Abstract
Introduction
Cancer disease is known due to its unregulated proliferation of cells that have evolved from the body’s regular cells. The disease develops as a result of epigenetic and genetic modifications, tumor suppressor gene inactivation, and oncogene activation. The present work describes an environmentally benign approach for the synthesis of manganese oxide nanoparticles (MnO2 NPs) using Gmelina arborea fruit extract (GAE) in an aqueous medium.
Methods
The study evaluated the formation of MnO2 NPs and their anticancer efficacy against MCF-7 breast cancer cell line.
Results
The formation of MnO2 NPs was confirmed through powder X-ray diffractometer (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HR-TEM). The crystalline nature of as-prepared MnO2 NPs was evident from XRD pattern. The morphology of the material was studied using SEM analysis, which suggested a rod-like nature with an average diameter of 50 nm. Further, the TEM and HR-TEM images confirmed the rod shape of the as-prepared MnO2 NPs with an interplanar distance of 0.271 nm. In addition, the concentric rings from selected area electron diffraction (SAED) analysis show the crystalline nature of the as-prepared material, which further supports the obtained XRD pattern. The anticancer efficacy of MnO2 NPs was evaluated against MCF-7 breast cancer cell line, which showed up to 96% inhibition of the cells at 400 µg/mL concentration.
Conclusion
Bio-conjugation of MnO2 NPs can provide enough scope for the therapeutic use of Gmelina arborea, assuming appropriate mechanistic evaluations are conducted.
Keywords: Gmelina arborea, MnO2 NPs, HR-TEM, MCF-7
Introduction
Cancer disease is known for its unregulated proliferation of cells that have evolved from the body’s regular cells. The disease develops as a result of epigenetic and genetic modifications, tumor suppressor gene inactivation, and oncogene activation.1 In developing countries, it has become of the leading causes of death. The MTT assay uses live cells to assess in vitro mammalian cellular development, survival, proliferation and even the cytotoxic potential of different compounds can be evaluated using this assay.2 The percentage of viable cells in a cell suspension is determined using cell viability assays.3 Membrane integrity has remained the most common metric for determination of cell viability.4
Since ancient times, Gmelina arborea (G. arborea) belonging to Verbenaceae family has been used in traditional medicinal practices such as Ayurveda.5 It is a tree that grows in Asia’s moist deciduous forests and was introduced to Africa and South America as a plantation tree species mainly for its timber-yielding qualities.6 The tree’s medicinal properties are also highly regarded. Almost every portion of the tree is used as medicine to treat fevers, skin issues and stomach problems. Flavonoids, iridoid glycosides, and lignans are among the phytochemical constituents present in this plant.7 All the organs of this plant have known medicinal value, and have been used for a long time as antimicrobial, anti-diabetic, anthelmintic, hepatoprotective, anti-aging, antiepileptic, analgesic and diuretic agents.8 As a thriving interdisciplinary field of exploration in an assortment of fields, nanotechnology can empower inventive applications in farming, like pesticide conveyance, nano-sensors, pesticide debasement, micronutrients for effective use, etc. A few inorganic and natural metal oxide nanomaterials, just as a few crossbreed nanomaterials, are progressively being utilized as elective antibacterial specialists in biomedical applications because of their strange uncommon nano-size structure, high surface-to-volume proportion and unrivaled physicochemical properties’ attributes.9 MnO2 NPs are non-toxic in nature that is relatively simple to obtain when compared to other different inorganic metal oxides.10 Recent advancements have resulted in notable improvements in materials and drugs, all of which have tremendous potential.11
Based on all the above-mentioned facts in the present investigation, the MnO2 NPs are synthesized from GAE and were characterized using XRD, SEM, TEM and HRTEM techniques. Further, as-prepared MnO2 NPs were screened for its tumoricidal effect against MCF-7 breast cancer cell line.
Materials and Methods
The precursor, manganese acetate tetrahydrate [Mn(OAc)2.4H2O] was obtained from Loba chemicals (Bangalore, India). Demineralized water was collected from an ELGA RO system and was used throughout the experiments (Elga Veolia, Lane End, UK). The crystalline phases were recorded on Bruker X-ray diffractometer with a scan range of 20–80° at a 2°/min scan rate using Cu Kα (1.5406 Å) radiation (Bruker, Karlsruhe, Germany). The morphology and elemental composition were studied using scanning electron microscopy (SEM) and énergy dispersive X-ray (EDX) mapping, respectively, which were recorded on a Zeiss microscope (Carl Zeiss, White Plains, NY, USA). Transmission electron microscopy (TEM) images and Selected Area Electron Diffraction (SAED) patterns were recorded on a JEOL 2100F FEG apparatus operating at 200 kV after casting a drop of MnO2 NPs for dispersion in ethanol over a Cu grid (Jeol, Akishima, Tokyo, Japan). Breast cancer cells (MCF-7) were procured from the ATCC and cultured in Dulbecco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS), penicillin (100 IU/mL), and streptomycin (100 μg/mL) in 5% CO2 at 37°C until confluence.
Collection and Preparation of Gmelina arborea Fruit Extract
Dr Chandrashekar S performed the unambiguous authentication of the specimen that was then deposited in the herbarium with the voucher specimen number GA01 in the Department of Biotechnology and Bioinformatics, JSS AHER, Mysore, Karnataka, India. The plant material was available in the University campus, the proper guidance was taken from the botanist while collecting the plant materials for the study. The fertile fruits of G. arborea were collected from the tropical forest of Malnad region in Karnataka, India. The collected plant material was surface sterilized 2–3 times with running tap water to remove adhered dust impurities followed by sterile double‐distilled water and then air‐dried at room temperature for 3–4 days. The yellow fruits were washed several times with demineralized water, sun-dried, finely ground, and 10% aqueous extracts were made as follows: Using a mortar and pestle, 1 gram of powder was mixed with 10 mL of water and thoroughly homogenized. The suspension was then centrifuged at 4°C for 15 minutes at 10,000 rpm. The supernatant was collected and stored in aliquots at a 20°C.12
Synthesis of MnO2 NPs from Gmelina arborea Fruit Extract
The MnO2 NPs were synthesized by reacting aqueous solution of manganese acetate (2 mm) with aqueous extract of G. arborea fruit (0.65 g) in 15 mL of deionized water. The above reaction mixture was stirred for 2.5 h at room temperature. The color change was observed from colorless to reddish brown, which primarily indicated the formation of MnO2 NPs via metal reduction. Upon allowing the above solution to age for 24 h, a black product indicating the complete stabilization of MnO2 NPs was obtained. The resulting product was centrifuged, filtered off, washed thrice with absolute ethanol and then with acetone to obtain pure MnO2 NPs. The obtained powder was dried at 80 °C for 6 h and used for further studies.
Measurement of Cell Viability Using MTT Assay
The cells were seeded in a 96-well flat-bottom microplate and held overnight at 37°C, 95% humidity, and 5% CO2. The samples were treated at various concentrations of GAE and MnO2 NPs (12.5, 25, 50, 100, 200 and 400 μg/mL). For another 24 h, the cells were incubated. The wells were washed twice with PBS before being filled with 20 μL of MTT staining solution and incubated at 37°C. After 4 h, 100 μL DMSO was applied to each well to dissolve the formazan crystals, and absorbance was measured at 570 nm using a microplate reader using the below-given formula.13
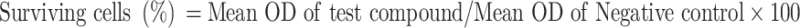 |
Results and Discussion
XRD (X-Ray Diffraction) Analysis
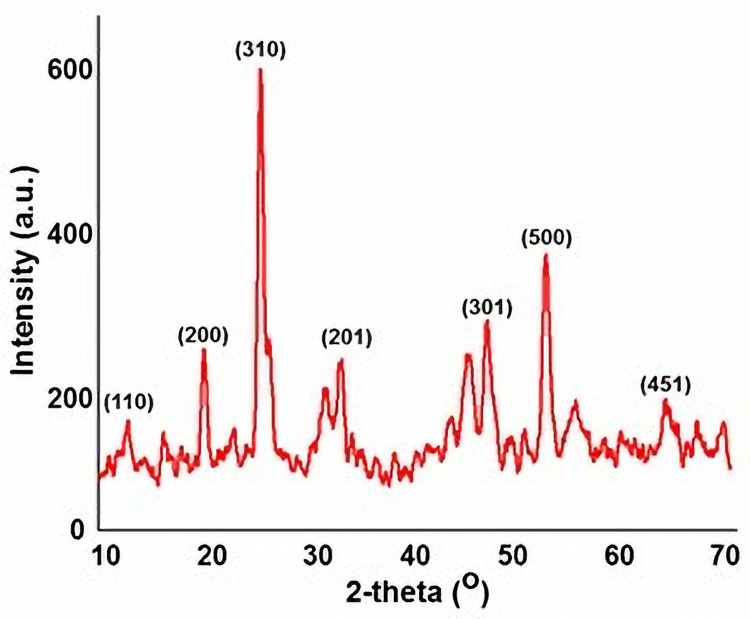
The XRD pattern of as-prepared MnO2 NPs is shown in Figure 1. The crystalline phases of MnO2 NPs revealed diffraction peaks at 2θ = 12.8°, 19.1°, 27.3°, 33.1°, 49.2°, 54.8°, and 68.2° corresponding to (110), (200), (310), (201), (301), (500) and (451) crystal planes. This further depicts the cubic structure of the as-obtained material with lattice parameter a = 4.46 Å. The indexed crystalline planes were in good agreement with the standard values JCPDS card PDF file no. 44–0141, further confirmed the crystalline nature of MnO2.14
Figure 1.
Powder X-ray diffraction pattern showing the crystalline planes of as-prepared MnO2 NPs.
SEM (Scanning Electron Microscopy) Analysis
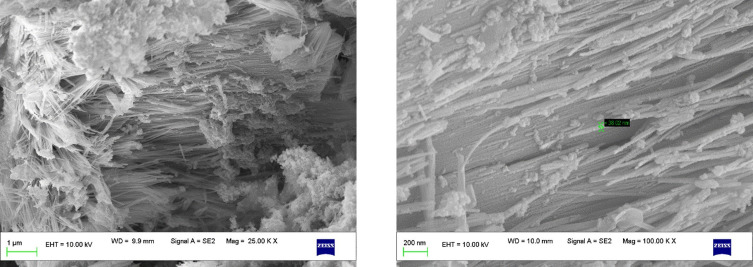
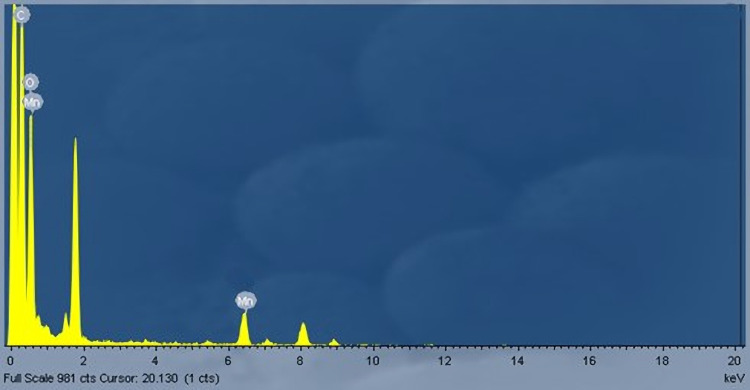
The morphology of as-prepared MnO2 NPs revealed that it is composed of nanorods with an average size ranging between 50 and 60 nm. It is seen from Figure 2 that the MnO2 NPs comprise the dense cloud and cluttered nanorods. Further, it can be seen from the magnified SEM image that the as-prepared MnO2 NPs microenvironment consists of furrowed nanorods. Furthermore, the EDX analysis of MnO2 NPs affirmed that the ratio of Mn:O as 1:2 (Figure 3). The uniform distribution of Mn and O are seen from the elemental mapping image. The intensity of oxygen particles was observed to be higher than that of Mn particles.15,16
Figure 2.
SEM images of as-prepared MnO2 NPs in different magnifications.
Figure 3.
Energy-dispersive X-ray (EDX) spectra of as-prepared MnO2 NPs.
TEM (Transmission Electron Microscopy) Analysis
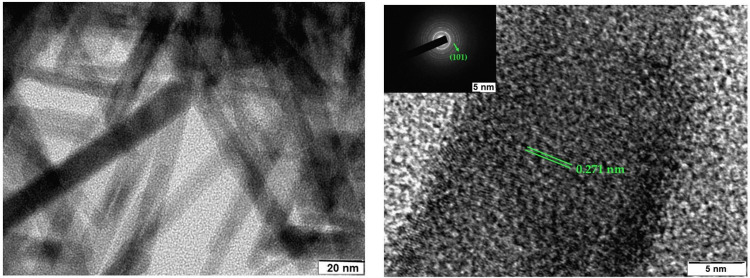
Transmission scanning electron microscopy (TEM) images of as-prepared MnO2 NPs are depicted in Figure 4. The average sizes of the MnO2 NPs are between 40 and 50 nm. The rod-shaped nature of MnO2 NPs for the sample is evident from the TEM image. Moreover, the aggregate blocks with the porous structure of as-prepared MnO2 NPs are seen from TEM image. Further, the HR-TEM image of as-prepared MnO2 NPs revealed that the interplanar spacing between two lattice fringes was 0.271 nm, corresponding to (101) lattice plane of MnO2 NPs. Furthermore, SAED pattern affirms the crystalline structure of as-prepared MnO2 NPs.
Figure 4.
TEM (left) and HR-TEM (right) micrographs with SAED patterns (inset) of as-prepared MnO2 NPs.
Cytotoxicity Effect of MnO2 NPs Against MCF-7 Breast Cancer Cell Line
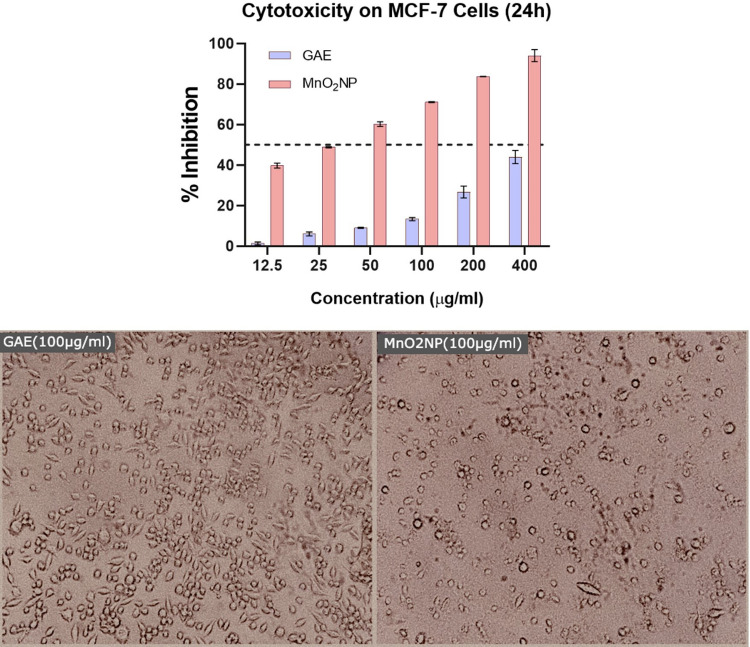
Both GAE and MnO2 NPs exhibited dose-dependent cytotoxicity on breast adenocarcinoma MCF-7 cell lines. However, the GAE coated with MnO2 NPs significantly showed better activity with respect to GAE alone (Figure 5). The cells were treated in hypoxic conditions for 24 h, with differential groupings of the tests going from 12.5, 25, 50, 100, 200 and 400 μg/mL. Moreover, the nanoparticle conveyance of GAE was found to reduce the dose required to inhibit the in vitro MCF-7 cell growth. The highest observed activity of GAE was 46% inhibition of MCF-7 cells at 400 μg/mL, while the MnO2 NPs inhibited up to 96% of the cells. Thereby implying that MnO2 NPs guided conveyance of G. arborea improves its antitumor action.17 Additionally, the treatment of MCF-7 cells with the GAE coated MnO2 NPs changed the cellular morphology to rounded, thereby indicating the normalization of cells.18,19
Figure 5.
Cytotoxicity of as-prepared MnO2 NPs against MCF-7 cells (top). Photomicrographs of MCF-7 cells treated with GAE (100 μg/mL) and MnO2 NPs (100 μg/mL).
Conclusions and Future Perspectives
In conclusion, MnO2 NPs from Gmelina arborea, a medicinally important plant (fruit extract) were successfully generated. The HRTEM analysis was used to determine spherical morphology and scale of freshly collected MnO2 NPs. The tentative efficacy of the MnO2 NPs as tumoricidal and antibacterial agents was carried out to confirm the bioactivities of MnO2 NPs were found to be substantially enhanced when they were bio-conjugated with GAE. Furthermore, bio-conjugation of MnO2 NPs can provide enough scope for the therapeutic use of Gmelina arborea, assuming appropriate mechanistic evaluations are conducted. Comprehensive evaluation of safety is an urgent problem for the clinical application of nanomaterials. Although MnO2 nanoparticles have excellent performance for enhanced cancer therapy, the detailed toxicity profiles and metabolic mechanism of MnO2-based nanomaterials should be further evaluated to ensure their future safe clinical applications. Improving the selectivity and specificity of MnO2-based nanoplatforms in cancer therapy will be another challenge in the future. Furthermore, in addition to the mostly explored tumor theranostics, these MnO2-based nanoplatforms are expected to have more applications in other biomedical fields, such as tissue engineering, anti-bacterial, diabetes, and even cardiovascular diseases. Those research directions, however, require further in-depth studies.
Acknowledgments
The authors thank the Director, Indian Institute of Science, Bengaluru for analytical facilities. SPK is grateful to the Director, Amrita Vishwa Vidyapeetham, Mysuru campus for infrastructure facility. SP, SKP and CS acknowledge the facility and infrastructure provided by the JSS Academy of Higher Education and Research (JSSAHER), Mysuru, India. The authors would like to thank Taif University, Taif, Saudi Arabia, for their support (Taif University Researchers Supporting Project number: TURSP-2020/80), Taif University, Taif, Saudi Arabia. The authors are grateful to the financial assistance from DUSMYTR-GRANT SANCTION LETTER; No;DU/HRM/2020-21/6045/Dated: 03-03-2021 Davangere University, Davangere, Karnataka India and Authors acknowledge the support and infrastructure provided by the Davangere University, Davangere, Karnataka, India.
Disclosure
The authors declare that there are no conflicts of interest.
References
- 1.Zhu X, Liu Y, Yuan G, et al. In situ fabrication of MS@MnO2 hybrid as nanozymes for enhancing ROS-mediated breast cancer therapy. Nanoscale. 2020;12(43):22317–22329. doi: 10.1039/D0NR03931D [DOI] [PubMed] [Google Scholar]
- 2.Sun P, Deng Q, Kang L, Sun Y, Ren J, Qu X. A smart nanoparticle-laden and remote-controlled self-destructive macrophage for enhanced chemo/chemodynamic synergistic therapy. ACS Nano. 2020;14(10):13894–13904. doi: 10.1021/acsnano.0c06290 [DOI] [PubMed] [Google Scholar]
- 3.Sharma GN, Dave R, Sanadya J, Sharma P, Sharma KK. Various types and management of breast cancer: an overview. J Adv Pharm Technol Res. 2010;1(2):109–126. [PMC free article] [PubMed] [Google Scholar]
- 4.Peng L, Xu T, Long T, Zuo H. Association between BRCA status and P53 status in breast cancer: a meta-analysis. Med Sci Monit. 2016;22:1939–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen WY. Patient education: factors that modify breast cancer risk in women (Beyond the Basics); 2015. Available from: http://www.uptodate.com/contents/factors-that-modify-breast-cancer-risk-in-women-beyond-the-basics. Accessed January 25, 2022.
- 6.Sahu A, Kwon I, Tae G. Improving cancer therapy through the nanomaterials-assisted alleviation of hypoxia. Biomaterials. 2020;228:119578. doi: 10.1016/j.biomaterials.2019.119578 [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Guo J, Huang L. Modulation of tumor microenvironment for immunotherapy: focus on nanomaterial-based strategies. Theranostics. 2020;10(7):3099–3117. doi: 10.7150/thno.42998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Hao Y, Popovtzer R, Feng L, Liu Z. Construction of enzyme nanoreactors to enable tumor microenvironment modulation and enhanced cancer treatment. Adv Healthcare Mater. 2020;10:2001167. doi: 10.1002/adhm.202001167 [DOI] [PubMed] [Google Scholar]
- 9.Gao F, Tang Y, Liu WL, et al. Intra/extracellular lactic acid exhaustion for synergistic metabolic therapy and immunotherapy of tumors. Adv Mater. 2019;31(51):1904639. doi: 10.1002/adma.201904639 [DOI] [PubMed] [Google Scholar]
- 10.Kollur SP, Alakananda P. Green synthesis of MnO2 nanorods using Phyllanthus amarus plant extract and their fluorescence studies. Green Process Synth. 2017;6:549–554. [Google Scholar]
- 11.Korrat A, Greiner T, Maurer M, Metz M, Fiebig HH. Gene signature-based prediction of tumor response to cyclophosphamide. Cancer Genomics Proteomics. 2007;4(3):187–195. [PubMed] [Google Scholar]
- 12.Essien ER, Atasie VN, Oyebanji TO, Nwude DO. Biomimetic synthesis of magnesium oxide nanoparticles using Chromolaena odorata (L.) leaf extract. Chem Pap. 2020;74(7):2101–2109. doi: 10.1007/s11696-020-01056-x [DOI] [Google Scholar]
- 13.Abdallah Y, Ogunyemi SO, Abdelazez A, et al. The green synthesis of MgO nano-flowers using Rosmarinus officinalis L. (Rosemary) and the antibacterial activities against Xanthomonas oryzae pv. oryzae. Biomed Res Int. 2019;1–8. doi: 10.1155/2019/5620989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang JW, Chen Y, Chen BZ. A synthesis method of MnO2/activated carbon composite for electrochemical supercapacitors. J Electrochem Soc. 2015;162:1654–1661. doi: 10.1149/2.0031509jes [DOI] [Google Scholar]
- 15.Nguta JM, Appiah-Opong R, Nyarko AK, et al. In vitro antimycobacterial and cytotoxic data on medicinal plants used to treat tuberculosis. Data Brief. 2016;7:1124–1130. doi: 10.1016/j.dib.2016.03.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumbar VM, Peram MR, Kugaji MS, et al. Effect of curcumin on growth, biofilm formation and virulence factor gene expression of Porphyromonas gingivalis. Odontology. 2021;109(1):18–28. doi: 10.1007/s10266-020-00514-y [DOI] [PubMed] [Google Scholar]
- 17.Shiva PK, Shashanka KP, Ravindra V, et al. Antitumor potential of green synthesized ZnONPs using root extract of withania somnifera against human breast cancer cell line. Separations. 2021;8(1):8. doi: 10.3390/separations8010008 [DOI] [Google Scholar]
- 18.Shiva PK, Shashanka KP, Sushma P, et al. Luteolin-fabricated ZnO nanostructures showed PLK-1 mediated anti-breast cancer activity. Biomolecules. 2021;11(3):385. doi: 10.3390/biom11030385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shashanka KP, Prashanth MV, Pushkal SR, Suma MN, Devananda D, Subbarao VM. Phytochemical fractions from annona muricata seeds and fruit pulp inhibited the growth of breast cancer cells through cell cycle arrest at G0/G1 phase. J Cancer Res Ther. 2020;16:1235–1249. doi: 10.4103/jcrt.JCRT_494_19 [DOI] [PubMed] [Google Scholar]