Abstract
Cognitive behavioral stress management (CBSM) improves quality of life and mitigates stress biology in patients with early-stage cancer, including men with localized prostate cancer. However, treatments for advanced prostate cancer like androgen deprivation therapy (ADT) can lead to significant symptom burden that may be further exacerbated by stress-induced inflammation and cortisol dysregulation. The aim of this study was to examine the effects of CBSM (versus an active health promotion control) on circulating inflammatory markers and cortisol in men with advanced prostate cancer. Methods: Men with stage III or IV prostate cancer (N = 192) who had undergone ADT within the last year were randomized to CBSM or health promotion. Both interventions were 10 weeks, group-based, and delivered online. Venous blood was drawn at baseline, 6 months, and 12 months to measure circulating levels of CRP, IL-6, IL8, IL-10, and TNF-α. Saliva samples were collected at awakening, 30 minutes after awakening, evening, and night for two consecutive days at baseline, 6-months, and 12-months to measure diurnal cortisol slopes. Results: Mixed modeling analyses demonstrated that changes in inflammatory markers and cortisol did not differ by intervention. Men in both CBSM and health promotion showed decreases in IL-10, IL-8, and TNF-α from baseline to 6 months (β=−3.85–5.04, p’s=.004–<.001). However, these markers generally demonstrated a rebound increase from 6 to 12 months (β=1.91–4.06, p’s=.06–<.001). Men in health promotion also demonstrated a flatter diurnal cortisol slope versus men in CBSM at 6 months (β=−2.27, p=.023), but not at 12 months. There were no intervention effects on CRP, IL-6, or overall cortisol output. Conclusions: Contrary to hypotheses, CBSM did not lead to changes in the circulating inflammatory markers and cortisol relative to health promotion. CBSM may be associated with healthy diurnal cortisol rhythm because of its focus on cognitive behavioral approaches to stress management. More research is needed to understand the impact of CBSM and health promotion on biomarkers among men with advanced prostate cancer.
Trial Registration ClinicalTrials.gov Identifier: NCT03149185
Keywords: cognitive behavioral stress management, prostate cancer, inflammation, cortisol, advanced cancer, metastatic
Prostate cancer is the most common non-cutaneous cancer in men and the second most deadly cancer in men.1,2 Although advancements in screening and early detection have increased the 5-year survival rate to nearly 100% for men with early stage prostate cancer, men with regionally advanced (stage III) or metastatic (stage IV) prostate cancer have a 5-year survival rate of 30%.3 Androgen deprivation therapy (ADT), also known as androgen suppression therapy, is standard of care for men with advanced prostate cancer.4,5 ADT has a wide range of side effects, including hot flashes, loss of bone density and muscle mass, insulin resistance, and weight gain, and commonly causes mood lability, fatigue, pain, and sexual and urinary dysfunction.6,7 As a result, men treated with ADT report the worst health-related quality of life among men with prostate cancer.8–14 ADT also is linked with significant long-term health risks, including risk for cardiovascular disease, diabetes, and thromboembolic disease.15–17
Cognitive behavioral stress management (CBSM) is a group-based, psychosocial intervention that incorporates cognitive behavioral therapy and relaxation techniques to help manage stress and reduce symptoms.18–21 Research demonstrates that CBSM confers numerous benefits for cancer survivors, including improved health-related quality of life, social support, relaxation, coping skills, and benefit finding, as well as reduced depressive symptoms, anxiety, and emotional distress.22–26 CBSM also mitigates stress-related biological changes, for example it has been shown to reverse upregulation of pro-inflammatory gene expression, reduce serum cortisol, and increase Th1 cytokine production in cancer survivors.25,27–30 This is particularly important as inflammation is implicated in tumor cell proliferation, angiogenesis, and metastasis 31–39 and poor diurnal cortisol rhythms are associated with worse mental and physical health and predict worse survival and health-related quality of life in individuals with cancer.40,41 Despite the fact that ADT leads to significant symptom burden that may be further exacerbated by inflammation and cortisol dysregulation, 42–44 no previous study has examined the effect of CBSM on cortisol and markers of circulating inflammation in men with advanced prostate cancer.
The primary aim of the current study was to examine the effects of CBSM versus an active health promotion control on circulating inflammatory markers and cortisol in men with advanced prostate cancer who received ADT within the last year. Previously published findings from this trial demonstrate that CBSM is acceptable and feasible in men with advanced prostate cancer.45,46Analyses examining primary psychosocial outcomes demonstrated no group differences in trajectories of health-related quality of life and symptom burden between men in CBSM and health promotion.47 However, based on evidence that CBSM mitigates stress-related biological changes among early-stage cancer survivors, 25,27–30 we hypothesized a priori that men with advanced prostate cancer in CBSM would demonstrate lower levels of inflammatory markers (CRP, IL-6, IL-8, IL-10, and TNF-α), steeper diurnal cortisol slopes, and lower overall cortisol output at 6 and 12 month follow-ups than men in the health promotion condition.
Method
Participants
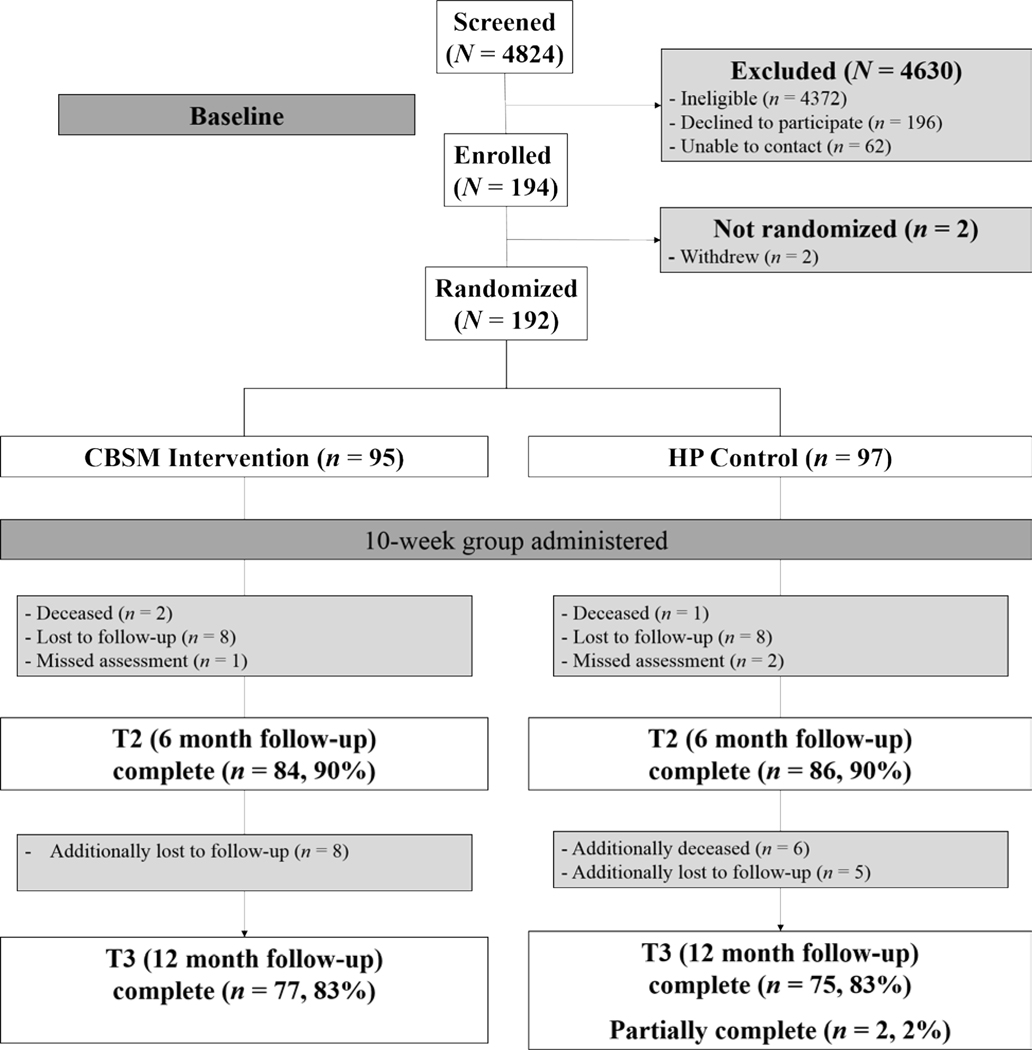
The study CONSORT diagram is shown in Figure 1. Men with advanced prostate cancer were recruited from Northwestern Memorial Hospital and the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Rush University Medical Center, the Jesse Brown Veterans Affairs Medical Center, and two Northwestern Medicine locations in Lake County, IL (Lake Forest Hospital and the Grayslake Outpatient Center). Participants were enrolled between January 2013 and November 2016.
Figure 1.
CONSORT Diagram
Eligible men were 50 years of age or older, fluent in English at the 6th grade level or higher, initially diagnosed with stage III or IV prostate cancer, and had undergone ADT within the 12 months immediately prior to study enrollment. Men were excluded if they: 1) had undergone treatment for any cancer other than prostate cancer or a non-melanoma skin cancer within the past five years, 2) had undergone inpatient psychiatric treatment for mental illness within the past six months or were displaying overt signs of severe psychopathology at the time of screening, 3) were experiencing active substance or alcohol dependence issues that would interfere with study participation, 4) had been diagnosed with an acute or chronic immune system condition (e.g., lupus or rheumatoid arthritis), 5) had an anticipated life expectancy < 12 months, or 6) received a score < 20 on the Mini Mental State Examination at the time of screening.48
Procedures
Institutional review board approval was received from each study site prior to enrollment. All participants provided informed consent prior to participation. Participants attended in-person appointments during which they completed a battery of psychosocial assessments via Assessment CenterSM (a secure, HIPAA-compliant online platform) at baseline (T1) as well as 6 months (T2) and 12 months (T3) post-baseline. Participants also provided biological specimens (detailed below). Participants were randomized (1:1) to the CBSM21 or health promotion condition. Groups were stratified by disease status (advanced versus metastatic disease) and were each comprised of four to ten men. Participants were not stratified by recruitment site as sites were added sequentially. Following each weekly group meeting, participants completed online assessments of group satisfaction and psychosocial functioning on a study-provided tablet (Samsung Galaxy 2 with 4G connectivity). Participants were compensated $100 for each in-person assessment and an additional $5 for each weekly post-session online assessment.
Study Conditions
The CBSM and health promotion conditions were both group-based, manualized, and delivered once per week over the course of 10 weeks via a HIPAA-compliant, web-based platform that was built within the Purple Development Environment.49 Sessions were facilitated by study team members who were master’s- or doctoral-level therapists. Participants received printed workbooks along with study tablets and headphones prior to the first group session. Workbooks included patient-facing intervention content for all ten sessions. Study staff also conducted tutorials with participants prior to the first group session to help them log onto WebEx using their study tablets, ensure they had access to intervention content, and check audio and video connection. Participants accessed the weekly groups and study-related content on their study tablet. During weekly group sessions, participants had the opportunity to interact with their group facilitator and fellow group members via WebEx video conferencing software. Between sessions, participants were instructed to complete weekly assessments of satisfaction and psychosocial functioning and review didactic material (e.g., session content) and expert videos (e.g., symptoms of ADT discussed by a urologist) on the program’s website. Direct links to each of these components were included on the home screen of participants’ study-provided tablet. Participants also were able to keep their printed workbooks throughout the study duration and beyond. Men in CBSM did not have access to the HP intervention content or workbook and vice versa.
CBSM Condition.
CBSM is a 10 session, group-based intervention that integrates cognitive-behavioral stress- and self-management skills (e.g., cognitive restructuring) with relaxation skills training (e.g., deep breathing) to improve health-related quality of life and reduce symptom burden.21 We adapted CBSM for men with advanced prostate cancer by including disease-relevant content (e.g., hot flashes, challenges with sexual functioning) and reviewing additional strategies relevant in the context of advanced disease (e.g., managing existential concerns, life review). See Table 1 for details of the CBSM condition content. Each weekly group session lasted approximately 90 minutes. Sessions began with practicing a relaxation technique (30 minutes) followed by discussion and practice of stress management techniques (60 minutes). Participants were encouraged to practice the skills taught each week between sessions by completing task-based homework assignments, which were reviewed in the following group session.
Table 1.
Description of Intervention Conditions
| Wk | Cognitive Behavioral Stress Management (CBSM) | Health Prom | |||
|---|---|---|---|---|---|
|
| |||||
| Relaxation | Stress Management | Linkage to APC | Topic |
S k_ Sa |
|
| 1 | Deep Breathing | My Health, Stress and Awareness | Disease/treatment-related issues/concerns | Living with APC | Understanding importance |
| 2 | Deep Breathing | Stress & Awareness | Awareness of thoughts, feelings, behaviors | Maintaining a Healthy Lifestyle | Positive adjustment |
| 3 | 7 Muscle Progressive Muscle Relaxation | Automatic Thoughts, Distortions & Thought Replacement | Bodily changes, impact of symptoms; fears over progression/death, negative outlook | Physical & Social Changes | Recognizing symptoms |
| 4 | 7 Muscle Progressive Muscle Relaxation | Cognitive Restructuring | Self-image as cancer survivor; adjusting expectations for self and others & to symptoms | Physical & Leisure Activity | Benefits active |
| 5 | Deep Breathing & 4 Muscle Progressive Muscle Relaxation | Effective Coping Skills | Body-changes: pain, fatigue, hot flashes; redefining intimacy; coping with symptoms | Healthy Eating | Appetite |
| 6 | Deep Breathing & 4 Muscle Progressive Muscle Relaxation | Sexuality & Intimacy | Loss of sexual desire and functioning; redefining sexual intimacy, negotiating intimacy/alternatives | Cognition & Memory | Cognitive |
| 7 | Imagery | Social Support | Loss of intimacy; interpersonal conflict; avoiding conversations about symptoms, progression/death | Intimacy & Family Relations | Role changes |
| 8 | Imagery | Anger Management | Interpersonal conflict; frustration with health care | HRQOL & Life Satisfaction | religious |
| 9 | Meditation | Assertiveness | Doctor-patient & intimate relationships; expressing needs adaptively and asking others for help | Information Overload | APC tracking |
| 10 | Meditation | Acceptance & Program Review | Generalizing skills to daily life, redefining roles | Review & Summary | Advance |
Note. APC = advanced prostate cancer; HRQOL = health-related quality of life
Health Promotion Condition.
The health promotion condition is a 10 session, group-based intervention that integrates didactic health-related presentations of both general health information and health information specific to living with advanced prostate cancer, including healthy lifestyle, physical and social changes, physical/leisure activities, healthy eating, cognition and memory, sexual intimacy, and social support. Men in the health promotion condition did not review any content related to the stress-management skills included in the CBSM condition. See Table 1 for details of the health promotion control condition content. Each group meeting lasted approximately 60 minutes.
Intervention Delivery
Group facilitators were master’s- or doctoral-level therapists who completed an in-person facilitator training on the manualized CBSM and health promotion interventions. Nine facilitators led CBSM groups and 12 facilitators led health promotion groups. Weekly group sessions were scheduled based on participants’ availability. All groups met on weekdays. Approximately half of groups met in the evening after 5pm (53%) and the other half met in the morning before 12pm (30%) or in the afternoon between noon and 5pm (17%). Sessions were audio and video recorded and reviewed in weekly supervision with licensed clinical psychologists who were trained in CBSM and health promotion.
Measures
Sociodemographic and Medical Characteristics.
Age, body mass index (BMI), stage of disease (stage III versus stage IV), prior radical retropubic prostatectomy, and time since diagnosis were collected at baseline via medical chart. Cancer treatment with radiation, chemotherapy, and ADT was collected at each assessment via medical chart review. Income, race, and ethnicity were collected at baseline via self-report. Comorbidities were self-reported at baseline and combined into a weighted index score using the weighting scheme from the Charlson Comorbidity Index.50
Inflammation.
Blood was drawn into a Serum Separator Tube (Becton-Dickinson) by antecubital venipuncture during laboratory visits at baseline, 6 months, and 12 months. Following the manufacturer’s instructions, the tube was centrifuged at 3,000 relative centrifugal force (RCF) for 15 minutes at 4°C, after which the serum was harvested, divided into aliquots, and frozen at −80 °C. At the end of the study, the samples were thawed, and five biomarkers of low-grade inflammation were measured: C-reactive protein (CRP), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α). CRP was measured in duplicate by high-sensitivity immunoturbidimetric assay on a Roche/Hitachi cobas c502 analyzer. The average intra- and inter-assay coefficients of variation were 2.5% and 5.6%, respectively. This assay’s lower limit of detection is 0.2 mg/L. The cytokines (IL-6, IL-8, IL-10, and TNF-α) were measured in duplicate by electrochemiluminescence on a SECTOR Imager 2400A (MesoScale Discovery) with a Human Pro-Inflammatory 4-Plex Ultra-Sensitive assay (MesoScale Discovery), following instructions provided by the manufacturer. The kit’s lower limits of detection range from .10 pg/mL (IL-8) to .80 pg/mL (IL-10). Across runs, the average intra-assay coefficients of variation for duplicate pairs were 3.45% (IL-6), 3.42% (IL-8), 3.44% (IL-10), and 4.61% (TNF-α).
Cortisol.
Participants were instructed to collect saliva samples upon awakening (Wake), 30 minutes after awakening (Wake +30min), in the evening between 4pm and 5pm (Evening), and at night between 9pm and 10pm (Night) for two consecutive days (four salivettes per day, eight salivettes total) at baseline, 6 months, and 12 months. Per standardized cortisol assay protocol from Cobas® by Roche Diagnostics, participants collected each saliva sample by removing the cotton swab from the salivette and chewing it vigorously between their premolars/molars for one minute until the cotton swab was saturated. Participants subsequently placed the cotton swab back into the salivette and secured the top. They were instructed not to eat, drink, or brush their teeth prior to the morning samples (Wake and Wake +30min) and for at least 30 minutes before the evening and night samples. In order to assess adherence to saliva collection instructions, participants provided date and time of collection for each saliva sample and indicated whether they had eaten, consumed caffeine, or exercised within the 30 minutes prior to a sample. If a kit was returned with unclear or illegible annotations, study staff contacted the participant by phone to clarify the response.
Samples were refrigerated until all samples across both days were collected and returned via an express mail delivery service or in person at their next scheduled study assessment if this assessment was within a few days of their collecting the saliva sample. Samples were returned to the laboratory within approximately one week of collection and were stored at −80°C upon receipt. Samples were thawed and subsequently centrifuged at 3800 rpm for 20 minutes at room temperature to separate saliva from any mucous. Samples were then transferred into assay cups by pipette and analyzed via ElectroChemiLuminescence (ECL) technology for immunoassay analysis, using Roche Cobas® e411 analyzer. The kit’s lower limit of detection is 1.00 nmol/L (= 0.036 μg/dL). Cortisol values at Wake, Wake +30min, Evening, and Night were averaged across the two consecutive days (for participants who only collected one day of samples, those values were retained). Area under the curve with respect to ground (AUC; (ug/dl)(hrs) was calculated at baseline, 6 months, and 12 months as a measure of overall cortisol output. The diurnal slope of the cortisol was calculated as the difference in Wake to Night values at baseline, 6 months, and 12 months.
Data Analysis
Two-level multilevel models were used to conduct intent-to-treat analyses comparing the effects of CBSM and health promotion on cortisol (i.e., AUC, diurnal slope) and markers of inflammation (i.e., TNFα, CRP, IL-6, IL-8, IL-10) over time in Stata version 15.1 (StataCorp LLC). These models tested group differences in cortisol and markers of inflammation at baseline, 6 months, and 12 months as well as within group changes from baseline to 6 months and 6 months to 12 months. Analyses controlled for cancer-specific covariates, including stage of disease (stage III versus stage IV), cancer treatment (surgery, radiation, chemotherapy, and ADT), and time since diagnosis. Men who underwent a radical retropubic prostatectomy did so prior to baseline. Given the stage of disease of this sample, treatment with radiation, chemotherapy, and/or ADT varied by individual and across time points. Therefore, the main effect of prostatectomy was a non-time-varying Level 2 covariate and main effects for radiation, chemotherapy, and ADT were included as time-varying Level 1 covariates. Analyses also controlled for key covariates that are known to influence immune and neuroendocrine functioning, 51 including age, BMI, comorbidities, socioeconomic status (income), race, and time between baseline and intervention completion. The following covariates were grand-mean centered to aid interpretation: age, years since diagnosis, BMI, and Charlson Comorbidity Index. 50 All values for markers of inflammation and cortisol were log transformed to correct for nonnormality. Final models included both a random intercept and slope for time at Level 1.
Results
Sample characteristics are presented in Table 2. On average, participants were 68 years old, married or cohabitating, had an annual household income greater than $35,000, and were currently unemployed. More than one-third of participants identified as Black. Participants were diagnosed with stage III (58%) and IV (42%) prostate cancer with an average time since diagnosis of less than 5 years. There were no statistically significant differences across groups on baseline sociodemographic or medical covariates. Most participants (81%) attended at least six of the ten weekly sessions, with an average attendance of more than seven sessions. Most participants in the CBSM condition attended at least seven sessions (74.7%) with over half (54.7%) attending at least nine sessions. Similarly, most participants in the health promotion condition attended at least seven sessions (78.3%) with over half (60.8%) attending at least nine sessions. Participants who were lost to follow-up prior to the 12 month follow-up were more likely to have stage IV (versus stage III) prostate cancer (χ2 (1) = 5.61, p = 0.018) and were more likely to have received some form of cancer treatment between the 6 month and 12 month follow-ups (χ2 (1) = 7.00, p = 0.008).
Table 2.
Sample Characteristics
| Overall (N = 192) | CBSM (n = 95) | HP (n = 97) | |
|---|---|---|---|
| Age, mean (SD) | 68.84 (8.87) | 68.81 (8.54) | 68.87 (9.23) |
| Years since diagnosis, mean (SD) | 4.70 (5.28) | 4.36 (5.16) | 5.05 (5.41) |
| # sessions completed, mean (SD) | 7.69 (2.97) | 7.47 (3.09) | 7.90 (2.86) |
| Days between session 10 and T2, mean (SD) | 76.43 (38.51) | 76.77 (38.81) | 76.10 (38.44) |
| Months since most recent ADT, mean (SD) | |||
| T1 - Baseline | 1.52 (1.53) | 1.61 (1.61) | 1.43 (1.44) |
| T2 – 6 months | 2.33 (2.46) | 2.58 (2.58) | 2.10 (2.32) |
| T3 – 12 months | 3.20 (3.86) | 3.60 (4.51) | 2.81 (3.09) |
| Race, n (%) | |||
| White | 113 (58.9) | 56 (58.9) | 57 (58.8) |
| Black | 69 (35.9) | 35 (36.8) | 34 (35.1) |
| Other | 10 (5.2) | 4 (4.3) | 6 (6.2) |
| Married or cohabitating, n (%) | 128 (66.7) | 67 (70.5) | 61 (62.9) |
| Family annual income > $35,000, n (%) | 125 (65.1) | 66 (69.5) | 59 (60.8) |
| Working full- or part-time; n (%) | 74 (38.5) | 31 (32.6) | 43 (44.3) |
| Metastatic (Stage IV), n (%) | 81 (42.2) | 37 (38.9) | 44 (45.4) |
| Prostate cancer treatment history, n (%) | |||
| Treatment within 6-months prior to T1 | 156 (81.3) | 80 (84.2) | 76 (78.4) |
| Treatment between T1 and T2 | 47 (24.5) | 22 (23.2) | 25 (25.8) |
| Treatment within T2 and T3 | 22 (11.5) | 10 (10.5) | 12 (12.4) |
| Recruitment Site, n (%) | |||
| Northwestern Memorial Hospital | 111 (57.8) | 58 (61.1) | 53 (54.6) |
| Jesse Brown VA | 42 (21.9) | 18 (18.9) | 24 (24.7) |
| Rush University Medical Center | 34 (17.7) | 19 (20.0) | 15 (15.5) |
| Other | 5 (2.6) | 0 (0.0) | 5 (5.1) |
Note. There were no statistically significant differences across groups on baseline sociodemographic or medical covariates. CBSM = Cognitive Behavioral Stress Management; HP = Health Promotion; SD = standard deviation; ADT = androgen deprivation therapy.
The number of participants who provided blood and saliva samples at baseline, 6 months, and 12 months as well as descriptive statistics for cortisol and markers of inflammation are reported in Table 3. Average CRP was greater than the established 3.0 cutoff for high cardiovascular risk at all time points in this sample. Of a possible 8 saliva samples (four salivettes per day across two days for eight salivettes total), most men submitted 7 or 8 viable samples at each time point. At baseline, 142 of 174 participants submitted 8 viable samples (81.6%) and 152 of 174 submitted at least 7 viable samples (87.4%). At 6 months, 118 of 151 participants submitted 8 viable samples (78.1%), and 131 of 151 submitted at least 7 viable samples (86.8%). At 12 months, 99 of 131 participants submitted 8 viable samples (75.6%), and 106 of 131 submitted at least 7 viable samples (80.9%). All viable samples contained sufficient saliva to be assayed were retained.
Table 3.
Descriptive Statistics for Cortisol and Markers of Inflammation at Baseline, 6 and 12 months
| Baseline (T1) | 6 months (T2) | 12 months (T3) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Overall | CBSM | HP | Overall | CBSM | HP | Overall | CBSM | HP | |
| Inflammation | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| TNF-α (pg/ml) | 3.68 (1.33) | 3.57 (1.28) | 3.79 (1.36) | 3.09 (.95) | 3.05 (.96) | 3.12 (.94) | 3.67 (1.36) | 3.57 (1.30) | 3.76 (1.42) |
| IL-10 (pg/ml) | 2.76 (4.85) | 2.91 (5.82) | 2.62 (3.70) | 2.27 (4.64) | 2.66 (6.04) | 1.90 (2.69) | 2.90 (7.31) | 3.52 (9.66) | 2.29 (3.67) |
| IL-8 (pg/ml) | 12.19 (11.82) | 12.78 (15.14) | 11.61 (7.28) | 10.32 (10.18) | 10.79 (12.67) | 9.87 (7.11) | 11.38 (11.70) | 12.22 (15.43) | 10.54 (6.04) |
| IL-6 (pg/ml) | 3.96 (5.21) | 2.97 (2.95) | 4.92 (6.61) | 3.12 (3.59) | 2.65 (2.29) | 3.57 (4.46) | 5.30 (13.39) | 3.24 (2.76) | 7.37 (18.57) |
| CRP (mg/l) | 4.83 (13.41) | 3.47 (4.81) | 6.17 (18.21) | 5.32 (14.35) | 3.63 (6.98) | 6.93 (18.78) | 6.87 (20.93) | 3.51 (5.51) | 10.22 (28.79) |
| Sample size, n | 186 | 92 | 94 | 158 | 77 | 81 | 146 | 73 | 73 |
| Cortisol | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Wake (ug/dl) | 1.21 (1.70) | 1.23 (1.85) | 1.19 (1.57) | 1.32 (2.35) | 1.17 (1.45) | 1.46 (2.95) | 1.06 (1.30) | .99 (.86) | 1.13 (1.65) |
| Wake+30min (ug/dl) | 1.18 (1.31) | 1.22 (1.46) | 1.15 (1.16) | 1.07 (1.38) | .99 (.79) | 1.14 (1.77) | 1.18 (1.58) | 1.10 (1.11) | 1.28 (1.96) |
| Evening (ug/dl) | .54 (.86) | .54 (.93) | .55 (.80) | .55 (.90) | .46 (.42) | .63 (1.19) | .46 (.44) | .46 (.45) | .46 (.42) |
| Night (ug/dl) | .50 (.60) | .55 (.52) | .46 (.66) | .59 (1.40) | .51 (.64) | .66 (1.85) | .47 (.51) | .40 (.26) | .54 (.69) |
| Sample size, n | 174 | 86 | 88 | 151 | 73 | 78 | 131 | 67 | 64 |
Note. CBSM = Cognitive Behavioral Stress Management; HP = Health Promotion; SD = standard deviation.
Intervention Effects
All results presented are intent-to-treat analyses.
Inflammation.
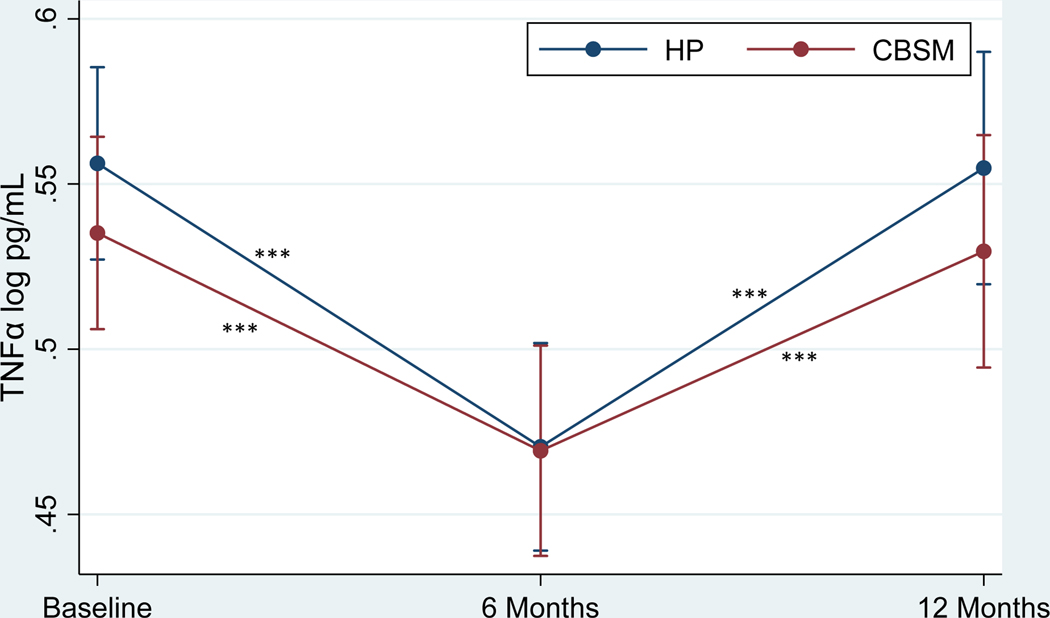
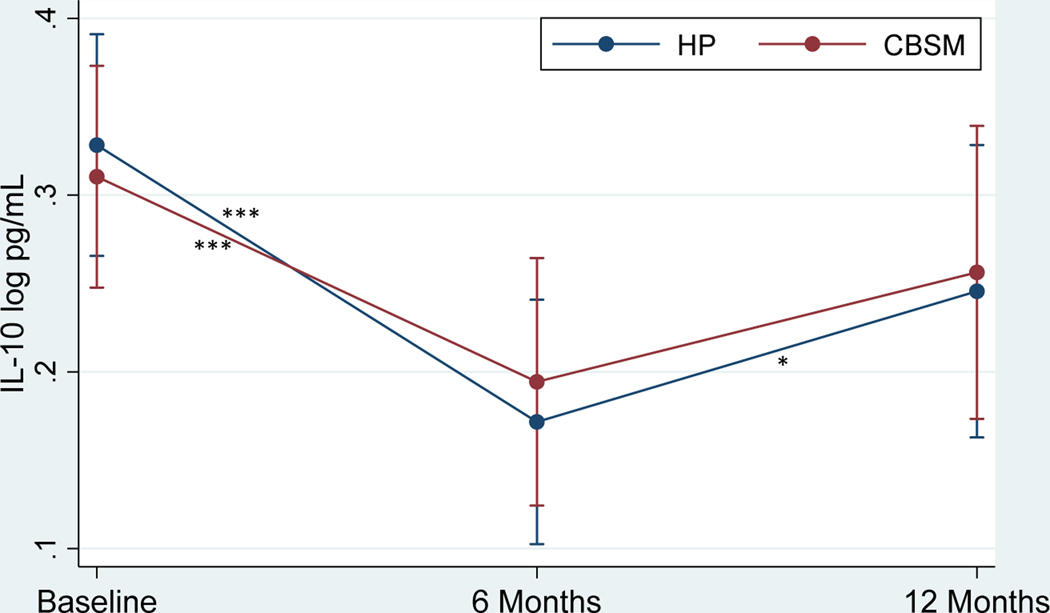
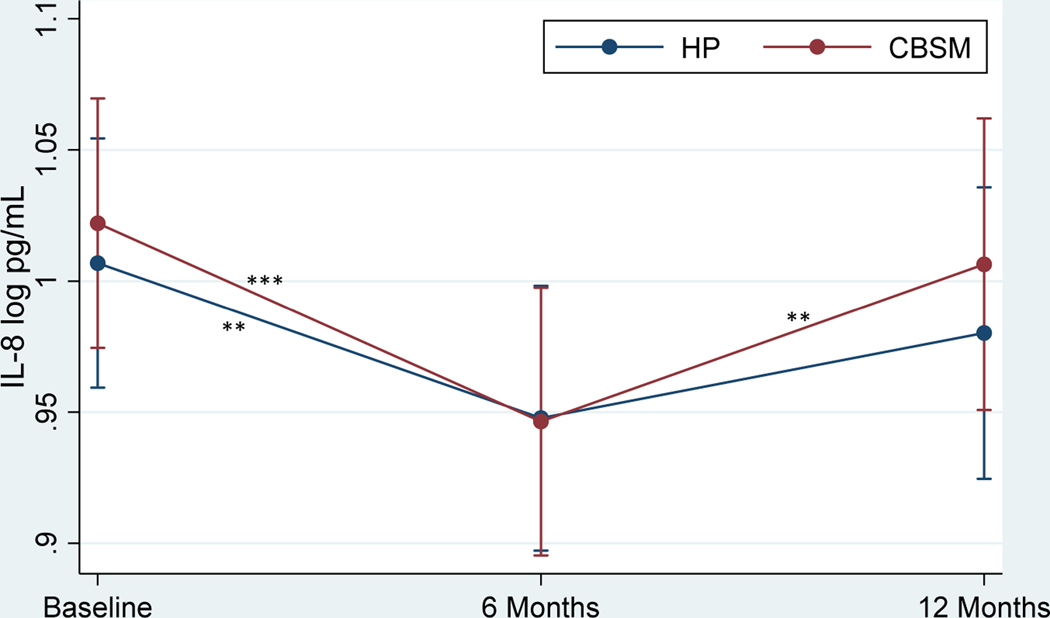
Table 4 shows the effects of CBSM and health promotion on markers of inflammation. There was no significant Group x Time interaction in TNF-α (Figure 2) or group differences at baseline, 6 months, or 12 months (p’s > .05). TNF-α decreased from baseline to 6 months (β = −4.37, p < .001, Cohen’s d = −.54 for CBSM; β = −5.04, p < .001, Cohen’s d = −.62 for health promotion) and increased from 6 to 12 months (β = 4.06, p < .001, Cohen’s d = .53 for CBSM; β = 5.24, p < .001, Cohen’s d = .69 for health promotion) for men in both conditions. There was no significant Group x Time interaction in IL-8 (Figure 3) or group differences at baseline, 6 months, or 12 months (p’s > .05). IL-8 decreased from baseline to 6 months (β = 4.09, p < .001, Cohen’s d = −.41 for CBSM; β = −2.88, p = .004, Cohen’s d = −.29 for health promotion) for men in both conditions and increased from 6 to 12 months for men in CBSM (β = 2.85, p = .004, Cohen’s d = .30), but not health promotion (p > .05). There was no significant Group x Time interaction in IL-10 (Figure 4) or group differences at baseline, 6 months, or 12 months (p’s > .05). IL-10 decreased from baseline to 6 months (β = −3.85, p < .001, Cohen’s d = −.43 for CBSM; β = −5.10, p < .001, Cohen’s d = −.57 for health promotion) for men in both conditions and increased from 6 to 12 months for men in health promotion (β = 2.29, p = .022, Cohen’s d = .28), and marginally for men in CBSM (β = 1.91, p = .056, Cohen’s d = .23). There was no significant Group x Time interaction in IL-6 or group differences at baseline or 6 months (p’s > .05), however men in CBSM had lower IL-6 than men in health promotion at 12 months (β = −2.04, p = .042, Cohen’s d = −.38). There was no change in IL-6 from baseline to 6 months for men in either CBSM or health promotion (p > .05). However, IL-6 increased from 6 to 12 months for men in both conditions (β = 2.70, p = .007, Cohen’s d = .42 for CBSM; β = 3.55, p < .001, Cohen’s d = .55 for health promotion). There was no significant Group x Time interaction in CRP or group differences at baseline, 6 months, or 12 months (p’s > .05). There also was no change in CRP from baseline to 6 months or 6 to 12 months for men in either CBSM or health promotion (p’s > .05).
Table 4.
Effect of Intervention and Covariates on Markers of Inflammation in Two-Level Mixed Models
| TNF-± | IL-10 | IL-8 | IL-6 | CRP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Coef. (SE) | β | P | Coef. (SE) | β | P | Coef. (SE) | β | P | Coef. (SE) | β | P | Coef. (SE) | β | P | |
| Intercept | .50 (.04) | 11.78 | .000 | .34 (.10) | 3.60 | .000 | .96 (.07) | 13.56 | .000 | .51 (.10) | 4.89 | .000 | .70 (.17) | 4.17 | .000 |
| Intervention | −.01 (.02) | −.69 | .492 | −.01 (.05) | −.13 | .894 | .02 (.04) | .54 | .592 | −.10 (.05) | −1.93 | .053 | −.10 (.09) | −1.10 | .270 |
| CBSM vs. HP | |||||||||||||||
| Time | |||||||||||||||
| 6 mo. vs. Baseline | −.08 (.02) | −5.04 | .000 | −.16 (.03) | −5.10 | .000 | −.06 (.02) | −2.88 | .004 | −.08 (.04) | −1.85 | .064 | −.01 (.07) | −.20 | .840 |
| 12 mo. vs. Baseline | .01 (.02) | .57 | .568 | −.08 (.04) | −2.01 | .044 | −.03 (.02) | −1.03 | .304 | .09 (.05) | 1.77 | .077 | .11 (.07) | 1.47 | .143 |
| Intervention*Time | |||||||||||||||
| CBSM | |||||||||||||||
| 6 mo. vs. Baseline | −.07 (.02) | −4.37 | .000 | −.12 (.03) | −3.85 | .000 | −.09 (.02) | −4.09 | .000 | −.07 (.05) | −1.55 | .121 | .00 (.07) | .04 | .972 |
| 12 mo. vs. 6 mo. | .07 (.02) | 4.06 | .000 | .06 (.03) | 1.91 | .056 | .06 (.02) | 2.85 | .004 | .13 (.05) | 2.70 | .007 | .05 (.07) | .66 | .511 |
| HP | |||||||||||||||
| 6 mo. vs. Baseline | −.08 (.02) | −5.04 | .000 | −.16 (.03) | −5.10 | .000 | −.06 (.02) | −2.88 | .004 | −.08 (.04) | −1.85 | .064 | −.01 (.07) | −.20 | .840 |
| 12 mo. vs. 6 mo. | .09 (.02) | 5.24 | .000 | .08 (.03) | 2.29 | .022 | .03 (.02) | 1.53 | .126 | .17 (.05) | 3.55 | .000 | .12 (.07) | 1.66 | .096 |
| CBSM vs. HP | |||||||||||||||
| at Baseline | −.01 (.02) | −.69 | .492 | −.01 (.05) | −.13 | .894 | .02 (.04) | .54 | .592 | −.10 (.05) | −1.93 | .053 | −.10 (.09) | −1.10 | .270 |
| at 6 mo. | −.01 (.02) | −.23 | .816 | .03 (.05) | .59 | .557 | −.01 (.04) | −1.20 | .839 | −.09 (.06) | −1.54 | .123 | −.08 (.09) | −.86 | .391 |
| at 12 mo. | −.03 (.03) | −1.00 | .316 | .02 (.06) | .27 | .784 | .02 (.04) | .53 | .596 | −.13 (.06) | −2.04 | .042 | −.15 (.10) | −1.56 | .119 |
| Age | .00 (.00) | 2.01 | .044 | .00 (.00) | .98 | .326 | .00 (.00) | 2.45 | .014 | .00 (.00) | 1.81 | .071 | .00 (.00) | −.84 | .402 |
| BMI | .00 (.00) | −1.35 | .178 | .00 (.00) | .28 | .776 | .00 (.00) | −.09 | .932 | .001 (.00) | 1.56 | .119 | .02 (.01) | 2.51 | .012 |
| Income (> 35k vs. <35k) | .01 (.02) | −.32 | .747 | −.03 (.06) | −.55 | .582 | −.01 (.04) | −.20 | .838 | −.09 (.06) | −1.54 | .123 | −.04 (.09) | −.38 | .706 |
| Race (White vs. Black/Other) | .02 (.02) | 1.10 | .273 | −.06 (.05) | −1.24 | .214 | .05 (.04) | 1.24 | .216 | −.02 (.05) | −.46 | .648 | −.12 (.08) | −1.36 | .173 |
| Metastasis (yes vs. no) | −.03 (.02) | −1.35 | .178 | .07 (.05) | 1.43 | .154 | −.01 (.04) | −.40 | .692 | .05 (.05) | 1.07 | .286 | −.09 (.08) | −1.04 | .300 |
| ADT (yes vs. no) | .03 (.02) | 1.68 | .094 | −.02 (.04) | −.50 | .616 | −.01 (.03) | −.32 | .752 | −.02 (.05) | −.32 | .750 | −.06 (.08) | −.68 | .496 |
| Radiation (yes vs. no) | −.00 (.01) | −.06 | .951 | .00 (.03) | .10 | .919 | .03 (.02) | 1.31 | .192 | .11 (.04) | 2.74 | .006 | .06 (.06) | .92 | .355 |
| Chemotherapy (yes vs. no) | −.09 (.03) | −2.87 | .004 | −.02 (.07) | −.27 | .790 | −.05 (.04) | −1.22 | .223 | −.12 (.09) | −1.39 | .164 | .09 (.13) | .69 | .493 |
| Prostatectomy (yes vs. no) | −.01 (.02) | −.27 | .789 | .04 (.05) | .65 | .518 | .05 (.04) | 1.10 | .273 | −.06 (.06) | −1.14 | .254 | −.27 (.09) | −2.91 | .004 |
| Years since diagnosis | .00 (.00) | 1.00 | .317 | .00 (.00) | −.46 | .649 | .00 (.00) | −1.44 | .149 | .00 (.00) | −.30 | .767 | .00 (.01) | −.16 | .875 |
| Charlson Comorbidities Index | .01 (.01) | 1.47 | .140 | .03 (.02) | 1.64 | .101 | .01 (.01) | .51 | .611 | .04 (.02) | 2.57 | .010 | .04 (.03) | 1.25 | .213 |
| Time from baseline to intervention completion | .01 (.01) | .89 | .372 | .00 (.02) | .13 | .899 | .00 (.02) | .25 | .806 | .01 (.02) | .61 | .539 | −.02 (.04) | −.50 | .617 |
Note. Reference categories in binary variables are listed second. CBSM = Cognitive Behavioral Stress Management; HP = Health Promotion; SD = standard deviation; ADT androgen deprivation therapy; BMI = body mass index; Coef. = coefficient; SE = standard error.
Figure 2.
Effect of Cognitive Behavioral Stress Management (CBSM) and Health Promotion (HP) on TNF-α
Note.*p < .05, **p < .01, ***p < .001. Standard error (SE) bars are shown.
Figure 3.
Effect of Cognitive Behavioral Stress Management (CBSM) and Health Promotion (HP) on IL-10
Note.*p < .05, **p < .01, ***p < .001. Standard error (SE) bars are shown.
Figure 4.
. Effect of Cognitive Behavioral Stress Management (CBSM) and Health Promotion (HP) on IL-8
Note. *p < .05, **p < .01, ***p < .001. Standard error (SE) bars are shown.
Cortisol.
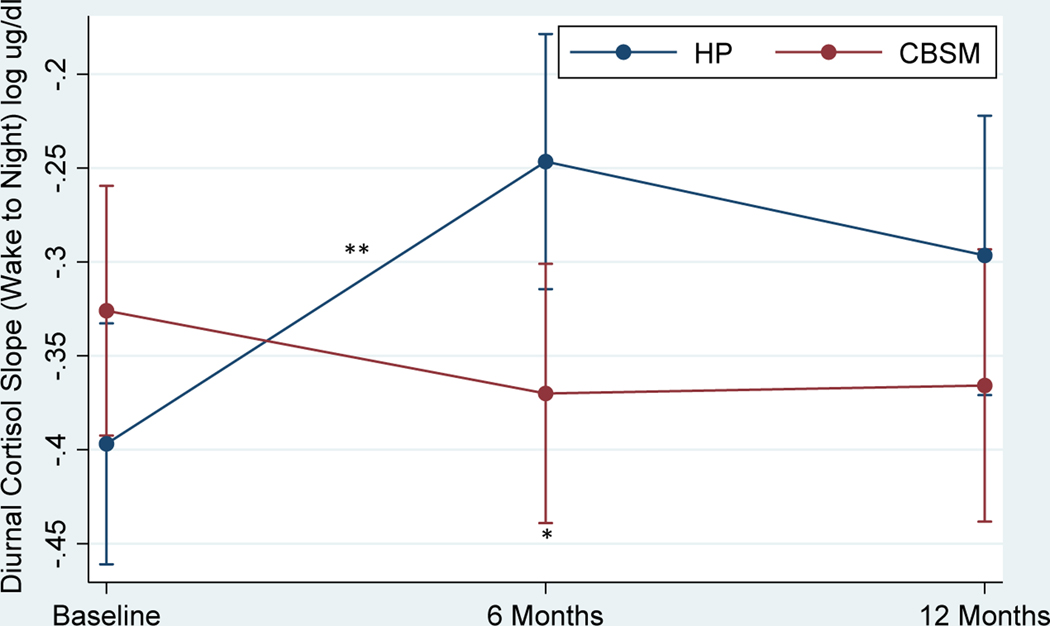
Table 5 shows the effects of CBSM and health promotion on diurnal cortisol slopes and overall cortisol output (AUC). There was no significant Group x Time interaction in diurnal cortisol slopes (Figure 5) or group differences at baseline or 12 months (p’s > .05). Men in CBSM demonstrated a steeper diurnal cortisol slope compared to men in health promotion at 6 months (β = −2.27, p = .023, Cohen’s d = −.37). Diurnal cortisol slopes became flatter from baseline to 6 months for men in health promotion (β = 3.66, p < .001, Cohen’s d = .57), but not for men in CBSM (p > .05). There was no change in diurnal cortisol slope from 6 to 12 months for men in either condition (p > .05). There was no significant Group x Time interaction in AUC or group differences at baseline, 6 months, or 12 months (p’s > .05). There also was no change in AUC from baseline to 6 months or 6 to 12 months for men in either CBSM or health promotion (p > .05).
Table 5.
Effect of Intervention and Covariates on Cortisol in Two-Level Mixed Models
| Diurnal Cortisol Slope | AUC | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Coef. (SE) | ² | P | Coef. (SE) | ² | P | |
| Intercept | −.47 (.09) | −5.41 | .000 | 7.22 (2.42) | 2.98 | .003 |
| Intervention | ||||||
| CBSM vs. HP | .08 (.05) | 1.62 | .106 | .06 (1.36) | .04 | .965 |
| Time | ||||||
| 6 mo. vs. Baseline | .16 (.04) | 3.66 | .000 | −.52 (1.23) | −.42 | .675 |
| 12 mo. vs. Baseline | .12 (.05) | 2.53 | .011 | −1.00 (1.33) | −.75 | .451 |
| Intervention*Time CBSM | ||||||
| 6 mo. vs. Baseline | −.04 (.04) | −.82 | .415 | −.47 (1.26) | −.38 | .707 |
| 12 mo. vs. 6 mo. | .02 (.05) | .44 | .658 | −.16 (1.32) | −.12 | .906 |
| HP | ||||||
| 6 mo. vs. Baseline | .16 (.04) | 3.66 | .000 | −.52 (1.23) | −.42 | .675 |
| 12 mo. vs. 6 mo. | −.04 (.05) | −.81 | .415 | −.49 (1.35) | −.36 | .719 |
| CBSM vs. HP | ||||||
| at Baseline | .08 (.05) | 1.62 | .106 | .06 (1.36) | .04 | .965 |
| at 6 mo. | −.11 (.05) | −2.27 | .023 | .10 (1.43) | .07 | .943 |
| at 12 mo. | .06 (.05) | −1.03 | .303 | .43 (1.53) | .28 | .776 |
| Age | .00 (.00) | −1.33 | .184 | .12 (.07) | 1.83 | .067 |
| BMI | .00 (.00) | .69 | .490 | .18 (.10) | 1.77 | .077 |
| Income (> 35k vs. <35k) | −.04 (.05) | −.85 | .398 | −.58 (1.29) | −.45 | .654 |
| Race (White vs. Black/Other) | −.04 (.04) | −1.07 | .285 | −.94 (1.16) | −.80 | .422 |
| Metastasis (yes vs. no) | .11 (.04) | 2.63 | .009 | −.37 (1.13) | −.33 | .744 |
| ADT (yes vs. no) | .01 (.05) | .31 | .760 | 1.78 (1.36) | 1.31 | .190 |
| Radiation (yes vs. no) | .01 (.04) | .23 | .821 | −1.15 (1.12) | −1.03 | .304 |
| Chemotherapy (yes vs. no) | −.03 (.08) | −.35 | .726 | −1.84 (2.33) | −.79 | .431 |
| Prostatectomy (yes vs. no) | −.05 (.05) | −1.17 | .242 | 1.07 (1.31) | .82 | .413 |
| Years since diagnosis | .01 (.00) | 2.13 | .033 | −.12 (.10) | −1.18 | .238 |
| Charlson Comorbidities Index | −.01 (.01) | −.58 | .564 | .18 (.38) | .47 | .640 |
| Time from baseline to _ _. . _ | ||||||
| intervention completion | .03 (.02) | 1.51 | .130 | .59 (.48) | 1.22 | .223 |
Note. Reference categories in binary variables are listed second. CBSM = Cognitive Behavioral Stress Management; HP = Health Promotion; AUC = area under the curve; SD = standard deviation; ADT = androgen deprivation therapy; BMI = body mass index; Coef. = coefficient; SE = standard error.
Figure 5.
Effect of Cognitive Behavioral Stress Management (CBSM) and Health Promotion (HP) on Diurnal Cortisol Slope
Note.*p < .05, **p < .01, ***p < .001. Standard error (SE) bars are shown.
Covariates
Older age was associated with higher levels of TNF-α (β = 2.01, p = .044, Cohen’s d = .01) and IL-8 (β = 2.45, p = .014, Cohen’s d = .02) and more comorbidities were associated with higher levels of IL-6 (β = 2.57, p = .010, Cohen’s d = .12). Radiation was associated with higher levels of IL-6 (β = 2.74, p = .006, Cohen’s d = .31), while prostatectomy and chemotherapy were associated with lower levels of CRP (β = −2.91, p = .004, Cohen’s d = −.53) and TNF-α (β = 2.87, p = .004, Cohen’s d = −.62), respectively. Metastatic disease versus regionally advanced, stage III disease (β = 2.63, p = .009, Cohen’s d = .35) and longer time since diagnosis (β = 2.13, p = .033, Cohen’s d = .03) were associated with flatter diurnal cortisol slopes.
Discussion
The primary aim of the current study was to examine the effects of CBSM versus an active health promotion control on circulating inflammatory markers and cortisol in men with advanced prostate cancer who received ADT within the last year. Similar to our previously published findings examining the effects of these interventions on health-related quality of life and psychosocial outcomes,47 changes in cortisol and markers of inflammation generally did not differ across CBSM and health promotion and there were no group by time interaction effects. Men in both CBSM and health promotion demonstrated a decrease in TNF-α, IL-8, and IL-10 at 6 months. However, these decreases were not sustained at 12 months and inflammatory markers generally showed a rebound increase. Analyses examining within group changes across time demonstrated that although the diurnal cortisol slopes became flatter for men in health promotion at 6 months, there were no changes for men in CBSM who demonstrated a steeper diurnal cortisol slope at 6 months than men in health promotion. Importantly, there was no group by time interaction effect and these differences were no longer present at 12 months. Therefore, these results should be interpreted with caution and await replication. Effect sizes for changes over time were small to moderate (Cohen’s d = .2 - .5), indicating modest decreases and subsequent increases in markers of inflammation. Changes in cortisol slopes also demonstrated small to moderate effect sizes.
Our findings suggest that CBSM and health promotion interventions may mitigate stress-related biological processes in men living with advanced prostate cancer. However, a third, usual care control is needed to truly disentangle intervention effects from the passage of time. If CBSM and health promotion have causal effects, decreases in inflammation could have clinical relevance given the significant symptom burden associated with undergoing ADT for advanced prostate cancer (i.e., hot flashes, loss of bone density and muscle mass, insulin resistance, weight gain, mood lability, fatigue, pain, and sexual and urinary dysfunction). 6–14 Furthermore, cytokines have been implicated in tumor cell proliferation, angiogenesis, and metastasis31–39 and decreases in inflammation could have meaningful consequences for men living with active, advanced stage prostate cancer. Flatter diurnal cortisol slopes also are associated with poor health (e.g., depression, frailty, anxiety, and worse cardiometabolic health) and predict worse survival and health-related quality of life in individuals with cancer.40,41 Given that cognitive behavioral strategies and progressive muscle relaxation have been shown to improve cortisol rhythms52–55 and our previous findings demonstrate that men in CBSM (but not health promotion) increase in their perceived ability to relax,47 it is possible that CBSM may have buffered the flattening in diurnal cortisol slope observed in men in health promotion. However, future research needs to include a third, usual care control in addition to an active control like health promotion in order to characterize typical trajectories of inflammatory markers and cortisol in men with advanced prostate cancer who have received ADT. Another limitation of the current study is the collection of saliva samples over two days as previous research demonstrates that more collection days may be needed to reliably estimate between-group differences in diurnal cortisol slopes and AUC.56
It is important to note that decreases in markers of inflammation were not sustained at 12 months. Our results are consistent with a recent systematic review57 that shows that psychological interventions that aim to improve health-related quality of life in individuals with chronic conditions often do not sustain benefits over long-term follow-up periods of 12 months or more. Furthermore, trials examining the effects of mind-body interventions on markers of inflammation among clinical populations, including cancer survivors, have shown mixed and null findings. 58 This pattern holds for studies that compare the effects of two active interventions (e.g., mindfulness meditation versus health education, cognitive behavioral therapy versus health education).59–62 In addition to including a third, usual care control, future research should examine how to bolster any possible beneficial effects of CBSM and health promotion in both the short- and long-term. Possible strategies that could be explored include booster sessions, varied dosing schemes, or approaches that maintain social contact and support beyond the weekly group sessions.
To our knowledge, this is the first randomized controlled trial to demonstrate a decrease in circulating inflammatory markers in the short-term among men with advanced prostate cancer who participated in CBSM and health promotion. Findings indicate that CBSM and health promotion could be viable adjunct interventions to mitigate common ADT side effects in men with advanced prostate cancer (e.g., insulin resistance, weight gain, and loss of bone density and muscle mass). Given the significant health-related challenges inherent to living with advanced prostate cancer, concerted research efforts are needed to understand whether behavioral intervention decrease inflammation in this population and to optimize interventions so that any conferred benefits and decreases in inflammation are sustained over long-term follow-up periods.
Highlights.
Inflammatory markers decreased among men in stress management and health promotion
Decreases in inflammatory markers were not sustained at 12 months
More research is needed to determine whether these interventions impact biomarkers
Acknowledgments:
This study was supported by a National Cancer Institute (NCI) grant (R01CA157809) awarded to Dr. Frank Penedo. Dr. Patricia Moreno was supported by a National Institute of Minority Health and Health Disparities (NIMHD) diversity supplement (R01MD010440). Drs. Patricia Moreno, Rina Fox, and Laura Oswald were supported by an NCI Training Grant (T32CA193193). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Molly Hermiller, Sara M. Goetzman, Alexandra R. Susi, Luke T. Smith, Jessica L. Thomas, and Amador Rosales for recruiting patients and collecting data and Katy Wortman for assistance with data management. We also thank the study participants for their time and contribution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review 1975–2016. National Cancer Institute. Published online 2019. Accessed November 1, 2019. https://seer.cancer.gov/csr/1975_2016/ [Google Scholar]
- 2.Prostate Cancer: Statistics | Cancer.Net. Accessed December 5, 2019. https://www.cancer.net/cancer-types/prostate-cancer/statistics
- 3.Survival Rates for Prostate Cancer. Accessed November 24, 2019. https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/survivalrates.html
- 4.National Comprehensive Cancer Network. NCCN Guidelines for Patients®: Prostate Cancer; 2013. [Google Scholar]
- 5.Lee R, S. MR Hormone Therapy for Prostate Cancer. In: Chabner B, Longo D, eds. Cancer Chemotherapy and Biotherapy: Principles and Practice. 5th ed. Wolters Kluwer: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 6.White WM, Sadetsky N, Waters WB, Carroll PR, Litwin MS. Quality of Life in Men With Locally Advanced Adenocarcinoma of the Prostate: An Exploratory Analysis Using Data From the CaPSURE Database. Journal of Urology. 2008;180(6):2409–2414. doi: 10.1016/j.juro.2008.08.079 [DOI] [PubMed] [Google Scholar]
- 7.Eton DT, Lepore SJ. Prostate cancer and health-related quality of life: A review of the literature. Psycho-Oncology. 2002;11(4):307–326. doi: 10.1002/pon.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adam S, Koch‐Gallenkamp L, Bertram H, et al. Health‐related quality of life in long‐term survivors with localised prostate cancer by therapy—Results from a population‐based study. European Journal of Cancer Care. 2019;28(5). doi: 10.1111/ecc.13076 [DOI] [PubMed] [Google Scholar]
- 9.Lee M, Jim HS, Fishman M, et al. Depressive symptomatology in men receiving androgen deprivation therapy for prostate cancer: A controlled comparison. Psycho-Oncology. 2015;24(4):472–477. doi: 10.1002/pon.3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YT, Li CC, Chou YH, Ke HL, Chen CY. Health-related quality of life of exposed versus non-exposed androgen deprivation therapy patients with prostate cancer: a crosssectional study. International Journal of Clinical Pharmacy. Published online August 1, 2019. doi: 10.1007/s11096-019-00854-y [DOI] [PubMed] [Google Scholar]
- 11.Pirl WF, Siegel GI, Goode MJ, Smith MR. Depression in men receiving androgen deprivation therapy for prostate cancer: A pilot study. Psycho-Oncology. 2002;11(6):518523. doi: 10.1002/pon.592 [DOI] [PubMed] [Google Scholar]
- 12.Holmstrom S, Naidoo S, Turnbull J, Hawryluk E, Paty J, Morlock R. Symptoms and Impacts in Metastatic Castration-Resistant Prostate Cancer: Qualitative Findings from Patient and Physician Interviews. Patient. 2019;12(1):57–67. doi: 10.1007/s40271-0180349-x [DOI] [PubMed] [Google Scholar]
- 13.Albertsen PC, Aaronson NK, Muller MJ, Keller SD, Ware JE. Health-related quality of life among patients with metastatic prostate cancer. Urology. 1997;49(2):207–217. doi: 10.1016/S0090-4295(96)00485-2 [DOI] [PubMed] [Google Scholar]
- 14.Chambers SK, Foley E, Clutton S, et al. The role of mindfulness in distress and quality of life for men with advanced prostate cancer. Quality of Life Research. 2016;25(12):3027–3035. doi: 10.1007/s11136-016-1341-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(27):4448–4456. doi: 10.1200/JCO.2006.06.2497 [DOI] [PubMed] [Google Scholar]
- 16.Fang F, Keating NL, Mucci LA, et al. Immediate risk of suicide and cardiovascular death after a prostate cancer diagnosis: Cohort study in the United States. Journal of the National Cancer Institute. 2010;102(5):307–314. doi: 10.1093/jnci/djp537 [DOI] [PubMed] [Google Scholar]
- 17.Bosco C, Bosnyak Z, Malmberg A, Adolfsson J, Keating NL, van Hemelrijck M. Quantifying Observational Evidence for Risk of Fatal and Nonfatal Cardiovascular Disease Following Androgen Deprivation Therapy for Prostate Cancer: A Meta-analysis. European Urology. 2015;68(3):386–396. doi: 10.1016/j.eururo.2014.11.039 [DOI] [PubMed] [Google Scholar]
- 18.Antoni MH, Smith Roselyn. Stress Management Intervention for Women with Breast Cancer. American Psychological Association; 2003. [Google Scholar]
- 19.Penedo FJ, Traeger L, Dahn J, et al. Cognitive behavioral stress management intervention improves quality of life in Spanish monolingual hispanic men treated for localized prostate cancer: results of a randomized controlled trial. International journal of behavioral medicine. 2007;14(3):164–172. doi: 10.1007/bf03000188 [DOI] [PubMed] [Google Scholar]
- 20.Penedo FJ, Molton I, Dahn JR, et al. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: Development of stress management skills improves quality of life and benefit finding. Annals of Behavioral Medicine. 2006;31(3):261–270. doi: 10.1207/s15324796abm3103_8 [DOI] [PubMed] [Google Scholar]
- 21.Penedo FJ, Antoni MH, Schneiderman N. Cognitive-Behavioral Stress Management for Prostate Cancer Recovery: Facilitator Guide Oxford University Press; 2008. doi: 10.1093/med:psych/9780195336979.001.0001 [DOI] [Google Scholar]
- 22.Spiegel D, Kraemer HC, Bloom JR, Gottheil E. EFFECT OF PSYCHOSOCIAL TREATMENT ON SURVIVAL OF PATIENTS WITH METASTATIC BREAST CANCER. The Lancet. 1989;334(8668):888–891. doi: 10.1016/S0140-6736(89)91551-1 [DOI] [PubMed] [Google Scholar]
- 23.Antoni MH, Lechner SC, Kazi A, et al. How Stress Management Improves Quality of Life After Treatment for Breast Cancer. Journal of Consulting and Clinical Psychology. 2006;74(6):1143–1152. doi: 10.1037/0022-006X.74.6.1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antoni MH, Wimberly SR, Lechner SC, et al. Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. American Journal of Psychiatry. 2006;163(10):1791–1797. doi: 10.1176/ajp.2006.163.10.1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antoni MH, Lechner S, Diaz A, et al. Cognitive behavioral stress management effects on psychosocial and physiological adaptation in women undergoing treatment for breast cancer. Brain, Behavior, and Immunity. 2009;23(5):580–591. doi: 10.1016/j.bbi.2008.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crosswell AD, Moreno PI, Raposa EB, et al. Effects of mindfulness training on emotional and physiologic recovery from induced negative affect. Psychoneuroendocrinology. 2017;86(August):78–86. doi: 10.1016/j.psyneuen.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antoni MH, Bouchard LC, Jacobs JM, et al. Stress management, leukocyte transcriptional changes and breast cancer recurrence in a randomized trial: An exploratory analysis. Psychoneuroendocrinology. 2016;74:269–277. doi: 10.1016/j.psyneuen.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antoni MH. Psychosocial intervention effects on adaptation, disease course and biobehavioral processes in cancer. Brain, behavior, and immunity. 2013;30 Suppl:S88–98. doi: 10.1016/j.bbi.2012.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips KM, Antoni MH, Lechner SC, et al. Stress management intervention reduces serum cortisol and increases relaxation during treatment for nonmetastatic breast cancer. Psychosomatic medicine. 2008;70(9):1044–1049. doi: 10.1097/PSY.0b013e318186fb27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antoni MH, Lutgendorf SK, Blomberg B, et al. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biological Psychiatry. 2012;71(4):366–372. doi: 10.1016/j.biopsych.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balkwill F. TNF-α in promotion and progression of cancer. Cancer and Metastasis Reviews. 2006;25(3):409–416. doi: 10.1007/s10555-006-9005-3 [DOI] [PubMed] [Google Scholar]
- 32.Wajant H. The role of TNF in cancer. Results and Problems in Cell Differentiation. 2009;49:1–15. doi: 10.1007/400_2008_26 [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacologica Sinica. 2008;29(11):1275–1288. doi: 10.1111/j.1745-7254.2008.00889.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheikhpour E, Noorbakhsh P, Foroughi E, Farahnak S, Nasiri R, Neamatzadeh H. A survey on the role of interleukin-10 in breast cancer: A narrative. Reports of Biochemistry and Molecular Biology. 2017;7(1):30–37. [PMC free article] [PubMed] [Google Scholar]
- 35.Dennis KL, Blatner NR, Gounari F, Khazaie K. Current status of interleukin-10 and regulatory T-cells in cancer. Current Opinion in Oncology. 2013;25(6):637–645. doi: 10.1097/CCO.0000000000000006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oft M. IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer immunology research. 2014;2(3):194–199. doi: 10.1158/23266066.CIR-13-0214 [DOI] [PubMed] [Google Scholar]
- 37.Mannino MH, Zhu Z, Xiao H, Bai Q, Wakefield MR, Fang Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Letters. 2015;367(2):103–107. doi: 10.1016/j.canlet.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 38.Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treatment Reviews. 2017;60:24–31. doi: 10.1016/j.ctrv.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 39.Waugh DJJ, Wilson C. The interleukin-8 pathway in cancer. Clinical Cancer Research. 2008;14(21):6735–6741. doi: 10.1158/1078-0432.CCR-07-4843 [DOI] [PubMed] [Google Scholar]
- 40.Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and metaanalysis. Psychoneuroendocrinology. 2017;83:25–41. doi: 10.1016/j.psyneuen.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29(8):1082–1092. doi: 10.1016/j.psyneuen.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 42.Jehn CF, Kuehnhardt D, Bartholomae A, et al. Biomarkers of depression in cancer patients. Cancer. 2006;107(11):2723–2729. doi: 10.1002/cncr.22294 [DOI] [PubMed] [Google Scholar]
- 43.Gold PW. The organization of the stress system and its dysregulation in depressive illness. Molecular Psychiatry. 2015;20(1):32–47. doi: 10.1038/mp.2014.163 [DOI] [PubMed] [Google Scholar]
- 44.Hoyt MA, Bower JE, Irwin MR, Weierich MR, Stanton AL. Sleep quality and depressive symptoms after prostate cancer: The mechanistic role of cortisol. Behavioral Neuroscience. 2016;130(3):351–356. doi: 10.1037/bne0000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouchard LC, Yanez B, Dahn JR, et al. Brief report of a tablet-delivered psychosocial intervention for men with advanced prostate cancer: Acceptability and efficacy by race. Translational Behavioral Medicine. 2019;9(4):629–637. doi: 10.1093/tbm/iby089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yanez B, McGinty HL, Mohr DC, et al. Feasibility, acceptability, and preliminary efficacy of a technology-assisted psychosocial intervention for racially diverse men with advanced prostate cancer. Cancer. 2015;121(24):4407–4415. doi: 10.1002/cncr.29658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox RS, Oswald LB, Moreno PI, et al. Technology-based psychosocial intervention to improve quality of life and reduce symptom burden in men with advanced prostate cancer : Results from a randomized controlled trial. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 49.Schueller SM, Begale M, Penedo FJ, Mohr DC. Purple: A modular system for developing and deploying behavioral intervention technologies. Journal of Medical Internet Research. 2014;16(7). doi: 10.2196/jmir.3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 51.O’Connor MF, Bower JE, Cho HJ, et al. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain, Behavior, and Immunity. 2009;23(7):887–897. doi: 10.1016/j.bbi.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chellew K, Evans P, Fornes-Vives J, Pérez G, Garcia-Banda G. The effect of progressive muscle relaxation on daily cortisol secretion. Stress. 2015;18(5):538–544. doi: 10.3109/10253890.2015.1053454 [DOI] [PubMed] [Google Scholar]
- 53.Pawlow LA, Jones GE. The impact of abbreviated progressive muscle relaxation on salivary cortisol and salivary immunoglobulin A (sIgA). Applied Psychophysiology Biofeedback. 2005;30(4):375–387. doi: 10.1007/s10484-005-8423-2 [DOI] [PubMed] [Google Scholar]
- 54.Phillips KM, Antoni MH, Lechner SC, et al. Stress management intervention reduces serum cortisol and increases relaxation during treatment for nonmetastatic breast cancer. Psychosomatic Medicine. 2008;70(9):1044–1049. doi: 10.1097/PSY.0b013e318186fb27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cruess DG, Antoni MH, Kumar M, Schneiderman N. Reductions in salivary cortisol are associated with mood improvement during relaxation training among HIV-seropositive men. Journal of Behavioral Medicine. 2000;23(2):107–122. doi: 10.1023/A:1005419917023 [DOI] [PubMed] [Google Scholar]
- 56.Segerstrom SC, Boggero IA, Smith GT, Sephton SE. Variability and reliability of diurnal cortisol in younger and older adults: Implications for design decisions. Psychoneuroendocrinology. 2014;49(1):299–309. doi: 10.1016/j.psyneuen.2014.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson N, Ozakinci G. Effectiveness of psychological interventions to improve quality of life in people with long-term conditions: Rapid systematic review of randomised controlled trials. BMC Psychology. 2018;6(1). doi: 10.1186/s40359-018-02254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bower JE, Irwin MR. Mind–body therapies and control of inflammatory biology: A descriptive review. Brain, Behavior, and Immunity. 2016;51:1–11. doi: 10.1016/j.bbi.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oken BS, Fonareva I, Haas M, et al. Pilot controlled trial of mindfulness meditation and education for dementia caregivers. Journal of Alternative and Complementary Medicine. 2010;16(10):1031–1038. doi: 10.1089/acm.2009.0733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janelsins MC, Davis PG, Wideman L, et al. Effects of Tai Chi Chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clinical Breast Cancer. 2011;11(3):161–170. doi: 10.1016/j.clbc.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malarkey WB, Jarjoura D, Klatt M. Workplace based mindfulness practice and inflammation: A randomized trial. Brain, Behavior, and Immunity. 2013;27(1):145–154. doi: 10.1016/j.bbi.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irwin MR, Olmstead R, Breen EC, et al. Tai Chi, cellular inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: A randomized controlled trial. Journal of the National Cancer Institute - Monographs. 2014;2014(50):295–301. doi: 10.1093/jncimonographs/lgu028 [DOI] [PMC free article] [PubMed] [Google Scholar]