Abstract
MET tyrosine kinase inhibitors, capmatinib and tepotinib, have been recently introduced for the treatment of advanced NSCLC with MET exon 14 skipping mutations. Although interstitial lung disease (ILD) induced by these drugs is reported, its optimal management and whether they can be rechallenged remain unclear. We report the first successful case of tepotinib treatment after capmatinib-induced ILD. Switching MET tyrosine kinase inhibitors after drug-induced ILD could be a clinical option, which warrants further investigation.
Keywords: MET exon 14 skipping, Capmatinib, Tepotinib, Interstitial lung disease, Case report
Introduction
In the past two decades, actionable genetic alterations such as EGFR mutations and EML4-ALK fusion have been identified in NSCLC. Among these alterations, MET exon 14 skipping mutation is found in 3% to 4% of patients with NSCLC.1 MET tyrosine kinase inhibitors (TKIs), capmatinib and tepotinib, were currently approved for patients with advanced NSCLC with MET exon 14 skipping mutations. These drugs were found to have considerable efficacy; the GEOMETRY mono-1 trial for capmatinib revealed 68% of objective response rate2 and the VISION trial for tepotinib revealed 46% of objective response rate.3
Drug-induced interstitial lung disease (ILD) is a potentially serious adverse effect of these TKIs. The incidences of ILD were reported to be 6.2% in the GEOMETRY mono-1 trial and 3.8% in the VISION trial. Nevertheless, optimal management for ILD owing to these specific TKIs has not been established.
Here, we present a case of lung adenocarcinoma treated with tepotinib successfully after capmatinib-induced ILD.
Case Presentation
A 75-year-old Japanese female never-smoker was presented with a small lung nodule in the right upper lobe, bilateral enlargement of mediastinal lymph nodes, and tiny lung nodule in the left lower lobe on a chest computed tomography (CT) scan. She had no previous lung disease. A transbronchial needle aspiration from the enlarged lymph nodes and additional examinations revealed clinical stage IVA (T1aN3M1a) lung adenocarcinoma without EGFR mutations, ALK translocations, nor ROS1 fusions. Programmed death-ligand 1 tumor proportion score for the specimens was 90% using the programmed death-ligand 1 IHC 22C3 pharmDx antibody (Dako Inc.). She received three lines of anticancer drug therapy serially for 4 years: first, pembrolizumab; second, carboplatin, pemetrexed, and bevacizumab; and third, carboplatin and nab-paclitaxel. Her lung cancer relapsed with single brain metastasis, which was treated with stereotactic irradiation, and then hilar lymph node metastases appeared. Fourth-line chemotherapy with tegaful, gimeracil, and oteracil potassium was introduced; however, it was discontinued 3 months after owing to grade 3 neutropenia despite its efficacy. Meanwhile, we undertook next-generation sequencing analysis of the biopsy specimens with FoundationOne CDx and found a MET exon 14 skipping mutation. She had not received any radiotherapy to the chest.
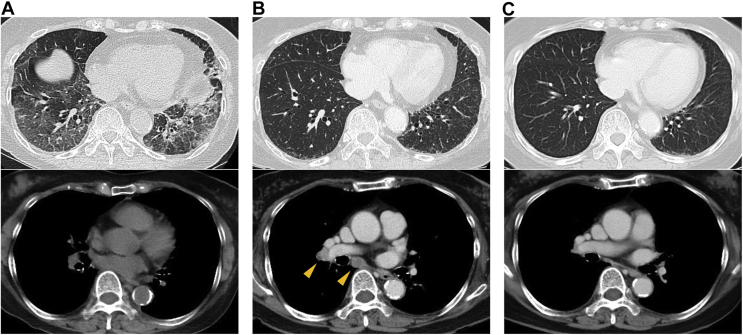
After 2 months from the discontinuation of the fourth-line chemotherapy, while the disease was stable in the chest, the brain metastasis became enlarged and serum carcinoembryonic antigen (CEA) level increased. She was treated with oral capmatinib at a dose of 400 mg twice daily. Nevertheless, after the treatment for 6 days, mild cough and dyspnea developed and a chest radiograph and CT scan revealed widespread ground-glass opacities in both lungs (Fig. 1A). Capmatinib was immediately discontinued with a clinical diagnosis of grade 2 capmatinib-induced ILD. Corticosteroids were not used at this point as her symptoms were very mild and her oxygen saturation level was consistently normal. After the discontinuation, her symptoms disappeared within several days and the radiological findings also improved. Meanwhile, the serum CEA level decreased from 14.7 ng/mL to 10.6 ng/mL.
Figure 1.
Chest computed tomography scans (A) at the time of capmatinib-induced ILD, (B) at the time of recurrence 2 months after discontinuing capmatinib, and (C) 7 weeks after starting tepotinib. Yellow arrowheads: enlarged hilar and mediastinal lymph nodes. ILD, interstitial lung disease.
After 2 months from the discontinuation of capmatinib, a chest CT scan revealed progressive disease with mediastinal lymphadenopathy (Fig. 1B) with the CEA level of 46.8 ng/mL. We administered tepotinib at a low starting dose of 250 mg once daily under careful observation. After 7 weeks, we confirmed its efficacy with shrinkage of the enlarged lymph nodes and decreased CEA level of 9.8 ng/mL without any side effect including interstitial pneumonitis (Fig. 1C). With the low dose of tepotinib, the response has still continued 6 months after the start of tepotinib. The brain metastatic lesion got bigger for the first 3 months and then shrank without any treatment change. It suggested that the transient increase in size was owing to radiation-induced brain necrosis.
Discussion
To the best of our knowledge, this is the first report of successful switch of MET TKIs after TKI-induced ILD. ILD is one of the most critical side effects of various TKIs.
Once it occurs, TKIs are principally discontinued to avoid extended lung toxicity. Nevertheless, they are sometimes rechallenged because they may be able to provide better and longer responses compared with alternative regimens with cytotoxic agents. There have been some case reports of rechallenges after TKI-induced ILD for other types of TKIs, such as gefitinib, erlotinib, and crizotinib, which have longer history for NSCLC treatment among various TKIs. Recently, two cases of successful treatment with lorlatinib after alectinib-induced ILD were also reported.4
Mechanisms of TKI-induced ILD in general are not well known. For MET, signaling by hepatocyte growth factor and its receptor MET is implicated in an antifibrotic role and a role in epithelial-mesenchymal transition in the alveolar epithelium. Nevertheless, whether these roles have any relation to the mechanisms of MET TKI-induced ILD has been completely unclear. In addition, dose intensity is generally thought to be irrelevant to the lung toxicities.
Although MET TKI-induced ILDs have been reported,2,3,5 whether MET TKIs can be rechallenged after MET TKI-induced ILDs remains unknown. In this case, we decided to switch from capmatinib to tepotinib after capmatinib-induced ILD because she still had a chance to get a survival benefit from the MET TKIs after a series of multiple anticancer regimens. In addition, we assumed that the risk of severe ILD owing to tepotinib was relatively low in consideration of the facts that her capmatinib-induced ILD did not have diffuse alveolar damage pattern, for which mortality rate is generally high,6 and that she recovered from capmatinib-induced ILD immediately after capmatinib discontinuation without any additional therapies including corticosteroid administration.
This is also the first report of a case that received two kinds of the same type Ib MET TKIs, capmatinib and tepotinib, for which differences of mechanisms of action and toxicity profiles are unclear. In this case, despite little knowledge on whether the dose determination for both drugs is related to their efficacy or side effects, we started tepotinib with a decreased dose on the patient’s strong request.
Conclusion
We experienced the first successful case of tepotinib challenge after capmatinib-induced ILD. Rechallenges of MET TKIs can be considered under strict informed consent and careful observation, which warrants further investigation.
CRediT Authorship Contribution Statement
Mizuha Haraguchi Hashiguchi: Investigation, Writing—original draft preparation, Visualization.
Takashi Sato: Conceptualization, Writing—review and editing, Supervision, Project administration.
Hiroki Yamamoto: Writing—review and editing, Visualization.
Rinako Watanabe: Investigation, Writing—review and editing.
Junko Kagyo, Tetsuya Shiomi: Writing—review and editing, Supervision.
Hideharu Domoto: Resources, Writing—review and editing.
Acknowledgment
Written informed consent to publish the patient’s case information and related images was obtained from her.
Footnotes
Disclosure: Dr. Hashiguchi reports receiving personal fees from AstraZeneca outside of the submitted work. Dr. Sato reports receiving personal fees from Chugai Pharmaceutical, Bristol-Myers Squibb, Boehringer Ingelheim, and Ono Pharmaceutical outside of the submitted work. Dr. Shiomi reports receiving personal fees from Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical, Pfizer, and Taiho Pharmaceutical outside of the submitted work. The remaining authors declare no conflict of interest.
Cite this article as: Hashiguchi MH, Sato T, Yamamoto H, et al. Successful tepotinib challenge after capmatinib-induced interstitial lung disease in a patient with lung adenocarcinoma harboring MET exon 14 skipping mutation: case report. JTO Clin Res Rep. 2022;3:100271.
References
- 1.Tsuta K., Kozu Y., Mimae T., et al. c-MET/phospho-MET protein expression and MET gene copy number in non-small cell lung carcinomas. J Thorac Oncol. 2012;7:331–339. doi: 10.1097/JTO.0b013e318241655f. [DOI] [PubMed] [Google Scholar]
- 2.Wolf J., Seto T., Han J.Y., et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383:944–957. doi: 10.1056/NEJMoa2002787. [DOI] [PubMed] [Google Scholar]
- 3.Paik P.K., Felip E., Veillon R., et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383:931–943. doi: 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myall N.J., Lei A.Q., Wakelee H.A. Safety of lorlatinib following alectinib-induced pneumonitis in two patients with ALK-rearranged non-small cell lung cancer: a case series. Transl Lung Cancer Res. 2021;10:487–495. doi: 10.21037/tlcr-20-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanemura H., Takeda M., Shimizu S., Nakagawa K. Interstitial lung disease associated with capmatinib therapy in a patient with non-small cell lung cancer harboring a skipping mutation of MET exon 14. Thorac Cancer. 2021;12:549–552. doi: 10.1111/1759-7714.13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endo M., Johkoh T., Kimura K., Yamamoto N. Imaging of gefitinib-related interstitial lung disease: multi-institutional analysis by the West Japan Thoracic Oncology Group. Lung Cancer. 2006;52:135–140. doi: 10.1016/j.lungcan.2006.02.002. [DOI] [PubMed] [Google Scholar]