Abstract
Cereals play an important role in global food security. Data from the UN Food and Agriculture Organization projects increased consumption of cereals from 2.6 billion tonnes in 2017 to approximately 2.9 billion tonnes by 2027. However, cereals are prone to contamination by toxigenic fungi, which lead to mycotoxicosis. The current methods for mycotoxin control involve the use of chemical preservatives. However, there are concerns about the use of chemicals in food preservation due to their effects on the health, nutritional quality, and organoleptic properties of food. Therefore, alternative methods are needed that are affordable and simple to use. The fermentation technique is based on the use of microorganisms mainly to impart desirable sensory properties and shelf-life extension. The lactic acid bacteria (LAB) are generally regarded as safe (GRAS) due to their long history of application in food fermentation systems and ability to produce antimicrobial compounds (hydroxyl fatty acids, organic acids, phenyllactic acid, hydrogen peroxide, bacteriocins, and carbon dioxide) with a broad range of antifungal activity. Hence, LAB can inhibit the growth of mycotoxin-producing fungi, thereby preventing the production of mycotoxins. Fermentation is also an efficient technique for improving nutrient bioavailability and other functional properties of cereal-based products. This review seeks to provide evidence of the potential of LAB from African fermented cereal-based products as potential biological agents against mycotoxin-producing fungi.
1. Introduction
Africa is an origin and the major producer of numerous cereals, including maize, finger millet, sorghum, pearl millet, rice, and teff [1]. Cereal consumption accounts for more than 50% of worldwide caloric daily intake [2], while in Eastern Africa, cereals consumption accounts for over 40% of the total human dietary calorie intake [3]. Maize is the major cereal crop produced and consumed in sub-Saharan Africa (SSA), with about 300 million people depending on it for livelihood [1]. It is the most important staple food crop relied upon by approximately 96% of Kenyan citizens [4]. On average, maize consumption in Kenya stands at 400 g per person per day, with 98 kg annual consumption per capita [5].
The chemical nature of cereal grains predisposes them to fungal contamination and infection, especially during production, processing, and storage [6]. Although earlier data from Food and Agricultural Organization (FAO) estimate mycotoxins contamination of food crops globally to be 25%, more recent studies from the European Food Safety Authority (EFSA) show the prevalence of detectable mycotoxins could be up to 60–80% [7]. Among the cereals, maize is the most prone to attack by potentially harmful opportunistic fungi, which may lead to mycotoxin contamination [8].
The growth of fungi lowers grain quality and yield leading nutritional and chemical changes. Specifically, it results in grain discolouration, a loss in dry matter due to carbohydrate utilization, protein, and lipid degradation, which result in changes in product digestibility and volatile metabolites production that gives off odour [9]. Furthermore, the production of heat and moisture in cereals during mould infestation contributes to product spoilage, which affects grain grade, marketing price, and customer satisfaction. The major concern is the production of toxic secondary fungal metabolites (mycotoxins) with adverse health effects on humans and animals [10]. Mycotoxins are mostly produced by fungi of genera Aspergillus, Penicillium, Alternaria, and Fusarium, affecting cereal grains during growth or after harvesting [11]. Genus Fusarium has been implicated as a cause of mycotoxin contamination in grapes through production of a variety of mycotoxin metabolites [12].
The extent of mycotoxin contamination in tropical regions can be attributed to the warm and moist weather, poor agronomic practices, inadequate conditions of crop storage, and weak regulatory systems during food handling and processing [13–15]. Aflatoxin is the most commonly reported mycotoxin in tropical developing countries [5] and is mainly produced by Aspergillus flavus and A. parasiticus [10], with significant public health importance due to its effects on human health [16].
The commonly used methods in mycotoxin control include chemical preservatives for the prevention of mould growth [17], decontamination of foods and feeds, and surveillance of mycotoxins in crops, foods, and feeds [18]. However, the use of chemical preservatives has raised food safety concerns. Furthermore, the lack of strong regulatory systems in most African countries has contributed to recurrent mycotoxicosis [15, 19]. This has triggered the investigation of novel antifungal agents produced by various lactic acid bacteria (LAB) strains as potential natural alternatives [20]. Among the strategies currently being considered for food, preservation is the use of microorganisms commonly occurring in fermented foods or their antimicrobial metabolites [21]. The use of natural food preservation methods is regarded as healthy, safe, and friendly by consumers, besides having a lower impact on food nutrition and organoleptic properties [22]. These technologies are cost-effective since they do not require advanced skills or equipment hence can be adopted in developing countries [21].
The use of LAB has shown the potential to control the growth of mycotoxins-producing fungi, thereby preventing mycotoxin production [23]. LAB are generally regarded as safe due to their long history in food fermentation [24]. They are known to produce nontoxic metabolites with broad-spectrum activity [25]. Fermented food can act as vehicles or reservoirs of beneficial LAB that produce antifungal products, which can help in food preservation and control of toxigenic fungi. Therefore, this review seeks to provide evidence of the potential of LAB from African fermented cereal-based products as potential biological agents against mycotoxins-producing fungi. This is important as it builds on indigenous knowledge in food production and preservation systems, which is in line with the African commission priority areas of Agenda 2063 framework and the United Nations sustainable development goals relating to improved food and nutrition security [26, 27].
2. Types and Distribution of Mycotoxins in Africa
Mycotoxins are secondary metabolites produced by fungi and are poisonous to both humans and animals. Worldwide, five mycotoxins are considered to be economically and toxicologically important: fumonisins (FUMs); aflatoxins (AF); ochratoxins A (OTA); deoxynivalenol (DON) and derivatives (DON-3-glucoside, monoacetyl-DONs, norDONs, and deepoxy-DON); zearalenone (ZEA) and derivatives [28]. Other mycotoxins of interest include patulin, T-2 toxin, moniliformin (MON), nivalenol (NIV), enniatins, beauvericin, and HT-2 toxin [28]. In Africa, the most prevalent mycotoxins are the AFs (43.75%), followed by FUMs (21.87%), OTA (12.5%), ZEA (9.375%), NIV, and beauvericin (both at 6.25%) [29], as indicated in Table 1. In SSA and specifically East Africa, aflatoxins are the most commonly reported mycotoxins, with previous episodes of AF poisoning having been documented in Kenya, Ethiopia, Uganda, and Tanzania [29–32].
Table 1.
Mycotoxins and related fungi with commonly contaminating crops and foodstuffs in different African countries.
| Mycotoxin | Foodstuffs | Related fungi | Country |
|---|---|---|---|
| Aflatoxins | Maize, milk, and animal feeds | Aspergillus flavus and A. parasiticus | Kenya |
| Aflatoxins | Groundnuts, cassava, millet, sorghum flour, and eshabwe sauce | A. flavus and A. parasiticus | Uganda |
| Aflatoxins | Meat products, spices, cereal grains, nuts and seeds, medicinal plants, milk, infant milk formula, cereals | A. flavus and A. parasiticus | Egypt |
| Nivalenol | Cereals and cereal products | Fusarium spp. | Tunisia and Morocco |
| Aflatoxins | Cereals and cereal products | A. flavus and A. parasiticus | |
| Ochratoxins A | Cereals and cereal products | A. ochraceus, Penicillium verrucosum | |
| Fumonisins | Cereals and cereal products | Fusarium spp. | |
| Fumonisins | Maize | Fusarium spp. | Tanzania |
| Aflatoxins | Maize | A. flavus and A. parasiticus | |
| Fumonisins | Maize | A. flavus and A. parasiticus | Zambia |
| Aflatoxins | Shiro, ground red pepper, sorghum, barley, teff and wheat sorghum, barley, and wheat | A. flavus and A. parasiticus | Ethiopia |
| Ochratoxins A | Sorghum | A. ochraceus, P. verrucosum | |
| Deoxynivalenol | Sorghum | Fusarium spp. | |
| Fumonisins | Fusarium spp. | ||
| Zearalenone | Gibberella spp. and Fusarium spp. | ||
| Aflatoxins | Sesame oil, groundnut oil, and peanuts butter | A. flavus and A. parasiticus | Sudan |
| Aflatoxins | Rice and weaning foods | A.flavus and A. parasiticus | Nigeria |
| Ochratoxins A | Rice | A. ochraceus, P. verrucosum | |
| Aflatoxins | Maize | A. flavus and A. parasiticus Fusarium spp. | Ghana |
| Fumonisins | Maize | ||
| Aflatoxins | Maize | A. flavus and A. parasiticus | Benin |
| Chips | |||
| Aflatoxins | Dried vegetables (baobab leaves, hot chili, and okra) | A. flavus and A. parasiticus | Benin, Mali, and Togo |
| Aflatoxins | Groundnuts | A. flavus and A. parasiticus | Burkina Faso |
| Fumonisins | Maize and compound feeds | Fusarium spp. | South Africa |
| Deoxynivalenol | Compound feeds | Fusarium spp. | |
| Zearalenone | Compound feeds | Gibberella spp. and Fusarium spp. | |
| Zearalenone | Sorghum beer | Gibberella spp. and Fusarium spp. | Lesotho |
Adapted from [29] with modifications.
Aflatoxins are a group of 20 related fungal metabolites that are classified into four main categories, namely, aflatoxins B1, B2, G1, and G2 [33]. Aflatoxins M1 and M2 are metabolites of aflatoxins B1 and B2, respectively, occurring in milk and milk products from animals consuming feeds or humans consuming aflatoxins B1 and B2 contaminated foods [34]. Maize, groundnuts, and tree nuts are the most common foods at risk of aflatoxins contamination [35], as shown in Table 1. Among the fumonisins, fumonisins B1, B2, and B3 are the major forms found in food [33]. The most common animal feeds harbouring mycotoxins include maize grain, wheat bran, pea hulls, and noug cake [18]. As indicated in Table 1, Aspergillus flavus, A. parasiticus, and A. nomius are some of the most common aflatoxigenic fungi [35]. Besides, other reported aflatoxigenic fungi include A. bombycis, A. ochraceus, and A. pseudotamari [35]. Aspergillus spp. are widely found in soil used for growing crops, processing facilities, storage areas, and the distribution systems for manufactured products. A. flavus strains have variable aflatoxin capabilities ranging from nontoxigenic to highly toxigenic but often produce more aflatoxin B1 than aflatoxin G1 [35]. On the other hand, there is less variation in toxigenicity among A. parasiticus strains and produce aflatoxin B1 and different amounts of aflatoxins B2, G1, and G2 [18]. Fusarium fungi are major sources of trichothecene mycotoxins, while other genera that produce trichothecene include Myrothecium, Stachybotrys, Cylindrocarpon, Trichothecium, Cephalosporium, Trichoderma, Verticimonosporium, and Phomopsis [36].
Zearalenone is a potent estrogenic metabolite produced by some Gibberella and Fusarium species such as F. graminearum, F. culmorum, F. verticillioides, F. incarnatum, F. semitectum, F. equiseti, F. oxysporum, and F. sporotrichioides [18]. Zearalenone often cooccurs with deoxynivalenol in cereal grains [7, 37]. Fumonisins B1 and B2 are naturally occurring mycotoxins produced by F. verticillioides. Aflatoxins and fumonisins are common contaminants of maize, but to a lesser extent of rice, sorghum, wheat, and cereal-based foods prepared from these commodities [33]. Cases of coexposure can either occur from the same food being contaminated with both mycotoxins or within the diet from different foods, each contaminated with one or the other [33].
Ochratoxins and citrinin are produced mainly by members of the genera Aspergillus and Penicillium. Ochratoxin A (OTA) is produced by two species: A. ochraceus and P. verrucosum. These fungi are ubiquitous and have great potential for contamination of animal feed and human food [38]. OTA often occurs in most cereal grains (wheat, oats, corn, and barley), cheese, peanuts, grapes/raisins, and dried fruits. Since it has a long half-life, OTA accumulates in the food chain. Citrinin cooccurs with OTA and often contaminates cereal grains (such as barley, corn, oats, wheat, and rice), peanuts, and fruits [38]. The fungal genera Aspergillus, Penicillium, Neotyphodium, and Claviceps can produce tremorgenic mycotoxins [39]. Fungi that produce tremorgens grow on dairy, stored grains and nuts (walnuts and peanuts), and forages [38, 39].
2.1. Aflatoxicosis Prevalence in Kenya
Episodes of aflatoxin contamination and poisoning have been reported in different parts of Kenya. The worst outbreak of aflatoxins poisoning was in 2004 when 317 cases were reported and 125 people died in the Makueni and Kitui districts in Eastern Kenya [40, 41]. There was another aflatoxicosis outbreak in 2005 in the same district of Makueni and Kitui affecting 75 people, resulting in 32 deaths [42]. Additionally, an aflatoxin outbreak occurred in 2010 during the season of abundant rainfall, which resulted in a good grain yield in Eastern Kenya [43]. The Kenyan Government banned the consumption and trade of approximately 200,000 tons of maize to protect the public from mycotoxicosis [43].
A study to compare aflatoxin prevalence in lowland and highland areas of Makueni county, Kenya, showed that low-altitude areas had more aflatoxin-contaminated maize than high-altitude areas [44]. About 50% of positive aflatoxin-contaminated maize samples in lowlands had aflatoxin levels exceeding 10 ppb, while in highlands, none of the maize had aflatoxin contamination exceeding 10 ppb [44]. An analysis of aflatoxin contamination in maize, millet, and sorghum across five counties of Kwale, Isiolo, Tharaka-Nithi, Kisii, and Bungoma indicated that about 20% of all maize samples had aflatoxin contamination levels above Kenyan legal limits of 10 ppb [45]. Consequently, the study revealed the mean aflatoxins level of 1.12 ppb in millet, hence the need to consider the potential role of traditional cereals such sorghum and millet in contributing to aflatoxin exposure in East Africa.
In a study to determine levels of aflatoxin-producing fungi and aflatoxin contamination of relief maize meal and a nutritional supplement of maize (Unimix) in Northern Kenya, the prevalence of Penicillium and Fusarium spp. occurred at exceptionally higher levels than the targeted aflatoxin-producing fungal spp.: A. parasiticus [46]. This finding implied that dietary coingestion of ochratoxins, fumonisins, trichothecenes, and zearalenone alongside aflatoxins is an unfortunate possibility among the residents of Northern Kenya. In children below the age of 5 years in Nandi and Makueni counties, the aflatoxin exposure rate from maize consumption was 0.011 and 0.49 ppb body weight per day, respectively. Consequently, aflatoxin exposure through milk was 4 × 10−4 and 1 × 10−4 ppb body weight per day in Nandi and Makueni counties, respectively [47]. Stunting growth and severe stunting growth rates associated with aflatoxin exposure in Makueni and Nandi were 28.7% and 18.5%; 30.7% and 16.5%, respectively. In 2004, the number of children who were wasting and fed on aflatoxin-contaminated flour in the Kisumu district was highly significant [47]. According to Tola and Kebede [18], the concentrated animal feedstuffs contain the highest level of mycotoxins. For example, 7 ppb was the lowest level of aflatoxin B1 contamination recorded from silage feed, but in concentrated animal feeds like wheat bran, noug cake, and sweet pea hull, the highest level of aflatoxin B1 contamination was about 419 ppb [18].
2.2. Aflatoxin Levels in Foods and Feeds in Kenya
Cereals are widely cultivated and consumed in Africa; however, they are most sensitive to aflatoxin contamination. A study by Sirma et al. [32] indicated that 60% of all maize samples (269) analysed from Laboret, Kilibwoni, and Chepkongony in Kenya were contaminated with aflatoxins ranging between 0.17 and 5.3 ppb. Similarly, 92.3% of all millet samples [39] collected from the study sites recorded aflatoxin levels ranging from 0.14 to 6.4 ppb. On the contrary, 37% of all sorghum samples [48] analysed recorded aflatoxin levels beyond the Kenya Bureau of Standards (KEBS) maximum tolerable limit of 10 ppb. Kilibwoni sublocation had the highest percentage (46%), while both Laboret and Chepkongony had 27.3%, each of samples exceeding the maximum tolerable limit of 10 ppb [32].
A study conducted in 2004 during the major aflatoxin outbreak in Kenya ascertained that 55% of maize products had aflatoxin levels greater than the Kenyan regulatory limit of 10 ppb, 35% had levels >100 ppb, and 7% had levels >1,000 ppb [49]. Locally obtained maize from the affected area was significantly more likely to have aflatoxin levels >10 ppb than maize bought from other regions of Kenya or other countries [49]. In a survey of aflatoxin contamination of milk and animal feeds (mostly cereal-based) across urban centres in Kenya, 86% of feed samples from farmers were positive for aflatoxin B1, while 81% of feed samples from feed millers and 87% from agrochemical shops were positive for AFB1 with 67%, 58%, and 66%, respectively [50]. Further, the positive samples exceeded the FAO/WHO level of 5 ppb. Standards for allowable aflatoxin limits in food for human consumption vary from country to country. For instance, the allowable level is 4 ppb in France and the Netherlands, 5–20 ppb in Canada and the USA, and 30 ppb in India, as reported by James et al. [51]. A range of 4–30 ppb is widely recognized as the acceptable limit of aflatoxin in food [52]; 20 ppb is the internationally recommended maximum limit of aflatoxin contamination. In Kenya, the maximum allowable limit in foods and feeds by the Kenya Bureau of Standards (KEBS) is 10 ppb and 20 ppb, respectively [53].
2.3. Mycotoxins Effects on Human and Animal Health
Mycotoxicoses in humans can be categorized as acute (rapid onset and a clear toxic response) or chronic (low dose exposure over a long period), leading to cancer and other health effects [28]. AFB1 is classified as a class 1 human carcinogen [54]. Chronic exposure to AFB1 has been linked to the development of liver cancer in humans [55]. Aflatoxin is the third most leading cause of hepatocellular carcinoma or liver cancer deaths globally, with about 83% of these reported deaths occurring in sub-Saharan Africa and East Asia [15, 56].
In Kenya, estimates for hepatocellular carcinoma (HCC) were associated with aflatoxin ranges from 1.05 to 39.9 persons per 100,000, but globally aflatoxin may play a causative role in 4.6–28.2% of all global HCC [57]. It is reported that aflatoxin can be metabolized by specific P450 enzymes in the liver into aflatoxin-8,9-epoxide (a reactive oxygen species) that then either binds to proteins and causes acute toxicity (aflatoxicosis) or binds to DNA leading to lesions that over time increase the risk of HCC [58]. Aflatoxin exposure (low to moderate levels) has been associated with stunting in children [16, 59], congenital malformations, and immunosuppression associated with teratogenic effects [52]. There is also strong evidence linking aflatoxin with reproductive disorders such as infertility, reduced sperm quality, and increased rate of stillbirths in both humans and animals [15, 60].
A study by Wakhisi et al. [61] found high incidences of oesophagal cancer in the North Rift valley that was associated with fumonisin exposure. Fumonisins, however, are thought to be possible cancer promoters of aflatoxin carcinogenicity [33]. Reports show some evidence and concern that additive or synergistic actions occur when there is coexposure of fumonisins and aflatoxins that potentially increase carcinogenicity [62]. The exposure of nursing children to aflatoxin through breast milk was 6 × 10−3 and 1 × 10−6 ppb per kg body weight per day in Makueni and Nandi, respectively, and children below the age of 30 months in Makueni had 1.4 times higher levels of aflatoxin M1 (AFM1) in urine than those of the same age in Nandi [47].
Health effects occur in animals, livestock, and poultry since aflatoxins are potent hepatotoxins, immunosuppressants, mutagens, and carcinogens [18]. It is reported that milk, beef, or wool reproduction and growth can be altered when ruminants consume mycotoxin-contaminated feed for extended periods [48]. In poultry, aflatoxins have been reported to cause liver damage, impaired productivity and reproductive efficiency, decreased egg production, inferior eggshell quality, inferior carcass quality, increased susceptibility to diseases, stunted growth, and death [33]. In pigs, aflatoxins are mainly associated with liver damage, whereas in cattle, it leads to reduced weight gain, liver and kidney damage, and reduced milk production [33]. Published information indicates that different forms of enzymes (such as cytochrome P450s and glutathione S-transferases), which metabolize aflatoxins, are considered responsible for the susceptibility of different animals to the toxic effects of aflatoxins [33]. Citrinin acts as a nephrotoxin in some animal species but with variation in acute toxicity in different species. The 50% lethal dose for ducks, chickens, and rabbits is 57 ppb, 95 ppb, and 134 ppb, respectively [35]. Citrinin can act synergistically with OTA to depress RNA synthesis in murine kidneys [35, 63]. Tremorgenic mycotoxins are known to elicit either intermittent or sustained tremors invertebrate species [39]. Zearalenone is linked to reproductive problems in cattle and sheep [64]. Dietary concentrations of zearalenone at 1,000 ppb can lead to hyperestrogenic syndromes in pigs, but higher concentrations can result in disrupted conception and abortion [35].
3. Control of Moulds' Contamination and Mycotoxins
3.1. Regular Surveillance of Mycotoxins in Food and Feedstuffs and Awareness Creation
Food grains and animal feeds need to be harvested correctly, dried completely, and stored properly. African countries need to strengthen nationwide surveillance and increase food and feed inspections to ensure food safety and local education and assistance [18]. Most smallholder farmers lack knowledge on mycotoxin contamination of staples and their health impacts in Africa [65, 66]. Surveys conducted in South Africa showed that people used fungi-contaminated staples for food, implying that they are not fully aware of the health hazards associated with the ingestion of mycotoxins [65]. Alberts et al. [67] noted that the lack of effective and sustained awareness and education of the threat of mycotoxins to human health hinders any mitigation strategy. To find appropriate means to prevent fungal infections of crops, education and training to raise awareness of smallholder farmers and consumers on mycotoxins are important [65, 66]. This could be achieved by creating awareness of the harmful effects of mycotoxins on human and animal health and productivity. The involvement of the government, private organizations, nongovernmental organizations, and national media networks and organizing seminars, workshops, and features in newspapers and magazines are needed to ensure local sustainability of mycotoxin interventions [68]. However, such important continuous efforts are impeded by inadequate research funding and technology in many research institutions that facilitate this surveillance in various African countries and the lack of adequate local experts on this problem [29]. Therefore, both local and international efforts must complement each other to overcome the mycotoxin menace in Africa.
3.2. Preharvest and Postharvest Prevention of Fungal Growth in Crops and Other Feedstuffs
Good Agricultural Practices (GAP) also play a critical role in reducing aflatoxin levels in foods. Proper land preparation, early planting, crop rotation, weeding, correct spacing, cleaning stores, sorting maize after shelling, use of wooden pallets, and stooking were indicated as GAP that led to reduced levels of aflatoxin [69]. Proper drying and storage of crops can effectively reduce mould growth and mycotoxin production. Diversification of crop varieties is also a recommended practice in reducing aflatoxin levels in harvested foods. A higher occurrence of aflatoxin was associated with monocropping systems, subhumid agroecological zone, and samples with broken kernels [43]. Prevention of mould growth can be achieved by following strict hygienic protocols during harvesting, storage, and processing of crops and feeds. It has been demonstrated that early harvesting of groundnuts resulted in lower aflatoxin levels [29]. Analysis of paired grain samples (visually sorted and unsorted) showed that sorting reduced fumonisin by 65%. For instance, an intervention study at subsistence farms conducted in the lower Kindia region of Guinea reported a 60% reduction in mean aflatoxin levels was achieved by thorough drying and proper storage of groundnuts [70]. These studies indicate that the timing of rainfall, rather than the total amount of rainfall, might be important in determining the spatial risk of aflatoxin accumulation.
In some African countries, a high percentage of calories come from maize, which is commonly contaminated by aflatoxins and fumonisins. Besides, the consumption of peanuts is another major source of exposure to aflatoxin [68]. The lack of dietary diversity is directly related to high levels of mycotoxin exposure [71]. Therefore, access to a wider variety of foods and the replacement of those at high risk of contamination will lower the risk of exposure [58].
3.3. Decontamination of Mycotoxin
3.3.1. Physical Approaches
Sorting, washing, and crushing combined with dehulling of maize grains, were effective in the removal of aflatoxin and fumonisin in Benin [72]. Traditional food processing methods are sustainable, practical, and inexpensive postharvest intervention strategies to reduce mycotoxin contamination and exposure [67]. Hand sorting and segregation of crops before cooking is a common practice in many countries in Africa [66, 67]. The postharvest practices for maize include shelling, winnowing, dehulling, and milling [67]. A significant reduction of fumonisins has been demonstrated in several African countries through effective hand sorting of maize [66]. A study of the acoustic-based screening method for mycotoxin contamination indicated a linear inverse relationship between aflatoxin contamination and the amplitude of the acoustic signal penetrating the nut and corn samples [73].
3.3.2. Chemical Approaches
Fungicides, such as cyproconazole, epoxiconazole, tebuconazole, oltipraz, prochloraz, propiconazole, azoxystrobin, and chlorophyllin, have been used to control Fusarium head blight for reduction of fumonisin and aflatoxin contamination [29]. Chemoprotection against aflatoxin has been employed with the use of several chemical compounds, such as oltipraz and chlorophyllin [29]. Dietary substances, including green tea and broccoli sprouts, have exhibited increased detoxification processes in animals and are being considered as effective approaches for reducing the health hazards caused by various mycotoxins [74]. For example, the aflatoxin-induced changes in the liver of mice were significantly reduced with the cotreatment of black tea extract [75]. The introduction of technologies for specific, efficient, and environmentally sound prevention of mycotoxins is inevitable [76]. For instance, the use of improved crop varieties such as maize genotypes with some resistance to fumonisin accumulation has been identified [77]. It is reported that transgenic Bt maize is less prone to insect damage and fumonisin accumulation than non-Bt hybrids [78], but the effectiveness of Bt maize in reducing aflatoxin contamination is still unclear [67].
3.3.3. Biological Methods
Most of the biological control strategies are promising and have shown reduced concentrations of aflatoxins, but the structure and toxicity of the detoxified products remain unclear [76]. Such strategies include breeding of maize-resistant cultivars, the introduction of biocontrol microorganisms, application of phenolic plant extracts, and expression of antifungal proteins and mycotoxins degrading enzymes in transgenic maize cultivars for the development of atoxigenic fungi that compete with toxigenic fungi in the environment [67]. The studies on microbial binding mainly focus on probiotics strains of lactic acid bacteria (LAB), including species of Lactobacillus, Lactococcus, Bifidobacterium spp., and Propionibacterium and yeast strains of Saccharomyces cerevisiae [76].
4. Degradation of Mycotoxins
Different approaches have been developed for the elimination and reduction of mycotoxins in foods and feeds, including physical, chemical, and biological methods. These methods work through modification and destruction of the toxins molecular structure, thereby inhibiting their transfer and absorption in the digestive system and thus reducing toxin's accessibility to the target tissue and eventually removing it [79, 80]. The physical methods include extrusion cooking, which is broadly used in the food industry, especially for cereals and cereal foodstuffs. It is a process that combines exposure to high temperature and high pressure in a very short time [81]. This method can destroy or inactivate aflatoxin and deoxynivalenol in maize flour [82]. The use of hydrated sodium calcium aluminosilicates is another novel and effective strategy to reduce the bioavailability of aflatoxins [83]. Microwave heating, ozone, gamma, and electron beam irradiation, ultraviolet and pulsed light, electrolyzed water, and cold plasma treatments are other good examples of physical methods for mycotoxins detoxification [84]. The drawbacks of physical methods include negative effects on food quality, safety, and human and animal health. The use of high temperature and pressure destroys nutrients in foods such as water-soluble vitamins and proteins.
The chemical strategies used in mycotoxins detoxification include ammoniation, which is regarded as the most advanced and economically practicable technique to detoxify aflatoxins in foodstuffs. Ammoniation treatment has been developed to detoxify ochratoxin A (OTA) in animal feed and alcoholic beverages [85]. Other chemicals that have shown maximum degradation rate in cereals include sodium sulfite, sodium hydrogen sulfate, sodium hydroxide, sodium hypochlorite, and hydrogen peroxide [86]. Neutral electrolyzed oxidizing water (NEW) is one of the two products produced during the process of electrolysis of pure water; this method could potently reduce the content of AFB1 in peanuts [87]. Unfortunately, chemical residues are generally unsafe and economically unrealistic for commercial applications [82].
The biological detoxification of mycotoxins involves fermenting microorganisms and/or enzymes to degrade or transform mycotoxins into less toxic compounds [88]. Various microorganisms have been reported to have the capacity for mycotoxins transformation and detoxification, for example, bacteria (e.g., LAB), yeast, and some moulds [89]. These microorganisms act in the intestinal tract of animals before resorption of the mycotoxins. Biological transformation reactions include acetylation, glycosylation, ring cleavage, hydrolysis, deamination, and decarboxylation [80]. The use of LAB in food fermentation is generally regarded as safe (GRAS) for consumption, they dominate many food fermentation systems and they produce metabolites with antifungal properties [17]. Therefore, this is the most encouraged method for mycotoxins biodetoxification of contaminated agricultural products [90]. Two mechanisms for AF detoxification by microbial methods, i.e., cell wall component adhesion and microbial enzymes, were studied and different LAB species were found to have varied effectiveness of AF detoxification in different food samples [91]. A combination of extrusion and fermentation with two LAB strains was confirmed as a promising innovative pretreatment for wheat bran by enhancing its nutrition value and safety [92].
4.1. LAB from African Fermented Cereal Foods
Traditionally, LAB are used as starter cultures for dairy, vegetable, cereal, and meat fermentations because of their contribution to flavour development and preservative potential [93, 94]. The commonly used LAB belong to the genera Lactobacillus, Lactococcus, Leuconostoc, Oenococcus, Pediococcus, Streptococcus, Tetragenococcus, Aerococcus, Carnobacterium, Enterococcus, Vagococcus, and Weissella [95]. The inhibitory activity of LAB over moulds may result from the production of metabolites such as organic acids (lactic, propionic, and acetic acids), carbon dioxide, ethanol, hydrogen peroxide, diacetyl, and other low-molecular-weight peptides [96, 97]. Other potential underlying mechanisms include competitive growth exclusion, a decrease in the pH caused by acid production, or a combination of these factors [96]. Various LAB are used in the fermentation of cereal-based African foods (Table 2 and Figure 1). The success in producing fermented functional food products is due to the industrial robustness of LAB. Industrially, the probiotic nature of LAB is supplemented with standard gene functions, which improve the activity of LAB under hostile processing conditions [106].
Table 2.
Common lactic acid-fermented cereal-based food products in Africa.
| Product | Substrate | LAB | Nature of use | Region | Reference |
|---|---|---|---|---|---|
| Seketeh | Maize | Lactobacillus plantarum, Lactococcus lactis | Alcohol | Nigeria | [98] |
| Sorghum beer | Sorghum, maize | LAB | Liquid drink, acidic, weakly alcoholic drink | South Africa | [98] |
| Tobwa | Maize | LAB | Nonalcoholic drink | Zimbabwe | [98] |
| Uji | Sorghum, maize, millet | L. paracasei | Nonalcoholic | Kenya, Uganda, Tanzania | [99] |
| Pito | Maize, sorghum, and millet | Leuconostoc mesenteroides | Alcoholic dark brown drink | Nigeria, Ghana | [100] |
| Ogi | Maize, millet sorghum | L. plantarum | Paste as staple, weaning food for babies alcoholic | Nigeria, West Africa | [100] |
| Nasha | Sorghum | LAB | Porridge | Sudan | [98] |
| Mutwiwa | Maize | LAB | Porridge | Zimbabwe | [98] |
| Merissa | Sorghum and millet | LAB | Alcoholic | Sudan | [98] |
| Mahewu | Maize | Lc. lactis subsp. lactis | Solid staple | Zimbabwe | [101] |
| Mawe | Maize | LAB | Basis for preparation of many dishes | South Africa | [98] |
| Kunun-zaki | Maize, sorghum, and millet | L. fermentum, L. leichimani, Streptococcus spp. | Paste used as a breakfast dish | Nigeria | [100] |
| Kisra | Sorghum | LAB | Staple as bread | Sudan | [100] |
| Koko | Maize | L. plantarum, Lb. brevis | Porridge as staple | Ghana | [98] |
| Kenkey | Maize | L. fermentum, L. reuteri | Mush | Ghana | [98] |
| Kaffir beer | Kaffir corn, rice | LAB | Alcoholic drink liquid | South Africa | [98] |
| Injera | Sorghum, tef, maize or wheat | LAB | Bread-like staple | Ethiopia | [102] |
| Ilambazi lokubilisa | Maize | LAB | Porridge as weaning food | Zimbabwe | [98] |
| Doro | Finger millet malt | LAB | Colloidal thick alcoholic drink | Zimbabwe | [98] |
| Banku | Maize | LAB | Dough as staple | Ghana | [98] |
| Burukutu | Sorghum | Leuc. Mesenteroides | Alcoholic drink | Nigeria, Benin, Ghana | [103] |
| Busaa | Rice, millet, maize | L. helveticus, Lb. salivarus, Lb. casei, Lb. brevis, L. plantarum, Lb. buchneri, | Alcoholic | Nigeria, Ghana | [98] |
| Fura | Maize and sorghum | L. plantarum, Pediococcus, Leuconostoc, Streptococcus, Enterococcus | Nigeria | [100] | |
| Agidi | Maize, sorghum and millet | LAB | Nigeria | [98, 100] | |
| Burukutu | Sorghum and millet | Leuc. mesenteroides | West Africa | [100] | |
| Bushera | Sorghum and millet | LAB | Nonalcoholic | Uganda | [104] |
| Tella | Barley, wheat, sorghum, millet, maize, and teff | LAB | Alcoholic | Ethiopia | [105] |
Figure 1.
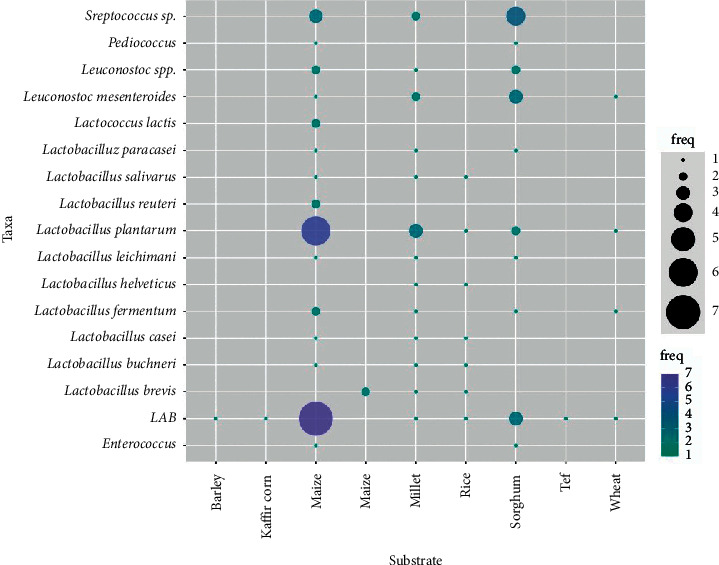
The most commonly used LAB for cereal-based fermentation in Africa (freq = frequency of usage). Adopted with modifications from [98–105].
A study by Moroni et al. [107] reported a broad spectrum of LAB belonging to the genera Lactobacillus, Pediococcus, and Leuconostoc in stable sourdoughs. Buckwheat and teff sourdoughs were dominated mainly by obligate or facultative heterofermentative LAB, which are commonly associated with traditional wheat or rye sourdoughs [108]. Similarly, the spontaneous fermentation of the gluten-free flours resulted in the selection of species such as Pediococcus pentosaceus, Leuconostoc holzapfelii, Lactobacillus gallinarum, L. vaginalis, L. sakei, L. graminis, and Weissella cibaria that are not endemic to traditional sourdoughs [107]. Moroni et al. [107] also reported L. plantarum and L. pontis as the dominant species in all buckwheat and teff sourdough, respectively. A study by Nwachukwu et al. [109] showed various LAB (L. plantarum, L. pentosus, L. cellbiosus, Pediococcus pentosaceus, and Leuconostoc mesenteroides) are involved in the spontaneous fermentation of maize, sorghum, and millet for the production of Nigerian indigenous foods (akamu). Moreover, W. cibaria was detected in both spontaneous and starter-induced type fermentations of buckwheat [107]. Pediococcus spp. are, however, regarded as endemic in Ethiopian traditional fermented foods (such as injera) produced from teff flour. Elsewhere, Corsetti et al. [110] isolated L. graminis from wheat grains.
Members of four LAB genera (Lactobacillus, Leuconostoc, Enterococcus, and Streptococcus) were reported in “kpètè-kpètè,” a traditional beer produced in Benin [111]. Moreover, five species of Lactobacillus (L. fermentum, L. divergens, L. bifermentum, L. fructivorans, L. casei, and L. acidophilus) were identified in the same study. In another study, species of the genus Lactobacillus (L. divergens, L. fermentum, L. bifermentum, L. fructivorans, L. viridescens, L. hilgardii, L. kandleri, and L. casei) were reported as dominant in traditional beer “tchoukoutou” [112]. According to Vieira-Dalodé et al. [113], L. fermentum was the most predominant species; however, also the presence of Leuconostoc mesenteroides was noted during the process of “gowé” fermentation. Likewise, L. fermentum was found to dominate in “bushera,” a Ugandan traditional fermented beverage [104].
In Africa, the fermentation technology is based on the ability of LAB to break down complex molecules (substrate) into simpler compounds as the preferred functional products (Table 2 and Figure 1). Maize, millet, and sorghum are the common substrates for the fermented product, with L. plantarum as the most preferred LAB, followed by Leuconostoc mesenteroides (Figure 1). Other LAB are also useful and mostly used in combination with other stable species to produce a blend of a functional product. Microbial fermentation coupled with biotechnology and biotechnological processing techniques provides a variety of tools to modify cereal products.
4.1.1. Mechanism of Mycotoxin Detoxification by LAB
Mycotoxins detoxification by LAB is achieved through either the viable cell, their enzymes, or the bioactive metabolites they produce, resulting in the limitation of the growth of fungi and prevention of the production of mycotoxins in food [114], as illustrated in Figure 2. The metabolites produced work via binding and absorption of mycotoxins onto the bacterial cell walls and utilizing them as primary sources of carbon, nitrogen, and phosphorus in the production of enzymes responsible for the degradation (Table 3) [136]. Aflatoxins are also removed by LAB through the biodegradation/bioadsorption mechanisms via direct binding based on probiotic affinity toward aflatoxins [137–139]. Biodegradation of modified AFB1 structure can result in undesirable metabolites (such as aflatoxin), which are probably harmful to the host [137–139]. The activities of aflatoxin toxicity are affected by two sites of furofuran and lactone rings. Any variations in the coumarin structure can change mutagenic properties of the aflatoxins; during aflatoxin detoxification, the difuran ring of the mycotoxin is cleaved usually by specific microorganisms such as Lactobacillus spp. and Bifidobacteria spp. or their enzymatic metabolites [138], as indicated in Figure 2. Previous reports indicate that AFB1 can be removed by probiotic bacteria through physical adhesion and binding to the carbohydrate components of the bacterial cells [140].
Figure 2.
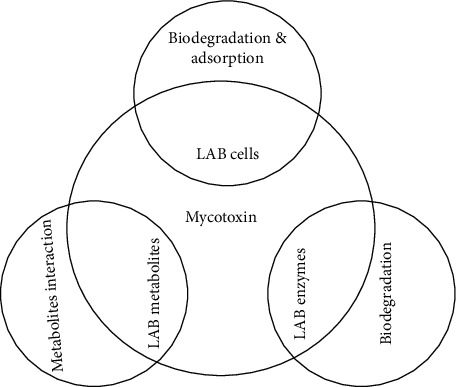
Mechanisms of mycotoxin detoxification by lactic acid bacteria.
Table 3.
Biodetoxification of mycotoxin by LAB strains.
| Mycotoxins | LAB | Mechanism of detoxification | Reference |
|---|---|---|---|
| AFB1 | L. plantarum MON03 | Binding | [115] |
| L. plantarum C88 | Binding | [116] | |
| L. kefiri KFLM3 | Adsorption and biotransformation | [117] | |
| L. acidophilus and L. brevis | Binding | [118] | |
| L. rhamnosus yoba 2012 | Binding | [119] | |
| L. fermentum TMU121 and Pediococcus pentosaceus TMU457 | Binding | [120] | |
| L. casei, L. plantarum, and L. fermentum | Binding | [121] | |
| L. reuteri ŁOCK 1096 and L. casei ŁOCK 0911 | Binding | [122] | |
| P. pentosaceus L6, L. plantarum L12, Leuconostoc mesenteroides L18, L. coryniformis subsp. coryniformis L47, L. brevis L52 | Binding and adsorption | [123] | |
| Enterococcus faecium M74 | Binding | [124] | |
| AFM1 | L. plantarum MON03 and L. rhamnosus GAF01 | Binding | [125] |
| L. delbrueckii spp. bulgaricus LB340, L. rhamnosus HOWARU, and Bifidobacterium lactis FLORA-FIT BI07 | Binding | [126] | |
| L. bulgaricus and Streptococcus thermophiles | Binding | [127] | |
| L. plantarum ATCC 10697, B. animalis ATCC 27672, and B. bifidum ATCC 35914 | Binding | [128] | |
| OTA | L. acidophilus VM 20 | Binding | [129] |
| L. rhamnosus CECT 278T and L. plantarum CECT 749 | Adsorption | [85] | |
| PAT | Bifidobacterium animalis VM 12 | Binding | [129] |
| Enterococcus faecium M74 | Binding | [124] | |
| DON | Various LAB | Adsorption | [130] |
| L. plantarum LP102 | Binding | [131] | |
| L. paracasei LHZ-1 | Adsorption | [132] | |
| ZEN | L. pentosus | Adsorption | [133] |
| L. plantarum | Binding | [134] | |
| L. rhamnosus GG/LC705 | Binding | [64] | |
| FB1/B2 | L. brevis, L. plantarum, L. paracasei, L. casei, L. pentosus, L. reuteri, and L. rhamnosus | Binding | [122] |
| L. plantarum B7 and L. pentosus X8 | Binding | [135] | |
| T-2 toxin | L. plantarum LP102 | Binding | [131] |
AFB1: aflatoxins B1; AFM1: aflatoxin M1; OTA: ochratoxin A; PAT: patulin; DON: deoxynivalenol; ZEN: zearalenone; FB1/B2: fumonisin B1/B2; T-2 toxin: trichothecenes-2 toxin [79].
Many LAB strains are safe microorganisms, easy to grow on a large scale, and a common byproduct of the food industry. They also have a great capacity for adsorption and/or uptake of various kinds of chemical contaminants that are present in an aqueous solution. The mechanisms of interaction of LAB with food contaminants are varied (Figure 2) and depend on the nature of the contaminant, the microbial strain, and the physicochemical conditions [141].
4.2. Natural Antifungal Compounds Produced by LAB
The growth of fungi and the production of mycotoxin on food and feed is the major cause of food poisoning and human health concerns. The use of fungicides and other chemicals in food preservation negatively affects health and the environment. However, biopreservative agents, especially from LAB, have been reported to be safe, effective, and biodegradable and can confer health benefits to consumers [142]. Furthermore, Shehata et al. [142] showed broad antimicrobial activity of cell-free supernatant of certain members of Lactobacillus spp. against toxigenic fungi. As shown in Table 4, various active antimicrobial compounds are produced by LAB during bacterial food fermentation, for example, hydroxyl fatty acids, lactic, benzoic, and acetic acids, ethanol, carbon dioxide, hydrogen peroxide, and bacteriocins [142]. Various members of Lactobacillus spp. have been reported to produce novel antimicrobial compound 3-phenyllactic acid (PLA) with broad-spectrum antifungal activity against different food spoilage and toxigenic moulds, such as Aspergillus flavus and Penicillium expansum [143]. LAB properties to inhibit pathogens have been associated with the low acidity of the fermentable substrates [166].
Table 4.
Lactic acid bacteria and their active compounds against mycotoxin-producing fungi.
| LAB strain | Activity spectrum | Active compounds | Reference |
|---|---|---|---|
| Lactobacillus casei, L. rhamnosus, L. fermentum, L. acidophilus, L. plantarum, L. sakei, and L. reuteri | Penicillium expansum and Aspergillus flavus | 3-Phenyllactic acid | [143] |
| L. sanfranciscensis CB1 | Fusarium spp., Penicillium spp., Aspergillus spp., Monilia spp. | Caproic acids, propionic acid, butyric acid, n-valeric acid | [144] |
| L. plantarum 21B | Broad-spectrum | 4-Hydroxy-phenyllactic acids | [145] |
| L. plantarum MiLAB 393 | Broad-spectrum | 3-Phenyllactic acid, cyclo(Phe-Pro), cyclo(Phe-OH-Pro) | [146] |
| L. coryniformis Si3 | Broad-spectrum | Cyclic dipeptides, phenyllactic acid, cyclo(Phe-Pro), cyclo(Phe-OH-Pro) | [147] |
| L. plantarum MiLAB 14 | Broad-spectrum | Hydroxy fatty acids | [148] |
| L. plantarum K35 | A. flavus and A. parasiticus | Lactic acid, 2-butyl-4-hexyloctahydro-1H-indene, oleic acid, palmitic acid, linoleic acid, 2,4-di-tert-butylphenol, stearic acid, 3-phenyllactic acid, and pyroglutamic acid | [149] |
| L. plantarum FST 1.7 | F. culmorum and F. graminearum | Lactic acid, phenyllactic acid, cyclic dipeptides cyclo(l-Leu-l-Pro), and cyclo (l-Phe-l-Pro) | [150] |
| L. plantarum AF1 | A. flavus | C12H22N2O2, 3,6-bis(2-methylpropyl)-2,5-piperazinedion | [151] |
| L. pentosus TV35b | Candida albicans | Pentocin TV35b | [152] |
| L. plantarum VE56, Weissella cibaria FMF4B16, and W. paramesenteroides LC11. | Broad-spectrum | Phenyllactic acid and 2-hydroxy-4-methylpentanoic acid | [153] |
| L. plantarum IMAU10014 | Broad-spectrum | 3-Phenyllactic acid, benzeneacetic acid, and 2-propenyl ester | [154] |
| L. casei AST18 | Penicillium sp. | Cyclo-(Leu-Pro), 2,6-diphenyl-piperidine, and 5,10-diethoxy-2,3,7,8-tetrahydro-1H, 6H-dipyrrolo[1,2-a; 1′,2′-d] pyrazine | [155] |
| L. hammesii DSM 16381 | Mucor plumbeus, A. niger, and P. roqueforti | Monohydroxy C18:1 fatty acid | [156] |
| L. reuteri ee1p | P. expansum, Trichophyton tonsurans | (S)-(-)-2-hydroxyisocaproic acid, hydrocinnamic acid, phenyllactic acid, decanoic acid, azelaic acid, 4-hydroxybenzoic acid, p-coumaric acid, vanillic acid, DL-b-hydroxyphenyllactic acid, and 3-hydroxydecanoic acid | [157] |
| L. amylovorus DSM 19280 | A. fumigatus and F. culmorum | Carboxylic acids, cinnamic acid derivatives (3-phenylpropanoic acid, p-coumaric, and (E)-2-methylcinnamic acid), 3-phenyllactic acid and its hydroxy derivative (3-(4-hydroxyphenyl), lactic acid, acetic acid, D-glucuronic acid and salicylic acid, nucleosides (cytidine and 2′-deoxycytidine), sodium decanoate, and cyclic dipeptides | [158] |
| L. plantarum LB1 and L. rossiae LB5 | P. roqueforti DPPMAF1 | Organic acids (formic acid) and four antifungal peptides | [159] |
| L. plantarum TE10 | A. flavus MD3 | Two antifungal peptides (VLHEPLF and ALKAAPSPA) | [160] |
| Lactobacillus sp. RM1 | A. parasiticus | 6-Octadecenoic acid methyl ester, hexadecanoic acid methyl ester, phenol, 2,4-bis(1,1-dimethylethyl), (Z)-7-hexadecenal, pentadecane, dotriacontane, and 2-methyldecane | [142] |
| L. plantarum UM55 | A. flavus, A. parasiticus, A. arachidicola, A. nomius, and A. minisclerotigenes | Lactic acid, phenyllactic acid, hydroxyphenyllactic acid, and indole lactic acid | [161] |
| L. reuteri | A. niger | n-Decanoic acid, 3- hydroxydecanoic acid and 3-hydroxydodecanoic acid | [162] |
| L. fermentum CRL 251 | A. niger, Penicillium sp., and F. graminearum | Lactic, acetic, and phenyllactic acids | [163] |
| Leuconostoc citreum L123, L. brevis Lu35, L. reuteri 5529, L. spicheri O15, and Propionibacterium freudenreichii LSaci68 | P. corylophilum and A. niger | Lactic, acetic, and propionic acids, ethanol and hydrogen peroxide, phenyllactic, hydroxyphenyllactic, azelaic, and caproic acids | [164] |
| L. plantarum UFG 121 | Broad-spectrum | Lactic acid and phenyllactic acid | [165] |
Studies by Ahlberg et al. [167] showed that Kenyan traditionally fermented maize and milk-based products contain various Lactobacillus strains with growth inhibitory activities against aflatoxin-producing A. flavus moulds. Literature indicated that L. plantarum 21B isolated from sourdough showed antifungal activity against different toxigenic and food spoilage moulds [145]. The study further showed that the antifungal compounds responsible for such antifungal activities included phenyllactic and 4-hydroxy-phenyllactic acids [145]. Additionally, various researchers have shown that L. coryniformis, L. casei, L. amylovorus, and L. plantarum produce antifungal cyclic dipeptides, such as cyclo(Phe-Pro), cyclo(Phe-OH-Pro), cyclo-(Leu-Pro), 2,6-diphenyl-piperidine, 5,10-diethoxy-2,3,7,8-tetrahydro-1H, and 6H-dipyrrolo[1,2-a; 1′,2′-d] pyrazine [150, 155]. They can also produce hydroxy fatty acids against a broad spectrum of food spoilage moulds [148, 149]. L. hammesii DSM 16381 was reported to produce monohydroxy C18:1 fatty acid with antifungal activities against Mucor plumbeus, A. niger, and P. roqueforti [156].
5. Conclusion and Recommendations
The harmful effects of mycotoxins in the food and feeds industry are numerous, ranging from health to economic losses. The mycotoxin menace has taken a toll on the African continent, as evidenced by the numerous episodes of aflatoxicosis aggravated by the warm weather and hence the need to come up with solutions that are native, economical, sustainable, and effective. LAB are naturally available in fermented foods in Africa and can be effective biological agents for controlling mycotoxins due to the wide variety of antifungal metabolites that they produce. The biological control of mycotoxins through LAB is a superior alternative over chemical and physical methods, which have negative health effects as LAB are generally regarded as safe, effective, and able to confer health benefits. LAB causes detoxification of mycotoxins through binding and absorption of the mycotoxins by the bacterial cell, where they are degraded and utilized as nutrient sources leading to the production of enzymes and other metabolites.
It is important that more strains of LAB are isolated from indigenous African foods and together with the known strains, they should be optimized for the detoxification of mycotoxins. More optimization also needs to be done to come up with the most effective consortia of LAB that are most effective against specific types of mycotoxins. An in-depth analysis of the LAB culture supernatants and separation of the compounds therein could give fractions of the compound that may be very effective against the mycotoxins in their pure state.
Data Availability
The reference data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Eliud N. Wafula contributed to the conceptualization. Eliud N. Wafula, Josiah O. Kuja, Eddy E. Owaga, Christabel N. Muhonja, Huxley M. Makonde, Julius M. Mathara, and Virginia W. Kimani wrote and prepared the original draft. Eliud N. Wafula and Josiah O. Kuja were responsible for the statistical review and graphical interpretation. Eliud N. Wafula, Josiah O. Kuja, Christabel N. Muhonja, and Virginia W. Kimani contributed to manuscript formatting and proofreading.
References
- 1.Macauley H., Ramadjita T. Feeding Africa: United Nations Economic Commission for Africa . United Nations Economic Commission for Africa; 2015. Cereal crops: rice, maize, millet, sorghum, wheat. [Google Scholar]
- 2.Awika J. M. ACS Symposium Series . Washington, DC, USA: American Chemical Society; 2011. Major cereal grains production and use around the world. https://pubs.acs.org/sharingguidelines . [Google Scholar]
- 3.Mkumbwa S. S. Cereal Food Commodities in Eastern Africa: Consumption–Production Gap Trends and Projections for 2020 . London, UK: MPRA; 2011. [Google Scholar]
- 4.MOALFI Agricultural. Sector Transformation and Growth Strategy: Towards Sustainable Agricultural Transformation and Food Security in Kenya . Pavia, Italy: MOALFI. Agricultural; 2018. [Google Scholar]
- 5.Kilonzo R. M., Imungi J. K., Muiru W. M., Lamuka P. O., Njage P. M. K. Household dietary exposure to aflatoxins from maize and maize products in Kenya. Food Additives & Contaminants: Part A . 2014;31(12):2055–2062. doi: 10.1080/19440049.2014.976595. [DOI] [PubMed] [Google Scholar]
- 6.Piotrowska M., Slizewska K., Biernasiak J. Soybean-Pest Resistance . West Palm Beach, FL, USA: InTech; 2013. Mycotoxins in cereal and soybean-based food and feed. http://www.intechopen.com/books/soybean-pest-resistance/mycotoxins-in-cereal-and-soybean-based-food-and-feed . [Google Scholar]
- 7.Eskola M., Kos G., Elliott C. T., Hajšlová J., Mayar S., Krska R. Worldwide contamination of food-crops with mycotoxins: validity of the widely cited “FAO estimate” of 25. Critical Reviews in Food Science and Nutrition . 2020;60(16) doi: 10.1080/10408398.2019.1658570. [DOI] [PubMed] [Google Scholar]
- 8.Kamala A., Ortiz J., Kimanya M., Haesaert G., Donoso S., Tiisekwa B. Multiple mycotoxin co-occurrence in maize grown in three agro-ecological zones of Tanzania. Food Control . 2015;54:208–215. doi: 10.1016/j.foodcont.2015.02.002. [DOI] [Google Scholar]
- 9.Orina I., Manley M., Williams P. J. Non-destructive techniques for the detection of fungal infection in cereal grains. Food Research International . 2017;100:74–86. doi: 10.1016/j.foodres.2017.07.069. [DOI] [PubMed] [Google Scholar]
- 10.Mwakinyali S. E., Ding X., Ming Z., Tong W., Zhang Q., Li P. Recent development of aflatoxin contamination biocontrol in agricultural products. Biological Control . 2019;128:31–39. doi: 10.1016/j.biocontrol.2018.09.012. [DOI] [Google Scholar]
- 11.Wagacha J. M., Muthomi J. W. Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies. International Journal of Food Microbiology . 2008 May;124(1):1–12. doi: 10.1016/j.ijfoodmicro.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Mikušová P., Šrobárová A., Sulyok M., Santini A. Fusarium fungi and associated metabolites presence on grapes from Slovakia. Mycotoxin Research . 2013;29(2):97–102. doi: 10.1007/s12550-013-0157-z. [DOI] [PubMed] [Google Scholar]
- 13.Chauhan Y. S., Wright G. C., Rachaputi N. C. Modelling climatic risks of aflatoxin contamination in maize. Australian Journal of Experimental Agriculture . 2008;48(3):p. 358. doi: 10.1071/ea06101. [DOI] [Google Scholar]
- 14.Cotty P. J., Jaime-Garcia R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. International Journal of Food Microbiology . 2007;119(1–2):109–15. doi: 10.1016/j.ijfoodmicro.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 15.Gong Y. Y., Watson S., Routledge M. N. Aflatoxin exposure and associated human health effects, a review of epidemiological studies. Food Safety . 2016;4:14–27. doi: 10.14252/foodsafetyfscj.2015026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owaga E., Muga R., Mumbo H., Aila F. Chronic dietary aflatoxins exposure in Kenya and emerging public health concerns of impaired growth and immune suppression in children. International Journal of Biological and Chemical Sciences . 2011;5(3):1325–1336. doi: 10.4314/ijbcs.v5i3.72287. [DOI] [Google Scholar]
- 17.Hassan Y. I., Zhou T., Bullerman L. B. Sourdough lactic acid bacteria as antifungal and mycotoxin-controlling agents. Food Science and Technology International . 2016;22:79–90. doi: 10.1177/1082013214565722. [DOI] [Google Scholar]
- 18.Tola M., Kebede B. Occurrence, importance and control of mycotoxins: a review. Cogent Food Agric . 2016;2(1):1–12. doi: 10.1080/23311932.2016.1191103. [DOI] [Google Scholar]
- 19.Ezekiel C., Ortega-Beltran A., Bandyopadhyay R. The need for integrated approaches to address food safety risk: the case of mycotoxins in Africa. Proceedings of the First FAO/WHO/AU International Food Safety Conference; February 2019; Addis Ababa, Ethiopia. [Google Scholar]
- 20.Ansari A., Aman A., Siddiqui N. N., Iqbal S., Ali ul Qader S. Bacteriocin (BAC-IB17): screening, isolation and production from Bacillus subtilis KIBGE IB-17. Pakistan Journal of Pharmaceutical Sciences . 2012;25(1):195–201. [PubMed] [Google Scholar]
- 21.Gálvez A., Abriouel H., Benomar N., Lucas R. Microbial antagonists to food-borne pathogens and biocontrol. Current Opinion in Biotechnology . 2010;21:142–148. doi: 10.1016/j.copbio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Ghanbari M., Jami M., Domig K. J., Kneifel W. Seafood biopreservation by lactic acid bacteria - a review. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology . 2013;54(2):315–324. doi: 10.1016/j.lwt.2013.05.039. [DOI] [Google Scholar]
- 23.Kagot V., Okoth S., De Boevre M., De Saeger S. Biocontrol of Aspergillus and Fusarium mycotoxins in Africa: benefits and limitations. Toxins . 2019;11(2):p. 109. doi: 10.3390/toxins11020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva J., Carvalho A. S., Teixeira P., Gibbs P. A. Bacteriocin production by spray-dried lactic acid bacteria. Letters in Applied Microbiology . 2002;34:77–81. doi: 10.1046/j.1472-765x.2002.01055.x. [DOI] [PubMed] [Google Scholar]
- 25.Bianchini A. Advances in Fermented Foods and Beverages: Improving Quality, Technologies and Health Benefits . Amsterdam, Netherlands: Elsevier; 2014. Lactic acid bacteria as antifungal agents. [Google Scholar]
- 26.United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development Goals . New York, NY, USA: 2015. https://sustainabledevelopment.un.org/post2015/transformingourworld/publication . [Google Scholar]
- 27.African Union. Agenda2063 Report of the Commission on the African Union Agenda 2063: The Africa We Want in 2063 . Addis Ababa, Ethiopia: African Uninon; 2015. https://au.int/en/agenda2063/overview . [Google Scholar]
- 28.Schaarschmidt S., Fauhl-Hassek C. The fate of mycotoxins during the processing of wheat for human consumption. Comprehensive Reviews in Food Science and Food Safety . 2018;17(3):556–593. doi: 10.1111/1541-4337.12338. [DOI] [PubMed] [Google Scholar]
- 29.Darwish W. S., Ikenaka Y., Nakayama S. M. M., Ishizuka M. An overview on mycotoxin contamination of foods in Africa. Journal of Veterinary Medical Science . 2014;76(6):789–797. doi: 10.1292/jvms.13-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elias N. K. S. Aflatoxins: a silent threat in developing countries. African Journal of Biotechnology . 2016;15(35):1864–1870. doi: 10.5897/ajb2016.15305. [DOI] [Google Scholar]
- 31.Smith L. E., Stasiewicz M., Hestrin R., Morales L., Mutiga S., Nelson R. J. Examining environmental drivers of spatial variability in aflatoxin accumulation in Kenyan maize: potential utility in risk prediction models. African Journal of Food, Agriculture, Nutrition and Development . 2016;16(3):11086–11105. doi: 10.18697/ajfand.75.ilri09. [DOI] [Google Scholar]
- 32.Sirma A. J., Ouko E. O., Murithi G., Mburugu C., Mapenay I., Ombui J. Prevalence of aflatoxin contamination in cereals from Nandi county, Kenya. International Journal of Agricultural Sciences and Veterinary Medicine . 2015;3(3):1–9. [Google Scholar]
- 33.Aflatoxins W. HO. Food Safety Digest . Geneva, Switzerland: WHO; 2018. https://www.who.int/foodsafety/FSDigest_Aflatoxins_EN.pdf . [Google Scholar]
- 34.Seid A., Mama A. Aflatoxicosis and occurrence of aflatoxin M1 (AFM1) in milk and dairy products: a review. Austin Journal of Veterinary Science & Animal Husbandry . 2019;1(1):1–12. [Google Scholar]
- 35.Bennett J. W., Klich M. Mycotoxins. Clinical Microbiology Reviews . 2003;16(3):497–516. doi: 10.1128/cmr.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Proctor R. H., McCormick S. P., Kim H.-S., et al. Evolution of structural diversity of trichothecenes, a family of toxins produced by plant pathogenic and entomopathogenic fungi. PLoS Pathogens . 2018;14(4):p. e1006946. doi: 10.1371/journal.ppat.1006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winter G., Pereg L. A review on the relation between soil and mycotoxins: effect of aflatoxin on field, food and finance. European Journal of Soil Science . 2019;70(4) doi: 10.1111/ejss.12813. [DOI] [Google Scholar]
- 38.Mostrom M. Encyclopedia of Food and Health . 1st. Addis Ababa, Ethiopia: Elsevier; 2016. Mycotoxins: classification. [Google Scholar]
- 39.Evans T. J., Gupta R. C. Veterinary Toxicology . Addis Ababa, Ethiopia: Elsevier; 2018. Tremorgenic mycotoxins. [DOI] [Google Scholar]
- 40.Barrett J. R. Liver cancer and aflatoxin: new information from the Kenyan outbreak. Environmental Health Perspectives . 2005;113 doi: 10.1289/ehp.113-a837. [DOI] [Google Scholar]
- 41.Gong Y. Y., Wilson S., Mwatha J. K., et al. Aflatoxin exposure may contribute to chronic hepatomegaly in Kenyan school children. Environmental Health Perspectives . 2012;120(6):893–896. doi: 10.1289/ehp.1104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mwihia J. T., Straetmans M., Ibrahim A., et al. Aflatoxin levels in locally grown maize from makueni district, Kenya. East African Medical Journal . 2008;85(7):311–317. doi: 10.4314/eamj.v85i7.9648. [DOI] [PubMed] [Google Scholar]
- 43.Mutiga S. K., Were V., Hoffmann V., Harvey J. W., Milgroom M. G., Nelson R. J. Extent and drivers of mycotoxin contamination: inferences from a survey of Kenyan maize mills. Phytopathology . 2014 Nov;104(11):1221–1231. doi: 10.1094/phyto-01-14-0006-r. [DOI] [PubMed] [Google Scholar]
- 44.Malusha J., Karama M., Makokha A. N. Comparative analysis of aflatoxin contamination of maize in two different physiographic zones and maize seasons in makueni county, Kenya. East African Medical Journal . 2015;92(3):126–135. [Google Scholar]
- 45.Sirma A. Aflatoxin B1 occurrence in millet, sorghum and maize from four agro-ecological zones in Kenya. African Journal of Food, Agriculture, Nutrition and Development . 2016;16 doi: 10.18697/ajfand.75.ilri03. [DOI] [Google Scholar]
- 46.Chebon S., Aila M. Mycological quality of relief maize meal and nutritional supplement of maize product (unimix) in moyale, northern Kenya. Microbiology Research Journal International . 2017;21(1):1–11. doi: 10.9734/mrji/2017/28684. [DOI] [Google Scholar]
- 47.Kang’ethe E. K., Gatwiri M., Sirma A. J., Ouko E. O., Mburugu-Musoti C. K., Kitala P. M. Exposure of Kenyan population to aflatoxins in foods with special reference to Nandi and Makueni counties. Food Quality and Safety . 2017;1(2):131–137. [Google Scholar]
- 48.Hussein H., Brasel J. M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology . 2001 Oct;167(2):101–134. doi: 10.1016/s0300-483x(01)00471-1. [DOI] [PubMed] [Google Scholar]
- 49.Lewis L., Onsongo M., Njapau H., et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and Central Kenya. Environmental Health Perspectives . 2005;113(12):1763–1767. doi: 10.1289/ehp.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang’Ethe E. K., Lang’A K. A. Aflatoxin B1 and M1 contamination of animal feeds and milk from urban centers in Kenya. African Health Sciences . 2009;9(4):218–226. [PMC free article] [PubMed] [Google Scholar]
- 51.James B., Adda C., Cardwell K., Annang D., Hell K., Korie S. Public information campaign on aflatoxin contamination of maize grains in market stores in Benin, Ghana and Togo. Food Additives & Contaminants . 2007;24:1283–1291. doi: 10.1080/02652030701416558. [DOI] [PubMed] [Google Scholar]
- 52.Williams J. H., Phillips T. D., Jolly P. E., Stiles J. K., Jolly C. M., Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. American Journal of Clinical Nutrition . 2004;80:1106–22. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- 53.Mutegi C. K., Wagacha J. M., Christie M. E., Kimani J., Karanja L. Effect of storage conditions on quality and aflatoxin contamination of peanuts (Arachis hypogaea L.) Int Acad Journals Int J AgriScience . 2013;3(10):746–758. [Google Scholar]
- 54.Ostry V., Malir F., Toman J., Grosse Y. Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Research . 2017;33(1):65–73. doi: 10.1007/s12550-016-0265-7. [DOI] [PubMed] [Google Scholar]
- 55.Fouad A. M., Ruan D., El Senousey H. A. K., Chen W., Jiang S., Zheng C. Harmful effects and control strategies of aflatoxin B1 produced by aspergillus flavus and aspergillus parasiticus strains on poultry. The Review . 2019;11 doi: 10.3390/toxins11030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bosetti C., Turati F., La Vecchia C. Hepatocellular carcinoma epidemiology. Best Practice & Research Clinical Gastroenterology . 2014;28(5):753–770. doi: 10.1016/j.bpg.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y., Wu F. Global burden of Aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environmental Health Perspectives . 2010;118(6):818–824. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groopman J. D., Kensler T. W., Wild C. P. Protective interventions to prevent aflatoxin-induced carcinogenesis in developing countries. Annual Review of Public Health . 2008;29(1):187–203. doi: 10.1146/annurev.publhealth.29.020907.090859. [DOI] [PubMed] [Google Scholar]
- 59.Gong Y., Hounsa A., Egal S., Turner P. C., Sutcliffe A. E., Hall A. J. Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, west Africa. Environmental Health Perspectives . 2004;112:1334–1338. doi: 10.1289/ehp.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borutova R., Aragon Y. A., Nährer K., Berthiller F. Co-occurrence and statistical correlations between mycotoxins in feedstuffs collected in the Asia–Oceania. Animal Feed Science and Technology . 2012;178 doi: 10.1016/j.anifeedsci.2012.09.015. [DOI] [Google Scholar]
- 61.Wakhisi J., Patel K., Buziba N., Rotich J. Esophageal cancer in north rift valley of western Kenya. African Health Sciences . 2005;5(2):157–163. [PMC free article] [PubMed] [Google Scholar]
- 62.Who. Co-exposure of Fumonisins with Aflatoxins . Geneva, Switzerland: WHO; 2018. [Google Scholar]
- 63.Jeswal P., Kumar D. Mycobiota and natural incidence of aflatoxins, ochratoxin A, and citrinin in Indian spices confirmed by LC-MS/MS. International Journal of Microbiology . 2015;2015:8. doi: 10.1155/2015/242486.242486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El-Nezami H., Polychronaki N., Salminen S., Mykkänen H. Binding rather than metabolism may explain the interaction of two food-grade Lactobacillus strains with zearalenone and its derivative ɑ́-zearalenol. Applied and Environmental Microbiology . 2002;68:3545–3549. doi: 10.1128/aem.68.7.3545-3549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mboya R. M., Kolanisi U. Subsistence farmers’ mycotoxin contamination awareness in the SADC region: implications on millennium development goal 1, 4 and 6. Journal of Human Ecology . 2014;46(1):21–31. doi: 10.1080/09709274.2014.11906702. [DOI] [Google Scholar]
- 66.Matumba L., Monjerezi M., Kankwamba H., et al. Knowledge, attitude, and practices concerning presence of molds in foods among members of the general public in Malawi. Mycotoxin Research . 2016;32:27–36. doi: 10.1007/s12550-015-0237-3. [DOI] [PubMed] [Google Scholar]
- 67.Alberts J. F., van Zyl W. H., Gelderblom W. C. Biologically based methods for control of fumonisin-producing Fusarium species and reduction of the fumonisins. Frontiers in Microbiology . 2016;7:p. 548. doi: 10.3389/fmicb.2016.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Misihairabgwi J. M., Ezekiel C. N., Sulyok M., Shephard G. S., Krska R. Mycotoxin contamination of foods in Southern Africa: a 10-year review. Critical Reviews in Food Science and Nutrition . 2007;59 doi: 10.1080/10408398.2017.1357003. [DOI] [PubMed] [Google Scholar]
- 69.Marete G. N., Kanja L. W., Mbaria J. M., et al. Effects of the use of good agricultural practices on aflatoxin levels in maize grown in Nandi county, Kenya. Science . 2020;2(4):p. 85. doi: 10.3390/sci2040085. [DOI] [Google Scholar]
- 70.Turner P. C., Sylla A., Gong Y. Y., et al. Reduction in exposure to carcinogenic aflatoxins by postharvest intervention measures in west Africa: a community-based intervention study. Lancet . 2005;365:1950–1956. doi: 10.1016/S0140-6736(05)66661-5. [DOI] [PubMed] [Google Scholar]
- 71.Wu F., Mitchell N. J., Male D., Kensler T. W. Reduced foodborne toxin exposure is a benefit of improving dietary diversity. Toxicological Sciences: An Official Journal of the Society of Toxicology . 2014;141:329–34. doi: 10.1093/toxsci/kfu137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fandohan P., Ahouansou R., Houssou P., Hell K., Marasas W. F., Wingfield M. J. Impact of mechanical shelling and dehulling on Fusarium infection and fumonisin contamination in maize. Food Additives and Contaminants . 2006;23:415–21. doi: 10.1080/02652030500442516. [DOI] [PubMed] [Google Scholar]
- 73.Juodeikiene G., Cernauskas D., Trakselyte-Rupsiene K., et al. Acoustic-based screening method for the detection of total aflatoxin in corn and biological detoxification in bioethanol production. Frontiers in Microbiology . 20205;11(4):p. 543. doi: 10.3389/fmicb.2020.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kensler T. W., Egner P. A., Wang J.-B., et al. Chemoprevention of hepatocellular carcinoma in aflatoxin endemic areas. Gastroenterology . 2004;127(5):S310–S318. doi: 10.1053/j.gastro.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 75.Jha A., Shah K., Verma R. J. Aflatoxin-induced biochemical changes in liver of mice and its mitigation by black tea extract. Acta Poloniae Pharmaceutica . 2012;69(5):851–857. [PubMed] [Google Scholar]
- 76.Guan S., Zhou T., Yin Y., Xie M., Ruan Z., Young J. Microbial strategies to control aflatoxins in food and feed. World Mycotoxin Journal . 2011;4(4):413–424. doi: 10.3920/wmj2011.1290. [DOI] [Google Scholar]
- 77.Santiago R., Cao A., Malvar R. A., Reid L. M., Butrón A. Assessment of corn resistance to fumonisin accumulation in a broad collection of inbred lines. Field Crops Research . 2013;149:193–202. doi: 10.1016/j.fcr.2013.04.011. [DOI] [Google Scholar]
- 78.Abbas H. K., Zablotowicz R. M., Weaver M. A., et al. Implications of Bt traits on mycotoxin contamination in maize: overview and recent experimental results in southern United States. Journal of Agricultural and Food Chemistry . 2013;61(48):11759–11770. doi: 10.1021/jf400754g. [DOI] [PubMed] [Google Scholar]
- 79.Afshar P., Shokrzadeh M., Raeisi S. N., Ghorbani-HasanSaraei A., Nasiraii L. R. Aflatoxins biodetoxification strategies based on probiotic bacteria. Toxicon: Official Journal of the International Society on Toxinology . 2020;178:50–58. doi: 10.1016/j.toxicon.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 80.Hathout A. S., Aly S. E. Biological detoxification of mycotoxins: a review. Annals of Microbiology . 2014;64(3):905–919. doi: 10.1007/s13213-014-0899-7. [DOI] [Google Scholar]
- 81.Cheftel J. C. Nutritional effects of extrusion-cooking. Food Chemistry . 1986;20(4):263–283. doi: 10.1016/0308-8146(86)90096-8. [DOI] [Google Scholar]
- 82.Peng Z., Chen L., Zhu Y., Huang Y., Hu X., Wu Q. Current major degradation methods for aflatoxins: a review. Trends in Food Science & Technology . 2018;80 doi: 10.1016/j.tifs.2018.08.009. [DOI] [Google Scholar]
- 83.Bingham A. K., Huebner H. J., Phillips T. D., Bauer J. E. Identification and reduction of urinary aflatoxin metabolites in dogs. Food and Chemical Toxicology . 2004;42 doi: 10.1016/j.fct.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 84.Pankaj S. K., Shi H., Keener K. M. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends in Food Science & Technology . 2018;71 doi: 10.1016/j.tifs.2017.11.007. [DOI] [Google Scholar]
- 85.Luz C., Ferrer J., Mañes J., Meca G. Toxicity reduction of ochratoxin A by lactic acid bacteria. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association . 2018;112:60–66. doi: 10.1016/j.fct.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 86.Mukendi N., Rollmann B., de Meester C. Detoxification of aflatoxin B1 by different chemical methods and evaluation of the effectiveness of the treatments applied. Journal de Pharmacie de Belgique . 1991;46 [PubMed] [Google Scholar]
- 87.Zhang Q., Xiong K., Tatsumi E., Li L., Liu H. Elimination of aflatoxin B1 in peanuts by acidic electrolyzed oxidizing water. Food Control . 2012;27 doi: 10.1016/j.foodcont.2012.02.029. [DOI] [Google Scholar]
- 88.Ji C., Fan Y., Zhao L. Review on biological degradation of mycotoxins. Animal Nutrition . 2016;2(3):127–133. doi: 10.1016/j.aninu.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tan H., Hu Y., He J., et al. Zearalenone degradation by two Pseudomonas strains from soil. Mycotoxin Research . 2014;30(4):191–196. doi: 10.1007/s12550-014-0199-x. [DOI] [PubMed] [Google Scholar]
- 90.Oluwafemi F., Kumar M., Bandyopadhyay R., Ogunbanwo T., Ayanwande K. B. Bio-detoxification of aflatoxin B1 in artificially contaminated maize grains using lactic acid bacteria. Toxin Reviews . 2010;29:115–122. doi: 10.3109/15569543.2010.512556. [DOI] [Google Scholar]
- 91.Nazhand A., Durazzo A., Lucarini M., Souto E. B., Santini A. Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. Foods . 2020;9(5):p. 644. doi: 10.3390/foods9050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bartkiene E., Zokaityte E., Lele V., et al. Combination of extrusion and fermentation with Lactobacillus plantarum and L. Uvarum strains for improving the safety characteristics of wheat bran. Toxins . 2021;13(2):p. 163. doi: 10.3390/toxins13020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fessard A., Remize F. Why are Weissella spp. not used as commercial starter cultures for food fermentation? Fermentatio . 2017;3 doi: 10.3390/fermentation3030038. [DOI] [Google Scholar]
- 94.Leroy F., De Vuyst L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends in Food Science & Technology . 2004;15(2):67–78. doi: 10.1016/j.tifs.2003.09.004. [DOI] [Google Scholar]
- 95.Vandamme P., De Bruyne K., Pot B. Lactic Acid Bacteria . Chichester, UK: John Wiley & Sons; 2014. Phylogenetics and systematics. [Google Scholar]
- 96.Bianchini A., Bullerman L. B. Biological Control of Molds and Mycotoxins in Foods 2010.
- 97.Bianchini A. Advances in Fermented Foods and Beverages . Amsterdam, Netherlands: Elsevier; 2015. Lactic acid bacteria as antifungal agents. [DOI] [Google Scholar]
- 98.Blandino A., Al-Aseeri M. E., Pandiella S. S., Cantero D., Webb C. Cereal-based fermented foods and beverages. Food Research International . 2003;36(6):527–543. doi: 10.1016/s0963-9969(03)00009-7. [DOI] [Google Scholar]
- 99.Onyango C., Bley T., Raddatz H., Henle T. Flavour compounds in backslop fermented uji (an East African sour porridge) European Food Research and Technology . 2004;218(6):579–583. doi: 10.1007/s00217-003-0870-5. [DOI] [Google Scholar]
- 100.Achi O. K., Asamudo N. U. Bioactive Molecules in Food, Reference Series in Phytochemistry . Berlin, Germany: Springer; 2019. Cereal-based fermented foods of Africa as functional foods. [Google Scholar]
- 101.Xiang H., Sun-Waterhouse D., Waterhouse G. I. N., Cui C., Ruan Z. Fermentation-enabled wellness foods: a fresh perspective. Food Science and Human Wellness . 2019;8(3):203–243. doi: 10.1016/j.fshw.2019.08.003. [DOI] [Google Scholar]
- 102.Desiye A., Abegaz K. Isolation, characterization and identification of lactic acid bacteria and yeast involved in fermentation of teff (Eragrostis tef) batter. International Journal of Advanced Research in Biological Sciences . 2013;1(May):36–44. [Google Scholar]
- 103.Iwuoha C. I., Eke O. S. Nigerian indigenous fermented foods: their traditional process operation, inherent problems, improvements and current status. Food Research International . 1996;29 doi: 10.1016/0963-9969(95)00045-3. [DOI] [Google Scholar]
- 104.Muyanja C. M. B. K., Narvhus J. A., Treimo J., Langsrud T. Isolation, characterisation and identification of lactic acid bacteria from bushera: a Ugandan traditional fermented beverage. International Journal of Food Microbiology . 2003;80(3):201–210. doi: 10.1016/s0168-1605(02)00148-4. [DOI] [PubMed] [Google Scholar]
- 105.Lee M., Regu M., Seleshe S. Uniqueness of Ethiopian traditional alcoholic beverage of plant origin, tella. Journal of Ethnic Foods . 2015;2(3):110–114. doi: 10.1016/j.jef.2015.08.002. [DOI] [Google Scholar]
- 106.Smid E. J., Hugenholtz J. Functional genomics for food fermentation processes. Annual Review of Food Science and Technology . 2010;1(1):497–519. doi: 10.1146/annurev.food.102308.124143. [DOI] [PubMed] [Google Scholar]
- 107.Moroni A. V., Arendt E. K., Bello F. D. Biodiversity of lactic acid bacteria and yeasts in spontaneously-fermented buckwheat and teff sourdoughs. Food Microbiology . 2011;28(3):497–502. doi: 10.1016/j.fm.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 108.De Vuyst L., Vrancken G., Ravyts F., Rimaux T., Weckx S. Biodiversity, ecological determinants, and metabolic exploitation of sourdough microbiota. Food Microbiology . 2009;26(7):666–675. doi: 10.1016/j.fm.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 109.Nwachukwu E., Achi O. K., Ijeoma I. O. Lactic acid bacteria in fermentation of cereals for the production of indigenous Nigerian foods. Journal, African Journal of Food Science and Technology . 2010;1(2):21–26. [Google Scholar]
- 110.Corsetti A., Settanni L., Valmorri S., Mastrangelo M., Suzzi G. Identification of subdominant sourdough lactic acid bacteria and their evolution during laboratory-scale fermentations. Food Microbiology . 2007;24(6):592–600. doi: 10.1016/j.fm.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 111.N’tcha C., Haziz S., Agbobatinkpo P., Vieira-Dalodé G., Boya B., Jtc C. Probiotic properties of lactic acid bacteria isolated from a beninese traditional beer’s ferment. International Journal of Applied Biology and Pharmaceutical Technology . 2016;7(2):314–331. [Google Scholar]
- 112.Kayodé A. P. P., Hounhouigan J. D., Nout M. J. R. Impact of brewing process operations on phytate, phenolic compounds and in vitro solubility of iron and zinc in opaque sorghum beer. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology . 2007;40(5):834–841. [Google Scholar]
- 113.Vieira-Dalodé G., Jespersen L., Hounhouigan J., Moller P. L., Nago C. M., Jakobsen M. Lactic acid bacteria and yeasts associated with gowé production from sorghum in Bénin. Journal of Applied Microbiology . 2007;103(2):342–349. doi: 10.1111/j.1365-2672.2006.03252.x. [DOI] [PubMed] [Google Scholar]
- 114.Muhialdin B. J., Saari N., Meor Hussin A. S. Review on the biological detoxification of mycotoxins using lactic acid bacteria to enhance the sustainability of foods supply. Molecules . 2020;19 doi: 10.3390/molecules25112655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jebali R., Abbès S., Salah-Abbès J. B., Younes R. B., Haous Z., Oueslati R. Ability of Lactobacillus plantarum Mon03 to mitigate aflatoxins (B 1 and M 1) immunotoxicities in mice. Journal of Immunotoxicology . 2015;12:290–299. doi: 10.3109/1547691x.2014.973622. [DOI] [PubMed] [Google Scholar]
- 116.Huang L., Duan C., Zhao Y., Gao L., Niu C., Xu J. Reduction of aflatoxin B1 toxicity by Lactobacillus plantarum C88: a potential probiotic strain isolated from Chinese traditional fermented food. PLoS One . 2017;12:p. e0170109. doi: 10.1371/journal.pone.0170109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Taheur F. B., Fedhila K., Chaieb K., Kouidhi B., Bakhrouf A., Abrunhosa L. Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. International Journal of Food Microbiology . 2017;251:1–7. doi: 10.1016/j.ijfoodmicro.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 118.Sadeghi A. R., Ebrahimi M., Sadeghi B. Effect of isolated Lactobacillus acidophilus and Lactobacillus brevis on growth of Aspergillus flavus and reduction of aflatoxin B1. Journal of Rafsanjan University of Medical Sciences . 2017;15 [Google Scholar]
- 119.Wacoo A. P., Mukisa I. M., Meeme R., et al. Probiotic enrichment and reduction of aflatoxins in a traditional african maize-based fermented food. Nutrients . 2019;11 doi: 10.3390/nu11020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bagherzadeh Kasmani F., Karimi Torshizi M. A., Allameh A. A., Shariatmadari F. Aflatoxin detoxification potential of lactic acid bacteria isolated from iranian poultry. Iranian Journal of Veterinary Research . 2012;13(2):152–155. [Google Scholar]
- 121.Fazeli M. R., Hajimohammadali M., Moshkani A., et al. Aflatoxin B1 binding capacity of autochthonous strains of lactic acid bacteria. Journal of Food Protection . 2009;72(1):189–192. doi: 10.4315/0362-028x-72.1.189. [DOI] [PubMed] [Google Scholar]
- 122.Chlebicz A., Śliżewska K. In Vitro detoxification of aflatoxin B1, deoxynivalenol, fumonisins, T-2 toxin and zearalenone by probiotic bacteria from genus Lactobacillus and Saccharomyces cerevisiae yeast. Probiotics Antimicrob Proteins . 2020;12 doi: 10.1007/s12602-018-9512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Asurmendi P., Gerbaldo G., Pascual L., Barberis L. Lactic acid bacteria with promising AFB 1 binding properties as an alternative strategy to mitigate contamination on brewers’ grains. Journal of Environmental Science and Health, Part B . 2020;55:1–7. doi: 10.1080/03601234.2020.1807834. [DOI] [PubMed] [Google Scholar]
- 124.Topcu A., Bulat T., Wishah R., Boyaci I. H. Detoxification of aflatoxin B1 and patulin by Enterococcus faecium strains. International Journal of Food Microbiology . 2010;139:202–205. doi: 10.1016/j.ijfoodmicro.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 125.Abbès S., Salah-Abbès J. B., Sharafi H., Jebali R., Noghabi K. A., Oueslati R. Ability of Lactobacillus rhamnosus GAF01 to remove AFM1 in vitro and to counteract AFM1 immunotoxicity in vivo. Journal of Immunotoxicology . 2013;10:279–286. doi: 10.3109/1547691X.2012.718810. [DOI] [PubMed] [Google Scholar]
- 126.Corassin C. H., Bovo F., Rosim R. E., Oliveira C. A. F. Efficiency of Saccharomyces cerevisiae and lactic acid bacteria strains to bind aflatoxin M1 in UHT skim milk. Food Control . 2013;31(1):80–83. doi: 10.1016/j.foodcont.2012.09.033. [DOI] [Google Scholar]
- 127.El Khoury A., Atoui A., Yaghi J. Analysis of aflatoxin M1 in milk and yogurt and AFM1 reduction by lactic acid bacteria used in Lebanese industry. Food Control . 2011;22(10):1695–1699. doi: 10.1016/j.foodcont.2011.04.001. [DOI] [Google Scholar]
- 128.Sevim S., Topal G. G., Tengilimoglu-Metin M. M., Sancak B., Kizil M. Effects of inulin and lactic acid bacteria strains on aflatoxin M1 detoxification in yoghurt. Food Control . 2019;100 doi: 10.1016/j.foodcont.2019.01.028. [DOI] [Google Scholar]
- 129.Fuchs S., Sontag G., Stidl R., Ehrlich V., Kundi M., Knasmüller S. Detoxification of patulin and ochratoxin A, two abundant mycotoxins, by lactic acid bacteria. Food and Chemical Toxicology . 2008;46(4):1398–1407. doi: 10.1016/j.fct.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 130.Franco T. S., Garcia S., Hirooka E. Y., Ono Y. S., dos Santos J. S. Lactic acid bacteria in the inhibition of Fusarium graminearum and deoxynivalenol detoxification. Journal of Applied Microbiology . 2011;111:739–48. doi: 10.1111/j.1365-2672.2011.05074.x. [DOI] [PubMed] [Google Scholar]
- 131.Zou Z.-Y., He Z.-F., Li H.-J., Han P.-F., Meng X., Zhang Y. In vitro removal of deoxynivalenol and T-2 toxin by lactic acid bacteria. Food Science and Biotechnology . 2012;21 doi: 10.1007/s10068-012-0223-x. [DOI] [Google Scholar]
- 132.Zhai Y., Hu S., Zhong L., et al. Characterization of deoxynivalenol detoxification by Lactobacillus paracasei LHZ-1 isolated from yogurt. Journal of Food Protection . 2011;82(8):1292–1299. doi: 10.4315/0362-028x.jfp-18-581. [DOI] [PubMed] [Google Scholar]
- 133.Sangsila A., Faucet-Marquis V., Pfohl-Leszkowicz A., Itsaranuwat P. Detoxification of zearalenone by Lactobacillus pentosus strains. Food Control . 2016;62 doi: 10.1016/j.foodcont.2015.10.031. [DOI] [Google Scholar]
- 134.Chen S.-W., Hsu J.-T., Chou Y.-A., Wang H.-T. The application of digestive tract lactic acid bacteria with high esterase activity for zearalenone detoxification. Journal of the Science of Food and Agriculture . 2018;98 doi: 10.1002/jsfa.8904. [DOI] [PubMed] [Google Scholar]
- 135.Zhao H., Wang X., Zhang J., Zhang J., Zhang B. The mechanism of Lactobacillus strains for their ability to remove fumonisins B1 and B2. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association . 2016;97:40–46. doi: 10.1016/j.fct.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 136.Adebiyi J. A., Kayitesi E., Adebo O. A., Changwa R., Njobeh P. B. Food fermentation and mycotoxin detoxification: an African perspective. Food Control . 2019;106(May):p. 106731. doi: 10.1016/j.foodcont.2019.106731. [DOI] [Google Scholar]
- 137.Luo Y., Liu X., Li J. Updating techniques on controlling mycotoxins - a review. Food Control . 2018;89:123–132. [Google Scholar]
- 138.Adebo O. A., Njobeh P. B., Gbashi S., Nwinyi O. C., Mavumengwana V. Review on microbial degradation of aflatoxins. Critical Reviews in Food Science and Nutrition . 2017;57:3208–3217. doi: 10.1080/10408398.2015.1106440. [DOI] [PubMed] [Google Scholar]
- 139.Solis-Cruz B., Hernandez-Patlan D., Hargis M., Tellez G. Mycotoxins - Impact and Management Strategies . London, UK: IntechOpen; 2019. Control of Aflatoxicosis in Poultry using probiotics and polymers. [Google Scholar]
- 140.Hernandez-Mendoza A., Garcia H. S., Steele J. L. Screening of Lactobacillus casei strains for their ability to bind aflatoxin B1. Food and Chemical Toxicology . 2009;47(6):1064–1068. doi: 10.1016/j.fct.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 141.Chiocchetti G. M., Jadán-Piedra C., Monedero V., Zúñiga M., Vélez D., Devesa V. Use of lactic acid bacteria and yeasts to reduce exposure to chemical food contaminants and toxicity. Critical Reviews in Food Science and Nutrition . 2019;59:1534–1545. doi: 10.1080/10408398.2017.1421521. [DOI] [PubMed] [Google Scholar]
- 142.Shehata M. G., Badr A. N., El Sohaimy S. A., Asker D., Awad T. S. Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Annals of Agricultural Science . 2019;64(1):71–78. doi: 10.1016/j.aoas.2019.05.002. [DOI] [Google Scholar]
- 143.Cortés-Zavaleta O., López-Malo A., Hernández-Mendoza A., García H. S. Antifungal activity of lactobacilli and its relationship with 3-phenyllactic acid production. International Journal of Food Microbiology . 2014;173 doi: 10.1016/j.ijfoodmicro.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 144.Corsetti A., Gobbetti M., Rossi J., Damiani P. Antimould activity of sourdough lactic acid bacteria: identification of a mixture of organic acids produced by Lactobacillus sanfrancisco CB1. Applied Microbiology and Biotechnology . 1998;50(2):253–256. doi: 10.1007/s002530051285. [DOI] [PubMed] [Google Scholar]
- 145.Lavermicocca P., Valerio F., Evidente A., Lazzaroni S., Corsetti A., Gobbetti M. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Applied and Environmental Microbiology . 2000;66(9):4084–4090. doi: 10.1128/aem.66.9.4084-4090.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ström K., Sjögren J., Broberg A., Schnürer J. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(l-phe-l-pro) and cyclo(l-phe-trans-4-OH-l-pro) and 3-phenyllactic acid. Applied and Environmental Microbiology . 2002;68(9):4322–4327. doi: 10.1128/AEM.68.9.4322-4327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Magnusson J., Stram K., Roos S., Sjagren J. r., Schnarer J. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiology Letters . 2003;219(1):129–135. doi: 10.1016/s0378-1097(02)01207-7. [DOI] [PubMed] [Google Scholar]
- 148.Sjögren J., Magnusson J., Broberg A., Schnürer J., Kenne L. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Applied and Environmental Microbiology . 2003;69(12):7554–7557. doi: 10.1128/AEM.69.12.7554-7557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sangmanee P., Hongpattarakere T. Inhibitory of multiple antifungal components produced by Lactobacillus plantarum K35 on growth, aflatoxin production and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Control . 2014;40(1):224–233. doi: 10.1016/j.foodcont.2013.12.005. [DOI] [Google Scholar]
- 150.Dal Bello F., Clarke C. I., Ryan L. A. M., et al. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. Journal of Cereal Science . 2007;45(3):309–318. doi: 10.1016/j.jcs.2006.09.004. [DOI] [Google Scholar]
- 151.Yang E. J., Chang H. C. Purification of a new antifungal compound produced by Lactobacillus plantarum AF1 isolated from kimchi. International Journal of Food Microbiology . 2010;139(1–2):56–63. doi: 10.1016/j.ijfoodmicro.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 152.Okkers D. J., Dicks L. M., Silvester M., Joubert J. J., Odendaal H. J. Characterization of pentocin TV35b, a bacteriocin-like peptide isolated from Lactobacillus pentosus with a fungistatic effect on Candida albicans. Journal of Applied Microbiology . 1999;87:726–34. doi: 10.1046/j.1365-2672.1999.00918.x. [DOI] [PubMed] [Google Scholar]
- 153.Ndagano D., Lamoureux T., Dortu C., Vandermoten S., Thonart P. Antifungal activity of 2 lactic acid bacteria of the Weissella genus isolated from food. Journal of Food Science . 2011;76:M305–11. doi: 10.1111/j.1750-3841.2011.02257.x. [DOI] [PubMed] [Google Scholar]
- 154.Wang H., Yan Y., Wang J., Zhang H., Qi W. Production and characterization of antifungal compounds produced by Lactobacillus plantarum IMAU10014. PLoS One . 2012;7(1):p. e29452. doi: 10.1371/journal.pone.0029452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li H., Liu L., Zhang S., Cui W., Lv J. Identification of antifungal compounds produced by Lactobacillus casei AST18. Current Microbiology . 2012;65(2):156–161. doi: 10.1007/s00284-012-0135-2. [DOI] [PubMed] [Google Scholar]
- 156.Black B. A., Zannini E., Curtis J. M., Gänzle M. G. Antifungal hydroxy fatty acids produced during sourdough fermentation: microbial and enzymatic pathways, and antifungal activity in bread. Applied and Environmental Microbiology . 2013;79(6):1866–1873. doi: 10.1128/aem.03784-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guo J., Mauch A., Galle S., Murphy P., Arendt E. K., Coffey A. Inhibition of growth of Trichophyton tonsurans by Lactobacillus reuteri. Journal of Applied Microbiology . 2011;111(2):474–483. doi: 10.1111/j.1365-2672.2011.05032.x. [DOI] [PubMed] [Google Scholar]
- 158.Ryan L. A. M., Zannini E., Dal Bello F., Pawlowska A., Koehler P., Arendt E. K. Lactobacillus amylovorus DSM 19280 as a novel food-grade antifungal agent for bakery products. International Journal of Food Microbiology . 2011;146(3):276–283. doi: 10.1016/j.ijfoodmicro.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 159.Rizzello C. G., Cassone A., Coda R., Gobbetti M. Antifungal activity of sourdough fermented wheat germ used as an ingredient for bread making. Food Chemistry . 2011;127:952–9. doi: 10.1016/j.foodchem.2011.01.063. [DOI] [PubMed] [Google Scholar]
- 160.Muhialdin B. J., Algboory H. L., Kadum H., Mohammed N. K., Saari N., Hassan Z. Antifungal activity determination for the peptides generated by Lactobacillus plantarum TE10 against Aspergillus flavus in maize seeds. Food Control . 2020;109:p. 106898. doi: 10.1016/j.foodcont.2019.106898. [DOI] [Google Scholar]
- 161.Guimarães A., Santiago A., Teixeira J. A., Venâncio A., Abrunhosa L. Anti-aflatoxigenic effect of organic acids produced by Lactobacillus plantarum. International Journal of Food Microbiology . 2018;264:31–38. doi: 10.1016/j.ijfoodmicro.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 162.Sadeghi A., Ebrahimi M., Raeisi M., Nematollahi Z. Biological control of foodborne pathogens and aflatoxins by selected probiotic LAB isolated from rice bran sourdough. Biological Control . 2019;130 doi: 10.1016/j.biocontrol.2018.09.017. [DOI] [Google Scholar]
- 163.Gerez C. L., Torres M. J., Font de Valdez G., Rollán G. Control of spoilage fungi by lactic acid bacteria. Biological Control . 2013;64(3):231–237. doi: 10.1016/j.biocontrol.2012.10.009. [DOI] [Google Scholar]
- 164.Le Lay C., Coton E., Le Blay G., Chobert J.-M., Haertlé T., Choiset Y. Identification and quantification of antifungal compounds produced by lactic acid bacteria and propionibacteria. International Journal of Food Microbiology . 2016;239 doi: 10.1016/j.ijfoodmicro.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 165.Russo P., Arena M. P., Fiocco D., Capozzi V., Drider D., Spano G. Lactobacillus plantarum with broad antifungal activity: a promising approach to increase safety and shelf-life of cereal-based products. International Journal of Food Microbiology . 2017;247:48–54. doi: 10.1016/j.ijfoodmicro.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 166.Bartkiene E., Lele V., Starkute V., et al. Plants and lactic acid bacteria combination for new antimicrobial and antioxidant properties product development in a sustainable manner. Foods . 2020;9(4):p. 433. doi: 10.3390/foods9040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ahlberg S., Joutsjoki V., Laurikkala S., Varmanen P., Korhonen H. Aspergillus flavus growth inhibition by Lactobacillus strains isolated from traditional fermented Kenyan milk and maize products. Archives of Microbiology . 2017;199(3):457–464. doi: 10.1007/s00203-016-1316-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The reference data used to support the findings of this study are included within the article.


