Abstract
Aims
The aim of this study was to investigate the pattern, causes, and predictors of all new hospitalizations in patients who underwent transcatheter aortic valve implantation (TAVI).
Methods and results
The nationwide Swedish TAVI registry was merged with other mandatory healthcare registries, which enabled the analysis of all TAVI procedures, new hospital admissions, and death between the years 2008 and 2017. A total of 2821 patients underwent TAVI with a mean of 2.5 hospitalizations during a mean follow-up of 2.2 years. Hospitalizations were associated with worse prognosis. Heart failure (HF) was the most common cause of hospitalization with 19% having at least one hospitalization due to HF causing, 16% of all-cause admissions, and 50% of cardiovascular admissions. Male gender, age >90 years, high Charlson Comorbidity Index, atrial fibrillation, present neurologic disease, severe renal impairment, peripheral vascular disease, New York Heart Association class IV, mild or moderate mean aortic valve gradients, and pulmonary hypertension were associated with an increased risk for all-cause hospitalizations or death. For cardiovascular hospitalization or death, the pattern was similar, with the addition of impaired systolic left ventricular function as a predictor.
Conclusion
Multiple hospitalizations after TAVI are common and are often caused by HF. Reducing the rate of HF hospitalizations is important to mitigate the burden on the healthcare system due to new hospitalizations after TAVI.
Keywords: TAVI, Hospitalization, Heart failure, Repeated events
Graphical Abstract
Graphical Abstract.
Introduction
Transcatheter aortic valve implantation (TAVI) has developed from a less invasive treatment option for patients with aortic stenosis to being favoured over open surgery in patients at high surgical risk.1 For patients at low surgical risk, open surgery is currently recommended although recent studies suggest that TAVI is non-inferior or superior in terms of mortality.2,3 Aortic stenosis is more prevalent among the elderly and most aortic valve replacement procedures are performed in patients over 65 years of age.4 As TAVI is considered mainly in patients at moderate or high surgical risk, the population usually have several underlying comorbidities. TAVI has been shown to be a cost-effective procedure compared to medical treatment in patients who are not accepted for open heart surgery, due to better survival and less hospitalizations.5,6 Some patients present with left ventricular hypertrophy and/or impaired left ventricular function and although studies have suggested improved function post-TAVI there are differences within the population and varying degrees of benefits.7,8 A better understanding on which patients that will benefit is desirable.
Readmission rates are considered important markers of both clinical outcome and cost-effectiveness.9 However, earlier studies on readmission rates after TAVI focus on time to event only ignoring repeated events.9 The Swedish registries,10,11 with nationwide complete coverage of deaths and hospitalizations over time offers a unique possibility to study the total burden of hospitalizations, hospitalization characteristics, and predictors of multiple admissions in a real-life setting.
Methods
Study population
Data in this study were collected from the Swedish Transcatheter Cardiac Intervention Registry (SWENTRY) which is part of the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated according to Recommended Therapies (SWEDEHEART) registry.11 SWENTRY was introduced in year 2010 to enable evaluation and follow-up of patients undergoing TAVI in Sweden. The nationwide registry holds 169 variables from eight different TAVI centres with pre-operative information on comorbidities, angiographic findings and findings from echocardiography as well as 40 variables on follow-up data. Swedish TAVI procedures is rapidly increasing and as of today, over 1000 new patients are reported each year.12,13
All patients who underwent a TAVI procedure for aortic stenosis in Sweden between the years 2008 to 2017 were included in the analysis. Patients with severe aortic regurgitation and patients who died at the day of procedure were excluded. No other exclusion criteria were adopted.
Data on mortality and new hospital admissions in the study population were obtained by merging SWEDEHEART with other national registries. All Swedish citizens have a unique personal identification number which is included in SWEDEHEART.10 Using the numbers, data from SWEDEHEART were merged with the National Cause of Death Register, which includes information about vital status of all Swedish citizens, and the National Patient Registry, which includes International Statistical Classification of Diseases and Related Health Problems—Tenth Revision (ICD-10) diagnoses for all hospital admissions in Sweden. Before analysis, data were pseudoanonymized. Unless stated otherwise, all variables came directly from the registries and main ICD-10 codes were used. Ethical approval was granted through the regional ethics committee.
Definitions and outcomes of interest
The primary outcomes of interest in this study were all-cause mortality and all-cause hospitalization. For hospitalization, a main ICD-10 diagnosis code starting with I20–I25, I3–I5, I7, and J819 [angina pectoris, acute coronary syndrome (ACS) with or without complications, aortic aneurysm, peri-/myocarditis, endocarditis, heart valve diseases, heart failure (HF)/pulmonary oedema, arrhythmias, peripheral vascular disease (PVD)] was considered as hospitalization from cardiovascular causes and was analysed in a subgroup analysis. For repeated hospitalizations, a censoring of two days from latest discharge was added to avoid double registration.
As in the registry, a critical procedure was defined as the presence of one of the following at the time of procedure: ventricular tachycardia, ventricular fibrillation, cardiopulmonary resuscitation, assisted ventilation, inotropic support, or acute renal failure. Atrial fibrillation (AF) was defined as both paroxysmal and permanent fibrillation. Neurologic disease was defined as difficulty moving as a result of neurological or neuromuscular disease estimated by the treating physician. Age, left ventricular function, mean aortic valve gradient, aortic-, and mitral regurgitation were treated as categorical variables.
Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) stage was calculated from register data with the R package ‘nephro’.14 A non-African ethnicity was assumed in the calculation.
For pulmonary hypertension, continuous values were only available from year 2012. As a consequence, a cut-off at 60 mmHg was applied on the continuous data and the complete variable was treated as dichotomous.
Charlson Comorbidity Index (CCI)15 was calculated on each patient based on ICD-10 diagnosis codes from the National Patient Registry where codes identifying myocardial infarction, congestive heart disease, chronic pulmonary disease, ulcer disease, liver disease, PVD, cerebrovascular disease, dementia, hemiplegia, tumours, connective tissue disease, diabetes with or without complications, and renal disease were included. AIDS was not included as those ICD-10 codes were not part of the register data.
Statistical analyses
The pattern of missing data was inspected and analysed. All register variables of interest with a missing frequency of less than 1% were included in the statistical analysis. Pulmonary hypertension had a higher missing frequency yet considered an important variable. To enable inclusion, multiple imputation was performed on the dataset and pulmonary hypertension was included. Imputation was performed based on Fully Conditional Specification, where each incomplete variable is imputed by a separate model. Predictive mean matching was used for numerical data, logistic regression for binary data and polytomous regression imputation for categorical data with more than two levels. Imputed data were checked and compared with non-imputed data.
A multistate model was used in the analysis of clinical outcomes which is presented in Supplementary material online, Appendix 1. Mortality and hospitalization were analysed as competing events. If an event occurred, time to next hospitalization or time to death from the latest hospitalization were analysed. The analysis included up to the three consecutive hospitalizations for each patient. In the case of no events, the patient contributed to the model up to censoring. When more than one TAVI procedure per patient was performed, only the first procedure was analysed.
The results were fitted using a multivariate-adjusted Cox proportional hazard regression model and the transition rates presented in forest plots. Time to first hospitalization or death was analysed separately and presented in a cumulative events plot. Furthermore, for the patient population, mean and median follow-up times and survival times were calculated. Baseline characteristics were stratified between patients with or without hospitalization and compared with the χ2 test. P < 0.05 was considered statistically significant. All statistical analyses were performed using the statistical software R v 4.0.2.
Results
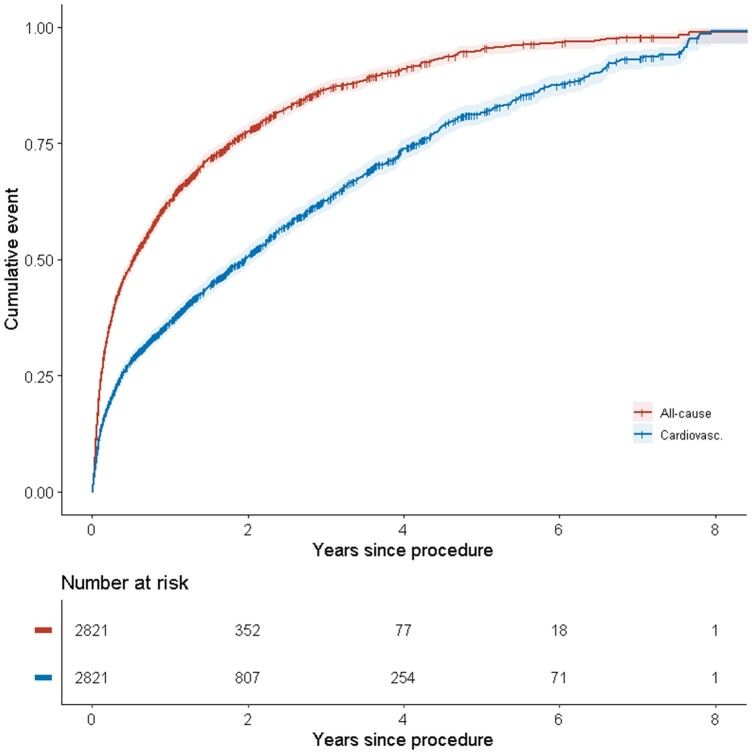
Three patients were removed due to obvious registration errors. All remaining patients (n = 2821) were included in the analysis. Baseline characteristics are shown in Supplementary material online, Table S1 in Appendix 1. Patients with at least one hospitalization had a higher rate of previous cardiac surgery and a lower rate of critical procedure. The follow-up time ranged from 0 to 3545 days (0–9.7 years) with a median of 626 days (1.7 years) and a mean of 805 days (2.2 years). During that time 7354 hospitalizations occurred, out of which 2351 were due to cardiovascular causes. For patients with follow-up time exceeding 6 years post-procedure, all were either hospitalized at least once or dead (Figure 1). The same time period for cardiovascular hospitalization or death was eight years. A total of 43% (1202) had a pre-procedural diagnosis of AF. Based on ICD-10 codes, 16% (261) patients of those without pre-existing AF (9% of the total study population) were diagnosed with AF post-procedure.
Figure 1.
Incidence of hospitalizations post-procedure grouped by all-cause hospitalization or death and cardiovascular hospitalization or death.
Hospitalization characteristics
The pattern of hospitalization is presented in Figure 2. The mean and median number of hospitalizations per patient were 2.5 and one respectively. For all-cause hospitalizations, 1001 different main diagnoses were registered. No significant decrease in rehospitalizations was observed during the investigated time period.
Figure 2.
Hospitalizations grouped by main ICD-10 diagnosis group.
HF was the most common rehospitalization reason, accounting for 16% (1178) of the total number of hospitalizations. A total of 19% (543) were hospitalized at least once due to HF. Infection was the second most common cause accounting for 7% of hospitalizations (525). At least one hospitalization due to infection occurred in 14% (390) of the study population. A total of 2% (52) of the hospitalizations were due to endocarditis. Full characteristics are presented in Table 1.
Table 1.
All-cause hospitalization pattern
| Total number of hospitalizations | Relative frequency | Individual patients with at least one hospitalization | Individual frequency | |
|---|---|---|---|---|
| Heart failure | 1178 | 16% | 543 | 19% |
| Infection | 525 | 7% | 390 | 14% |
| Pneumonia | 321 | 4% | 257 | 9% |
| Acute coronary syndrome/angina pectoris | 266 | 4% | 173 | 6% |
| Atrial fibrillation | 254 | 3% | 174 | 6% |
| Stroke | 213 | 3% | 177 | 6% |
| Chronic obstructive pulmonary disease | 174 | 2% | 73 | 3% |
| Renal failure | 169 | 2% | 102 | 4% |
| Chest pain | 165 | 2% | 113 | 4% |
| Bleeding | 100 | 1% | 91 | 3% |
| Other | 3989 | 54% | 1560 | 54% |
For hospitalizations from cardiovascular causes, totally 92 different main diagnoses were registered. HF was most common, accounting for 50% of the total number of cardiovascular hospitalizations. ACS or angina pectoris was the second most common cause, accounting for 15% of the cardiovascular hospitalizations and with 7% (212) admitted at least once. AF accounted for 11% of admissions (254) and 6% (174) of patients were admitted at least once. Further details are presented in Table 2.
Table 2.
Hospitalization pattern from cardiovascular causes
| Total number of hospitalizations | Relative frequency | Individual patients with at least one hospitalization | Individual frequency | |
|---|---|---|---|---|
| Heart failure | 1178 | 50% | 543 | 19% |
| Acute coronary syndrome/angina pectoris | 342 | 15% | 212 | 7% |
| Atrial fibrillation | 254 | 11% | 174 | 6% |
| Atherosclerosis/PVD | 172 | 7% | 99 | 3% |
| Atrioventricular/bundle block | 68 | 3% | 68 | 2% |
| Endocarditis | 52 | 2% | 49 | 2% |
| Aortic aneurysm | 27 | 1% | 23 | 1% |
| Other arrhythmia | 23 | 1% | 21 | 1% |
| Mitral regurgitation | 18 | 1% | 13 | 0% |
| Ventricular tachycardia/cardiac arrest | 13 | 1% | 13 | 0% |
| Other | 204 | 9% | 167 | 6% |
PVD, peripheral vascular disease.
Predictors of hospitalization or death
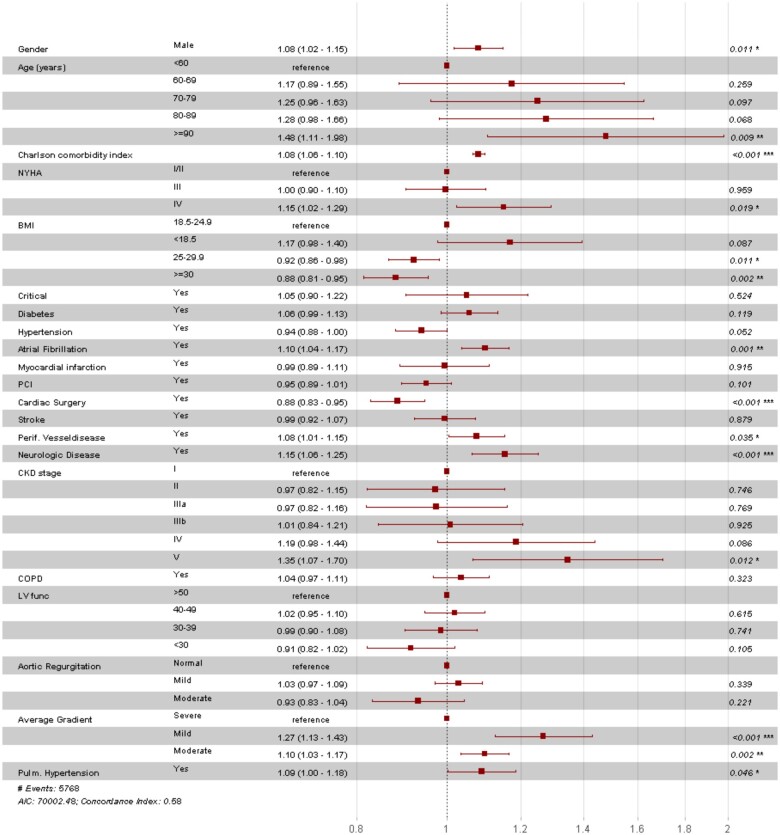
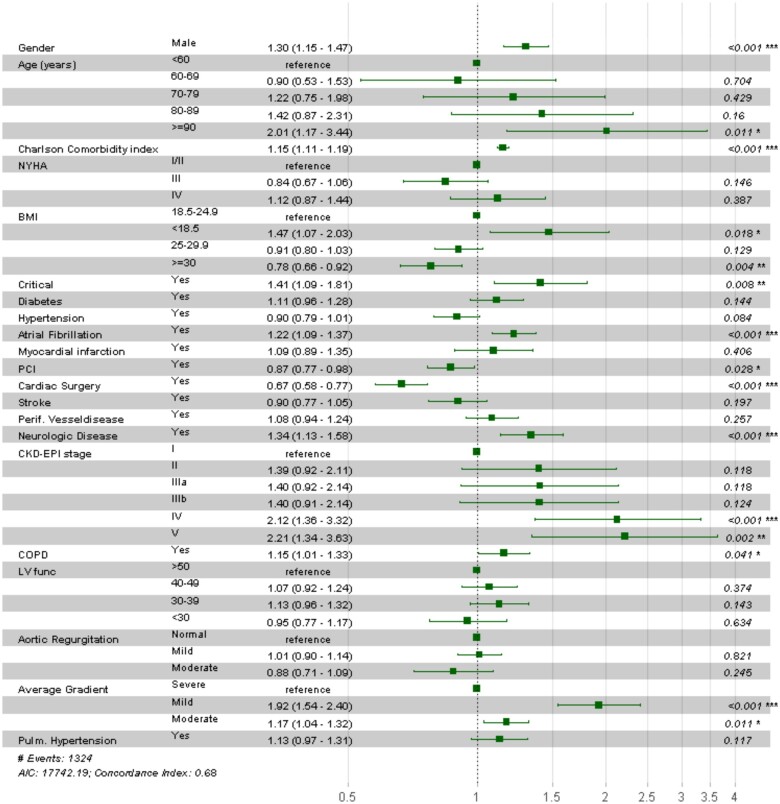
As presented in Figure 3; male gender, age >90 years, high CCI, AF, present neurologic disease, PVD, CKD-EPI stage V, New York Heart Association (NYHA) class IV, mild mean aortic valve gradient and pulmonary hypertension were associated with increased risk for all-cause hospitalizations or death. Instead, body mass index (BMI) >25, female gender, previous cardiac surgery and moderate (25–50 mmHg) or severe (>50 mmHg) mean aortic valve gradient were associated with lower risk for all-cause hospitalizations or death.
Figure 3.
Hazard ratio for all-cause hospitalization or death. Column three represents estimates with 95% confidence intervals between round brackets. P <0.05 was considered significant. Aortic regurgitation levels: mild = <25%, moderate = 25–69%; Average gradient levels: severe = >40 mmHg, Mild = 0–20 mmHg, Moderate = 20–40 mmHg; BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; Critical, one of the following at the time of procedure: ventricular tachycardia, ventricular fibrillation, cardiopulmonary resuscitation, assisted ventilation, inotropic support or acute renal failure; LV func, systolic left ventricular function; Neurologic disease, difficulty moving as a result of neurological- or neuromuscular disease estimated by the surgeon; NYHA class, New York Heart Association class; PCI, percutaneous coronary intervention; Pulmonary hypertension, ≥60 mmHg; PVD, peripheral vascular disease.
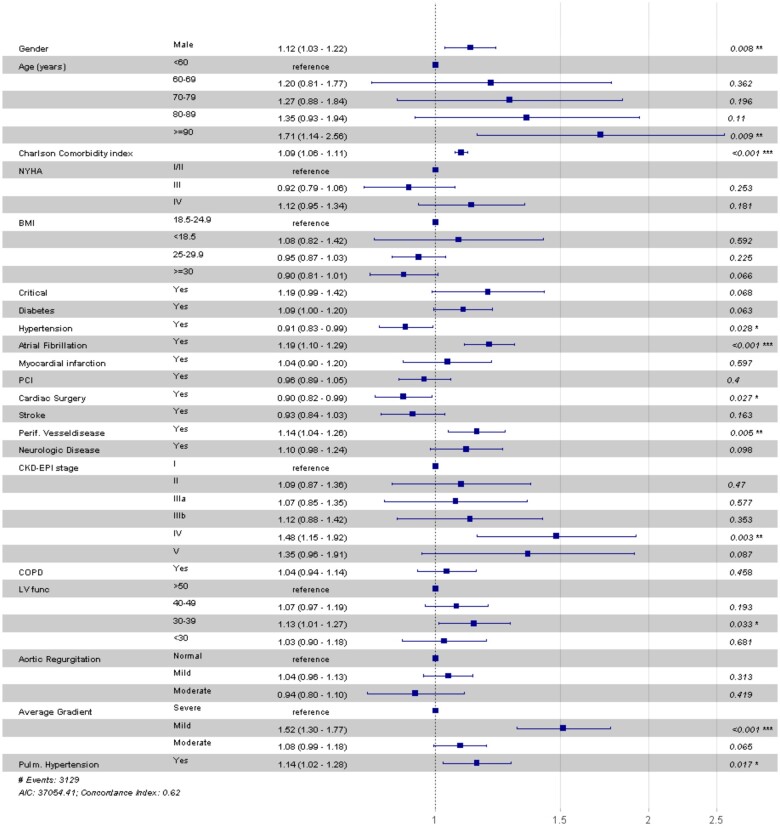
Predictors of cardiovascular hospitalizations are presented in Figure 4. Male gender, age >90 years, high CCI, AF, CKD-EPI stage IV, PVD, decreased left ventricular function, mild mean aortic valve gradient, and pulmonary hypertension were associated with increased risk for cardiovascular hospitalizations or death. Female gender, hypertension and prior cardiac surgery were associated with lower risk for cardiovascular hospitalizations or death.
Figure 4.
Hazard ratio for cardiovascular hospitalization or death. Column three represents estimates with 95% confidence intervals between round brackets. P < 0.05 was considered significant. Aortic regurgitation levels: mild = <25%, moderate = 25–69%; Average gradient levels: severe = >40 mmHg, mild = 0–20 mmHg, moderate = 20–40 mmHg. BMI, body mass index; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; Critical, one of the following at the time of procedure: ventricular tachycardia, ventricular fibrillation, cardiopulmonary resuscitation, assisted ventilation, inotropic support, or acute renal failure; LV func, systolic left ventricular function; Neurologic disease, difficulty moving as a result of neurological- or neuromuscular disease estimated by the surgeon; NYHA class, New York Heart Association class; PCI, percutaneous coronary intervention; Pulmonary hypertension, ≥60 mmHg; PVD, peripheral vascular disease.
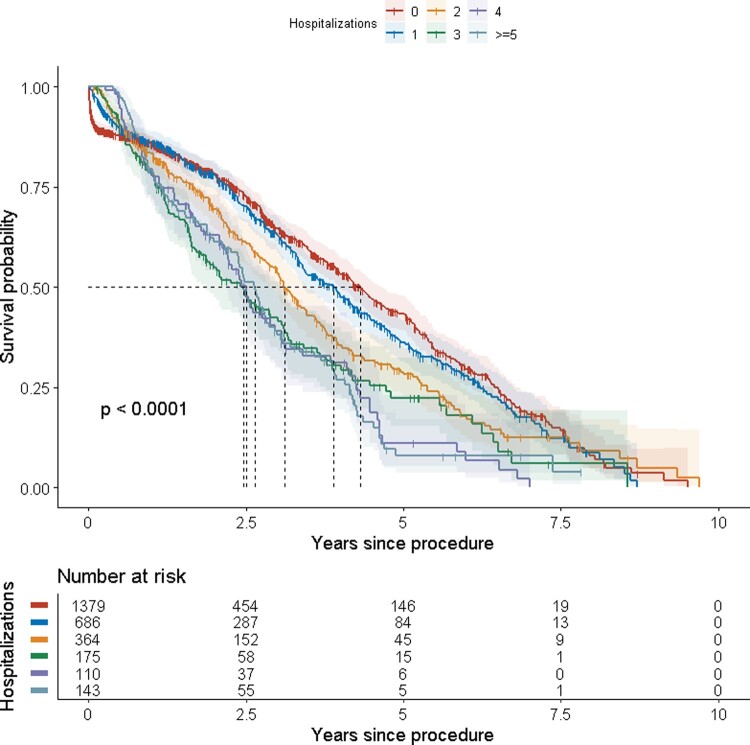
As seen in Figure 5, expected median survival decreased with an increasing number of hospitalizations.
Figure 5.
Survival grouped on number of hospitalizations within one year from procedure.
Risk factors for early death independent of hospitalizations were analysed (Figure 6) and were the same as in the model including all-cause hospitalizations with the exceptions of critical procedure, chronic obstructive pulmonary disease, and prior percutaneous coronary intervention being significant while NYHA class IV, PVD and pulmonary hypertension was not.
Figure 6.
Hazard ratio for early death. Column three represents estimates with 95% confidence intervals between round brackets. P < 0.05 was considered significant. Aortic regurgitation levels: mild = <25%, moderate = 25–69%; Average gradient levels: severe = >40 mmHg; mild = 0–20 mmHg; moderate = 20–40 mmHg; BMI, body mass index; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; Critical, one of the following at the time of procedure: ventricular tachycardia, ventricular fibrillation, cardiopulmonary resuscitation, assisted ventilation, inotropic support, or acute renal failure; LV func, systolic left ventricular function; Neurologic disease, difficulty moving as a result of neurological- or neuromuscular disease estimated by the surgeon; NYHA class, New York Heart Association class; PCI, percutaneous coronary intervention; Pulmonary hypertension, ≥60 mmHg; PVD, peripheral vascular disease.
Discussion
When analysing all patients undergoing TAVI in Sweden with complete follow-up on all hospitalizations we found that repeat hospitalizations were very frequent based on multiple different diagnoses. Many hospitalizations were associated with worse survival. HF was most common as main diagnosis, accounting for 16% of the total hospitalizations and half of cardiovascular hospitalizations. The numbers are consistent with earlier studies on both short-term and long-term hospitalization9,16 and suggest that HF represent an important cause of disability after TAVI.
Pre-procedural NYHA class was not a significant marker for repeated hospitalizations, whilst impaired left ventricular function was. The presence of pulmonary hypertension did indicate an increased risk for repeated cardiovascular hospitalizations or death. In earlier studies, pulmonary hypertension at baseline has been reported as a predictor of mortality.17 A high mean aortic valve gradient was associated with decreased risk for repeated hospitalizations or death. However, only moderate or high mean aortic valve gradient with preserved left ventricular function was found to be prognostic for decreased risk for hospitalizations whilst no such effect was found for patients with concomitant impaired left ventricular function. Patients with low-flow low-gradient aortic stenosis are known to have worse long-term outcomes and in earlier publications low flow were an independent risk factor regardless of left ventricular function in terms of mortality and this is a plausible explanation.18 Echocardiography is an important tool for risk assessment and findings from this study suggest that in terms of risk for hospitalization, the intervention is most useful while the left ventricular systolic function is intact although intervention as such is proven superior to medical treatment in all relevant subgroups.19 However, full classification including flow characteristics would have been valuable to include in the analysis, which was not possible with this material.
Pre-procedural AF was present in almost half of the patients whilst earlier reported numbers ranges between 16% and 51%.20 For new-onset AF, the numbers in the literature vary from 1% up to 32%.20 The variation might be explained by inconsistent presentation, for instance some studies reporting percentage of those without known AF and others percentage of the whole study population. The findings in this study was within the known range and significantly lower than for surgical aortic valve replacement, where numbers up to 40% are reported.20 AF is a known risk factor for mortality and HF,21 the latter as shown above accountable for a large proportion of hospitalizations after TAVI. As a result, this group is a target for further investigation.
Previous studies on renal function and TAVI suggests a strong relationship between impaired renal function and mortality.22 In the referenced study,22 acute periprocedural kidney injury was closely related to PVD, a variable that was also significant in terms of repeated cardiovascular hospitalizations in this material.
For repeated hospitalizations, an important finding is that nonagenarians were at higher risk whilst earlier studies on TAVI have described favourable outcomes.12,23 Age is a risk factor for hospitalization24 and one might argue that, despite more frequent hospitalization, nonagenarians will still benefit from the procedure. A careful patient selection in this age group is needed and a study where hospitalization rates for elderly TAVI patients are compared with rates for patients from the general population would be ideal to gain further knowledge.
ACS/angina pectoris accounted for a higher number than in previous studies where levels around a few percent are reported.9,16 A plausible explanation might be that in the previous studies, only ACS is accounted for and if stable angina pectoris is added, the numbers might be more similar. Furthermore, since many previous studies only register the first hospitalization, they do not have the possibility to find patients with repeated hospitalizations due to both HF and ACS/angina pectoris which might affect the proportions. The relatively high frequency highlights the question of timing of revascularization, an area where further research is needed.25
Endocarditis post-procedure is a minor but clinically important complication. In our complete national database, 2% of cardiovascular hospitalizations were due to endocarditis, or less than 1% of the total number of rehospitalizations which is in agreement with earlier studies.26
For all-cause hospitalization, high BMI was protective in terms of repeated hospitalizations or death. This finding is in agreement with previous studies both on TAVI and other cardiovascular diseases and known as the obesity paradox.27
Male gender was associated with increased risk which is in line with previous studies where female gender was associated with lower mortality rates.28 As high BMI in this study was associated with lower risk of hospitalization, an interesting question is whether gender matters in terms of BMI? This question was not addressed in this study.
For all-cause hospitalizations or death, previous cardiac surgery was a predictor of lower risk. In baseline characteristics, the group with at least one hospitalization had higher rate of prior cardiac surgery. A plausible explanation to why cardiac surgery was protective in terms of repeated hospitalizations or death yet had a higher incidence in the group with at least one hospitalization is due to longer follow-up time as a consequence of the group being younger.
Mitral regurgitation was common in the study population which is in agreement with earlier publications. The relevance is disputed, with limited studies suggesting both impaired prognosis and the opposite.29
Limitations
This study had several limitations. All register-based research comes with inherent weaknesses due to human errors or factors built in into the registry. Measurements have shown a high degree of data agreement in the SWEDEHEART registry11 and few missing data points were found in the dataset. Still, for some variables, the levels were higher, with the result that they had to be excluded or imputed.
Hospitalization data were based on main diagnosis codes and as a result dependent on correct diagnosis at the time of hospitalization.
Another limitation was the lack of a control group from the general population. Ideally, the number of rehospitalizations and its characteristics would have been compared with a matched cohort, something which was not possible with this material.
Conclusions
Frequent hospitalizations from many different causes were found in this nationwide database, with complete follow-up on all Swedish TAVI patients between the years 2008 to 2017. On average, each patient was hospitalized 2.5 times after a mean follow-up time of 2.2 years. HF was the most common cause of hospitalization. Several traditional risk factors were associated with an increase in hospitalizations and mortality. Pre-existing AF as well as new onset AF post-procedure was common in the population and is subject of further investigation. Given the frequent HF hospitalizations, a decreased rate is important to reduce the burden on the health care system due to new hospitalizations after TAVI.
Supplementary material
Supplementary material is available at European Heart Journal – Quality of Care and Clinical Outcomes online.
Supplementary Material
Data availability
The data underlying this article cannot be shared due to ethical reasons.
Conflict of interest: Two authors report financial support unrelated to the present study. Remaining authors report no conflict of interest.
References
- 1. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ. et al. ; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 2. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M. et al. ; PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 3. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D. et al. ; Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 4. Otto CM, Prendergast B.. Aortic-valve stenosis—from patients at risk to severe valve obstruction. N Engl J Med 2014;371:744–756. [DOI] [PubMed] [Google Scholar]
- 5. Freeman PM, Protty MB, Aldalati O, Lacey A, King W, Anderson RA. et al. Severe symptomatic aortic stenosis: medical therapy and transcatheter aortic valve implantation (TAVI)—a real-world retrospective cohort analysis of outcomes and cost-effectiveness using national data. Open Heart 2016;3:e000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG. et al. ; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 7. Puri R, Iung B, Cohen DJ, Rodés-Cabau J.. TAVI or No TAVI: identifying patients unlikely to benefit from transcatheter aortic valve implantation. Eur Heart J 2016;37:2217–2225. [DOI] [PubMed] [Google Scholar]
- 8. Kupari M, Turto H, Lommi J.. Left ventricular hypertrophy in aortic valve stenosis: preventive or promotive of systolic dysfunction and heart failure? Eur Heart J 2005;26:1790–1796. [DOI] [PubMed] [Google Scholar]
- 9. Li Y-M, Mei F-Y, Yao Y-J, Tsauo J-Y, Peng Y, Chen M.. Causes and predictors of readmission after transcatheter aortic valve implantation: a meta-analysis and systematic review. Herz; doi:10.1007/s00059-019-04870-6. Published online ahead of print 2019. [DOI] [PubMed] [Google Scholar]
- 10. Ludvigsson JF, Almqvist C, Bonamy A-KE, Ljung R, Michaëlsson K, Neovius M. et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31:125–136. [DOI] [PubMed] [Google Scholar]
- 11. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A. et al. The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart Br Heart 2010;96:1617–1621. [DOI] [PubMed] [Google Scholar]
- 12. Årsrapport 2019. https://www.ucr.uu.se/swedeheart/dokument-sh/arsrapporter-sh (9 April 2021).
- 13. Bakgrund och historia—SWEDEHEART. https://www.ucr.uu.se/swedeheart/om-swentry/bakgrund-och-historia-swentry (9 April 2021).
- 14. CRAN - Package nephrology. https://cran.r-project.org/web/packages/nephro/index.html (9 April 2021).
- 15. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA.. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004;57:1288–1294. [DOI] [PubMed] [Google Scholar]
- 16. Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, Webb JG. et al. ; PARTNER 2 Investigators. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med 2020;382:799–809. [DOI] [PubMed] [Google Scholar]
- 17. Kokkinidis DG, Papanastasiou CA, Jonnalagadda AK, Oikonomou EK, Theochari CA, Palaiodimos L. et al. The predictive value of baseline pulmonary hypertension in early and long term cardiac and all-cause mortality after transcatheter aortic valve implantation for patients with severe aortic valve stenosis: a systematic review and meta-analysis. Cardiovasc Revasc Med 2018;19:859–867. [DOI] [PubMed] [Google Scholar]
- 18. Baron SJ, Arnold SV, Herrmann HC, Holmes DR, Szeto WY, Allen KB. et al. Impact of ejection fraction and aortic valve gradient on outcomes of transcatheter aortic valve replacement. J Am Coll Cardiol 2016;67:2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S. et al. ; PARTNER trial investigators. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2485–2491. [DOI] [PubMed] [Google Scholar]
- 20. Tarantini G, Mojoli M, Urena M, Vahanian A.. Atrial fibrillation in patients undergoing transcatheter aortic valve implantation: epidemiology, timing, predictors, and outcome. Eur Heart J 2017;38:1285–1293. [DOI] [PubMed] [Google Scholar]
- 21. Stewart S, Hart CL, Hole DJ, McMurray JJV.. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 22. Sinning J-M, Ghanem A, Steinhäuser H, Adenauer V, Hammerstingl C, Nickenig G. et al. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovasc Interv 2010;3:1141–1149. [DOI] [PubMed] [Google Scholar]
- 23. Noguchi M, Tabata M, Obunai K, Shibayama K, Ito J, Watanabe H. et al. Clinical outcomes of transcatheter aortic valve implantation (TAVI) in nonagenarians from the optimized catheter valvular intervention-TAVI registry. Catheter Cardiovasc Interv 2021;97:E113–E120. [DOI] [PubMed] [Google Scholar]
- 24. Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM. et al. ; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ochiai T, Yoon S-H, Flint N, Sharma R, Chakravarty T, Kaewkes D. et al. Timing and outcomes of percutaneous coronary intervention in patients who underwent transcatheter aortic valve implantation. Am J Cardiol 2020;125:1361–1368. [DOI] [PubMed] [Google Scholar]
- 26. Bjursten H, Rasmussen M, Nozohoor S, Götberg M, Olaison L, Rück A. et al. Infective endocarditis after transcatheter aortic valve implantation: a nationwide study. Eur Heart J 2019;40:3263–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boon RMA, van der Chieffo A, Dumonteil N, Tchetche D, Van Mieghem NM, Buchanan GL. et al. Effect of body mass index on short- and long-term outcomes after transcatheter aortic valve implantation. Am J Cardiol 2013;111:231–236. [DOI] [PubMed] [Google Scholar]
- 28. Williams M, Kodali SK, Hahn RT, Humphries KH, Nkomo VT, Cohen DJ, Douglas PS, Mack M. et al. Sex-related differences in outcomes after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis: insights from the PARTNER Trial (Placement of Aortic Transcatheter Valve). J Am Coll Cardiol 2014;63:1522–1528. [DOI] [PubMed] [Google Scholar]
- 29. Szymański P, Hryniewiecki T, Dąbrowski M, Sorysz D, Kochman J, Jastrzębski J. et al. Mitral and aortic regurgitation following transcatheter aortic valve replacement. Heart Br Card Soc 2016;102:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared due to ethical reasons.
Conflict of interest: Two authors report financial support unrelated to the present study. Remaining authors report no conflict of interest.