Abstract
Background
Local consolidative therapy (LCT) has emerged as a treatment option in patients with oligometastatic non‐small cell lung cancer (NSCLC) undergoing chemotherapy or targeted therapy. However, the current literature lacks evidence as to whether LCT improves survival in NSCLC patients receiving immunotherapy. Our study aimed to assess whether LCT combined with pembrolizumab ± chemotherapy could improve the survival of patients with synchronous oligometastatic NSCLC.
Methods
Patients with NSCLC, without EGFR or ALK genetic aberrations, who were treated with first‐line pembrolizumab ± chemotherapy, were included in the study. Survival analysis of the LCT and non‐LCT groups was compared.
Results
A total of 231 patients were included in the study. The median follow‐up time was 15.24 months. Median progression‐free survival (PFS) and overall survival (OS) of the entire cohort were 12.00 and 23.43 months, respectively. Of the 231 patients included, 76 patients received LCT combined with pembrolizumab ± chemotherapy (LCT group) while 155 patients received pembrolizumab ± chemotherapy alone (non‐LCT group). Of note, the PFS of the LCT and non‐LCT groups was 13.97 and 10.08 months (p = 0.016), respectively. The OS were 30.67 and 21.97 months (p = 0.011), respectively. The PFS and OS were significantly improved with LCT for patients with brain or lung metastases but not bone metastases. No significant increase in treatment‐related toxicity was observed in the LCT group.
Conclusions
The present study shows that LCT to metastatic sites is an option for consideration in patients with synchronous oligometastatic NSCLC during first‐line pembrolizumab treatment, with significantly improved PFS and OS compared with systemic treatment alone.
Keywords: local consolidative therapy, non‐small cell lung cancer, pembrolizumab
LCT combined with pembrolizumab ± chemotherapy provides better PFS and OS. Patients who receive LCT only to part lesions could also benefit from LCT

INTRODUCTION
Non–small cell lung cancer (NSCLC) accounts for 85% of lung cancer, and ~50% of NSCLC patients presenting with metastatic disease (TNM stage IV) at diagnosis. 1 , 2 Oligometastatic NSCLC refers to an intermediate state between limited primary and polymetastatic NSCLC. 3 , 4 , 5 In addition, local consolidative therapy (LCT) strategies are frequently offered to patients diagnosed with oligometastatic disease. 6 Although different study protocols including patient selection (e.g., patients with epidermal growth factor receptor [EGFR] mutation or without mutation), disease status (e.g., synchronous diseases and metachronous diseases) and definition of oligometastatic metastases (e.g., up to three or five metastases) were used in different trials, they have consistently demonstrated that LCT provided to metastatic sites in oligometastatic NSCLC patients led to significant survival benefits compared to systemic therapy alone. 7 , 8 , 9 , 10 , 11 , 12
In recent years, pembrolizumab, an anti–programmed death‐1 (PD‐1) monoclonal antibody, has dramatically altered treatment strategies especially first‐line therapy for patients with advanced NSCLC without targetable EGFR or anaplastic lymphoma kinase (ALK) genetic aberrations. 13 , 14 The US Food and Drug Administration (FDA) approved pembrolizumab for use in NSCLC patients based on the outstanding outcomes of relevant clinical trials. 15 , 16 However, these clinical trials have not shed light on whether patients with oligometastatic NSCLC should be exposed to LCT. In clinical practice, there is a highly controversial treatment option for treatment‐naïve patients without targetable EGFR or ALK genetic aberrations diagnosed with oligometastatic NSCLC; that is, standard systemic treatment (pembrolizumab alone or combined with platinum‐based chemotherapy [pembrolizumab ± chemotherapy]) in combination with LCT. A single‐arm clinical trial has revealed that pembrolizumab after LCT for oligometastatic NSCLC led to a dramatic improvement in PFS and an immature but promising OS compared with historical data. However, the trial had some limitations, including bias in single‐arm design (no pembrolizumab control group alone was designed for direct comparison), bias in patient selection (only patients who had completed LAT to all sites of tumor were included in the trial), bias in previous treatment (most patients were heavily‐treated and had received radiotherapy before enrollment) and the limitation of small sample size (N = 45). Therefore, more studies are needed to provide stronger evidence, in order to provide a more tailored therapy in the future.
Herein, we collected the clinical data of patients with synchronous oligometastatic NSCLC without targetable EGFR or ALK genetic aberrations and compared the efficacy of LCT plus pembrolizumab ± chemotherapy with pembrolizumab ± chemotherapy alone as first‐line therapy in a specific population.
METHODS
Patients
The medical records of NSCLC patients without targetable EGFR or ALK genetic aberrations treated with first‐line pembrolizumab ± chemotherapy at the Shanghai Chest Hospital between March 1, 2015 and December 30, 2020 were screened. The inclusion criteria were as follows: (i) Stage IV NSCLC patients with synchronous oligometastases. Synchronous oligometastatic NSCLC was defined as NSCLC with 1–5 metastases in 1–3 organs, and all metastases were detected at the time of diagnosis of the primary tumor. 3 , 4 , 17 , 18 The status of oligometastasis was evaluated by 18F‐fluorodeoxyglucose‐positron emission tomography (18F‐FDG‐PET‐CT) and brain magnetic resonance imaging (MRI) at the time of initial staging.3 (ii) Patients without EGFR/ALK sensitive mutation. (iii) First‐line treatment with pembrolizumab ± chemotherapy following standard treatment guidelines. (iv) The interval between pembrolizumab ± chemotherapy and LCT should not exceed 3 months.19 (v) Eastern Cooperative Oncology Group performance status (ECOG PS) 0–1. The exclusion criteria were as follows: (i) Patients with metachronous oligometastatic NSCLC (metastasis developed during follow‐up); (ii) incorporation of LCT after disease progression and (iii) patients with PFS≤2 months were excluded due to large selection bias. The patient selection procedure is shown in Figure S1. This study was approved by the Institutional Review Board of Shanghai Chest Hospital and performed following the declaration of Helsinki.
Treatment and clinical response evaluation
Drugs were administered following standard NCCN guidelines. Pembrolizumab was administered 200 mg intravenously every 3 weeks. Patients with adenocarcinoma received AC (pemetrexed plus carboplatin for four cycles and pemetrexed as maintenance) and patients with squamous NSCLC received TC (nab‐paclitaxel plus carboplatin for four cycles and nab‐paclitaxel as maintenance). LCT, including surgery (local resection of oligometastases) and radiotherapy (stereotactic body radiotherapy, stereotactic radiosurgery) was performed in all patients with metastases under evaluation of the physician. Staging of the disease was determined using the eighth edition of the American Joint Committee on Cancer (AJCC) tumor‐node‐metastasis (TNM) classification. Enhanced chest computed tomography (CT) scan and abdominal ultrasound scan were performed every 4 weeks for therapeutic response evaluation. Enhanced brain magnetic resonance imaging (MRI) was performed every 4–6 weeks if there was evidence of existing brain metastasis and 4–6 months if there was no baseline lesion and no symptoms thereafter. Efficacy was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Detection of gene and programmed death ligand 1 (PD‐L1) tumor proportion score (TPS)
Biopsy of tissue samples was performed at the time of initial diagnosis. The amplification refractory mutation system (ARMS) or next‐generation sequencing (NGS) was used for EGFR detection. Immunohistochemistry (IHC) and break‐apart fluorescence in situ hybridization (FISH) were used for ALK rearrangement detection. PD‐L1 TPS was detected by the PD‐L1 IHC 22C3 pharmDx assay and was classified into TPS < 0%, 1%–49% and ≥50%.
Statistical analysis
The categorical variables were compared by χ2 test. The endpoints were PFS (calculated from disease diagnosis to disease progression or the last follow‐up), ORR (the ratio of complete and partial response) and OS (from disease diagnosis to death or the last follow‐up). The median PFS and OS was estimated using the Kaplan–Meier method and compared by the log‐rank test. Hazard ratio (HR) and 95% confidence intervals were estimated by a stratified Cox proportional‐hazards model. All statistical analyses were performed using SPSS version 22.0 (IBM Corporation).
RESULTS
Patient characteristics
A total of 231 patients with oligometastatic NSCLC who met the inclusion criteria were included in the study. The patient characteristics are detailed in Table 1. The majority of patients were male (201/231, 87%) and smokers (160/231, 69.3%). A total of 45.9% of patients had T1‐2 stage, 48.1% had N3 stage, 60.6% had histology of adenocarcinoma. The majority of patients who received PD‐L1 detection had a tumor proportion score of 50% or higher. A total of 84.4% (195/231) patients received combination therapy (pembrolizumab + chemotherapy) and (15.6%, 36/231) received pembrolizumab monotherapy. With respect to the number of oligometastatic lesions in the 231 patients, 219 (94.8%) had 1–3 metastatic lesions, while 12 (5.2%) had 4–5 metastatic lesions. With regard to the oligometastatic sites, 118 (51.1%) patients had metastatic lesions involving multiple organs. In patients with only a single organ involved, the most common site was lung, followed by bone and brain. Overall, 31 patients received LCT to a primary lung tumor, 32 to brain metastasis, 28 to contralateral lung,12 to bone metastasis, three to chest wall metastasis, one to cervical lymph nodes and one to adrenal gland. Treatment details are reported in Table 1 and Figure S2.
TABLE 1.
Clinical characteristics of 231 patients
| Characteristics | No. of patients (%) |
|---|---|
| Median (range), years | 63 (29–82) |
| Age (years) | |
| <65 | 130 (56.3) |
| ≥65 | 101 (43.7) |
| Sex | |
| Male | 201 (87) |
| Female | 30 (13) |
| Smoking history | |
| No | 71 (30.7) |
| Yes | 160 (69.3) |
| Recurrence after surgery | |
| No | 195 (84.4) |
| Yes | 36 (15.6) |
| T stage | |
| T 1–2 | 106 (45.9) |
| T 3–4 | 94 (40.7) |
| Unavailable | 31 (13.4) |
| N stage | |
| N0‐N2 | 101 (43.7) |
| N3 | 111 (48.1) |
| Unavailable | 19 (8.2) |
| Number of metastatic lesions | |
| 1–3 | 219 (94.8) |
| 4–5 | 12 (5.2) |
| Metastatic organs | |
| Brain only | 22 (9.5) |
| Lung only | 55 (23.8) |
| Bone only | 32 (13.9) |
| Other single organ | 4 (1.7) |
| Two or more organs | 118 (51.1) |
| Pathology | |
| Adenocarcinoma | 140 (60.6) |
| Squamous cell carcinoma | 91 (39.4) |
| PD‐L1 TPS (%) | |
| 0 | 47 (20.3) |
| 1%–49% | 36 (15.6) |
| ≥50% | 64 (27.7) |
| Unavailable | 84 (36.4) |
| Treatment | |
| Pembrolizumab + chemotherapy | 195 (84.4) |
| Pembrolizumab | 36 (15.6) |
| LCT for primary tumor | 31 |
| Radiotherapy | 29 (93.5) |
| Surgery | 2 (6.5) |
| LAT for oligometastasis | 77 |
| Brain | 32 |
| SRS | 9 (28.1) |
| Whole brain irradiation | 22 (68.8) |
| Surgery | 1 (3.1) |
| Contralateral lung | 28 |
| Radiotherapy | 20 (71.4) |
| Surgery | 6 (21.4) |
| Radio frequency ablation | 2 (7.2) |
| Bone | 12 |
| Radiotherapy | 12 (100) |
| Chest wall | 3 |
| Radiotherapy | 3 (100) |
| Cervical lymph nodes | 1 |
| Radiotherapy | 1 (100) |
| Adrenal gland | 1 |
| Surgery | 1 (100) |
In brief, a total of 32.9% (76/231) patients received systemic therapy and LCT, while 67.1% (155/231) patients received systemic therapy without LCT. The baseline characteristics were well balanced between the two groups except for the slight difference in T stage (p = 0.047). The baseline characteristics of the LCT and non‐LCT groups are shown in Table 2. After disease progression, 28 (64%) of 44 patients in the LCT group and 68 (71%) of 96 in the non‐LCT group received subsequent treatment, most of which were chemotherapy and antiangiogenic therapy.
TABLE 2.
Clinical characteristics of local consolidative therapy (LCT) group and non‐LCT group
| Characteristics | LCT (n = 76)No. (%) | Non‐LCT (n = 155)No. (%) | p‐value |
|---|---|---|---|
| Age (years) | 0.729 | ||
| <65 | 44 (57.9) | 86 (55.5) | |
| ≥65 | 32 (42.1) | 69 (44.5) | |
| Sex | 0.107 | ||
| Male | 70 (92.1) | 131 (84.5) | |
| Female | 6 (7.9) | 24 (15.5) | |
| Smoking history | 0.308 | ||
| No | 20 (26.3) | 51 (32.9) | |
| Yes | 56 (73.7) | 104 (67.1) | |
| Recurrence after surgery | 0.405 | ||
| No | 62 (81.6) | 133 (85.8) | |
| Yes | 14 (18.4) | 22 (14.2) | |
| T stage | 0.047 | ||
| T 1–2 | 34 (44.7) | 72 (46.4) | |
| T 3–4 | 26 (34.2) | 68 (43.9) | |
| Unavailable | 16 (21.1) | 15 (9.7) | |
| N stage | 0.612 | ||
| N0–N2 | 36 (47.4) | 65 (41.9) | |
| N3 | 33 (43.4) | 78 (50.3) | |
| Unavailable | 7 (9.2) | 12 (7.8) | |
| Number of metastatic lesions | 0.728 | ||
| 1–3 | 71 (93.4) | 148 (95.5) | |
| 4–5 | 5 (6.6) | 7 (4.5) | |
| Pathology | 0.761 | ||
| Adenocarcinoma | 45 (59.2) | 95 (61.3) | |
| Squamous cell carcinoma | 31 (40.8) | 60 (38.7) | |
| PD‐L1 TPS (%) | 0.981 | ||
| 0 | 15 (19.7) | 32 (20.7) | |
| 1%–49% | 11 (14.5) | 25 (16.1) | |
| ≥50% | 22 (29) | 42 (27.1) | |
| Unavailable | 28 (36.8) | 56 (36.1) | |
| Treatment | 0.272 | ||
| Pembrolizumab + chemotherapy | 67 (88.2) | 128 (82.6) | |
| Pembrolizumab | 9 (11.8) | 27 (17.4) |
Survival analysis of the overall population
The median follow‐up time was 15.24 months (range: 2.10–73.30 months). For the entire cohort, the median PFS was 12.00 months (95% CI: 10.17–13.83) and median OS was 23.43 months (95% CI: 20.04–26.82) (Figure S3). Univariate analysis identified LCT treatment (HR: 0.45, 95% CI: 0.64–0.92, p = 0.016) and 1–3 metastatic lesions (HR: 0.22, 95% CI: 0.44–0.88, p = 0.020) as being significantly associated with better PFS. Multivariate analysis further revealed LCT treatment (HR: 0.45, 95% CI: 0.65–0.93, p = 0.020) and 1–3 metastatic lesions (HR: 0.23, 95% CI: 0.45–0.9, p = 0.020) as independent predictive factors for better PFS. With regard to overall survival, univariate analysis identified LCT treatment (HR: 0.32, 95%CI: 0.53–0.87, p = 0.012), 1–3 metastatic lesions (HR: 0.19, 95%CI: 0.39–0.82, p = 0.012), PD‐L1 1–49% (HR: 0.19, 95% CI: 0.41–0.9, p = 0.025) and PD‐L1 ≥ 50% (HR: 0.22, 95% CI: 0.41–0.76, p = 0.005) as being significantly associated with better OS. Multivariate analysis further confirmed the LCT treatment (HR: 0.32, 95% CI: 0.53–0.87, p = 0.013), 1–3 metastatic lesions (HR: 0.17, 95% CI: 0.35–0.74, p = 0.006), PD‐L1 1–49% (HR: 0.18, 95% CI: 0.38–0.84, p = 0.016) and PD‐L1 ≥ 50% (HR: 0.2, 95% CI: 0.38–0.71, p = 0.003) as independent predictive factors for better OS (Table 3).
TABLE 3.
Univariable and multivariable analysis of covariables associated with progression‐free survival (PFS) and overall survival (OS)
| Characteristics | Category | Progression‐free survival (n = 231) | Overall survival (n = 231) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||
| Hazard ratio | p‐value | Hazard ratio | p‐value | Hazard ratio | p‐value | Hazard ratio | p‐value | ||
| LCT | Yes vs. no | 0.45 (0.64–0.92) | 0.016 | 0.45 (0.65–0.93) | 0.020 | 0.32 (0.53–0.87) | 0.012 | 0.32 (0.53–0.87) | 0.013 |
| Age | <65 vs. >65 years | 0.63 (0.87–1.22) | 0.426 | 0.55 (0.84–1.29) | 0.433 | ||||
| Sex | Male vs. female | 0.46 (0.72–1.13) | 0.152 | 0.62 (1.17–2.21) | 0.631 | ||||
| Smoking history | Yes vs. no | 0.56 (0.79–1.12) | 0.182 | 0.82 (1.3–2.07) | 0.272 | ||||
| Recurrence after surgery | Yes vs. no | 0.54 (0.85–1.36) | 0.508 | 0.56 (0.98–1.71) | 0.934 | ||||
| T stage | T3‐4 versus T1‐2 | 0.68 (0.97–1.39) | 0.862 | 0.68 (1.07–1.69) | 0.755 | ||||
| Unavailable vs. T1‐2 | 0.56 (0.95–1.6) | 0.849 | 0.35 (0.73–1.5) | 0.387 | |||||
| N stage | N3 vs. N0‐2 | 0.57 (0.89–1.38) | 0.920 | 0.62 (0.98–1.53) | 0.920 | ||||
| Unavailable vs. N0‐2 | 0.45 (1–2.23) | 0.262 | 0.32 (0.66–1.37) | 0.262 | |||||
| Number of metastatic lesions | 1–3 vs. 4–5 | 0.22 (0.44–0.88) | 0.020 | 0.23 (0.45–0.9) | 0.020 | 0.19 (0.39–0.82) | 0.012 | 0.17 (0.35–0.74) | 0.006 |
| Pathology | Adenocarcinoma vs. squamous cell carcinoma | 0.58 (0.82–1.14) | 0.238 | 0.53 (0.81–1.25) | 0.344 | ||||
| PD‐L1 TPS (%) | 1%–49% vs. 0 | 0.41 (0.72–1.25) | 0.237 | 0.19 (0.41–0.9) | 0.025 | 0.18 (0.38–0.84) | 0.016 | ||
| ≥50% vs. 0 | 0.4 (0.65–1.06) | 0.083 | 0.22 (0.41–0.76) | 0.005 | 0.2 (0.38–0.71) | 0.003 | |||
| Unavailable vs. 0 | 0.46 (0.72–1.14) | 0.162 | 0.38 (0.65–1.11) | 0.115 | 0.38 (0.65–1.1) | 0.110 | |||
| Treatment | P + C vs. P | 0.53 (0.82–1.27) | 0.371 | 0.4 (0.69–1.18) | 0.173 | ||||
Survival analysis of the LCT and non‐LCT groups
Progression events occurred in 44 of 76 (57.9%) patients in the LCT group and 96 of 155 (61.9%) patients in the non‐LCT group. The pattern of progression of the two groups are shown in Figure S4. The proportion of progression in both local regional and distant sites was slightly higher in the non‐LCT compared with the LCT group. Median PFS was 13.97 months (95% CI: 12.04–15.89) in the LCT arm versus 10.08 months (95% CI: 7.90–12.27) in the non‐LCT arm (p = 0.016) (Figure 1). Progression‐survival free rates differed at different time points between the two groups (Figure S5A). For example, the PFS rate at 12 months was 62.0% in the LCT arm and 43.4% in the non‐LCT arm.
FIGURE 1.
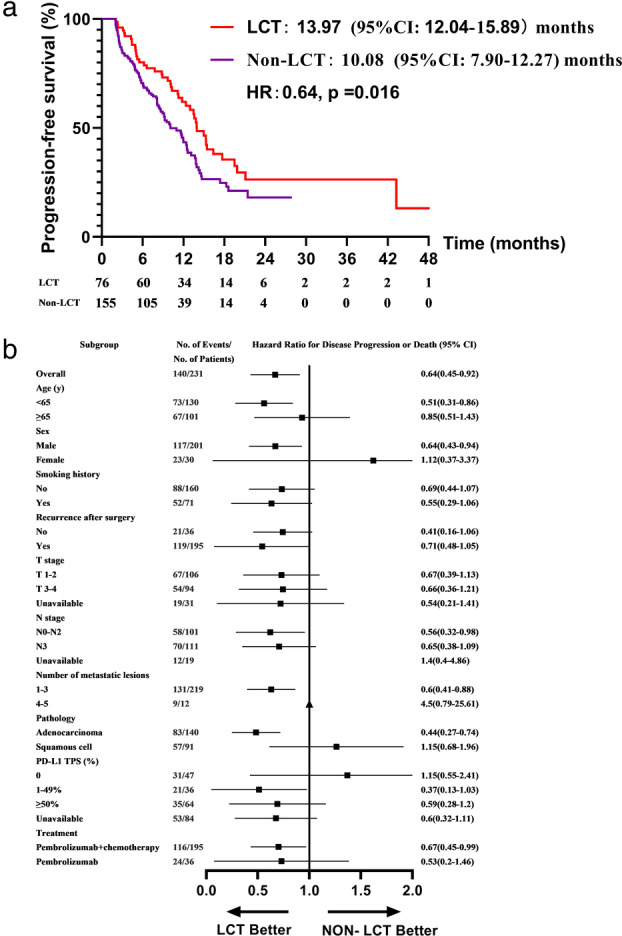
Kaplan–Meier estimates of progression‐free survival in the LCT and non‐LCT groups (a) and an analysis of progression‐free survival in key subgroups (b). LCT, local consolidative therapy
Death events occurred in 24 of 76 (31.6%) patients in the LCT group and 62 of 155 (40%) patients in the non‐LCT group. Median OS was 30.67 months (95% CI: 22.20–39.14) in the LCT arm versus 21.97 months (95% CI: 18.64–25.30) in the non‐LCT arm (p = 0.011) (Figure 2). Survival rates also differed at different time points between the two groups (Figure S5B). For example, the OS rates of the LCT and non‐LCT arms at 12 months were 83.9 and 76.4%, the 24 month OS rates were 60.3% and 37.0%, respectively.
FIGURE 2.
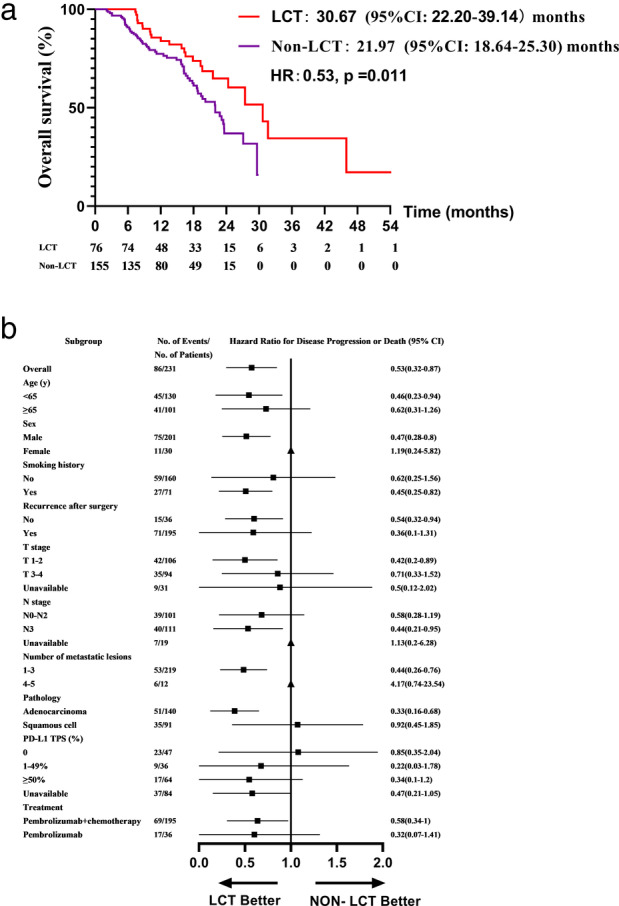
Kaplan–Meier estimates of overall survival in the LCT and non‐LCT groups (a) and an analysis of overall survival in key subgroups (b). LCT, local consolidative therapy
With regard to response rate, 42 patients (55.3%) in the LCT group and 70 patients (45.2%) in the non‐LCT group had an objective response (ORR). The change from baseline in the sum of the longest diameters of target lesions is shown in Figure S6.
To further clarify the beneficial populations from LCT, we subdivided patients by metastatic sites. The PFS and OS were significantly improved by LCT in patients with brain metastases (Figure S7C, D) and lung metastases (Figure S7E, F). However, there was no significant difference in patients with bone metastases (Figure S7G, H). In addition, LCT for primary tumor also failed to improve survival compared with others.
Survival analysis of the all‐LCT, part‐LCT and non‐LCT groups
To better understand the effect of the LCT on survival, we subdivided patients to all‐LCT group (patients received LCT to both primary tumor and all oligometastatic sites, n = 24), part‐LCT group (patients received LCT but not to all lesions, n = 52) and non‐LCT (patients not received LCT, n = 155). The clinical characteristics were well balanced between the three groups, except that patients in the all‐LCT group were more likely to be T3–4 stage (Table 4). The PFS in the all‐LAT, part‐LAT, and non‐LAT groups was 16.40 months (95% CI: 11.76–20.04), 13.6 months (95% CI: 10.86–16.34), and 10.08 months (95% CI: 7.90–12.27), respectively (p (All‐LCT vs. Non‐LCT) = 0.031) (Figure 3a). The OS was 31.63 months (95% CI: 17.25–46.01), 27.43 months (95% CI: 20.56–34.30), and 21.97 months (95% CI: 18.64–25.30), respectively (p (All‐LCT vs. Non‐LCT) = 0.025) (Figure 3b).
TABLE 4.
Clinical characteristics of all‐LCT, part‐LCT and non‐LCT groups
| Characteristics | All‐LCT (n = 24 (%) | Part‐LCT (n = 52 (%) | Non‐LCT (n = 155 (%) | p‐value |
|---|---|---|---|---|
| Age (years) | 0.604 | |||
| <65 | 12 (50) | 32 (61.5) | 86 (55.5) | |
| ≥65 | 12 (50) | 20 (38.5) | 69 (44.5) | |
| Sex | 0.220 | |||
| Male | 23 (95.8) | 47 (90.4) | 131 (84.5) | |
| Female | 1 (4.2) | 5 (9.6) | 24 (15.5) | |
| Smoking history | 0.464 | |||
| Yes | 5 (20.8) | 15 (28.8) | 51 (32.9) | |
| No | 19 (79.2) | 37 (71.2) | 104 (67.1) | |
| Recurrence after surgery | 0.667 | |||
| Yes | 19 (79.2) | 43 (82.7) | 133 (85.8) | |
| No | 5 (20.8) | 9 (17.3) | 22 (14.2) | |
| T stage | 0.016 | |||
| T 1–2 | 6 (25) | 28 (53.8) | 72 (46.5) | |
| T 3–4 | 10 (41.7) | 16 (30.8) | 68 (43.9) | |
| Unavailable | 8 (33.3) | 8 (15.4) | 15 (9.7) | |
| N stage | 0.615 | |||
| N0‐N2 | 9 (37.5) | 27 (51.9) | 65 (41.9) | |
| N3 | 13 (54.2) | 20 (38.5) | 78 (50.3) | |
| Unavailable | 2 (8.3) | 5 (9.6) | 12 (7.7) | |
| Number of metastatic lesions | 0.112 | |||
| 1–3 | 24 (100) | 47 (90.4) | 148 (95.5) | |
| 4–5 | 0 (0) | 5 (9.6) | 7 (4.5) | |
| Pathology | 0.792 | |||
| Adenocarcinoma | 13 (54.2) | 32 (61.5) | 95 (61.3) | |
| Squamous cell carcinoma | 11 (45.8) | 20 (38.5) | 60 (38.7) | |
| PD‐L1 TPS (%) | ||||
| 0 | 6 (25) | 9 (17.3) | 32 (20.6) | |
| 1%–49% | 3 (12.5) | 8 (15.4) | 25 (16.1) | |
| ≥50% | 6 (25) | 16 (30.8) | 42 (27.1) | |
| Unavailable | 9 (37.5) | 19 (36.5) | 56 (36.1) | |
| Treatment | 0.167 | |||
| Pembrolizumab + chemotherapy | 23 (95.8) | 44 (84.6) | 128 (82.6) | |
| Pembrolizumab | 1 (4.2) | 8 (15.4) | 27 (17.4) |
FIGURE 3.
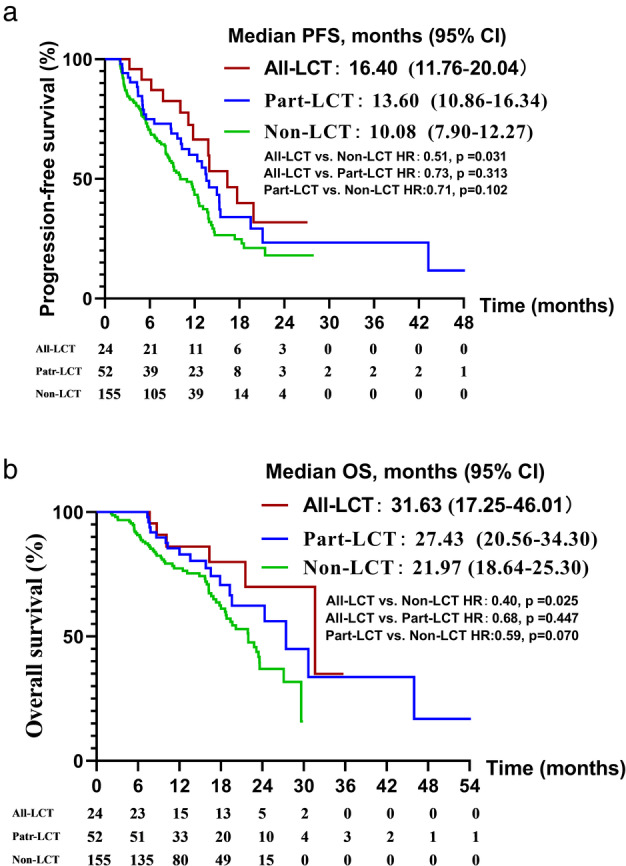
Progression‐free survival (a) and overall survival (b) in the all‐LCT, part‐LCT and non‐LCT groups. LCT, local consolidative therapy
Adverse events
The most common adverse events reported were nausea, diarrhea, neutropenia, skin rash and fatigue. The majority of toxicities were grade 1 to 2. Adverse events of grade 3 or higher included neutropenia, abnormal liver function, pneumonitis, rash, and thyroid dysfunction. There was no significant difference in adverse events between the two groups. A total of 3% of patients discontinued immunotherapy due to pneumonitis. Rates of grade 3 or higher pneumonitis were slightly higher in the LCT group versus the non‐LCT group (7.89% vs. 3.87%) but were not statistically significant (p = 0.350). Eighty‐three percent of patients with grade 3 or higher pneumonitis received corticosteroid therapy with a median duration of treatment of 18 days. No grade 5 toxicity was recorded.
DISCUSSION
To the best of our knowledge, this retrospective study is the first large‐scale study to demonstrate the efficacy of LCT in patients with synchronous oligometastatic NSCLC who received pembrolizumab as first‐line treatment. In this report, the PFS and OS were increased in the LCT group compared with the non‐LCT group, but toxicity was not significantly increased. Among the non‐LCT group of 155 patients in our study, the median PFS and OS was 10.08 and 21.97 months, similar to the KEYNOTE‐189 and KEYNOTE‐407 studies. The addition of LCT reduced the risk of disease progression by 39% and reduced the risk of death by 47% in our study.
At present, the benefits of local therapy combined with targeted therapy or chemotherapy in patients with oligometastatic NSCLC have been confirmed by many studies, including randomized clinical trials and retrospective studies. 10 , 20 , 21 , 22 , 23 However, studies on the combination of local therapy and immunotherapy are still scarce, and should urgently be further explored in the era of immunotherapy.
Narek et al. revealed that previous radiotherapy in patients of KEYNOTE001 resulted in better PFS and OS outcomes with pembrolizumab treatment than that seen in patients who had not had previous radiotherapy, with no reduction in quality of life. These interesting results propose a new hypothesis of the potential of radiotherapy to convert immunotherapy nonresponders to immunotherapy responders. 24 The PACIFIC trial has also partially confirmed this hypothesis by reporting on the long‐term survival benefit of immunotherapy after chemoradiotherapy in patients with stage III NSCLC. 25 Recently, a single arm phase II clinical trial aimed to evaluate whether the addition of pembrolizumab after locally ablative therapy (completed LAT to all known sites of disease) improved outcomes for patients with oligometastatic NSCLC (≤4 metastatic sites) regardless of their prior treatment. 26 Of interest, they found that PFS and OS from the start of LAT were 18.7 and 41.6 months for oligometastatic NSCLC, which is nearly four times greater than the PFS (4 months) and OS (12.7 months) of KEYNOTE010. 27 In addition, PEMBRO‐RT, a randomized phase II study, demonstrated the augmenting effect of SBRT on the response to pembrolizumab in patients with metastatic NSCLC regardless of their prior treatment. 28 The ORR of the experimental group (SBRT + pembrolizumab) was significantly improved compared with the control group (pembrolizumab alone). In addition, the PFS (6.6 vs. 1.9 months, p = 0.19) and OS (15.9 vs. 7.6 months, p = 0.16) were dramatically prolonged in the experimental group but there was no significant difference, which may have been due to the small sample size. However, the MDACC trial evaluated the efficacy of pembrolizumab in combination with concurrent radiotherapy for NSCLC with lung and liver lesions. However, they found no differences in PFS between the groups and the OS results in this study are not yet mature. 29 However, a pooled analysis including PEMBRO‐RT and MDACC trials found that the addition of radiotherapy to pembrolizumab immunotherapy significantly increased responses and outcomes. 30 In addition, a meta‐analysis described a trend towards achieving higher ORR and longer survival in the ICI‐SABR combination group than SABR alone. The above studies are of great significance, but undeniable biases exist, such as no restriction on the previous treatment, and small sample size which resulted in a limited guiding effect on precision therapy. Therefore, in this study we focused on patients with synchronous oligometastatic NSCLC who received pembrolizumab as first‐line therapy and found that LCT combined with pembrolizumab might have a synergistic effect on survival in the specific population with no increase in toxicity. The safety profile observed in our study was consistent with previous pembrolizumab studies combined with LCT treatment for patients with advanced NSCLC. 26 , 28
Few studies have revealed the efficacy of LCT by specific sites of metastases. Here, we reported for the first time that patients with brain and pulmonary metastases receiving immunotherapy had survival benefit from LCT but patients with bone metastases do not, which might help to identify the beneficial populations from LCT. To better understand the effect of the LCT on survival, patients were subdivided into all‐LCT, part‐LCT and non‐LCT groups. The present study showed that LCT to all lesions could significantly reduce the risk of disease progression and death compared with non‐LCT, which was consistent with a previous study on patients with EGFR mutation. 22 LCT to part lesions failed to improve PFS compared with non‐LCT. However, part‐LCT failed to improve PFS compared with non‐LCT. OS was greater in the part‐LCT (27.43 months) than in the non‐LCT group (21.97 months) although the difference was not statistically significant (HR, 0.59; p = 0.070), which might be due to the small sample size. To the best of our knowledge, most clinical trials have only included patients who received LCT to all target lesions. Therefore, outcomes of clinical trials have not shed light on whether patients who receive LCT to only part lesions could improve survival. Here, our study demonstrates for the first time that there are survival benefits for pembrolizumab‐treated patients who receive LCT only to part lesions.
Recently, more and more studies have suggested that radiation therapy could stimulate an immune response, increase the production of neoantigens and transform nonimmunogenic tumors (cold tumors) into highly‐immunogenic tumors (hot tumors), known as the abscopal effect. 31 , 32 In addition, the abscopal effect may lead to a survival benefit of the combination of radiotherapy and immunotherapy. However, the mechanism of the abscopal effect remains unclear and needs further study. Many studies have confirmed that the response rate of pembrolizumab‐treated patients with advanced NSCLC is dependent on the PD‐L1 expression level. 16 , 26 , 28 , 33 Consistent with their findings, our study also showed a trend toward improved outcomes in patients with high PD‐L1 expression. In addition to PD‐L1 expression level, we found that the number of metastatic lesions was an independent prognostic factor for survival. The more lesions involved, the heavier the tumor burden, which may account for poor survival.
To our knowledge, our study is the largest of this research topic. However, there are some limitations. First, it was a single center research with a relatively short median follow‐up of 15.24 months. Second, selection bias existence was inevitable because of the retrospective nature of the studies. For example, patients with rapidly progressing disease were not appropriate candidates for local therapy. Thus, patients with PFS ≤2 months were excluded to reduce selection bias. In addition, the baseline clinical characteristics of patients were balanced well between groups, indicating that no large selection bias existed. Third, the details of local treatment were not available, such as the operative type, radiation dose, etc.
In conclusion, the present study highlights the potential role of LCT in patients with synchronous oligometastatic NSCLC during first‐line pembrolizumab treatment, with significant improvement in PFS and OS compared to systemic treatment alone.
CONFLICT OF INTEREST
All authors have completed the ICMJE uniform disclosure form. The authors have no conflicts of interest to declare.
Supporting information
Figure S1 Patient selection flow‐chart.
Figure S2 Treatment details of different organs.
Figure S3 Progression‐free survival (A)and overall survival (B) of the entire cohort.
Figure S4 The different patterns of progression between the LCT and non‐LCT groups. LCT: local consolidative therapy.
Figure S5 Progression‐free survival rate (A) and overall survival rate (B) in the LCT and non‐LCT groups. LCT: local consolidative therapy.
Figure S6 The objective response rate is shown as a percent change of target lesions from baseline in LCT and non‐LCT groups (A) and histograms showing the response rate (B).
Figure S7 The PFS by consolidative LCT sites. (A) Primary tumor. (C) Brain metastases. (E) Lung metastases. (G) Bone metastases. The OS by consolidative LAT sites. (B) Primary tumor. (D) Brain metastases. (F) Lung metastases. (H) Bone metastases. PFS: progression free survival; LCT: local consolidative therapy; OS: overall survival.
ACKNOWLEDGMENTS
The authors thank all the patients and their families for their contributions to this study.
Chen Y, Wang Y, Yang Z, Hu M, Lu J, Zhang Y, et al. Local consolidative therapy for synchronous oligometastatic non‐small cell lung cancer treated with first‐line pembrolizumab: A retrospective observational study. Thorac Cancer. 2022;13:732–741. 10.1111/1759-7714.14312
Ya Chen, Yanan Wang and Zhengyu Yang share first authorship.
Baohui Han, Wei Zhang and Kai Wang contribute equally to the research as corresponding authors.
Funding information The program of system biomedicine innovation center from Shanghai Jiao Tong University, Grant/Award Number: 15ZH4009; Shanghai Jiao Tong University School of Medicine, Grant/Award Number: 15ZH1008; The foundation of Shanghai Chest Hospital, Grant/Award Number: YJXT20190102
Contributor Information
Kai Wang, Email: wkvet0406@163.com.
Wei Zhang, Email: zhwei2002@hotmail.com.
Baohui Han, Email: 18930858216@163.com.
REFERENCES
- 1. Walters S, Maringe C, Coleman MP, Peake MD, Butler J, Young N, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population‐based study, 2004–2007. Thorax. 2013;68(6):551–64. 10.1136/thoraxjnl-2012-202297 [DOI] [PubMed] [Google Scholar]
- 2. Testa U, Castelli G, Pelosi E. Lung cancers: molecular characterization, clonal heterogeneity and evolution, and cancer stem cells. Cancers (Basel). 2018;10(8):248. 10.3390/cancers10080248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dingemans AC, Hendriks LEL, Berghmans T, Levy A, Hasan B, Faivre‐Finn C, et al. Definition of synchronous oligometastatic non‐small cell lung cancer‐A consensus report. J Thorac Oncol. 2019;14(12):2109–19. 10.1016/j.jtho.2019.07.025 [DOI] [PubMed] [Google Scholar]
- 4. Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8(6):378–82. 10.1038/nrclinonc.2011.44 [DOI] [PubMed] [Google Scholar]
- 5. Zhou Y, Yu F, Zhao Y, Zeng Y, Yang X, Chu L, et al. A narrative review of evolving roles of radiotherapy in advanced non‐small cell lung cancer: from palliative care to active player. Translational lung cancer research. 2020;9(6):2479–93. 10.21037/tlcr-20-1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zeng Y, Ni J, Yu F, Zhou Y, Zhao Y, Li S, et al. The value of local consolidative therapy in Osimertinib‐treated non‐small cell lung cancer with oligo‐residual disease. Radiat Oncol. 2020;15(1):207. 10.1186/s13014-020-01651-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tibdewal A, Agarwal J, Mummudi N, Noronha V, Prabhash K, Patil V, et al. Protocol for a phase II randomised controlled trial of TKI alone versus TKI and local consolidative radiation therapy in patients with oncogene driver‐mutated oligometastatic non‐small cell lung cancer. BMJ Open. 2021;11(2):e041345. 10.1136/bmjopen-2020-041345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long‐term results of the SABR‐COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830–8. 10.1200/JCO.20.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitchell KG, Farooqi A, Ludmir EB, Corsini EM, Zhang J, Sepesi B, et al. Improved overall survival with comprehensive local consolidative therapy in synchronous oligometastatic non‐small‐cell lung cancer. Clin Lung Cancer. 2020;21(1):37–46.e7. 10.1016/j.cllc.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 10. Elamin YY, Gomez DR, Antonoff MB, Robichaux JP, Tran H, Shorter MK, et al. Local Consolidation Therapy (LCT) After First Line Tyrosine Kinase Inhibitor (TKI) for Patients With EGFR Mutant Metastatic Non‐small‐cell Lung Cancer (NSCLC). Clin Lung Cancer. 2019;20(1):43–7. 10.1016/j.cllc.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 11. Horner‐Rieber J, Bernhardt D, Blanck O, Duma M, Eich HT, Gerum S, et al. Long‐term follow‐up and patterns of recurrence of patients with oligometastatic nsclc treated with pulmonary SBRT. Clin Lung Cancer. 2019;20(6):e667–77. 10.1016/j.cllc.2019.06.024 [DOI] [PubMed] [Google Scholar]
- 12. Buglione M, Jereczek‐Fossa BA, Bonu ML, Franceschini D, Fodor A, Zanetti IB, et al. Radiosurgery and fractionated stereotactic radiotherapy in oligometastatic/oligoprogressive non‐small cell lung cancer patients: Results of a multi‐institutional series of 198 patients treated with "curative" intent. Lung Cancer. 2020;141:1–8. 10.1016/j.lungcan.2019.12.019 [DOI] [PubMed] [Google Scholar]
- 13. Ichihara E, Harada D, Inoue K, Sato K, Hosokawa S, Kishino D, et al. The impact of body mass index on the efficacy of anti‐PD‐1/PD‐L1 antibodies in patients with non‐small cell lung cancer. Lung Cancer. 2020;139:140–5. 10.1016/j.lungcan.2019.11.011 [DOI] [PubMed] [Google Scholar]
- 14. Liang H, Lin G, Wang W, Huang J, Yang Y, Lan Y, et al. Feasibility and safety of PD‐1/L1 inhibitors for non‐small cell lung cancer in front‐line treatment: a Bayesian network meta‐analysis. Translational lung cancer research. 2020;9(2):188–203. 10.21037/tlcr.2020.02.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. 10.1016/s0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 16. Gandhi L, Rodriguez‐Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378(22):2078–92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 17. Parikh RB, Cronin AM, Kozono DE, Oxnard GR, Mak RH, Jackman DM, et al. Definitive primary therapy in patients presenting with oligometastatic non‐small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;89(4):880–7. 10.1016/j.ijrobp.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 18. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre‐Finn C, et al. Metastatic non‐small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2018;29(Suppl 4):iv192–237. 10.1093/annonc/mdy275 [DOI] [PubMed] [Google Scholar]
- 19. Hu F, Xu J, Zhang B, Li C, Nie W, Gu P, et al. Efficacy of local consolidative therapy for oligometastatic lung adenocarcinoma patients harboring epidermal growth factor receptor mutations. Clin Lung Cancer. 2019;20(1):e81–90. 10.1016/j.cllc.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 20. Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative radiotherapy for limited metastatic non‐small‐cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4(1):e173501. 10.1001/jamaoncol.2017.3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu HA, Sima CS, Huang J, Solomon SB, Rimner A, Paik P, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR‐mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8(3):346–51. 10.1097/JTO.0b013e31827e1f83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu Q, Zhou F, Liu H, Jiang T, Li X, Xu Y, et al. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first‐line EGFR‐TKIs. J Thorac Oncol. 2018;13(9):1383–92. 10.1016/j.jtho.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 23. Palma DA, Olson R, Harrow S, Veruttipong D, Goldman JW, Formenti SC, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR‐COMET): a randomised, phase 2, open‐label trial. The Lancet. 2019;393(10185):2051–8. 10.1016/s0140-6736(18)32487-5 [DOI] [PubMed] [Google Scholar]
- 24. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non‐small‐cell lung cancer: a secondary analysis of the KEYNOTE‐001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903. 10.1016/S1470-2045(17)30380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Three‐year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC‐update from PACIFIC. J Thorac Oncol. 2019;15(2):288–293. 10.1016/j.jtho.2019.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bauml JM, Mick R, Ciunci C, Aggarwal C, Davis C, Evans T, et al. Pembrolizumab after completion of locally ablative therapy for oligometastatic non‐small cell lung cancer: a phase 2 trial. JAMA Oncol. 2019;5(9):1283–90. 10.1001/jamaoncol.2019.1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herbst RS, Baas P, Kim DW, Felip E, Pérez‐Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 28. Theelen W, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, JGJV A, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non‐small cell lung cancer: results of the PEMBRO‐RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1276–82. 10.1001/jamaoncol.2019.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Welsh J, Menon H, Chen D, Verma V, Tang C, Altan M, et al. Pembrolizumab with or without radiation therapy for metastatic non‐small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer. 2020;8(2):e001001. 10.1136/jitc-2020-001001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Theelen W, Chen D, Verma V, Hobbs BP, HMU P, JGJV A, et al. Pembrolizumab with or without radiotherapy for metastatic non‐small‐cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021;9(5):467–75. 10.1016/s2213-2600(20)30391-x [DOI] [PubMed] [Google Scholar]
- 31. Ko EC, Raben D, Formenti SC. The integration of radiotherapy with immunotherapy for the treatment of non‐small cell lung cancer. Clin Cancer Res. 2018;24(23):5792–806. 10.1158/1078-0432.CCR-17-3620 [DOI] [PubMed] [Google Scholar]
- 32. Frak M, Krawczyk P, Kalinka E, Milanowski J. Molecular and clinical premises for the combination therapy consisting of radiochemotherapy and immunotherapy in non‐small cell lung cancer patients. Cancers (Basel). 2021;13(6):1222. 10.3390/cancers13061222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paz‐Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A randomized, placebo‐controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol‐specified final analysis of KEYNOTE‐407. J Thorac Oncol. 2020;15(10):1657–69. 10.1016/j.jtho.2020.06.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Patient selection flow‐chart.
Figure S2 Treatment details of different organs.
Figure S3 Progression‐free survival (A)and overall survival (B) of the entire cohort.
Figure S4 The different patterns of progression between the LCT and non‐LCT groups. LCT: local consolidative therapy.
Figure S5 Progression‐free survival rate (A) and overall survival rate (B) in the LCT and non‐LCT groups. LCT: local consolidative therapy.
Figure S6 The objective response rate is shown as a percent change of target lesions from baseline in LCT and non‐LCT groups (A) and histograms showing the response rate (B).
Figure S7 The PFS by consolidative LCT sites. (A) Primary tumor. (C) Brain metastases. (E) Lung metastases. (G) Bone metastases. The OS by consolidative LAT sites. (B) Primary tumor. (D) Brain metastases. (F) Lung metastases. (H) Bone metastases. PFS: progression free survival; LCT: local consolidative therapy; OS: overall survival.


