Abstract
Nine-banded armadillos (Dasypus novemcinctus) are naturally infected with Mycobacterium leprae and are implicated in the zoonotic transmission of leprosy in the United States. In Mexico, the existence of such a reservoir remains to be characterized. We describe a wild armadillo infected by M. leprae in the state of Nuevo León, Mexico.
Keywords: leprosy, Mycobacterium leprae, nine-banded armadillo, Dasypus novemcinctus, whole-genome sequencing, Nuevo León, Mexico, tuberculosis and other mycobacteria, zoonoses, Hansen disease
Nine-banded armadillos (Dasypus novemcinctus) can be naturally infected with Mycobacterium leprae and have been implicated in the zoonotic transmission of leprosy in the US states of Texas, Louisiana, Alabama, Georgia, and Florida (1,2). Despite Mexico falling within the armadillos’ natural geographic habitat and the report of 182 new human leprosy cases in Mexico in 2019 (3), only 1 report of an armadillo infected with acid-fast bacilli has occurred since 1984, and the bacterial species in that case was never fully characterized (4).
In 2019, a nine-banded armadillo with ataxia, dyspnea, and adynamia was captured along the Pilon River in Montemorelos in the state of Nuevo León, Mexico. The animal was euthanized, and necropsy revealed granulomatous lesions in diverse organs and tissues (Appendix Figure 1). Histopathologic examination identified acid-fast bacilli in the liver, lung, heart, striated muscle, and ear; the bacilli were especially abundant in the spleen (Figure; Appendix Figure 2). We confirmed the presence of M. leprae in tissue by PCR testing of DNA extracted from the ear, liver, and lung by using the specific repetitive element RLEP (5) (Appendix). We used bacterial DNA extracted from the liver of the infected armadillo (strain A1), harboring the highest bacilli number by microscopy, for library preparation, followed by targeted enrichment using hybridization capture and whole-genome sequencing using NextSeq 500 (Illumina, https://www.illumina.com) (Appendix). The mean read coverage of 87× was sufficient for further comparative analysis at the single nucleotide level with other M. leprae isolates (Appendix Table 1). The armadillo-derived A1 strain belongs to genotype 3I-2, similar to other M. leprae isolates from the United States, Venezuela, Brazil, and Mexico (1).
Figure.
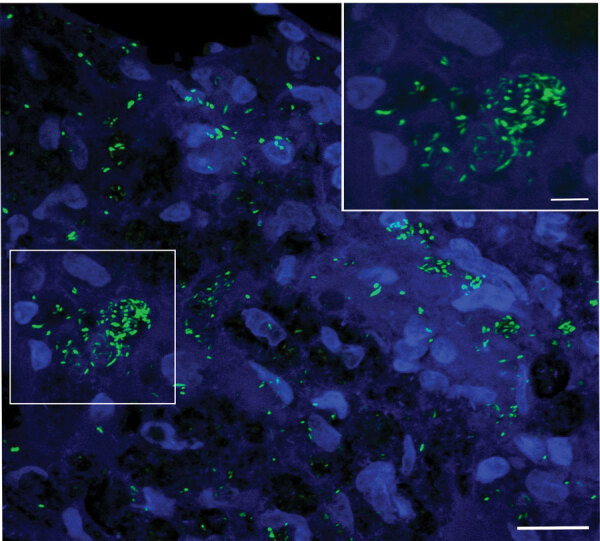
Identification and characterization of leprosy and Mycobacterium leprae acid-fast bacilli in the tissue in the wild nine-banded armadillo (Dasypus novemcinctus), Nuevo León, Mexico. SYBR gold staining shows a high density of bacilli in the spleen tissue organized in globi (boxed area at left and inset at right). Image is a merger of 16 images, 0.33 µm apart, in a z-stack taken with a 100× objective lens. Scale bars represent 20 µm (main image) and 5 µm (inset).
Phylogenetically, A1 branches between the US human (NHDP-98) and animal–human (I30, NHDP-63, NHDP-55) M. leprae strains and closely clusters with EGG (6), a strain isolated in 2014 from a 70 year-old man with leprosy living in Nuevo León, Mexico (Appendix Figure 4, 5). Strains A1 and EGG share 9 polymorphisms when compared with the whole-genome sequences from 295 other M. leprae isolates and differed from each other by only 5 single-nucleotide polymorphisms (SNPs) (Appendix Figure 6).
We submitted DNA from M. leprae isolates recovered from the biopsies of additional leprosy patients from the states of Nuevo León (n = 9) and Jalisco (n = 2), Mexico, to partial whole-genome sequencing (n = 4) and PCR genotyping (n = 7) (Appendix Table 2, Figure 5). We deciphered their clustering from previously described positions specific to genotypes 3I-1 and 3I-2 (1) as well as new informative SNPs specific to EGG and A1 (Appendix Table 2, Figure 6). Partial genome reconstruction for all 11 isolates revealed that 4 of them belong to genotype 3I-1, whereas 7 belong to genotype 3I-2. Within genotype 3I-1, isolates F2, F6, and F11 belong to a similar cluster, named 3I-1-c2 (Appendix Figures 4, 5). Within genotype 3I-2, 4 isolates (F1, F8, F14, and F23) belong to the same cluster, named 3I-2-c3, which also encompasses A1 and EGG. Of these isolates, only F1 shared an additional common SNP with A1 (Appendix Figures 4, 6). All patients infected with an M. leprae isolate from cluster 3I-2-c3 live in close vicinity (radius of ≈100 km) to the city of Montemorelos, where the infected armadillo was captured (Appendix Figure 5).
We describe the identification and genetic characterization of Mycobacterium leprae in a wild nine-banded armadillo in Mexico. In addition, we show that M. leprae strains belonging to different clusters are circulating in patients in Mexico. The state of Nuevo León, Mexico, shares a border with the US state of Texas, where a high density of leprosy-infected nine-banded armadillos have been reported (4,7). Nine-banded armadillos expanded their range into the United States in the mid-1800s from Mexico (8).
The M. leprae armadillo isolate from Mexico we describe belongs to the same genotype as patients and armadillo isolates from the United States but clusters separately. Isolate A1 further clusters with human isolates exclusively identified in Mexico thus far, with which it displays similar low genetic variation as observed between animal and human isolates in the United States (1). Therefore, our results raise concerns that wild-banded armadillos may, similarly to the situation in the United States, serve as reservoirs for the leprosy bacillus in the state of Nuevo León and call for additional surveillance across Mexico to assess the spread of the disease in the animal population and evaluate zoonosis risks associated with human contact with armadillos.
The existence of an animal reservoir hosting the leprosy bacillus in Mexico threatens the goal of leprosy elimination. In light of our results, we propose that interventions based on a One Health approach may be more efficient in achieving eradication of the disease.
Additional information about Mycobacterium leprae infection in a wild nine-banded armadillo, Nuevo León, Mexico.
Acknowledgments
We are grateful to all the patients and clinical staff who participated in the study. We thank Mark Stenglein, Marylee Kapuscinski, Mikaela Samsel for Illumina sequencing and technical support, and Dan Sloan for facilitating access to his laboratory.
This work was supported by the Fondation Raoul Follereau (grants to M.J. and C.A.), the Heiser Program of the New York Community Trust for Research in Leprosy (grant no. P18-000250 to J.S.S. and C.A.), a Fulbright Scholar to Brazil award 2019–2020 (grant to J.S.S.), the Association de Chimiothérapie Anti-Infectieuse of the Société Française de Microbiologie, the European Union’s Horizon 2020 Research and Innovation Program (Marie Sklodowska-Curie grant no. 845479 to C.A.), the National Institutes of Health (grant no. 1 S10 RR023735 [Zeiss LSM 510 Laser Scanning Microscope] to M.G.J.), and the Programa de Apoyo a la Investigación Científica y Tecnológica of the Universidad Autonoma de Nuevo León (grant no. SA-1900-21).
Biography
Dr. Vera-Cabrera is a professor of dermatology at the Faculty of Medicine in University of Nuevo León, Monterrey, Mexico. His research interests include immune response against intracellular infectious agents and pathogenic mechanisms of bacteria of the genus Mycobacterium and Nocardia.
Footnotes
Suggested citation for this article: Vera-Cabrera L, Ramos-Cavazos CJ, Youssef NA, Pearce CM, Molina-Torres CA, Avalos-Ramirez R, et al. Mycobacterium leprae infection in a wild nine-banded armadillo, Nuevo León, Mexico. Emerg Infect Dis. 2022 Mar [date cited]. https://doi.org/10.3201/eid2803.211295
References
- 1.Truman RW, Singh P, Sharma R, Busso P, Rougemont J, Paniz-Mondolfi A, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364:1626–33. 10.1056/NEJMoa1010536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma R, Singh P, Loughry WJ, Lockhart JM, Inman WB, Duthie MS, et al. Zoonotic leprosy in the southeastern United States. Emerg Infect Dis. 2015;21:2127–34. 10.3201/eid2112.150501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global leprosy (Hansen disease) update, 2019: time to step-up prevention initiatives. Wkly Epidemiol Rec. 2020;36:417–40. [Google Scholar]
- 4.Ploemacher T, Faber WR, Menke H, Rutten V, Pieters T. Reservoirs and transmission routes of leprosy; A systematic review. PLoS Negl Trop Dis. 2020;14:e0008276. 10.1371/journal.pntd.0008276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braet S, Vandelannoote K, Meehan CJ, Brum Fontes AN, Hasker E, Rosa PS, et al. The repetitive element RLEP is a highly specific target for detection of Mycobacterium leprae. J Clin Microbiol. 2018;56:e01924–17. 10.1128/JCM.01924-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjak A, Avanzi C, Singh P, Loiseau C, Girma S, Busso P, et al. Phylogenomics and antimicrobial resistance of the leprosy bacillus Mycobacterium leprae. Nat Commun. 2018;9:352. 10.1038/s41467-017-02576-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng X, Papeş M. Ecological niche modelling confirms potential north-east range expansion of the nine-banded armadillo (Dasypus novemcinctus) in the USA. J Biogeogr. 2015;42:803–7. 10.1111/jbi.12427 [DOI] [Google Scholar]
- 8.Taulman JF, Robbins LW. Range expansion and distributional limits of the nine-banded armadillo in the United States: an update of Taulman & Robbins (1996). J Biogeogr. 2014;41:1626–30. 10.1111/jbi.12319 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information about Mycobacterium leprae infection in a wild nine-banded armadillo, Nuevo León, Mexico.


