Abstract
Aims
To clarify the real-world clinical status and prognosis of elderly and very elderly non-valvular atrial fibrillation (NVAF) patients, more than 30 000 elderly patients with NVAF aged ≥75 years were enrolled in the ANAFIE Registry.
Methods and results
This multicentre, prospective, observational study followed elderly NVAF patients in Japan for ∼2 years. Among 32 275 patients (mean age, 81.5 years; men, 57.3%; mean CHA2DS2-VASc score, 4.5), 2445 (7.6%) were not receiving oral anticoagulants (OACs) and 29 830 (92.4%) were given OACs. Of these, 21 585 (66.9%) were receiving direct OACs (DOACs) and 8233 (25.5%), warfarin (mean time in therapeutic range: ∼75%). In total, the 2-year incidence rate was 3.01% for stroke/systemic embolic events (SEE); 2.00%, major bleeding; and 6.95%, all-cause death. When compared with the warfarin group, the DOAC group had a lower hazard ratio (HR) for stroke/SEE, major bleeding, and all-cause death after adjusting for confounders. The group without OACs had a higher HR for stroke/SEE and all-cause death, with a lower HR for major bleeding. History of falls within 1 year at enrolment and of catheter ablation were positive and negative independent risk factors, respectively, for stroke/SEE, major bleeding, and all-cause death.
Conclusion
In Japan, a large proportion of elderly and very elderly NVAF patients were receiving DOACs, which was significantly associated with lower rates of stroke/SEE, major bleeding, and all-cause death vs. well-controlled warfarin. History of falls and of catheter ablation were independently associated with stroke/SEE, major bleeding, and all-cause death.
Keywords: Anticoagulants, Atrial fibrillation, Elderly patients, Stroke, Systemic embolism, DOACs
Introduction
The ageing population continues to grow rapidly worldwide, particularly in developed countries. The number of people aged 65 years or older is projected to double to 1.5 billion and that aged 80 years or older to triple to 0.4 billion in 2050.1 The prevalence of non-valvular atrial fibrillation (NVAF) will increase with this global ageing. A recent epidemiological study2 predicted that elderly atrial fibrillation (AF) patients ≥65 years in the European Union would increase by 89% in 2060. Of note, very elderly (i.e. ≥80 years of age) AF patients represented 51.2% of the total AF population in 2016, but this percentage is expected to increase up to 65.2% in 2060. The management of elderly and very elderly NVAF patients is gaining more significance for our societies because older age is associated with an increased risk of stroke and heart failure, particularly among patients with NVAF.3 However, clinical trials on NVAF4–7 have not specifically targeted the enrolment of these patients. Even observational data on the background and clinical outcomes of these patients remain limited.
Some studies reported that oral anticoagulants (OACs) are underused among elderly patients in real-world clinical practice.8,9 Such OAC underuse is mainly attributed to concerns of a higher risk of complications related to multimorbidity among elderly patients,8,10 which is even more marked in the very elderly. Other studies have shown that populations at higher risk of embolic events and bleeding reap important benefits from anticoagulation.11,12 More information is required for improving anticoagulation for elderly patients, especially very elderly NVAF patients, for whom physicians are prone to be reluctant to use OACs.
Japan has one of the largest and fastest growing ageing populations in the world.13 At present, the prevalence of NVAF in Japan is less than 1% in the overall population, but the prevalence increases sharply among patients aged 80 years and older.14 Thus, based on the clinical practices applied to the increasing number of elderly and very elderly NVAF patients, data from Japan can aid other regions undergoing similar ageing population growth. The ANAFIE Registry15,16 is the world’s largest registry, with more than 30 000 NVAF patients aged ≥75 years. The main objectives were to clarify the clinical status of elderly and very elderly NVAF patients, including their anticoagulant therapeutic regimens and their outcomes. Herein, we report the 2-year outcomes of this registry.
Methods
Study design
The rationale and full study design of the ANAFIE registry have been published,15 as have the baseline data.16 Briefly, the ANAFIE Registry was a multicentre, prospective, observational study of elderly patients (aged ≥75 years) with NVAF, irrespective of OAC use. A total of 1273 medical facilities participated in the study throughout Japan, which was conducted from October 2016 to January 2020 and included a 2-year follow-up, with data collected at baseline, and at 1 and 2 years.
Ethical considerations of the study
The study complied with the Declaration of Helsinki, and local requirements for registries and ethical guidelines for clinical studies in Japan were met. Ethics committee approvals were obtained. Written informed consent was obtained from all patients (or their family members for patients with communication disorders, such as aphasia, or cognitive impairment).
Patients
Men and women ≥75 years of age at the time of informed consent, with a definite diagnosis of NVAF (by electrocardiogram), who were able to attend hospital visits were included. Patients were excluded if any of the following criteria were met: currently participating or planning to participate in an interventional study; definite diagnosis of mitral stenosis; artificial heart valve replacement (either mechanical or tissue valve prostheses); very recent history of cardiovascular events including stroke, myocardial infarction (MI), cardiac intervention, heart failure requiring hospitalization, or any bleeding leading to hospitalization within 1 month prior to enrolment; life expectancy of <1 year; or participation was deemed inappropriate by treating physicians.
Study endpoints
The primary endpoint was the incidence of stroke/systemic embolic events (SEE). The secondary endpoints were the incidence of the following events: major bleeding; stroke; SEE; ischaemic stroke; haemorrhagic stroke; intracranial haemorrhage; cardiovascular events (stroke, MI, cardiac intervention for ischaemic disease other than MI, and heart failure requiring hospitalization); death from cardiovascular diseases; and all-cause death. Net clinical outcomes were defined as the composite of stroke/SEE, major bleeding, and all-cause death. All endpoint events were adjudicated by cerebrovascular, cardiac, and bleeding event evaluation committees, consisting of neurologists, cardiologists, and haematologists, respectively, who were blind to the anticoagulation treatment. Major bleeding was classified according to the International Society on Thrombosis and Haemostasis (ISTH) definition. Additional disease classifications (including the type of AF) and assessments [including the target range of prothrombin time-international normalized ratio (PT-INR) of 1.6–2.6 for Japanese patients] were based on AF guidelines published by the Japanese Circulation Society.17 PT-INR monitoring was conducted using the results obtained from outpatient blood tests; data from patients who had three or more measurements in 6 months were averaged. All patients underwent follow-up from enrolment until the end of the study, death, or withdrawal from the study.
Statistical analysis
The analysis was performed using the full analysis set, which included all enrolled patients but excluded patients with protocol violation (i.e. not meeting all of the inclusion criteria or meeting any of the exclusion criteria), lack of follow-up visits after obtaining informed consent, and other reasons. Categorical variables were analysed using frequency tables. Summary statistics, including n, mean, standard deviation, minimum value, median, and maximum value, were calculated for continuous variables. Baseline variables were compared among no anticoagulation (No OAC), warfarin, and direct oral anticoagulant (DOAC) groups, using the χ2 test for categorical variables and one-way analysis of variance for continuous variables. Outcomes were assessed at 2 years from obtaining informed consent, and the effect of anticoagulation on outcomes was analysed by type of anticoagulation therapy at the enrolment. The occurrence of primary and secondary endpoints was analysed with Kaplan–Meier curves and also described as rate per 100 person-years with 95% confidence interval (CI). For the comparison of incidence rates among types of anticoagulation therapy, the Cox proportional hazards model was used, where the hazard ratios (HRs) and 95% CIs were calculated using the warfarin group as a reference. In this analysis, the variables possibly associated with selection of anticoagulant therapy or incidence of outcomes were entered in the statistical model. The variables, other than anticoagulation therapy, were also determined as independent risk factors for stroke/SEE, major bleeding, intracranial haemorrhage, and all-cause death. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Tokyo, Japan).
Role of the funding source
Daiichi Sankyo Co., Ltd., supported the ANAFIE Registry. The sponsor was involved in the study design, planning of the data analysis, data interpretation, and decision to submit the manuscript for publication, but was not directly involved in data management, direct access, or statistical analysis. The corresponding author had full access to all data and was responsible for the submission for publication.
Results
Patient disposition and characteristics
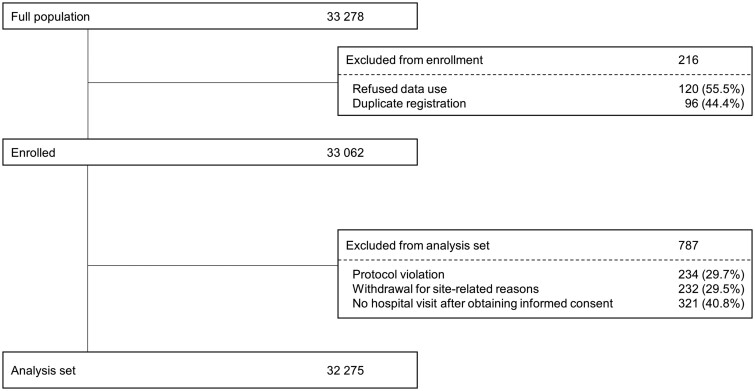
Of the 33 062 patients enrolled, 787 were excluded, and a total of 32 275 patients were included in the present analysis (Figure 1). There were 1109 (3.4%) patients lost to follow-up; the proportion did not differ among the No OAC, warfarin, and DOAC groups (P = 0.29). Meanwhile, 762 (2.4%) discontinued the study due to withdrawal of consent and other reasons; the proportion of patients who discontinued differed slightly but significantly among the three treatment groups (P = 0.005, Supplementary material online, Table S1). The mean follow-up period was 1.88 years.
Figure 1.
Patient disposition.
Table 1 provides a summary of the main baseline characteristics of the patients by anticoagulant use. Overall, 57.3% of patients were men, the mean body mass index (BMI) was 23.3 kg/m2, and the mean age was 81.5 years. Most patients were between 75 and 79 years of age (40.0%) or between 80 and 89 years of age (43.5%), and 6.5% were aged over 90 years. Paroxysmal AF was the most common type of AF, followed by persistent AF and long-standing persistent/permanent AF. The most common comorbidities were hypertension (75.3%) and heart failure (37.5%).
Table 1.
Patient characteristics
| Total (N = 32 275) | No OAC (n = 2445) | OAC | ||||
|---|---|---|---|---|---|---|
| All (n = 29 818) | Warfarin (n = 8233) | DOAC (n = 21 585) | P-valuea | |||
| Men | 18 482 (57.3) | 1273 (52.1) | 17 200 (57.7) | 5109 (62.1) | 12 091 (56.0) | <0.001 |
| Age, years | 81.5 ± 4.8 | 82.3 ± 5.5 | 81.4 ± 4.7 | 81.9 ± 4.9 | 81.2 ± 4.7 | <0.001 |
| ≥75 to <80 | 12 895 (40.0) | 893 (36.5) | 11 995 (40.2) | 2986 (36.3) | 9009 (41.7) | <0.001 |
| ≥80 to <85 | 10 961 (34.0) | 725 (29.7) | 10 232 (34.3) | 2863 (34.8) | 7369 (34.1) | |
| ≥85 to <90 | 6295 (19.5) | 547 (22.4) | 5747 (19.3) | 1743 (21.2) | 4004 (18.5) | |
| ≥90 to <95 | 1848 (5.7) | 224 (9.2) | 1624 (5.4) | 556 (6.8) | 1068 (4.9) | |
| ≥95 to <100 | 265 (0.8) | 51 (2.1) | 214 (0.7) | 82 (1.0) | 132 (0.6) | |
| ≥100 | 11 (<0.1) | 5 (0.2) | 6 (0.0) | 3 (<0.1) | 3 (<0.1) | |
| BMI, kg/m2 | 23.3 ± 3.6 | 22.7 ± 3.5 | 23.4 ± 3.6 | 23.4 ± 3.6 | 23.4 ± 3.6 | <0.001 |
| SBP, mmHg | 127.4 ± 17.0 | 130.0 ± 17.5 | 127.1 ± 17.0 | 125.8 ± 17.1 | 127.7 ± 16.9 | <0.001 |
| DBP, mmHg | 70.6 ± 11.6 | 70.4 ± 11.5 | 70.7 ± 11.6 | 69.9 ± 11.8 | 71.0 ± 11.6 | <0.001 |
| Creatinine clearance, mL/min | 48.6 ± 22.0 | 45.6 ± 18.2 | 48.6 ± 18.2 | 44.7 ± 18.3 | 50.1 ± 18.0 | <0.001 |
| CHADS2 score | 2.9 ± 1.2 | 2.6 ± 1.2 | 2.9 ± 1.2 | 3.0 ± 1.2 | 2.8 ± 1.2 | <0.001 |
| CHA2DS2-VASc score | 4.5 ± 1.4 | 4.3 ± 1.4 | 4.5 ± 1.4 | 4.5 ± 1.4 | 4.4 ± 1.4 | <0.001 |
| HAS-BLED score | 1.9 ± 0.9 | 2.0 ± 0.9 | 1.9 ± 0.9 | 2.0 ± 0.9 | 1.8 ± 0.8 | <0.001 |
| History of major bleeding | 1439 (4.5) | 170 (7.0) | 1268 (4.3) | 387 (4.7) | 881 (4.1) | <0.001 |
| AF type | ||||||
| Paroxysmal | 13 586 (42.1) | 1720 (70.3) | 11 857 (39.8) | 2431 (29.5) | 9426 (43.7) | <0.001 |
| Persistent | 5336 (16.5) | 255 (10.4) | 5080 (17.0) | 1279 (15.5) | 3801 (17.6) | |
| Long-standing persistent/permanent | 13 353 (41.4) | 470 (19.3) | 12 881 (43.2) | 4523 (55.0) | 8358 (38.7) | |
| History of non-pharmacological therapy for AF | 5677 (17.6) | 580 (23.7) | 5096 (17.1) | 1342 (16.3) | 3754 (17.4) | <0.001 |
| Catheter ablation | 2970 (9.2) | 376 (15.4) | 2594 (8.7) | 500 (6.1) | 2094 (9.7) | <0.001 |
| Electrical defibrillation | 715 (2.2) | 40 (1.6) | 675 (2.3) | 186 (2.3) | 489 (2.3) | 0.13 |
| ICD | 151 (0.5) | 17 (0.7) | 134 (0.4) | 54 (0.7) | 80 (0.4) | 0.001 |
| Pacemaker | 2358 (7.3) | 189 (7.7) | 2169 (7.3) | 713 (8.7) | 1456 (6.7) | <0.001 |
| Others | 112 (0.3) | 13 (0.5) | 98 (0.3) | 39 (0.5) | 59 (0.3) | <0.001 |
| Comorbidities | ||||||
| Hypertension | 24 312 (75.3) | 1821 (74.5) | 22 482 (75.4) | 6159 (74.8) | 16 323 (75.6) | 0.21 |
| Diabetes mellitus | 8733 (27.1) | 580 (23.7) | 8150 (27.3) | 2416 (29.3) | 5734 (26.6) | <0.001 |
| Chronic kidney disease | 6705 (20.8) | 441 (18.0) | 6261 (21.0) | 2150 (26.1) | 4111 (19.0) | <0.001 |
| Myocardial infarction | 1851 (5.7) | 138 (5.6) | 1712 (5.7) | 614 (7.5) | 1098 (5.1) | <0.001 |
| Heart failure | 12 116 (37.5) | 742 (30.3) | 11 372 (38.1) | 3592 (43.6) | 7780 (36.0) | <0.001 |
| History of cerebrovascular disease | 7303 (22.6) | 468 (19.1) | 6833 (22.9) | 1873 (22.7) | 4960 (23.0) | <0.001 |
| Gastrointestinal diseases | 9467 (29.3) | 805 (32.9) | 8661 (29.0) | 2336 (28.4) | 6325 (29.3) | <0.001 |
| Active cancer | 3569 (11.1) | 284 (11.6) | 3283 (11.0) | 846 (10.3) | 2437 (11.3) | 0.03 |
| Dementia | 2512 (7.8) | 248 (10.1) | 2264 (7.6) | 574 (7.0) | 1690 (7.8) | <0.001 |
| Fall within 1 year | 2347 (7.3) | 167 (6.8) | 2178 (7.3) | 660 (8.0) | 1518 (7.0) | 0.01 |
Data in the table are presented as n (%) or mean ± standard deviation.
AF, atrial fibrillation; BMI, body mass index; DBP, diastolic blood pressure; ICD, implantable cardioverter-defibrillator; OAC, oral anticoagulants; SBP, systolic blood pressure.
Comparison among no OAC, warfarin and DOAC groups.
Of 32 275 patients, 12 were using parenteral anticoagulants and were therefore excluded from further analyses according to types of anticoagulation. In total, 2445 (7.6%) patients were not receiving OACs (No OAC group), and 29 830 (92.4%) were receiving OACs (OAC group). Of the latter, 8233 (25.5%) were using warfarin, and 21 585 (66.9%) were using DOACs. In the warfarin group, the mean prothrombin time-international normalized ratio was 1.98 at enrolment, and the mean time in the therapeutic range (PT-INR 1.6–2.6) was 75.5% during the 6 months just before the enrolment. In the DOAC group, apixaban, rivaroxaban, edoxaban, and dabigatran was used in 8045 (24.9% of the total), 6403 (19.8%), 4790 (14.8%), and 2347 (7.3%) patients, respectively. The percentage of patients prescribed a reduced dose of each DOAC was 61.1%, and 27.5% of those were treated with doses below those recommended in the package insert (Supplementary material online, Table S2).
The mean age of the No OAC group was the highest, followed in order by the warfarin and DOAC groups (Table 1). Patients receiving DOACs tended to have a higher creatinine clearance and tended to be more frequently diagnosed with paroxysmal AF than warfarin groups. Patients using warfarin tended to have higher proportions of heart failure, diabetes mellitus, chronic kidney disease, and MI than those in the No OAC and DOAC groups. Non-pharmacological therapy for AF, including catheter ablation was more common in the No OAC group.
Primary and secondary endpoints
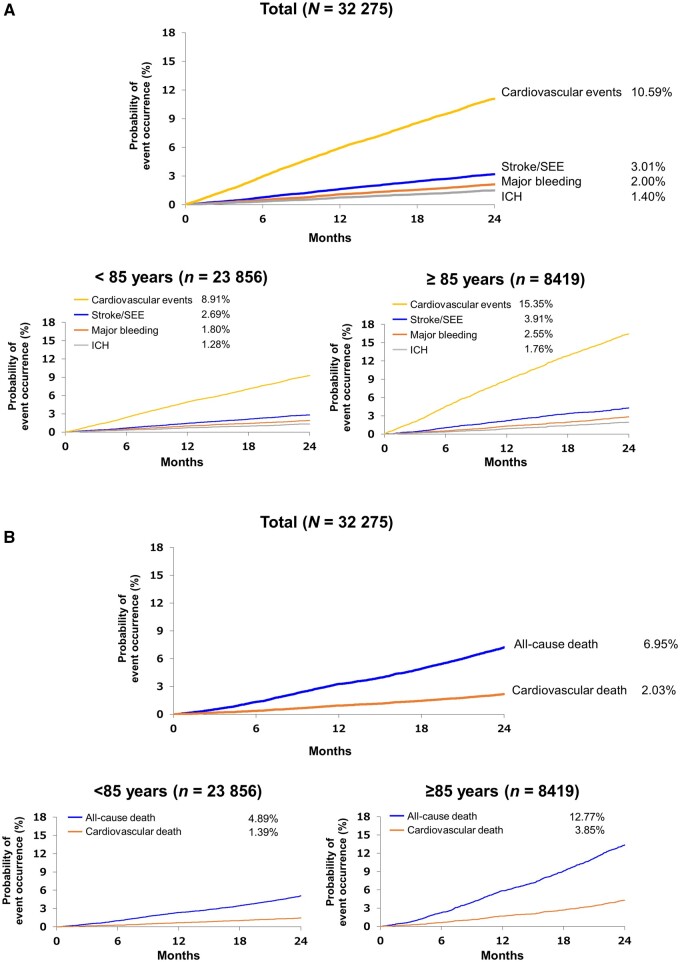
Kaplan–Meier curves are shown in Figure 2. Among 32 275 patients, the 2-year incidence of the primary endpoint of stroke/SEE was 3.01% and was lower in patients aged <85 years than in those aged ≥85 years (Figure 2A). The incidence of major bleeding and intracranial haemorrhage was 2.00% and 1.40%, respectively, and was higher in patients aged ≥85 years compared with those aged <85 years (Figure 2A). The incidence of cardiovascular events was 10.59%, and nearly two-fold higher for those aged ≥85 years compared with those aged <85 years (Figure 2A). The 2-year incidence of all-cause death was 6.95%, and that of cardiovascular death was 2.03%, which nearly tripled in patients aged ≥85 years compared with those aged <85 years (Figure 2B). Incidence rates of endpoints according to types of anticoagulation are shown in Table 2.
Figure 2.
Kaplan–Meier curves of the total population and by age group, showing stroke/SEE, major bleeding, cardiovascular events, and ICH (A), and deaths (B). ICH, intracranial haemorrhage; SEE, systemic embolic events.
Table 2.
Incidence rates of clinical events
| Variable | Total (N = 32 275) | No OAC (n = 2445) | Warfarin (n = 8233) | DOAC (n = 21 585) |
|---|---|---|---|---|
|
Event number Incidence rate/ 100 person-years (95% CI) |
||||
| Stroke/SEE |
970 1.62 (1.52–1.73) |
88 2.00 (1.58–2.41) |
298 1.98 (1.75–2.20) |
584 1.45 (1.33–1.57) |
| Stroke |
945 1.58 (1.48–1.68) |
86 1.95 (1.54–2.36) |
287 1.90 (1.68–2.13) |
572 1.42 (1.31–1.54) |
| Ischaemic stroke |
743 1.24 (1.15–1.33) |
77 1.74 (1.35–2.13) |
227 1.50 (1.31–1.70) |
439 1.09 (0.99–1.19) |
| Haemorrhagic stroke |
201 0.33 (0.29–0.38) |
10 0.22 (0.09–0.36) |
60 0.39 (0.29–0.49) |
131 0.32 (0.27–0.38) |
| SEE |
28 0.05 (0.03–0.06) |
2 0.04 (−0.02–0.11) |
13 0.09 (0.04–0.13) |
13 0.03 (0.01–0.05) |
| Major bleeding |
645 1.08 (0.99–1.16) |
40 0.90 (0.62–1.18) |
216 1.43 (1.24–1.62) |
389 0.96 (0.87–1.06) |
| All bleeding |
2557 4.40 (4.23–4.57) |
116 2.65 (2.17–3.14) |
749 5.12 (4.76–5.49) |
1690 4.32 (4.11–4.53) |
| ICH |
453 0.75 (0.68–0.82) |
27 0.61 (0.38–0.83) |
156 1.03 (0.87–1.19) |
270 0.67 (0.59–0.75) |
| GI bleeding |
1139 1.92 (1.81–2.03) |
59 1.34 (1.00–1.68) |
316 2.11 (1.88–2.34) |
763 1.91 (1.78–2.05) |
| Cardiovascular disease |
3417 5.91 (5.71–6.11) |
259 6.03 (5.29–6.76) |
1140 7.92 (7.46–8.38) |
2018 5.16 (4.94–5.39) |
| Cardiovascular death |
654 1.08 (1.00–1.17) |
73 1.63 (1.26–2.01) |
231 1.51 (1.32–1.71) |
350 0.86 (0.77–0.95) |
| All-cause death |
2242 3.71 (3.56–3.87) |
235 5.26 (4.58–5.93) |
728 4.77 (4.43–5.12) |
1278 3.14 (2.97–3.32) |
| Net clinical outcomea |
3273 5.51 (5.32–5.70) |
313 7.13 (6.34–7.92) |
1034 6.91 (6.49–7.33) |
1925 4.81 (4.59–5.02) |
DOAC, direct oral anticoagulant; GI, gastrointestinal; ICH, intracranial haemorrhage; OAC, oral anticoagulant; SEE, systemic embolic events.
Stroke/SEE/major bleeding/all-cause death.
Comparison of anticoagulation therapy
Table 3 shows the crude and adjusted HRs of primary and secondary endpoints according to anticoagulation therapy with the warfarin group serving as the reference. The risk of stroke/SEE, stroke, and ischaemic stroke was higher in the No OAC group but lower in DOAC group as compared with the warfarin group. The risk of major bleeding events and intracranial haemorrhage was lower in the No OAC and DOAC groups than in the warfarin group. However, all bleeding and gastrointestinal bleeding was comparable between the warfarin and DOAC groups. All-cause death and net clinical outcome were significantly higher in the No OAC group and lower in the DOAC group compared with the warfarin group. Similar results were observed when excluding off-label dosing (Supplementary material online, Tables S3 and S4).
Table 3.
Analysis of each event by crude and adjusted hazard ratios for oral anticoagulant treatment group vs. warfarin
| Variable | No OAC vs. warfarin | DOAC vs. warfarin | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude HR (95% Cl) | P-value | Adjusted HRa (95% Cl) | P-value | Crude HR (95% Cl) | P-value | Adjusted HRa (95% Cl) | P-value | |
| Stroke/SEE | 1.01 (0.80–1.28) | 0.94 | 1.31 (1.02–1.68) | 0.03 | 0.73 (0.64–0.84) | <0.001 | 0.82 (0.71–0.95) | <0.001 |
| Stroke | 1.02 (0.80–1.30) | 0.85 | 1.32 (1.03–1.70) | 0.03 | 0.75 (0.65–0.86) | <0.001 | 0.83 (0.72–0.96) | 0.01 |
| Ischaemic stroke | 1.16 (0.90–1.50) | 0.26 | 1.62 (1.24–2.13) | <0.001 | 0.72 (0.62–0.85) | <0.001 | 0.82 (0.70–0.97) | 0.02 |
| Haemorrhagic stroke | 0.57 (0.29–1.11) | 0.10 | 0.59 (0.29–1.17) | 0.13 | 0.82 (0.60–1.11) | 0.20 | 0.85 (0.62–1.17) | 0.31 |
| SEE | 0.52 (0.12–2.32) | 0.40 | 0.77 (0.16–3.58) | 0.74 | 0.38 (0.17–0.81) | 0.01 | 0.53 (0.24–1.17) | 0.11 |
| Major bleeding | 0.63 (0.45–0.88) | 0.01 | 0.67 (0.48–0.95) | 0.03 | 0.67 (0.57–0.80) | <0.001 | 0.73 (0.62–0.87) | <0.001 |
| All bleeding | 0.52 (0.43–0.63) | 0.007 | 0.53 (0.43–0.65) | <0.001 | 0.84 (0.77–0.92) | <0.001 | 0.92 (0.84–1.01) | 0.07 |
| ICH | 0.59 (0.39–0.89) | 0.01 | 0.62 (0.40–0.94) | 0.02 | 0.65 (0.53–0.79) | <0.001 | 0.68 (0.55–0.83) | <0.001 |
| GI bleeding | 0.63 (0.48–0.84) | 0.001 | 0.65 (0.49–0.86) | 0.003 | 0.91 (0.79–1.03) | 0.14 | 1.00 (0.87–1.14) | 0.98 |
| Cardiovascular disease | 0.76 (0.66–0.87) | <0.001 | 0.95 (0.83–1.10) | 0.50 | 0.65 (0.61–0.70) | <0.001 | 0.83 (0.77–0.89) | <0.001 |
| Cardiovascular death | 1.08 (0.83–1.40) | 0.57 | 1.41 (1.07–1.86) | 0.01 | 0.57 (0.48–0.67) | <0.001 | 0.81 (0.68–0.96) | 0.01 |
| All-cause death | 1.10 (0.95–1.28) | 0.19 | 1.29 (1.11–1.51) | 0.001 | 0.66 (0.60–0.72) | <0.001 | 0.85 (0.77–0.93) | <0.001 |
| Net clinical outcomeb | 1.03 (0.91–1.17) | 0.63 | 1.24 (1.09–1.42) | 0.001 | 0.69 (0.64–0.75) | <0.001 | 0.85 (0.78–0.91) | <0.001 |
CI, confidence interval; DOAC, direct oral anticoagulant; GI, gastrointestinal; HR, hazard ratio; ICH, intracranial haemorrhage; OAC, oral anticoagulant; SEE, systemic embolism.
Adjusted by sex, body mass index history of bleeding, type of AF, systolic blood pressure, severe hepatic disease, diabetes, hyperuricaemia, heart failure and/or reduced left ventricular ejection fraction, myocardial infarction, cerebrovascular disease, thromboembolic disease, active cancer, dementia, fall within 1 year, history of catheter ablation, creatinine clearance, digestive diseases, polypharmacy, and use of antiarrhythmic drugs, anti-platelet agents, proton pump inhibitors, P-glycoprotein inhibitors, and anti-hyperlipidaemia drugs.
Stroke/SEE/major bleeding/all-cause death.
Analysis of risk factors for stroke/SEE, major bleeding, and all-cause death
Table 4 shows the results of Cox multivariate regression analysis for variables other than the type of anticoagulation therapy for stroke/SEE, major bleeding, and all-cause death. Independent risk factors for stroke/SEE were older age ≥85 years, history of cerebrovascular diseases, persistent and long-standing persistent/permanent AF, higher systolic blood pressure, higher HbA1c, other thromboembolic disease, creatinine clearance <30 mL/min, and history of falls within 1 year. Conversely, history of catheter ablation emerged as a factor associated with lower risk of stroke/SEE. As for major bleeding, older age, severe liver function disorder, history of major bleeding, history of cerebrovascular diseases, active cancer, multiple (≥5) drug use, and history of falls within 1 year were significant risk factors. BMI ≥25 kg/m2, history of catheter ablation and antiarrhythmic drug use were associated with lower major bleeding events. Independent risk factors for all-cause death varied widely and included factors other than those for stroke/SEE and major bleeding. In addition to the known risk factors, history of falls within 1 year was also associated with higher all-cause death, whereas female sex, BMI ≥25 kg/m2, dyslipidaemia, digestive disease, and history of catheter ablation were associated with lower all-cause death. Risk factors identified for intracranial haemorrhage were similar to those associated with major bleeding (Supplementary material online, TableS5).
Table 4.
Multivariate analysis on Stroke/SEE, major bleeding and all-cause death
| Factor | Stroke/SEE | Major bleeding | All-cause death | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | ||||
| Sex | Mena | — | — | — | |||||
| Women | 1.01 (0.88–1.16) | 0.91 | 0.92 (0.78–1.09) | 0.33 | 0.61 (0.55–0.67) | <0.001 | |||
| Age | <85 yearsa | — | — | — | |||||
| ≥85 years | 1.28 (1.11–1.48) | 0.001 | 1.27 (1.06–1.52) | 0.01 | 1.81 (1.66–1.99) | <0.001 | |||
| Body mass index | <18.5 kg/m2 | 1.12 (0.86–1.44) | 0.40 | 1.32 (0.99–1.76) | 0.06 | 1.84 (1.62–2.09) | <0.001 | ||
| ≥18.5, <25.0 kg/m2a | — | — | — | ||||||
| ≥25.0 kg/m2 | 1.03 (0.88–1.21) | 0.74 | 0.74 (0.60–0.91) | 0.005 | 0.85 (0.75– 0.95) | 0.006 | |||
| History of major bleeding | Yes | 1.18 (0.91—1.51) | 0.21 | 1.75 (1.32–2.32) | <0.001 | 1.18 (1.00–1.39) | 0.06 | ||
| Noa | — | — | — | ||||||
| Types of AF | Paroxysmala | — | — | — | |||||
| Persistent | 1.64 (1.36–1.98) | <0.001 | 1.03 (0.82–1.30) | 0.78 | 1.29 (1.14–1.46) | <0.001 | |||
| Long–standing persistent/permanent | 1.68 (1.44–1.96) | <0.001 | 0.98 (0.81–1.17) | 0.79 | 1.22 (1.10–1.35) | <0.001 | |||
| Systolic blood pressure | <130 mmHga | — | — | — | |||||
| ≥130, <140 mmHg | 1.08 (0.92–1.28) | 0.35 | 0.97 (0.79–1.19) | 0.79 | 0.88 (0.78–0.98) | 0.02 | |||
| ≥140 mmHg | 1.31 (1.12–1.54) | 0.001 | 1.14 (0.93–1.39) | 0.20 | 0.91 (0.81–1.02) | 0.12 | |||
| Severe liver function disorderb | Yes | 1.60 (0.94–2.72) | 0.08 | 2.26 (1.32–3.85) | 0.003 | 1.79 (1.30– 2.47) | <0.001 | ||
| Noa | — | — | — | ||||||
| Diabetes mellitus | HbA1c: <6.0% | 1.11 (0.82–1.51) | 0.50 | 0.95 (0.64–1.42) | 0.81 | 1.07 (0.88–1.31) | 0.50 | ||
| HbA1c: ≥6.0% | 1.18 (1.01–1.40) | 0.04 | 1.00 (0.81–1.23) | 0.98 | 1.04 (0.93–1.16) | 0.52 | |||
| Nonea | — | — | — | ||||||
| Hyperuricaemia | Yes | 0.91 (0.78–1.07) | 0.27 | 1.06 (0.88–1.28) | 0.55 | 1.11 (1.01–1.22) | 0.04 | ||
| Noa | — | — | — | ||||||
| Heart failure, reduced LVEF | Yes | 0.95 (0.83–1.09) | 0.46 | 1.13 (0.95–1.34) | 0.16 | 1.33 (1.21–1.45) | <0.001 | ||
| Noa | — | — | — | ||||||
| Myocardial infarction | Yes | 1.18 (0.91–1.55) | 0.22 | 1.08 (0.78–1.49) | 0.64 | 1.41 (1.21–1.64) | <0.001 | ||
| Noa | — | — | — | ||||||
| Cerebrovascular diseasec | Yes | 2.25 (1.97–2.58) | <0.001 | 1.25 (1.05–1.49) | 0.01 | 1.13 (1.02–1.24) | 0.02 | ||
| Noa | — | — | — | ||||||
| Other thromboembolic disease | Yes | 1.38 (1.13–1.67) | 0.001 | 1.17 (0.91–1.51) | 0.21 | 1.16 (1.01–1.32) | 0.04 | ||
| Noa | — | — | — | ||||||
| Active cancer | Yes | 1.00 (0.82–1.22) | 1.00 | 1.34 (1.08–1.68) | 0.009 | 1.54 (1.38–1.73) | <0.001 | ||
| Noa | — | — | — | ||||||
| Dementia | Yes | 1.11 (0.90–1.36) | 0.34 | 1.05 (0.81–1.38) | 0.70 | 1.78 (1.59–2.00) | <0.001 | ||
| Noa | — | — | — | ||||||
| Fall within 1 year | Yes | 1.38 (1.12–1.69) | 0.002 | 1.94 (1.54–2.44) | <0.001 | 1.47 (1.29–1.67) | <0.001 | ||
| Noa | — | — | — | ||||||
| Catheter ablation | Yes | 0.58 (0.42–0.79) | <0.001 | 0.66 (0.47–0.94) | 0.02 | 0.55 (0.44–0.69) | <0.001 | ||
| Noa | — | — | — | ||||||
| Antiarrhythmic agents | Yes | 0.99 (0.87–1.13) | 0.91 | 0.84 (0.72–0.99) | 0.04 | 0.97 (0.89–1.06) | 0.54 | ||
| Noa | — | — | — | ||||||
| Antiplatelet agents | Yes | 0.89 (0.74–1.06) | 0.19 | 0.99 (0.80–1.22) | 0.91 | 1.09 (0.98–1.22) | 0.12 | ||
| Noa | — | — | — | ||||||
| Proton pump inhibitors | Yes | 0.88 (0.76–1.01) | 0.08 | 1.00 (0.84–1.19) | 1.00 | 1.06 (0.97–1.17) | 0.18 | ||
| Noa | — | — | — | ||||||
| P-gp inhibitors | Yes | 1.08 (0.67–1.76) | 0.74 | 0.84 (0.43–1.63) | 0.60 | 1.34 (1.01–1.78) | 0.04 | ||
| Noa | — | — | — | ||||||
| Dyslipidaemia | Yes | 0.91 (0.79–1.04) | 0.16 | 0.93 (0.79–1.10) | 0.42 | 0.75 (0.69–0.83) | <0.001 | ||
| Noa | — | — | — | ||||||
| Creatinine clearance | <30 mL/min, severe renal disease, dialysis | 1.34 (1.07–1.67) | 0.01 | 1.10 (0.84–1.43) | 0.51 | 2.56 (2.22–2.96) | <0.001 | ||
| ≥30, <50mL/min | 1.09 (0.92–1.29) | 0.31 | 0.91 (0.74–1.12) | 0.37 | 1.42 (1.25–1.61) | <0.001 | |||
| ≥50 mL/mina | — | — | — | ||||||
| Digestive disease | Yes | 0.97 (0.84–1.12) | 0.67 | 0.94 (0.78–1.12) | 0.47 | 0.82 (0.75–0.90) | <0.001 | ||
| Noa | — | — | — | ||||||
| Polypharmacy | <5 medicinesa | — | — | — | |||||
| ≥5 medicines | 1.01 (0.86–1.19) | 0.90 | 1.30 (1.05–1.61) | 0.02 | 1.28 (1.13–1.44) | <0.001 | |||
Type of anticoagulants were included in the multivariate analysis model as an explanatory factor. The results of univariate and multivariate analysis for type of anticoagulants are separately shown in Table 2 to avoid duplication. Unknown category of body mass index, blood pressure, HbA1c, creatinine clearance, and fall within 1 year were not shown.
AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; HbA1c, glycosylated haemoglobin; HR, hazard ratio; LVEF, left ventricular ejection fraction; P-gp, glycoprotein; SBP, systolic blood pressure; SEE, systemic embolic events.
Reference.
Severe liver dysfunction was based on physician’s decision with no criteria.
Cerebrovascular disease included stroke, TIA, and other cerebral diseases.
Discussion
The present results are clinically relevant because clinical trial data on the efficacy and safety of DOACs for elderly and very elderly patients are limited. Illness, treatment response, and complications tend to manifest differently in elderly patients compared with younger patients owing to the ageing process itself and reduced physiological functions.18,19 Furthermore, elderly patients generally present several comorbidities and are likely to be taking multiple medications, which increases the risk of drug interactions.20 All these factors highlight the complexity involved in treating elderly NVAF patients. The risk of bleeding and stroke increase with increasing age. Thus, the prospectively collected data on the safety of anticoagulants in this large-scale study will help to inform the selection of treatment in this particular population to achieve optimal anticoagulation with fewer complications.
Major findings of the present study
At the 2-year final assessment of the elderly and very elderly NVAF patients (mean age of 81.5 years) in the ANAFIE Registry, 92.4% of patients were receiving OACs, and only 7.6% of patients did not receive OACs. Among those receiving OACs, three-quarters were receiving DOACs, and those remaining were receiving warfarin. During the 2-year observation period, the overall incidences of stroke/SEE, major bleeding, and all-cause death were 3.01%, 2.00%, and 6.95%, respectively. These incidences were lower in the DOAC group than in the warfarin group, but higher in the No OAC group, except for major bleeding. Most of the risk factors for stroke/SEE, major bleeding, and all-cause death were consistent with previous reports,21–23 but several novel risk factors were identified; for instance, fall within 1 year was associated with increased risk, and history of catheter ablation was associated with a decreased risk for stroke/SEE, major bleeding, and all-cause death.
Clinical characteristics of patients in the ANAFIE Registry
In the ANAFIE Registry, the proportion of patients receiving OACs was >90%, which seems high. However, a previous study from Canada24 has reported that more than 70% of octogenarians with AF received anticoagulation therapy and that the proportion increased with lower Clinical Frailty Scale scores. Additionally, the Atrial Fibrillation in Octogenarians (OCTOFA) study25 in France reported that a similar proportion of elderly patients was receiving OACs. Considering that the patients in our registry could visit the hospital and that the development of DOACs has progressively contributed to the widespread use of anticoagulation, the proportion of patients receiving OACs would be comparable to these previous reports.
Among the anticoagulated patients, patients receiving DOACs tended to have a higher creatinine clearance and tended to be more frequently diagnosed with paroxysmal AF, while patients receiving warfarin tended to be older and to have higher proportions of heart failure, diabetes mellitus, chronic kidney disease, and MI. This is consistent with the results of the OCTOFA study, in which most patients receiving vitamin K antagonists had similar characteristics to those in our analysis.25
In contrast, the No OAC group patient profile was complex. Although they were characterized by older age, low BMI, decreased creatinine clearance, and a higher proportion of history of major bleeding, they had relatively low CHADS2 and CHA2DS2-VASc scores, and a relatively high proportion of paroxysmal AF and history of catheter ablation. The former indicated an increased risk for bleeding, which may have led to hesitation from clinicians to prescribe anticoagulation. In contrast, the latter may suggest a relatively low risk for stroke/SEE.26 From these characteristics, the group might comprise subgroups that could be classified by reasons for not using anticoagulation. However, the fact that elderly patients, with lower AF burdens with/without a history of catheter ablation, were included in the same group as very elderly persistent/permanent AF patients, at a high risk of stroke/SEE, might have distorted the patient profiles and clinical outcomes of the No OAC group.
Incidences of clinical events
In the ANAFIE Registry, the incidence rate of stroke/SEE (1.62/100 person-years) was mostly comparable to that in the PREFER in AF trial (<85 years, 2.3%/year)27 and those in other clinical trials (mostly 2–2.5%/year).28–30 While a remarkable increase in stroke/SEE was observed for very elderly patients aged >85 years in the PREFER in AF trial (4.8%/year),27 such an increase was not evident in the ANAFIE Registry. The incidence rate of all-cause death in the ANAFIE Registry (3.71/100 person-years) was much lower than that in previous studies, reflecting an inherent high life expectancy in Japanese elderly NVAF patients than in Caucasian patients.9 The incidence rate of major bleeding in the ANAFIE Registry (1.08/100 person-years) was extremely low compared with previous reports on elderly NVAF patients (aged ≥75 years), which were mostly over 4%/year.27–30
Comparison of event rates among anticoagulation treatment groups
In the ANAFIE Registry, patients in the DOAC group had favourable outcomes for stroke/SEE, major bleeding, all-cause death, and other endpoints compared with those in the warfarin group. These findings would primarily be explained by the advantages of the pharmacological profiles of DOACs, more stable and predictable effects, and less intracranial haemorrhages than warfarin; all of these could also be applied to elderly and very elderly NVAF patients.
Patients receiving DOACs tended to have a higher creatinine clearance, at least in part, because of the prescribing recommendations that prohibit the use for patients with severely impaired renal function. Accordingly, they were more frequently diagnosed with paroxysmal AF. In contrast, patients receiving warfarin tended to be older and have higher proportions of heart failure, diabetes mellitus, chronic kidney disease, and MI. These differences might contribute to the apparently improved prognosis in the DOAC group through unknown confounding factors even after adjustment.
Risk factors associated with primary and secondary endpoints
Most of the identified risk factors for outcome events were consistent with previous reports.21–23 In addition to the well-known risk factors included in the components of CHA2DS2-VASc score, AF types and creatinine clearance <30 mL/min were extracted as independent risks for stroke/SEE. A recent prospective, observational registry study, which included >10 000 patients with AF, also identified the type of AF as a risk factor for the incidence of stroke events and suggested that the AF diagnosis should be considered during therapeutic decision making.31 As for major bleeding, in addition to the components of HAS-BLED score, polypharmacy was an independent risk factor, while BMI >25 kg/m2 was identified as a negative risk factor. Risk factors for all-cause death were various, including age >85 years, BMI <18.5 kg/m2, dementia, and creatinine clearance <30 mL/min. In general, these risk factors are consistent with previous reports.20,21
This registry also identified lesser-known positive and negative risk factors, which were a history of falls within 1 year and history of catheter ablation. The former was associated with both stroke/SEE and major bleeding, and further associated with all-cause death. A history of falls represents the presence of frailty in elderly patients, and it is associated with the increased risk of all-cause death. Moreover, as history of falls is associated with a high risk for future falls, repeated falls could lead to fall-induced major bleeding. The apparent relationships of fall to stroke/SEE remain controversial. In contrast to a report from the ARISTOTLE study, in which a history of falls was not associated with stroke/SEE,32 the Loire Valley Atrial Fibrillation Project demonstrated that it was independently associated with thromboembolism.33 The direct association would be unlikely, and the history of falls, which is common in elderly individuals, might be a surrogate marker applied only to elderly patients.
History of catheter ablation was negatively associated with stroke/SEE, major bleeding, and all-cause death. Reducing AF burden by catheter ablation would contribute to reducing the risk of stroke/SEE and other cardiovascular events leading to lower cardiovascular deaths.26 Although anticoagulation may not be discontinued after catheter ablation, reducing AF burden would allow lowering of the dose or transient interruption of anticoagulation as necessary, and may lead to a reduced risk of major bleeding.
Limitations
This study had some limitations. First, the registry targeted a specific group of Japanese elderly patients. Therefore, the results should not be applied to other populations, particularly inpatients, patients visited by home doctors, or residents of nursing homes. Second, the present analysis did not take into account OAC changes during the follow-up. Control with warfarin had been determined for 6 months just preceding the enrolment, but not during the follow-up period. Third, in the design of this study, not only newly diagnosed AF patients or new users of anticoagulants, but established NVAF patients or those who were receiving anticoagulants prior to enrolment were allowed to participate. Such patients were associated with low incidence of clinical events. However, even in those populations, stroke/SEE, major bleeding, and all-cause death were significantly lower in the DOAC group than in the well-controlled warfarin group. Fourth, as in many observational studies, the proportions of patients lost to follow-up and who withdrew consent were relatively high as compared with randomized controlled trials. While the frequency of patients lost to follow-up did not differ among the treatment groups that of withdrawal of consent differed among the groups. Continued follow-up, particularly in very elderly patients and frail patients, was difficult. Fifth, the incidence of major bleeding was lower than we expected. We used the ISTH definition, which explains that a bleeding event with a fall in haemoglobin level of 2 g/dL (1.24 mmol/L) or more or leading to transfusion of two or more units of whole blood or red cells, should be classified as major bleeding if it is not a surgical situation and not in a critical area or organ. A possible reason for the low incidence of major bleeding is that the haemoglobin level may not have been well evaluated before and after the bleeding events occurred, or the amounts of transfusions may be smaller for elderly patients in the real-world setting compared with that in clinical trial settings. Finally, multivariate analysis was performed with the obtained factors that were expected to be associated with each event according to current guidelines. Nonetheless, some unknown confounders might have affected the present results.
Conclusion
Currently, in Japan, a large proportion of elderly and very elderly NVAF patients were treated with DOACs. The rates of stroke/SEE, major bleeding, and all-cause death were observed less frequently in patients receiving DOACs as compared with patients well-controlled with warfarin. No anticoagulation was associated with worse outcomes, except for major and all bleeding events compared with warfarin. Moreover, history of falls within 1 year at enrolment and history of catheter ablation were identified as positive and negative independent risk factors, respectively, for stroke/SEE, major bleeding, and all-cause death. These results can help inform appropriate management of the growing elderly and very elderly NVAF patient population worldwide.
Supplementary material
Supplementary material is available at European Heart Journal – Quality of Care and Clinical Outcomes online.
Supplementary Material
Acknowledgements
The authors wish to thank Keyra Martinez Dunn, MD, of Edanz Pharma for providing medical writing support, which was funded by Daiichi Sankyo Co., Ltd.
Notes
Baseline data were presented at the 83rd Annual Scientific Meeting of the Japanese Circulation Society; 29–31 March 2019, Yokohama, Japan
Funding
This work was supported by Daiichi Sankyo Co., Ltd.
Conflict of interest: T.Yamashita received research funding from Bristol-Myers Squibb, Bayer, and Daiichi Sankyo, manuscript fees from Daiichi Sankyo and Bristol-Myers Squibb, and remuneration from Daiichi Sankyo, Bayer, Pfizer Japan, and Bristol-Myers Squibb. S.S. received research funding from Daiichi Sankyo, and remuneration from Bristol-Myers Squibb and Daiichi Sankyo. H.I. received remuneration from Daiichi Sankyo, Bayer, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. M.A. received research funding from Bayer and Daiichi Sankyo, and remuneration from Bristol-Myers Squibb, Nippon Boehringer Ingelheim, Bayer, and Daiichi Sankyo. H.A. received remuneration from Daiichi Sankyo. T.I. received research funding from Daiichi Sankyo and Bayer, and remuneration from Daiichi Sankyo, Bayer, Nippon Boehringer Ingelheim, and Bristol-Myers Squibb. K.O. received remuneration from Nippon Boehringer Ingelheim, Daiichi Sankyo, Johnson & Johnson, and Medtronic. YK received remuneration from Daiichi Sankyo, Bayer, and Nippon Boehringer Ingelheim. W.S. received research funding from Bristol-Myers Squibb, Daiichi Sankyo, and Nippon Boehringer Ingelheim, and patent royalties/licensing fees from Daiichi Sankyo, Pfizer Japan, Bristol-Myers Squibb, Bayer, and Nippon Boehringer Ingelheim. H.T. received research funding from Daiichi Sankyo and Nippon Boehringer Ingelheim, remuneration from Daiichi Sankyo, Bayer, Nippon Boehringer Ingelheim, and Pfizer Japan, scholarship funding from Daiichi Sankyo, and consultancy fees from Pfizer Japan, Bayer, and Nippon Boehringer Ingelheim. K.T. received remuneration from Daiichi Sankyo, Bayer, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. A.H. participated in a course endowed by Boston Scientific Japan, has received research funding from Daiichi Sankyo and Bayer, and remuneration from Bayer, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. M.Y. received research funding from Nippon Boehringer Ingelheim, and remuneration from Nippon Boehringer Ingelheim, Daiichi Sankyo, Bayer, Bristol-Myers Squibb, and Pfizer Japan. T.Yamaguchi acted as an Advisory Board member of Daiichi Sankyo and received remuneration from Daiichi Sankyo and Bristol-Myers Squibb. S.T. received research funding from Nippon Boehringer Ingelheim and remuneration from Daiichi Sankyo. T.K., J.K., and A.T. are employees of Daiichi Sankyo.
Data availability
1. Will the individual deidentified participant data (including data dictionaries) be shared?
→Yes
2. What data in particular will be shared?
→Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices).
3. Will any additional, related documents be available? If so, what is it? (e.g. study protocol, statistical analysis plan, etc.)
→Study Protocol
4. When will the data become available and for how long?
→Ending 36 months following article publication.
5. By what access criteria will the data be shared (including with whom)?
→Access criteria for data sharing (including proposals) may be reviewed by a committee led by Daiichi-Sankyo.
6. For what types of analyses, and by what mechanism will the data be available?
→Any purpose; proposals should be directed to yamt-tky@umin.ac.jp
To gain access, data requestors will need to sign a data access agreement.
References
- 1. World Population Ageing 2019 Highlight. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/worldpopulationageing2019-highlights.pdf (12 April 2021).
- 2. Di Carlo A, Bellino L, Consoli D, Mori F, Zaninelli A, Baldereschi M. et al. ; National Research Program: Progetto FAI. La Fibrillazione Atriale in Italia. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: the FAI Project. Europace 2019;21:1468–1475. [DOI] [PubMed] [Google Scholar]
- 3. Wolf PA, Abbott RD, Kannel WB.. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 4. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A. et al. ; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 5. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W. et al. ; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 6. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M. et al. ; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 7. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL. et al. ; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 8. Potpara TS, Lip GY.. Oral anticoagulant therapy in atrial fibrillation patients at high stroke and bleeding risk. Prog Cardiovasc Dis 2015;58:177–194. [DOI] [PubMed] [Google Scholar]
- 9. Senoo K, An Y, Ogawa H, Lane DA, Wolff A, Shantsila E. et al. Stroke and death in elderly patients with atrial fibrillation in Japan compared with the United Kingdom. Heart 2016;102:1878–1882. [DOI] [PubMed] [Google Scholar]
- 10. Batey M, Hecht J, Callahan C, Wahl W.. Direct oral anticoagulants do not worsen traumatic brain injury after low-level falls in the elderly. Surgery 2018;164:814–819. [DOI] [PubMed] [Google Scholar]
- 11. Cavallari I, Patti G.. Efficacy and safety of oral anticoagulation in elderly patients with atrial fibrillation. Anatol J Cardiol 2018;19:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shinohara M, Wada R, Yao S, Yano K, Akitsu K, Koike H. et al. Evaluation of oral anticoagulants in atrial fibrillation patients over 80 years of age with nonsevere frailty. J Arrhythmia 2019;35:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tse HF, Wang YJ, Ahmed Ai-Abdullah M, Pizarro-Borromeo AB, Chiang CE, Krittayaphong R. et al. Stroke prevention in atrial fibrillation—an Asian stroke perspective. Heart Rhythm 2013;10:1082–1088. [DOI] [PubMed] [Google Scholar]
- 14. Inoue H, Fujiki A, Origasa H, Ogawa S, Okumura K, Kubota I. et al. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol 2009;137:102–107. [DOI] [PubMed] [Google Scholar]
- 15. Inoue H, Yamashita T, Akao M, Atarashi H, Ikeda T, Okumura K. et al. Prospective observational study in elderly patients with non-valvular atrial fibrillation: rationale and design of the All Nippon AF In the Elderly (ANAFIE) Registry. J Cardiol 2018;72:300–306. [DOI] [PubMed] [Google Scholar]
- 16. Koretsune Y, Yamashita T, Akao M, Atarashi H, Ikeda T, Okumura K. et al. Baseline demographics and clinical characteristics in the All Nippon AF in the Elderly (ANAFIE) Registry. Circ J 2019;83:1538–1545. [DOI] [PubMed] [Google Scholar]
- 17. JCS Joint Working Group. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013). Circ J 2014;78:1997–2021. [DOI] [PubMed] [Google Scholar]
- 18. Navaratnarajah A, Jackson SHD.. The physiology of ageing. Medicine 2017;45:6–10. [Google Scholar]
- 19. Steenman M, Lande G.. Cardiac aging and heart disease in humans. Biophys Rev 2017;9:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Benedetti G, Neccia M, Agati L.. Direct oral anticoagulants use in elderly patients with non valvular atrial fibrillation: state of evidence. Minerva Cardioangiol 2018;66:301–313. [DOI] [PubMed] [Google Scholar]
- 21. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 22. Pokorney SD, Piccini JP, Stevens SR, Patel MR, Pieper KS, Halperin JL. et al. Cause of death and predictors of all-cause mortality in anticoagulated patients with nonvalvular atrial fibrillation: data from ROCKET AF. J Am Heart Assoc 2016;5:e002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steinberg JS, Sadaniantz A, Kron J, Krahn A, Denny DM, Daubert J. et al. Analysis of cause-specific mortality in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Circulation 2004;109:1973–1980. [DOI] [PubMed] [Google Scholar]
- 24. Roca F, Bahri O, Chassagne P.. Frailty and anticoagulation prescription rate for atrial fibrillation in the elderly. Can J Cardiol 2016;32:270.e9. [DOI] [PubMed] [Google Scholar]
- 25. Blacher J, Sorbets E, Guedj Meynier D, Huberman JP, Gauthier J, Cohen S. et al. Determinants of antithrombotic treatment for atrial fibrillation in octogenarians: results of the OCTOFA study. Clin Drug Investig 2019;39:891–898. [DOI] [PubMed] [Google Scholar]
- 26. Friberg L, Tabrizi F, Englund A.. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. Eur Heart J 2016;37:2478–2487. [DOI] [PubMed] [Google Scholar]
- 27. Patti G, Lucerna M, Pecen L, Siller-Matula JM, Cavallari I, Kirchhof P. et al. Thromboembolic risk, bleeding outcomes and effect of different antithrombotic strategies in very elderly patients with atrial fibrillation: a sub-analysis from the PREFER in AF (PREvention oF Thromboembolic Events-European Registry in Atrial Fibrillation). J Am Heart Assoc 2017;6:e005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Halperin JL, Hankey GJ, Wojdyla DM, Piccini JP, Lokhnygina Y, Patel MR. et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation 2014;130:138–146. [DOI] [PubMed] [Google Scholar]
- 29. Halvorsen S, Atar D, Yang H, De Caterina R, Erol C, Garcia D. et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J 2014;35:1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kato ET, Giugliano RP, Ruff CT, Koretsune Y, Yamashita T, Kiss RG. et al. Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the ENGAGE AF-TIMI 48 trial. J Am Heart Assoc 2016;5:e003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cho S, Kim J, Kim JB, Park J, Park JK, Kang KW, Shim J. et al. The difference of burden of ectopic beats in different types of atrial fibrillation and the effect of atrial fibrillation type on stroke risk in a prospective cohort of patients with atrial fibrillation (CODE-AF registry). Sci Rep 2020;10:6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rao MP, Vinereanu D, Wojdyla DM, Alexander JH, Atar D, Hylek EM. et al. Clinical outcomes and history of fall in patients with atrial fibrillation treated with oral anticoagulation: insights from the ARISTOTLE trial. Am J Med 2018;131:269–275. [DOI] [PubMed] [Google Scholar]
- 33. Banerjee A, Clementy N, Haguenoer K, Fauchier L, Lip GY.. Prior history of falls and risk of outcomes in atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Am J Med 2014;127:972–978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
1. Will the individual deidentified participant data (including data dictionaries) be shared?
→Yes
2. What data in particular will be shared?
→Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices).
3. Will any additional, related documents be available? If so, what is it? (e.g. study protocol, statistical analysis plan, etc.)
→Study Protocol
4. When will the data become available and for how long?
→Ending 36 months following article publication.
5. By what access criteria will the data be shared (including with whom)?
→Access criteria for data sharing (including proposals) may be reviewed by a committee led by Daiichi-Sankyo.
6. For what types of analyses, and by what mechanism will the data be available?
→Any purpose; proposals should be directed to yamt-tky@umin.ac.jp
To gain access, data requestors will need to sign a data access agreement.