Abstract
The World Health Organization (WHO) declared COVID-19 as a pandemic on March 11, 2020, because of its widespread transmission and infection rates. The unique severe disease was found in Wuhan, China, since December 2019, and swiftly spread throughout the world. Natural chemicals derived from herbal medicines and medicinal mushrooms provide a significant resource for the development of novel antiviral drugs. Many natural drugs have been proven to have antiviral properties against a variety of virus strains, such as the coronavirus and the herpes simplex virus (HSV).. In this research, successful dietary treatments for different COVID illnesses were compared to potential of mushroom products in its therapy. In Google Scholar, Science Direct, PubMed, and Scopus, search keywords like COVID, COVID-19, SARS, MERS, mushrooms, and their compounds were utilized. In this review of the literature we foucsed popular mushrooms such as Agaricus subrufescens Peck, Agaricus blazei Murill, Cordyceps sinensis (Berk.) Sacc., Ganoderma lucidum (Curtis.) P. Karst., Grifola frondosa (Dicks.) Gray, Hericium erinaceus (Bull.) Pers., Inonotus obliquus (Arch. Ex Pers.) Pilát., Lentinula edodes (Berk.) Pegler, Pleurotus ostreatus (Jacq.) P. Kumm., Poria cocos F.A. Wolf, and Trametes versicolor (L.) Lloyd.,. Changed forms of β-Glucan seem to have a good impact on viral replication suppression and might be used in future studies. However, the results seems terpenoids, lectins, glycoproteins, lentinan, galactomannan, and polysaccharides from mushrooms are promising prophylactic or therapeutic agents against COVID-19.
Keywords: Mushrooms, Immunomodulatory effect, Antiviral activity, COVID-19, β-glucan
1. Introduction
SARS (Severe Acute Respiratory Syndrome) outbreak in 2003 and the MERS (Middle East respiratory syndrome) in 2012 persuaded severe human diseases (World Health Organization, 2020). SARS and MERS are both zoonotic diseases that originated in bats. The capacity of these viruses to evolve quickly and adapt to a new host is one of their most distinguishing characteristics. These viruses' zoonotic origins allow them to move from host to host (Cui et al., 2019). After seven years, SARS-CoV-2 (Severe Acute Respiratory Syndrome – Coronavirus-2) was detected in December 31, 2019 in Wuhan, the capital of Central China's Hubei Province (World Health Organization, 2020) causing the pneumonia disease named as coronavirus disease 2019 (COVID-19) (formerly 2019-nCoV) by WHO. In comparison to SARS and MERS, COVID-19 causes an extreme acute respiratory syndrome in humans and animals, resulting in massive alveolar damage and progressive respiratory action stoppage, posing significant challenges to the medical and health communities (Velavan & Meyer, 2020).
The SARS-CoV-2 has emerged as the source of a global pandemic and after that WHO proclaimed the worldwide pandemic on March 11, 2020 due to the novel type of coronavirus (nCoV). Many of the early instances were linked to the Huanan market in Wuhan, but a comparable number were linked to other markets, and some were not linked to any markets at all. Transmission among the wider community in December might explain for cases not linked to the Huanan market, which, together with the occurrence of early cases not linked to the market, could indicate that the Huanan market was not the outbreak's genesis (World Health Organization, 2021). Eventhough, since then, it has rapidly spread across China and in other countries, raising major global concerns.
1.1. Epidemiology
The nCoV is a polyadenylated, enveloped, positive, single-stranded big RNA virus with a size of 27–32 kb that belongs to the genus beta coronavirus and is 96% genetically identical to the bat SARS-alike coronavirus. Coronaviruses are spherical virions with a core shell and outward projections that resemble a solar corona, therefore the name (corona means "crown" in Latin). There are various subtypes of coronaviruses that can infect humans, such as Alpha (B.1.1.7 and Q lineages, identified in Kent, UK), Beta (B.1.351 and descendent lineages, identified in South Africa), Gamma (P.1 and descendent lineages, identified in Brazil), Epsilon (B.1.427 and B.1.429), Eta (B.1.525), Iota (B.1.526), Kappa (B.1.617.1), 1.617.3, Mu (B.1.621, B.1.621.1), Zeta (P.2), Delta (B.1.617.2 and AY lineages, identified in India). The Omicron variant (Pango lineages B.1.1.529, BA.1, BA.2 and BA.3. BA.1, first identified in South Africa) with the variant being monitored (VBM) and a variant of concern (VOC), respectively (Centers for Disease Control, 2021; Divya et al., 2020; Velavan & Meyer, 2020; Wolter et al., 2021).
1.2. Infection
It affects patients of all ages, albeit fewer occurrences have been documented in children, with an estimated time between infection, illness progression, and hospitalization in patients ranging from 9.1 to 12.5 days (Divya et al., 2020; Fauci et al., 2020) and that affected more than 224 countries and territories with over 422 million infected cases and deaths of 5.89 lakhs as of today (https://www.worldometers.info/coronavirus/and accessed on 19 February 2022). It mainly infects the lungs by exploiting the ACE2 (Angiotensin-converting enzyme II) receptor for receptor-mediated endocytosis into host lung alveolar epithelial cells (Velavan & Meyer, 2020) activating both innate and adaptive immune responses in the host, leading to uncontrolled inflammatory innate and impaired adaptive immunological responses. In COVID-19 patients, the number of CD4+ T, CD8+ T, B, and natural killer (NK) cells, as well as the proportion of monocytes, eosinophils, and basophils, is significantly decreased. The number of neutrophils, the natural killer inhibitory receptor CD94/NK group 2 member A (NKG2A) on cytotoxic lymphocytes (NK and CD8+ T cells) are all increased in infected individuals (Cao, 2020).
The infection by nCoV increased the levels of pro-inflammatory cytokines such as IL-2, IL-6, IL-8, IL-1, IL-17, G-CSF, GM-CSF, IP10, MCP1, MIP1 (CCL3), and TNF, as well as the levels of C- reactive protein and D- dimer, which leads to cytokine storm (Cao, 2020). The TLR (Toll-like receptors) identifies viral particles and activates the NF-κB pathway, which leads to the transcription of cytokines like IFN-γ and IL-6. However, in the case of COVID-19, the virus inhibits the NF-κB-TLR4 pathway, delaying IFN- γ production and allowing the virus to replicate uncontrolled. While when COVID-19 virus particles connect to TLRs on macrophages, neutrophils, and dendritic cells, pro-IL-1 is produced, which is subsequently cleaved by caspase-1, culminating in the production of IL-1, a mediator of lung inflammation, fever, and fibrosis (Conti et al., 2020).
As a result, there are three stages to the COVID-19 infection: early (phase one), pulmonary (phase two), and hyper-inflammation (phase three). The first phase is characterized by fever and cough caused by viral replication in the respiratory epithelium, whereas the second phase is marked by hypoxia. Phase three is characterized by the emergence of acute respiratory distress syndrome, septic shock, cytokine release syndrome, cardiac problems, and secondary hemophagocytic syndrome, which may appear between 9 and 12 days after the onset of the illness (Khadke et al., 2020). Inflammatory variables were found in COVID-19 patients, including an increase in (IL)−6, IL-8, tumor necrosis factor (TNF)-α and IL-1β in non-survival groups compared to survival groups, according to the study. It is critical to understand how to block the Cytokine Storm and when to begin anti-inflammation medication to lower the chance of mortality from COVID-19 (Del Valle et al., 2020). Suppression of pro-inflammatory cytokines TNF-α, IL-1β and IL-6 have been proven to improve a variety of inflammatory disorders, including viral infections which could be considered in the management and treatment of COVID-19 (Zhang et al., 2020a).
1.3. Prevention and treatments
Among the antiviral drugs that are prescribed to treat viral infections, some antivirals were designed to fight specific viruses, while others are made to fight a broad spectrum of viruses. Some of these are Remdesivir (Veklury), AT-527, EIDD-2801, Favipiravir (Avidan), Fluvoxamine, Kaletra, Merimepodib (VX-497), Niclosamide, Umifenovir (Arbidol), and monoclonal antibodies from AstraZeneca and Celltrion. Immune modulators like dexamethasone, medicines like lopinavir and ritonavir and tocilizumab, an IL-6 receptor-targeted monoclonal antibody, have been linked to reduction in fever and improved respiratory function in the treatment of COVID-19 (Qomara et al., 2021). Conversely, WHO recommended suspending hydroxychloroquine and lopinavir/ritonavir treatment for COVID-19 patients (Harris et al., 2020).
Therefore, the key goals in COVID-19 treatment are the creation of vaccinations and preventive agents. Several vaccinations to fight viral spread were produced within a year of the pandemic. Some of these vaccinations are being rolled out over the world to help reduce infection, illness severity, and death. The vaccinations produced against nCoV are split into four types: whole virus, protein subunit, viral vector, and nucleic acid (RNA and DNA) vaccines. Some try to inject the antigen into the circulation, while others, like the messenger RNA (mRNA) vaccination, employ the body's cells to produce the viral antigen (World Health Organization, 2020). Many institutes have been involved in this research. Moderna/National Institutes of Health, Sanofi/Translate Bio and Pfizer (collaborated with German biotech business BioNTech and Chinese pharmaceutical Fosun Pharma) produced the mRNA vaccine. While CanSino Biologics created a potential vaccine that employs an adenovirus known as Ad5 to deliver coronavirus proteins into cells. Gamaleya Research Institute produced Trusted Source, a vaccination that combines two adenoviruses, Ad5 and Ad26 (Xiaoni et al., 2021). AstraZeneca and the University of Oxford in collaboration developed vaccination based on a chimp adenovirus that transfers coronavirus proteins into cells. Sanofi and GSK have based on recombinant vaccine candidate. The Novavax vaccine is made up of virus proteins coupled to tiny particles. In Brazil, Indonesia, and Turkey, the Wuhan Institute of Biological Products/Sinopharm, Sinopharm and Beijing Institute of Biological Products, Sinovac Biotech, and Bharat Biotech, the Indian Council of Medical Research, and the Indian National Institute of Virology developed an inactivated virus vaccine. Furthermore, the DRDO's Institute of Nuclear Medicine and Allied Sciences Laboratory, in collaboration with Dr. Reddy's Laboratories in India, has developed an oral antiviral drug for COVID-19 called 2-DG (2-Deoxy-d-glucose) (Kimble et al., 2021; Lai et al., 2021).
Inspite of this, in January 2022, WHO has approved the following vaccines after complete the evaluation criteria about their safety and efficacy which are including AstraZeneca/Oxford vaccine, Johnson and Johnson, Moderna, Pfizer/BionTech, Sinopharm, Sinovac, COVAXIN, Covovax, Nuvaxovid (World Health Organization, 2022).
1.4. Importance of alternative therapies for COVID-19
Despite the fact that the number of COVID-19 positive cases and deaths is steadily growing, COVID-19 infection spreads swiftly and varies from previous SARS-CoV infections due to structural differences in ‘S’ proteins. Each variant brings new benefits to its family over the previous's and the new variant Omicron has a strong growth advantage over Delta, resulting in fast community dispersion and greater occurrence rates than that in prior pandemics (Callaway, 2021). Hence we have yet to find safe, highly effective, and widely available therapies for coronavirus disease 2019 (COVID-19), and even as the vaccine begins to roll out in many countries, there are still more questions than answers about the best method to treat COVID-19 and post COVID-19 symptoms.
As well as, since these treatments do not provide a full cure or are unsuccessful for any one infected individual, and because of the rising number of human setbacks, research has focused on better understanding the disease's concept in order to design convincing cures. COVID-19 has yet to be identified with a particular therapy. Immunization is at a critical juncture, with many individuals currently undergoing clinical trials (Fauci et al., 2020). Whereas, different pharmaceuticals have now been found and created as instruments for treating and smothering incendiary crises, using steroids, nonsteroidal relaxation medications, and immunosuppressants (Fauci et al., 2020). As a consequence, a powerful antiviral with increased effectiveness for the prevention and treatment of COVID-19 illness is urgently needed.
In practice, the goal is to build a medicine from a basic feasible component that is more adequate. Increased use of these treatments, however, is associated with negative side effects such as ulceration, gastric aggravation, angioedema, hepatic disappointment, migraine, hemolytic fragility, hyperglycemia, immunodeficiency-related difficulties, and others (Fauci et al., 2020). As a consequence, conventional medicinal treatments that are widely deemed to be safe as a type of optional therapy are being explored, as well as possible antiviral and immune booster herbal medications, extracts, or formulations.
Traditional or alternative remedies are frequently the only therapeutic options accessible in impoverished nations. Traditional, complementary, and integrative medicine (TCIM) strategies appear to have sparked interest in the quest for conventional medical therapies for COVID-19. TCIM methods frequently emphasize prevention, and the immune-supporting benefits of several TCIM medicines are expected to help patients better fight against infection and after symptoms (Nugraha et al., 2020; Pergolizzi et al., 2021). Natural products (medicinal plants/mushrooms) and their compounds could have the potential for prevention and treatment against COVID-19 since many phytocompounds have been recoganized as potential prophylactic and therapeutic agents for viral infections.
ACE2 has been discovered as a host cell surface receptor for the entrance of COVID-19 into humans, making it a promising target for novel treatments (Cheke et al., 2021). Some popular plants, including cinnamon, pepper, olive, black nightshade, hawthorn, passion fruit, and grapes, were shown to have ACE2 inhibitory action and have been intensively explored with in silico, pre-clinical, and clinical models. Alkaloids, flavonols, flavonones, terpenes, limonoids, lignans, terpenoids, tannins, phenolic acids, and fatty acids are all examples of ACE2 inhibitors found in plant extracts (Abubakar et al., 2021; Cheke et al., 2021; da Silva Antonio et al., 2020; Joshi et al., 2021).
In addition to using natural medicine to treat COVID-19, antiquated medicinal structures in Asia, such as Traditional Chinese Medicine (TCM) and Indian Ayurveda medicine, rely on the use of plants and therapeutic mushrooms in traditional medical systems and even regularly for various diseases such as influenza, hepatitis B, diarrhea, throat infection, and also against various viruses such as hepatitis B, dengue, chikungunya, polio, and so on (Pergolizzi et al., 2021).
2. Baseline of the study
Therefore, the purpose of this paper was to look into the anti-COVID-19 properties of regularly used and linked medicinal mushrooms. The mushrooms, which are extensively used in food and have numerous reports of improving immunity response, were used as a backdrop for this research since the primary concern from nCoV is the downregulation of the immune system and the overexpression of cytokines. As a result, in light of the COVID-19 study's focus on natural products, this evaluation suggests that mushrooms have the potential to be effective against nCoV based on existing scientific data. It also proposes that potential COVID-19 infection research targets for mushrooms should be researched and prioritized based on their antiviral, immunomodulatory, and other relevant properties. The selection criteria included PubMed/Google Scholar indexed studies or books on the immunomodulatory, anti-inflammatory, and antiviral activities of these mushrooms, as well as the primary term COVID-19.
3. The use of medicinal mushrooms as adjuvant therapy against COVID-19
Many medicinal mushrooms utilized in Traditional Chinese and Indian medical systems for over 2000 years have been discovered to have antiviral, immunomodulatory, and anti-allergic/anti-asthmatic effects (Patwardhan et al., 2005). As well as, it have previously been reported to have anti-infectious, anti-inflammatory, anti-tumor, and immune-modulating properties and as a result, there is an increasing interest in the medicinal use of mushroom nutraceuticals (Akramienė et al., 2007; Pelizon et al., 2005).
Mushroom immunomodulators activate both the innate and adaptive immune systems. By proliferating and activating innate immune system components such as natural killer (NK) cells, neutrophils, and macrophages, they increase the development and release of cytokines. These cytokines subsequently promote B cell antibody production while also boosting T cell differentiation into T helper (Th) 1 and Th2 cells, which mediate cell and humoral immunity, respectively (Zhao et al., 2020).
Due to their large molecular weight, mushroom polysaccharides are unable to infiltrate immune cells and activate them directly. Dectin-1, Complement receptor 3 (CR3), Lactosylceramide (LacCer), and Toll-like receptor (TLR)2 are among the cell receptors implicated in polysaccharide stimulation. Polysaccharide effectiveness in these settings is determined by its affinity for immune cell receptors (Rangsinth et al., 2021).
Since the dawn of time, mushrooms have been used to prevent and treat infection and sickness. The various reports on the immune-boosting abilities of a range of medicinal mushrooms may looked at the treatment of COVID-19 (Rangsinth et al., 2021). According to Hetland et al. (2021), basidiomycetes mushrooms may be effective as preventative or therapeutic add-on therapies in COVID-19 infection, as well as for the immunological overreaction and harmful inflammation associated with COVID-19 infection. Six low-toxic/nontoxic chemicals in mushrooms demonstrated SARS-CoV-2 protease inhibitor action by Rangsinth et al. (2021). Similarly, Chaga mushrooms (which grow largely on the bark of birch trees in Northern Europe, Siberia, Russia, Korea, Northern Canada, and Alaska) with a powerful enzymatic system and a strong defense system due to their parasitic way of life have demonstrated promising benefits in the decrease of COVID-19 related inflammatory responses in a study (Shahzad et al., 2020).
Apart from this, the β-glucans from the edible shiitake mushroom have been found to protect against a wide range of viral infections and may assist to reduce the key cytokines implicated in the cytokine storm observed in severe COVID-19 instances. The beta-glucans, carbohydrates, found in the cell walls of saprophytes, lichens, and plants are most oftenly prescribed for heart disease and excessive cholesterol. According to recent study, medicinal mushrooms may aid with asthma and lung infections, meaning that they can also help with COVID-19. In this sense, medicinal mushrooms may have a preventive effect by strengthening the immune system's tolerance to COVID-19 (Murphy et al., 2020).
According to Saxe, who led the MACH-19 trials with a combination of two mushrooms: turkey tail (Trametes versicolor) and agarikon (Fomitopsis officinalis), which are both accessible as over-the-counter supplements, the mushrooms offer physiologically plausible immune-modulating capabilities against SARS-CoV-2. Interacting to receptors on immune cells is one of the ways fungus interact as part of the gut microbiome. T lymphocytes, for instance, have receptors that bind mushroom polysaccharides. This is one route by which mushrooms might change the behavior of our immune cells, which could help us to fight against SARS-CoV-2 (Slomski, 2021).
4. Antiviral and immunomodulatory effects of medicinal mushrooms
A wide rage of mushrooms have been reported with antiviral effects against various viruses as well as anti-inflammatory and immunomodulatory effects in various experiments Table 1. shows the potential of medicinal mushrooms for the creation of broad-spectrum antiviral, anti-inflammatory, and immunomodulatory therapies, as well as the implications for COVID-19 therapy. Because of its nutritional and pharmacological characteristics, the sun mushroom Agaricus brasiliensis (synonyms: Agaricus blazei SS. Heinem) is frequently consumed, particularly as tea. Aqueous (AqE) and ethanol (EtOHE) extracts from the fruiting body of A. brasiliensis, as well as an isolated polysaccharide (PLS), were tested in HEp-2 cells for antiviral activity against poliovirus type 1. With selectivity lists (SI) of 5.4, 9.9, and 12.3, respectively, the quantity of plaques fell by half, 67 and 88% when AqE, PLS, and EtOHE were administered shortly after infection immunization (time 0 h) (Faccin et al., 2007).
Table 1.
Antiviral activities of mushrooms and their chemical constituents.
| Species name | Family | Extract/compound | Model | Key results | Refs. |
|---|---|---|---|---|---|
| Agaricus blazei Murrill | Agaricaceae | Polysaccharides (ABP – AW1) | Agaricus blazei mycelium significantly reduced the CPE of Western equine encephalitis (WEE) virus, herpes simplex virus, and poliovirus in Vero cells.In the early phases of herpesvirus and enterovirus reproduction, basidiomycetes extract contains inhibitory chemicals. | Sorimachi et al. (2001) | |
| Agaricus brasiliensis Fr., | Agaricaceae | Sulfated polysaccharides | HSV-1 [KOS and 29R (acyclovir-resistant) strains] and HSV-2 strain 333, | With selectivity indices (IC50) of 439, 208, and 562, a sulfated derivative (MI-S) of a polysaccharide isolated from A. brasiliensis mycelia demonstrated inhibitory efficacy against HSV-1 [KOS and 29R (acyclovir-resistant) strains] and HSV-2 strain 333.MI-S suppressed HSV-1 and HSV-2 binding, penetration, and cell-to-cell dissemination, as well as the expression of HSV-1 ICP27, UL42, gB, and gD proteins. When MI-S was coupled with acyclovir, it showed a synergistic antiviral effect. | De Sousa Cardozo et al. (2014) |
| polysaccharide (PLS) | antiviral activity against poliovirus type 1 in HEp-2 cells. | With a selectivity index (SI) of 5.4, 9.9, and 12.3, respectively, AqE, PLS, and EtOHE were lowered by 50%, 67%, and 88%, respectively. | Faccin et al. (2007) | ||
| polysaccharides β-(1→2)‑gluco-β-(1→3)-mannan | The antiherpetic efficacy of MI-S was assessed in murine ocular, cutaneous, and genital infection models of HSV. herpes simplex virus (HSV) attachment, | By day 9, mice that had been infected on the skin and administered MI-S orally had significantly reduced illness scores (p 0.05), showing that healing had been hastened. | Cardozo et al. (2013) | ||
| Agaricus bisporus (J.E. Lange) Imbach | Agaricaceae | Tyrosinases - protein | antiviral activity against the Hepatitis C virus | Tyrosinases may represent a potential antiviral inhibitory strategy by catalysing the selective hydroxylation of crucial position tyrosine residues in viral proteases.Tyrosinases derived directly from fresh mushrooms (which contained both tyrosinases) had equal antiviral efficacy, implying that they could be used to treat hepatitis C at a low cost. | Lopez-Tejedor et al. (2021) |
| Ganoderma lucidum (Curtis) P. Karst., | Polyporaceae | GLPG (Ganoderma lucidum proteoglycan) | antiviral activities against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) were investigated by the cytopathic effect (CPE) inhibition assay in cell culture | Polysaccharide reduced the cytopathic impact in HSV-infected cells in a dose-dependent manner, with no cytotoxic effects even at a dosage of 2000 µg/ml.The antiviral activity of GLPG was demonstrated in cells treated with the compound before, during, and after infection, with virus titers in the supernatant of cell culture 48 h later assessed using the TCID(50) assay.Pre-treatment and treatment during virus infection revealed stronger antiviral effects of GLPG than post-infection therapy. | Liu et al. (2004) |
| Lentinula edodes (Berk.) Pegler, | Omphalotaceae | mycelia (LEM) solid culture extracts | anti-influenza virus activity of LEM in vitro (influenza virus growth) and in vivo (infected mice) | Infected mice who were given LEM orally had a longer median survival duration.Infected mice developed mild bronchiolitis after receiving LEM by mouth, and the amount of alveolitis was dramatically reduced.The LEM-administered mice demonstrated a rapid stimulation of IFN-γ gene expression after being infected with influenza virus.In a mouse model, LEM's immunopotentiation activity on the type I IFN pathway prevents virus propagation from peribronchiolar to distal alveolar areas. | Kuroki et al. (2018) |
| Lentinula edodes (Berk.) Pegler, | Omphalotaceae | polysaccharide (LeP) | Poliovirus type 1 (PV-1) and bovine herpes virus type 1 (BoHV-1). | AqE and LeP were more effective when added at 0 h after infection; however, EtOHE was more effective at 1 h and 2 h after infection. AqE, EtOHE, and LeP demonstrated modest virucidal efficacy, and viral adsorption was not significantly inhibited. | Rincão et al. (2012) |
| aqueous (AqE) and ethanol (EtOHE) extracts and polysaccharide (LeP) | Inhibition of viral adsorption. Plaque assay. Poliovirus type 1 (PV-1), vaccinal strain, bovine herpesvirus type 1 (BoHV-1) | Adding the extract at concentrations of 3.1, 6.3, 12.5, and 25 mg/ml at the time of infection (0 h) resulted in viral inhibition of 1.8, 17.5, 41.1, and 82.5%, respectively.The percentages of inhibition were 9.2, 12.1, 24.5, and 60.2% one hour after infection (1 h). | Rincão et al. (2012) | ||
| Lentinus edodes (Berk.) Singer, | Polyporaceae | peptidomannan, KS-2, extracted from culture mycelia | influenza A2 (H2N2) virus, | In mice infected intranasally (IN) with influenza A2 (H2N2) virus, KS-2 was revealed to exhibit important protective qualities.The agent's efficacy was established by an increase in the number of survivors, a longer mean survival time, a reduction in viral titer in lung tissues, and an inhibition of lung consolidation produced by the viral infection.Oral and intraperitoneal KS-2 administrations protected mice from infection, and both prophylactic and chemotherapeutic administrations had substantial antiviral efficacy. | Suzuki et al. (1979) |
| Ganoderma pfeifferi Bres., | Polyporaceae | triterpenes | Herpes simplex virus. | Inhibitory activity of the herpes simplex virus in ganoderone A, lucialdehyde B, and ergosta-7,22‑dien-3-ol. | Niedermeyer et al. (2005) |
| Lentinus edodes (Berk.) Singer, | Polyporaceae | lentinan (LNT-I) | IHNV infection | In pre-addition, co-addition, and post-addition to IHNV, direct inactivation and antiviral capability were 62.34%, 39.60%, 53.63%, and 82.38%, respectively, under 100 g/mL of LNT-I. (MOI of 0.05).The principal antiviral mechanisms of LNT-I are direct inactivation and suppression of viral replication.LNT-I dramatically decreased the expression of TNF-, IL-2, and IL-11 after being exposed to IHNV while considerably raising the expression of IFN-1 and IFN-γ. | Ren et al. (2018) |
| Coriolus versicolor (L.) Quél., | Polyporaceae | Polysaccharide, peptide | anti-human immunodeficiency virus type 1 (HIV-1) | It stopped HIV-1 gp 120 from interacting with an immobilised CD4 receptor (IC50 = 150 µg/ml), recombinant HIV-1 reverse transcriptase (IC50 = 6.25 µg/ml), and a glycohydrolase enzyme involved in viral glycosylation (IC50 = 6.25 µg/ml). | Collins and Ng (1997) |
| Ganoderma lucidum (Curtis) P. Karst. | Polyporaceae | triterpenoids (GLTs), Lanosta-7,9(11),24-trien-3-one,15;26-dihydroxy (GLTA) and Ganoderic acid Y (GLTB), | EV71 infection | By connecting with the viral particle and blocking virus adsorption to the cells, GLTA and GLTB prevent EV71 infection.The interactions between the EV71 virion and the chemicals were predicted using molecular docking, which revealed that GLTA and GLTB bind to the viral capsid protein at a ydrophobic pocket (F site), blocking EV71 uncoating.EV71 replication is inhibited by GLTA and GLTB, which impede EV71 uncoating and consequently viral RNA (vRNA) replication. | Zhang et al. (2014) |
| GLPG (Ganoderma lucidum proteoglycan) | antiviral activities against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) | GLPG decreased cell death in HSV-infected cells in a dose-dependent manner.GLPG had no cytotoxic effect even at 2 mg/ml.To test GLPG's antiviral action, cells were infected with the virus and the viral titer in the cell culture supernatant was evaluated before, during, and after infection. A TCID ((50)) assay was used to assess 48 h after infection. | Li et al. (2005) | ||
| Pleurotus ostreatus (Jacq.) P. Kumm.,, Fomes fomentarius (L.) Fr., , Auriporia aurea (Peck) Ryvarden, and Trametes versicolor (L.) Lloyd, | Pleurotaceae, Polyporaceae, Fomitopsidaceae, | HSV-2 strain BH in RK-13 cells - inhibition as for influenza | The mycelium of T. versicolor 353 was discovered to have a high therapeutic index (324.67) | Filippova et al. (2013) | |
| Boletus edulis Bull.,Pleurotus ostreatus (Jacq.) P. Kumm., andLentinus edodes (Berk.) Singer, | Boletaceae, Pleurotaceae, Polyporaceae | Polysaccharide fraction | herpes simplex virus type 1 (HSV-1) | The mushrooms water extracts strongly suppressed viral reproduction in vitro, with IC50 values ranging from 26.69 mg/ml-1 to 35.12 mg/ml-1.Compounds with a high molecular weight (HMW) are responsible for antiviral activity.The antiviral activity of B. edulis compounds (likely chitin-binding lectins) was discovered to be correlated with the presence of b-glucans in the polysaccharide fractions, which were found to have stronger antiviral activity than the whole water extracts. | Santoyo et al. (2012) |
| Cordyceps militaris (L.) Link, | Cordycipitaceae | acidic polysaccharide | bronchoalveolar lavage fluid and the lung of mice infected with influenza A virus | When given intranasally, the polysaccharide lowered virus titers in bronchoalveolar lavage fluid and the lungs of mice infected with influenza A virus and enhanced survival rates.APS increased TNF-alpha and IFN-gamma levels in mice.APS enhanced NO production and promoted iNOS mRNA and protein expression in RAW 264.7 murine macrophage cells.There was also an increase in the expression of cytokine mRNA, such as IL-1beta, IL-6, IL-10, and TNF-alpha. | Ohta et al. (2007) |
| Rozites caperatus (Pers.) P. Karst., | Cortinariaceae | protein RC28 | herpes simplex virus-1 in Vero cells and in a herpes simplex virus-1 mouse keratitis model |
|
Yan et al. (2015) |
| Phellinus linteus (Berk. & M.A. Curtis) Teng | Hymenochaetaceae | inotilone (1) and 4-(3,4 dihydroxyphenyl)−3-buten-2-one (2) | Recombinant influenza A virus H1N1 (rvH1N1). | The activity of H1N1 neuraminidase was reduced in a dose-dependent manner by compounds 1 and 2, with IC50 values of 29.1 and 125.6 M, respectively.They also showed antiviral effectiveness in a viral cytopathic impact reduction experiment employing MDCK cells. | Hwang et al. (2014) |
| Phellinus baumii Pilát, | Hymenochaetaceae | Polyphenols (hispidin,hypholomine B,inoscavin A,davallialactone, andphelligridin D) | Influenza A virus(H1N1, H5N1, andH3N2) | All drugs inhibited noncompetitively H1N1, H5N1, and H3N2 neuraminidase activity and reduced the quantity of virally-induced cytopathic effect in an MDCK cell-based experiment (CPE). | Hwang et al. (2015) |
| Phellinus igniarius (L.) Quél., | Hymenochaetaceae | eudesm-1β, 6α, 11-triol, compound 1), one ergosta −4, 6, 8(14), 22-tetraen-3-one (compound 2), four polyphenols (compounds 3, 4, 5, 6), and one pyrone (3‑hydroxy-2-methyl-4-pyrone, compound 7) | H5N1 influenza A virus | Antiviral activity against H5N1 influenza is demonstrated by these drugs. An MTT colorimetric assay method was used to analyze a virus in Madin-Darby canine kidney cells.Compound 1 has a great ability to inhibit the influenza virus, according to the data. The 50% effective concentration was 0.14 0.04 M. According to molecular modeling, the anti-influenza virus activity of this drug was also linked to interactions of hydroxyl groups with an amino acid residue (Asn 170) of neuraminidase (NA) at the binding site.In addition, at a concentration of 0.657 0.325 mg/mL, compound 1 inhibited the NA enzyme by 50%, indicating that compound 1 is likely to interfere with the NA enzyme. | Song et al. (2014) |
| Porodaedalea pini (Brot.) Murrill | Hymenochaetaceae | polymers, EP-AV1 and EP-AV2 | plaque formation in Vero cells caused by herpes simplex virus 1 (HSV-1) |
|
Lee et al. (2010) |
| Inonotus obliquus (Ach. ex Pers.) Pilát, | Hymenochaetaceae | Inonotus obliquus polysaccharides (IOP), | herpes simplex virus (HSV) | AEIO showed a significant reduction in herpes simplex virus (HSV) infection (the 50% inhibitory concentration in the plaque reduction assay was 3.82 g/mL, and 12.29 g/mL in the HSV-1/blue assay), as well as protection in Vero cells (the 50% cellular cytotoxicity was > 1 mg/mL, and the selection index was > 80).The mechanism of anti-HSV efficacy against the early stages of viral infection was revealed utilizing a time course test, successful stage analysis, and fusion inhibition assay to prevent viral-induced membrane fusion.AEIO, unlike nucleoside analogue a, was able to effectively block HSV-1 entry by acting on viral glycoproteins, preventing membrane fusion. | Pan et al. (2013) |
| hepatitis C virus | After both preventive (24 h before infection) and therapeutic administration, fungus extracts demonstrated antiviral properties (during infection of porcine embryo kidney cells).The findings reveal that birch fungal extracts suppress infective virus production in pig embryo kidney cells. | Shibnev et al. (2011) | |||
| Human immunodeficiency virus type 1 (HIV-1). | In lymphoblastoid cells culture MT-4, low toxic extracts were combined with the virus at a dosage of 5.0 g/ml and demonstrated antiviral action.The extract of the birch fungus can be used to make new antiviral medications and HIV-replication inhibitors when used as single pharmaceuticals or as part of a complicated treatment. | Shibnev et al. (2015) | |||
| Inonotus obliquus f. sterilis (Vanin) Balandaykin et Zmitr., | Hymenochaetaceae | Polysaccharides (IOPs) | HIV-1 | IOP was found to have wide antiviral activity against feline herpesvirus 1, feline influenza viruses H3N2 and H5N6, feline panleukopenia virus, and feline infectious peritonitis virus, all of which can cause respiratory and gastrointestinal illnesses in cats.According to these findings, IOP could be a viable broad-spectrum antiviral therapy for feline infections. | Tian et al. (2017) |
| Pleurotus cystidiosus O.K. Mill., | Pleurotaceae | polysaccharide | HIV-1 reverse transcriptase |
|
Wang et al. (2007) |
| Pleurotus citrinopileatus Singer, | Pleurotaceae | Polysaccharopeptide(modified withchlorosulfonic acid) | HIV Reverse transcriptase andglycohydrolase |
|
Li et al. (2008) |
| Pleurotus ostreatus (Jacq.) P. Kumm., | Pleurotaceae | Ubiquitin-like Protein | HIV-1 reverse transcriptase inhibitory activity |
|
Wang et al. (2000) |
| Pleurotus nebrodensis (Inzenga) Quél., | Pleurotaceae | hemolysin | anti-HIV-1 effects | Hemolysin was discovered to exhibit anti-HIV-1 action in CEM cell culture. | Lv et al. (2008) |
| Russula paludosa Britzelm., | Russulaceae | Peptide | HIV-1 reverse transcriptase inhibitory activity |
|
Wang et al. (2007) |
| Tricholoma giganteum Massee, | Tricholomataceae | Laccase | HIV-1 |
|
Wang et al. (2004) |
| Grifola frondosa (Dicks.) Gray | Grifolaceae | G. frondosa polysaccharide (GFP1) | GFP1 was examined for anti-EV71 efficacy in cultured cells, and it was discovered that it inhibited EV71 viral replication, as well as viral VP1 protein expression and genomic RNA synthesis.GFP1 inhibited caspase-3 cleavage and IB downregulation produced by EV71, indicating apoptotic and other activities.The findings reveal that the novel G. frondosa polysaccharide exhibits antiviral action, implying that it could be used as a new anti-EV71 treatment. | Zhao et al. (2016) | |
| protein | herpes simplex virus type 1 (HSV-1) replication in vitro | GFAHP inhibits HSV-1 penetration into Vero cells by directly inactivating HSV-1. | Gu et al. (2007) | ||
| D-fraction extracted from Grifola frondosa (GF-D) | inhibitory effect on hepatitis B virus (HBV) | The combination of GF-D and IFN reduced HBV replication synergistically in 2.2.15 cells. In the presence of 0.45 mg/ml GF-D, the apparent IC50 for IFN was 154 IU/ml.The antiviral activity of IFN was boosted 9-fold, suggesting that GF-D might be used in conjunction with IFN.These data show that using GF-D in combination with IFN could be a good way to treat persistent HBV infections. | Gu et al. (2006) | ||
| β-glucan | HIV infection patients | Twenty patients had an increase of 1.4–1.8 fold in CD4+ cell counts, whereas eight had a drop of 0.8–0.5 fold. The viral load increased in 9 cases and dropped in 10 others.However, 85% of respondents reported an increased sense of well-being in terms of numerous symptoms and secondary diseases caused by HIV.These results suggest that Maitake D-Fraction has a positive impact on HIV patients. | Nanba et al. (2000) |
4.1. Immunomodulatory effects of mushrooms
Immunomodulatory and perhaps cancer-fighting activities of the extracts of Agaricus blazei mushroom have also been discovered. To assess leukocyte homing and enactment, mice were given (99 m) Tc-radiolabeled leukocytes, which showed enhanced leukocyte migration to the spleen and heart of animals treated with A. blazei enhancements. In the spleen, researchers detected increased initiation of neutrophils, NKT cells, and monocytes, as well as enhanced production of TNF-α and IFN-γ. Circulating NKT cells and monocytes were also more stimulated as a result of the enhanced gathering. The atherosclerotic sore patches in the aorta of mice were improved, with more macrophages and neutrophils counts. After 12 weeks of supplementation, A. blazei induced transcriptional activation of genes associated to macrophage initiation (CD36, TLR4), neutrophil chemotaxis (CXCL1), leukocyte adhesion (VCAM-1), and plaque vulnerability (MMP9) (Gonçalves et al., 2012).
4.2. Antiviral effects of mushrooms
According to the literature, the related medicinal mushrooms, Agaricus blazei Murill, Hericium erinaceus, Grifola frondosa and Inonotus obliquus might have value as prophylactic or therapeutic add-on remedies in COVID-19 infection, particularly as countermeasures against pneumococcal superinfection, even when caused by multiresistant bacteria, and for the immune overreaction and damaging inflammation that occurs with COVID-19 attack (Hetland et al., 2021; Shahzad et al., 2020).
Senthil Kumar et al. (2021) reported that Antcins from Antrodia cinnamomea and Antrodia salmonea inhibit Angiotensin-Converting Enzyme 2 (ACE2) in Epithelial Cells which could be a potential compound against COVID-19 as prophylactic agents. Herein antcin-I show inhibitory effects on ACE2 in cultured human epithelial cells. As a result, there's a lot of curiosity about whether or if mushroom compounds can be used to combat the epidemic. Our goal was to write a short narrative assessment of the many sorts of mushrooms antiviaral and immunomodulatory effects and how they've been employed to against COVID-19.
The in-vitro cytotoxicity and hostile to HIV-1 activities of Cordyceps sinensis aqueous extract were investigated using the CCK-8 and TZM-bl pseudovirus tests. C. sinensis extract displayed anti-HIV-1 activities in vitro, while an aqueous extract from the fresh fungus inhibited opposite transcriptase more effectively. Furthermore, a significant relationship between the novel stroma concentrate and the Vif protein was observed. According to the study, limiting converse transcriptase movement and partnering with Vif protein might impede the in vitro anti-HIV-1 effect of C. sinensis watery concentrates (Zhu et al., 2016).
Grifola frondosa polysaccharide (GFP1) has a 1,6-d-glucan spine with a single 1,3-d-fucopyranosyl side-spreading unit and was isolated from Grifola frondosa mycelia. Enterovirus 71 is the germ that causes hand, foot, and mouth disease (EV71). GFP1 was evaluated for anti-EV71 action in cultivated cells, and it was discovered that EV71 viral replication was reduced, as well as viral VP1 protein articulation and genotoxicity. Furthermore, by masking EV71-induced caspase-3 cleavage and IB downregulation, GFP1 demonstrated apoptotic and other actions. The results reveal that the unique G. frondosa polysaccharide possesses antiviral action, suggesting that it might be effective as a new anti-EV71 therapeutic chemical in the future (Zhao et al., 2016).
Lignosus rhinocerotis, Pleurotus giganteus, Hericium erinaceus, Schizophyllum commune, and Ganoderma lucidium were used in the in vitro hostile to dengue infection serotype 2 (DENV-2) experiments. Hot water (HAEs), ethanol (EEs), hexane (HSEs), ethyl acetic acid derivation (ESEs), and aqueous extraction (ASEs) were used to extract the mushrooms. The anti-DENV-2 activity of the extracts was assessed in three ways: simultaneous, attachment, and penetration testing utilizing plaque reduction assays and RT-qPCR. The influence of expansion time on viral replication was explored during expansion tests, and a virucidal test was done to assess the immediate impact of each mushroom on DENV-2. The HAEs and ASEs of L. rhinocerotis, P. giganteus, H. erinaceus, and S. commune had a low toxic effect on Vero cells and demonstrated anti-DENV2 activity, according to the findings. During synchronous therapy, the ASEs' half inhibitory focus (IC50) was estimated to be between 399.2 and 637.9 µg/mL, while the HAEs' IC50 was estimated to be between 312.9 and 680.6 µg/mL. ASEs (IC50: 226.3–315.4 µg/mL) and HAEs (IC50: 943.1–2080.2 µg/mL) were also identified as major dengue-fighting agents in the penetration evaluation. In addition, in the ASEs and HAEs' simultaneous and penetration experiments, the expression levels of the ENV and NS5 genes were measured. Anti-DENV2 activity was strongest when the mushroom extracts were added after infection adsorption, according to time-of-addition experiments. In any of the concentrations, there was no indication of virucidal action (Ellan et al., 2019).
In cell-based experiments, IOP (Inonotus obliquus polysaccharides) therapy showed anti-Feline calicivirus (FCV) (strain F9) activity and minimal cytotoxicity. According to an analysis of the compound's mechanism of action, IOP treatment incites its inhibitory actions directly on infection particles by blocking viral binding/absorptions. IOP also exhibited antiviral effectiveness against the Feline herpesvirus 1, Feline influenza viruses H3N2 and H5N6, cat panleukopenia infection, and Feline influenza virus irresistible peritonitis infection, which may all cause feline respiratory and gastrointestinal diseases. These data showed that IOP might be used to treat Feline infections as a broad-spectrum antiviral (Tian et al., 2017).
Shibnev et al. (2011) discovered that fractions of the aqueous extract of the Inonotus obliquus fungus had a virucidal impact on the hepatitis C virus, lowering its infective characteristics by 100-fold in under 10 min. Similarly, its antiviral action against the human immunodeficiency virus type 1 (HIV-1) was demonstrated for both aqueous and water-alcohol extracts of the fungus. The grouping of 5.0 µg/mL demonstrated antiviral activity with a minimal harmful effect when used in conjunction with infection in the lymphoblastoid cell culture MT-4. Thus, the birch fungus could be utilized in novel antiviral treatments and HIV replication inhibitors either employed as single pharmaceutical or as part of a complicated therapy (Shibnev et al., 2015).
One of the most well-known medicinal fungus species, Ganoderma lucidum, has been used to treat a number of diseases. Triterpenoids and polysaccharides have been found as the main pharmacologically active components in G. lucidum (Boh et al., 2007 ). Compounds from G. lucidum have reported anti-tumor ( Cherian et al., 2009 ), anti-microbial ( Chen et al., 2012a), anti-atherosclerotic, anti-inflammatory, hypolipidemic ( Chen et al., 2005 ), anti-diabetic, anti-oxidative, radical-scavenging, and anti-aging properties. Along with this, antiviral effectiveness of G. lucidum triterpenoids has been shown against a range of pathogenic viruses, including herpes simplex virus types 1 (HSV-1 and HSV-2), influenza A virus (Flu A), vesicular stomatitis virus (VSV), and human immunodeficiency virus (HIV) ( El-Mekkawy et al., 1998 ; Eo et al., 1999 ) and siginificant inhibitory effects against dengue virus (DENV) NS2B-NS3 protease in in vitro assay. Also in silico analysis of four triterpenoids of G. lucidum namely Ganodermanontriol, Lucidumol A, Ganoderic acid C2, and Ganosporeric acid A recorded viral protease inhibition effects when compared to the reference inhibitor 1,8-Dihydroxy-4,5-dinitroanthraquinone. Thus the G. lucidum may beneficially use in the development of novel medications to treat DENV-related illnesses (Bharadwaj et al., 2019).
The antiviral effects of two protein-bound polysaccharides were isolated from G. lucidum, a neutral protein-bound polysaccharide (NPBP) and an acidic protein-bound polysaccharide (APBP), were examined utilizing a plaque reduction test against herpes simplex virus types 1 (HSV-1) and 2 (HSV-2). APBP surpassed NPBP in terms of antiviral activity against HSV-1 and HSV-2 at a 50% effective concentration (EC50 of 300–520 µg/mL). APBP blocked HSV-1 and HSV-2 binding to Vero cells at doses of 100 and 90 µg/mL, respectively, and both forms of HSV were prevented from entering Vero cells (Eo et al., 2000).
In the face of the infectious haematopoietic necrosis virus (IHNV), the antiviral activity of Lentinan (LNT-1), a powder made from Lentinus edodes mycelia (shiitake) mushroom, was investigated. According to the results, LNT-1 has a sub-atomic burden of 3.79 × 105 Da and has a β—(1 3)—glucan backbone with β—(1 6)—glucosyl side-branching units terminated by mannosyl and galactosyl residues. LNT-1 demonstrated antiviral efficacy against INHV, and its main antiviral mechanisms were due to the immediate inactivation and viral replication limitation, according to the research (Lee et al., 2009; Rincão et al., 2012).
The anti- herpes simplex Virus-1 activity was found in the aqueous extracts of Fomes fomentarius (EC50, 11.22 mg/mL; SI > 4.46), Phellinus igniarius (EC50, 9.71 mg/mL; SI > 5.15), and P. pini (EC50, 7.16 mg/mL; SI > 6.98) (Doğan et al., 2018).
In another in vitro study in MDCK cells, the antiviral activity of higher mushroom mycelia was examined against influenza A (serotype H1N1) and herpes simplex infection type 2 (HSV-2), strain BH. Whereas another experiment in RK-13 cells reported, Pleurotus ostreatus, Fomes fomentarius, Auriporia aurea, and Trametes versicolor viable against HSV-2 strain BH, with comparable levels of flu suppression. In this study, A. aurea exhibited resistance to the flu and antiherpetic workouts. The high regenerative file (324.67) of T. versicolor 353 mycelium suggests that it might be a potential material for the pharmaceutics as an antiviral and antiherpetic specialist with low toxicity (Krupodorova et al., 2014).
In a rat experiment, an antiviral protein derived from Grifola frondosa (GFAHP) inhibited HSV1 multiplication in vitro and lowered the severity of the viral infection (Gu et al., 2007). HSV1 infection was similarly inhibited by polysaccharides from Agaricus blazei Murill in HEp2 cell cultures (Minari et al., 2011; Yamamoto et al., 2013). By reducing infection attachment, segment, and cell to cell transmission, A. blazei mycelium polysaccharide decreased ocular, cutaneous, and vaginal (HSV2) illnesses in mice, as indicated by plaque reduction (Cardozo et al., 2013). Surprisingly, this was caused by a blockage of early viral penetration (Minari et al., 2011). In vitro assays, G. frondosa polysaccharide was found to inhibit replication of enterovirus 71 (EV71), the major causative for foot, hand, and mouth infection, smother viral protein articulation and induce apoptosis (Zhao et al., 2016).
One study found that A. blazei metabolites had a direct antiviral effect against flu infection in vitro and inhibited H1N1 flu infection in a plaque structure test after viral infiltration of host cells (Avtonomova & Krasnopolskaya, 2014). Hericium erinaceus has also been demonstrated antiviral characteristics by restoring mucosal tolerance in ducklings that had been damaged by the Muscovy duck reovirus (Wu et al., 2018). Furthermore, H. erinaceus was assumed to be capable of neutralizing Dengue virus contamination in vitro, as shown by plaque reduction experiments that showed inhibition of adhesion and penetration, as well as a decrease in viral output articulation (Ellan et al., 2019).
Against influenza A virus (H1N1pdm), polysaccharide fractions of water and ethanol extracts of Pleurotus pulmonarius showed antiviral activity (Ilyicheva et al., 2020). After being introduced to cells, polysaccharide fractions inhibited or prevented viral multiplication, assembly, and release.
Pleurotus abalonus, Coriolus versicolor, Agaricus bisporus, Pleurotus citrinopileatus, Lentinula edodes, Pleurotus ostreatus, Rassula paludosa, and Tricholoma giganteum have all developed anti-HIV mushroom compounds. The polysaccharide, polysaccharopeptide, lectin, lentin, ubiquitin-like protein, peptide, and laccase have been reported to primarily target HIV reverse transcriptase as well as by reducing viral entrance, replication, viral enzyme, viral protein production, and cellular proteins, they claim to improve immunity against HSV-1, HSV-2, influenza A virus, HIV, HCV, FCV, and EV71. Polysaccharide, lectin, lentin, and laccase from P. abalonus, P. citrinopileatus, L. edodes, and T. giganteum had IC50 of 0.1–2.2 M against HIV-1. While, anti-HIV drugs like AZT, dideoxycytidine, and dideoxyinosine have IC50 of 0.03–0.5 M, 0.5–1.5 M, and 2–10 M against HIV-1, respectively. As a result, mushrooms-derived compounds may be used as antiviral agents (Adotey et al., 2011; Collins & Ng, 1997; Eguchi et al., 2017; Li et al., 2008; Lv et al., 2009; Ngai & Ng, 2003; Seo & Choi, 2021; Wang & Ng, 2000, 2004; Wang et al., 2011, 2007).
Mushroom compounds' antiviral mechanisms have been well established against enveloped viruses; however, non-enveloped viruses like NoV and enteroviruses are yet to be investigated. Therefore, bioactive metabolites from mushrooms might be employed as antiviral options against DNA and RNA viruses like nCoV causing COVID-19 (Seo & Choi, 2021). The more details of the antiviral studies’ results are depicted in Table 1.
4.3. Mushroom bioactive compounds as immunomodulators
Immunomodulators are important components in advanced health and wellness industries, matching the invulnerable framework's role as the principal infection-prevention barrier. In clinical practice, they are usually classed as immunological suppressants, immune stimulants, or immune adjuvants. For healthy persons, they are also utilized as prodrugs or preventive medication. Over 50 mushrooms have been recognized as containing natural mixes that are invulnerable to handle and have extraordinarily extended subatomic weight and structure. Almost no mushrooms with immunomodulator properties have workouts that improve both inborn and flexible invulnerable frameworks. They increase cytokine articulation and emission, malignant development, and incurable disorders by multiplying and activating inborn safe framework components such as Natural Killer (NK) cells, neutrophils, and macrophages, as well as enhancing cytokine articulation and emission (Zhao et al., 2020). Many mushroom compounds act as the potential immunotherapeutic and immunomodulators in various research, and the results depict that it could be possibly use in the treatment of COVID–19.
Some of these important mushrooms and their compounds include Grifola frondosa (Dicks: Fr) S.F. Gray (ergosterol), Ganoderma lucidum (Curtis) P. Karst (ganoderiol F, ganodermanontriol, ganoderic acids (A, B, D, F, G, H, Z), ganosporeric acid A, ganopoly), Ganoderma pfeifferi Bres (ganodermadiol, lucidadiol and applanoxidic acid G), Ganoderma annulare (Pers.) Bres. (Applanoxidic acid A), Hericium coralloides (Scop.) Pers. (Erinacin E), Inonotus hispidus (Bull.) P. Karst. (Hispolon and hispidin), Lentinula edodes (Berk.) Pegler (eritadenine), Laricifomes officinalis (Vill.) Kotl. & Pouzar (Dehydrotrametenolic acid), Lenzites betulina (L.) Fr. (Betulinan A), Scutiger ovinus (Scutigeral), Scutiger confluens (Albaconol), Wolfiporia cocos (F.A. Wolf) (Dehydrotrametenolic acid), Trametes versicolor (L.:Fr.) Quel. (Coriolan) and Schizophyllum commune Fries (Schizophyllan) (Hazama et al., 1995; Liua, 2002; Ali et al., 1996; Lee et al., 1996; Mothana et al., 2003; Saito et al., 1998; Sato et al., 2002; Smania et al., 2003; Szallasi et al., 1999; Zhang et al., 2002). These compounds were described and their structures depicted in Fig. 2.
Fig. 2.
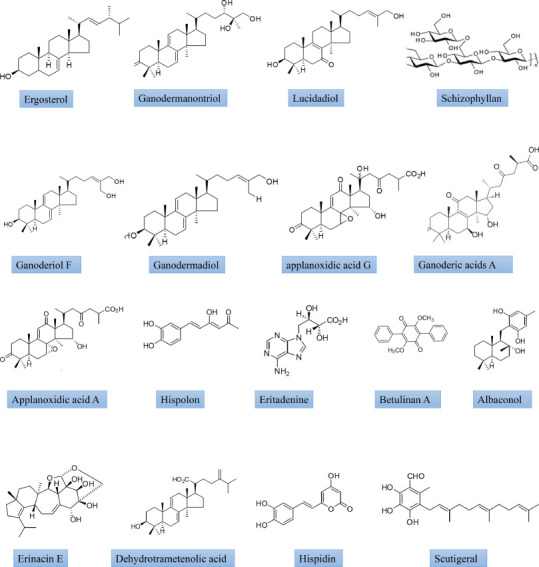
Some of the significant immunomodulatory effective compounds from mushrooms.
These compounds promote B cell growth for antibody production while also inciting T cell dissociation to T partner cells, which combat cell and humoral invulnerability independently. Immune-modulatory lectins, immunological-modulatory terpenes and terpenoids, immune-modulatory polysaccharides, and fungal immune-modulatory proteins (FIPs) are the four primary categories of mushroom immune modulators that have been proven to effectively reduce cytokine production (Zhao et al., 2020).
TML-1 and TML-2, two lectins isolated from Leucocalocybe mongolica (syn. Tricholoma mongolicum), showed anticancer and immune-modulating activities through raise the production of macrophage-activating factors and so impede the proliferation of mouse lymphoblast-like (p815) mastocytoma cells by increasing nitrite and tumor necrosis factor (TNF-α). Interferon (IFN-γ) and other cytokines, such as interleukin (IL-1β) and transforming growth factor (TGF), are influenced by up-regulation of inducible nitric oxide synthase (iNOS) (Zhao et al., 2020).
Even at extremely low doses, the lectin from Grifola frondosa has been demonstrated to have a significant cytotoxic impact on HeLa cells in vitro. Ricin-B-like lectin, a 15.9-kDa homodimeric lactose-binding lectin, isolated from Clitocybe nebularis with anti-proliferative action against human leukemic T cells by binding to carbohydrate receptors (Pohleven et al., 2009), causes the maturation and activation of dendritic cells (DCs), as well as the activation of many pro-inflammatory cytokines (Pohleven et al., 2012).
LNT-1, Lentinan powder made from Lentinus edodes mycelia (shiitake) mushroom, caused a significant decrease in the outflow of pro-inflammatory cytokines like TNF-α, IL-2, and IL-11, as well as an increase in the expression of antiviral, antiproliferative, and immunomodulatory cytokines like IFN-1 and IFN- γ (Lee et al., 2009; Rincão et al., 2012). The usual safe response, as previously stated, is a crucial determinant in the severity of COVID-19 disease and infection result. Therefore, the effects of LNT-1 could be evaluated against this disease, since SARS-COV-2 patients have high levels of flaming cytokines (Chaisuwan et al., 2021).
Similarly in another study using peptidomannan extracted from Cordyceps militaris, the mice infected with influenza A virus (H2N2) were administered orally and intraperitoneally with peptidomannan, which enhanced survival and elevated IFN levels in the blood (Suzuki et al., 1979). In mouse lung tissue, it also reduced lung consolidation and virus titer. In vitro, however, peptididomannan had no impact on the virus. According to the study results, peptidomannan seemed to reduce viral infection via immunological strengthening (Hwang et al., 2014).
4.3.1. Immunomodulatory effects of mushrooms terpenes
Modified terpenes (terpenoids or isoprenoids) are discovered in macrofungi with biological properties that might be employed in medicine. Ganoderma sp. mushrooms, such as G. lucidum and G. applanatum, are known for high quantities of triterpenoids such as lanostane, which possesses immunomodulating and anti-infective activities (Chakraborty et al., 2019). Whereas, the triterpenoids from G. lucidum and G. lingzhi may aid to minimize drug-induced nephrotoxicity and inflammation. The activation of the nuclear factor (NF-κB) pathway and mitogen-activated protein kinases has been shown to possess antitumor, immune-modulatory, and/or anti-infective properties of G. lucidum, G. lingzhi, and G. applanatum (Ina et al., 2013; Jeong et al., 2008).
Their diversified behaviors, on the other hand, suggest that they have a lot of promise for research and clinical treatment applications, despite the fact that their behavior processes and structure-activity correlations are still little understood. Some G. lucidum terpenes have been demonstrated to protect against medication nephrotoxicity and inflammation, hinting that they might be used in medicine (Zhao et al., 2020).
4.3.2. Immunomodulatory effects of polysaccharides
In recent times Yin et al. (2021) have been reviewed and reported that, edible mushrooms polysaccharides immunomodulatory effects. Many mushroom polysaccharides enhanced the immune system by the activation of inflammatory cytokines and other mediators. F. velutipes (PVPB1), enhanced the pro-inflammatory cytokines levels (IL-1, IL-6, TNF-α) in the RAW 264.7 cells. On the other hand, increased IgM, IgG levels and IL-10 in mouse B lymphocytes by the activation of ERK1/2, NF-κB pathways. I. obliquus (IP3a) increased the cytokines (IL-2, IL-12, IL-6, TNF-α) in mice experiment. G. frondosa polysaccharide (GP11) and fruiting body stimulate mRNA expression to produce NO, TNF-α and IL-1β, IL-6, IFN-γ, MIP-1β, MIP-1α, and MIP-2 in the RAW 264.7 cells. H. erinaceus (HEP-S) increased the levels of NO, IL-6, TNF-α by activating the mRNA protein expression in RAW 264.7 cells. A. biporus increased NO production and iNOS, COX-2 expressions as well as mRNA levels by increasing cytokines like IL-1β, IL-6, TNF-α. Similarly activated ERK/MAPK, NF-κB/IκB and ERK1/2 signaling pathways in RAW 264.7 cells. P. eryngii (EPA-1), A. mella (AAMP), C. militaris (CMPB-91) and S. commune (F2) mushroom polysaccharides were increased the production of NO, IL-1, IL-6, TNF-α by the activation of p38, ERK, JNK, MAPK and NF-κB, TLR-2 signaling pathways in RAW 264.7 cells. In this review study authors compiled in vitro, in vivo experiments of mushroom polysaccharides immunomodulatory effects (Yin et al., 2021).
Because the polysaccharides' large molecular weight prevents them from entering immune cells, they attach to cell receptors instead. The inciting component of polysaccharides contain many cell receptors including Dectin-1, Complement Receptor 3 (CR3), Lactosylceramide (LacCer), and Toll-Like Receptor (TLR)2. In these cases, the adequacy of polysaccharides is governed by their low affinity for invulnerable cell receptors. They have immunomodulatory effects as well, although with a delayed immune system activation mechanism. Low molecular weight polysaccharides, on the other hand, may enter immune cells and activate them as a result of their simple structural shape, hence regulating immunity (Barbosa et al., 2021).
The amount and length of short branched chains in mushroom polysaccharides may have a significant influence on their bioactivity in the immune system. In immunologically active polysaccharides, the degree of branching number (DB) is generally between 20% and 40%. The polysaccharide fraction of G. frondosa extract with the best immunomodulatory activity was discovered to have a molecular weight of around 800 kDa (Adachi et al., 1990).
Although a high DB number is normally linked with enhanced activity, in certain situations, debranching polysaccharides may also boost their bioactivity. The activity of a largely debranched pachymaran from Poria cocos, for example, was greater than the natural form (Chihara et al., 1970). Even with the well-studied lentinan, maximal immune-modulating and anticancer activities were attained at a DB of 32%, and there was a negative relationship between biological activity and DB levels between 32 and 40% (Bae et al., 2013).
Polysaccharide conformation, in addition to main-chain structure and branching pattern, may alter bioactivity via changing the structure's stability. Mushroom polysaccharides with a random coil shape may have anticancer and immune-modulating effects (Zhao et al., 2020). Increasing the biological activity of polysaccharides by chemical modification is a common and effective strategy. From mushroom polysaccharides, this approach has been utilized to manufacture a range of immune modulators. These alterations include carboxymethylation, hydroxylation, formyl-methylation, amino-ethylation, and sulfation. The inclusion of such chemical groups might enhance the probable interaction between modified polysaccharides and immune cell receptors through hydrogen bonding and/or electrostatic attraction, hence boosting the immunological response. The sulfated cell wall glucan from Lentinus edodes has stronger immune-modulatory and anticancer effects than native polysaccharides. The enhanced impact of sulfation may be owing to greater solubility, as demonstrated in the hyperbranched glucan TM3b. As a result, the molecular weight, branching, chemical composition, and chemical modification of polysaccharides from mushrooms, when combined, may have a major influence on their bioactivity (Chen et al., 2012b; Zhang & Cheung, 2002).
In recent years, it has been established that mushrooms produce a novel class of protein immune modulators known as FIPs (fungal immune-modulatory protein) (Zhao et al., 2020). The FIPs are capable of a broad variety of tasks. By connecting to Toll-like receptors, it stimulates antigen-presenting cells and produces cytokines like NO and IL-12 (TLRs). By stimulating phosphorylation of p38/MAPK, it may stimulate the proliferation and differentiation of helper T cells (Th0) into Th1 and Th2 cells, activate macrophages and B cells, and enhance the formation of NF-κB. FIP from Flammulina velutipes, for example, may stimulate Th1 cells to create IL-2 and IFN-γ, and therefore perform its immune-modulatory impact via phosphorylating p38/MAPK, which up regulates the expression of intercellular adhesion molecules on the T cell surface (Wang et al., 2004).
FIP, from Volvariella volvacea, can stimulate Th1 cells and enhance IL-2, TNF-α and IFN-γ transformations, as well as induce Th2 cells to generate IL-4, B cell differentiation, immunoglobulin transformation, and antibody IgE development. FIP can also activate other signaling pathways beside p38/MAPK and NF-κB by interacting with TLRs, according to several reports. FIP from Ganoderma tsugae (FIP-gts), for example, can activate the PI3K/Akt signaling pathway and induce human peripheral blood monocytes to produce IFN-γ (Hsiao et al., 2008).
FIPs is present in modest quantities in their native mushrooms, which has impeded its study and adoption. As a consequence, strategies for improving the generation of recombinant FIPs in other species, such as the yeast Pichia pastoris and the bacterium E. coli, are being developed at a fast pace. For example, the LZ-8 gene from Ganoderma lucidum has been expressed in P. pastoris to produce recombinant LZ-8 protein (rLZ-8). Despite the fact that the recombinant protein lacks the carbohydrate component of the natural protein, it has similar IL-2 induction bioactivity. The FIP protein has also been expressed effectively in E. coli (Zhao et al., 2020).
Poria cocos has a long history of medical usage in China. The adjuvant mobility of PCP-II, a polysaccharide from Poria cocos, was studied in mice co-vaccinated with H1N1 flu vaccination and hepatitis B surface antigen (HBsAg). According to the results, PCP-II raised antigen-explicit counteracting agent levels in mice vaccinated with influenza antibodies. PCP-II induced larger titers of anti-HBsAg antibodies than HBsAg-alum vaccinated animals. PCP-II enhanced the production of IL-12p70 and TNF-α in dendritic cells and macrophages, respectively (Rincão et al., 2012). These findings suggested that PCP-II-adjuvanted vaccines improved humoral and cell resistance, which may be used as an adjuvant in both human and animal antibodies (Wu et al., 2016).
The acidic polysaccharide from Cordyceps militaris was reported to increase immunological function by decreased influenza A virus (H1N1) titers in the bronchoalveolar area and lung of mice while raising TNF-α and interferon (IFN) levels in the blood, bronchoalveolar lavage fluid, and lung. It also elevated NO, inducible nitric oxide synthase, interleukin (IL)−10, and proinflammatory cytokines such IL-1β, IL-6, and TNF-α in RAW 264.7 cells (Ohta et al., 2007). According to the study results, acidic polysaccharides seemed to reduce viral infection via immunological strengthening (Hwang et al., 2014).
5. Potential applications of mushrooms to combat COVID-19
The viral load in COVID-19 (severe coronavirus disease) by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection peaks early and past after symptom onset. COVID-19 is marked by altered innate and adaptive immune responses, as well as an atypical cytokine profile and multiorgan system dysfunction that persists long after the virus has been eradicated. As a consequence, a treatment strategy based primarily on antivirals may be inefficient, and antiviral medicines may not be therapeutic later in the disease. Immunomodulatory approaches being studied include corticosteroids, cytokine and anti-cytokine medications, small molecule inhibitors, and cellular therapies (Mingyi et al., 2019).
In a randomized, controlled study, dexamethasone was the only medication that showed a survival advantage for COVID-19 patients. However, it is unknown which patients may benefit the most and if long-term dangers, such as secondary infections, will be present. The immunological dysregulation of severe COVID-19 is discussed here, as well as the existing evidence for medicinal mushrooms' immunomodulatory effects. For a number of reasons, natural polysaccharides, particularly mushroom polysaccharides, have received a lot of attention recently and have become a frequent dietary component (Mingyi et al., 2019).
Various research discussed here showed that mushroom polysaccharides may improve the function of immunological organs and cells, hence boost immunity (Zhang et al., 2020b). As a consequence, discovering a natural immunoregulator in mushrooms to improve immunity is a current scenario. Beside the specific polysaccharides, mushroom biomolecules have previously been shown to have antiviral and anti-inflammatory properties, making them promising candidates for new antiviral therapeutics (Fig. 1 ). As a consequence, natural compounds with the potential to be effective innovative COVID-19 therapies may seem to be attractive sources (Orhan & Deniz, 2020).
Fig. 1.
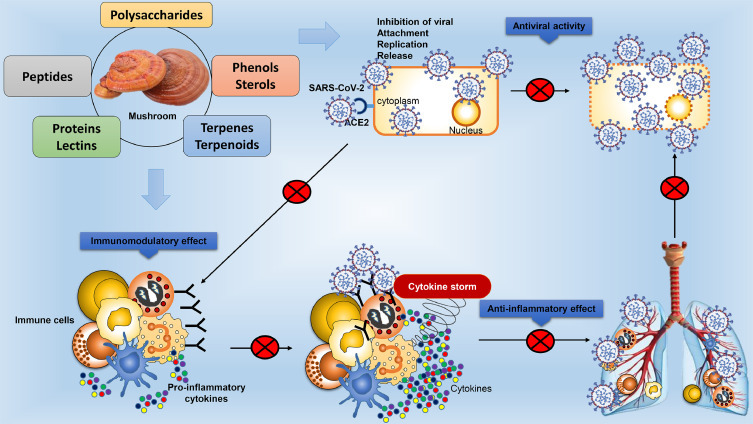
Schematic diagram representing potential candidates originating from mushrooms, such as polysaccharides, lectins, terpenes, polyphenols, and lipids, can be investigated for possession of high degree antiviral, anti-inflammatory and immunomodulatory effects for prevention and therapy of SARS-CoV-2.
Polysaccharide extracts from Cordyceps (Ophiocordyceps sinensis/Cordyceps militaris), Coriolus (Trametes versicolor), Shiitake (Lentinula edodes), and Maitake (Grifola frondosa) mushrooms have all been shown to have an inhibitory effect on flu infection in vivo. Different extracts from Zhu Ling (Polyporus umbellatus), Sun Agaric (Agaricus subrufescens), Reishi (Ganoderma lucidum), and Cordyceps have also shown promise in the treatment of Hepatitis B in clinical trials (Gao et al., 2002; Hsu et al., 2008; Liu et al., 2001; Yan, 1988). In individuals with recurrent genital herpes, supplementing with Coriolus extract resulted in enhanced invulnerability and fewer days off, and clinical research have demonstrated that Coriolus, Reishi, and mixed mushroom polysaccharide derivatives all help to prolong the freedom of high-risk HPV strains (Couto & da Silva, 2008; Donatini, 2014; Kawana & Hashido, 1988).
In an in vivo study, using mushroom extracts to guard against influenza antibodies increased protective invulnerability, while FVe, a protein from Enokitake (Flammulina velutipes), was shown to fundamentally prolong the anti-tumor protection afforded by HPV-16 vaccination in another sample (Ding et al., 2009; Ichinohe et al., 2010). Several mushrooms have chemicals with direct antiviral action, and two in particular stand out for their potential advantages in the present epidemic.
Cordycepin (3′-deoxyadenosine) from Cordyceps organisms has been proven to limit viral replication in a number of investigations (Qin et al., 2019). It also has a powerful calming movement and has been demonstrated to successfully protect the lungs from severe harm caused by the sort of provocative insusceptible response observed in more extreme COVID-19 virus illnesses (Lei et al., 2018). At dosages of 10, 30, and 60 mg/kg/day, Ophiocordyceps sinensis was shown to protect mice against severe lung damage, with higher doses giving more substantial protection (Fu et al., 2019).
Reishi triterpenes have also been demonstrated to have a considerable mitigating impact, inhibiting viral replication and reducing viral replication. Angiotensin-converting enzyme activity has been reported to be inhibited by triterpenes and proteins from Reishi, blocking the conversion of ACE-1 to ACE-2, the sort of chemical via which COVID-19 reaches cells. Although mushrooms clearly aid in the development of our immune systems and the prevention of infection, they are not a cure (Ansor et al., 2013; Morigiwa et al., 1986).
Finally, some interesting mushrooms studied here (Agaricus subrufescens, Agaricus blazei Murill, Cordyceps sinensis, Ganoderma lucidum, Grifola frondosa, Hericium erinaceus, Inonotus obliquus, Lentinula edodes, Pleurotus ostreatus, Poria cocos and Trametes versicolor) also showed pleiotropic effects that could provide a multimodal approach to COVID-19 management through antiviral, anti-inflammatory, and immunomodulatory effects as they had reduced the cytokines production which is considered as most fatal in COVID-19. As a result, dietary supplements and nutraceuticals based on ethnomycological mushroom expertise have showed promise in preventing and treating the present epidemic (Slomski, 2021).
Based on prior experiences with coronavirus outbreaks, seasonal epidemics produced by other viruses, and the efficiency of natural products in the treatment of HIV, HCV, and Influenza, mushrooms and their phytoconstituents might be developed as a possible medication candidate against COVID-19 (Shahzad et al., 2020). Despite this, there is not enough proof of direct antiviral actions specific to the COVID-19 virus. As a result, further study into the anti-viral property with anti-inflammatory and immunomodulatory effects, as well as the quality and safety of herbal drugs, is required to identify their role in COVID-19 therapy. Mushroom derived biochemical compounds must have anti-inflammatory, antioxidant, antiviral, and immunomodulatory effects to be an effective therapy in the treatment of COVID-19. Despite the renin-angiotensin system being involved in COVID-19, with ACE-2 as the main target, to be an effective therapy in the treatment of COVID-19 (Attah et al., 2021; Brendler et al., 2021; Rangsinth et al., 2021).
6. Limitations of the study
-
•
Mushrooms are mostly employed as nutritional supplements or functional meals at the moment. However, special precautions should be taken in terms of preparation, application, dose, and harmful consequences.
-
•
In addition, to establish the mushroom's impact against coronavirus infection, preclinical and clinical laboratory experiments, standardization, and scientific validation must be considered with legal authorization.
-
•
Establishing the safety of medicinal mushroom-derived food items for therapeutic use via the development of clinical study approaches.
-
•
Strengthening the reverse pharmacology process by identifying the primary molecule using cutting-edge technologies.
7. Future perspective and conclusion
The continued proliferation of COVID-19 necessitates the public exposure of alternate therapeutic procedures. Natural remedies have been utilized in a variety of methods since ancient times. They've been dubbed "intense clinical specialists" against a broad spectrum of viral diseases because to their antiviral capabilities. The body's protective frameworks help to fight against infections and pathogens development as well as a number of other disorders. Many allopathic medications are available to assist us to improve our immune systems, but we all know that they come with a lengthy list of negative effects and are costly. As a consequence, we seek for other sources such as Traditional Chinese Medicine and Ayurvedic products containing medicinal mushrooms, which offer a healthy environment for the body while also strengthening the immune system without producing any negative side effects. Several studies have shown that those with excellent immunity have a greater recovery rate in the event of a COVID-19 pandemic. Phytochemicals found in medicinal mushrooms include terpenoids, alkaloids, flavonoids, phenols, tannins, polyphenols, polysaccharides, proteins, lipids, and peptides, all of which have potent antiviral properties in terms of preventing viral invasion, penetration, replication, expression, assembly, and release. Furthermore, since the illness's emergence, restorative medicinal mushrooms and their culinary elements have emerged as the most promising options for preventing or treating infection and disease transmission. Regardless, they are now being evaluated in vitro in order to acquire quick verification of COVID-19 patients' positive strength.
In the present paper, which explained the possible role of natural definitions to treat COVID-19, we outlined the progressing preliminaries of medicinal mushrooms and their biomolecules against this hazardous sickness. Nonetheless, the assays for determining COVID-19 resistance by several medicinal mushroom taxa are inadequate or not pointed out but they are in one or other way effective for its symptoms. Regardless, research is beginning to identify their potential benefits, and we hope that future studies will be undertaken in a more thorough manner. Several researchers are continuing to work on these investigations in order to produce an effective antiviral agent for this virus. Combining these studies with persuasive creativity and study, protein denaturation of receptor proteins and parts of certain proteases chemicals may be regarded in the future as a manner of proving their involvement in obstructing the life pattern of this infection.
CRediT authorship contribution statement
Karuppusamy Arunachalam: Conceptualization, Methodology, Data curation, Writing – original draft. Sreeja Puthanpura Sasidharan: Visualization, Investigation, Writing – review & editing. Xuefei Yang: Supervision, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
KA appreciates the President's International Fellowship from the Chinese Academy of Sciences (PIFI-CAS) for awarded postdoctoral research fellowship (Reference no. 2020PB0112). The Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, funded this research via the herbal medicine inventory and database development project, as well as the "Belt and Road Countries (2018FY100700)" (Y4ZK111B01).
References
- Abubakar M.B., Usman D., Batiha G.E.S., Cruz-Martins N., Malami I., Ibrahim K.G., et al. Natural products modulating angiotensin converting enzyme 2 (ACE2) as potential COVID-19 therapies. Frontiers in Pharmacology. 2021;12:12:–629935. doi: 10.3389/fphar.2021.629935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi Y., Ohno N., Ohsawa M., Oikawa S., Yadomae T. Change of biological activities of (1→ 3)-β-d-glucan from Grifola frondosa upon molecular weight reduction by heat treatment. Chemical and Pharmaceutical Bulletin. 1990;38(2):477–481. doi: 10.1248/cpb.38.477. [DOI] [PubMed] [Google Scholar]
- Adotey G., Quarcoo A., Holliday J., Fofie S., Saaka B. Effect of immunomodulating and antiviral agent of medicinal mushrooms (immune assist 24/7 TM) on CD4+ T-lymphocyte counts of HIV-infected patients. International Journal of Medicinal Mushrooms. 2011;13(2):109–113. doi: 10.1615/IntJMedMushr.v13.i2.20. [DOI] [PubMed] [Google Scholar]
- Akramienė D., Kondrotas A., Didžiapetrienė J., Kėvelaitis E. Effects of ß-glucans on the immune system. Medicina. 2007;43(8):597. [PubMed] [Google Scholar]
- Ali N.A., Lüdtke J., Pilgrim H., Lindequist U. Inhibition of chemiluminescence response of human mononuclear cells and suppression of mitogen-induced proliferation of spleen lymphocytes of mice by hispolon and hispidin. Die Pharmazie. 1996;51(9):667–670. [PubMed] [Google Scholar]
- Ansor N.M., Abdullah N., Aminudin N. Anti-angiotensin converting enzyme (ACE) proteins from mycelia of Ganoderma lucidum (Curtis) P. Karst. BMC Complementary and Alternative Medicine. 2013;13(1):1–8. doi: 10.1186/1472-6882-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attah A.F., Fagbemi A.A., Olubiyi O., Dada-Adegbola H., Oluwadotun A., Elujoba A., et al. Therapeutic potentials of antiviral plants used in traditional African medicine with COVID-19 in focus: A Nigerian perspective. Frontiers in pharmacology. 2021;12 doi: 10.3389/fphar.2021.596855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avtonomova A.V., Krasnopolskaya L.M. Antiviral properties of basidiomycetes metabolites. Antibiotiki i Khimioterapiia= Antibiotics and Chemoterapy [sic] 2014;59(7–8):41–48. [Article in Russian] [PubMed] [Google Scholar]
- Bae I.Y., Kim H.W., Yoo H.J., Kim E.S., Lee S., Park D.Y., et al. Correlation of branching structure of mushroom β-glucan with its physiological activities. Food Research International. 2013;51(1):195–200. doi: 10.1016/j.foodres.2012.12.008. [DOI] [Google Scholar]
- Barbosa J.R., de Carvalho Junior R.N. Polysaccharides obtained from natural edible sources and their role in modulating the immune system: Biologically active potential that can be exploited against COVID-19. Trends in Food Science & Technology. 2021 doi: 10.1016/2Fj.tifs.2020.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj S., Lee K.E., Dwivedi V.D., Yadava U., Panwar A., Lucas S.J., et al. Discovery of Ganoderma lucidum triterpenoids as potential inhibitors against dengue virus NS2B-NS3 protease. Scientific Reports. 2019;9(1):1–12. doi: 10.1038/s41598-019-55723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boh B., Berovic M., Zhang J., Zhi-Bin L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnology Annual Review. 2007;13:265–301. doi: 10.1016/S1387-2656(07)13010-6. [DOI] [PubMed] [Google Scholar]
- Brendler T., Al-Harrasi A., Bauer R., Gafner S., Hardy M.L., Heinrich M., et al. Botanical drugs and supplements affecting the immune response in the time of COVID-19: Implications for research and clinical practice. Phytotherapy Research. 2021;35(6):3013–3031. doi: 10.1002/ptr.7008. [DOI] [PubMed] [Google Scholar]
- Callaway E. COVID vaccine boosters: The most important questions. Nature. 2021;596(7871):178–180. doi: 10.1038/d41586-021-02158-6. [DOI] [PubMed] [Google Scholar]
- Cao X. COVID-19: Immunopathology and its implications for therapy. Nature Reviews Immunology. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo F.T.G.S., Larsen I.V., Carballo E.V., Jose G., Stern R.A., Brummel R.C., et al. In vivo anti-herpes simplex virus activity of a sulfated derivative of Agaricus brasiliensis mycelial polysaccharide. Antimicrobial Agents and Chemotherapy. 2013;57(6):2541–2549. doi: 10.1128/AAC.02250-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisuwan W., Phimolsiripol Y., Chaiyaso T., Techapun C., Leksawasdi N., Jantanasakulwong K., et al. The antiviral activity of bacterial, fungal, and algal polysaccharides as bioactive ingredients: Potential uses for enhancing immune systems and preventing viruses. Frontiers in Nutrition. 2021;8:8–772033. doi: 10.3389/fnut.2021.772033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty I., Sen I.K., Mondal S., Rout D., Bhanja S.K., Maity G.N., et al. Bioactive polysaccharides from natural sources: A review on the antitumor and immunomodulating activities. Biocatalysis and Agricultural Biotechnology. 2019;22 doi: 10.1016/j.bcab.2019.101425. [DOI] [Google Scholar]
- Cheke R.S., Narkhede R.R., Shinde S.D., Ambhore J.P., Jain P.G. Natural product emerging as potential SARS spike glycoproteins-ACE2 inhibitors to combat COVID-19 attributed by in-silico investigations. Biointerface Research in Applied Chemistry. 2021;11:10628–10639. [Google Scholar]
- Chen H., Ju Y., Li J., Yu M. Antioxidant activities of polysaccharides from Lentinus edodes and their significance for disease prevention. International Journal of Biological Macromolecules. 2012;50(1):214–218. doi: 10.1016/j.ijbiomac.2011.10.027. [DOI] [PubMed] [Google Scholar]
- Chen S., Xu J., Liu C., Zhu Y., Nelson D.R., Zhou S., et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nature Csommunications. 2012;3(1):1–9. doi: 10.1038/ncomms1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.Q., Luo S.H., Ll H.Z., Yang H. Effects of Ganoderma lucidum polysaccharides on serum lipids and lipoperoxidation in experimental hyperlipidemic rats. Zhongguo Zhong yao za zhi= Zhongguo Zhongyao zazhi= China Journal of Chinese Materia Medica. 2005;30(17):1358–1360. [PubMed] [Google Scholar]
- Cherian E., Sudheesh N.P., Janardhanan K.K., Patani G. Free radical scavenging and mitochondrial antioxidant activities of Reishi-Ganoderma lucidum (Curt: Fr) P. Karst and Arogyapacha-Trichopus zeylanicus Gaertn extracts. Journal of Basic and Clinical Physiology and Pharmacology. 2009;20(4):289–308. doi: 10.1515/JBCPP.2009.20.4.289. [DOI] [PubMed] [Google Scholar]
- Chihara G., Hamuro J., Maeda Y., Arai Y., Fukuoka F. Antitumour polysaccharide derived chemically from natural glucan (pachyman) Nature. 1970;225(5236):943–944. doi: 10.1038/225943a0. [DOI] [PubMed] [Google Scholar]
- Collins R.A., Ng T.B. Polysaccharopeptide from Coriolus versicolor has potential for use against human immunodeficiency virus type 1 infection. Life Sciences. 1997;60(25):PL383–PL387. doi: 10.1016/S0024-3205(97)00294-4. [DOI] [PubMed] [Google Scholar]
- Conti P., Ronconi G., Caraffa A.L., Gallenga C.E., Ross R., Frydas I., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. Journal of Biological Regulators & Homeostatic Agents. 2020;34(2):1. doi: 10.23812/conti-e. [DOI] [PubMed] [Google Scholar]
- Couto J.S., da Silva D.P. Proceedings of the 20th European congress of obstetrics and gynaecology. 2008. Coriolus versicolor supplementation in HPV patients. [Poster] March 7th. [Google Scholar]
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nature Reviews Microbiology. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Antonio A., Wiedemann L.S.M., Veiga-Junior V.F. Natural products' role against COVID-19. RSC Advances. 2020;10(39):23379–23393. doi: 10.1039/d0ra03774e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sousa Cardozo, Camelini C.M., Leal P.C., Kratz J.M.…Nunes R.J., et al. Antiherpetic mechanism of a sulfated derivative of Agaricus brasiliensis fruiting bodies polysaccharide. Intervirology. 2014;57:375–383. doi: 10.1159/000365194. [DOI] [PubMed] [Google Scholar]
- Del Valle D.M., Kim-Schulze S., Huang H.H., Beckmann N.D., Nirenberg S., Wang B., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nature Medicine. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Seow S.V., Huang C.H., Liew L.M., Lim Y.C., Kuo I.C., et al. Coadministration of the fungal immunomodulatory protein FIP-Fve and a tumour-associated antigen enhanced antitumour immunity. Immunology. 2009;128(1pt2):e881–e894. doi: 10.1111/j.1365-2567.2009.03099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divya M., Vijayakumar S., Chen J., Vaseeharan B., Durán-Lara E.F. South Indian medicinal plants can combat deadly viruses along with COVID-19?-A review. Microbial Pathogenesis. 2020;148 doi: 10.1016/j.micpath.2020.104277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doğan H.H., Karagöz S., Duman R. In vitro evaluation of the antiviral activity of some mushrooms from Turkey. International Journal of Medicinal Mushrooms. 2018;20(3) doi: 10.1615/IntJMedMushrooms.2018025468. [DOI] [PubMed] [Google Scholar]
- Donatini B. Control of oral human papillomavirus (HPV) by medicinal mushrooms, trametes versicolor and Ganoderma lucidum: A preliminary clinical trial. International Journal of Medicinal Mushrooms. 2014;16(5):497–498. doi: 10.1615/IntJMedMushrooms.v16.i5.80. [DOI] [PubMed] [Google Scholar]
- Eguchi N., Fujino K., Thanasut K., Taharaguchi M., Motoi M., Motoi A., et al. In vitro anti-influenza virus activity of Agaricus brasiliensis KA21. Biocontrol Science. 2017;22(3):171–174. doi: 10.4265/bio.22.171. [DOI] [PubMed] [Google Scholar]
- Ellan K., Thayan R., Raman J., Hidari K.I., Ismail N., Sabaratnam V. Anti-viral activity of culinary and medicinal mushroom extracts against dengue virus serotype 2: An in-vitro study. BMC Complementary and Alternative Medicine. 2019;19(1):1–12. doi: 10.1186/s12906-019-2629-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mekkawy S., Meselhy M.R., Nakamura N., Tezuka Y., Hattori M., Kakiuchi N., et al. Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum. Phytochemistry. 1998;49(6):1651–1657. doi: 10.1016/S0031-9422(98)00254-4. [DOI] [PubMed] [Google Scholar]
- Eo S.K., Kim Y.S., Lee C.K., Han S.S. Antiviral activities of various water and methanol soluble substances isolated from Ganoderma lucidum. Journal of Ethnopharmacology. 1999;68(1–3):129–136. doi: 10.1016/S0378-8741(99)00067-7. [DOI] [PubMed] [Google Scholar]
- Eo S.K., Kim Y.S., Lee C.K., Han S.S. Possible mode of antiviral activity of acidic protein bound polysaccharide isolated from Ganoderma lucidum on herpes simplex viruses. Journal of Ethnopharmacology. 2000;72(3):475–481. doi: 10.1016/S0378-8741(00)00266-X. [DOI] [PubMed] [Google Scholar]
- Faccin L.C., Benati F., Rincão V.P., Mantovani M.S., Soares S.A., Gonzaga M.L., et al. Antiviral activity of aqueous and ethanol extracts and of an isolated polysaccharide from Agaricus brasiliensis against poliovirus type 1. Letters in Applied Microbiology. 2007;45(1):24–28. doi: 10.1111/j.1472-765X.2007.02153.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2022). Weekly epidemiological update on COVID-19 - 25 January 2022 [WWW Document], n.d. URL https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---25-january-2022 (accessed 3.7.22).
- Fauci, A.S., Lane, H.C., & Redfield, R.R. (2020). COVID-19-navigating the uncharted. 382:1268–1269. doi: 10.1056/NEJMe2002387 [DOI] [PMC free article] [PubMed]
- Fu S., Lu W., Yu W., Hu J. Protective effect of Cordyceps sinensis extract on lipopolysaccharide-induced acute lung injury in mice. Bioscience Reports. 2019;39(6) doi: 10.1042/BSR20190789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Zhou S., Chen G., Dai X., Ye J., Gao H. A phase I/II study of a Ganoderma lucidum (Curt.: Fr.) P. Karst.(Ling Zhi, Reishi Mushroom) extract in patients with chronic hepatitis В. International Journal of Medicinal Mushrooms. 2002;4(4) doi: 10.1615/IntJMedMushr.v4.i4.50. [DOI] [Google Scholar]
- Gonçalves J.L., Roma E.H., Gomes-Santos A.C., Aguilar E.C., Cisalpino D., Fernandes L.R., et al. Pro-inflammatory effects of the mushroom Agaricus blazei and its consequences on atherosclerosis development. European Journal of Nutrition. 2012;51(8):927–937. doi: 10.1007/s00394-011-0270-8. [DOI] [PubMed] [Google Scholar]
- Gu C.Q., Li J.W., Chao F.H. Inhibition of hepatitis B virus by D-fraction from Grifola frondosa: Synergistic effect of combination with interferon-α in HepG2 2.2. 15. Antiviral research. 2006;72(2):162–165. doi: 10.1016/j.antiviral.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Gu C.Q., Li J.W., Chao F., Jin M., Wang X.W., Shen Z.Q. Isolation, identification and function of a novel anti-HSV-1 protein from Grifola frondosa. Antiviral Research. 2007;75(3):250–257. doi: 10.1016/j.antiviral.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Harris M., Bagozzi D. WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19 [WWW Document], n.d. URL https://www.who.int/news/item/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19 (accessed 3.7.22) World Heal Organ News Releas. 2020 [Google Scholar]
- Hazama S., Oka M., Yoshino S., Iizuka N., Wadamori K., Yamamoto K., Masaki Y. Clinical effects and immunological analysis of intraabdominal and intrapleural injection of lentinan for malignant ascites and pleural effusion of gastric carcinoma. Gan to Kagaku Ryoho. Cancer & Chemotherapy. 1995;22(1):1595–1597. [PubMed] [Google Scholar]
- Hetland G., Johnson E., Bernardshaw S.V., Grinde B. Can medicinal mushrooms have prophylactic or therapeutic effect against COVID-19 and its pneumonic superinfection and complicating inflammation? Scandinavian Journal of Immunology. 2021;93(1):e12937. doi: 10.1111/sji.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao Y.M., Huang Y.L., Tang S.C., Shieh G.J., Lai J.Y., Wang P.H., et al. Effect of a fungal immunomodulatory protein from Ganoderma tsugae on cell cycle and interferon-gamma production through phosphatidylinositol 3-kinase signal pathway. Process Biochemistry. 2008;43(4):423–430. doi: 10.1016/j.procbio.2008.01.005. [DOI] [Google Scholar]
- Hsu C.H., Hwang K.C., Chiang Y.H., Chou P. The mushroom Agaricus blazei Murill extract normalizes liver function in patients with chronic hepatitis B. The Journal of Alternative and Complementary Medicine. 2008;14(3):299–301. doi: 10.1089/acm.2006.6344. [DOI] [PubMed] [Google Scholar]
- Hwang B.S., Lee I., Choi H.J., Yun B. Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg. Med. Chem. Lett. 2015;25:3256–3260. doi: 10.1016/j.bmcl.2015.05.081. [DOI] [PubMed] [Google Scholar]
- Hwang B.S., Lee M.S., Lee S.W., Lee I.K., Seo G.S., Choi H.J., et al. Neuraminidase inhibitors from the fermentation broth of Phellinus linteus. Mycobiology. 2014;42(2):189–192. doi: 10.5941/MYCO.2014.42.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T., Ainai A., Nakamura T., Akiyama Y., Maeyama J.I., Odagiri T., et al. Induction of cross-protective immunity against influenza A virus H5N1 by an intranasal vaccine with extracts of mushroom mycelia. Journal of Medical Virology. 2010;82(1):128–137. doi: 10.1002/jmv.21670. [DOI] [PubMed] [Google Scholar]
- Ilyicheva T.N., Teplyakova T.V., Svyatchenko S.V., Asbaganov S.V., Zmitrovich I.V., Vlasenko A.V. Antiviral activity of total polysaccharide fraction of water and ethanol extracts of Pleurotus pulmonarius against the influenza A virus. Current Research in Environmental & Applied Mycology (Journal of Fungal Biology) 2020;10(1):224–235. doi: 10.5943/cream/10/1/22. [DOI] [Google Scholar]
- Ina K., Kataoka T., Ando T. The use of lentinan for treating gastric cancer. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 2013;13(5):681–688. doi: 10.2174/1871520611313050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y.T., Yang B.K., Jeong S.C., Kim S.M., Song C.H. Ganoderma applanatum: A promising mushroom for antitumor and immunomodulating activity. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2008;22(5):614–619. doi: 10.1002/ptr.2294. [DOI] [PubMed] [Google Scholar]
- Joshi G., Sindhu J., Thakur S., Rana A., Sharma G., Poduri R. Recent efforts for drug identification from phytochemicals against SARS-CoV-2: Exploration of the chemical space to identify druggable leads. Food and Chemical Toxicology. 2021;152 doi: 10.1016/j.fct.2021.112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawana T., Hashido M. Treatment of recurrent genital herpes with PSK. International Journal of Immunopharmacology. 1988;10:152. [Google Scholar]
- Khadke S., Ahmed N., Ahmed N., Ratts R., Raju S., Gallogly M., et al. Harnessing the immune system to overcome cytokine storm and reduce viral load in COVID-19: A review of the phases of illness and therapeutic agents. Virology Journal. 2020;17(1):1–18. doi: 10.1186/s12985-020-01415-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble C., Coustasse A., Maxik K. Considerations on the distribution and administration of the new COVID-19 vaccines. International Journal of Healthcare Management. 2021;14(1):306–310. [Google Scholar]
- Krupodorova T., Rybalko S., Barshteyn V. Antiviral activity of Basidiomycete mycelia against influenza type A (serotype H1N1) and herpes simplex virus type 2 in cell culture. Virologica Sinica. 2014;29(5):284–290. doi: 10.1007/s12250-014-3486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki T., Lee S., Hirohama M., Taku T., Kumakura M., Haruyama T.…Kawaguchi Inhibition of influenza virus infection by Lentinus edodes mycelia extract through its direct action and immunopotentiating activity. Frontiers in microbiology. 2018;9:1164. doi: 10.3389/fmicb.2018.01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Chen I.T., Chao C.M., Lee P.I., Ko W.C., Hsueh P.R. COVID-19 vaccines: Concerns beyond protective efficacy and safety. Expert Review of Vaccines. 2021;20(8):1013–1025. doi: 10.1080/14760584.2021.1949293. [DOI] [PubMed] [Google Scholar]
- Lee H.H., Lee J.S., Cho J.Y., Kim Y.E., Hong E.K. Study on immunostimulating activity of macrophage treated with purified polysaccharides from liquid culture and fruiting body of Lentinus edodes. Journal of Microbiology and Biotechnology. 2009;19(6):566–572. doi: 10.4014/jmb.0809.541. [DOI] [PubMed] [Google Scholar]
- Lee I.K., Yun B.S., Cho S.M., Kim W.G., Kim J.P., Ryoo I.J., Yoo I.D. Betulinans A and B, two benzoquinone compounds from Lenzites betulina. Journal of Natural Products. 1996;59(11):1090–1092. doi: 10.1021/np960253z. [DOI] [PubMed] [Google Scholar]
- Lee S.M., Kim S.M., Lee Y.H., Kim W.J., Park J.K., Park Y.I.…Synytsya A. Macromolecules isolated from Phellinus pini fruiting body: chemical characterization and antiviral activity. Macromolecular Research. 2010;18(6):602–609. [Google Scholar]
- Lei J., Wei Y., Song P., Li Y., Zhang T., Feng Q., et al. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. European Journal of Pharmacology. 2018;818:110–114. doi: 10.1016/j.ejphar.2017.10.029. [DOI] [PubMed] [Google Scholar]
- Li Y.R., Liu Q.H., Wang H.X., Ng T.B. A novel lectin with potent antitumor, mitogenic and HIV-1 reverse transcriptase inhibitory activities from the edible mushroom Pleurotus citrinopileatus. Biochimica et Biophysica Acta (BBA)-General Subjects. 2008;1780(1):51–57. doi: 10.1016/j.bbagen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Li Z, Liu J, Zhao Y. Possible mechanism underlying the antiherpetic activity of a proteoglycan isolated from the mycelia of Ganoderma lucidum in vitro. J Biochem Mol Biol. 2005;38(1):34–40. doi: 10.5483/bmbrep.2005.38.1.034. [DOI] [PubMed] [Google Scholar]
- Liu J., McIntosh H., Lin H. Chinese medicinal herbs for chronic hepatitis B: A systematic review. Liver. 2001;21(4):280–286. doi: 10.1034/j.1600-0676.2001.021004280.x. [DOI] [PubMed] [Google Scholar]
- Liu J., Yang F., Ye L.B., Yang X.J., Timani K.A., Zheng Y.I., Wang Y.H. Possible mode of action of antiherpetic activities of a proteoglycan isolated from the mycelia of Ganoderma lucidum in vitro. Journal of ethnopharmacology. 2004;95(2–3):265–272. doi: 10.1016/j.jep.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Liua W.K., Ho J.C.K., Ng T.B. Suppression of Cell Cycle Progression by a Fungal Lectin: Activation of Cyclin-dependent Kinase Inhibitors. Biochem. Pharmacol. 2001;61:33–37. doi: 10.1016/s0006-2952(00)00533-5. [DOI] [PubMed] [Google Scholar]
- Lopez-Tejedor D., Clavería-Gimeno R., Velazquez-Campoy A., Abian O., Palomo J.M. Tyrosinase from mushroom Agaricus bisporus as an inhibitor of the Hepatitis C virus. bioRxiv. 2021:2020–2022. doi: 10.3390/ph14080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H., Kong Y., Yao Q., Zhang B., Leng F.W., Bian H.J., et al. Nebrodeolysin, a novel hemolytic protein from mushroom Pleurotus nebrodensis with apoptosis-inducing and anti-HIV-1 effects. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2009;16(2–3):198–205. doi: 10.1016/j.phymed.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Lv H., Kong Y., Yao Q., Zhang B., Leng F., Bian H.…Bao J. Nebrodeolysin, a novel hemolytic protein from mushroom Pleurotus nebrodensis with apoptosis-inducing and anti-HIV-1 effects. Phytomedicine. 2009;16:198–205. doi: 10.1016/j.phymed.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Minari M.C., Rincão V.P., Soares S.A., Ricardo N.M.P.S., Nozawa C., Linhares R.E.C. Antiviral properties of polysaccharides from Agaricus brasiliensis in the replication of bovine herpesvirus 1. Acta Virologica. 2011;55(3):255–259. doi: 10.4149/av_2011_03_255. [DOI] [PubMed] [Google Scholar]
- Mingyi Y., Belwal T., Devkota H.P., Li L., Luo Z. Trends of utilizing mushroom polysaccharides (MPs) as potent nutraceutical components in food and medicine: A comprehensive review. Trends in Food Science & Technology. 2019;92:94–110. doi: 10.1016/j.tifs.2019.08.009. [DOI] [Google Scholar]
- Morigiwa A., Kitabatake K., Fujimoto Y., Ikekawa N. Angiotensin converting enzyme-inhibitory triterpenes from Ganoderma lucidim. Chemical and Pharmaceutical Bulletin. 1986;34(7):3025–3028. doi: 10.1248/cpb.34.3025. [DOI] [PubMed] [Google Scholar]
- Mothana R.A.A., Ali N.A., Jansen R., Wegner U., Mentel R., Lindequist U. Antiviral lanostanoid triterpenes from the fungus Ganoderma pfeifferi. Fitoterapia. 2003;74(1–2):177–180. doi: 10.1016/s0367-326x(02)00305-2. [DOI] [PubMed] [Google Scholar]
- Murphy E.J., Masterson C., Rezoagli E., O'Toole D., Major I., Stack G.D., et al. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects-implications for coronavirus disease (COVID-19) immunotherapies. Science of the Total Environment. 2020;732 doi: 10.1016/j.scitotenv.2020.139330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba H., Kodama N., Schar D., Turner D. Effects of maitake (Grifola frondosa) glucan in HIV-infected patients. Mycoscience. 2000;41(4):293–295. [Google Scholar]
- Ngai P.H., Ng T.B. Lentin, a novel and potent antifungal protein from shitake mushroom with inhibitory effects on activity of human immunodeficiency virus-1 reverse transcriptase and proliferation of leukemia cells. Life Sciences. 2003;73(26):3363–3374. doi: 10.1016/j.lfs.2003.06.023. [DOI] [PubMed] [Google Scholar]
- Niedermeyer T.H., Lindequist U., Mentel R., Gördes D., Schmidt E., Thurow K., Lalk M. Antiviral Terpenoid constituents of Ganoderma p feifferi. Journal of Natural Products. 2005;68(12):1728–1731. doi: 10.1021/np0501886. [DOI] [PubMed] [Google Scholar]
- Nugraha R.V., Ridwansyah H., Ghozali M., Khairani A.F., Atik N. Traditional herbal medicine candidates as complementary treatments for COVID-19: A review of their mechanisms, pros and cons. Evidence-Based Complementary and Alternative Medicine. 2020;2020::–2560645. doi: 10.1155/2020/2560645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y., Lee J.B., Hayashi K., Fujita A., Park D.K., Hayashi T. In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. Journal of Agricultural and Food Chemistry. 2007;55(25):10194–10199. doi: 10.1021/jf0721287. [DOI] [PubMed] [Google Scholar]
- Orhan I.E., Deniz F.S.S. Natural products as potential leads against coronaviruses: Could they be encouraging structural models against SARS-CoV-2? Natural Products and Bioprospecting. 2020;10(4):171–186. doi: 10.1007/s13659-020-00250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H.H., Yu X.T., Li T., Wu H.L., Jiao C.W., Cai M.H., Peng T. Aqueous extract from a Chaga medicinal mushroom, Inonotus obliquus (higher basidiomyetes), prevents herpes simplex virus entry through inhibition of viral-induced membrane fusion. International journal of medicinal mushrooms. 2013;15(1):29–38. doi: 10.1615/intjmedmushr.v15.i1.40. [DOI] [PubMed] [Google Scholar]
- Patwardhan B., Warude D., Pushpangadan P., Bhatt N. Ayurveda and traditional Chinese medicine: A comparative overview. Evidence-Based Complementary and Alternative Medicine. 2005;2(4):465–473. doi: 10.1093/ecam/neh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelizon A.C., Kaneno R., Soares A.M.V.C., Meira D.A., Sartori A. Immunomodulatory activities associated with β-glucan derived from Saccharomyces cerevisiae. Physiological Research. 2005;54(5):557–564. [PubMed] [Google Scholar]
- Pergolizzi J.V., LeQuang J.A., Magnusson P., Varrassi G. Traditional, complementary and integrative medicine approaches to COVID-19: A narrative review. OBM Integrative and Complementary Medicine. 2021;6(3):1. [Google Scholar]
- Pohleven J., Obermajer N., Sabotič J., Anžlovar S., Sepčić K., Kos J., et al. Purification, characterization and cloning of a ricin B-like lectin from mushroom Clitocybe nebularis with antiproliferative activity against human leukemic T cells. Biochimica et Biophysica Acta (BBA)-General Subjects. 2009;1790(3):173–181. doi: 10.1016/j.bbagen.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohleven J., Renko M., Magister Š., Smith D.F., Künzler M., Štrukelj B., et al. Bivalent carbohydrate binding is required for biological activity of Clitocybe nebularis lectin (CNL), the N, N′-Diacetyllactosediamine (GalNAcβ1–4GlcNAc, LacdiNAc)-specific lectin from basidiomycete C. nebularis. Journal of Biological Chemistry. 2012;287(13):10602–10612. doi: 10.1074/jbc.M111.317263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P., Li X., Yang H., Wang Z.Y., Lu D. Therapeutic potential and biological applications of cordycepin and metabolic mechanisms in cordycepin-producing fungi. Molecules. 2019;24(12):2231. doi: 10.3390/molecules24122231. (Basel, Switzerland) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qomara W.F., Primanissa D.N., Amalia S.H., Purwadi F.V., Zakiyah N. Effectiveness of remdesivir, lopinavir/ritonavir, and favipiravir for COVID-19 treatment: A systematic review. International Journal of General Medicine. 2021;14:8557. doi: 10.2147/IJGM.S332458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangsinth P., Sillapachaiyaporn C., Nilkhet S., Tencomnao T., Ung A.T., Chuchawankul S. Mushroom-derived bioactive compounds potentially serve as the inhibitors of SARS-CoV-2 main protease: An in silico approach. Journal of Traditional and Complementary Medicine. 2021;11(2):158–172. doi: 10.1016/j.jtcme.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., Xu L., Lu T., Yin J. Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. International journal of biological macromolecules. 2018;115:1202–1210. doi: 10.1016/j.ijbiomac.2018.04.132. [DOI] [PubMed] [Google Scholar]
- Rincão V.P., Yamamoto K.A., Ricardo N.M.P.S., Soares S.A., Meirelles L.D.P., Nozawa C., et al. Polysaccharide and extracts from Lentinula edodes: Structural features and antiviral activity. Virology Journal. 2012;9(1):1–6. doi: 10.1186/1743-422X-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Aoki F., Hirai H., Inagaki T., Matsunaga Y., Sakakibara T., et al. Erinacine E as a kappa opioid receptor agonist and its new analogs from a basidiomycete, Hericium ramosum. The Journal of Antibiotics. 1998;51(11):983–990. doi: 10.7164/antibiotics.51.983. [DOI] [PubMed] [Google Scholar]
- Sato M., Tai T., Nunoura Y., Yajima Y., Kawashima S., Tanaka K. Dehydrotrametenolic acid induces preadipocyte differentiation and sensitizes animal models of noninsulin-dependent diabetes mellitus to insulin. Biological and Pharmaceutical Bulletin. 2002;25(1):81–86. doi: 10.1248/bpb.25.81. [DOI] [PubMed] [Google Scholar]
- Senthil Kumar K.J., Gokila Vani M., Hsieh H.W., Lin C.C., Wang S.Y. Antcins from antrodia cinnamomea and antrodia salmonea inhibit angiotensin-converting enzyme 2 (ACE2) in epithelial cells: Can be potential candidates for the development of SARS-CoV-2 prophylactic agents. Plants. 2021;10(8):1736. doi: 10.3390/plants10081736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D.J., Choi C. Antiviral bioactive compounds of mushrooms and their antiviral mechanisms: A review. Viruses. 2021;13(2):350. doi: 10.3390/v13020350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad F., Anderson D., Najafzadeh M. The antiviral, anti-inflammatory effects of natural medicinal herbs and mushrooms and SARS-CoV-2 infection. Nutrients. 2020;12(9):2573. doi: 10.3390/nu12092573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibnev V.A., Garaev T.M., Finogenova M.P., Kalnina L.B., Nosik D.N. Antiviral activity of aqueous extracts of the birch fungus Inonotus obliquus on the human immunodeficiency virus. Voprosy Virusologii. 2015;60(2):35–38. [PubMed] [Google Scholar]
- Shibnev V.A., Mishin D.V., Garaev T.M., Finogenova N.P., Botikov A.G., Deryabin P.G. Antiviral activity of Inonotus obliquus fungus extract towards infection caused by hepatitis C virus in cell cultures. Bulletin of Experimental Biology and Medicine. 2011;151(5):612. doi: 10.1007/s10517-011-1395-8. [DOI] [PubMed] [Google Scholar]
- Slomski A. Trials test mushrooms and herbs as anti–COVID-19 agents. JAMA. 2021;326(20):1997–1999. doi: 10.1001/jama.2021.19388. [DOI] [PubMed] [Google Scholar]
- Smania E., Delle Monache F., Smania A., Yunes R.A., Cuneo R.S. Antifungal activity of sterols and triterpenes isolated from Ganoderma annulare. Fitoterapia. 2003;74(4):375–377. doi: 10.1016/s0367-326x(03)00064-9. [DOI] [PubMed] [Google Scholar]
- Song A., Sun X., Kong C., Zhao C., Qin D., Huang F., Yang S. Discovery of a new sesquiterpenoid from Phellinus ignarius with antiviral activity against influenza virus. Arch. Virol. 2014;159:753–760. doi: 10.1007/s00705-013-1857-6. [DOI] [PubMed] [Google Scholar]
- Sorimachi K., Akimoto K., Ikehara Y., Inafuku K., Okubo A., Yamazaki S. Secretion of TNF-α, IL-8 and nitric oxide by macrophages activated with Agaricus blazei Murill fractions in vitro. Cell structure and function. 2001;26(2):103–108. doi: 10.1247/csf.26.103. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Suzuki C., Shimomura E., Maeda H., Fujii T., Ishida N. Antiviral and interferon-inducing activities of a new peptidomannan, KS-2, extracted from culture mycelia of Lentinus edodes. The Journal of Antibiotics. 1979;32(12):1336–1345. doi: 10.7164/antibiotics.32.1336. [DOI] [PubMed] [Google Scholar]
- Szallasi A., Biro T., Szabo T., Modarres S., Petersen M., Klusch A., et al. A non-pungent triprenyl phenol of fungal origin, scutigeral, stimulates rat dorsal root ganglion neurons via interaction at vanilloid receptors. British Journal of Pharmacology. 1999;126:1351–1358. doi: 10.1038/sj.bjp.0702440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Hu X., Liu D., Wu H., Qu L. Identification of inonotus obliquus polysaccharide with broad-spectrum antiviral activity against multi-feline viruses. International Journal of Biological Macromolecules. 2017;95:160–167. doi: 10.1016/j.ijbiomac.2016.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velavan T.P., Meyer C.G. The COVID-19 epidemic. Tropical Medicine & International Health. 2020;25(3):278. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.R., Ng T.B., Li L., Fang J.C., Jiang Y., Wen T.Y., et al. Isolation of a polysaccharide with antiproliferative, hypoglycemic, antioxidant and HIV-1 reverse transcriptase inhibitory activities from the fruiting bodies of the abalone mushroom Pleurotus abalonus. Journal of Pharmacy and Pharmacology. 2011;63(6):825–832. doi: 10.1111/j.2042-7158.2011.01274.x. [DOI] [PubMed] [Google Scholar]
- Wang H.X., Ng T.B. Isolation of a novel ubiquitin-like protein from Pleurotus ostreatus mushroom with anti-human immunodeficiency virus, translation-inhibitory, and ribonuclease activities. Biochemical and Biophysical Research Communications. 2000;276(2):587–593. doi: 10.1006/bbrc.2000.3540. [DOI] [PubMed] [Google Scholar]
- Wang H.X., Ng T.B. Isolation of a novel ubiquitin-like protein from Pleurotus ostreatus mushroom with anti-human immunodeficiency virus, translation-inhibitory, and ribonuclease activities. Biochemical and Biophysical Research Communications. 2000;276(2):587–593. doi: 10.1006/bbrc.2000.3540. [DOI] [PubMed] [Google Scholar]
- Wang H.X., Ng T.B. Purification of a novel low-molecular-mass laccase with HIV-1 reverse transcriptase inhibitory activity from the mushroom Tricholoma giganteum. Biochemical and Biophysical Research Communications. 2004;315(2):450–454. doi: 10.1016/j.bbrc.2004.01.064. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang H.X., Ng T.B. A peptide with HIV-1 reverse transcriptase inhibitory activity from the medicinal mushroom Russula paludosa. Peptides. 2007;28(3):560–565. doi: 10.1016/j.peptides.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Wang P.H., Hsu C.I., Tang S.C., Huang Y.L., Lin J.Y., Ko J.L. Fungal immunomodulatory protein from Flammulina velutipes induces interferon-γ production through p38 mitogen-activated protein kinase signaling pathway. Journal of Agricultural and Food Chemistry. 2004;52(9):2721–2725. doi: 10.1021/jf034556s. [DOI] [PubMed] [Google Scholar]
- Wolter, N., Jassat, W., Walaza, S., Welch, R., Moultrie, H., Groome, M. et al. (2021). Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa. medRxiv 2021.12.21.21268116; 10.1101/2021.12.21.21268116 [DOI] [PMC free article] [PubMed]
- World Health Organization. (2021). WHO-convened global study of origins of SARS-CoV-2: China Part [WWW Document], n.d. URL https://www.who.int/publications-detail-redirect/who-convened-global-study-of-origins-of-sars-cov-2-china-part (accessed 3.7.22).
- World Health Organization . Origin of SARS-CoV-2, 26 March 2020. World Health Organization. https://apps.who.int/iris/handle/10665/332197. License: CC BY-NC-SA 3.0 IGO. World Health Organization; 2020. Origin of sars-CoV-2. 26 march 2020. no. WHO/2019-nCoV/FAQ/Virus_origin/2020.1. [Google Scholar]
- Wu Y., Jiang H., Zhu E., Li J., Wang Q., Zhou W., et al. Hericium erinaceus polysaccharide facilitates restoration of injured intestinal mucosal immunity in Muscovy duck reovirus-infected Muscovy ducklings. International Journal of Biological Macromolecules. 2018;107:1151–1161. doi: 10.1016/j.ijbiomac.2017.09.092. [DOI] [PubMed] [Google Scholar]
- Wu Y., Li S., Li H., Zhao C., Ma H., Zhao X., et al. Effect of a polysaccharide from Poria cocos on humoral response in mice immunized by H1N1 influenza and HBsAg vaccines. International Journal of Biological Macromolecules. 2016;91:248–257. doi: 10.1016/j.ijbiomac.2016.05.046. [DOI] [PubMed] [Google Scholar]
- Xiaoni C., Pengxiang W., Zhun W. Emergency use of COVID-19 vaccines recommended by the World Health Organization (WHO) as of June 2021. Drug Discoveries & Therapeutics. 2021;1:P1–P3. doi: 10.5582/ddt.2021.01064. [DOI] [PubMed] [Google Scholar]
- Yamamoto K.A., Galhardi L.C.F., Rincão V.P., de Aguiar Soares S., Vieira Í.G.P., Ricardo N.M.P.S., et al. Antiherpetic activity of an Agaricus brasiliensis polysaccharide, its sulfated derivative and fractions. International Journal of Biological Macromolecules. 2013;52:9–13. doi: 10.1016/j.ijbiomac.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Yan N., He F., Piraino F.F., Xiang H., Chen J., Wang Y., Liu X. Antiviral activity of a cloned peptide RC28 isolated from the higher basidiomycetes mushroom Rozites caperata in a mouse model of HSV-1 keratitis. Int. J. Med. Mushrooms. 2015;17:819–828. doi: 10.1615/intjmedmushrooms.v17.i9.20. [DOI] [PubMed] [Google Scholar]
- Yan S.C. Clinical and experimental research on Polyporus umbellatus polysaccharide in the treatment of chronic viral hepatitis. Zhong xi yi jie he za zhi= Chinese Journal of Modern Developments in Traditional Medicine. 1988;8(3):141–143. [PubMed] [Google Scholar]
- Yin Z., Liang Z., Li C., Wang J., Ma C., Kang W. Immunomodulatory effects of polysaccharides from edible fungus: A review. Food Science and Human Wellness. 2021;10(4):393–400. [Google Scholar]
- Zhang P., Cheung P.C. Evaluation of sulfated Lentinus edodes α-(1→ 3)-d-glucan as a potential antitumor agent. Bioscience, Biotechnology, and Biochemistry. 2002;66(5):1052–1056. doi: 10.1271/bbb.66.1052. [DOI] [PubMed] [Google Scholar]
- Zhang W., Tao J., Yang X., Yang Z., Zhang L., Liu H.…Wu J. Antiviral effects of two Ganoderma lucidum triterpenoids against enterovirus 71 infection. Biochemical and biophysical research communications. 2014;449(3):307–312. doi: 10.1016/j.bbrc.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Zhang W.N., Gong L.L., Liu Y., Zhou Z.B., Wan C.X., Xu J.J., et al. Immunoenhancement effect of crude polysaccharides of Helvella leucopus on cyclophosphamide-induced immunosuppressive mice. Journal of Functional Foods. 2020;69 doi: 10.1016/j.jff.2020.103942. [DOI] [Google Scholar]
- Zhang X., Tan Y., Ling Y., Lu G., Liu F., Yi Z., et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2021). https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html (accessed 3.7.22).
- Zhao, C., Gao, L., Wang, C., Liu, B., Jin, Y., & Xing, Z. (2016). Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydrate Polymers, 144, 382–389. 10.1016/j.carbpol.2015.12.005 [DOI] [PubMed]
- Zhao S., Gao Q., Rong C., Wang S., Zhao Z., Liu Y., et al. Immunomodulatory effects of edible and medicinal mushrooms and their bioactive immunoregulatory products. Journal of Fungi. 2020;6(4):269. doi: 10.3390/jof6040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Ma L., Hu Q., Li J., Chen Y., Jia R., et al. In vitro anti-HIV-1 activity of cordyceps sinensis extracts. Bing Du Xue Bao= Chinese Journal of Virology. 2016;32(4):417–422. [PubMed] [Google Scholar]
- Filippova E.I., Mazurkova N.A., Kabanov A.S., Teplyakova T.V., Ibragimova Z.B., Makarevich E.V.…Shishkina L.N. Antiviral Properties Of Aqueous Extracts Isolated From Higher Basidiomycetes As Respect To Pandemic Influenza Virus А (Шш) 2009. Biological Science. 2013;130 [Google Scholar]
- Santoyo, S., Ramírez-Anguiano, A.C., Aldars-García, L., Reglero, G. and Soler-Rivas, C., 2012. Antiviral activities of Boletus edulis, Pleurotus ostreatus and Lentinus edodes extracts and polysaccharide fractions against Herpes simplex virus type 1. Journal of food and nutrition research. 51. 4, 225-235


