 0.93–11.67]; p = 0.082) and intermediate score (OR 1.62 [CI: 0.51–6.20]; p = 0.435) groups were at no higher risk for 60-day mortality compared to the low score group (Fig. 2).
Fig. 2.
Kaplan-Meier 60-Day Survival Curve with Univariable Odds Ratios in COVID-19 Patients by H2FpEF Group
*Low, intermediate, and high score groups defined as H2FPEF scores from 0 to 2, 3–5, and 6–9, respectively.
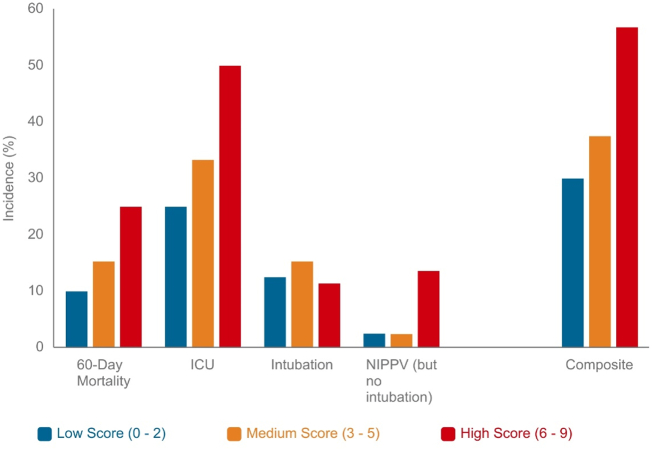
In comparing secondary outcomes, the high score group was at greatest risk of requiring the intensive care unit (50.0%), while the low score group was at the lowest risk (25.0%, p = 0.048; Table 4). There was no significant difference between the groups in mechanical ventilation requirements, in-hospital mortality, inpatient length of stay, and 60-day readmission among the low, intermediate, and high score groups. When comparing individual 60-day complications, the high score group was at the highest risk of acute heart failure exacerbation (11.4%, p = 0.009). Otherwise, the incidence of other complications, including life-threatening arrhythmia, venous thromboembolism, and requiring renal replacement therapy, was similar across the groups. However, event counts were low within the individual outcomes. The occurrence of overall MACE between the groups was also not statistically different, with incidences of 5.0%, 6.9%, and 13.6% in the low, intermediate, and high score groups, respectively (Fig. 3).
Table 4.
Secondary outcomes by H2FPEF score group.
| Low score | Intermediate score* | High score⁎ | p-Value | |
|---|---|---|---|---|
| n | 40 | 72 | 44 | |
| During initial admission | ||||
| In-hospital mortality (%) | 3 (7.5) | 7 (9.7) | 8 (18.2) | 0.250 |
| ICU level of care (%) | 10 (25.0) | 24 (33.3) | 22 (50.0) | 0.048 |
| Mechanical ventilation (%) | 5 (12.5) | 11 (15.3) | 5 (11.4) | 0.818 |
| Length of stay (median (IQR)) | 4.00 [2.00, 10.00] | 6.00 [3.00, 10.00] | 7.50 [3.75, 15.25] | 0.118 |
| 60-day outcomes | ||||
| Readmission (%) | 7 (17.5) | 21 (29.2) | 17 (38.6) | 0.102 |
| Life-threatening arrhythmia (%) | 2 (5.0) | 5 (6.9) | 3 (6.8) | 0.914 |
| DVT (%) | 0 (0.0) | 2 (2.8) | 2 (4.5) | 0.415 |
| HF exacerbation† (%) | 0 (0.0) | 1 (1.4) | 5 (11.4) | 0.009 |
| Requiring RRT (%) | 2 (5.0) | 5 (6.9) | 4 (9.1) | 0.764 |
| PE (%) | 1 (2.5) | 2 (2.8) | 1 (2.3) | 0.986 |
| Total MACE (%) | 2 (5.0) | 5 (6.9) | 6 (13.6) | 0.304 |
| Myocardial injury‡ (%) | 1 (2.5) | 4 (5.6) | 4 (9.1) | 0.431 |
| Stroke (%) | 0 (0.0) | 0 (0.0) | 1 (2.3) | 0.278 |
| Cardiovascular death (%) | 1 (2.5) | 2 (2.8) | 2 (4.5) | 0.835 |
ICU = intensive care unit; IQR = interquartile range; MACE = Major adverse cardiac events; HF = heart failure; RRT = renal replacement therapy.
Low, intermediate, and high score groups defined as H2FPEF scores from 0 to 2, 3–5, and 6–9, respectively.
Defined as having the signs and symptoms consistent with a heart failure exacerbation.
Myocardial injury defined as cardiac troponin greater than the upper limit of normal.
Fig. 3.
Incidence of the composite outcome and individual components of the composite by H2FPEF Score Level
Abbreviations: ICU = intensive care unit; NIPPV = non-invasive positive pressure ventilation.
4. Discussion
4.1. Severe COVID-19 infection in HFpEF patients
A high H2FPEF score of 6–9, indicating a greater than 90% probability of having HFpEF, was a predictor of severe COVID-19 infection, which included (1) 60-day mortality or illness requiring (2) intensive care unit, (3) intubation, or (4) non-invasive positive pressure ventilation when compared to the low score group. To our knowledge, this study is the first to use the H2FPEF score, an estimator of the probability of a patient having HFpEF, to demonstrate the association of HFpEF and COVID-19 outcomes.
Emerging theories suggest that COVID-19 causes significant morbidity and mortality through direct mechanisms such as viral entry into cells and indirectly through cytokine storm, hypoxemia, immune system dysregulation, and increased procoagulant activity [14]. The accelerated production of inflammatory cytokines in COVID-19 can mediate atherosclerosis, destabilization of vascular plaques, procoagulant activation, and hemodynamic instability leading to ischemia and thrombosis [15]. The confluence of all of these mechanisms can lead to arrhythmias, cardiogenic shock, and sudden cardiac death [3], [16]. Patients with HFpEF are thought to be at increased risk for severe illness and complications due to their general frailty and reduced hemodynamic capacity at baseline [10]. Moreover, the widespread systemic inflammatory response and increased metabolic demand associated with severe COVID-19 infections requires enhanced cardiac performance and high cardiac output, which is difficult for patients with heart failure. In particular, patients with HFpEF may have a relatively fixed stroke volume that cannot be augmented with increased metabolic stress requirements. Given the finding in a previous study that showed patients with higher H2FPEF scores had significantly higher incidence of cardiovascular and cerebrovascular related events as compared to lower H2FPEF scores [17], it is possible that the added insult of COVID-19 and its systemic manifestations might overwhelm their already reduced hemodynamic capabilities.
One prior study showed that a baseline echocardiographic finding of elevated E/e′ ratio, a surrogate marker of LV filling pressures, was significantly associated with increased mortality in COVID-19 infection [1]. Although an elevated E/e′ ratio is often a hallmark feature in HFpEF, its measurement alone is not accurate in identifying patients with HFpEF [18]. Scoring systems such as the H2FPEF score are thought to give a more accurate estimation of the probability of a patient have HFpEF.
However, it must be noted that the relationship between COVID-19 severity and H2FPEF score may be associated with the complex network of overlapping cardiometabolic risk profiles, given that some of the individual components of the H2FPEF score are considered independent risk factors for worse COVID-19 prognosis themselves. HFpEF is currently believed to be a systemic disorder driven primarily by comorbidities, including obesity, HTN, and advanced age [19], [20], [21], with many of the same cardiometabolic comorbidities being linked to worse outcomes in patients with COVID-19. Emerging models suggest that these comorbidities induce a proinflammatory state that leads to systemic microvascular endothelial inflammation, cardiac muscle inflammation, and oxidative stress promoting global cardiomyocyte hypertrophy and coronary microvascular inflammation [19]. Given the widespread systemic inflammatory response and cytokine storm associated with COVID-19 infection, the underlying inflammatory milieu of HFpEF patients may predispose them to exaggerated deleterious effects of COVID-19.
4.2. HF exacerbations in HFpEF patients with COVID-19 infection
A high H2FPEF score of 6–9 was also a significant predictor for the occurrence of acute HF exacerbation within 60 days. The mechanisms responsible for acute decompensation in HFpEF patients are likely multifactorial, involving the systemic inflammatory response, shifts in fluid balance, injury to the myocardium itself, and increases in pulmonary pressures. The severe inflammatory storm seen in COVID-19 leads to increased metabolic demand, which can lead to cardiac depression and acute decompensation of chronic HF [22].
One study showed that 20% of COVID-19 patients who experienced clinical decompensation had deteriorated right ventricular parameters related to increased pulmonary pressures [1]. This increase in pulmonary pressures can be precipitated through many conditions seen in COVID-19, including pulmonary embolism, hypoxic pulmonary vasoconstriction, increased pulmonary vascular resistance, decreased lung volumes, excessive positive end-expiratory pressure, pneumonia, hypercarbia, elevated left atrial pressures, volume overload, and ARDS [1], [10]. Derangements in fluid balance are partly due to the increased incidence of acute kidney injury and renal dysfunction reported in COVID-19 [23], as well as increased capillary permeability related to localized microvascular inflammation and oxidative stress on endothelial cells [24]. These contributors to worsening pulmonary hypertension can then potentiate right ventricular dysfunction and right heart failure [25]. Patients with high H2FPEF scores may already have elevated E/e′ and pulmonary artery systolic pressure at baseline and are unlikely to be able to accommodate even further increases in pulmonary pressures, thus predisposing them to clinical decompensation. In addition to these complications seen in the acute phase of the illness, there are also the long-term sequelae of chronic pulmonary disease in COVID-19, which is thought to cause adverse cardiac remodeling and subsequent pulmonary hypertension and left ventricular diastolic dysfunction [12]. Longer longitudinal studies are required to investigate the possible longstanding effects of COVID-19, specifically in the context of cardiac manifestations, as it may unmask previously subclinical HFpEF and trigger the development of new HFpEF.
4.3. Complications and Major Adverse Cardiac Events (MACE) in HFpEF patients with COVID-19
Except for HF exacerbation, there was no difference in the occurrence of the 60-day complications and overall MACE between the low, intermediate, and high H2FPEF score groups. Myocardial injury is known to occur in up to 60% of hospitalized COVID-19 patients through various mechanisms, including direct viral entry into the cell via ACE2 receptors, coronary microvascular thrombi, stress cardiomyopathy, and supply-demand mismatch leading to plaque rupture and myocardial infarction [1], [26]. Coagulation dysfunction due to endothelial dysfunction, inflammation, oxidative stress, and platelet activation can lead to arterial and venous thrombi and subsequent DVT, PE, MI, and stroke [27], [28]. Our study suggests that the H2FPEF score was not a significant predictor for MACE in COVID-19. This finding is not entirely surprising as many studies have shown higher cardiac biomarker levels in patients with HF than those without HF, but similar outcomes among patients with HFpEF and HFrEF [29]. However, one study showed that troponin levels were positively associated with the E/e′ ratio, a component of the H2FPEF score [1].
4.4. Clinical implications
This analysis of the prognostic impact of the H2FPEF score on COVID-19 outcomes holds significant clinical implications. First, to minimize the spread of COVID-19 and prevent overwhelming healthcare resources, screening and diagnostic tools can be used to triage and clinically manage patients appropriately. Since patients with COVID-19 can rapidly progress to ARDS, septic shock, and multiorgan failure, early recognition of vulnerable populations is paramount. This study demonstrates the prognostic utility of the H2FPEF score in identifying high-risk patients and the potential for unfavorable clinical outcomes. The H2FPEF score can be used to risk-stratify patients during telemedicine or clinic visits in patients with a prior echocardiogram and help guide the threshold for admission. The simplicity of the H2FPEF score system and ease of calculation allows for quick application to patients. Since it remains unclear how prevalent COVID-19 will be in the coming years, the implementation of exercise, weight loss, and medical management to address CVD risk factors may play a role in optimizing patients in a vulnerable population to mitigate severe outcomes. Furthermore, the H2FPEF score may help guide decision-making and initiate earlier therapies such as corticosteroids, anticoagulation, and antiviral therapies typically reserved for more severe COVID-19 cases.
4.5. Study limitations
The key limitation of this paper is the significantly different populations included in this study when compared to the original H2FPEF score manuscript by Reddy et al. While Reddy et al. assessed patients with unexplained dyspnea, our study included patients admitted for COVID-19 with an echocardiogram within 1 year before admission, only 45.5% of whom had dyspnea as a symptom during their admission. Therefore, our study used a highly pre-selected group, which could alter the predictive ability of the H2FPEF score in estimating whether a patient has HFpEF or not.
Other limitations are also present in this study. First, our inclusion criteria likely led to an underestimation of COVID-19 patients with HFpEF who had not had recent TTEs and subclinical HFpEF patients with no echocardiogram on record. Second, we do not know the number of patients in our cohort who had a previous diagnosis of HFpEF though this can be a challenging diagnosis to make and is in part addressed by the H2FPEF score. Third, the inclusion of only hospitalized COVID-19 patients with HFpEF may have overestimated the incidence of severe COVID infection, 60-day complications, and overall MACE. Furthermore, readmission rates and post-discharge mortality were only captured for patients who had a subsequent hospitalization to a health care system in or around Chicago that also uses the EPIC electronic medical record. Lastly, the observed relationship between high H2FPEF score and outcomes may merely reflect the adverse effects of the individual score variables themselves and does not delineate which factors contribute and the extent of their contribution. Larger multicenter studies are required to verify and further elucidate the relationship.
5. Conclusion
A high H2FPEF score was an independent risk factor for severe COVID-19 infection, which included (1) 60-day mortality or illness requiring, (2) intensive care unit, (3) intubation, or (4) non-invasive positive pressure ventilation compared to those with a with an intermediate or low H2FPEF score. A high H2FPEF score was also a significant predictor for the occurrence of acute HF exacerbation within 60 days. The H2FPEF score may hold prognostic value in risk stratifying patients and guiding triage and management in COVID-19.
The following is the supplementary data related to this article.
Individual H2FPEF score groups matched to a similar group lacking the prior echocardiogram.
Sources of support
The authors received no financial support for the research, authorship, or publication of this manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Szekely Y., Lichter Y., Taieb P., et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142(4):342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Driggin E., Madhavan M.V., Bikdeli B., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murthy S., Archambault P.M., Atique A., et al. Characteristics and outcomes of patients with COVID-19 admitted to hospital and intensive care in the first phase of the pandemic in Canada: a national cohort study. CMAJ Open. 2021;9(1):E181–E188. doi: 10.9778/cmajo.20200250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinberg B.A., Zhao X., Heidenreich P.A., et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. Jul 2012;126(1):65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 10.Bader F., Manla Y., Atallah B., Starling R.C. Heart failure and COVID-19. Heart Fail Rev. 2020:1–10. doi: 10.1007/s10741-020-10008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeFilippis E.M., Reza N., Donald E., Givertz M.M., Lindenfeld J., Jessup M. Considerations for heart failure care during the COVID-19 pandemic. JACC Heart Fail. 2020;8(8):681–691. doi: 10.1016/j.jchf.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freaney P.M., Shah S.J., Khan S.S. COVID-19 and heart failure with preserved ejection fraction. JAMA. 2020 doi: 10.1001/jama.2020.17445. [DOI] [PubMed] [Google Scholar]
- 13.YNV Reddy, Carter R.E., Obokata M., Redfield M.M., Borlaug B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138(9):861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020;38(7):1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonow R.O., Fonarow G.C., O'Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5(7):751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 16.Clerkin K.J., Fried J.A., Raikhelkar J., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141(20):1648–1655. doi: 10.1161/circulationaha.120.046941. [DOI] [PubMed] [Google Scholar]
- 17.Sueta D., Yamamoto E., Nishihara T., et al. H2FPEF score as a prognostic value in HFpEF patients. Am. J. Hypertens. 2019;32(11):1082–1090. doi: 10.1093/ajh/hpz108. [DOI] [PubMed] [Google Scholar]
- 18.Santos M., Rivero J., McCullough S.D., et al. E/e' ratio in patients with unexplained dyspnea: lack of accuracy in estimating left ventricular filling pressure. Circ Heart Fail. Jul 2015;8(4):749–756. doi: 10.1161/CIRCHEARTFAILURE.115.002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redfield M.M. Heart failure with preserved ejection fraction. N. Engl. J. Med. Nov 2016;375(19):1868–1877. doi: 10.1056/NEJMcp1511175. [DOI] [PubMed] [Google Scholar]
- 20.Mentz R.J., Kelly J.P., von Lueder T.G., et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J. Am. Coll. Cardiol. Dec 2014;64(21):2281–2293. doi: 10.1016/j.jacc.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah S.J., Kitzman D.W., Borlaug B.A., et al. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. Jul 2016;134(1):73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Court O., Kumar A., Parrillo J.E. Clinical review: myocardial depression in sepsis and septic shock. Crit. Care. Dec 2002;6(6):500–508. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wazny V., Siau A., Wu K.X., Cheung C. Vascular underpinning of COVID-19. Open Biol. 2020;10(8):200208. doi: 10.1098/rsob.200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H., Liu G., Zhou W., Zhang W., Wang K., Zhang J. Neprilysin inhibitor-angiotensin II receptor blocker combination therapy (sacubitril/valsartan) suppresses atherosclerotic plaque formation and inhibits inflammation in apolipoprotein E- deficient mice. Sci. Rep. 2019;9(1):6509. doi: 10.1038/s41598-019-42994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang J.P., Wang X., Moura F.A., Siddiqi H.K., Morrow D.A., Bohula E.A. A current review of COVID-19 for the cardiovascular specialist. Am. Heart J. Aug 2020;226:29–44. doi: 10.1016/j.ahj.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tajbakhsh A., Gheibi Hayat S.M., Taghizadeh H., et al. COVID-19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev. Anti. Infect. Ther. 2021;19(3):345–357. doi: 10.1080/14787210.2020.1822737. [DOI] [PubMed] [Google Scholar]
- 28.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez-Garcia J., Lee S., Gupta A., et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J. Am. Coll. Cardiol. 2020;76(20):2334–2348. doi: 10.1016/j.jacc.2020.09.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual H2FPEF score groups matched to a similar group lacking the prior echocardiogram.