Abstract
I. Introduction
Torsemide is a loop diuretic that inhibits the Na+/K+/2Cl− cotransporter type 2 in the thick ascending loop of Henle, leading to increased excretion of urinary sodium and chloride and associated diuresis. While furosemide remains the dominant diuretic utilized in current practice, increasing evidence supports potential advantages of torsemide in heart failure (HF) and/or renal disease.
II. Areas covered
This narrative review covers the evidence for use of torsemide in HF and renal disease. Comparative effectiveness with regards to clinical outcomes is reviewed, as well as the ongoing multicenter trial, TRANSFORM-HF, comparing the effect of torsemide versus furosemide among patients with HF.
III. Expert opinion
Compared with furosemide, torsemide has favorable pharmacodynamics/pharmacokinetics including higher bioavailability, longer duration of effect, minor renal excretion, decreased kaliuresis, and enhanced natriuresis/diuresis. These properties may be further supported by differential effects on RAAS regulation and fibrosis modulation as compared with other diuretics. The limited current body of evidence indicates that torsemide may be superior to furosemide with respect to improving HF functional status and reducing HF hospitalization, and there are mixed data regarding effect on reducing overall cardiovascular (CV) hospitalizations/mortality. Further randomized data are necessary to definitively determine if torsemide can reduce risk of mortality and hospitalization among patients with HF.
Keywords: torsemide, diuretics, heart failure, renal disease, drug profile, comparative analysis
1. Introduction
Heart failure (HF) is a clinical syndrome marked by signs and symptoms of congestion that affects over ~6 million Americans and costs the health system over $30 billion per year.[1-4] Worldwide, heart failure prevalence is estimated at over 64 million.[5] HF accounts for a heavy burden of hospitalizations with over 800,000 hospital discharges in the U.S. each year.[1] These hospitalizations are generally driven by worsening congestion often related to volume overload, and associated with particularly high rates of post-discharge death and hospital readmission. Notably, non-cardiac comorbidities are playing an increasingly important role in the management of HF as well as clinical outcomes, particularly in heart failure with preserved ejection fraction (HFpEF).[6] Renal disease, in particular, is a pervasive and impactful co-morbidity in HF and dramatically impacts the efficacy and safety of volume management.[7,8] In this context, maintenance of euvolemia in chronic HF and effective decongestion in decompensated HF are increasingly critical components of HF care. Loop diuretics remain the primary pharmacologic therapy to achieve these two objectives in HF management, as supported by recent guidelines.[9] Commonly utilized loop diuretics include furosemide, torsemide, and bumetanide, while ethacrynic acid serves an alternative for patients with hypersensitivity reactions to sulfonamide-containing loop diuretics. Notably, established guidelines do not specify differential recommendations or preference for particular loop diuretics.[9]
Since its approval in 1966, furosemide has served as the dominant loop diuretic for HF and non-cardiac volume overload while torsemide (initial approval in 1993) and bumetanide (initial approval in 1983) are used in a minority of cases. Indeed, in a recent analysis of patients with HFrEF using loop diuretics in contemporary US clinical practice, 83% of patients were prescribed furosemide, 10% were prescribed torsemide, and 7% were prescribed bumetanide.[10] Nonetheless, in the early 2000s, generic forms have been developed for torsemide and bumetanide, and, as a result, cost has become a less significant driver of use. Furosemide’s dominant use in clinical practice may be driven more now by the fact that it was first to market, and the inertia that develops as a result of experience with the drug over decades. This trend may also have roots in the classic teaching of loop diuretics having a class effect with dose-responsiveness at a certain threshold level. Still, there is a growing body of literature that will be covered in this narrative review that supports the potential benefits of torsemide over furosemide and other loop diuretics, as well as ongoing research studying the potential clinical benefits of torsemide over furosemide for clinical outcomes.
2. Torsemide/loop diuretic pharmacodynamics and pharmacokinetics
As a drug class, loop diuretics inhibit the Na+/K+/2Cl− cotransporter type 2 in the thick ascending loop of Henle, leading to increased excretion of urinary sodium and chloride and associated diuresis.[11] Torsemide, a diuretic of the pyridine-sulfonylurea class, works by the same mechanism and undergoes predominant hepatic metabolism (80%) with minor renal excretion (20%).[12,13] This lack of reliance on renal function provides several benefits including a lower risk of otoxicity.[12] Torsemide demonstrates 90% bioavailability, 3.5 hour half-life, and 6-16 hour duration of effect, as compared with furosemide’s wide range of bioavailability (10-100%), 2 hour half-life, and 6-8 hour duration of effect. Notably, torsemide’s absorption is unaffected by food intake while meal-time use of furosemide may reduce its bioavailability by 30%.[12] Torsemide’s extended duration of effect is particularly useful in reducing rebound post-diuretic sodium chloride retention, which is more prominent with furosemide.[12] Further, torsemide’s half-life is extended significantly more in states of heart failure (6 hours) and hepatic dysfunction (8 hours) compared to furosemide.[14] This, in part, drives improved absorption and efficacy in these populations; accordingly, torsemide has been shown to be less affected by the clinical HF state whereas furosemide and bumetanide demonstrate reduced absorption and thereby reduced diuresis in HF.[12,15-17] In patients with varying degrees of renal dysfunction, torsemide clearance and half-life do not change, unlike furosemide.[18] However, renal clearance of torsemide is attenuated in patients with renal insufficiency; the greatest magnitude of reduction is seen in patients with most significant renal disease.[18] In this population, increased doses may be necessary to deliver sufficient diuretic to the urinary site of action.[18] For patients with renal disease, remaining nephron tubules respond appropriately to diuretic exposure, once achieved, as compared to nephrons of patients with normal renal function.[19] Extended-release (ER)/prolonged-release (PR) formulations of torsemide have also been recently developed and have demonstrated extended periods of effective serum concentrations, increased fluid + sodium excretion, and less reduction in glomerular filtration rate compared to immediate-release torsemide, although these products are not yet available for prescription in the United States at present.[20] Lastly, it should be noted that there is some evidence that differences in genetic polymorphisms may drive some of the variability in efficacy among loop diuretics (up to 1/6 of variation in urinary electrolyte excretion and 15% variation in urinary volume), but these findings require further investigation to determine their clinical implications.[11,21]
3. Torsemide and potential effects on RAAS inhibition and myocardial fibrosis
Preclinical and early clinical data from the 1980s and early 1990s established torsemide as a generally safe and effective diuretic. Indeed, these early data demonstrated more potent diuresis and natriuresis, enhanced blood pressure control, and less significant hypokalemia.[22-24] Later studies in the early 1990s demonstrated improvements in weight, edema, and NYHA functional class and led to torsemide’s FDA approval in 1993.[12,25]
This early scientific investigation in the 1980s and 1990s also demonstrated that, while diuretic usage (namely furosemide) relieved symptoms of HF, usage came at the potential cost of stimulating renin-angiotensin-aldosterone system (RAAS).[26,27] On the other hand, researchers demonstrated some early pre-clinical data that torsemide inhibited aldosterone binding and aldosterone secretion in the 1990s.[28,29] As a result, after torsemide’s FDA approval, pre-clinical and clinical research shifted to focus on investigating the potential down-stream clinical effects of diuretics on inhibition of the RAAS and myocardial fibrosis.
In 2004, McCurley et al published one of the first prospective pre-clinical studies of diuretic use and RAAS/LV function effects.[30] This study demonstrated in a porcine model that not only did those treated with furosemide have higher aldosterone levels, but they also more quickly developed LV dysfunction and demonstrated altered calcium current handling. In contrast, Veeraveedu et al showed that torsemide was associated with improvement in myocardial functional parameters, LV fibrosis, and mortality in a rat model,[23] and that torsemide reduced progression of myocarditis to cardiomyopathy in a rat model as well.[31] This was supported by several clinical studies that demonstrated favorable RAAS effects of torsemide including signs of inhibition of transcardiac extraction of aldosterone in HF patients,[32] as well as a randomized 6-month, open-label trial (n=50) that demonstrated improved LV echocardiographic parameters, increased renin/aldosterone (supporting the aldosterone receptor antagonism mechanism), and decreased BNP, compared to furosemide.[33] More recently, in 2013, these prior data were contrasted by an in-depth study of mineralocorticoid receptor (MR) cellular/genetic-level effects of torsemide compared to a MR antagonist (MRA) ‘standard’ (spironolactone) and found that torsemide did not act as an MRA by this analytical approach.[34]
Clinical studies regarding torsemide and myocardial fibrosis have also demonstrated discordant results over the years. From 2004-2007, Lopez et al published seminal work that tested the hypothesis and mechanisms behind torsemide’s anti-fibrotic effects with biopsy-sampled collagen volume fraction (CVF) and serum markers/enzymes of collagen synthesis and degradation: carboxy-terminal peptide of procollagen type 1 (PIP), carboxy-terminal telopeptide of collagen type I (CITP), procollagen type I carboxy-terminal proteinase (PCP), and carboxy-terminal propeptide of procollagen type I (PICP).[35,36] These studies demonstrated that, compared with furosemide, torsemide significantly decreased CVF, PIP (but not CITP), the ratio of active PCP, and PICP; these findings demonstrated torsemide’s ability to reverse/reduce myocardial fibrosis, potentially through the mechanism of decreased activation of the collagen-generating enzyme, PCP. This hypothesis was also supported by work from Kasama et al (n=40), which demonstrated improvements in LV remodeling parameters (LVEDV and LVESV) as well as improvement in NYHA functional class.[37]
These studies were followed by the TORAFIC trial and the DROP-PIP trial, both of which challenged the anti-fibrotic mechanisms of torsemide.[38,39] TORAFIC was a multicenter, open-label study that randomized 155 hypertensive, chronic HF patients to either prolonged-release (PR) torsemide or furosemide. The study did not meet its primary endpoint of reducing serum PICP. The authors noted several potential contributing factors to these neutral results including a milder heart failure population (lower NYHA class, higher ejection fraction), low serum PICP baseline levels, and higher than expected within-subject variability of PICP levels. DROP-PIP studied a similar population (35 HFpEF patients with T2DM) in a randomized, single-center, double-blind study and found no significant difference in mean change in PIP between torsemide and furosemide-treated patients. Again, the authors highlighted several considerations for this neutral result including the small nature of the study with 17% drop-out rate, the unique pathophysiology of fibrosis in T2DM patients, lower dosing of torsemide utilized (vs Lopez et al studies and TORAFIC), differential baseline patient characteristics (sex, HF hospitalization history, and baseline PIP levels), and focus on a single biomarker with differential inclusion criteria based on left atrial size. While the limitations to these two neutral studies are notable, these conflicting data leave questions regarding torsemide’s antifibrotic efficacy and mechanisms.
4. Morbidity/mortality - comparative effectiveness studies
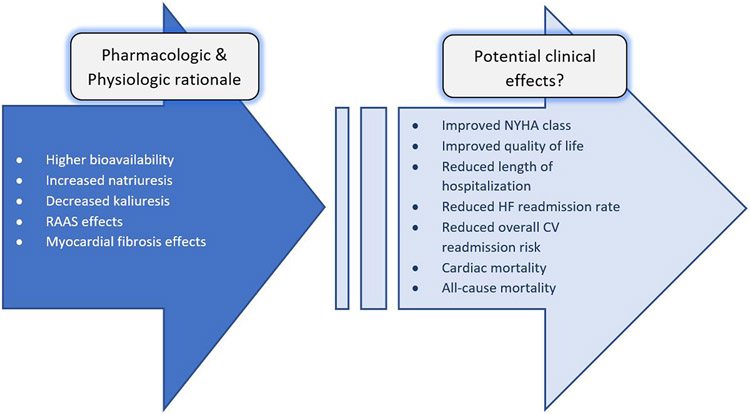
The early 2000s brought the results of multiple modestly-sized comparative effectiveness trials with torsemide and furosemide, elevating the discussion of potential benefits from pharmacologic and physiologic rationale to morbidity and mortality (see Figure 1). Two of the first studies, based in the US (Murray et al)[40] and in Switzerland (Muller et al)[41], were both randomized, open-label, prospective trials comparing torsemide and furosemide and each involving ~235 patients each. The results were concordant in that each showed no statistically significant difference in mortality. Muller et al also demonstrated improved quality of life with use of torsemide through assessment with specifically designed questionnaire (i.e. urination frequency/urgency and sense of lifestyle restriction). On the other hand, some outcomes differed in that the US study demonstrated a significant reduction in HF/CV readmissions while Muller et al showed no difference. Several caveats and key differences in patient population and background therapy between the studies could explain this discordance. For instance, Muller et al enrolled below its projected sample size (projected n=240, randomized n=237 randomized; complete follow-up n=194) and had relatively few mortality outcomes (14 total deaths). Further, in Murray et al, patients in the torsemide group actually had significantly more HF hospitalizations in the year prior to enrollment, which makes the demonstrated reduction in HF/CV readmissions more intriguing given this baseline imbalance suggests the torsemide group included patients at potentially higher risk.
Figure 1:
Rationale for torsemide compared to other diuretics: From pharmacologic/physiologic evidence to potential mortality and morbidity
In contrast to these two RCTs, the TOrasemide In Congestive Heart Failure (TORIC) Study was an open-label, non-randomized study, designed primarily for post-market safety and tolerability surveillance and published in 2002.[42] TORIC initially enrolled 2303 patients and, given a signal for differential mortality, a post-hoc analysis was pursued. This analysis included 1,377 patients, representing the largest study directly comparing torsemide and furosemide/’other diuretics’ (88% furosemide, 12% other). At baseline, there appeared to be few differences between study groups, although it was notable that the furosemide group was more likely to be on other ‘concurrent’ diuretic therapy compared to the torsemide group (7.4% vs 0.9%). Results from the torsemide group were significantly different with higher rates of improvement in NYHA functional class (45.8% vs. 37.2%, p<0.001), 59.7% relative risk reduction in cardiovascular mortality, and 51.5% relative risk reduction in overall mortality. Importantly, these observational data are inherently unable to definitively prove cause-effect relationships and may be best considered hypothesis generating. This was also a primarily rural, non-hospital-based study performed in Spain and use of goal-directed medical therapy was fairly low (beta blocker use - 9.5%, ACEi use 30%); therefore, these findings may not be generalizable to other populations.
Another more recent study, TORNADO (TORasemide oN hemodynAmic and Neurohormonal Stress, and carDiac remOdeling in Heart Failure), also randomized patients to furosemide vs torsemide and published initial results in 2019.[43] This study was limited by its size (n=40), unblinded design, and narrow geographic scope (two centers in Poland). Within these limitations, the study did demonstrate a statistically significant improvement in its composite outcome (improvement in NYHA class, improvement of 6-minute walk test (6MWT) length, and decrease in fluid retention), although none of these individual subcomponents demonstrate significant improvements alone and the composite outcome was largely driven by improved 6MWT.
5. Torsemide in renal disease
The use of diuretics including torsemide in patients with chronic kidney disease (CKD) is well-established as generally safe and effective means of maintaining euvolemia. As renal function deteriorates, providers typically utilize escalating high-doses of intravenous furosemide or torsemide, often along with intermittent thiazide-like diuretics, although bioavailability remains a challenge. Renal insufficiency was quite common in the comparative effectiveness studies of HF populations described above; ~87% of patients in Muller et al had some element of CKD, and average creatinine in Murray et al was ~1.6 mg/dL.[40,41] Therefore, conclusions from these studies (namely, reduction in hospitalization days with torsemide use, a possible reduction in HF/CV readmissions, and no clear difference in mortality) may be reasonably extrapolated to patients with comorbid CKD. Data specifically comparing diuretics based on renal function are relatively sparse, but several small studies address this issue. Vasavada et al performed a double-blind, randomized, crossover study, comparing torsemide and furosemide in 14 patients with stage 2-3 CKD through both inpatient and outpatient care.[44] They found no difference in urinary sodium excretion or ambulatory blood pressure reduction. Another small but unique study was performed by Hein et al as a prospective, controlled, single-center, open-label, randomized trial of 29 patients recovering renal function after continuous renal replacement therapy (CRRT).[45] The majority of outcome measures in this study demonstrated equivalence between furosemide and torsemide as urine output, 24 hour fluid balance, central venous pressure, serum creatinine clearance, and renin/aldosterone concentrations were similar between the two groups. The authors noted some subtle differences in urine output and laboratory trends: there was a significant decrease in urine output in the furosemide group over the course of the study, although this may have been affected by numerically lower urine output pre-intervention (436 mL vs. 660 mL at 6 hours pre-study start, p=0.07) in this group and a subsequent large initial increase in urine output with initial furosemide dosing, followed by a decline. There was also a significant increase in serum creatinine and BUN in the furosemide group, which may have reflected less robust renal filtration and excretion. Lastly, the authors suggested that there may have been a more “dose-dependent” urine output response with torsemide, although this was not formally statistically quantified by study investigators. However, this suggestion is supported by findings from pharmacokinetic studies of torsemide in renal disease.[18]
6. Meta-analyses
In light of the modest size of most prior comparative effectiveness studies, several meta-analyses have been performed in recent years in an attempt to collate these data and refine estimates of torsemide effectiveness. Methodology in these analyses has varied widely, including a range of 2-19 studies (with associated trade-offs between sample size and heterogeneity) and with some analyses including randomized studies alone versus a mix of randomized and observational studies (see Table 1).[12,46-51] Still, there are several key findings that are consistent across all or most of these meta-analyses. First, there is no clear difference in all-cause mortality between patients treated with torsemide vs. furosemide, although heterogeneity was often high; only one analysis studied cardiovascular mortality specifically and found it to be significantly lower in torsemide-treated patients, although these data included only 3 studies.[50] Second, the majority of analyses demonstrated improved NYHA functional class with the use of torsemide with relatively low heterogeneity, with exception of an early analysis from 2013.[46] Conclusions regarding hospitalization rates were variable with 3 analyses demonstrating an improvement in HF re-hospitalization rate and 2 analysis noting improvement in CV readmission rate. Meanwhile, Kido et al[52] found no difference in HF or CV re-hospitalization and Abraham et al[50] found only a non-significant, numerically lower risk of HF hospitalization with torsemide.
Table 1:
Summary of meta-analyses
| Study | # of studies analyzed* |
Torsemide superior (+), neutral (←→) or not assessed (n/a) | |||
|---|---|---|---|---|---|
| NYHA |
HF
readmissions |
CV
readmissions |
All-cause
mortality |
||
| DiNicolantonio et al, 2012[12] | 2 RCTs | n/a | + | + | ←→ |
| Bikdeli et al, 2013[46] | 5 RCTs / 1 otherǂ | ←→ | n/a | n/a | ←→ |
| Shah et al, 2018[47] | 3 RCTs | n/a | + | + | ←→ |
| Kido et al, 2019[48] | 2 RCTs / 3 otherǂ | + | ←→ | ←→ | ←→ |
| Miles et al, 2019[49] | 10 RCTs / 4 other | + | + | n/a | ←→ |
| Abraham et al, 2020[50] | 9 RCTs / 10 otherǂ | + | ←→ ¶ | n/a | ←→ § |
| Sherif et al, 2020[51] | 14 RCTs | n/a | n/a § | n/a | ←→ |
Represents total number of studies in meta-analysis; analyses of specific outcome measures (i.e. NYHA, HF/CV readmission, and mortality) typically included subsets of these total number of studies
Includes observational studies, prospective cohort studies, post hoc analyses of RCTs
Trend towards lower risk of HF hospitalization (odds ratio 0.72 [0.51, 1.03], p =0.07)
Did not assess readmissions but did note lower “hospital stay”
Analysis of cardiovascular mortality did demonstrate significant reduction with torsemide
7. Ongoing trials
Given this pre-clinical and modest randomized clinical data supporting potential benefits of torsemide compared with furosemide (see Figure 2), there remained an unmet need for a large, randomized clinical trial to definitively determine the comparative effects of torsemide on clinical outcomes. In this setting, investigators developed and initiated the TRANSFORM-HF (Torsemide Comparison with Furosemide for Management of Heart Failure) trial in 2018.[53] TRANSFORM-HF is a pragmatic, event-driven, comparative effectiveness trial that will randomize up to 6,000 patients hospitalized for HF across approximately 50 US sites. The primary outcome of the trial is all-cause mortality. Secondary outcomes include the composite of all-cause mortality or all-cause readmission over 30 days and 12 months, total number of hospitalizations over 12 months, health-related quality of life over 12 months (as measured by the Kansas City Cardiomyopathy Questionnaire [KCCQ]), and symptoms of depression over 12 months (as measured by the Patient Health Questionnaire-2 [PHQ-2]). Consistent with its pragmatic design and reflecting care in routine clinical practice, the inclusion and exclusion criteria are purposely broad and inclusive. Notably, the trial will include patients across the spectrum of ejection fraction, and patients are eligible irrespective of renal function so long as not with end-stage renal disease on dialysis.
Figure 2:
Summary of currently available evidence for torsemide relative to furosemide and other loop diuretics
8. Conclusion
Compared with furosemide, the current body of evidence suggests torsemide has superior pharmacodynamics and pharmacokinetics, including higher bioavailability, longer duration of effect, minor renal excretion, decreased kaliuresis, and enhanced natriuresis/diuresis. These properties may be further supported by differential effects on RAAS regulation and fibrosis modulation as compared with other diuretics. There is limited evidence that torsemide may be superior to furosemide with regards to NYHA functional status and reducing risk for heart failure hospitalization, and there are mixed data regarding its impact on overall cardiovascular re-admissions and mortality. Further randomized data are necessary to determine if torsemide can conclusively alter risk of hospitalization and mortality among patients with HF.
9. Expert opinion
Chronic kidney disease frequently overlaps with the pathophysiology and epidemiology of heart failure and can complicate the management of edema either alone or in combination with heart failure.[7,8] Specifically, renal insufficiency increases the likelihood of diuretic resistance and residual volume overload, which in turn have been consistently associated with worse patient outcomes.
While furosemide remains a commonly utilized first-line diuretic in the practice of the authors, we have a low threshold to consider initiating or switching to torsemide among patients where volume status is particularly difficult to manage and/or with repeated hospitalizations for worsening HF. Our rationale for this approach stems from the higher bioavailability, enhanced natriuresis/diuresis, longer duration of effect, and decreased kaliuresis of torsemide, as compared with furosemide. We also recognize the potential favorable effects on RAAS inhibition and cardiac fibrosis suggested by some studies, but acknowledge that there are conflicting studies demonstrating neutral effects compared with the more well-established and reliably proven pharmacodynamic/pharmacokinetic advantages of torsemide.
In terms of comparative effectiveness for clinical outcomes, the Murray et al and Muller et al RCTs from the early 2000s (along with the non-randomized TORIC analysis) have laid the hypothesis-generating foundation to consider torsemide’s potential for improved hospitalization and mortality outcomes in HF. Still, these RCTs were relatively small and had key differences in patient population and trial execution, thereby producing variable results for hospitalization and mortality outcomes. As a result, there is a critical need for results from a large, randomized clinical trial. In this setting, the ongoing TRANSFORM-HF trial, randomizing up to 6,000 patients across approximately 50 US sites, aims to provide definitive data on whether a direct-to-torsemide approach or switching from furosemide to torsemide is justified in our routine management of HF patients with hypervolemia. In the interim before results from this trial, judicious consideration of patients suitable for treatment with torsemide in clinical practice is recommended, and further research into the mechanistic RAAS and fibrosis effects of torsemide will complement the primary findings from TRANSFORM-HF on clinical outcomes.
ARTICLE HIGHLIGHTS:
Torsemide is a loop diuretic prescribed for a minority of patients with heart failure and renal disease, despite growing evidence for its potential advantages.
Torsemide demonstrates superior pharmacodynamics and pharmacokinetics compared with furosemide.
There is some evidence that torsemide modulates the renin-angiotensin-aldosterone system and cardiac fibrosis processes.
The current body of evidence indicates that torsemide may be superior to furosemide with respect to improving New York Heart Association functional class and reducing HF hospitalization, although data are limited.
Further randomized data are necessary to definitively determine if torsemide can conclusively reduce risk of mortality and hospitalization among patients with HF.
Footnotes
Declaration of Interest
A Peters is supported by the National Heart Lung and Blood Institute (T32HL069749). RJ Mentz has received research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Eli Lilly, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Innolife, Medtronic, Merck, Novartis, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. T DeWald has received research support from Novartis. S Greene has received research support from the Duke University Department of Medicine Chair’s Research Award, the American Heart Association, Amgen, AstraZeneca, Bristol Myers Squibb, Cytokinetics, Merck, Novartis, and Pfizer; has served on advisory boards for Amgen, AstraZeneca, Birstoly Myers Squibb, and Cytokinetics; and serves as a consultant for Amgen, Bayer, Bristol Myers Squibb, Merck, and Vifor.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
REFERENCES:
- [1].Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation. 2021. [DOI] [PubMed] [Google Scholar]
- [2].Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the united states a policy statement from the american heart association. Circ Hear Fail. 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Metra M, Teerlink JR. Heart failure. Lancet. 2017;390:1981–1995. [DOI] [PubMed] [Google Scholar]
- [4].Murphy SP, Ibrahim NE, Januzzi JL. Heart Failure with Reduced Ejection Fraction: A Review. JAMA - J Am Med Assoc. 2020;324:488–504. [DOI] [PubMed] [Google Scholar]
- [5].James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vergaro G, Ghionzoli N, Innocenti L, et al. Noncardiac Versus Cardiac Mortality in Heart Failure With Preserved, Midrange, and Reduced Ejection Fraction. J Am Heart Assoc. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ahmed A, Campbell RC. Epidemiology of Chronic Kidney Disease in Heart Failure. Heart Fail Clin. 2008;4:387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hebert K, Dias A, Delgado MC, et al. Epidemiology and survival of the five stages of chronic kidney disease in a systolic heart failure population. Eur J Heart Fail. 2010;12:861–865. [DOI] [PubMed] [Google Scholar]
- [9].Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol [Internet]. 2013;62:e147–e239. Available from: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- [10].Khan MS, Greene SJ, Hellkamp AS, DeVore AD, Shen X, Sanghera N, Albert NM, Patterson JH, Spertus JA, Thomas LE, Williams F, Hernandez AF, Fonarow GC, Butler J. Diuretic Changes, Healthcare Resource Utilization, and Clinical Outcomes for Heart Failure with Reduced Ejection Fraction: From the CHAMP-HF Registry . Circ Hear Fail. 2021;In press. [DOI] [PubMed] [Google Scholar]
- [11].Buggey J, Mentz RJ, Pitt B, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J [Internet]. 2015;169:323–333. Available from: 10.1016/j.ahj.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dinicolantonio JJ. Should torsemide be the loop diuretic of choice in systolic heart failure? Future Cardiol. 2012;8:707–728. [DOI] [PubMed] [Google Scholar]
- [13].Knauf H, Mutschler E. Clinical Pharmacokinetics and Pharmacodynamics of Torasemide. Clin Pharmacokinet. 1998;34:1–24. [DOI] [PubMed] [Google Scholar]
- [14].Wargo KA, Banta WM. A comprehensive review of the loop diuretics: Should furosemide be first line? Ann Pharmacother. 2009;43:1836–1847. [DOI] [PubMed] [Google Scholar]
- [15].Vasko MR, Cartwright DB, Knochel JP, et al. Furosemide absorption altered in decompensated congestive heart failure. Ann Intern Med. 1985;102:314–318. [DOI] [PubMed] [Google Scholar]
- [16].Brater DC, Day B, Burdette A, et al. Bumetanide and furosemide in heart failure. Kidney Int. 1984;26:183–189. [DOI] [PubMed] [Google Scholar]
- [17].Bleske BE, Welage LS, Kramer WG, et al. Pharmacokinetics of torsemide in patients with decompensated and compensated congestive heart failure. J Clin Pharmacol. 1998;38:708–714. [DOI] [PubMed] [Google Scholar]
- [18].Gehr TWB, Rudy DW, Matzke GR, et al. The pharmacokinetics of intravenous and oral torsemide in patients with chronic renal insufficiency. Clin Pharmacol Ther. 1994;56:31–38. [DOI] [PubMed] [Google Scholar]
- [19].Rudy DW, Gehr TWB, Matzke GR, et al. The pharmacodynamics of intravenous and oral torsemide in patients with chronic renal insufficiency. Clin Pharmacol Ther. 1994;56:39–47. [DOI] [PubMed] [Google Scholar]
- [20].Shah S, Pitt B, Brater DC, et al. Sodium and fluid excretion with torsemide in healthy subjects is limited by the short duration of diuretic action. J Am Heart Assoc. 2017;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vormfelde S V, Brockmöller J The genetics of loop diuretic effects. Pharmacogenomics J. 2012;12:45–53. [DOI] [PubMed] [Google Scholar]
- [22].Lambe R, Kennedy O, Kenny M, et al. Study of the tolerance and diuretic properties of torasemide following oral or intravenous administration to healthy volunteers. Eur J Clin Pharmacol. 1986;31:9–14. [DOI] [PubMed] [Google Scholar]
- [23].Veeraveedu PT, Watanabe K, Ma M, et al. Comparative effects of torasemide and furosemide in rats with heart failure. Biochem Pharmacol. 2008;75:649–659. [DOI] [PubMed] [Google Scholar]
- [24].Broekhuysen J, Deger F, Douchamps J, et al. Torasemide, a new potent diuretic - Double-blind comparison with furosemide. Eur J Clin Pharmacol. 1986;31:29–34. [DOI] [PubMed] [Google Scholar]
- [25].Goebel KM. Six-week study of torsemide in patients with congestive heart failure. Clin Ther [Internet]. 1993;15:1051–1059. Available from: http://europepmc.org/abstract/MED/8111802. [PubMed] [Google Scholar]
- [26].Bayliss J, Norell M, Canepa-Anson R, et al. Untreated heart failure: Clinical and neuroendocrine effects of introducing diuretics. Br Heart J. 1987;57:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Francis GS, Benedict C, Johnstone DE, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure: A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation. 1990;82:1724–1729. [DOI] [PubMed] [Google Scholar]
- [28].Uchida T, Yamanaga K, Nishikawa M, et al. Anti-aldosteronergic effect of torasemide. Eur J Pharmacol. 1991;205:145–150. [DOI] [PubMed] [Google Scholar]
- [29].Goodfriend TL, Ball DL, Oelkers WVB. Torsemide Inhibits Aldosterone Secretion In Vitro. Life Sci. 1998;63:45–50. [DOI] [PubMed] [Google Scholar]
- [30].McCurley JM, Hanlon SU, Wei SK, et al. Furosemide and the progression of left ventricular dysfunction in experimental heart failure. J Am Coll Cardiol [Internet]. 2004;44:1301–1307. Available from: 10.1016/j.jacc.2004.04.059. [DOI] [PubMed] [Google Scholar]
- [31].Veeraveedu PT, Watanabe K, Ma M, et al. Torasemide, a long-acting loop diuretic, reduces the progression of myocarditis to dilated cardiomyopathy. Eur J Pharmacol. 2008;581:121–131. [DOI] [PubMed] [Google Scholar]
- [32].Tsutamoto T, Sakai H, Wada A, et al. Torasemide inhibits transcardiac extraction of aldosterone in patients with congestive heart failure [3]. J Am Coll Cardiol [Internet]. 2004;44:2252–2253. Available from: 10.1016/j.jacc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- [33].Yamato M, Sasaki T, Honda K, et al. Effects of torasemide on left ventricular function and neurohumoral factors in patients with chronic heart failure. Circ J. 2003;67:384–390. [DOI] [PubMed] [Google Scholar]
- [34].Gravez B, Tarjus A, Jimenez-Canino R, et al. The Diuretic Torasemide Does Not Prevent Aldosterone-Mediated Mineralocorticoid Receptor Activation in Cardiomyocytes. PLoS One. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].López B, Querejeta R, González A, et al. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol. 2004;43:2028–2035. [DOI] [PubMed] [Google Scholar]
- [36].López B, González A, Beaumont J, et al. Identification of a Potential Cardiac Antifibrotic Mechanism of Torasemide in Patients With Chronic Heart Failure. J Am Coll Cardiol. 2007;50:859–867. [DOI] [PubMed] [Google Scholar]
- [37].Kasama S, Toyama T, Hatori T, et al. Effects of torasemide on cardiac sympathetic nerve activity and left ventricular remodelling in patients with congestive heart failure. Heart. 2006;92:1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].The Torafic Investigators Group. Effects of prolonged-release torasemide versus furosemide on myocardial fibrosis in hypertensive patients with chronic heart failure: A randomized, blinded-end point, active-controlled study. Clin Ther. 2011;33:1204–1213.e3. [DOI] [PubMed] [Google Scholar]
- [39].Trippel TD, Van Linthout S, Westermann D, et al. Investigating a biomarker-driven approach to target collagen turnover in diabetic heart failure with preserved ejection fraction patients. Effect of torasemide versus furosemide on serum C-terminal propeptide of procollagen type I (DROP-PIP trial). Eur J Heart Fail. 2018;20:460–470. [DOI] [PubMed] [Google Scholar]
- [40].Murray MD, Deer MM, Ferguson JA, et al. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111:513–520. [DOI] [PubMed] [Google Scholar]
- [41].Müller K, Gamba G, Jaquet F, et al. Torasemide vs. furosemide in primary care patients with chronic heart failure NYHA II to IV - Efficacy and quality of life. Eur J Heart Fail. 2003;5:793–801. [DOI] [PubMed] [Google Scholar]
- [42].Cosín J, Díez J. Torasemide in chronic heart failure: Results of the TORIC study. Eur J Heart Fail. 2002;4:507–513. [DOI] [PubMed] [Google Scholar]
- [43].Balsam P, Ozierański K, Marchel M, et al. Comparative effectiveness of torasemide versus furosemide in symptomatic therapy in heart failure patients: Preliminary results from the randomized tornado trial. Cardiol J. 2019;26:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vasavada N, Saha C, Agarwal R. A double-blind randomized crossover trial of two loop diuretics in chronic kidney disease. Kidney Int. 2003;64:632–640. [DOI] [PubMed] [Google Scholar]
- [45].Hein OV, Staegemann M, Wagner D, et al. Torsemide versus furosemide after continuous renal replacement therapy due to acute renal failure in cardiac surgery patients. Ren Fail. 2005;27:385–392. [DOI] [PubMed] [Google Scholar]
- [46].Bikdeli B, Strait KM, Dharmarajan K, et al. Dominance of furosemide for loop diuretic therapy in heart failure: Time to revisit the alternatives? J Am Coll Cardiol [Internet]. 2013;61:1549–1550. Available from: 10.1016/j.jacc.2012.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shah P, Patel H, Mithawala P, et al. Torsemide versus furosemide in heart failure patients: A meta-analysis of randomized controlled trials. Eur J Intern Med. 2018;57:e38–e40. [DOI] [PubMed] [Google Scholar]
- [48].Kido K, Shimizu M, Hashiguchi M. Comparing torsemide versus furosemide in patients with heart failure: A meta-analysis. J Am Pharm Assoc [Internet]. 2019;59:432–438. Available from: 10.1016/j.japh.2019.01.014. [DOI] [PubMed] [Google Scholar]
- [49].Miles JA, Hanumanthu BK, Patel K, et al. Torsemide versus furosemide and intermediate-term outcomes in patients with heart failure: An updated meta-analysis. J Cardiovasc Med. 2019;20:379–388. [DOI] [PubMed] [Google Scholar]
- [50].Abraham B, Megaly M, Sous M, et al. Meta-Analysis Comparing Torsemide Versus Furosemide in Patients With Heart Failure. Am J Cardiol [Internet]. 2020;125:92–99. Available from: 10.1016/j.amjcard.2019.09.039. [DOI] [PubMed] [Google Scholar]
- [51].Sherif NA, Morra ME, Thanh L Van, et al. Torasemide versus furosemide in treatment of heart failure: A systematic review and meta-analysis of randomized controlled trials. J Eval Clin Pract. 2020;26:842–851. [DOI] [PubMed] [Google Scholar]
- [52].Kido K, Shimizu M, Hashiguchi M. Comparing torsemide versus furosemide in patients with heart failure: A meta-analysis. J Am Pharm Assoc. 2019;59:432–438. [DOI] [PubMed] [Google Scholar]
- [53].Greene SJ, Velazquez EJ, Anstrom KJ, et al. Pragmatic Design of Randomized Clinical Trials for Heart Failure: Rationale and Design of the TRANSFORM-HF Trial. JACC Hear Fail. 2021;9:325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]