Abstract
Background
Rotator cuff tears and glenoid loosening remain the two most common causes for revision after anatomic total shoulder arthroplasty. Oversizing of the humeral head leads to increased contact force across the glenohumeral joint and is hypothesized to contribute to clinical and radiographic failure. The purpose of this study is to compare the rate of radiographic overstuffing between standard short humeral heads and newer extra-short heads with decreased lateral offset.
Methods
Fifty-five consecutive anatomic total shoulder arthroplasties performed using extra-short humeral heads were retrospectively reviewed and compared with age- and sex-matched controls receiving standard short heads. A total of 110 postoperative radiographs were analyzed using the Iannotti's perfect circle method to compare the prosthesis' center of rotation (COR) with the native humeral head COR. A difference in the COR of >3.0 mm was considered malpositioned. Malpositioning medially was considered overstuffed, and malpositioning laterally was considered understuffed. The direction of displacement of malpositioned prostheses was categorized using a quadrant system. Furthermore, we used a novel method to evaluate medial and superior overstuffing by measuring the displacement between the anatomic and prosthetic head positions along perpendicular axes.
Results
Using the Iannotti's perfect circle method, 56% of heads were malpositioned. Overstuffing occurred more frequently with short heads compared with extra-short heads (47% vs. 4%, P < .001). Conversely, understuffing occurred more frequently with extra-short heads (47% vs. 15%, P = .001). Malpositioned extra-short heads were most frequently placed in the inferomedial quadrant (93% vs. 24%, P < .001), whereas malpositioned short heads were most commonly placed in the superomedial quadrant (56% vs. 7%, P < .001). Our novel measurement method demonstrated that extra-short heads reduced medial overstuffing (2.8 ± 2.8 mm vs. 0.3 ± 2.0 mm, P < .001). Both extra-short and short heads had similar rates of superior malpositioning (1.6 ± 2.2 mm vs. 1.4 ± 1.5 mm, P = .683).
Conclusion
Routine use of extra-short humeral head sizes reduces the rate of medial glenohumeral joint overstuffing but not superior malpositioning. This is hypothesized to improve clinical outcomes, but future studies are needed to assess the relationship between improved humeral head fit and clinical outcomes.
Keywords: Shoulder replacement, ATSA, Extra-short, Malposition, Radiograph, Loosening, Humeral head
The goal of anatomic total shoulder arthroplasty (ATSA) is to improve pain and function by replicating normal anatomy with prosthetic components. ATSA is generally successful, with good patient outcomes and quality of life improvements.4,9,12,14,21 Proper implant selection and placement are crucial to achieving these good outcomes. Inaccurate humeral head sizing and or positioning can lead to glenohumeral joint overstuffing, which has been associated with overtensioning of the shoulder musculature and soft tissues, reduced range of motion and strength, and increased glenohumeral joint reaction forces with subsequent glenoid-sided wear and loosening.13,20,22 Alolabi et al2 reported an overstuffing rate of 31%, with most of these occurring secondary to improper humeral head implant size selection.
Several manufacturers now offer an array of humeral head component sizes with varying head heights to allow surgeons to better recreate patients' native glenohumeral anatomy (Table I). Recently, extra-short humeral heads were designed to provide 2- to 3-mm less medial offset with the same humeral cut surface coverage. This is hypothesized to reduce the incidence of overstuffing and thereby better recreate the native center of rotation (COR), moment arms of the surrounding musculature, and soft tissue tensioning of the glenohumeral joint. In 2014, Alolabi et al2 described a method of assessing humeral head component size and positioning of the prosthesis COR relative to native anatomic landmarks after ATSA. This method, commonly referred to as “Iannotti's perfect circle” (IPC), uses a single Grashey radiograph and three preserved anatomic landmarks to assess joint overstuffing.
Table I.
Humeral head heights available for commonly used ATSA implant systems.
| Manufacturer | Implant system | Available head heights |
|---|---|---|
| Arthrex | Univers Apex System | 17-26 mm |
| DePuy Synthes | Global Advantage Shoulder Arthroplasty System | 15-21 mm |
| Global AP Shoulder Arthroplasty System | 15-21 mm | |
| Global Unite Anatomic Platform Shoulder System | 12-21 mm | |
| Exactech, Inc. | Equinoxe Shoulder System | 16-28 mm |
| Equinoxe Stemless Shoulder System | 13-20 mm | |
| Stryker | ReUnion Shoulder System | 14-28 mm |
| Wright Medical/Tornier | Aequalis Perform Shoulder System | 13-24 mm |
| SIMPLICITI Shoulder System | 14-23 mm | |
| Zimmer Biomet | Anatomical Shoulder Domelock System | 12-24 mm |
| Anatomical Shoulder Combined System | 14-23 mm | |
| Comprehensive Total Shoulder System | 18-27 mm | |
| Sidus Stem-Free Shoulder | 13-23 mm |
ATSA, anatomic total shoulder arthroplasty.
No previous study has used the IPC method to evaluate radiographical outcomes after ATSA performed with extra-short humeral heads. Therefore, the purpose of this study was to use the IPC method to compare the incidence of glenohumeral joint overstuffing between extra-short and short humeral heads. We hypothesized that extra-short head sizes would lead to a lower incidence of joint overstuffing. Our secondary aim was to assess whether superior or medial overstuffing is more radiographically prevalent with each head type.
Materials and methods
We conducted a retrospective review of the shoulder arthroplasty database at a large tertiary care academic medical center to identify all patients undergoing primary ATSA between January 1, 2004 and December 1, 2016. Inclusion criteria consisted of patients aged 18-90 years old who received stemmed implants with either a short or extra-short humeral head from a single implant system (Exactech Equinoxe, Gainesville, FL). All surgeries were performed by one of four fellowship-trained shoulder surgeons. Shoulders with post-traumatic arthritis or oncologic diagnoses were excluded. Two patients with an intraoperative fracture were also excluded because of concerns about altering the native proximal humeral anatomy. Fifty-five ATSAs performed with extra-short humeral heads met inclusion criteria. These were then age- and sex-matched in a 1:1 ratio to a cohort of patients treated with short head sized humeral components.
All ATSAs were performed with a deltopectoral approach and lesser tuberosity osteotomy. The humeral head cut was routinely made with the goal of cutting directly adjacent to the fibers of the superior rotator cuff in the patient's native version. This cutting technique was standard across the study period, regardless of head size selection.
Medical records of all included patients were reviewed. Postoperative x-rays were routinely obtained at 2 weeks, 3 months, and annually after surgery. All radiographs were evaluated, and the best Grashey view available of the humeral head component in the profile was assessed using IPC technique. The displacement between the COR of the implanted head and the native anatomic position determined using the IPC method was calculated.2
Radiographic evaluation techniques
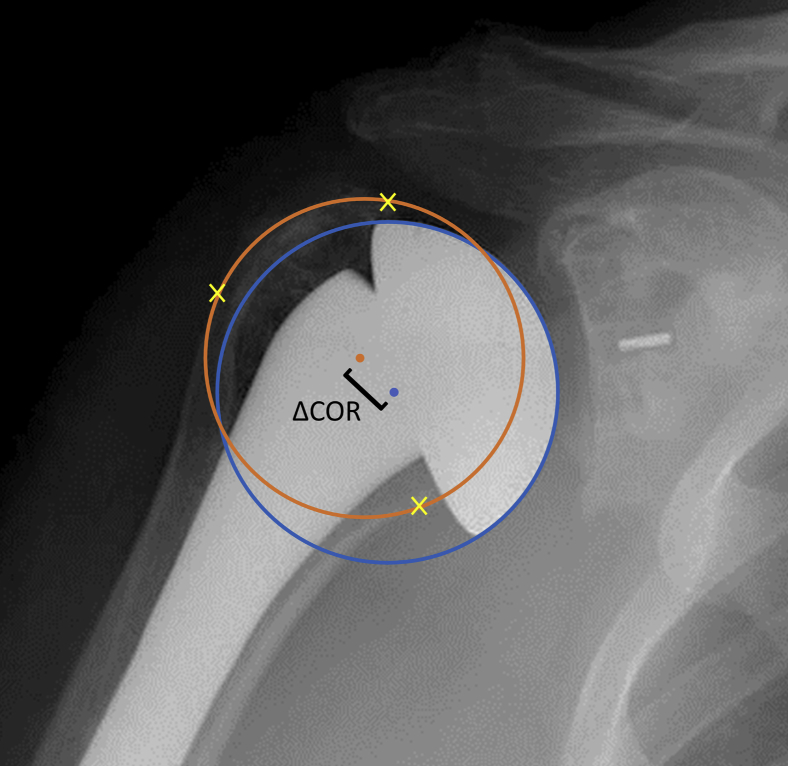
All radiographs were calibrated to the known size of the humeral heads to ensure measurement accuracy. As originally described,2 a best fit circle was drawn around the humeral head component and calibrated. The COR of the humeral head component circle was then recorded. IPC was then drawn as a second circle contacting the lateral cortex of the greater tuberosity, the medial calcar at the inflection point of the articular surface, and the medial edge of the supraspinatus insertion on the proximal humerus. The COR of IPC was then identified, and the distance and direction between the two CORs were measured (Fig. 1).
Figure 1.
Demonstration of the Iannotti's perfect circle (IPC) method for radiographic evaluation of humeral head size. The  represents the best fit of the humeral head, the
represents the best fit of the humeral head, the  mark preserved anatomic landmarks, and the
mark preserved anatomic landmarks, and the  represents IPC. The
represents IPC. The  represent each circle's COR, and the [ demonstrates measuring the distance between the two CORs. COR, center of rotation.
represent each circle's COR, and the [ demonstrates measuring the distance between the two CORs. COR, center of rotation.
The distance between the COR of the humeral head best fit circle and IPC was then classified as being matched, overstuffed, or understuffed depending on its value. When the COR of IPC and the humeral head was within 3.0 mm, the humeral component was considered matched. If the COR of the implanted humeral head was displaced laterally compared with the COR of IPC by more than 3.0 mm, the humeral head component was considered understuffed. Finally, if the COR of the implanted humeral head was displaced medially compared with the COR of IPC by more than 3.0 mm, the humeral head component was considered overstuffed. The 3.0-mm threshold value was selected because it has been previously established as the lowest amount of COR malposition that is anticipated to negatively influence shoulder biomechanics.2
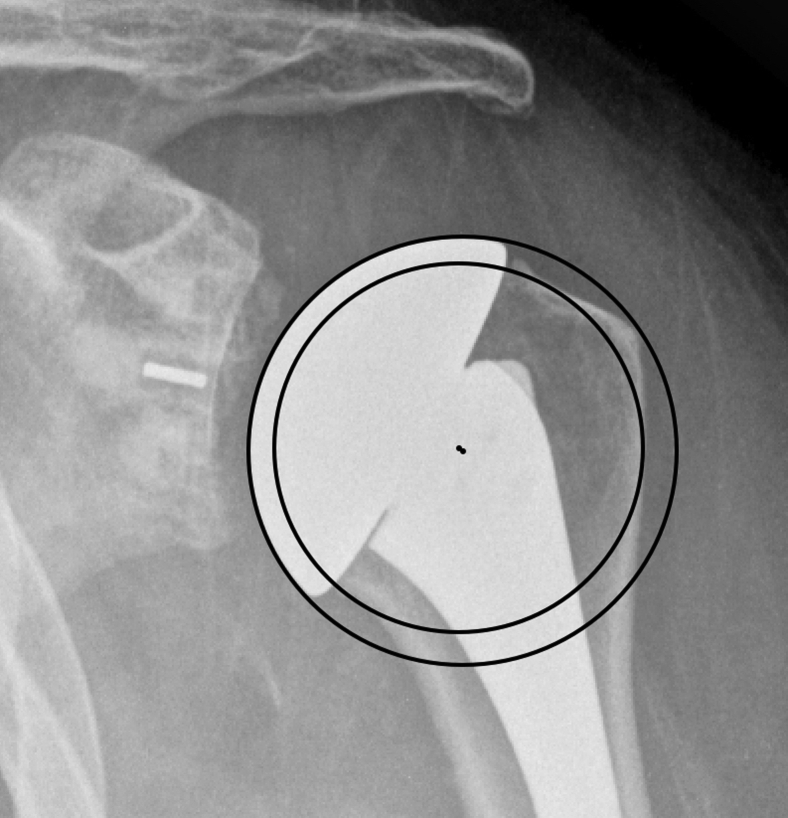
Importantly, the IPC technique does not account for cases where overstuffing can occur despite a relatively well-matched COR, as can occur with correctly positioned but incorrectly sized humeral head implants (Fig. 2). Therefore, to more clearly evaluate the magnitude of overstuffing, we developed two additional measurements to evaluate superior and medial overstuffing. These were chosen based on the most common failure modes of ATSA: superior malpositioning can lead to impingement causing attritional rotator cuff tearing, and medial overstuffing can over-tension the subscapularis repair and increase joint contact forces.
Figure 2.
Radiograph demonstrating an overstuffed humeral head due to the oversized implant, despite a center of rotation nearly identical to that of IPC. IPC, Iannotti's perfect circle.
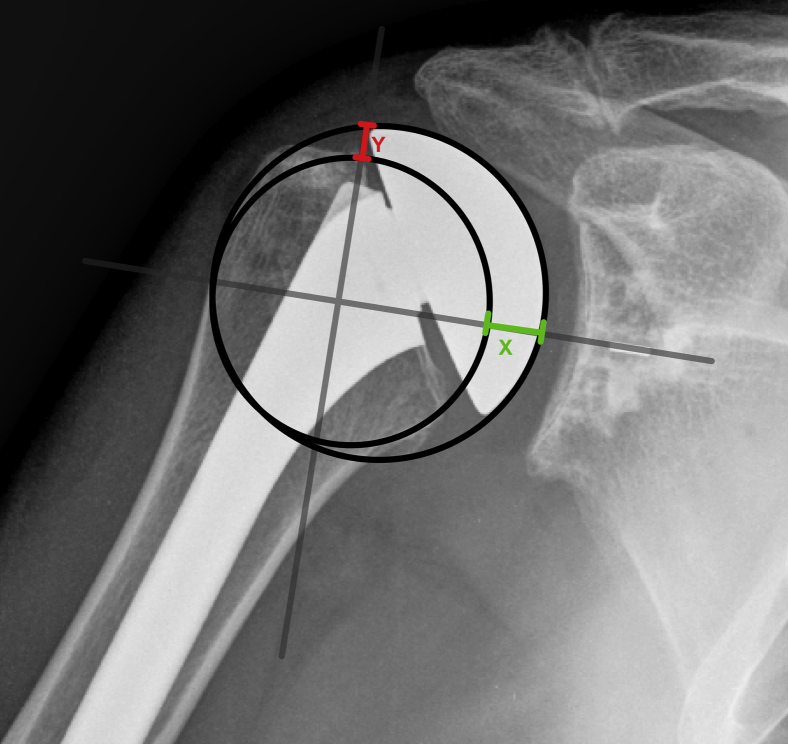
To more accurately quantify the degree and direction of malpositioning, we used a novel technique using two additional measurements along an x-y axis. First, a horizontal line was drawn collinear to the central peg of the glenoid component to establish an x-axis. Then, a perpendicular y-axis was drawn in line with the medial footprint of the supraspinatus. The resulting four quadrants were used to identify directionality of COR displacement: superomedial, superolateral, inferomedial, and inferolateral. The difference between the two circles was then calculated along the x-axis to assess medial overstuffing. In a similar fashion, the difference along the y-axis was calculated to evaluate the amount of superior malpositioning (Fig. 3). All measurements were made by an attending or resident orthopedic surgeon and rechecked by two shoulder and elbow fellowship-trained orthopedic surgeons.
Figure 3.
Demonstration of our novel measurement technique. A line is drawn parallel to the glenoid central peg and another perpendicular to it at the medial edge of the supraspinatus insertion. The difference between IPC and the humeral head best fit circle is measured along each axis. The prosthesis in this image is overstuffed superomedially. IPC, Iannotti's perfect circle.
Statistics
Chi-square and unpaired two-sided t-tests were used where appropriate. Significant interactions were followed by a Bonferroni post hoc test for pairwise comparisons. Fisher's exact test was used when count data in any category were fewer than 5. All statistical analyses were performed using R Software (version 3.6.3; R Core Team, Vienna, Austria), and significance was set at a P value of .05.
Results
Radiographs from 110 ATSAs were reviewed (55 extra-short heads and 55 short heads). Implanted humeral head diameter did not differ between cohorts receiving extra-short vs. short heads (46.7 ± 2.1 vs. 46.1 ± 3.2, P = .295). There was no correlation between humeral head diameter and displacement of the COR using the IPC method (R = .049, P = .612).
Iannotti perfect circle analysis
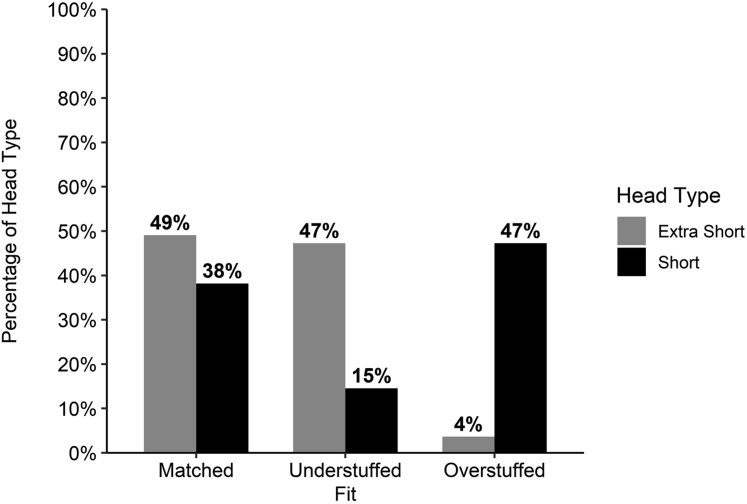
The mean magnitude of deviation for the entire cohort between the prosthetic and anatomic COR was 3.5 ± 2.0 mm and was comparable between extra-short and short heads (3.5 ± 2.2 mm vs. 3.5 ± 1.8 mm, P = .855). The COR of the prosthetic head was displaced >3.0 mm from the anatomic COR in 28 extra-short and 34 short heads (56% total). Of these 62 malpositioned heads, 55% (34) of heads were displaced medially (overstuffed) and 45% (28) were displaced laterally (understuffed). Humeral head prosthesis fit differed between ATSAs performed using extra-short and short heads (P < .001) (Fig. 4). On post hoc pairwise analysis, a greater proportion of short heads were considered overstuffed than extra-short heads (47% [26] vs. 4% [2], P < .001). Conversely, a greater proportion of extra-short heads were understuffed (47% [26] vs. 15% [8], P = .001). A similar proportion of extra-short and short heads were matched (49% [27] vs. 38% [21], P = 1).
Figure 4.
Percentage of extra-short and short heads that were a match (within 3.0 mm), overstuffed (>3.0 mm displaced medially), and understuffed (>3.0 mm displaced laterally).
Quadrant analysis
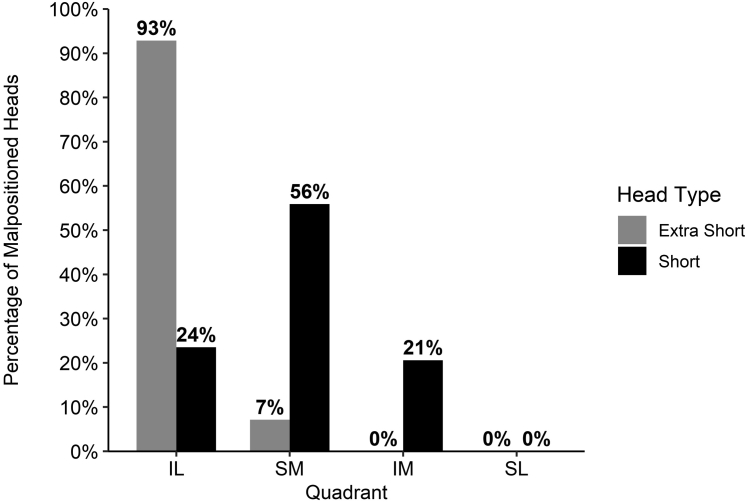
Malpositioned extra-short and short humeral heads were displaced in different quadrants (P < .001) (Fig. 5). On post hoc pairwise analysis, malpositioned extra-short heads were more often positioned in the inferolateral quadrant than short heads (93% [26] vs. 24% [8], P < .001). Conversely, malpositioned short heads were more often positioned in the superomedial quadrant (56% [19] vs. 7% [2], P < .001). A greater proportion of malpositioned short heads were in the inferomedial quadrant; however, this was not statistically significant (21% [7] vs. 0% [0], P = .086).
Figure 5.
Percentage of malpositioned extra-short (N = 28) and short (N = 34) heads that were placed in the inferolateral (IL), superomedial (SM), inferomedial (IM), and superolateral (SL) quadrants.
Medial and superior displacement
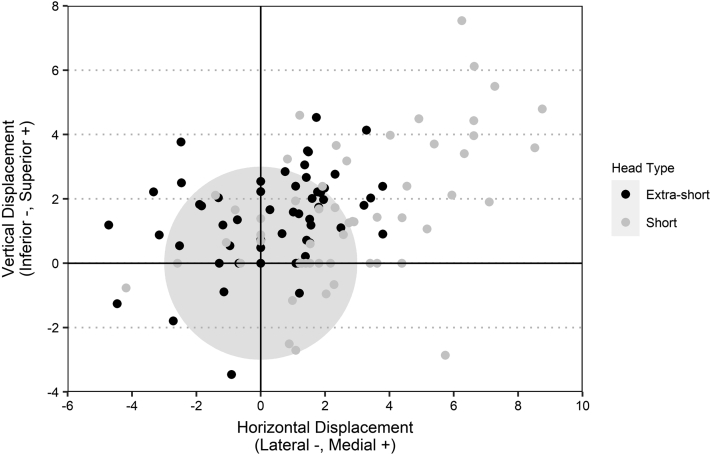
Using our novel measuring method, the overall medial displacement of the prosthetic head from the anatomic head position was 1.6 ± 2.7 mm. Short heads were more medially displaced than extra-short heads (2.8 ± 2.8 mm vs. 0.3 ± 2.0 mm, P < .001) (Fig. 6). The overall superior displacement was 1.5 ± 1.9 mm and did not differ between short and extra-short heads (1.6 ± 2.2 mm vs. 1.4 ± 1.5 mm, P = .683).
Figure 6.
Scatter plot showing the direction and magnitude of the displacement between IPC and the humeral head best fit circle measured along each axis using our novel method. The shaded circle has a radius of 3.0 mm. IPC, Iannotti's perfect circle.
Discussion
Although ATSA can have good long-term outcomes,4,9,12,14,21 concern remains regarding implant survival because of high rates of glenoid component loosening at mid-term follow-up.1,3,5,7,16,19 Appropriate sizing and positioning of the humeral head component is an important modifiable risk factor for ATSA failure, but even skilled shoulder surgeons often experience difficulty.2 Using the IPC method, this study found that routine use of extra-short humeral heads reduced the rate of glenohumeral overstuffing but increased the rate of glenohumeral understuffing. Malpositioned extra-short heads were more frequently positioned in the inferolateral quadrant, whereas malpositioned short heads were more frequently placed in the superomedial quadrant. When assessing implant positioning using our novel method, short heads had a higher rate of medial displacement than extra-short heads, but superior displacement was similar between implants. Glenohumeral overstuffing has been identified as the primary reason for poor anatomy restoration in total shoulder arthroplasty and is thought to negatively impact shoulder function.10,11 For this reason, the results of this study support the routine use of extra-short humeral heads to decrease the risk of overstuffing. This practice has been adopted by the senior author.
Our study is the first to compare postoperative radiographic outcomes between conventional short and newer extra-short humeral head prostheses in ATSA. The use of extra-short humeral heads reduced the incidence of glenohumeral joint overstuffing, with a simultaneous increase in the rate of understuffing. We hypothesize that understuffing is preferable to overstuffing. In theory, understuffing may reduce strain at the glenoid bone-implant interface, the subscapularis repair site, and the supraspinatus insertion, although there is a paucity of data on the subject. In addition, several millimeters of polyethylene are added in the standard ATSA glenoid designs, so slight humeral component understuffing may be prudent to prevent glenohumeral joint overstuffing from the addition of polyethylene on the glenoid side. Future clinical studies are needed to evaluate whether the reduced rate of overstuffing achieved by using extra-short humeral heads is accompanied by improved clinical outcomes.
Prior studies have reported radiographic results of ATSA using the IPC method with results similar to ours. Alolabi et al2 reported that 31.2% of stemmed ATSA cases were displaced >3.0 mm, with 53.8% of those being medially displaced. Although our overall rate of malpositioning in short heads was greater (62%), we found a similar rate of medial displacement among malpositioned short heads (47%). In contrast, extra-short heads were medially displaced at a much lower rate of 4%. Gallacher et al9 reported 24% malpositioning in their series of stemless ATSAs, with 51.9% of those being displaced superomedially. This overall rate of displacement is again lower than found in our study, although with a similar rate of superomedial displacement in malpositioned short heads (56%). Extra-short heads were displaced superomedially at a much lower rate (7%). Taken together, our findings agree with and elaborate on current literature by demonstrating a frequency of medial displacement with short heads that is significantly higher than with extra-short heads.
The displacement between the COR of the anatomic head using the IPC method and the prosthetic head component may not accurately assess the amount of overstuffing of the joint, particularly if the COR of both circles is nearly identical despite markedly different humeral head diameters. To more accurately assess this, we used an axis-based quadrant system to measure direction and magnitude of overstuffing. Using this method, we found that short heads were displaced medially more frequently than extra-short heads, but displacement superiorly was equivalent.
Superior displacement of the humeral head prosthesis COR in ATSA is thought to increase the risk of subacromial impingement, reduce range of motion and strength, and possibly negatively impact implant survival.6,13,15,20,22 However, these conclusions are primarily drawn from cadaveric and simulation studies. In addition, there is a paucity of literature evaluating the incidence of superior displacement retrospectively in patients who underwent ATSA. In a study of 136 ATSAs, Franta et al8 found humeral component malpositioning ≥2.5 mm in 64% of shoulders, most commonly due to superior displacement. In our study using our novel measurement method, the prosthesis COR was displaced ≥2.5 mm from the anatomic position in 18% of extra-short heads and 27% of short heads. The significantly higher incidence described by Franta et al can likely be explained by their inclusion of only unsatisfactory shoulders. The lack of a widely accepted standard method of assessing component malpositioning also makes direct comparison challenging. Still, it is apparent that superior displacement of the humeral head occurs frequently and merits further study.
Although this study demonstrated that routine use of extra-short humeral heads can more closely imitate native glenohumeral joint anatomy, it is not yet clear whether this results in improved clinical outcomes. Geervliet et al10 demonstrated a predictive relationship between glenohumeral joint overstuffing and an increased probability of revision surgery after resurfacing hemiarthroplasty, with a cutoff point of 5.8 mm. Studies of the potential clinical implications of glenohumeral joint overstuffing in ATSA are limited to cadaveric and simulation studies. An early cadaveric study demonstrated that superior displacement of the humeral head relative to the anatomic position may limit range of motion and abduction strength and cause overload of the subscapularis tendon.15 Superior translation of the humeral head by 4 mm in a cadaver study and 2.5 mm in a simulation study has been shown to increase impingement and restrict range of motion.6,22 Conversely, inferior translation of the humeral head by 4 mm in a cadaver study led to subacromial impingement and reduced range of motion.13 Similarly, a modeling study found that humeral head malpositioning of 5 mm inferiorly led to impingement and limited abduction, whereas 5 mm superiorly increased the risk of subluxation.20 These studies indicate that there are biomechanical consequences to humeral head malpositioning. Given the decreased rate of malpositioning with extra-short humeral heads, it would be expected that they would minimize these biomechanical aberrations and thus may have superior clinical outcomes. Future studies with long-term follow-up are needed to formally assess clinical benefit.
This study is limited by its retrospective nature and exclusive radiographic evaluation. Conclusions regarding the clinical implications of overstuffing such as component loosening and rotator cuff tearing cannot be made on the basis of our results. In addition, only one type of implant was used, and the results may not be generalizable to other implants. In all cases, the surgeon attempted to make the humeral head cut along the anatomic neck; however, deviations in varus, valgus, and humeral head height invariably occurred in both groups. The Equinoxe system uses a replicator plate to allow adjustments of version and inclination along with an eccentric head to correct for such case-specific deviations. In all cases, the goal was to cut at the anatomic neck regardless of whether the surgeon's plan was to preferentially use the extra-short head. Although the calibrated measurement technique used in this study has been shown to be very precise when using plain radiographs,18 interobserver reliability was shown to be low when this method was used to assess glenohumeral joint overstuffing on plain radiographs after resurfacing hemiarthroplasty.17 However, neither interobserver reliability nor test-retest reliability for the IPC method has been previously evaluated for ATSA when using plain radiographs. To reduce potential error, all measurements were performed by a single orthopedic surgeon and were verified by two other senior surgeons. Corrections were made if most surgeons disagreed on measurements. The quadrant method is based on the glenoid, so superior humeral head subluxation could errantly be recorded as superior overstuffing. Surgeons should ensure an adequately reduced glenohumeral joint before using this method. The IPC method does not consider the influence of inappropriate humeral head diameter selection on glenohumeral overstuffing. For this reason, we supplemented the IPC method with our novel measurement method, which accounts for both malposition of the COR and humeral head size. Finally, humeral head mismatch does not consider joint lateralization from the polyethylene liner or factor in glenoid malpositioning. Despite its limitations, this study provides important radiographical evidence supporting the routine use of extra-short humeral heads in ATSA and serves as a foundation for future clinical studies.
Conclusions
Extra-short humeral heads provide 2- to 3-mm less medial offset with the same humeral cut surface coverage than short heads. Extra-short heads significantly reduce the incidence of glenohumeral joint overstuffing and thus are thought to maintain more normal shoulder biomechanics. This is hypothesized to reduce the prevalence of complications such as glenoid loosening and impingement, although further studies are needed to assess the relationship between humeral head fit and clinical outcomes. Based on these data, surgeons should consider the routine use of extra-short heads in ATSA.
Disclaimers
Funding: This study received no outside funding.
Conflicts of interest: Dr King is a paid consultant for Exactech and LinkBio Corps. Dr Wright reports royalties from Exactech, Wolters Kluwer Health – Lippincott Williams & Wilkins and is a paid consultant for Exactech. Dr Schoch is a paid consultant for Exactech and reports royalties from Exactech. The other authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Approval for this study was received from the University of Florida Institutional Review Board (# IRB201802995).
References
- 1.Aibinder W.R., Schoch B., Schleck C., Sperling J.W., Cofield R.H. Revisions for aseptic glenoid component loosening after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:443–449. doi: 10.1016/j.jse.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Alolabi B., Youderian A.R., Napolitano L., Szerlip B.W., Evans P.J., Nowinski R.J., et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1740–1746. doi: 10.1016/j.jse.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Bonnevialle N., Melis B., Neyton L., Favard L., Molé D., Walch G., et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22:745–751. doi: 10.1016/j.jse.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Boorman R.S., Kopjar B., Fehringer E., Churchill R.S., Smith K., Matsen F.A. The effect of total shoulder arthroplasty on self-assessed health status is comparable to that of total hip arthroplasty and coronary artery bypassgrafting. J Shoulder Elbow Surg. 2003;12:158–163. doi: 10.1067/mse.2003.18. [DOI] [PubMed] [Google Scholar]
- 5.Castagna A., Garofalo R. Journey of the glenoid in anatomic total shoulder replacement. Shoulder Elb. 2019;11:140–148. doi: 10.1177/1758573218790119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Favre P., Moor B., Snedeker J.G., Gerber C. Influence of component positioning on impingement in conventional total shoulder arthroplasty. Clin Biomech. 2008;23:175–183. doi: 10.1016/j.clinbiomech.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Franklin J.L., Barrett W.P., Jackins S.E., Matsen F.A. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3:39–46. doi: 10.1016/s0883-5403(88)80051-2. [DOI] [PubMed] [Google Scholar]
- 8.Franta A.K., Lenters T.R., Mounce D., Neradilek B., Matsen F.A. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16:555–562. doi: 10.1016/j.jse.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Gallacher S., Williams H.L.M., King A., Kitson J., Smith C.D., Thomas W.J. Clinical and radiologic outcomes following total shoulder arthroplasty using Arthrex Eclipse stemless humeral component with minimum 2 years' follow-up. J Shoulder Elbow Surg. 2018;27:2191–2197. doi: 10.1016/j.jse.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 10.Geervliet P.C., Willems J.H., Sierevelt I.N., Visser C.P.J., van Noort A. Overstuffing in resurfacing hemiarthroplasty is a potential risk for failure. J Orthop Surg. 2019;14:474. doi: 10.1186/s13018-019-1522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grubhofer F., Muniz Martinez A.R., Haberli J., Selig M.E., Ernstbrunner L., Price M.D., et al. Does computerized CT-based 3D planning of the humeral head cut help to restore the anatomy of the proximal humerus after stemless total shoulder arthroplasty? J Shoulder Elbow Surg. 2021;30:e309–e316. doi: 10.1016/j.jse.2020.08.045. [DOI] [PubMed] [Google Scholar]
- 12.Hawi N., Tauber M., Messina M.J., Habermeyer P., Martetschläger F. Anatomic stemless shoulder arthroplasty and related outcomes: a systematic review. BMC Musculoskelet Disord. 2016;17:376. doi: 10.1186/s12891-016-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iannotti J.P., Spencer E.E., Winter U., Deffenbaugh D., Williams G. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 Suppl S):S111–S121. doi: 10.1016/j.jse.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Nelson C.G., Brolin T.J., Ford M.C., Smith R.A., Azar F.M., Throckmorton T.W. Five-year minimum clinical and radiographic outcomes of total shoulder arthroplasty using a hybrid glenoid component with a central porous titanium post. J Shoulder Elbow Surg. 2018;27:1462–1467. doi: 10.1016/j.jse.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Nyffeler R.W., Sheikh R., Jacob H.A.C., Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction: an in vitro study. J Bone Joint Surg Am. 2004;86:575–580. doi: 10.2106/00004623-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Pinkas D., Wiater B., Wiater J.M. The glenoid component in anatomic shoulder arthroplasty. J Am Acad Orthop Surg. 2015;23:317–326. doi: 10.5435/JAAOS-D-13-00208. [DOI] [PubMed] [Google Scholar]
- 17.Sandau N., Brorson S., Olsen B.S., Sørensen A.K., Jensen S.L., Schantz K., et al. Low inter-observer agreement among experienced shoulder surgeons assessing overstuffing of glenohumeral resurfacing hemiarthroplasty based on plain radiographs. J Orthop Surg. 2018;13:299. doi: 10.1186/s13018-018-1008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savin D.D., Piponov H., Goldstein J., Youderian A.R. Humeral head sizing using extra-articular landmarks on conventional radiographs. Surg Radiol Anat SRA. 2017;39:999–1004. doi: 10.1007/s00276-017-1833-z. [DOI] [PubMed] [Google Scholar]
- 19.Schoch B., Werthel J.D., Schleck C.D., Harmsen W.S., Sperling J., Sánchez-Sotelo J., et al. Optimizing follow-up after anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:997–1002. doi: 10.1016/j.jse.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Terrier A., Ramondetti S., Merlini F., Pioletti D.D., Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19:1184–1190. doi: 10.1016/j.jse.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Werthel J.-D., Lonjon G., Jo S., Cofield R., Sperling J.W., Elhassan B.T. Long-term outcomes of cemented versus cementless humeral components in arthroplasty of the shoulder: a propensity score-matched analysis. Bone Joint J. 2017;99-B:666–673. doi: 10.1302/0301-620X.99B5.BJJ-2016-0910.R1. [DOI] [PubMed] [Google Scholar]
- 22.Williams G.R., Wong K.L., Pepe M.D., Tan V., Silverberg D., Ramsey M.L., et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10:399–409. doi: 10.1067/mse.2001.116871. [DOI] [PubMed] [Google Scholar]