Abstract
Background
There is an ongoing controversy regarding the nonoperative treatment of lateral epicondylitis. Given that the evidence surrounding the use of various treatment options for lateral epicondylitis has expanded, an overall assessment of nonoperative treatment options is required. The purpose of this systematic review and meta-analysis was to compare physiotherapy (strengthening), corticosteroids (CSIs), platelet-rich plasma (PRP), and autologous blood (AB) with no active treatment or placebo control in patients with lateral epicondylitis.
Methods
MEDLINE, Embase, and Cochrane were searched through till March 8, 2021. Additional studies were identified from reviews. All English-language randomized trials comparing nonoperative treatment of patients >18 years of age with lateral epicondylitis were included.
Results
A total of 5 randomized studies compared physiotherapy (strengthening) with no active treatment. There were no significant differences in pain (mean difference: −0.07, 95% confidence interval [CI]: −0.56 to 0.41) or function (standardized mean difference [SMD]: −0.08, 95% CI: −0.46 to 0.30). Seven studies compared CSI with a control. The control group had statistically superior pain (mean difference: 0.70, 95% CI: 0.22 to 1.18) and functional scores (SMD: −0.35, 95% CI: −0.54 to −0.16). Two studies compared PRP with controls, and no differences were found in pain (SD: −0.15, 95% CI: −1.89 to 1.35) or function (SMD: 0.14, 95% CI: −0.45 to 0.73). Three studies compared AB with controls, and no differences were observed in pain (0.49, 95% CI: −2.35 to 3.33) or function (−0.07, 95% CI: −0.64 to 0.50).
Discussion
The available evidence does not support the use of nonoperative treatment options including physiotherapy (strengthening), CSI, PRP, or AB in the treatment of lateral epicondylitis.
Keywords: Lateral epicondylitis, Nonoperative treatment, Physiotherapy, Corticosteroids, Platelet-rich plasma, Autologous blood, Systematic review, Meta-analysis
Lateral epicondylitis is common, affecting 1% to 3% of the population.5 The optimal management of lateral epicondylitis in the high-functioning patient remains controversial. Despite a lack of high-level evidence to inform clinical decision-making, nonoperative management represents first-line treatment. Nonoperative treatment may include no active treatment, physiotherapy, and injections including corticosteroids (CSIs), platelet-rich plasma (PRP), and autologous blood (AB).11,16,38 Surgery may be considered when nonoperative treatment fails.3
Although there is consensus that nonoperative management should represent first-line treatment, guidelines informing the optimal approach to nonsurgical treatment are not well established.11,12,30 Evidence is lacking regarding the superiority of one nonoperative treatment option over another, and past systematic reviews have not reached definitive conclusions.7,34 Past systematic reviews have often concentrated on various injection treatments without considering other common forms of treatment such as physiotherapy.13,16 The study by Houck et al found that AB products such as AB and PRP improved pain and elbow function in the intermediate term, and CSI injections relieved pain and improved elbow function in the short term.16 In contrast, a recent meta-analysis reported that injections did not confer any treatment benefits compared with placebo, whereas physiotherapy improved pain and functional scores.18 A meta-analysis by Weber et al concluded that there was insufficient evidence to support physiotherapy for the treatment of tennis elbow.39 These inconsistent findings make interpretation of the literature difficult and cloud clinicians’ ability to counsel patients effectively.
The uncertainty surrounding the efficacy of various available nonoperative interventions makes the selection of appropriate treatments difficult. With no consistent consensus in the literature, the specific nonoperative management of lateral epicondylitis remains highly variable with various options commonly used. The aim of this systematic review and meta-analysis was to compare the functional and pain outcomes of physiotherapy (strengthening), CSI injections, PRP, and AB with no active treatment or placebo control.
Methods
Inclusion and exclusion
We identified English-language randomized controlled trials (RCTs) in any setting comparing nonoperative treatment with a control in patients aged 18 years or older with lateral epicondylitis. Studies with a minimum follow-up duration of 6 months after the first intervention were considered.
This study adheres to the standards of the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) statement32 and was registered at the PROSPERO registry of systematic reviews (CRD42021268775).
Study eligibility criteria
We established the review eligibility criteria based on the PICOS (Population-Intervention-Comparators-Outcomes-Study design) framework. Primary studies were included that met the following criteria:
-
•
Population: Studies enrolling adult patients aged 18-75 years with lateral epicondylitis receiving nonoperative treatment for their condition were sought.
-
•Interventions:
-
1)Physiotherapy (must include strengthening exercises and passive treatment such as stretching, and other modalities including laser therapy, extracorporeal shock wave therapy, massage, and acupuncture were excluded).
-
2)CSI.
-
3)PRP (methodology used for included studies involved a standard protocol of collecting 15 mL of venous blood from the cephalic vein, centrifugation of venous blood for 5 minutes, use of a kit syringe to collect one-third of the original sample of 4-6 mL, and injection of PRP at the site of greatest pain at the extensor origin of the lateral epicondyle).
-
4)AB (methodology involved collection of 3 mL of venous blood and injection with a 22-G or 23-G needle to the extensor tendon origin of the lateral epicondyle).
-
1)
-
•
Comparators: No active treatment or placebo control for interventions 2, 3, or 4 above.
-
•Outcomes: End points of interest included the following with a minimum of 6-month follow-up.
-
1)Postintervention pain (visual analog scale for pain).
-
2)Functional outcomes (eg, Patient-Rated Tennis Elbow Evaluation, Disabilities of the Arm, Shoulder and Hand).
-
1)
Information sources and search strategy
The search strategies were developed and tested through an iterative process by an experienced medical information specialist in consultation with the review team. Using the OVID platform, we searched Ovid MEDLINE, including Epub Ahead of Print and In-Process & Other Non-Indexed Citations, Embase Classic + Embase, and the Cochrane Library. The latest search was conducted on March 8, 2021.
Three different search strategies were used for physiotherapy, CSI, and PRP/AB, respectively. We used a combination of controlled vocabulary (eg, “lateral epicondylitis”) and keywords (eg, “randomized controlled trial, physiotherapy”). Results were filtered using headings for systematic reviews, RCTs, and non-RCTs as applicable for each database. Vocabulary and syntax were adjusted across databases. The search was restricted to English-language studies with no date restrictions on any of the searches, but when possible, animal-only and opinion pieces were removed from the results. The three search strategies can be found in Supplementary Appendix S1.
The bibliographies of published systematic reviews were inspected to confirm that no relevant studies had been missed. No attempt was made to contact content experts to obtain information on unknown or ongoing studies.
Screening and data extraction
Screening was performed in two stages via two reviewers working independently and in duplicate against eligibility criteria established a priori. Stage 1 screening was based on review of the abstracts and titles identified from the electronic search, whereas stage 2 screening considered full-text review of the articles deemed potentially relevant during stage 1. At stage 1, two reviewers independently assessed the titles and abstracts for eligible studies using the liberal accelerated method17 where only one reviewer was required to include citations for further assessment at full-text screening and two reviewers were needed to exclude a citation. At stage 2, full-text articles of potentially relevant citations were retrieved for full-text screening and the same two reviewers independently assessed the article for relevancy. Disagreements between reviewers were resolved via consensus. The study selection process was reported using a PRISMA flow diagram.25 References of all included studies were scanned for inclusion by one reviewer (P.L.). Study authors were consulted where necessary for verifying eligibility and for missing or unclear information on studies (and information was included if received in a timely manner). When multiple reports of the same study cohort were published, we used the most complete set and excluded repeated publications.
A standardized data extraction form in Microsoft Excel (Microsoft Corporation, Seattle, WA, USA) was used for collecting key study information that included all prespecified data items. After piloting the data extraction form on a small number of studies, two reviewers extracted the data independently and any discrepancies were resolved by discussion or a third person. Information from each study was recorded that included (but not be limited to) the following: publication characteristics (eg, authors’ names, publication year, and journal), study design traits (cited trial design, clinical setting, duration of follow-up, number of patients randomized and number analyzed for each outcome, occurrence of dropouts, funding source, authors’ conflict of interest, etc), study population details (patient inclusion and exclusion criteria, age, sex, and body mass index), comorbidities, and prior treatments. Intervention and comparator specifics (type of treatment) and outcome data (including reported outcome definitions and summary data related to treatment effects (eg, mean change and the corresponding standard error for continuous outcomes, and number of events, and number of total patients for dichotomous outcomes), and reported scales for evaluating the outcomes). Means and measures of dispersion were approximated from figures in the primary studies using online tools. We contacted authors for any missing or additional data of interest. Authors’ defined prespecified outcomes of interest were extracted and grouped accordingly for analyses.
Outcomes and prioritization
We explored the Core Outcome Measures in Effectiveness Trials (COMET) initiative but did not locate a core outcome set for the lateral epicondylitis.24 As such, the end points of interest for this review were selected via consultation with our clinical experts. The primary outcome of interest was pain, whereas the secondary outcome of interest included postintervention function. In terms of time of assessment for the end points of interest, we considered preintervention outcome data compared with the interval occurring between baseline and 24 months post-treatment. However, if insufficient data were reported across primary studies, we collected outcome data at various time points such as 6 months and 12 months post-treatment if possible.
Risk of bias assessment
We used the Cochrane Risk of Bias Tool for RCTs15 to evaluate the risk of bias of each included trial. Two reviewers carried out assessments independently and resolved disagreements via consensus or third-party adjudication. All domains of the Cochrane Risk of Bias tool for RCTs were considered, including selection bias (sequence generation and allocation sequence concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), and reporting bias (selective reporting). Trials were scored as low risk, moderate risk, or high risk based on the study methodology. The Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach was used to evaluate the quality of the included studies.2,14 The GRADE approach rates RCTs as “high” quality but can be graded down due to risk of bias, imprecision, inconsistency, indirectness, or publication bias.14 An overall grade is assigned (high, moderate, low, or very low) based on the aforementioned considerations and graded as per the population, intervention, comparator, and outcome framework described above and was applied to each outcome.
Approaches to evidence syntheses
Criteria for quantitative synthesis
As noted earlier, separate sets of analyses pertaining to comparisons of interventions were performed. Initially, we inspected the characteristics of included studies such as patients’ clinical characteristics (age, sex) and methodologic homogeneity (eg, risk of bias, study design), and we summarized them accordingly. A pairwise meta-analysis for each intervention comparison was pursued to explore statistical heterogeneity (based on the I2 statistic) if data permitted.
Statistical analysis and assessment of heterogeneity
Data for patient-reported pain and function were pooled using Revman 5.4.9 Mean visual analog scale for pain and standardized mean difference (SMD) were used across related functional scales to maximize usage of available data. All pairwise comparisons between interventions were expressed with 95% credible intervals. Cohen’s effect sizes were used as a guide to interpretation of the SMDs,8 with an SMD <0.2 considered as a small effect, 0.2 to 0.8 considered a moderate effect, and >0.8 considered a large effect. An SMD of 0.5 was considered a clinically significant improvement in function.27
In addition to inspection of the forest plots, the I2 statistic was used to detect the presence of heterogeneity (<40%, low heterogeneity and >75% substantial heterogeneity). Fixed effects models were used in the presence of low or absent heterogeneity, and mixed effects models were used if heterogeneity was detected (I2 > 40%).
We relied on pairwise meta-analyses of each of the main outcomes of interest as outlined in the population, intervention, comparator, and outcome framework described previously.
Results
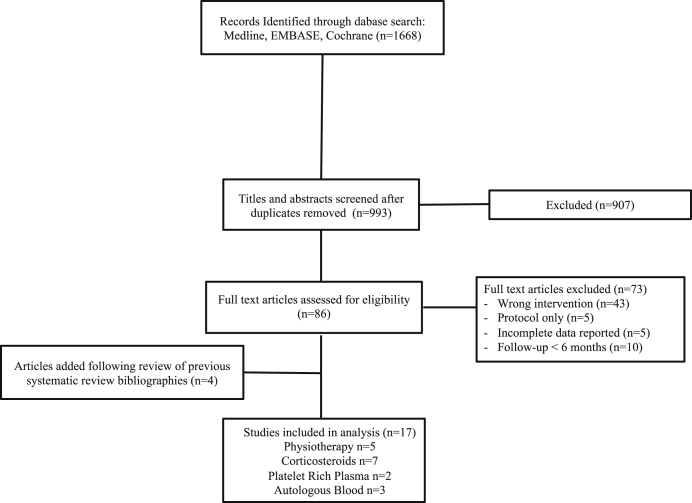
The search for studies of nonoperative treatment of lateral epicondylitis identified 1668 potential articles, and 993 articles after duplicates were removed. These were reviewed as full abstracts. Of these, 86 articles were reviewed as full texts and 73 articles were excluded. Four additional articles were added after inspection of past systematic reviews. Seventeen trials were included in the review that compared nonoperative treatment of lateral epicondylitis with a control. The study flow is summarized in Figure 1. The sample sizes of individual studies ranged from 18 to 132 patients. Follow-up time was most commonly 12 months but ranged from 6 to 12 months. Study characteristics are summarized in Table I.
Figure 1.
Flow diagram of search strategy.
Table I.
Study characteristics.
| Total number of trials | 17 |
| Age, yr, mean (range) | Aggregate: 48 (43-62) Physiotherapy vs. control: 46 (43-48) Corticosteroid vs. control: 49 (46-62); PRP vs. control: 47 (46-49); AB vs. control: 49 (46-52) |
| Duration of follow-up, months, median (range) | 12 (6-12) |
| Outcome measures | |
| Clinical Outcome Scores | Global Improvement Scale: 1 DASH: 6 PRFE: 1 Roles and Maudsley: 1 PFFQ: 4 Tennis Elbow Score: 1 Not reported: 3 |
| Pain | VAS: 16 Nirschl/Petrone pain score:1 |
PRP, platelet-rich plasma; AB, autologous blood; VAS, visual analog scale; DASH, Disabilities of the Arm, Shoulder and Hand.
Physiotherapy
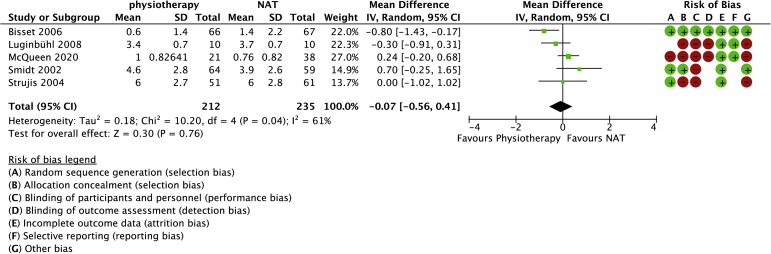
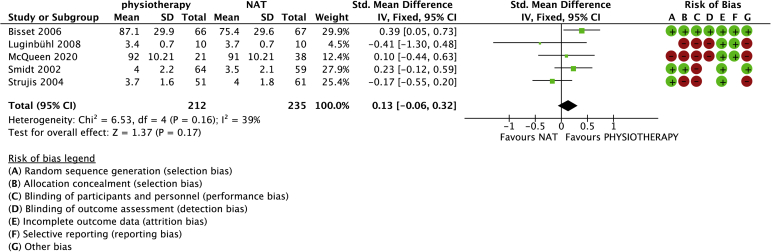
Physiotherapy (strengthening) versus no active treatment was compared with data in 5 studies (447 randomized patients) (Figs. 2 and 3).4,22,23,35,36 One study was included in which the control group was treated with a band only.22 One study used a brace in the control group.36 The brace was approximately 6 cm wide and fastened with Velcro around the forearm below the elbow. The mean age was 46 years (range: 43-48 years). No differences in pain (−0.07, 95% confidence interval [CI]: −0.56 to 0.41) or function (−0.08, 95% CI: −0.46 to 0.30) were observed between physiotherapy (strengthening) and control groups. Heterogeneity (I2) was 61% and 53% for pain and function, respectively.
Figure 2.
Forest plot of physiotherapy versus no active treatment for pain. Risk of bias legend: red dot = high risk of bias; no color = unclear risk; green dot = low risk of bias.
Figure 3.
Forest plot of physiotherapy versus no active treatment for function. Risk of bias legend: red dot = high risk of bias; no color = unclear risk; green dot = low risk of bias.
An additional subgroup analysis was conducted in which trials that allowed stretching, a band, or a brace in the no active treatment group were omitted from the analysis. We found no differences in patient-reported pain (mean difference: 0.38 [95% CI −1.12, 0.37]) or function (95% CI 0.61 [−0.13, 1.36]) between groups.
Corticosteroids
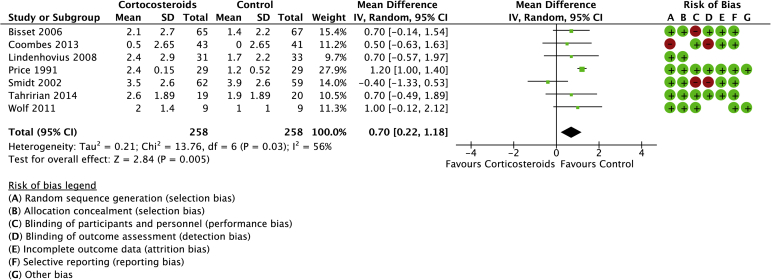
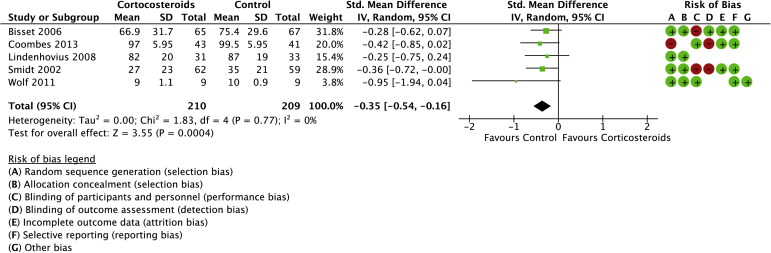
Seven studies were included in the systematic review of CSI compared with a placebo control or no active treatment with a total of 416 randomized patients (Figs. 4 and 5).4,10,20,29,35,37,40 The mean age was 50 years (range: 46-62 years). There was a statistical difference in favor of the control group for pain (mean difference: 0.70, 95% CI: 0.22 to 1.18). Statistically higher functional scores were found in favor of controls (SMD: −0.35, 95% CI: −0.54 to −0.16).
Figure 4.
Forest plot of corticosteroids versus no active treatment for pain. Risk of bias legend: red dot = high risk of bias; no color = unclear risk; green dot = low risk of bias.
Figure 5.
Forest plot of corticosteroids versus no active treatment for function. Risk of bias legend: red dot = high risk of bias; no color = unclear risk; green dot = low risk of bias.
No heterogeneity was detected for function, but significant heterogeneity was detected for pain (I2 = 56%).
Platelet-rich plasma
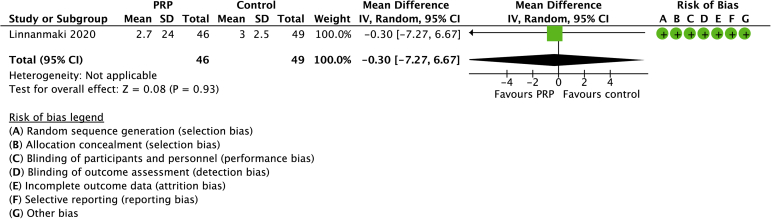
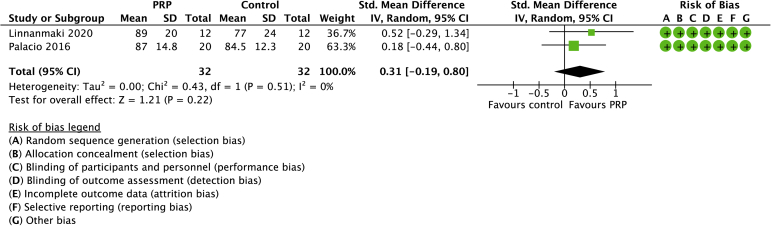
Two studies compared PRP with controls with a total of 64 randomized patients (Figs. 6 and 7).21,28 The mean age was 47 years (range: 46-49 years). No differences between groups were found in pain (SD −0.15, 95% CI −1.89 to 1.35) or function (SMD 0.14, 95% CI −0.45 to 0.73). No heterogeneity was detected across the included studies.
Figure 6.
Forest plot of PRP versus no active treatment for pain. Risk of bias legend: red dot = high risk of bias; no color = unclear risk; green dot = low risk of bias.
Figure 7.
Forest plot of PRP versus no active treatment for function. Risk of bias legend: red dot = high risk of bias; no color = unclear risk; green dot = low risk of bias.
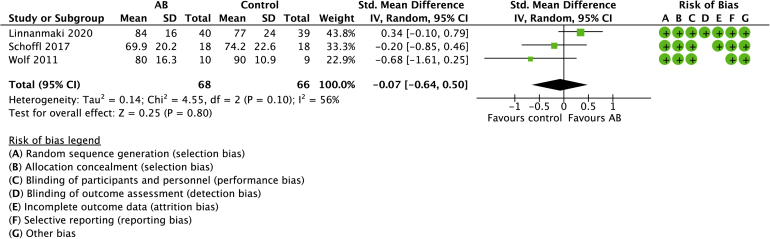
Autologous blood
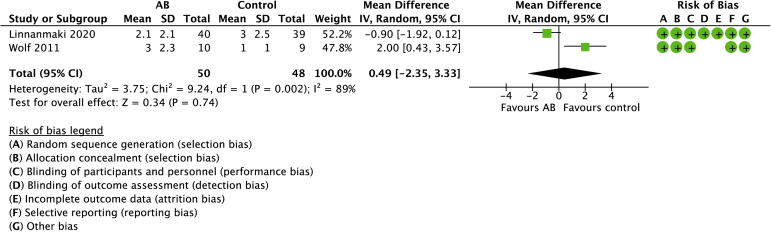
Three studies compared AB with controls with a total of 134 randomized patients (Figs. 8 and 9).21,31,40 The mean age was 49 years (range: 46-52 years). No differences were observed in pain (0.49, 95% CI: −2.35 to 3.33) or function (−0.07, 95% CI: −0.64 to 0.50). Significant heterogeneity was detected for pain (I2 = 89%) and function (I2 = 56%), respectively.
Figure 8.
Forest plot of AB versus no active treatment for pain. Risk of bias legend: red dot = high risk of bias; no color = unclear risk; green dot = low risk of bias.
Figure 9.
Forest plot of AB versus no active treatment for function. Risk of bias legend: red dot = high risk of bias; no color = unclear risk; green dot = low risk of bias.
Risk of bias
Using the Cochrane Risk of Bias tool, 4 of 5 studies (80%) included in the physiotherapy review were found to have a moderate or high risk of bias (Figs. 2 and 3). There was complete agreement among reviewers (P.L. and A.A.). Table II, Table III, Table IV, Table V contain the GRADE summary of findings, as well as the level of certainty for each comparison. The certainty of the GRADE assessments was downgraded in most cases most commonly due to methodological concerns related to lack of blinding and lack of concealment of allocated treatment. Most studies in the CSI review were graded as “moderate” risk of bias mainly due to concerns related to blinding of participants and outcome assessors (Fig. 3 and Table III). The risk of bias was graded as “low” in the PRP review (Fig. 4 and Table IV). There were no serious methodological concerns in the review on AB (Fig. 5 and Table V).
Table II.
Physiotherapy compared to no active treatment for tennis elbow.
| Certainty assessment |
Number of patients |
Effect |
Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Physiotherapy | No active treatment | Relative (95% CI) | Absolute (95% CI) | |
| Pain (scale from: 0 to 10) | |||||||||||
| 5 | randomised trials | serious∗,† | serious‡ | not serious | not serious | None | 212 | 235⊕ | - | MD 0.07 VAS lower (0.56 lower to 0.41 higher) | ⊕⊕◯◯ Low |
| Function | |||||||||||
| 5 | randomised trials | serious∗,† | not serious | not serious | not serious | None | 212 | 235 | - | SMD 0.13 SD higher (0.06 lower to 0.32 higher) | ⊕⊕⊕◯ Moderate |
CI, confidence interval; MD, mean difference; VAS, visual analog scale; SMD, standardized mean difference.
treatment allocation was not concealed.
Blinding of participants did not occur in any study.
I2 = 61% in this comparison.
Table III.
Corticosteroids compared to control for health problem and/or population.
| Certainty assessment |
Number of patients |
Effect |
Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Corticosteroids | Control | Relative (95% CI) | Absolute (95% CI) | |
| Pain (follow-up: range 2 months to 12 months) | |||||||||||
| 7 | randomised trials | serious∗,†,‡ | serious§ | not serious | not serious | None | 258 | 258 | - | MD 0.7 VAS higher (0.22 higher to 1.18 higher) | ⊕⊕◯◯ Low |
| Function (follow-up: range 2 months to 12 months) | |||||||||||
| 5 | randomised trials | serious∗,†,‡ | not serious | not serious | not serious | None | 211 | 208 | - | SMD 0.19 SD lower (0.4 lower to 0.22 higher) | ⊕⊕⊕◯ Moderate |
CI, confidence interval; MD, mean difference; VAS, visual analog scale; SMD, standardized mean difference.
In one study, it was unclear whether treatment allocation was truly random.
blinding of participants did not occur in two studies.
blinding of outcome assessments not done with two studies.
I2 value = 56%.
Table IV.
PRP compared to no active treatment for tennis elbow.
| Certainty assessment |
Number of patients |
Effect |
Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | PRP | No active treatment | Relative (95% CI) | Absolute (95% CI) | |
| Pain | |||||||||||
| 1 | randomised trials | not serious | not serious | not serious | not serious | none | 46 | 49 | - | MD 0.3 VAS lower (7.27 lower to 6.67 higher) | ⊕⊕⊕⊕ High |
| Function | |||||||||||
| 2 | randomised trials | not serious | not serious | not serious | not serious | none | 32 | 32 | - | MD 0.31 VAS higher (0.19 lower to 0.8 higher) | ⊕⊕⊕⊕ High |
PRP, platelet-rich plasma; CI, confidence interval; MD, mean difference; VAS, visual analog scale.
Table V.
Question: Autologous blood compared to control for tennis elbow.
| Certainty assessment |
Number of patients |
Effect |
Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Autologous blood | Control | Relative (95% CI) | Absolute (95% CI) | |
| Pain | |||||||||||
| 2 | randomised trials | not serious | not serious | not serious | Serious∗ | none | 50 | 48 | - | MD 0.49 VAS higher (2.35 lower to 3.33 higher) | ⊕⊕⊕◯ Moderate |
| Function | |||||||||||
| 3 | randomised trials | not serious | not serious | not serious | not serious | none | 68 | 66 | - | SMD 0.07 SD lower (0.64 lower to 0.5 higher) | ⊕⊕⊕⊕ High |
CI, confidence interval; MD, mean difference; VAS, visual analog scale; SMD, standardized mean difference.
Only two studies reported pain. Treatment effect estimate range is large due to small number of patients.
Discussion
This systematic review and meta-analysis included 17 trials comparing the nonoperative treatment of lateral epicondylitis to no active treatment or a placebo control. This study finds that pain and functional scores were similar between groups for nonoperative treatment including physiotherapy (strengthening), PRP, and AB compared with controls. The comparison of CSI with placebo control revealed that both pain scores (−0.35, 9% CI: −0.54 to −0.16) and functional scores (0.7, 95% CI: 0.22 to 1.18) favored controls.
The findings of the present study are consistent with a recent systematic review and meta-analysis of RCTs by Kim et al that compared nonoperative treatment in lateral epicondylitis.18 The latter study found that injections did not improve patient-reported outcomes. Our findings indicate that both pain and function were statistically worse in the CSI group. In contrast to the present study, however, Kim et al reported that both physiotherapy and electrophysiotherapy improved pain outcomes. One possible explanation for the difference in results may be related to the broader inclusion criteria by Kim et al in which studies were included comparing extracorporeal shock wave therapy6 and microcurrent therapy1 in the physiotherapy group, making interpretation of the results difficult. A systematic review by Lian et al found that injected CSIs resulted in better pain outcomes compared with placebo in patients with lateral epicondylitis.19 The inclusion criteria in the latter study were not as restrictive as in the current review because studies were included that allowed rehabilitation exercises in the control group,26 whereas in the current review, we only included studies comparing CSI to placebo. The current review also included a greater number of studies comparing CSI with a control than the review by Lian et al.
In a systematic review of overlapping meta-analyses, Houck et al reported that most previous systematic reviews found that PRP and AB were effective treatment options in the short term (12-26 weeks).16 In addition, CSIs were found to be effective in the short term (<12 weeks). The results of the current review contrast sharply with the study by Houck et al and do not demonstrate any benefit of injectable treatment over placebo with follow-up of >6 months.
Few prospective randomized trials have been published comparing PRP injections with placebo. The results of the present study are similar to a recently published systematic review and meta-analysis by Simental-Mendia et al33 which reported comparable results between PRP and placebo in pain and functional scores. The present study found similar outcomes with the addition of one further study to the meta-analysis.
We provide an updated analysis of the lateral epicondylitis literature. Our findings of no added benefit to the nonoperative treatments studied provide further confidence in the lack of effectiveness in these treatment options.
A strength of our review of nonoperative treatment of lateral epicondylitis is that it focused exclusively on RCTs to limit the risk of bias. A further strength was the strict inclusion criteria. Only studies that compared physiotherapy with a strengthening program compared with no active treatment were included. All other physiotherapy modalities were excluded from the review, which allows a clear interpretation of the treatment effect of strengthening alone. Similarly, only studies comparing CSI, PRP, and AB with a placebo control group were included, whereas previous systematic reviews included studies that comprised additional treatment modalities such as exercise programs19 in addition to the allocated treatment which may lead to confounding. The strict inclusion criteria allowed us to confidently interpret the treatment effect of these individual modalities in isolation.
One limitation of the present study is related to the studies included in the review; methodologic quality was not uniformly high and design limitations were identified in most trials. The relatively small number of patients in many of the trials limited conclusions that may be drawn by these individual studies. Most studies had an end point of 12 months, and therefore, there is a lack of data on the long-term durability of all nonsurgical options. Nonsurgical treatment approaches need to be further explored through rigorous comparative research with longer term follow-up. In addition, the comparison of physiotherapy to no active treatment was isolated to strengthening exercises, and therefore, the conclusions only pertain to this treatment modality.
Conclusion
This meta-analysis demonstrates that the highest quality available evidence does not support the use of exercise-based physiotherapy, CSI injections, PRP, or AB injections in the treatment of lateral epicondylitis. Furthermore, high-quality trials with longer term follow-up should focus on other forms of physiotherapy interventions other than exercise therapy.
Disclaimers
Funding: No funding was disclosed by the authors.
Conflicts of interest: The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Acknowledgments
The authors would like to thank Katie McIlquham, clinical research coordinator for assistance with Prospero registration and administrative details. The authors would also like to acknowledge special contributor Risa Shorr for literature search support.
Footnotes
Institutional review board approval was not required for this systematic review.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jseint.2021.11.010.
Supplementary data
References
- 1.Ammar T. Pulsed electromagnetic field versus microcurrent electrical nerve stimulation in patients with lateral epicondylopathy. Int J Ther Rehabil. 2016;23:519–523. doi: 10.12968/ijtr.2016.23.11.519. [DOI] [Google Scholar]
- 2.Balshem H., Helfand M., Schunemann H.J., Oxman A.D., Kunz R., Brozek J., et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Bateman M., Littlewood C., Rawson B., Tambe A.A. Surgery for tennis elbow: a systematic review. Shoulder Elbow. 2019;11:35–44. doi: 10.1177/1758573217745041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisset L., Beller E., Jull G., Brooks P., Darnell R., Vicenzino B. Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: randomised trial. BMJ. 2006;333:939. doi: 10.1136/bmj.38961.584653.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan B., Varacallo M. StatPearls [Internet] 2019. Tennis elbow (Ltaeral epicondylitis) Treasure Island, FL: StatPearls Publishing LLC. [Google Scholar]
- 6.Capan N., Esmaeilzadeh S., Oral A., Basoglu C., Karan A., Sindel D. Radial extracorporeal shock wave therapy is not more effective than placebo in the management of lateral epicondylitis: a double-blind, randomized, placebo-controlled trial. Am J Phys Med Rehabil. 2016;95:495–506. doi: 10.1097/PHM.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 7.Chesterton L.S., Mallen C.D., Hay E.M. Management of tennis elbow. Open Access J Sports Med. 2011;2:53–59. doi: 10.2147/OAJSM.S10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen J. Rev. ed. Academic Press; New York: 1977. Statistical power analysis for the behavioural sciences. [Google Scholar]
- 9.Collaboration T.C. 2020. Rev Manager (Revman) [Computer program]https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/reasons-downloading-revman-5 Cochran Informatics and Technology Services (IT). Available at: Accessed February 1, 2022. [Google Scholar]
- 10.Coombes B.K., Bisset L., Brooks P., Khan A., Vicenzino B. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia: a randomized controlled trial. JAMA. 2013;309:461–469. doi: 10.1001/jama.2013.129. [DOI] [PubMed] [Google Scholar]
- 11.Coombes B.K., Bisset L., Vicenzino B. Management of lateral elbow tendinopathy: one size does not Fit all. J Orthop Sports Phys Ther. 2015;45:938–949. doi: 10.2519/jospt.2015.5841. [DOI] [PubMed] [Google Scholar]
- 12.Degen R.M., Conti M.S., Camp C.L., Altchek D.W., Dines J.S., Werner B.C. Epidemiology and disease burden of lateral epicondylitis in the USA: analysis of 85,318 patients. HSS J. 2018;14:9–14. doi: 10.1007/s11420-017-9559-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong W., Goost H., Lin X.B., Burger C., Paul C., Wang Z.L., et al. Injection therapies for lateral epicondylalgia: a systematic review and Bayesian network meta-analysis. Br J Sports Med. 2016;50:900–908. doi: 10.1136/bjsports-2014-094387. [DOI] [PubMed] [Google Scholar]
- 14.Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Higgins J.P., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houck D.A., Kraeutler M.J., Thornton L.B., McCarty E.C., Bravman J.T. Treatment of lateral epicondylitis with autologous blood, platelet-rich plasma, or corticosteroid injections: a systematic review of overlapping meta-analyses. Orthop J Sports Med. 2019;7 doi: 10.1177/2325967119831052. 2325967119831052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khangura S., Konnyu K., Cushman R., Grimshaw J., Moher D. Evidence summaries: the evolution of a rapid review approach. Syst Rev. 2012;1:10. doi: 10.1186/2046-4053-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y.J., Wood S.M., Yoon A.P., Howard J.C., Yang L.Y., Chung K.C. Efficacy of nonoperative treatments for lateral epicondylitis: a systematic review and meta-analysis. Plast Reconstr Surg. 2021;147:112–125. doi: 10.1097/PRS.0000000000007440. [DOI] [PubMed] [Google Scholar]
- 19.Lian J., Mohamadi A., Chan J.J., Hanna P., Hemmati D., Lechtig A., et al. Comparative efficacy and safety of nonsurgical treatment options for enthesopathy of the extensor carpi radialis brevis: a systematic review and meta-analysis of randomized placebo-controlled trials. Am J Sports Med. 2019;47:3019–3029. doi: 10.1177/0363546518801914. [DOI] [PubMed] [Google Scholar]
- 20.Lindenhovius A., Henket M., Gilligan B.P., Lozano-Calderon S., Jupiter J.B., Ring D. Injection of dexamethasone versus placebo for lateral elbow pain: a prospective, double-blind, randomized clinical trial. J Hand Surg. 2008;33:909–919. doi: 10.1016/j.jhsa.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Linnanmaki L., Kanto K., Karjalainen T., Leppanen O.V., Lehtinen J. Platelet-rich plasma or autologous blood do not reduce pain or improve function in patients with lateral epicondylitis: a randomized controlled trial. Clin Orthop Relat Res. 2020;478:1892–1900. doi: 10.1097/CORR.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luginbühl R., Brunner F., Schneeberger A.G. No effect of forearm band and extensor strengthening exercises for the treatment of tennis elbow: a prospective randomised study. Chir Organi Mov. 2008;91:35–40. doi: 10.1007/s12306-007-0006-3. [DOI] [PubMed] [Google Scholar]
- 23.McQueen K.S., Powell R.K., Keener T., Whalley R., Calfee R.P. Role of strengthening during nonoperative treatment of lateral epicondyle tendinopathy. J Hand Ther. 2020;34:619–626. doi: 10.1016/j.jht.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Measures in effectiveness trials initiative (COMET). Available at: https://www.comet-initiative.org. Accessed December 1, 2020.
- 25.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newcomer K.L., Laskowski E.R., Idank D.M., McLean T.J., Egan K.S. Corticosteroid injection in early treatment of lateral epicondylitis. Clin J Sport Med. 2001;11:214–222. doi: 10.1097/00042752-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Norman G.R., Sloan J.A., Wyrwich K.W. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 28.Palacio E.P., Schiavetti R.R., Kanematsu M., Ikeda T.M., Mizobuchi R.R., Galbiatti J.A. Effects of platelet-rich plasma on lateral epicondylitis of the elbow: prospective randomized controlled trial. Rev Bras Ortop. 2016;51:90–95. doi: 10.1016/j.rboe.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price R., Sinclair H., Heinrich I., Gibson T. Local injection treatment of tennis elbow--hydrocortisone, triamcinolone and lignocaine compared. Br J Rheumatol. 1991;30:39–44. doi: 10.1093/rheumatology/30.1.39. [DOI] [PubMed] [Google Scholar]
- 30.Sanders T.L., Jr., Maradit Kremers H., Bryan A.J., Ransom J.E., Smith J., Morrey B.F. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43:1066–1071. doi: 10.1177/0363546514568087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoffl V., Willauschus W., Sauer F., Kupper T., Schoffl I., Lutter C., et al. Autologous conditioned plasma versus placebo injection therapy in lateral epicondylitis of the elbow: a double blind, randomized study. Sportverletz Sportschaden. 2017;31:31–36. doi: 10.1055/s-0043-101042. [DOI] [PubMed] [Google Scholar]
- 32.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 33.Simental-Mendia M., Vilchez-Cavazos F., Alvarez-Villalobos N., Blazquez-Saldana J., Pena-Martinez V., Villarreal-Villarreal G., et al. Clinical efficacy of platelet-rich plasma in the treatment of lateral epicondylitis: a systematic review and meta-analysis of randomized placebo-controlled clinical trials. Clin Rheumatol. 2020;39:2255–2265. doi: 10.1007/s10067-020-05000-y. [DOI] [PubMed] [Google Scholar]
- 34.Sims S.E., Miller K., Elfar J.C., Hammert W.C. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (N Y) 2014;9:419–446. doi: 10.1007/s11552-014-9642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smidt N., van der Windt D.A., Assendelft W.J., Devillé W.L., Korthals-de Bos I.B., Bouter L.M. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359:657–662. doi: 10.1016/s0140-6736(02)07811-x. [DOI] [PubMed] [Google Scholar]
- 36.Struijs P.A., Kerkhoffs G.M., Assendelft W.J., Van Dijk C.N. Conservative treatment of lateral epicondylitis: brace versus physical therapy or a combination of both-a randomized clinical trial. Am J Sports Med. 2004;32:462–469. doi: 10.1177/0095399703258714. [DOI] [PubMed] [Google Scholar]
- 37.Tahririan M.A., Moayednia A., Momeni A., Yousefi A., Vahdatpour B. A randomized clinical trial on comparison of corticosteroid injection with or without splinting versus saline injection with or without splinting in patients with lateral epicondylitis. J Res Med Sci. 2014;19:813–818. [PMC free article] [PubMed] [Google Scholar]
- 38.Vaquero-Picado A., Barco R., Antuna S.A. Lateral epicondylitis of the elbow. EFORT Open Rev. 2016;1:391–397. doi: 10.1302/2058-5241.1.000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber C., Thai V., Neuheuser K., Groover K., Christ O. Efficacy of physical therapy for the treatment of lateral epicondylitis: a meta-analysis. BMC Musculoskelet Disord. 2015;16:223. doi: 10.1186/s12891-015-0665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf J.M., Ozer K., Scott F., Gordon M.J., Williams A.E. Comparison of autologous blood, corticosteroid, and saline injection in the treatment of lateral epicondylitis: a prospective, randomized, controlled multicenter study. J Hand Surg Am. 2011;36:1269–1272. doi: 10.1016/j.jhsa.2011.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.