Abstract
Gemcitabine has been extensively applied in treating various solid tumors. Nonetheless, the clinical performance of gemcitabine is severely restricted by its unsatisfactory pharmacokinetic parameters and easy deactivation mainly because of its rapid deamination, deficiencies in deoxycytidine kinase (DCK), and alterations in nucleoside transporter. On this account, repeated injections with a high concentration of gemcitabine are adopted, leading to severe systemic toxicity to healthy cells. Accordingly, it is highly crucial to fabricate efficient gemcitabine delivery systems to obtain improved therapeutic efficacy of gemcitabine. A large number of gemcitabine pro-drugs were synthesized by chemical modification of gemcitabine to improve its biostability and bioavailability. Besides, gemcitabine-loaded nano-drugs were prepared to improve the delivery efficiency. In this review article, we introduced different strategies for improving the therapeutic performance of gemcitabine by the fabrication of pro-drugs and nano-drugs. We hope this review will provide new insight into the rational design of gemcitabine-based delivery strategies for enhanced cancer therapy.
Keywords: Gemcitabine, Pro-drug, Nano-drug, Cancer therapy
1. Introduction
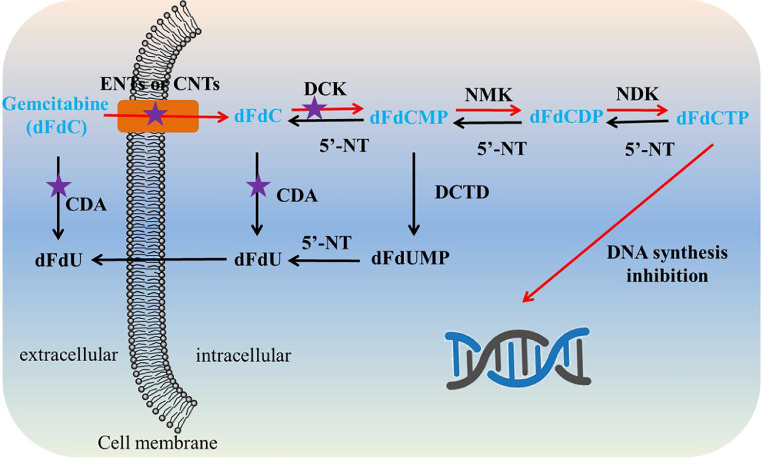
Gemcitabine is an S-phase-specific deoxycytidine analog (Fig. 1), which has been applied as the first-line therapy of pancreatic cancer approved by the US FDA since 1996 [1]. After that, FDA approved the clinical applications of gemcitabine for non-small cell lung cancer, ovarian cancer, and breast cancer [2]. Gemcitabine exerts its activity against cancer cells after it is metabolized to gemcitabine triphosphate (dFdCTP), which can integrate into DNA as a false nucleoside, inhibiting DNA replication and inducing DNA damage. In detail, the activation process of gemcitabine includes a sequence of phosphorylations. Gemcitabine is primarily phosphorylated to gemcitabine monophosphate (dFdCMP) by deoxycytidine kinase (DCK), subsequently turning to gemcitabine diphosphate (dFdCDP) and dFdCTP by catalysis of other pyrimidine kinases (Fig. 2).
Fig. 1.
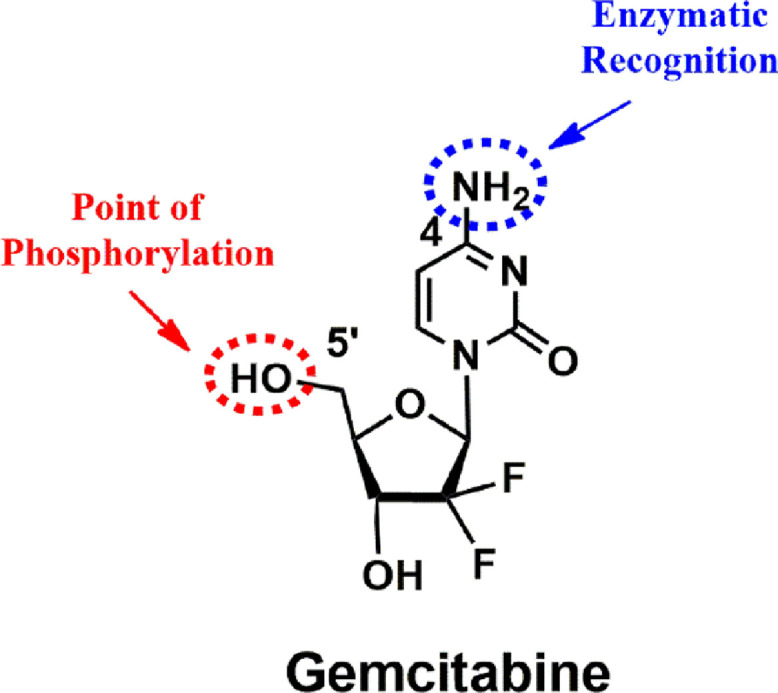
Chemical structure of gemcitabine.
Fig. 2.
Intracellular phosphorylation and metabolic pathways of gemcitabine. Red arrow: mechanisms of action, purple pentacle: main cause of resistance.(CNTs, concentrative nucleoside transporters; NMK, nucleoside monophosphate kinase; NDK, nucleoside diphosphate kinase; dFdUMP, 2′-deoxy-2′,2′-difluorouridine monophosphate).
However, many kinds of cancers appear resistant to gemcitabine rapidly following initial sensitivity, a feature that essentially characterizes this disease [2]. Specifically, after intravenous injection, gemcitabine can be rapidly deaminated to 2′,2′-difluorodeoxyuridine (dFdU) by deoxycytidine deaminase (CDA) present in blood and liver and then subjected to a fast renal clearance [3]. As a hydrophilic drug, gemcitabine cannot cross cell membranes by passive diffusion and thus is actively internalized through nucleoside transporters [4]. Resistance can hence come from the alterations in the nucleoside transporter. Inside cells, deficiency in DCK activity is one of the causes of resistance since the first step of gemcitabine phosphorylation is the rate-limiting step in the whole process of phosphorylation to activate pharmacological gemcitabine [5]. Resistance towards gemcitabine can also derive from deamination of gemcitabine and dFdCMP catalyzed by up-expression of intracellular CDA and deoxycytidylate deaminase (DCTD), respectively. In addition, resistance is associated with dephosphorylations catalyzed by intracellular 5′-nucleotidase (5′-NT), which occurs in phosphorylated metabolites of gemcitabine, leading to a decreased concentration of activated dFdCTP [2,5]. But generally, up-expression of CDA, deficiencies in DCK, and alterations in nucleoside transporter were considered the main reasons for the resistance of gemcitabine [2]. Owing to the short plasma half-life (∼10 min) and low bioavailability of gemcitabine, frequent administration is required, which results in severe myelosuppression, hepatotoxicity, and nephrotoxicity but still with poor therapeutic efficacy [6]. Therefore, it is necessary to develop efficient drug delivery strategies to circumvent resistance phenomena of gemcitabine, ultimately improving the therapeutic efficacy and reducing adverse effects.
In the field of drug delivery, chemical modification of drugs (pro-drug) and drug encapsulation using nanocarriers (nano-drug) are two interesting research directions. Pro-drugs, as drug derivatives, can be metabolized or activated in the body to release or generate the active drugs with improved physicochemical, biopharmaceutical and/or pharmacokinetic properties [7,8]. Chemical modification of anticancer drugs has been widely utilized for surmounting the disadvantages, such as water insolubility, chemical instability, and high toxicity of the parent drug. For instance, the camptothecin analog 7-ethyl-10-hydroxy camptothecin (SN38) was covalently conjugated to 4 arm-PEG, obtaining a hydrophilic pro-drug of SN38 to increase its water solubility and stabilize its lactone form [9]. Doxorubicin was modified with phosphorylcholine to increase its accumulation in tumor tissues and reduce its cardiotoxicity [10,11]. While nanocarriers not only make anticancer drugs selectively accumulate at tumor sites via the enhanced permeability and retention (EPR) effect, they also enable anticancer drugs to enter the cancer cells by endocytosis with improved intracellular drug concentration, ultimately enhancing the bioavailability and therapeutic effect. In this review, in particular, we introduced the recent advances in gemcitabine delivery strategies, including chemical modification of gemcitabine (pro-drug) to overcome chemical instability and nanocarrier loading (nano-drug) to improve its cellular uptake and accumulation in tumor tissues. This well-defined gemcitabine delivery could maximize therapeutic efficacy while minimizing systemic toxicity.
2. Pro-drug strategies by chemical modification of gemcitabine
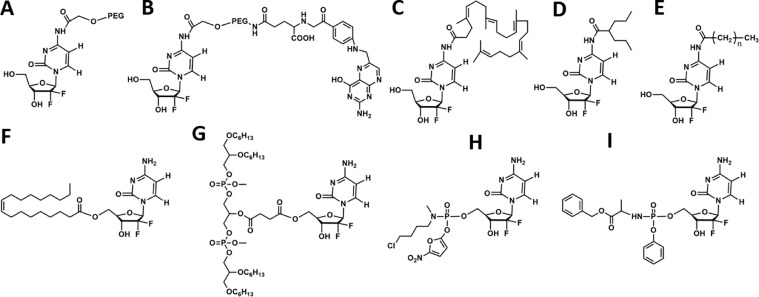
A variety of gemcitabine-based pro-drug-related chemical modifications was already reported. The modification sites of gemcitabine were exclusively based on two functional groups (4-NH2 and 5′-OH, Fig. 1). These novel gemcitabine pro-drugs will be promising to enhance therapeutic benefit as they can (1) guard the amine function and thus block deamination, (2) enhance storage stability in cytosolic fractions, (3) prolong intracellular release, (4) promote cellular uptake, and (5) initiate activation pathway of gemcitabine [2]. Some typical gemcitabine pro-drugs were summarized in Fig. 3. The gemcitabine pro-drugs can be classified into three types: ester-type gemcitabine pro-drugs, amide-type gemcitabine pro-drugs, and phosphoramidate-type gemcitabine pro-drugs. The ester-type (5′-OH modification) and amide-type (4-NH2 modification) gemcitabine pro-drugs can inhibit deamination and improve stability in vivo. The phosphoramidate-type (5′-OH modification) gemcitabine pro-drugs can be directly metabolized to monophosphate gemcitabine in the absence of DCK. Since the formation of monophosphate gemcitabine is the rate-limiting step for the whole process of phosphorylation to activate pharmacological gemcitabine and deficiency in DCK activity is one of the reasons for drug resistance, phosphoramidate-type gemcitabine pro-drugs can not only improve in vivo stability but also be very beneficial to overcome drug resistance.
Fig. 3.
Gemcitabine pro-drugs and the mass fraction of gemcitabine in the pro-drugs. Chemical modification at 4-NH2 sites: (A) PEG-gemcitabine (5.0 wt%), (B) folate-PEG-gemcitabine (4.8 wt%), (C) squalenoyl-gemcitabine (40.8 wt%), (D) LY2334737 (67.6 wt%), (E) 4-(N)-acyl-gemcitabine (82.4 wt%); 5′-OH sites: (F) CP-4126 (49.9 wt%), (G) NEO6002 (23.7 wt%), (H) 5′-(2′-deoxy-2′,2′-difluorocytidyl) 5-nitrofurfuryl N-methyl-N-(4-chlorobutyl) phosphoramidate (47.2 wt%), (I) NUC-1031 (45.3 wt%).
Poly (ethylene glycol) (PEG), identified as generally recognized as safe (GRAS) by the FDA, is extensively employed to modify therapeutic molecules. This modification approach, also called PEGylation, can decrease clearance by macrophages in the reticuloendothelial system (RES), decrease kidney clearance, reduce metabolic degradation by enzymes, prolong blood circulation time as well as decrease immunogenicity, and consequently, ameliorate the pharmacokinetic and pharmacodynamic effects of therapeutics [12,13]. Accordingly, PEGylated gemcitabine, PEG-gemcitabine (Fig. 3A) was synthesized by linking the N-hydroxy succinimide derivative of PEG to 4-NH2 of gemcitabine via an amide bond [14]. After intravenous injection, the amidic-linkage in gemcitabine pro-drug would be catalytically degraded by overexpressed lysosomal cathepsin B, leading to the release of parent gemcitabine. The bioavailability of PEG-gemcitabine increased to 1.7, 21, 37 times compared to free gemcitabine at 0.5, 1, 6 h, respectively. Due to the enhanced permeation and prolonged localization, PEG-gemcitabine demonstrated remarkably higher antiproliferative and apoptosis-inducing activity compared to the parent gemcitabine in human pancreatic cancer cell (PANC-1) and human pancreatic cancer cell (MIA PaCa-2). Targeting ligand folic acid was then introduced to PEG-gemcitabine to enhance the cytotoxicity and selectivity of gemcitabine (Fig. 3B) [15]. As is known, overexpression of folate receptor on plentiful tumor cells surface is related to advanced disease stage and negative prognostic factor and it is regarded as a potential target for various therapeutics [16,17]. The pharmacokinetic studies demonstrated the folic acid targeting approach increased to ∼3 times the affinity to the folate receptor overexpressed cell. Cell viability assays revealed that targeted PEG-gemcitabine conjugating with folic acid showed higher cytotoxicity and selectivity than the non-targeted ones against human nasopharyngeal epidermal cancer cell (KB-3-1) overexpressing folate receptors. Meanwhile, these targeted conjugates showed decreased cytotoxicity in human colon adenocarcinoma cell (HT-29) without overexpression of folate receptors since receptor-mediated endocytosis is of requirement towards cell penetration [15].
Additionally, covalently coupling 1,1’2-tris-nor-squalenoic acid at 4-NH2 of gemcitabine (Fig. 3C) is an efficient way to improve the pharmacological activity of gemcitabine through gemcitabine squalenoylation. Squalene, a natural triterpene with significant dietary benefits, biocompatibility, inertness, etc., has been utilized to construct squalene-drug conjugates [18,19]. It is worthy to note that squalene is reported to contribute to cancer therapy directly or indirectly despite the inhibition effect on the tumor is low [18,20]. A study using squalenoylation strategy to overcome the chemoresistance and rapid inactivation of gemcitabine on pancreatic cancer models was performed. Cell viability and apoptosis assay proved that squalenoylation significantly enhanced gemcitabine cytotoxicity and antiproliferation to sensitive human pancreatic cancer cell (BxPc-3), human pancreatic cancer cell (Capan-1), and particularly on chemo-resistant PANC-1 cells as a result of more efficient inhibition of DNA synthesis, higher S-phase accumulation, and more marked apoptotic induction. Subcutaneous tumor models (PANC-1 and Capan-1) and orthotopic tumors (PANC-1) were adopted to investigate in vivo antitumor effect. Compared to gemcitabine, squalene-gemcitabine showed higher antitumor efficacy, attenuated cellular invasion, and extended survival duration in both pancreatic tumor models [21]. Additionally, the distribution of the gemcitabine pro-drug is largely affected by the modified molecule. Since squalene can be well absorbed orally, it could be used for enhancing the oral delivery efficiency of gemcitabine. Parent gemcitabine was found to achieve fast absorption and rapid clearance from the plasma after oral administration. But squalene-gemcitabine showed slower absorption and progressive accumulation in tissues. Thus, squalene-gemcitabine exerted strikingly enhanced accumulation in the stomach and intestinal tissues. Yet its accumulation in the organs like liver, spleen, lung, pancreas, and thymus after 1 h of treatment was similar to that of gemcitabine [22]. The mechanisms underlying were to avoid deamination of CDA. Free gemcitabine had weak cytotoxicity against murine leukemic cell (RNK-16 LGL) due to its degradation from deamination, whereas squalene-gemcitabine could resist deamination, showing enhanced cytotoxicity by ∼83-fold. Besides, squalene-gemcitabine displayed higher intracellular accumulation and retention in comparison to free gemcitabine since its cellular access was not affected. After oral administration, squalene-gemcitabine, different from free gemcitabine, had much shower absorptions but displayed enhanced pharmacokinetics and tissue distribution profiles, especially in the major metastasis organs, namely lymphoid organs [23].
On the basis of exclusively frequent intravenous administration in clinical, oral administration of gemcitabine is more likely to improve the therapeutic efficacy further. However, extensive and rapid first-pass metabolism by CDA to dFdU results in low systemic exposure of gemcitabine with gastrointestinal toxicity. As described earlier, squalene-gemcitabine pro-drug could be a potential candidate for oral administration. Consequently, an oral gemcitabine pro-drug (LY2334737, Fig. 3D) was designed accordingly. LY2334737 was obtained by covalently conjugating valproic acid to 4-NH2 of gemcitabine, enabling it to block the site of deamination, thus preventing the first-pass metabolism which happened in free gemcitabine [2]. Orally administered LY2334737 could be slowly hydrolyzed to parent gemcitabine by highly expressed carboxylesterase 2 (CES2) in the liver and gastrointestinal tract. In cancer cells, CES2 could also metabolize intracellular LY2334737 to gemcitabine with a guarantee of sensitivity to LY2334737. The tumors expressed CES2 might respond to the oral metronomic dosage of LY2334737 because of its systemic and intracellular generated gemcitabine, thus further improving therapeutic efficacy. However, further studies of LY2334737 were suspended because of its hepatic toxicities observed in patients in Phase I trials [24].
The 5′-OH of gemcitabine is another important reaction site to synthesize gemcitabine pro-drugs. The fatty acid, elaidic-acid, was esterified to the 5′-OH group of gemcitabine to make it less hydrophilic. This gemcitabine derivative, CP-4126 (Fig. 3F), could be converted to the parent gemcitabine in the presence of esterases, followed by phosphorylation [25]. The CEM cells and its dCK- variant exhibited a similar conversion rate of nearly 40% from CP-4126 to gemcitabine. Moreover, their uptake and retention of CP-4126 were not affected by equilibrative nucleoside transporters (ENT)-inhibitor dipyridamole. CP-4126 was mainly distributed in membrane and cytosol, resulting in long retention. On the contrary, the cells exposed to free gemcitabine presented increased intracellular drug concentration and rapidly reduced to be undetectable. In the deoxycytidine-kinase deficient (dCK)-cells, no metabolism of gemcitabine could be observed. The concentration of dFdCTP arrived at a peak after incubating with the drug and then reduced dramatically after removing the drug. However, if exposed to CP-4126, dFdCTP levels continued to increase till 120 min after removal of CP-4126. Additionally, the level of dFdCTP was much higher than that of the free gemcitabine [26].
Apart from elaidic acid, a lipid named cardiolipin was grafted towards 5′-OH of gemcitabine through a succinate linker. Cardiolipin, as the hallmark phospholipid of mitochondrial, is involved in sophisticated cell signals, including mitophagy, apoptosis, and innate and adaptive immunity [27]. This gemcitabine-cardiolipin called NEO6002 (Fig. 3G) was first synthesized by NeoPharm [28]. The release of gemcitabine from NEO6002 in vivo could be implemented by hydrolysis of the succinate ester function. NeoPharm's sulforhodamine B assay on NEO6002 demonstrated dose-dependent cytotoxicity against a series of cancer cells, including human non-small cell lung cancer cell (A549), BxPC-3, HT-29, human breast cancer cell (MX-1), human acute lymphatic leukemia cell (CCRF-CEM), and murine leukemia cell (P388). Moreover, NEO6002 was testified to enter the cells through a nucleoside transporter-independent route, which could potentially overcome nucleoside transporter-independent-induced gemcitabine resistance. The therapeutic efficiency of NEO6002 was quite encouraging. The median survival rate of CD2F1 mice bearing P338 cells receiving treatment of 27 μmol/kg NEO6002 rose to 73%, while all the mice receiving gemcitabine died under the same condition because of the gemcitabine toxicity. At a dosage of 18 μmol/kg, NEO6002 could effectively inhibit tumor growth with 52% tumor inhibition, while gemcitabine merely achieved 32% tumor inhibition. It was suggested that NEO6002 could be a potential candidate with improved tolerability for treating nucleoside transporter-deficient and gemcitabine-resistant tumors [29].
As mentioned earlier, the rate of the whole process of phosphorylation that metabolizes gemcitabine to dFdCTP is determined by that of primary phosphorylation. Therefore, to bypass the primary phosphorylation of gemcitabine, strategies of chemical modification of gemcitabine by linking a phosphate function protecting group for selective release of the phosphorylated gemcitabine have been studied extensively [30]. Accordingly, a gemcitabine phosphoramidate pro-drug was developed for the delivery of gemcitabine-monophospate (Fig. 3H). Although the synthesized pro-drug showed lower activity than gemcitabine in wild-type cells, it exhibited much higher cytotoxicity than parent gemcitabine in the dCK variants and kept the cytotoxicity even with transport inhibitors. Therefore, DCK and nucleoside transporters were not the prerequisite for pro-drug activity, which suggested that the designed pro-drug can overcome dCK deficiency-induced chemoresistance [31]. Similarly, it was capable of obstructing the key cancer resistance mechanisms by modifying gemcitabine with phosphoramidate. A gemcitabine phosphoramidate pro-drug, NUC-1031 (Fig. 3I), was developed accordingly through “ProTide” technology [32]. NUC-1031 revealed a better (2-4-fold increase) biological activity against MIA PaCa-2 cells and BxPC-3 cells than gemcitabine. Furthermore, NUC-1031 achieved a much more tumor volume reduction in MIA PaCa-2 xenograft models. NUC-1031 has verified clear clinical activity signs to various patients with advanced and rapidly progressing diseases, and relevant phase III trials were underway [33,34].
In addition, antibody-drug conjugates serve as a classical strategy for establishing a gemcitabine pro-drug with the targeting ability to increase potency while simultaneously reduce the dose-limited sequela of gemcitabine. Gemcitabine-(5′-phosphoramidate)-[anti-IGF-1R] was obtained by grafting monoclonal anti-IGF-1R immunoglobulin towards gemcitabine-(5′-phosphorylimidazolide), which was the first report about covalent gemcitabine-monophosphate immune-chemo therapeutic. The gemcitabine molar incorporation index for the target gemcitabine pro-drug was calculated as 2.67:1. The cytotoxicity of the pro-drug against pulmonary adenocarcinoma (A549) measured by MTT assay increased dramatically when the concentration increased [35]. Furthermore, gemcitabine was coupled with therapeutic monoclonal antibodies trastuzumab with potent toxins through acid-labile silyl ether linkers for targeting cancer cells and controlled drug release [36].
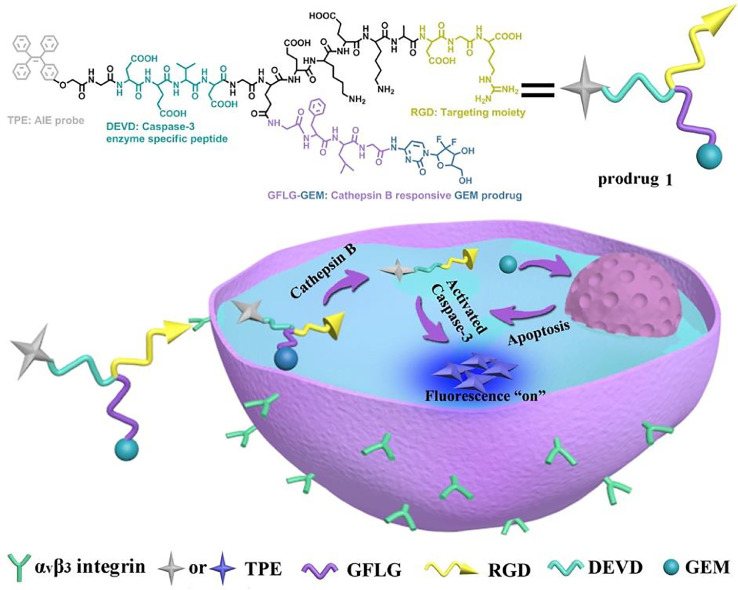
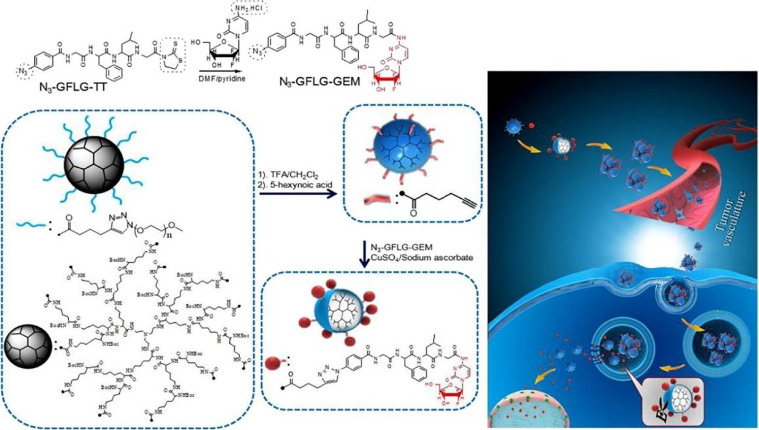
Different from extensively investigated anticancer drugs such as doxorubicin, gemcitabine is essentially non-fluorescent. As a result, it is difficult to track the drug delivery or release process. Multifunctional gemcitabine pro-drugs with diagnosis and therapy were hence developed [17,[37], [38], [39]]. A novel theranostic pro-drug, the gemcitabine-coumarin-biotin conjugate, was reported, and its cytotoxicity and intracellular imaging function were studied. Upon adding glutathione (GSH) to the gemcitabine-coumarin-biotin conjugate, the disulfide bond was cleaved with the release of parent gemcitabine and simultaneous fluorescence enhancement, which provided a new and potent strategy for tumor drug delivery and intracellular imaging [37]. Yet, the traditional fluorescent dyes, coumarin as mentioned earlier, for example, suffered from aggregation-caused quenching, which led to decreased signal-to-noise ratio for biosensing and bioimaging [40,41]. Alternatively, a novel category of fluorescent probes with aggregation-induced emission (AIE) characteristics was developed [42], [43], [44]. These AIE-based probes present relatively low fluorescent intensity in the molecularly dissolved condition while exhibited very strong fluorescence when the probes were in the aggregated state [45,46]. Given the unique AIE behavior, it provided a powerful strategy to establish fluorescence light-up probe-based AIE molecules, which were especially crucial for monitoring intracellular biological processes and tracking numerous diseases. Recently, we developed a theranostic pro-drug for targeted delivery of gemcitabine and in situ evaluation of the therapeutic efficacy (Fig. 4) [38]. This pro-drug entered the BxPC-3 cells by αvβ3 integrin-mediated endocytosis, followed by simultaneously releasing gemcitabine and the cell apoptotic probe because of the cleavage of cathepsin B-sensitive GFLG sequence. The released gemcitabine caused apoptosis, which induced the activation of the intracellular caspase-3. The activated caspase-3 was a specific enzyme to cut off the DEVD peptide sequence as the cell apoptotic probe, and the strong fluorescence of TPE could be observed for evaluating its therapeutic effects. This gemcitabine pro-drug-based theranostic system activated by cascade enzymatic reaction could be utilized as a versatile platform for simultaneously eliminating cancer cells and real-time monitoring the therapeutic responses.
Fig. 4.
The targeted gemcitabine pro-drug was designed as an AIE-based intracellular light-up apoptotic probe for simultaneous eliminating cancer cells and real-time monitoring the therapeutic responses. Reprinted with permission from [38], Copyright 2017 Royal Society of Chemistry.
3. Nano-drug strategies using nanocarriers
Nanocarrier-based gemcitabine delivery systems are able to enhance the bioavailability of gemcitabine since they can selectively accumulate in tumor sites by the EPR effect [47], [48], [49]. Besides, they can carry gemcitabine into the cancer cells by endocytosis rather than through nucleoside transports that are few in drug-resistant cells. This refined gemcitabine delivery could maximize therapeutic benefits while minimizing side effects.
3.1. Gemcitabine-loaded liposomes
Liposomes consist of phospholipids, which orientate themselves in spheres of lipid bilayers with an aqueous core in an aqueous buffer [50,51]. They can load both lipophilic and hydrophilic drugs in their lipid bilayers and inner core, respectively, which are considered universal drug delivery systems [52], [53], [54], [55]. Gemcitabine-loaded liposomes possess great potential in clinical applications since they can prevent gemcitabine from CDA degradation in plasma. Moreover, they can selectively accumulate in tumor sites with reduced distribution in normal tissues. Therefore, liposomes enable an increased gemcitabine concentration in tumor tissues with a decrease in non-specific systemic toxicity.
Gemcitabine, a hydrophilic drug, or its lipophilic formation can be entrapped into the aqueous inner core or the lipid bilayers, respectively [56], [57], [58]. The lipophilic gemcitabine can be obtained by linking different alkyl chains (Fig. 3E) to 4-NH2 of gemcitabine as mentioned above [59]. Compared to the hydrophilic gemcitabine that is packed in the aqueous inner core of liposomes, a pro-drug formation, lipophilic gemcitabine could achieve a higher biostability in the lipid bilayers. This increased biological character can avoid gemcitabine deactivation by CDA, and therefore, pharmacological activity and antitumor efficacy are greatly enhanced. Furthermore, the lipophilic gemcitabine spread through the bilayer more rapidly than its hydrophilic form since lipophilic gemcitabine can be retained within the lipid bilayer [60].
One of the major limitations of liposomes is their relatively short circulation time and low physiological stability in vivo. To overcome these drawbacks, PEG is utilized for coating the surface of the liposomes. These PEGylated liposomes are also called stealth liposomes. Accordingly, a stealth liposome formulation, GemPo, was reported [61]. By encapsulating gemcitabine with sterically stabilized liposomes made up of 2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000] (DSPE-PEG2000), cholesterol, and phosphatidylcholine (1:5:10 mass ratio), GemPo was more effective in inhibiting cell proliferation both in sensitive murine breast cancer cell (4T1) and recalcitrant human breast cancer cell (MDA-MB-231) than free gemcitabine. Meanwhile, GemPo was found to have the ability to significantly reverse the “promigratory” which occurred in gemcitabine-resistant cells. GemPo was further demonstrated to have superior antitumor activity and increased mice survival in mice bearing 4T1. Its antitumor effect was close to that of gemcitabine/paclitaxel combination, whereas GemPo decreased systemic toxicity, highlighting its clinical potential [61]. Furthermore, PEGylated liposomes that consisted of DSPE-PEG2000, cholesterol, and 1,2-dioleoyl-sn-glycero-3-phosphocholine (1:2:2 molar ratio) were applied to load hydrophobic coordination nanoparticle that co-loading gemcitabine monophosphate and oxaliplatin (NCP-1) for pancreatic cancer management. The synergistic effect of gemcitabine monophosphate and oxaliplatin against AsPc-1 cells and BxPc-3 cells was verified, and NCP-1 obtained much better therapeutic effects in vivo than monotherapy NCPs or the combination of free drugs [62].
Another formulation of PEGylated liposomes is L-GEM, which is composed of DSPE-PEG 2000, cholesterol, and 1,2-dipalmitoyl-sn-glycero-3-phospocho monohydrate (DPPC) (1:3:6 molar ratio) and prepared by the pH remote loading method with high and stable loading of gemcitabine in the aqueous core [63]. A similar lipid form of doxorubicin, Doxil®, has been approved by FDA [64]. The L-GEM revealed cell proliferation inhibition of human anaplastic thyroid carcinoma cell (ARO) after only 12 h by inducing apoptotic pathways. In comparison, the free gemcitabine showed reduced cell viability up to 72 h. The L-GEM achieved the same cell proliferation inhibition with a concentration 100 times lower than free gemcitabine, which was ascribed to a more effective gemcitabine uptake [65]. To further investigate the antitumor effect of L-GEM, immunodeficient nonobese diabetic/severe combined immunodeficiency (NOD-SCID) mice bearing ARO xenograft were adopted. Interestingly, after 4 weeks’ therapy, L-GEM (5 mg/kg) showed similar antitumor effects with 50 mg/kg of free gemcitabine, which meant the same antitumor effect could be obtained by using 10 times lower concentration of gemcitabine in the liposomal formulation. This fact was of great importance since the lower drug dose used in L-GEM could reduce the side effects of gemcitabine, particularly hematological toxicity, while simultaneously retaining its antitumor efficacy [63].
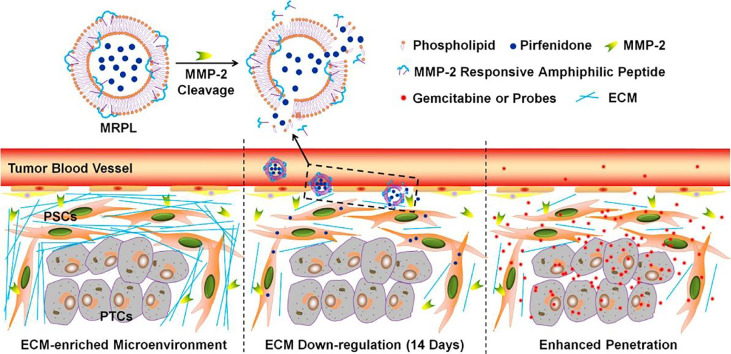
Pancreatic cancer has rich stroma, and this abundant and dense desmoplastic stroma could hamper the delivery and penetration of gemcitabine into lesions [66,67]. A strategy of stromal depletion, combined with gemcitabine-loaded liposome therapy, was thus developed. The liposomes that loaded nitric oxide (NO) donor S-nitroso-N-acetylpenicillamine (SNAP) (Lip-SNAP) were first administrated to inhibit the stroma production in pancreatic cancer, followed by treatment of gemcitabine-loaded liposomes, Lip-GEM. Lip-GEM could be penetrated into the tumor more effectively owing to the stromal depletion by the pre-treatment of NO and hence critically improved the gemcitabine delivery efficiency, exerting enhanced therapeutic efficacy of gemcitabine to a greater extent [68]. Furthermore, taking advantage of the pathological microenvironment of overexpressed matrix metalloproteinase-2 (MMP-2), the pirfenidone-loaded MMP-2 responsive peptide-hybrid liposome could specifically release pirfenidone and down-regulate the stroma production at the pancreatic tumor site (Fig. 5). A significantly increased penetration and therapeutic efficacy of gemcitabine for pancreatic cancer therapy were achieved [69]. These nano-drugs that can achieve synergistic therapy, especially possess the functions of destroying dense stroma and killing cancer cells, are preferred means for pancreatic cancer treatment.
Fig. 5.
The designed pirfenidone-loaded MMP-2 responsive peptide-hybrid liposome and its proposed mechanism for extracellular matrix down-regulation in pancreatic tumors. Reprinted with permission from [69], Copyright 2017 American Chemical Society.
For the sake of enhancing site-specific delivery of gemcitabine-loaded liposomes, an active targeting strategy was adopted by decorating their surface with targeting ligands like antibodies (CD133, epidermal growth factor-EGF), or hyaluronic acid (HA) [70]. Overexpression of EGF receptor (EGFR), which is 10-1000-fold higher than it in normal cells, is related to advanced cancer and poor prognosis, and EGFR is a substantiated target for cancer treatment [71]. The EGF-conjugated stearoyl gemcitabine (GemC18) nanoparticles (GemC18-NPs) had more than a 2-fold increased accumulation of GemC18-NPs in EGFR overexpressing human breast cancer cell (MDA-MB-468) tumor-bearing mice than the untargeted GemC18-NPs. Meanwhile, EGF-conjugated GemC18-NPs showed higher antitumor activity against EGFR overexpressing MDA-MB-468 tumor-bearing mice than untargeted GemC18-NPs, which could be ascribed to the more antiproliferative, antiangiogenic, and proapoptotic functions of EGF-conjugated GemC18-NPs [72]. As is known, HA has been extensively utilized for drug delivery in consequence of its biocompatibility, biodegradability, non-toxicity, and non-immunogenicity. Furthermore, HA can effectively bind to CD44 receptor overexpressed cancer cells [73,74]. HA-liposomes loading lipophilic gemcitabine pro-drugs were demonstrated to be taken up into the CD44-expressing that MIA PaCa-2 cells through lipid raft-mediated endocytosis. In vivo results verified MIA PaCa-2 tumors upon treatment with all the liposome formulations containing gemcitabine grew significantly slower than those with the treatment of free gemcitabine. Among all the liposome formulations, the 12 kDa HA-liposomes were the most efficient, while the 4.8 kDa HA-liposomes and non-conjugated liposomes showed similar activity [70].
3.2. Polymeric nanoparticles
Polymeric nanoparticles we discuss here mainly refer to dendrimer, chitosan, albumin, which themselves are nanoscopic and thus can be used as drug carriers directly. As a result of narrowly distributed and nanoscopic structure, these polymeric nanoparticles can attain improved delivery efficiency and enhanced therapeutic efficiency of gemcitabine [75], [76], [77], [78].
Dendrimers have unique three-dimensional spherical polymer structures with many attractive features including nanoscale size, narrow polydispersity index, excellent molecular structure control, easy functionalization, and hydrophobic cavities for drug loading [79,80]. Dendrimers can be used for the encapsulation of gemcitabine. As an example, gemcitabine conjugated lysine peptide dendrimer (Dendrimer-GEM) was synthesized. Dendrimer-GEM was prepared through conjugating gemcitabine to PEG modified dendrimer by the GFLG linkage and self-assembled to nanoparticles with the capability of enzyme-triggered gemcitabine release. Compared to free gemcitabine, Dendrimer-GEM significantly improved the anticancer effect with reduced systemic toxicity, which was attributed to their high accumulation into tumors (Fig. 6) [81].
Fig. 6.
The preparation of Dendrimer-GEM nanoparticles as well as tumor accumulation and enzyme-responsive drug release for improved in vivo antitumor efficacy Reprinted with permission from [81], Copyright 2017 Elsevier.
Chitosan is also extensively applied in drug delivery because of its excellent biocompatibility, relatively low toxicity, and excellent biodegradability. Chitosan is the only one exhibiting a cationic character in contrast with all other biodegradable polymers. The distinguishing cationic feature based on its primary amino groups is in charge of various properties and its following use in drug delivery systems [82]. For example, chitosan-based GemChit nanoparticles were prepared by coacervation. Different from the conventional surface adsorption strategy, chitosan nanoparticles were used to entrap gemcitabine during the coacervation procedure, which led to a higher drug loading efficiency and much slower drug release behavior. Such chitosan nanoparticles were very promising for intracellular delivery of gemcitabine. The therapeutic efficacy of GemChit nanoparticles was proved to be superior to that of free gemcitabine for tumor treatments [83]. Further, anti-human epidermal growth factor receptor-2 (anti-HER2) was applied for conjugating chitosan-based nanoparticles in the targeted gemcitabine-delivery systems, which resulted in improved therapeutic performance. Specific anti-HER2 mediated targeting and enhanced internalization of these nanoparticles led to enhanced cytotoxicity of gemcitabine, thereby enhancing the apoptosis of pancreatic cancer cells.
As the most enriching protein in plasma, albumin has superior biocompatibility, non-immunogenicity, and prominently long circulating time in blood. It has gained wide recognition as a versatile drug carrier. Besides, albumin possesses many hydrophobic cavities and therefore can be used as an ideal carrier of numerous small molecule therapeutics and diagnostic agents. In addition, due to the active -NH2 residues on human serum albumin (HSA), targeting ligands, imaging probes, as well as drugs, could be covalently grafted to HSA by simple chemical reactions [84]. In consequence of these favorable advantages, HSA is considered as a versatile drug carrier. Gemcitabine covalently binding to HSA was reported to possess 21-fold greater bioavailability and 8-fold higher gemcitabine accumulation in tumors than free gemcitabine, which exhibited profound in vivo antitumor effect [85]. We developed a simple, safe, imageable, and versatile theranostic nanocomplexes, HSA-GEM/IR780, by covalently coupling gemcitabine to HSA, and then simply complexed with near-infrared (NIR) dye IR780. HSA-GEM/IR780 was effectively uptaken by BxPC-3 cells. Moreover, compared with free IR780, HSA-GEM/IR780 could be effectively accumulated in tumor tissue and exhibited long-term retention capability. Because of the conjugation of gemcitabine to HSA, the deactivation of gemcitabine to dFdU was substantially inhibited while the activation of gemcitabine to dFdCTP in tumor sites was prominently increased. Therefore, HSA-GEM exhibited excellent tumor inhibition capability and very low side effects [86].
3.3. Polymeric micelles
Polymeric micelles have nano-sized core-shell structures, which are prepared by the self-assembly of amphiphilic polymers [87], [88], [89]. For gemcitabine polymeric micelles, gemcitabine or its lipophilic pro-drug formulation is conjugated to or loaded in polymeric micelles. Consequently, the drug can be released in a controlled behavior and exhibited improved stability in plasma as the gemcitabine is in the core or under the protection of hydrophilic segment such as PEG of the micelles, thereby preventing its rapid metabolism from CDA and improving its biostability. It is noted that the gemcitabine micelles with a narrow distribution of nanoscopic size are specifically beneficial for EPR-involved body disposition.
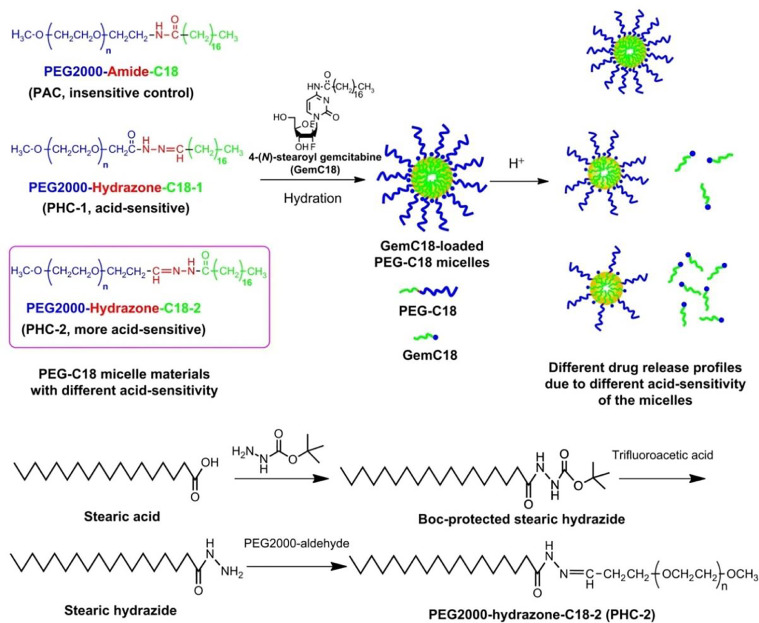
Since hydrophilic gemcitabine could not be directly loaded into polymeric micelles, hydrophobic gemcitabine derivates are usually utilized to prepare micelles. Lipophilic gemcitabine pro-drug, GemC18, was incorporated into pH-sensitive micelles that self-assembled from an amphiphilic polymer by conjugating stearic acid and PEG using the acid-responsive hydrazone linkage. GemC18 in the pH-sensitive micelles showed higher cytotoxicity to murine melanoma cell (B16-F10) and BxPC-3 cells than in the pH-insensitive micelles due to the pH-responsive release of GemC18. Moreover, GemC18 in the pH-sensitive micelles had higher cytotoxicity than GemC18 solution in consequence of the lysosomal delivery of GemC18. In B16-F10-bearing mice, GemC18-loaded pH-responsive micelles exhibited a distinctly more robust antitumor ability than gemcitabine, GemC18, and GemC18 in the pH-insensitive micelles [90]. Furthermore, more pH-sensitive micelles were designed and synthesized using the same PEG and stearic acid derivative but exchanging the positions of PEG and stearic acid derivative around the hydrazine bond (Fig. 7). These highly pH-sensitive micelles could achieve an enhanced pH-responsive release of GemC18. Consequently, gemcitabine was rapidly activated from the released GemC18 in the lysosomes, which was vital to effectively down-regulate ribonucleotide reductase subunit M1 (RRM1) expression that was associated with gemcitabine resistance, increase the intracellular concentration of dFdCTP, and finally kill RRM1-overexpressed cancer cells. This strategy of lipophilic gemcitabine pro-drug and pH-sensitive micelles delivery provides a possibility to enhance the anticancer activity of gemcitabine [91].
Fig. 7.
Chemical structures of the three micelles with different pH-responsive release profiles of GemC18 Reprinted with permission from [91], Copyright 2013 Elsevier.
The dense stroma of pancreatic cancer hampers drug diffusion and T cell infiltration, resulting in an immunosuppressive microenvironment. However, the stroma can prevent the spread of cancer cells by a neighboring suppression effect. Therefore, it is extremely important to develop a pancreatic cancer treatment strategy for selectively destroying the inner but maintaining the outer layer of the stroma. Taking advantage of the unique microenvironment, paclitaxel and phosphorylated gemcitabine co-loaded nanoparticles were developed to destruct the central stroma while retaining the external stroma through tumor central stroma target and the microenvironment (pH differences) stimuli. Moreover, the tumor immunosuppressive microenvironment was modulated with increased cytotoxic T cells but decreased T regulatory cells. Combining stroma targeting with the delivery of stimuli-responsive polymeric nanoparticles could prevent tumor cell metastasis while killing cancer cells [92].
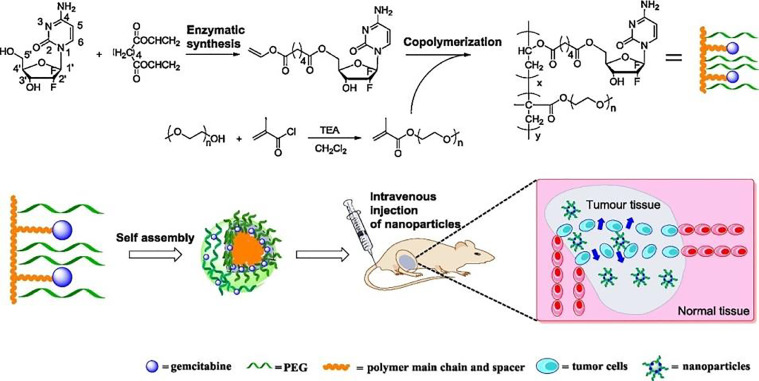
Gemcitabine could also be conjugated to the polymer to obtain gemcitabine-polymer conjugate micelle with extremely high drug loading, enabling gemcitabine to prevent rapid plasma degradation [93], prolong cytotoxicity [94], and enhance antitumor efficacy [95]. In a typical example, gemcitabine and PEG were grafted to comb-like amphiphilic polymers, which could further self-assemble to obtain micelles (Fig. 8). These PEGylated polymeric micelles had effective anticancer activities against A549 cells due to efficient internalization. In vivo studies using A549 tumor borne nude mice verified an improved antitumor efficiency of gemcitabine-containing micelles compared to free gemcitabine owing to the prevention of rapid plasma degradation of gemcitabine [93]. However, the release of parent gemcitabine was usually restricted from these gemcitabine conjugated micelles with an ester bond or amide bond. Therefore, we developed reduction-sensitive gemcitabine pro-drug micelles to solve this problem. The biodegradable and reduction-cleavable gemcitabine pro-drug micelles could implement a quick release of gemcitabine in a reductive tumor microenvironment, but few release of gemcitabine in the normal physiological environment. These reduction-sensitive pro-drug micelles could be effectively uptaken by BxPC-3 cells and killed the cancer cells [96,97]. As is known, the biocompatibility and the nature of the interaction between the drug delivery systems and their surrounding tissues are rather crucial. Polyamino acid itself had outstanding biocompatibility with low cytotoxicity. Therefore, we further designed and fabricated polyamino acid-based micelles with biocompatible, reduction-sensitive, and active targeting characteristics for effective delivery of gemcitabine [98].
Fig. 8.
Schematic illustration of chemoenzymatic synthesis, self-assembly, and antitumor activity of comb-like random copolymer containing gemcitabine and PEG side chains. Reprinted with permission from [93], Copyright 2016 Elsevier.
Mixed polymeric micelles that are obtained by the self-assembly of two or more copolymers enable the concurrent integration of multiple functions in one system, which is very challenging for conventional micelles [99]. DSPE-PEG and D-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS) mixed micelles loading GemC18 were reported to improve the pharmacokinetic characteristics of gemcitabine, thus having a pronouncedly enhanced in vivo antitumor ability in BxPC-3 xenograft mice. This augmented delivery of micellar GemC18 at a much lower dosage to the tumor by EPR effect could implement delayed deamination, prolonged circulation time, and elevated concentration (3-fold) of gemcitabine in vivo, thus leading to maximizing therapeutic effect and minimizing debilitating normal tissue toxicity [100]. Mixed micelles make it possible to construct multifunctional micelles simply by using several dissimilar copolymers as Lego building blocks. An EGFR-targeting gemcitabine polymeric mixed micelle was further developed for pancreatic cancer treatment. GE11-linked mixed micelles could deliver gemcitabine to EGFR-expressing pancreatic cancer cells with high specificity and efficiency, leading to remarkable inhibition of the growth of orthotopic pancreatic tumors. The results suggested GE11-linked mixed micelles showed great promise in delivering gemcitabine to EGFR-expressing cancers [101].
3.4. Inorganic nanoparticles
Inorganic nanocarriers can be used as nanocarriers for the delivery of gemcitabine with controlled drug-release characteristics [102]. At the nanoscale, inorganic materials as nanocarriers can achieve targeted gemcitabine delivery to tumor tissues via the EPR effect, resulting in an improved therapeutic performance with decreased systemic toxicity. Most importantly, the physicochemical properties of most inorganic nanoparticles were very different from their corresponding bulk counterparts due to their quantum size effects [102]. These size-dependent optoelectronic properties, which are usually not found in conventional nanocarriers like liposomes, polymeric micelles, dendrimers, etc., can be employed in a variety of biomedical applications, including imaging and therapy. Moreover, the surface of inorganic nanoparticles can be easily tailored, which is another important feature of inorganic nanocarriers. The inorganic nanoparticles are very promising to load biomolecules such as enzymes, antibodies by covalent bonds, or noncovalent interactions. Therefore, multifunctional drug delivery systems can be easily prepared using inorganic nanoparticles. Moreover, inorganic nanoparticles with different shapes can be prepared with shape-dependent optoelectronic characteristics, which shows great potential in biomedical applications [102,103].
3.4.1. Carbon-based nanoparticles
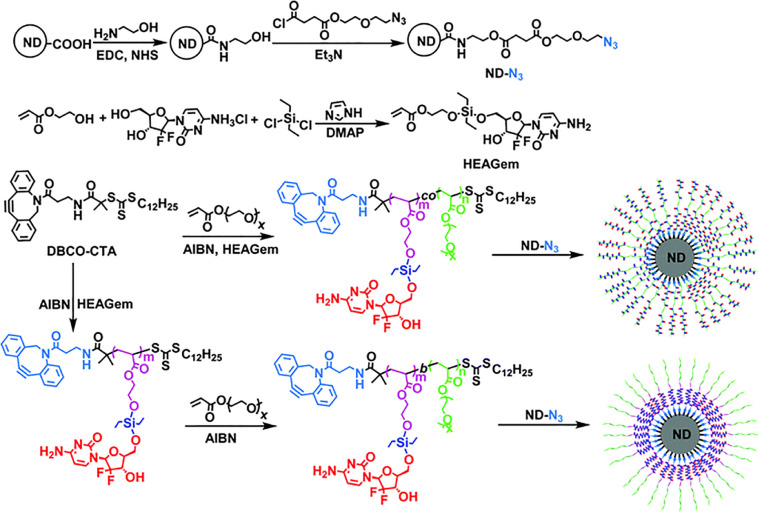
The motivations and reasons for using nanocarbons for gemcitabine delivery are extremely attractive. Many nanocarbons, including carbon nanotubes (CNTs), carbon dots (Cdots), nanodiamonds (NDs), and graphene derivatives, exhibit important innate optical properties such as fluorescence for imaging and sensing [104], [105], [106], [107]. A study showed hydrophilic multi-walled CNTs (MN-MWNTs) could be preferentially accumulated at lymph vessels and then moved to lymph nodes with the guidance of an external magnetic field. After 24 h of injection, the concentration of gemcitabine in lymph nodes reached a maximum level. The concentration of gemcitabine in MN-MWNTs under magnetic field guidance group was more than 9 μg/g, while that of gemcitabine group was just 1 μg/g. MN-MWNTs showed high efficiency of gemcitabine delivery into the lymphatic system if a magnetic field was used [108]. Furthermore, triple-functionalized CNTs containing gemcitabine as the drug, folic acid as the targeting group, and fluorescein isothiocyanate (FITC) as the bio-probe were prepared. The gemcitabine covalently grafted onto CNTs maintained its antitumor activity. CNTs loaded with gemcitabine entered cancer cells by passive diffusion since the functionalized nanotubes could be internalized by folic acid-positive and negative cells [109]. Nitrogen-doped Cdots (N-Cdots) were synthesized by the hydrothermal method, followed by conjugating quinic acid and loading gemcitabine to ultimately obtain multifunctional theranostic agents, i.e. quinic acid conjugated, gemcitabine loaded N-Cdots. These complexes with high gemcitabine loading had high cytotoxicity to human breast cancer cell (MCF-7) and superior fluorescent performance for in vivo non-invasive imaging [110]. Additionally, polymer-coated NDs using a “grafting onto” method was developed (Fig. 9). The NDs were found to release gemcitabine much faster at a lower pH and thus considered stimuli-sensitive drug delivery agents for pancreatic cancer management [111]. The NDs could also be gemcitabine nanocarriers which self-assembled the polyelectrolytes, polyethyleneimine, and polyacrylic acid on their surface by electrostatic forces and then attached enzyme-sensitive gemcitabine pro-drug [112]. Graphene derivatives, specifically graphene oxide (GO) with fucose modification as an intelligently targeted nanocarrier, were also reported for packing hydrophilic gemcitabine to achieve high charge, controlled release, and targeted delivery to fucose-receptor-overexpressing MDA-MB-231 cells and A549 cells [113].
Fig. 9.
Schematic illustration of the “grafting onto” approach of gemcitabine pro-drug-based polymer-coated NDs. Reprinted with permission from [111], Copyright 2016 Royal Society of Chemistry.
3.4.2. Silica-based nanoparticles
Silica-based nanoparticles for drug delivery and biomedical application have been well documented [114], [115], [116], [117]. Hydrophilic gemcitabine can not be encapsulated directly in mesoporous silica nanoparticles (MSNs), but its lipophilic pro-drug formation can. It has been reported that lauroyl-gemcitabine could be effectively loaded into MSNs to protect gemcitabine from rapid plasmatic metabolization physically and chemically. However, compared to gemcitabine, MSNs loading lauroyl-gemcitabine showed less cytotoxicity to MDA-MB-231 cells and human ovarian cancer cell (A2780 cells) due to the slow and gradual drug release [118]. There is another approach to load gemcitabine: MSNs carry free gemcitabine with bilayer coatings such as HSA and poly(acrylic acid-co-itaconic acid), lipid [119,120], or supramolecular nanovalves [121]. The bilayer structure coatings constructed with HSA and poly (acrylic acid-co-itaconic acid) around MSNs could achieve controlled release of gemcitabine in a pH-sensitive manner. The maximum release occurred at pH 5.5 in endosomes, thus obtaining enhanced cytotoxicity in murine fibroblasts cell (L929). In a similar approach, MSNs were used to co-delivery gemcitabine and LY364947, a kind of TGF-β inhibitor [120]. Such co-delivery system exhibited inhibition of the tumor stroma, suppression of the gemcitabine-inactivating CDA expression, and decreased pericytic coverage, thus overcoming stromal resistance and enhancing in vivo therapeutic efficacy of pancreatic cancer [119,120]. A multi-drug delivery system, based on MSNs and functionalized with acid-sensitive bonds and monoferrocene modified β-cyclodextrin, was further developed to carry and programmedly release multiple drugs. Zero premature release could be achieved in a physiological environment with two different drug release behaviors. One is to release encapsulated drugs in the presence of voltage and acidic pH. The release behavior of gemcitabine (time and dose) can be easily controlled by an external voltage. The pH-sensitive release of doxorubicin can be triggered by the cleavage of ketal groups. Another modality is to release gemcitabine and doxorubicin directly in acidic pH. The cell viability studies verified that this multidrug delivery system had significantly improved cytotoxicity against MCF-7 cells compared to single doxorubicin- or gemcitabine-loaded MSNs [121].
3.4.3. Metal-based nanoparticles
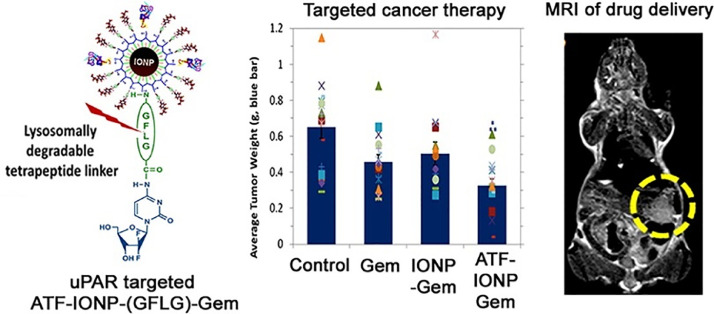
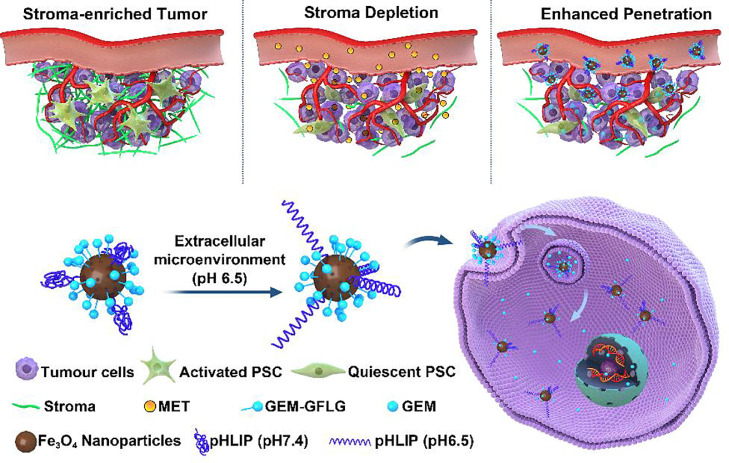
Metal-based nanoparticles including iron oxide nanoparticles (IONPs) [122], gold nanoparticles [123], and quantum dots (QDs) [124] have been extensively used in drug delivery due to the unique physicochemical characteristics. IONPs is an attractive theranostic nanoplatform with integrated capacities as a contrast agent in MRI and a drug nanocarrier. To conquer the physical barrier of tumor stroma, urokinase plasminogen activator receptor (uPAR) targeted IONPs were reported for targeted gemcitabine delivery and controlled intratumoral drug release (Fig. 10). The amino-terminal fragment (ATF) peptides modified IONPs could specifically target uPAR-overexpressing pancreatic cancer cells and stromal cells. Then these theranostic nanoparticles enabled intracellular release of gemcitabine after cellular uptake to optimize drug performance, reduced systemic effects, and provided contrast enhancement in magnetic resonance imaging (MRI) of tumors. More importantly, the conjugation of gemcitabine to nanocarriers could protect gemcitabine from deactivation. Furthermore, the prepared theranostic nanoparticles could remarkably inhibit the tumor growth in an orthotopic pancreatic cancer model after intravenous injection. Taking advantage of IONPs, the residual tumors after therapy could be detected by MRI, providing an important way to track drug delivery and assess the therapeutic performance via MRI [125]. Recently, we developed gemcitabine-loaded IONPs for targeted pancreatic cancer therapy via a two-step sequential delivery approach (Fig. 11). At first, metformin was used to disrupt the dense tumor stroma. The tumor penetration of pH(low) insertion peptide (pHLIP) and gemcitabine co-modified IONPs could then be greatly improved. A significantly improved therapeutic efficacy was verified on both subcutaneous and orthotopic tumor models [126]. For gold nanoparticles, 10 nm biomimetic Au@BSA nanospheres were developed using BSA (bio-template) and hydrazine hydrate (reducer). Au@BSA nanospheres were utilized for the effective delivery of gemcitabine and simultaneous CT imaging. Moreover, the Au@BSA nanocomposites were validated to have the potential for CT imaging because of the prominent X-ray attenuation ability [127]. Based on CdSe/ZnS QDs, we fabricated dual enzymatic reaction-assisted gemcitabine nanocarriers for the targeted treatment of pancreatic cancer. Gemcitabine could be grafted to the nanocarriers and released in cathepsin B-overexpressing BxPC-3 cells. Thanks to the dual enzymatic reaction, the deactivation of gemcitabine to dFdU was significantly inhibited, whereas the activated dFdCTP in tumor sites was remarkably increased. Besides, the PEG shell could be detached when the nanoparticles were exposed to matrix metalloproteinase-9 (MMP-9) which was overexpressed in the tumor microenvironment, leading to the exposure of targeting ligand RGD and thus achieving enhanced cellular internalization [128]. Therefore, the contradictions between long circulation time and high cellular internalization of carriers could be addressed. Drug leakage during circulation can be avoided with efficient drug release in cancer cells. Such cascade enzymatic reactions presenting with reduced gemcitabine deactivation during blood circulation, high drug accumulation in tumor sites, enhanced cancer cell internalization, and specific intracellular gemcitabine release, therefore exhibiting excellent tumor inhibition capability with minimal adverse effects [129].
Fig. 10.
Theranostic nanoparticles with controlled release of gemcitabine for MRI and targeted therapy of pancreatic cancer. Reprinted with permission from [125], Copyright 2013 American Chemical Society.
Fig. 11.
Schematic illustration of MET-induced stromal depletion for improving the penetration of the nanocarriers and cathepsin B-triggered release of gemcitabine loaded by Fe3O4 nanoparticles in the lysosome of PADC cells. Reprinted with permission from [126], Copyright 2020 American Chemical Society.
4. Conclusion and future perspective
Gemcitabine has been widely used for cancer therapy since the 1990s [130]. Unfortunately, its clinical performance is greatly limited by its unsatisfactory pharmacokinetic parameters and deactivation after systematic injection. This is because of its rapid metabolization into the inactive formation, deficiencies in DCK, and alterations in nucleoside transporter. In consequence, it is inevitable to adopt repeated injections with a high concentration of gemcitabine, ending with severe systemic toxicity to healthy cells and limited clinical improvement. It is imperative to develop new gemcitabine delivery strategies for efficient therapeutic schemes [131,132]. It seemed to be preferred methods by pro-drug strategies using a chemical modification to avoid rapid metabolization and improve the pharmacokinetic parameters of gemcitabine [2]. For instance, modification at 4-NH2 of gemcitabine could protect its amine function and thus block CDA degradation. Besides, phosphorylation modification at the 5′-OH of gemcitabine provided numerous monophosphate gemcitabine, which could bypass the rate-limiting step of activation—primary phosphorylation. All these gemcitabine pro-drugs have improved the antitumor effect by decreasing the CDA metabolism, altering the internalization ways, increasing the active monophosphate gemcitabine, thereby enhancing cytotoxicity. However, for most pro-drugs, site-specific delivery, efficient hydrolysis and release of the parent drug is still a big challenge. Nano-drug strategies using nanovectors loaded with gemcitabine or lipophilic gemcitabine physically and chemically appeared to be a more efficient manner since they could achieve enhanced biodistribution profile and tumor-targeting drug delivery due to the EPR effects. More importantly, adjustable co-delivery in nanocarriers with other therapeutic agents such as siRNA and paclitaxel could implement a synergistic effect during the treatment. However, the improvement in the therapeutic efficacy of nano-drug seems to be limited in clinical, despite a significantly reduced non-specific side effect.
Due to the lack of standard methods for evaluating the safety of biomaterials, the long-term toxicity of most nanomaterials requires particular attention. Site-specific delivery of gemcitabine has emerged as an important research field. A stimuli-responsive release of gemcitabine for both pro-drugs and nano-drugs in tumor tissues can be considered as “targeting” ability since they can release the drug in target sites specifically. Additionally, the active targeting strategy can improve the therapeutic performance of gemcitabine significantly. However, one issue needs to be paid attention to. Receptor expression levels vary among different kinds of cancers, or even among different patients with the same type of cancer. Therefore, when applied in clinical, specific target agents should be carefully selected for patients. Overall, there present various gemcitabine delivery strategies, but a direct comparison among them remains challenging. However, by summarizing various novel delivery strategies in this review, gemcitabine is confirmed to receive benefits including higher in vivo stability, longer half-life, and improved bioavailability. Therefore, the future of different pro-drug and nano-drug approaches to improve the therapeutic performance of gemcitabine is very promising, and relevant gemcitabine products may clinically success one day.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgment
This project was supported by the Natural Science Foundation of China (Grant Nos. 52022090, 22005265, 82070739, 81870641), National Key R&D Program of China (Grant No. 2018YFC1106104), Key Research and Development Program of Zhejiang Province (Grant No. 2020C03035), Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ20E030011), Zhejiang Medical Health Science and Technology Program (Grant No. 2021RC061), and Zhejiang Provincial Ten Thousand Talents Program (2018R52001).
Contributor Information
Qiao Jin, Email: jinqiao@zju.edu.cn.
Jian Ji, Email: jijian@zju.edu.cn.
Ke Yao, Email: xlren@zju.edu.cn.
References
- 1.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. New Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moysan E., Bastiat G., Benoit J.P. Gemcitabine versus modified gemcitabine: a review of several promising chemical modifications. Mol Pharm. 2013;10:430–444. doi: 10.1021/mp300370t. [DOI] [PubMed] [Google Scholar]
- 3.Abbruzzese J.L., Grunewald R., Weeks E.A., Gravel D., Adams T., Nowak B., et al. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991;9:491–498. doi: 10.1200/JCO.1991.9.3.491. [DOI] [PubMed] [Google Scholar]
- 4.Celia C., Cosco D., Paolino D., Fresta M. Gemcitabine-loaded innovative nanocarriers vs GEMZAR: biodistribution, pharmacokinetic features and in vivo antitumor activity. Expert Opin Drug Deliv. 2011;8:1609–1629. doi: 10.1517/17425247.2011.632630. [DOI] [PubMed] [Google Scholar]
- 5.Dubey R.D., Saneja A., Gupta P.K., Gupta P.N. Recent advances in drug delivery strategies for improved therapeutic efficacy of gemcitabine. Eur J Pharm Sci. 2016;93:147–162. doi: 10.1016/j.ejps.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Khare V., Kour S., Alam N., Dubey R.D., Saneja A., Koul M., et al. Synthesis, characterization and mechanistic-insight into the anti-proliferative potential of PLGA-gemcitabine conjugate. Int J Pharm. 2014;470:51–62. doi: 10.1016/j.ijpharm.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Rautio J., Kumpulainen H., Heimbach T., Oliyai R., Oh D., Järvinen T., et al. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., Cheetham A.G., Angacian G., Su H., Xie L., Cui H. Peptide-drug conjugates as effective prodrug strategies for targeted delivery. Adv Drug Deliv Rev. 2017;110-111:112–126. doi: 10.1016/j.addr.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao H., Rubio B., Sapra P., Wu D., Reddy P., Sai P., et al. Novel prodrugs of SN38 using multiarm poly(ethylene glycol) linkers. Bioconjug Chem. 2008;19:849–859. doi: 10.1021/bc700333s. [DOI] [PubMed] [Google Scholar]
- 10.Wang H., Liu X., Wang Y., Chen Y., Jin Q., Ji J. Doxorubicin conjugated phospholipid prodrugs as smart nanomedicine platforms for cancer therapy. J Mater Chem B. 2015;3:3297–3305. doi: 10.1039/c4tb01984a. [DOI] [PubMed] [Google Scholar]
- 11.Bildstein L., Dubernet C., Couvreur P. Prodrug-based intracellular delivery of anticancer agents. Adv Drug Deliv Rev. 2011;63:3–23. doi: 10.1016/j.addr.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X., Si J., Huang D., Li K., Xin Y., Sui M. Application of star poly(ethylene glycol) derivatives in drug delivery and controlled release. J Control Release. 2020;323:565–577. doi: 10.1016/j.jconrel.2020.04.039. [DOI] [PubMed] [Google Scholar]
- 14.Vandana M., Sahoo S.K. Long circulation and cytotoxicity of PEGylated gemcitabine and its potential for the treatment of pancreatic cancer. Biomaterials. 2010;31:9340–9356. doi: 10.1016/j.biomaterials.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Pasut G., Canal F., Dalla V.L., Arpicco S., Veronese F.M., Schiavon O. Antitumoral activity of PEG-gemcitabine prodrugs targeted by folic acid. J Control Release. 2008;127:239–248. doi: 10.1016/j.jconrel.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Du X., Li L., Wei S., Wang S., Li Y. A tumor-targeted, intracellular activatable and theranostic nanodiamond drug platform for strongly enhanced in vivo antitumor therapy. J Mater Chem B. 2020;8:1660–1671. doi: 10.1039/c9tb02259g. [DOI] [PubMed] [Google Scholar]
- 17.Yang Z., Lee J.H., Jeon H.M., Han J.H., Park N., He Y., et al. Folate-based near-infrared fluorescent theranostic gemcitabine delivery. J Am Chem Soc. 2013;135:11657–11662. doi: 10.1021/ja405372k. [DOI] [PubMed] [Google Scholar]
- 18.Reddy L.H., Couvreur P. Squalene: a natural triterpene for use in disease management and therapy. Adv Drug Deliv Rev. 2009;61:1412–1426. doi: 10.1016/j.addr.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Sobot D., Mura S., Yesylevskyy S.O., Dalbin L., Cayre F., Bort G., et al. Conjugation of squalene to gemcitabine as unique approach exploiting endogenous lipoproteins for drug delivery. Nat Commun. 2017;8:15678. doi: 10.1038/ncomms15678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatewaki N., Konishi T., Nakajima Y., Nishida M., Saito M., Eitsuka T., et al. Squalene Inhibits ATM-dependent signaling in γIR-induced DNA damage response through induction of wip1 phosphatase. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Réjiba S., Reddy L.H., Bigand C., Parmentier C., Couvreur P., Hajri A. Squalenoyl gemcitabine nanomedicine overcomes the low efficacy of gemcitabine therapy in pancreatic cancer. Nanomedicine. 2011;7:841–849. doi: 10.1016/j.nano.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Harivardhan Reddy L., Ferreira H., Dubernet C., Mouelhi S.L., Desmaële D., Rousseau B., et al. Oral absorption and tissue distribution of a new squalenoyl anticancer nanomedicine. J Nanopartical Res. 2008;10:887–891. [Google Scholar]
- 23.Reddy L.H., Ferreira H., Dubernet C., Mouelhi S.L., Desmaele D., Rousseau B., et al. Squalenoyl nanomedicine of gemcitabine is more potent after oral administration in leukemia-bearing rats: study of mechanisms. Anticancer Drugs. 2008;19:999–1006. doi: 10.1097/CAD.0b013e3283126585. [DOI] [PubMed] [Google Scholar]
- 24.Cavaliere A., Probst K.C., Westwell A.D., Slusarczyk M. Fluorinated nucleosides as an important class of anticancer and antiviral agents. Future Med Chem. 2017;9:1809–1833. doi: 10.4155/fmc-2017-0095. [DOI] [PubMed] [Google Scholar]
- 25.Galmarini C.M., Myhren F., Sandvold M.L. CP-4055 and CP-4126 are active in ara-C and gemcitabine-resistant lymphoma cell lines. Br J Haematol. 2009;144:273–275. doi: 10.1111/j.1365-2141.2008.07467.x. [DOI] [PubMed] [Google Scholar]
- 26.Adema A.D., Smid K., Losekoot N., Honeywell R.J., Verheul H.M., Myhren F., et al. Metabolism and accumulation of the lipophilic deoxynucleoside analogs elacytarabine and CP-4126. Invest New Drugs. 2012;30:1908–1916. doi: 10.1007/s10637-011-9756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maguire J.J., Tyurina Y.Y., Mohammadyani D., Kapralov A.A., Anthonymuthu T.S., Qu F., et al. Known unknowns of cardiolipin signaling: the best is yet to come. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:8–24. doi: 10.1016/j.bbalip.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali S.M., Khan A.R., Ahmad M.U., Chen P., Sheikh S., Ahmad I. Synthesis and biological evaluation of gemcitabine-lipid conjugate (NEO6002) Bioorg Med Chem Lett. 2005;15:2571–2574. doi: 10.1016/j.bmcl.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 29.Chen P., Chien P.Y., Khan A.R., Sheikh S., Ali S.M., Ahmad M.U., et al. In-vitro and in-vivo anti-cancer activity of a novel gemcitabine-cardiolipin conjugate. Anticancer Drugs. 2006;17:53–61. doi: 10.1097/01.cad.0000185182.80227.48. [DOI] [PubMed] [Google Scholar]
- 30.Pradere U., Garnier-Amblard E.C., Coats S.J., Amblard F., Schinazi R.F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem Rev. 2014;114:9154–9218. doi: 10.1021/cr5002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu W., Sigmond J., Peters G.J., Borch R.F. Synthesis and biological activity of a gemcitabine phosphoramidate prodrug. J Med Chem. 2007;50:3743–3746. doi: 10.1021/jm070269u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slusarczyk M., Lopez M.H., Balzarini J., Mason M., Jiang W.G., Blagden S., et al. Application of ProTide technology to gemcitabine: a successful approach to overcome the key cancer resistance mechanisms leads to a new agent (NUC-1031) in clinical development. J Med Chem. 2014;57:1531–1542. doi: 10.1021/jm401853a. [DOI] [PubMed] [Google Scholar]
- 33.Blagden S.P., Rizzuto I., Stavraka C., O'Shea D., Suppiah P., Patel M., et al. A first in human Phase I/II study of NUC-1031 in patients with advanced gynecological cancers. J Clin Oncol. 2015;33:2547. [Google Scholar]
- 34.Blagden S.P., Rizzuto I., Stavraka C., O'Shea D., Suppiah P., Patel M., et al. Final results of ProGem1, the first in-human phase I/II study of NUC-1031 in patients with solid malignancies. J Clin Oncol. 2015;33:2514. [Google Scholar]
- 35.Coyne C.P., Narayanan L. Gemcitabine-(5′-phosphoramidate)-[anti-IGF-1R]: molecular design, synthetic organic chemistry reactions, and antineoplastic cytotoxic potency in populations of pulmonary adenocarcinoma (A549) Chem Biol Drug Des. 2017;89:379–399. doi: 10.1111/cbdd.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finniss M.C., Chu K.S., Bowerman C.J., Luft J.C., Haroon Z.A., DeSimone J.M. A versatile acid-labile linker for antibody–drug conjugates. MedChemComm. 2014;5:1355–1358. [Google Scholar]
- 37.Maiti S., Park N., Han J.H., Jeon H.M., Lee J.H., Bhuniya S., et al. Gemcitabine-coumarin-biotin conjugates: a target specific theranostic anticancer prodrug. J Am Chem Soc. 2013;135:4567–4572. doi: 10.1021/ja401350x. [DOI] [PubMed] [Google Scholar]
- 38.Han H., Teng W., Chen T., Zhao J., Jin Q., Qin Z., et al. A cascade enzymatic reaction activatable gemcitabine prodrug with an AIE-based intracellular light-up apoptotic probe for in situ self-therapeutic monitoring. Chem Commun. 2017;53:9214–9217. doi: 10.1039/c7cc04872f. (Camb) [DOI] [PubMed] [Google Scholar]
- 39.Han H., Jin Q., Wang Y., Chen Y., Ji J. The rational design of a gemcitabine prodrug with AIE-based intracellular light-up characteristics for selective suppression of pancreatic cancer cells. Chem Commun. 2015;51:17435–17438. doi: 10.1039/c5cc06654a. (Camb) [DOI] [PubMed] [Google Scholar]
- 40.Ding D., Li K., Liu B., Tang B.Z. Bioprobes based on AIE fluorogens. Acc Chem Res. 2013;46:2441–2453. doi: 10.1021/ar3003464. [DOI] [PubMed] [Google Scholar]
- 41.Han H., Jin Q., Wang H., Teng W., Wu J., Tong H., et al. Intracellular dual fluorescent lightup bioprobes for image-guided photodynamic cancer therapy. Small. 2016;12:3870–3878. doi: 10.1002/smll.201600950. [DOI] [PubMed] [Google Scholar]
- 42.Qin A., Lam J.W.Y., Tang B.Z. Luminogenic polymers with aggregation-induced emission characteristics. Prog Polym Sci. 2012;37:182–209. [Google Scholar]
- 43.Wang H., Liu G. Advances in luminescent materials with aggregation-induced emission (AIE) properties for biomedical applications. J Mater Chem B. 2018;6:4029–4042. doi: 10.1039/c8tb00674a. [DOI] [PubMed] [Google Scholar]
- 44.Feng H.T., Li Y., Duan X., Wang X., Qi C., Lam J., et al. Substitution activated precise phototheranostics through supramolecular assembly of AIEgen and calixarene. J Am Chem Soc. 2020;142:15966–15974. doi: 10.1021/jacs.0c06872. [DOI] [PubMed] [Google Scholar]
- 45.Ren B., Li K., Liu Z., Liu G., Wang H. White light-triggered zwitterionic polymer nanoparticles based on an AIE-active photosensitizer for photodynamic antimicrobial therapy. J Mater Chem B. 2020;8:10754–10763. doi: 10.1039/d0tb02272a. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y., Han H., Tong H., Chen T., Wang H., Ji J., et al. Zwitterionic phosphorylcholine–TPE conjugate for pH-responsive drug delivery and AIE active imaging. ACS Appl Mater Interfaces. 2016;8:21185–21192. doi: 10.1021/acsami.6b06071. [DOI] [PubMed] [Google Scholar]
- 47.Chen D., Zhu X., Tao W., Kong Y., Huag Y., Zhang Y., et al. Regulation of pancreatic cancer microenvironment by an intelligent gemcitabine@nanogel system via in vitro 3D model for promoting therapeutic efficiency. J Control Release. 2020;324:545–559. doi: 10.1016/j.jconrel.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Jin Q., Deng Y., Chen X., Ji J. Rational design of cancer nanomedicine for simultaneous stealth surface and enhanced cellular uptake. ACS Nano. 2019;13:954–977. doi: 10.1021/acsnano.8b07746. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X., Detering L., Sultan D., Luehmann H., Li L., Heo G.S., et al. CC chemokine receptor 2-targeting copper nanoparticles for positron emission tomography-guided delivery of gemcitabine for pancreatic ductal adenocarcinoma. ACS Nano. 2021;15:1186–1198. doi: 10.1021/acsnano.0c08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDougall I.R. Liposomes and vesicles. Scott Med J. 1978;23:6–8. doi: 10.1177/003693307802300103. [DOI] [PubMed] [Google Scholar]
- 51.Peng Y., Chen L., Ye S., Kang Y., Liu J., Zeng S., et al. Research and development of drug delivery systems based on drug transporter and nano-formulation. Asian J Pharm Sci. 2020;15:220–236. doi: 10.1016/j.ajps.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pattni B.S., Chupin V.V., Torchilin V.P. New developments in liposomal drug delivery. Chem Rev. 2015;115:10938–10966. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 53.Naumenko V.A., Vlasova K.Y., Garanina A.S., Melnikov P.A., Potashnikova D.M., Vishnevskiy D.A., et al. Extravasating neutrophils open vascular barrier and improve liposomes delivery to tumors. ACS Nano. 2019;13:12599–12612. doi: 10.1021/acsnano.9b03848. [DOI] [PubMed] [Google Scholar]
- 54.Zhang R., Zhang Y., Zhang Y., Wang X., Gao X., Liu Y., et al. Ratiometric delivery of doxorubicin and berberine by liposome enables superior therapeutic index than DoxilⓇ. Asian J Pharm Sci. 2020;15:385–396. doi: 10.1016/j.ajps.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sonju J.J., Dahal A., Singh S.S., Jois S.D. Peptide-functionalized liposomes as therapeutic and diagnostic tools for cancer treatment. J Control Release. 2021;329:624–644. doi: 10.1016/j.jconrel.2020.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Federico C., Morittu V.M., Britti D., Trapasso E., Cosco D. Gemcitabine-loaded liposomes: rationale, potentialities and future perspectives. Int J Nanomed. 2012;7:5423–5436. doi: 10.2147/IJN.S34025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tucci S.T., Kheirolomoom A., Ingham E.S., Mahakian L.M., Tam S.M., Foiret J., et al. Tumor-specific delivery of gemcitabine with activatable liposomes. J Control Release. 2019;309:277–288. doi: 10.1016/j.jconrel.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim D.H., Im B.N., Hwang H.S., Na K. Gemcitabine-loaded DSPE-PEG-PheoA liposome as a photomediated immune modulator for cholangiocarcinoma treatment. Biomaterials. 2018;183:139–150. doi: 10.1016/j.biomaterials.2018.08.052. [DOI] [PubMed] [Google Scholar]
- 59.Immordino M.L., Brusa P., Rocco F., Arpicco S., Ceruti M., Cattel L. Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing lipophilic gemcitabine prodrugs. J Control Release. 2004;100:331–346. doi: 10.1016/j.jconrel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 60.Brusa P., Immordino M.L., Rocco F., Cattel L. Antitumor activity and pharmacokinetics of liposomes containing lipophilic gemcitabine prodrugs. Anticancer Res. 2007;27:195–199. [PubMed] [Google Scholar]
- 61.Papa A.L., Sidiqui A., Balasubramanian S.U., Sarangi S., Luchette M., Sengupta S., et al. PEGylated liposomal Gemcitabine: insights into a potential breast cancer therapeutic. Cell Oncol. 2013;36:449–457. doi: 10.1007/s13402-013-0146-4. (Dordr) [DOI] [PubMed] [Google Scholar]
- 62.Poon C., He C., Liu D., Lu K., Lin W. Self-assembled nanoscale coordination polymers carrying oxaliplatin and gemcitabine for synergistic combination therapy of pancreatic cancer. J Control Release. 2015;201:90–99. doi: 10.1016/j.jconrel.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paolino D., Cosco D., Racanicchi L., Trapasso E., Celia C., Iannone M., et al. Gemcitabine-loaded PEGylated unilamellar liposomes vs GEMZAR: biodistribution, pharmacokinetic features and in vivo antitumor activity. J Control Release. 2010;144:144–150. doi: 10.1016/j.jconrel.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 64.Barenholz Y. Doxil®–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 65.Celia C., Calvagno M.G., Paolino D., Bulotta S., Ventura C.A., Russo D., et al. Improved in vitro anti-tumoral activity, intracellular uptake and apoptotic induction of gemcitabine-loaded pegylated unilamellar liposomes. J Nanosci Nanotechnol. 2008;8:2102–2113. doi: 10.1166/jnn.2008.065. [DOI] [PubMed] [Google Scholar]
- 66.Erkan M., Hausmann S., Michalski C.W., Fingerle A.A., Dobritz M., Kleeff J., et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 67.Ding J., Chen J., Gao L., Jiang Z., Zhang Y., Li M., et al. Engineered nanomedicines with enhanced tumor penetration. Nano Today. 2019;29 [Google Scholar]
- 68.Chen X., Jia F., Li Y., Deng Y., Huang Y., Liu W., et al. Nitric oxide-induced stromal depletion for improved nanoparticle penetration in pancreatic cancer treatment. Biomaterials. 2020;246 doi: 10.1016/j.biomaterials.2020.119999. [DOI] [PubMed] [Google Scholar]
- 69.Ji T., Lang J., Wang J., Cai R., Zhang Y., Qi F., et al. Designing liposomes to suppress extracellular matrix expression to enhance drug penetration and pancreatic tumor therapy. ACS Nano. 2017;11:8668–8678. doi: 10.1021/acsnano.7b01026. [DOI] [PubMed] [Google Scholar]
- 70.Dalla P.E., Lerda C., Costanzo C., Donadelli M., Dando I., Zoratti E., et al. Targeting gemcitabine containing liposomes to CD44 expressing pancreatic adenocarcinoma cells causes an increase in the antitumoral activity. Biochim Biophys Acta Mol Cell Biol Lipids. 2013;1828:1396–1404. doi: 10.1016/j.bbamem.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 71.LeMaistre C.F., Meneghetti C., Howes L., Osborne C.K. Targeting the EGF receptor in breast cancer treatment. Breast Cancer Res Treat. 1994;32:97–103. doi: 10.1007/BF00666210. [DOI] [PubMed] [Google Scholar]
- 72.Sandoval M.A., Sloat B.R., Lansakara-P D.S., Kumar A., Rodriguez B.L., Kiguchi K., et al. EGFR-targeted stearoyl gemcitabine nanoparticles show enhanced anti-tumor activity. J Control Release. 2012;157:287–296. doi: 10.1016/j.jconrel.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dosio F., Arpicco S., Stella B., Fattal E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv Drug Deliv Rev. 2016;97:204–236. doi: 10.1016/j.addr.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 74.Zhong L., Liu Y., Xu L., Li Q., Zhao D., Li Z., et al. Exploring the relationship of hyaluronic acid molecular weight and active targeting efficiency for designing hyaluronic acid-modified nanoparticles. Asian J Pharm Sci. 2019;14:521–530. doi: 10.1016/j.ajps.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qu Q., Zhang J., Chen X., Ravanbakhsh H., Tang G., Xiong R., et al. Triggered release from cellulose microparticles inspired by wood degradation by fungi. ACS Sustain Chem Eng. 2021;9:387–397. [Google Scholar]
- 76.Tang G., Xiong R., Lv D., Xu R.X., Braeckmans K., Huang C., et al. Gas-shearing fabrication of multicompartmental microspheres: a one-step and oil-free approach. Adv Sci. 2019;6 doi: 10.1002/advs.201802342. (Weinh) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y., Li Z., Shmidov Y., Carrazzone R.J., Bitton R., Matson J.B. Crescent-shaped supramolecular tetrapeptide nanostructures. J Am Chem Soc. 2020;142:20058–20065. doi: 10.1021/jacs.0c09399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yorseng K., Siengchin S., Ashok B., Rajulu A.V. Nanocomposite egg shell powder with in situ generated silver nanoparticles using inherent collagen as reducing agent. J Bioresour Bioproduct. 2020;5:101–107. [Google Scholar]
- 79.Carvalho M.R., Reis R.L., Oliveira J.M. Dendrimer nanoparticles for colorectal cancer applications. J Mater Chem B. 2020;8:1128–1138. doi: 10.1039/c9tb02289a. [DOI] [PubMed] [Google Scholar]
- 80.Kesharwani P., Jain K., Jain N. Dendrimer as nanocarrier for drug delivery. Prog Polym Sci. 2014;39:268–307. [Google Scholar]
- 81.Zhang C., Pan D., Li J., Hu J., Bains A., Guys N., et al. Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomater. 2017;55:153–162. doi: 10.1016/j.actbio.2017.02.047. [DOI] [PubMed] [Google Scholar]
- 82.Bernkop-Schnürch A., Dünnhaupt S. Chitosan-based drug delivery systems. Eur J Pharm Biopharm. 2012;81:463–469. doi: 10.1016/j.ejpb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 83.Arias J.L., Reddy L.H., Couvreur P. Superior preclinical efficacy of gemcitabine developed as chitosan nanoparticulate system. Biomacromolecules. 2011;12:97–104. doi: 10.1021/bm101044h. [DOI] [PubMed] [Google Scholar]
- 84.Liu Z., Chen X. Simple bioconjugate chemistry serves great clinical advances: albumin as a versatile platform for diagnosis and precision therapy. Chem Soc Rev. 2016;45:1432–1456. doi: 10.1039/c5cs00158g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang H., Wang K., Na K., Li D., Li Z., Zhao D., et al. Striking a balance between carbonate/carbamate linkage bond- and reduction-sensitive disulfide bond-bearing linker for tailored controlled release: in situ covalent-albumin-binding femcitabine prodrugs promote bioavailability and tumor accumulation. J Med Chem. 2018;61:4904–4917. doi: 10.1021/acs.jmedchem.8b00293. [DOI] [PubMed] [Google Scholar]
- 86.Han H., Wang J., Chen T., Yin L., Jin Q., Ji J. Enzyme-sensitive gemcitabine conjugated albumin nanoparticles as a versatile theranostic nanoplatform for pancreatic cancer treatment. J Colloid Interface Sci. 2017;507:217–224. doi: 10.1016/j.jcis.2017.07.047. [DOI] [PubMed] [Google Scholar]
- 87.Han H., Yin Q., Tang X., Yu X., Gao Q., Tang Y., et al. Development of mucoadhesive cationic polypeptide micelles for sustained cabozantinib release and inhibition of corneal neovascularization. J Mater Chem B. 2020;8:5143–5154. doi: 10.1039/d0tb00874e. [DOI] [PubMed] [Google Scholar]
- 88.Cabral H., Miyata K., Osada K., Kataoka K. Block copolymer micelles in nanomedicine applications. Chem Rev. 2018;118:6844–6892. doi: 10.1021/acs.chemrev.8b00199. [DOI] [PubMed] [Google Scholar]
- 89.Ghosh B., Biswas S. Polymeric micelles in cancer therapy: state of the art. J Control Release. 2021;332:127–147. doi: 10.1016/j.jconrel.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 90.Zhu S., Lansakara-P D.S., Li X., Cui Z. Lysosomal delivery of a lipophilic gemcitabine prodrug using novel acid-sensitive micelles improved its antitumor activity. Bioconjug Chem. 2012;23:966–980. doi: 10.1021/bc2005945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu S., Wonganan P., Lansakara-P D.S., O'Mary H.L., Li Y., Cui Z. The effect of the acid-sensitivity of 4-(N)-stearoyl gemcitabine-loaded micelles on drug resistance caused by RRM1 overexpression. Biomaterials. 2013;34:2327–2339. doi: 10.1016/j.biomaterials.2012.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen X., Zhou W., Liang C., Shi S., Yu X., Chen Q., et al. Codelivery nanosystem targeting the deep microenvironment of pancreatic cancer. Nano Lett. 2019;19:3527–3534. doi: 10.1021/acs.nanolett.9b00374. [DOI] [PubMed] [Google Scholar]
- 93.Wang J., Zhang X., Cen Y., Lin X., Wu Q. Antitumor gemcitabine conjugated micelles from amphiphilic comb-like random copolymers. Colloids Surf B Biointerfaces. 2016;146:707–715. doi: 10.1016/j.colsurfb.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 94.Joubert F., Martin L., Perrier S., Pasparakis G. Development of a gemcitabine-polymer conjugate with prolonged cytotoxicity against a pancreatic cancer cell line. ACS Macro Lett. 2017;6:535–540. doi: 10.1021/acsmacrolett.7b00160. [DOI] [PubMed] [Google Scholar]
- 95.Chitkara D., Mittal A., Behrman S.W., Kumar N., Mahato R.I. Self-assembling, amphiphilic polymer-gemcitabine conjugate shows enhanced antitumor efficacy against human pancreatic adenocarcinoma. Bioconjug Chem. 2013;24:1161–1173. doi: 10.1021/bc400032x. [DOI] [PubMed] [Google Scholar]
- 96.Han H., Wang H., Chen Y., Li Z., Wang Y., Jin Q., et al. Theranostic reduction-sensitive gemcitabine prodrug micelles for near-infrared imaging and pancreatic cancer therapy. Nanoscale. 2016;8:283–291. doi: 10.1039/c5nr06734k. [DOI] [PubMed] [Google Scholar]
- 97.Chen X., Teng W., Jin Q., Ji J. One-step preparation of reduction-responsive cross-linked gemcitabine prodrug micelles for intracellular drug delivery. Colloids Surf B Biointerfaces. 2019;181:94–101. doi: 10.1016/j.colsurfb.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 98.Teng W., Jia F., Han H., Qin Z., Jin Q., Ji J. Polyamino acid-based gemcitabine nanocarriers for targeted intracellular drug delivery. Polym Chem. 2017;8:2490–2498. [Google Scholar]
- 99.Ebrahim Attia A.B., Ong Z.Y., Hedrick J.L., Lee P.P., Ee P.L.R., Hammond P.T., et al. Mixed micelles self-assembled from block copolymers for drug delivery. Curr Opin Colloid Interface Sci. 2011;16:182–194. [Google Scholar]
- 100.Wang Y., Fan W., Dai X., Katragadda U., Mckinley D., Teng Q., et al. Enhanced tumor delivery of gemcitabine via PEG-DSPE/TPGS mixed micelles. Mol Pharm. 2014;11:1140–1150. doi: 10.1021/mp4005904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mondal G., Kumar V., Shukla S.K., Singh P.K., Mahato R.I. EGFR-targeted polymeric mixed micelles carrying gemcitabine for treating pancreatic cancer. Biomacromolecules. 2016;17:301–313. doi: 10.1021/acs.biomac.5b01419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mukherjee P. Targeted delivery using inorganic nanosystem preface. Adv Drug Deliv Rev. 2010;62:283. doi: 10.1016/j.addr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y., Yang X., Liu T., Li Z., Leskauskas D., Liu G., et al. Molecular-level control over plasmonic properties in silver nanoparticle/self-assembling peptide hybrids. J Am Chem Soc. 2020;142:9158–9162. doi: 10.1021/jacs.0c03672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Z., Liang X.J. Nano-carbons as theranostics. Theranostics. 2012;2:235–237. doi: 10.7150/thno.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chung Y.J., Kim J., Park C.B. Photonic carbon dots as an emerging nanoagent for biomedical and healthcare applications. ACS Nano. 2020;14:6470–6497. doi: 10.1021/acsnano.0c02114. [DOI] [PubMed] [Google Scholar]
- 106.Faysal Hossain M.D., Akther N., Zhou Y. Recent advancements in graphene adsorbents for wastewater treatment: current status and challenges. Chin Chem Lett. 2020;31:2525–2538. [Google Scholar]
- 107.Lai W., Wong W. Use of graphene-based materials as carriers of bioactive agents. Asian J Pharm Sci. 2020 doi: 10.1016/j.ajps.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang D., Yang F., Hu J., Long J., Wang C., Fu D., et al. Hydrophilic multi-walled carbon nanotubes decorated with magnetite nanoparticles as lymphatic targeted drug delivery vehicles. Chem Commun. 2009:4447–4449. doi: 10.1039/b908012k. (Camb) [DOI] [PubMed] [Google Scholar]
- 109.Ménard-Moyon C., Ali-Boucetta H., Fabbro C., Chaloin O., Kostarelos K., Bianco A. Controlled chemical derivatisation of carbon nanotubes with imaging, targeting, and therapeutic capabilities. Chemistry. 2015;21:14886–14892. doi: 10.1002/chem.201501993. [DOI] [PubMed] [Google Scholar]
- 110.Samimi S., Ardestani M.S., Dorkoosh F.A. Preparation of carbon quantum dots- quinic acid for drug delivery of gemcitabine to breast cancer cells. J Drug Deliv Sci Technol. 2021;61 [Google Scholar]
- 111.Lai H., Lu M., Lu H., Stenzel M.H., Xiao P. pH-Triggered release of gemcitabine from polymer coated nanodiamonds fabricated by RAFT polymerization and copper free click chemistry. Polym Chem. 2016;7:6220–6230. [Google Scholar]
- 112.Ye W., Han H., Li H., Jin Q., Wu Y., Chakrabortty S., et al. Polymer coated nanodiamonds as gemcitabine prodrug with enzymatic sensitivity for pancreatic cancer treatment. Prog Nat Sci. 2020;30:711–717. [Google Scholar]
- 113.Gupta N., Jangid A.K., Singh M., Pooja D., Kulhari H. Designing two-dimensional nanosheets for improving drug delivery to fucose-receptor-overexpressing cancer cells. ChemMedChem. 2018;13:2644–2652. doi: 10.1002/cmdc.201800575. [DOI] [PubMed] [Google Scholar]
- 114.Singh R.K., Patel K.D., Leong K.W., Kim H. Progress in nanotheranostics based on mesoporous silica nanomaterial platforms. ACS Appl Mater Interfaces. 2017;9:10309–10337. doi: 10.1021/acsami.6b16505. [DOI] [PubMed] [Google Scholar]
- 115.Abeer M.M., Rewatkar P., Qu Z., Talekar M., Kleitz F., Schmid R., et al. Silica nanoparticles: a promising platform for enhanced oral delivery of macromolecules. J Control Release. 2020;326:544–555. doi: 10.1016/j.jconrel.2020.07.021. [DOI] [PubMed] [Google Scholar]
- 116.Liong M., Lu J., Kovochich M., Xia T., Ruehm S.G., Nel A.E., et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang X., Li C., Fan N., Li J., Zhang H., Shang L., et al. Amino functionalized chiral mesoporous silica nanoparticles for improved loading and release of poorly water-soluble drug. Asian J Pharm Sci. 2019;14:405–412. doi: 10.1016/j.ajps.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Malfanti A., Miletto I., Bottinelli E., Zonari D., Blandino G., Berlier G., et al. Delivery of gemcitabine prodrugs employing mesoporous silica nanoparticles. Molecules. 2016;21:522. doi: 10.3390/molecules21040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meng H., Wang M., Liu H., Liu X., Situ A., Wu B., et al. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano. 2015;9:3540–3557. doi: 10.1021/acsnano.5b00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meng H., Zhao Y., Dong J., Xue M., Lin Y.S., Ji Z., et al. Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS Nano. 2013;7:10048–10065. doi: 10.1021/nn404083m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang T., Sun G., Wang M., Zhou B., Fu J. Voltage/pH-driven mechanized silica nanoparticles for the multimodal controlled release of drugs. ACS Appl Mater Interfaces. 2015;7:21295–21304. doi: 10.1021/acsami.5b05619. [DOI] [PubMed] [Google Scholar]
- 122.Dadfar S.M., Roemhild K., Drude N.I., Von Stillfried S., Knüchel R., Kiessling F., et al. Iron oxide nanoparticles: diagnostic, therapeutic and theranostic applications. Adv Drug Deliv Rev. 2019;138:302–325. doi: 10.1016/j.addr.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu J., Han H., Jin Q., Li Z., Li H., Ji J. Design and proof of programmed 5-aminolevulinic acid prodrug nanocarriers for targeted photodynamic cancer therapy. ACS Appl Mater Interfaces. 2017;9:14596–14605. doi: 10.1021/acsami.6b15853. [DOI] [PubMed] [Google Scholar]
- 124.Zhou J., Yang Y., Zhang C.Y. Toward biocompatible semiconductor quantum dots: from biosynthesis and bioconjugation to biomedical application. Chem Rev. 2015;115:11669–11717. doi: 10.1021/acs.chemrev.5b00049. [DOI] [PubMed] [Google Scholar]
- 125.Lee G.Y., Qian W.P., Wang L., Wang Y.A., Staley C.A., Satpathy M., et al. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano. 2013;7:2078–2089. doi: 10.1021/nn3043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Han H., Hou Y., Chen X., Zhang P., Kang M., Jin Q., et al. Metformin-induced stromal depletion to enhance the penetration of gemcitabine-loaded magnetic nanoparticles for pancreatic cancer targeted therapy. J Am Chem Soc. 2020;142:4944–4954. doi: 10.1021/jacs.0c00650. [DOI] [PubMed] [Google Scholar]
- 127.Wang Z., Chen L., Chu Z., Huang C., Huang Y., Jia N. Gemcitabine-loaded gold nanospheres mediated by albumin for enhanced anti-tumor activity combining with CT imaging. Mater Sci Eng C Mater Biol Appl. 2018;89:106–118. doi: 10.1016/j.msec.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 128.Han H., Gao Y., Chai M., Zhang X., Liu S., Huang Y., et al. Biofilm microenvironment activated supramolecular nanoparticles for enhanced photodynamic therapy of bacterial keratitis. J Control Release. 2020;327:676–687. doi: 10.1016/j.jconrel.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 129.Han H., Valdepérez D., Jin Q., Yang B., Li Z., Wu Y., et al. Dual enzymatic reaction-assisted gemcitabine delivery systems for programmed pancreatic cancer therapy. ACS Nano. 2017;11:1281–1291. doi: 10.1021/acsnano.6b05541. [DOI] [PubMed] [Google Scholar]
- 130.Burris H.R., Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R., et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 131.Liu X., Jiang J., Meng H. Transcytosis - an effective targeting strategy that is complementary to "EPR effect" for pancreatic cancer nano drug delivery. Theranostics. 2019;9:8018–8025. doi: 10.7150/thno.38587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu X., Lin P., Perrett I., Lin J., Liao Y.P., Chang C.H., et al. Tumor-penetrating peptide enhances transcytosis of silicasome-based chemotherapy for pancreatic cancer. J Clin Invest. 2017;127:2007–2018. doi: 10.1172/JCI92284. [DOI] [PMC free article] [PubMed] [Google Scholar]