Abstract
Background
Cancer‐related cachexia is a major cause of treatment resistance and poor prognosis, which is characterized by anorexia and skeletal muscle depletion. To date, there have been no reports on the relationship between IL‐35 and cancer‐related cachexia in patients with stage IV non‐small cell lung cancer.
Methods
Serum IL‐35 levels in 86 patients with stage IV NSCLC were measured and statistically analyzed based on patients' clinicopathological parameters. Serum albumin levels, C‐reactive protein, and skeletal muscle index (SMI) of the patients were also determined. In vivo studies using a mouse model were also conducted by subcutaneously injecting immunodeficiency (SCID) mice with overexpressing IL‐35 cell lines and determining their daily food intake, bodyweight and muscle atrophy. Cachexia indicators were measured again after administering the mice with an anti‐IL35 neutralizing antibody.
Results
Patients with stage IV NSCLC had significantly higher serum IL‐35 levels than the healthy controls. Similarly, circulating IL‐35 levels were significantly higher in patients with cachexia than those without. The SMI values of patients with high serum IL‐35 levels were significantly lower than those with low serum IL‐35 levels. Mice subcutaneously injected with LLC PLV‐IL‐35 cell lines exhibited anorexia, weight loss, and muscle atrophy. Moreover, these symptoms were significantly reduced after administering the mice with an anti‐IL35 neutralizing antibody.
Conclusions
This study reveals that high serum IL‐35 expression is associated with non‐small cell lung cancer cachexia and skeletal muscle atrophy. These findings highlight its potential as a biomarker and therapeutic target for controlling cachexia of advanced lung cancer.
Keywords: cachexia, IL‐35, skeletal muscle atrophy, SMI, stage IV NSCLC
Serum IL‐35 level was increased in stage IV NSCLC and induced cancer‐related cachexia and skeletal muscle atrophy.

INTRODUCTION
Cancer‐associated cachexia is reported to occur in 50%–80% of cancer patients, leading to direct deaths of 20% of the patients, especially those with lung and gastrointestinal tumors. 1 Cachexia is a metabolic syndrome characterized by depletion of the skeletal muscle, leading to progressive functional impairment, treatment‐related complications, poor life quality, and mortality. 2 Non‐small cell lung cancer (NSCLC) patients often suffer from cachexia, characterized by anorexia, early satiety, fatigue, and gradual weight loss. 3 Despite its significant negative effects and high prevalence in cancer patients, there are only a few therapeutic strategies that exist for its treatment, with no standard management. 4
Interleukin‐35 (IL‐35) is a member of the interleukin‐12 family cytokines originally secreted by the regulatory T cells (Tregs). 5 IL‐35 exhibits strong immunosuppressive effects comparable to those of IL‐10 and the transforming growth factor‐β. 6 It exerts a protective role against inflammation and autoimmune diseases, such as experimental autoimmune encephalitis, autoimmune raffinitis, rheumatoid arthritis, and primary biliary liver. 7 , 8 , 9 However, tumor‐derived IL‐35 plays an unfavorable role in tumor diseases, promoting tumor cell metastasis, angiogenesis, and drug resistance. 10 , 11
Serum IL‐35 is also elevated in many cancers, especially in the advanced stages. 12 , 13 , 14 , 15 To date, the systemic effects of serum IL‐35 in advanced cancer stages remain unclear. This study investigated the clinical significance of serum IL‐35 to identify its role in cancer‐associated cachexia in stage IV NSCLC.
METHODS
Patients
Eighty‐six patients admitted to Tianjin Cancer Hospital with stage IV non‐small cell lung cancer between October 2019, and October 2020 were enrolled in this study. Their treatment information, height, weight, age, gender, serum albumin and CRP levels were subsequently obtained from their medical records. An additional 50 age‐ and sex‐matched healthy individuals were enrolled as the healthy controls. The patients' information and clinical samples were collected prospectively. The written informed consent was obtained from patients and healthy controls. The study was approved by the ethics committee of Tianjin Medical University Cancer Institute and Hospital.
Analysis of blood sample
Heparin blood samples from 50 healthy controls and 86 lung cancer patients were centrifuged at 400 x g for 5 min immediately upon collection, followed by immediate plasma freezing at 80°C until analysis. The IL‐35 level was determined using the enzyme‐linked immunosorbent assay (ELISA) kit (Cusabio Biotech), following the manufacturer's instructions.
Cachexia and skeletal muscle mass index (SMI)
The standard diagnostic criterion used for cachexia was more than 5% weight loss within 6 months. A more than 2% weight loss in individuals already exhibiting depletion based on the current bodyweight and height (body‐mass index [BMI] <20 kg/m2) or skeletal muscle mass (sarcopenia) was also used as a diagnostic criterion. 16 SMI (cm2/m2) was the mean value of the skeletal muscle area, including psoas, erector spinae, quadratus lumborum, transversus abdominis, external, internal obliques, and rectus abdominis, of two consecutive CT scans of the third lumbar spine, normalized by height (m2).
Cell culture and overexpression of IL‐35
Lewis lung cancer (LLC) cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco's modified Eagle's medium (DMEM) in a humidified incubator at 37°C and 5% CO2. The DMEM was enriched with 10% fetal bovine serum (FBS, Gibco) and 100 units/ml of penicillin–streptomycin. IL‐35 overexpression in the LLC cells, lentivirus‐mediated plasmid was performed using the PLV‐cDNA system (Biosettia) following the manufacturer's instructions. The medium containing lentivirus was collected, filtered, and transferred onto the LLC cells after transfection. An empty vector was transfected into the same cell lines to act as a PLV‐control.
Establishment of the tumor model
Severe combined immunodeficiency (SCID) female mice aged 4–5 weeks were maintained in a barrier facility on high‐efficiency particulate air (HEPA)‐filtered racks. The tumor cells were harvested by trypsinization, washed with PBS, and then resuspended in Matrigel. The resuspended cells (1 × 106 cells) were then subcutaneously injected into each SCID mice to develop the xenograft tumors. Tumor implantation caused anorexia, whose progress was assessed daily by registering the food intake of the mice by collecting and weighing all the remaining food, including the spilled pellets in the cage. The bodyweight of the mice was also recorded daily. The animals were sacrificed 3 weeks post tumor implantation. Mice in the anti‐IL35 antibody treatment group were intravenously injected with anti‐IL35 (25 μg) antibodies every 3 days from the seventh day (totalling six injection times) when the tumor's long axis reached about 5 mm.
Reverse transcription PCR assays
Total RNA was extracted from the LLC cells and gastrocnemius of the mice using TRIzol reagent (Invitrogen). The RNA samples were subsequently transcribed to cDNA using the first‐strand synthesis kit and then used as templates for PCR and real‐time PCR. Real‐time PCR was used to quantify 1 μg cDNA sample using the SYBR Green PCR Master Mix (Takara) and appropriate primer pairs. Each sample was processed in triplicate. Primers for Ebi3 were forward: 5′‐GCTCCCTACGTGCTCAATGT‐3′, reverse 5′‐CCCTGACGCTTGTAACGG AT‐3′; and p35: forward 5′‐TCCTCCCTTGAAGAACCGGA‐3′, reverse 5′‐TGACAACGGTTTGGAGGGAC‐3′. Primers for Atrogin‐1 were forward: 5′‐CTTTCAACAGACTGGACTTCTCGA‐3′ and reverse: 5′‐CAGCTCCAACAGCCTTACTACGT‐3′; primers for MuRF1 were forward: 5′‐AACCTGGAGAAGCAGCTGAT‐3′ and reverse: 5′‐GATTCGCAGCCTGGAAGATG‐3′; and primers for β‐actin were forward: 5′‐CAGAGCAAGAGAGGCATCC‐3′ and reverse: 5′‐CTGGGGTGTTGAAGGTCTC‐3′.
Western blotting
Whole‐cell protein extracts were prepared by lysing the LLC cells with sodium dodecyl sulfate lysis buffer supplemented with protease inhibitor cocktails (Sigma). The protein extracts were then quantified using the Pierce protein assay kit (Pierce). Sodium dodecyl sulfate‐polyacrylamide gel electrophoresis was done to separate the protein lysates (20 g), followed by detection of the target proteins through western blotting analysis using special primary antibodies against MuRF1 (Proteintech 55 456‐1‐AP [1:1000]), and Atrogin‐1 (Proteintech, 12 866‐1‐AP [1:1000]). The monoclonal β‐tubulin antibody (Ray Antibody, RM2003 [1:5000]) was used as a loading control.
Histochemistry and cross‐sectional area analyses
Morphological changes of the skeletal muscle tissue were microscopically observed after conventional hematoxylin and eosin staining. Transverse serial sections of gastrocnemius muscle from the paraffin‐embedded tissue samples were dewaxed and stained with hematoxylin and eosin, followed by several washing steps. At least eight randomly selected x20 magnification images for each section were quantified using ImageJ software.
Statistical analysis
All statistical analyses were performed using SPSS v21.0 software (IBM SPSS Statistics), and the data (serum IL‐35 levels) were expressed as means ± SD. Analysis of variance was employed to analyze the differences among means of two groups with continuous variables. In the same line, a Spearman's rank correlation coefficient test was carried out to test the association between ordinal variables. Student's t‐test was used to compare the mean values of the serum IL‐35 levels, followed by determining the best cutoff point value using the receiver operating characteristic (ROC) analysis. The significance threshold was set at p < 0.05 (*p < 0.05, **p < 0.01, and ***p < 0.001).
RESULTS
Positive correlation between cachexia and elevation of serum IL‐35 level in stage IV NSCLC
Stage IV NSCLC patients had a significant increase in serum IL‐35 levels compared with those in the control group (14.44 ± 6.91 vs. 76.27 ± 26.5 pg/ml: p < 0.001: Figure 1a). An assessment of the relationships between serum IL‐35 levels of stage IV NSCLC patients and the clinicopathological parameters revealed insignificant differences in serum IL‐35 levels among different genders, ages, treatment regimes, and BMI groups (Table 1). However, circulating IL‐35 levels were significantly higher in patients with cachexia (84.07 ± 27.01 pg/ml) than in those without (63.09 ± 19.13 pg/ml; p < 0.001; Table 1 and Figure 1b). These findings suggested that elevated serum IL‐35 level was associated with cachexia. These results were further used to divide the 86 NSCLC patients into two groups based on plasma IL‐35 level. The cutoff value for serum IL‐35 levels for patients with cachexia was determined to be 77.5 pg/ml and above using the receiver operating characteristic (ROC) curve analysis, with a 0.82 area under the curve (p < 0.001; Figure 1c). An analysis of the association of serum IL‐35 levels with albumin and C‐reactive protein (CRP), the two diagnostic markers of cachexia, revealed that the expression of serum IL‐35 was significantly positively correlated with serum albumin (p = 0.037) and CRP (p = 0.024; Table 2). These results suggested that IL‐35 is highly expressed and associated with cachexia in advanced NSCLC.
FIGURE 1.
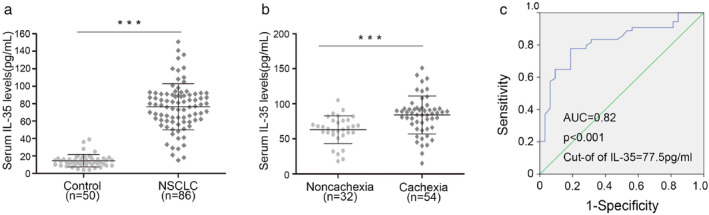
Serum IL‐35 levels were elevated in patients with stage IV NSCLC and cachexia. (a). Serum IL‐35 levels in stage IV NSCLC patients and health control were measured by ELISA. (b). Serum IL‐35 levels in stage IV NSCLC patients with or without cachexia. (c). Receiver operating characteristic curves (ROC) of serum IL‐35 between cachexia and noncachexia patients. ***p < 0.001
TABLE 1.
Patient characteristics and serum IL‐35 levels (n = 86,mean ± SD)
| Variable | n (%) | IL‐35 (pg/ml) | p‐value |
|---|---|---|---|
| Sex | 0.1595 | ||
| Male | 62 | 78.18 ± 26.06 | |
| Female | 24 | 69.79 ± 20.13 | |
| Age | 0.4085 | ||
| <65 | 36 | 73.22 ± 30.72 | |
| ≥65 | 50 | 77.72 ± 19.43 | |
| Treatment | ≥0.0755 | ||
| Chemotherapy | 35 | 68.48 ± 24.03 | |
| Targeted therapy | 24 | 77.08 ± 18.89 | |
| Supportive care | 27 | 80.66 ± 27.88 | |
| BMI(kg/m 2 ) | ≥0.1509 | ||
| Underweight | 7 | 63.86 ± 17.97 | |
| Normal | 44 | 75.98 ± 25.9 | |
| Overweight/obesity | 35 | 78.06 ± 24.25 | |
| Cachexia | <0.001*** | ||
| No | 32 | 63.09 ± 19.13 | |
| Yes | 54 | 84.07 ± 27.01 |
Abbreviations: BMI, body mass index.
p < 0.001.
TABLE 2.
Correlation between serum IL‐35 levels and cachexia, albumin, CRP and SMI among NSCLC patients
| Serum IL‐35 levels(pg/ml) | p‐value | ||
|---|---|---|---|
| <77.5 (n = 42) | ≥77.5 (n = 44) | ||
| ALB (g/l) | 0.037* | ||
| <35 | 13 | 23 | |
| ≥35 | 29 | 21 | |
| CRP (g/l) | 0.024* | ||
| <10 | 19 | 10 | |
| ≥10 | 23 | 34 | |
Abbreviations: ALB, albumin; CRP, C‐reactive protein.
p < 0.05.
High levels of IL‐35 associated with skeletal muscle wasting
Skeletal muscle depletion is the most typical characteristic of cancer cachexia. Evaluation of the skeletal muscle area of the third lumbar spine through computed tomography (CT) or magnetic resonance imaging is the preferred method for analyzing the muscle mass in cancer patients (Figure 2a).
FIGURE 2.
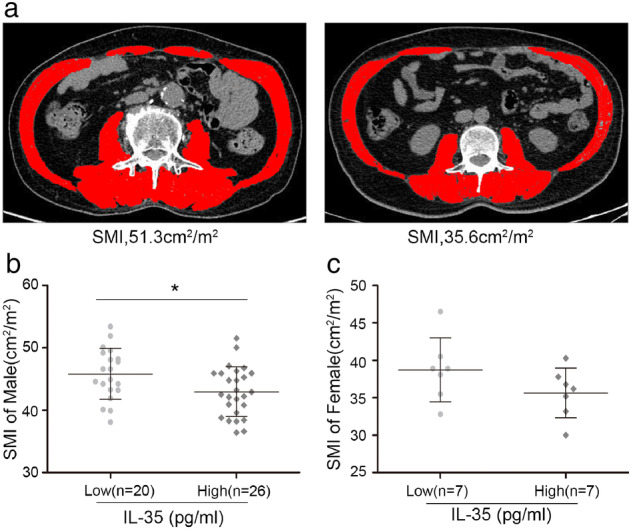
Serum IL‐35 levels were are associated with skeletal muscle index (SMI) and induced muscle wasting. A. Representative axial computed tomography images of the third lumbar vertebra region with skeletal muscle highlighted inred (Houns eldunits [HU]). (b–c) scatterplot highlights the relationship between serum IL‐35 levels and SMI and HU in male and female stage IV NSCLC patients. Results are plotted as mean ± standard deviation
An assessment of the skeletal muscle mass index (SMI) values of 46 male and 14 female patients who fulfilled the evaluation criteria was thus done to determine the association between SMI and serum IL‐35 level. The assessment was done separately because of differences in sarcopenia standards (43.1 cm2/m2 for males and 37.8 cm2/m2 for females). 17 The SMI value of the patients with low IL‐35 levels was significantly higher than that of patients with high IL‐35 levels among the 46 male patients (45.8 ± 4.09 vs. 43.0 ± 3.96 cm2/m2; p = 0.022; Figure 2b). Notably, 53.8% of the male patients in the high IL‐35 expression group met the sarcopenia criteria, while only 20% in the low IL‐35 expression group met the criteria. Similar results were obtained among the 14 female patients, but the difference was insignificant (38.74 ± 4.27 vs. 35.64 ± 3.32 cm2/m2; p = 0.155; Figure 2c). These results suggested that high serum IL‐35 levels are associated with skeletal muscle depletion and a higher incidence of sarcopenia.
Overexpression of IL‐35 induces cachexia and muscle wasting in SCID mice
IGFBP2 stable overexpression cell lines were established using PLV‐IL‐35 stable transfection, followed by a determination of the mRNA levels of EBI3 and IL‐12p35, and the secreted‐IL35 in the supernatant of the growth medium (Figure 3a,b). The contribution of increased serum IL‐35 levels to cachexia and muscle wasting was subsequently examined using LLC cells stably expressing PLV‐IL‐35 in a xenograft mouse model. PLV‐IL‐35 and PLV‐Control cells were injected subcutaneously into the flank of 4–5 weeks‐old SCID mice, followed by a daily recording of food intake and weight changes. The mice were euthanized 3 weeks later to examine the IL‐35 levels from their terminal peripheral blood samples. The levels of circulating IL‐35 in the PLV‐IL‐35 group were significantly increased (Figure 3c; p < 0.001), with obvious typical characteristics of cachexia, such as anorexia (Figure 3d, p = 0.03) and weight loss (Figure 3e, p = 0.02). HE staining of the gastrocnemius muscle sections of the two groups revealed muscle atrophy in the PLV‐IL‐35 group (Figure 3f), with a significantly lower cross‐sectional area of muscle fibers than that of the PLV‐Control group (Figure 3g, 1550 ± 250 vs. 954 ± 439.98 μm2; p < 0.001). These results showed that implanting mice with PLV‐IL‐35 cells elevated the circulating IL‐35 levels, leading to anorexia, weight loss, and muscle atrophy.
FIGURE 3.
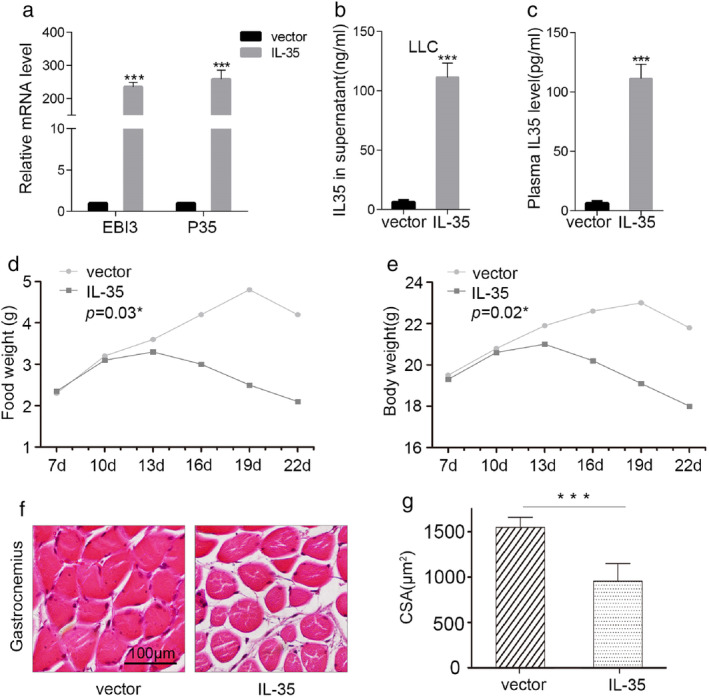
Overexpression of IL‐35 induced cachexia and muscle wasting in SCID mice. (a–b) IL‐35 mRNA and protein levels were detected by RT‐PCR and ELISA in stable transfection cell lines. (c) Terminal peripheral blood samples were collected, and IL‐35 levels in serum measured. (d–e). A week after subcutaneous tumor transplantation, bodyweight and food intake were measured every day. (f–g) The gastrocnemius muscles was determined by hematoxylin and eosin staining (×20), and the cross‐sectional area (CSA) were quantitated. Results are shown as mean ± standard deviation
Overexpression of IL‐35 elevated MuRF1 and Atrogin‐1 expression in SCID mice
Skeletal muscle loss is usually accompanied by accelerated protein degradation, leading to increased expression of two muscle‐restricted ubiquitin ligase proteins, MuRF1 and Atrogin‐1. MuRF‐1 and Atrogin‐1 expression in the gastrocnemius muscle of mice in PLV‐IL‐35 and PLV‐Control groups was thus determined to verify whether serum IL‐35 led to skeletal muscle atrophy by accelerating protein degradation. Quantitative reverse transcription PCR and western blot analysis revealed a significant increase in the mRNA and protein expressions of MuRF‐1 and Atrogin‐1 in mice in the PLV‐35 group than those in the PLV‐Control group (Figure 4a, b). These results demonstrated that elevated serum IL‐35 levels potentially induce muscle wasting by regulating MuRF1 and Atrogin‐1.
FIGURE 4.
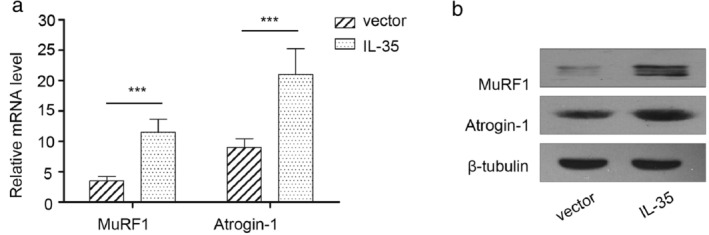
IL‐35 induced muscle wasting via regulating muscle RING finger1 (MuRF1) and atrogin‐1 in SCID mice. (a). MuRF1and atrogin‐1mRNA expression level in gastrocnemius muscles were detected by RT‐PCR. (b). MuRF1 and atrogin‐1 protein expression in gastrocnemius muscles were detected by western blotting
Anti‐IL35 neutralizing antibody exerts an anticachexia effect in vivo
This study further investigated whether anti‐IL‐35 treatment could relieve cachexia symptoms on the background that overexpression of IL‐35 aggravated cachexia in the xenograft mouse model. SCID mice were first subcutaneously injected with the LLC cells to develop the xenograft tumors. An anti‐IL35 antibody was then used to neutralize the IL‐35. IL‐35 neutralization significantly increased the bodyweight of the mice, food intake, and cross‐sectional area of muscle fibers (Figure 5a–c). Moreover, IL‐35 neutralization decreased MuRF‐1 and Atrogin‐1 expression in the gastrocnemius muscle of mice (Figure 5d). These results suggested that IL‐35 is a promising therapeutic target for cachexia.
FIGURE 5.
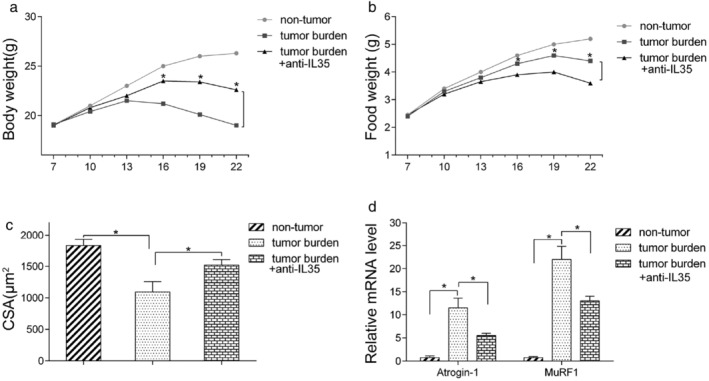
Anti‐IL35 neutralizing antibody showed an anticachexia effect in vivo. LLC cells were subcutaneously transplanted to the SCID mice. From the second week, IgG control and anti‐IL35 antibody were administered via tail vein three times a week. (a–b). Bodyweight and food intake were measured every day. (c) The cross‐sectional area (CSA) of the gastrocnemius muscles was quantitated. (d) MuRF1 and atrogin‐1mRNA expression level in gastrocnemius muscles were detected by RT‐PCR
DISCUSSION
Lung cancer is one of the major causes of cancer‐related deaths globally, with only an 18.2% 5‐year survival rate for NSCLC patients. 18 Cancer‐cachexia is a major cause of treatment resistance and poor prognosis, affecting 70% of lung cancer patients and 45.6% of patients with advanced NSCLC. 19 Approximately 22% of deaths among lung cancer patients are related to cachexia. However, there are no effective biomarkers nor interventions to manage or control cachexia. 20 Cachexia is characterized by anorexia, progressive weight loss, and abnormal fat and protein metabolism. Skeletal muscle loss is its most powerful prognostic factor. 21 Numerous clinical trials conducted in recent years postulate that ghrelin receptor anamorelin ameliorates cachexia symptoms, such as anorexia and weight loss. However, further studies are required to determine whether long‐term use of anamorelin is beneficial because the alamoline group showed similar motor function and overall survival to the placebo group in several studies. 1 , 22 Currently, there are no objective biomarkers for cachexia. The majority of patients diagnosed with cachexia are already in the refractory stage of cachexia, which cannot be reversed by nutritional therapy. These developments necessitate the detection of biomarkers for cancer cachexia.
IL‐35 is the fourth member of the IL‐12 family and consists of two subunits, EBI3 and p35. 6 It is a cytokine primarily produced by the regulatory T cells and protects cancer cells against apoptosis, thereby facilitating the development and progression of a variety of cancers, such as pancreatic ductal adenocarcinoma, acute myeloid leukemia, and NSCLC. 23 It has been reported that IL‐35 increases serum IL‐10 levels. 24 In this study, serum IL‐35 was highly expressed in patients with advanced NSCLC and was positively correlated with cachexia. Notably, the SMI values of patients with high serum IL‐35 levels were significantly lower than those with low IL‐35 levels, but with a higher incidence of sarcopenia. These findings strongly suggested that IL‐35 is potentially involved in cachexia and muscle atrophy. This assertion was further verified through animal experiments using a mouse model. Mice subcutaneously injected with overexpressing IL‐35 cell lines had significantly higher serum IL‐35 levels than those in the control group. They also exhibited typical signs of cachexia, such as anorexia and muscle atrophy. However, cachexia symptoms were significantly reduced when IL‐35 neutralizing antibody was administered. This study postulates that high serum IL‐35 expression is associated with non‐small cell lung cancer cachexia and skeletal muscle atrophy. IL‐35 is thus a potential biomarker and therapeutic target for controlling advanced lung cancer cachexia.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by Tianjin Natural Science Foundation (grant no. 19JCYBJC26200).
Li Z, Zhu L, Zheng H, Jiang W, Wang Y, Jiang Z, et al. Serum IL‐35 levels is a new candidate biomarker of cancer‐related cachexia in stage IV non‐small cell lung cancer. Thorac Cancer. 2022;13:716–723. 10.1111/1759-7714.14307
Funding information Natural Science Foundation of Tianjin City, Grant/Award Number: 19JCYBJC26200
Contributor Information
Zengxun Li, Email: zhjiang@tmu.edu.cn.
Zhansheng Jiang, Email: zhjiang@tmu.edu.cn.
Jie Xu, Email: xujie508@126.com.
REFERENCES
- 1. Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non‐small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double‐blind, phase 3 trials. Lancet Oncol. 2016;17:519–31. 10.1016/S1470-2045(15)00558-6 [DOI] [PubMed] [Google Scholar]
- 2. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer‐associated cachexia. Nat Rev Dis Prim. 2018;4:17105. [DOI] [PubMed] [Google Scholar]
- 3. Chlebowski RT, Palomares MR, Lillington L, Grosvenor M. Recent implications of weight loss in lung cancer management. Nutrition. 1996;12:S43–7. [DOI] [PubMed] [Google Scholar]
- 4. Nishie K, Yamamoto S, Nagata C, Koizumi T, Hanaoka M. Anamorelin for advanced non‐small‐cell lung cancer with cachexia: systematic review and meta‐analysis. Lung Cancer. 2017;112:25–34. 10.1016/j.lungcan.2017.07.023 [DOI] [PubMed] [Google Scholar]
- 5. Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL‐35‐mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–101. 10.1038/ni.1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL‐35 contributes to regulatory T‐cell function. Nature. 2007;450:566–9. 10.1038/nature06306 [DOI] [PubMed] [Google Scholar]
- 7. Haller S, Duval A, Migliorini R, Stevanin M, Mack V, Acha‐Orbea H. Interleukin‐35‐producing CD8alpha(+) dendritic cells acquire a Tolerogenic state and regulate T cell function. Front Immunol. 2017;8:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsuda M, Zhang W, Yang GX, Tsuneyama K, Ando Y, Kawata K, et al. Deletion of interleukin (IL)‐12p35 induces liver fibrosis in dominant‐negative TGFbeta receptor type II mice. Hepatology. 2013;57:806–16. 10.1002/hep.25829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kang M, Choi JK, Jittayasothorn Y, Egwuagu CE. Interleukin 35‐producing Exosomes suppress Neuroinflammation and autoimmune uveitis. Front Immunol. 2020;11:1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang C, Li N, Li Z, Chang A, Chen Y, Zhao T, et al. Tumour‐derived interleukin 35 promotes pancreatic ductal adenocarcinoma cell extravasation and metastasis by inducing ICAM1 expression. Nat Commun. 2017;8:14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang C, Li Z, Li N, Li Y, Chang A, Zhao T, et al. Interleukin 35 expression correlates with microvessel density in pancreatic ductal adenocarcinoma, recruits monocytes, and promotes growth and angiogenesis of Xenograft tumors in mice. Gastroenterology. 2018;154:675–88. 10.1053/j.gastro.2017.09.039 [DOI] [PubMed] [Google Scholar]
- 12. Gu JH, Wang XG, Wang LQ, Zhou LN, Tang M, Li P, et al. Serum level of interleukin‐35 as a potential prognostic factor for gastric cancer. Asia Pac J Clin Oncol. 2021;17:52–9. 10.1111/ajco.13403 [DOI] [PubMed] [Google Scholar]
- 13. Wang YN, Lou DF, Li DY, Jiang W, Dong J, Gao W, et al. Elevated levels of IL‐17A and IL‐35 in plasma and bronchoalveolar lavage fluid are associated with checkpoint inhibitor pneumonitis in patients with non‐small cell lung cancer. Oncol Lett. 2020;20:611–22. 10.3892/ol.2020.11618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin L, Xu X, Ye B, Pan M, Shi Z, Hu Y. Elevated serum interleukin‐35 levels correlate with poor prognosis in patients with clear cell renal cell carcinoma. Int J Clin Exp Med. 2015;8:18861–6. [PMC free article] [PubMed] [Google Scholar]
- 15. Jin P, Ren H, Sun W, Xin W, Zhang H, Hao J. Circulating IL‐35 in pancreatic ductal adenocarcinoma patients. Hum Immunol. 2014;75:29–33. 10.1016/j.humimm.2013.09.018 [DOI] [PubMed] [Google Scholar]
- 16. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. 10.1016/S1470-2045(10)70218-7 [DOI] [PubMed] [Google Scholar]
- 17. Zhang S, Tan S, Jiang Y, Xi Q, Meng Q, Zhuang Q, et al. Sarcopenia as a predictor of poor surgical and oncologic outcomes after abdominal surgery for digestive tract cancer: a prospective cohort study. Clin Nutr. 2019;38:2881–8. 10.1016/j.clnu.2018.12.025 [DOI] [PubMed] [Google Scholar]
- 18. DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–71. [DOI] [PubMed] [Google Scholar]
- 19. Zhu R, Liu Z, Jiao R, Zhang C, Yu Q, Han S, et al. Updates on the pathogenesis of advanced lung cancer‐induced cachexia. Thorac Cancer. 2019;10:8–16. 10.1111/1759-7714.12910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Argiles JM, Lopez‐Soriano FJ, Busquets S. Mechanisms and treatment of cancer cachexia. Nutr Metab Cardiovasc Dis. 2013;23(Suppl 1):S19–24. [DOI] [PubMed] [Google Scholar]
- 21. Sjoblom B, Gronberg BH, Wentzel‐Larsen T, et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non‐small cell lung cancer. Clin Nutr. 2016;35:1386–93. 10.1016/j.clnu.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 22. Katakami N, Uchino J, Yokoyama T, Naito T, Kondo M, Yamada K, et al. Anamorelin (ONO‐7643) for the treatment of patients with non‐small cell lung cancer and cachexia: results from a randomized, double‐blind, placebo‐controlled, multicenter study of Japanese patients (ONO‐7643‐04). Cancer. 2018;124:606–16. 10.1002/cncr.31128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yazdani Z, Rafiei A, Golpour M, Zafari P, Moonesi M, Ghaffari S. IL‐35, a double‐edged sword in cancer. J Cell Biochem. 2020;121:2064–76. 10.1002/jcb.29441 [DOI] [PubMed] [Google Scholar]
- 24. Fujita H, Soyka MB, Akdis M, Akdis CA. Mechanisms of allergen‐specific immunotherapy. Clin Transl Allergy. 2012;2:2. 10.1186/2045-7022-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


