Abstract
Dispersal plays a vital role in the geographical distribution, population genetic structure, quantity dynamics, and evolution of a species. Sex‐biased dispersal is common among vertebrates and many studies have documented a tendency toward male‐biased dispersal in mammals and female‐biased dispersal in birds. However, dispersal patterns in reptiles remain poorly understood. In this study, we explored the genetic diversity and dispersal patterns of the widely distributed Asian pitviper Protobothrops mucrosquamatus. In total, 16 polymorphic microsatellite loci were screened in 150 snakes (48 males, 44 females, 58 samples without sex information) covering most of their distribution. Microsatellite analysis revealed high genetic diversity in P. mucrosquamatus. Bayesian clustering of population assignment identified two major clusters for all populations, somewhat inconsistent with the mitochondrial DNA phylogeny of P. mucrosquamatus reported in previous research. Analyses based on 92 sex‐determined and 37 samples of P. mucrosquamatus from three small sites in Sichuan, China (Mingshan, Yibin, and Zizhong) consistently suggested female‐biased dispersal in P. mucrosquamatus, which is the first example of this pattern in snakes. The female‐biased dispersal patterns in P. mucrosquamatus may be explained by local resource competition.
Keywords: genetic diversity, microsatellites, Protobothrops mucrosquamatus, sex‐biased dispersal, snake
In this study, we explored the genetic diversity and dispersal patterns of the widely distributed Asian pitviper Protobothrops mucrosquamatus. Microsatellite analysis revealed high genetic diversity in P. mucrosquamatus. Analyses based on sex‐determined samples suggested female‐biased dispersal in P. mucrosquamatus, which is the first example of this pattern in snakes. The female‐biased dispersal patterns in P. mucrosquamatus may be explained by local resource competition.

1. INTRODUCTION
Dispersal plays a vital role in the life history of a species by influencing population structure, quantity dynamics, genetic diversity, and species evolution (Guerrini et al., 2014; Ronce, 2007; Trochet et al., 2016). While movement may entail substantial costs in terms of death and unknown future habitat (Greenwood & Harvey, 1982; Howard, 1960), immigrant individuals gain certain benefits, such as inbreeding avoidance and increased breeding opportunities. In vertebrates, individuals of one sex often disperse more or further than individuals of the other sex, i.e., sex‐biased dispersal. Currently, 257 species have been reported to show sex‐dispersal patterns, including seven species of invertebrate arthropods, 118 species of birds, 110 species of mammals, four species of fish, 14 species of reptiles, and four species of amphibians (Trochet et al., 2016). Many studies had documented a tendency toward male‐biased dispersal in mammals and female‐biased dispersal in birds (Corrales & Höglund, 2012; Costello et al., 2008; Greenwood, 1980; Nemesházi et al., 2018; Paplinska et al., 2009; Song et al., 2015; Vangestel et al., 2013). Based on mammalian and bird studies, several hypotheses have been proposed to explain sex‐biased dispersal, including resource competition (Greenwood, 1980), local mate competition (Dobson, 1982; Perrin & Mazalov, 2000), and inbreeding avoidance (Perrin & Mazalov, 2000; Pusey, 1987). However, compared with birds and mammals, comparatively fewer studies have been conducted on dispersal patterns in reptiles (Dubey et al., 2008; Hofmann et al., 2012; Johansson et al., 2008; Keogh et al., 2007; Qi et al., 2013; Ujvari et al., 2008; Urquhart et al., 2009; Wang et al., 2019).
Protobothrops mucrosquamatus (Cantor, 1839) (Figure 1) is a medium‐sized Asian pitviper distributed in southwest and southeast China, Laos, northern Bangladesh, Vietnam, northern Myanmar, and northeastern India (Zhao, 2006). Due to the wide distribution of P. mucrosquamatus, it is easy to be encountered in the field. Thus, it is a very ideal species to explore its genetics, evolution, and ecology. Zhong et al. (2017) examined and morphologically compared 142 specimens of P. mucrosquamatus and identified sexual dimorphism within the species but no significant morphological differences among the populations, despite their wide distribution. Based on two mitochondrial DNA fragments and two nuclear genes, Guo et al. (2019) explored the genetic diversity and population evolutionary history of P. mucrosquamatus and found five geographically structured and well‐supported mtDNA matrilineal lineages within the species. However, due to the limited genes, the DNA sequences did not provide much additional information on population structure.
FIGURE 1.

The photo of Protobothrops mucrosquamatus in life
Microsatellites, also known as simple sequence repeats (SSR), are recurring motifs of 1–6 nucleotides found in the genomes of eukaryotes (Selkoe & Toonen, 2006). In comparison to other polymerase chain reaction (PCR)‐based methods, including inter‐simple sequence repeat (ISSR), randomly amplified polymorphic DNA (RAPD), and amplified fragment length polymorphism (AFLP), microsatellites represent a powerful marker due to their codominant inheritance and high polymorphism, and have been widely used in phylogeographic, population, and parental analyses (Guichoux et al., 2011; Hodel et al., 2016; Qin et al., 2017). In this study, based on microsatellite markers, we explored the genetic diversity and population genetic structure of P. mucrosquamatus, and determined whether sex‐biased dispersal exists in this species.
2. MATERIALS AND METHODS
2.1. Sampling and RAD sequencing
In total, 150 P. mucrosquamatus snakes covering most of their range were collected between 1994 and 2018 through fieldwork or tissue loans from colleagues and museums (Figure 2 and Table 1). Liver and muscle tissue samples were taken and preserved in 90% ethanol. Whole genomic DNA was extracted using a TIANamp Genomic DNA kit (Tiangen Biotech (Beijing) Co., Ltd.) following the manufacturer's protocols.
FIGURE 2.
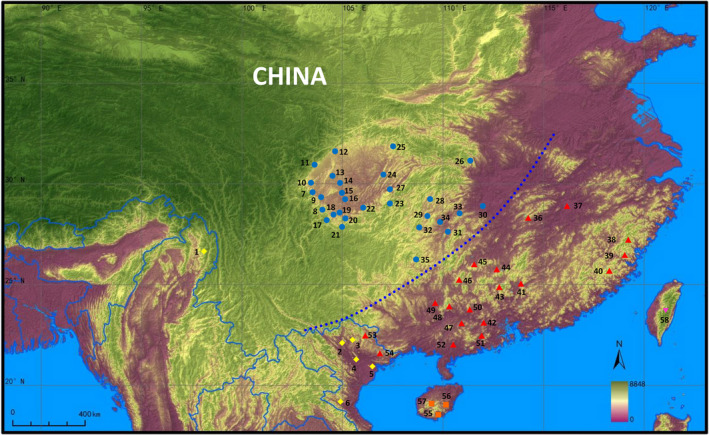
Topographic map of China and adjoining countries showing sampling localities for Protobothrops mucrosquamatus across 58 localities. Numbers indicate specimen localities numbered in Table 1. Blue dotted line separates two clusters detected in STRUCTURE; filled circles: SWC (blue); diamonds: VM (yellow); squares: HN (orange); inverted triangles: TW (purple); triangles: SCV (red)
TABLE 1.
Sample information for Protobothrops mucrosquamatus analyzed in this study (CAS: California Academy of Science, San Francisco; ROM: Royal Ontario Museum, Toronto; AM: Anita Malhotra catalogue number; GP: Guo Peng, own catalogue number)
| Individual ID | Location | Location No | Population | Sex |
|---|---|---|---|---|
| CAS224693 | KaChin State, Myanmar | 1 | VM | |
| CAS232934 | KaChin State, Myanmar | 1 | VM | |
| ROM6551 | Tuyen Quang, Vietnam | 2 | VM | |
| ROM6809 | Tuyen Quang, Vietnam | 2 | VM | |
| ROM14465 | Bac Thai, Vietnam | 3 | VM | |
| AMB744 | Vinh Phuc, Tam Dao, Vietnam | 4 | VM | |
| AMB746 | Vinh Phuc, Tam Dao, Vietnam | 4 | VM | |
| AMB748 | Vinh Phuc, Tam Dao, Vietnam | 4 | VM | |
| ROM14489 | Vinh Phu, Tam Dao, Vietnam | 4 | VM | |
| ROM18207 | Vinh Phu, Tam Dao, Vietnam | 4 | VM | |
| ROM24163 | Hia Duong, Vietnam | 5 | VM | |
| ROM25111 | Hia Duong, Vietnam | 5 | VM | |
| ROM25716 | Nghe An, Vietnam | 6 | VM | |
| ROM25715 | Nghe An, Vietnam | 6 | VM | |
| GP4510 | Tianquan, Sichuan, China | 7 | SWC | |
| GP4682 | Leshan, Sichuan, China | 8 | SWC | M |
| GP4683 | Leshan, Sichuan, China | 8 | SWC | F |
| GP31 | Liujiang, Hongya, Sichuan | 9 | SWC | |
| GP2057 | Mingshan, Sichuan, China | 10 | SWC | F |
| GP2065 | Mingshan, Sichuan, China | 10 | SWC | M |
| GP2066 | Mingshan, Sichuan, China | 10 | SWC | M |
| GP2068 | Mingshan, Sichuan, China | 10 | SWC | F |
| GP2428 | Mingshan, Sichuan, China | 10 | SWC | M |
| GP1381 | Mingshan, Sichuan, China | 10 | SWC | M |
| GP2067 | Mingshan, Sichuan, China | 10 | SWC | M |
| GP2058 | Mingshan, Sichuan, China | 10 | SWC | |
| GP2426 | Mingshan, Sichuan, China | 10 | SWC | M |
| GP2427 | Mingshan, Sichuan, China | 10 | SWC | M |
| GP2422 | Mingshan, Sichuan, China | 10 | SWC | F |
| GP2425 | Mingshan, Sichuan, China | 10 | SWC | M |
| GP2543 | Dujiangyan, Sichuan, China | 11 | SWC | |
| GP1041 | Anxian, Sichuan, China | 12 | SWC | |
| GP1575 | Jianyang, Sichuan, China | 13 | SWC | M |
| GP314 | Longquan, Sichuan, China | 13 | SWC | |
| GP1578 | Jianyang, Sichuan, China | 13 | SWC | F |
| GP1579 | Jianyang, Sichuan, China | 13 | SWC | F |
| GP1580 | Jianyang, Sichuan, China | 13 | SWC | M |
| GP1209 | Ziyang, Sichuan, China | 14 | SWC | M |
| GP2172 | Zizhong, Sichuan, China | 15 | SWC | F |
| GP2173 | Zizhong, Sichuan, China | 15 | SWC | M |
| GP2174 | Zizhong, Sichuan, China | 15 | SWC | M |
| GP2175 | Zizhong, Sichuan, China | 15 | SWC | M |
| GP2176 | Zizhong, Sichuan, China | 15 | SWC | M |
| GP2177 | Zizhong, Sichuan, China | 15 | SWC | M |
| GP2178 | Zizhong, Sichuan, China | 15 | SWC | F |
| GP2179 | Zizhong, Sichuan, China | 15 | SWC | M |
| GP2180 | Zizhong, Sichuan, China | 15 | SWC | F |
| GP2181 | Zizhong, Sichuan, China | 15 | SWC | F |
| GP2182 | Zizhong, Sichuan, China | 15 | SWC | M |
| GP2183 | Zizhong, Sichuan, China | 15 | SWC | F |
| GP2184 | Zizhong, Sichuan, China | 15 | SWC | F |
| GP2185 | Zizhong, Sichuan, China | 15 | SWC | F |
| GP2319 | Zigong, Sichuan, China | 16 | SWC | F |
| GP2329 | Zigong, Sichuan, China | 16 | SWC | M |
| GP2331 | Zigong, Sichuan, China | 16 | SWC | M |
| GP2453 | Pingshan, Sichuan, China | 17 | SWC | F |
| GP426 | Hengjiang, Sichuan, China | 18 | SWC | M |
| GP427 | Hengjiang, Sichuan, China | 18 | SWC | M |
| GP2470 | Yibin, Sichuan, China | 19 | SWC | M |
| GP2669 | Yibin, Sichuan, China | 19 | SWC | F |
| GP523 | Yibin, Sichuan, China | 19 | SWC | M |
| GP1380 | Yibin, Sichuan, China | 19 | SWC | M |
| GP2487 | Yibin, Sichuan, China | 19 | SWC | F |
| GP2658 | Yibin, Sichuan, China | 19 | SWC | M |
| GP5663 | Yibin, Sichuan, China | 19 | SWC | F |
| GP5559 | Yibin, Sichuan, China | 19 | SWC | M |
| GP5059 | Yibin, Sichuan, China | 19 | SWC | F |
| GP5109 | Yibin, Sichuan, China | 19 | SWC | F |
| GP5110 | Yibin, Sichuan, China | 19 | SWC | M |
| GP5494 | Yibin, Sichuan, China | 19 | SWC | M |
| GP5683 | Yibin, Sichuan, China | 19 | SWC | F |
| GP1677A | Yibin, Sichuan, China | 19 | SWC | M |
| GP659 | Changning, Sichuan, China | 20 | SWC | F |
| GP2758 | junlian, Sichuan, China | 21 | SWC | F |
| GP2759 | junlian, Sichuan, China | 21 | SWC | F |
| GP5342 | junlian, Sichuan, China | 21 | SWC | |
| GP5355 | junlian, Sichuan, China | 21 | SWC | |
| GP4368 | junlian, Sichuan, China | 21 | SWC | F |
| GP4367 | junlian, Sichuan, China | 21 | SWC | F |
| GP3358 | junlian, Sichuan, China | 21 | SWC | F |
| GP1767 | Hejiang, Sichuan, China | 22 | SWC | |
| GP965 | Hejiang, Sichuan, China | 22 | SWC | F |
| GP968 | Hejiang, Sichuan, China | 22 | SWC | F |
| GP1080 | Nanchuang, Chongqing, China | 23 | SWC | F |
| GP2764 | Guang'an, Sichuan, China | 24 | SWC | F |
| GP135 | Tongjiang, Sichuan, China | 25 | SWC | F |
| GP138 | Tongjiang, Sichuan, China | 25 | SWC | F |
| GP777 | Yichang, Hubei, China | 26 | SWC | |
| GP849 | Yichang, Hubei, China | 26 | SWC | |
| GP4726 | Yidu, Hubei, China | 26 | SWC | |
| GP5107 | Yichang, Hubei, China | 26 | SWC | M |
| GP4883 | Beibei, Chongqing, China | 27 | SWC | |
| GP4719 | Qijiang, Chongqing, China | 27 | SWC | |
| GP424 | Laifeng, Hubei, China | 28 | SWC | |
| GP2001 | Xiushan, Chongqing, China | 29 | SWC | M |
| GP2009 | Xiushan, Chongqing, China | 29 | SWC | M |
| GP887 | Taoyuan, Hunan, China | 30 | SWC | |
| GP886 | Luxi, Hunan, China | 31 | SWC | |
| GP892 | Luxi, Hunan, China | 31 | SWC | |
| GP2948 | Jiangkou, Guizhou, China | 32 | SWC | |
| GP2968 | Yinjiang, Guizhou, Sichuan | 32 | SWC | M |
| GP2976 | Yinjiang, Guizhou, Sichuan | 32 | SWC | |
| GP2013 | Huaihua, Hunan, China | 33 | SWC | M |
| GP4930 | Guzhang, Hunan, China | 34 | SWC | |
| GP4931 | Yongshun, Hunan, China | 34 | SWC | |
| GP4928 | Guzhang, Hunan, China | 34 | SWC | |
| GP2012 | Huaihua, Hunan, China | 34 | SWC | F |
| GP2476 | Pingyang, Guizhou, China | 35 | SWC | F |
| GP2472 | Pingyang, Guizhou, China | 35 | SWC | M |
| GP2916 | Liuyang, Hunan, China | 36 | SCV | F |
| GP2689 | Liuyang, Hunan, China | 36 | SCV | |
| GP3858 | Shangrao, Jiangxi, China | 37 | SCV | F |
| GP4990 | Cangnan, Zhejiang, China | 38 | SCV | M |
| GP2694 | Fuzhou, Fujian, China | 39 | SCV | M |
| GP2430 | Dehua, Fujian, China | 40 | SCV | F |
| GP2431 | Dehua, Fujian, China | 40 | SCV | F |
| GP2217 | Shixing, Guangdong, China | 41 | SCV | F |
| GP2218 | Shixing, Guangdong, China | 41 | SCV | M |
| GP2040 | Conghua, Guangdong, China | 42 | SCV | |
| GP2237 | Conghua, Guangdong, China | 42 | SCV | F |
| GP2035 | Fuzhou, Fujian, China | 43 | SCV | |
| GP749 | Ruyuan, Guangdong, China | 43 | SCV | M |
| GP1585 | Chenzhou, Hunan, China | 44 | SCV | M |
| GP1586 | Yongzhou, Hunan, China | 45 | SCV | F |
| GP1588 | Yongzhou, Hunan, China | 45 | SCV | M |
| GP1589 | Yongzhou, Hunan, China | 45 | SCV | F |
| GP1590 | Yongzhou, Hunan, China | 45 | SCV | F |
| GP3799 | Xing'an, Guangxi, China | 46 | SCV | |
| GP3800 | Xing'an, Guangxi, China | 46 | SCV | |
| GP3954 | Xing'an, Guangxi, China | 46 | SCV | |
| GP3986 | Xing'an, Guangxi, China | 46 | SCV | |
| GP4414 | Cenxi, Guangxi, China | 47 | SCV | M |
| GP4872 | Hezhou, Guangxi, China | 48 | SCV | F |
| GP745 | Jinxiu, Guangxi, China | 49 | SCV | |
| GP2542 | Jinxiu, Guangxi, China | 49 | SCV | |
| GP4434 | Wuzhou, Guangxi, China | 50 | SCV | F |
| GP4433 | Wuzhou, Guangxi, China | 50 | SCV | F |
| GP2055 | Guangzhou, China | 51 | SCV | |
| GP1622 | Maoming, Guangzhou, China | 52 | SCV | F |
| IEKB2492 | Lang Son, Vietnam | 53 | SCV | |
| ROM26695 | Cao Bang, Vietnam | 54 | SCV | |
| ROM26696 | Cao Bang, Vietnam | 54 | SCV | |
| GP2121 | Diaoluoshan, Hainan, China | 55 | HN | |
| AMB753 | Qiongzhong, Hainan, China | 56 | HN | |
| AMB754 | Qiongzhong, Hainan, China | 56 | HN | |
| GP4639 | Jianfenglin, Hainan, China | 57 | HN | |
| AMA211 | Taiwan, China | 58 | TW | |
| AMA231 | Taiwan, China | 58 | TW | |
| AMA232 | Taiwan, China | 58 | TW | |
| AMB537 | Taiwan, China | 58 | TW |
Bold represents sex‐determined individuals from the three sites from Sichuan which were used to test dispersal pattern.
High‐quality DNA was transferred to Novogene Bioinformatics Technology Co., Ltd. for restriction site‐associated DNA sequencing (RAD‐seq) according to the standard protocols, in which total genomic DNA was digested with MseI restriction enzymes. The generated library was sequenced on the Illumina HiSeq 2000 platform to produce paired‐end reads. The quality of the raw reads was assessed using FastQC v.0.11.9 (Brown et al., 2017). High‐quality reads were clustered using CD‐HIT‐EST v. 4.8.1 (Li & Godzik, 2006) and assembled into contigs using Velvet v.1.2.10 (Namiki et al., 2012).
2.2. Microsatellite amplification and genotyping
After quality filtering, the high‐throughput sequencing data were screened to locate tetra‐nucleotide perfect repeat microsatellite loci using MSDB v.2.4.2 software (Du et al., 2012). Primer pairs were designed using Primer v.3.0 (Untergasser et al., 2012), with amplicon size ranging from 100 to 250 bp. In total, 25 microsatellite markers were randomly selected for optimization, and 16 markers were subsequently used to evaluate the genetic diversity and dispersal patterns of P. mucrosquamatus.
2.3. Diversity assessment
The successfully optimized microsatellites were used to evaluate the genetic diversity of P. mucrosquamatus. PCR was performed in a 25 µl volume containing 30 ng of genomic DNA, 1 µl of each primer (10 µM), 12.5 µl of 2 × T5 Super PCR Mix (PAGE) (Beijing Tsingke Biotech Co., Ltd.), and 10 µl of nuclease‐free water. The cycling conditions included a hot start pre‐denaturation of 95°C for 4 min, followed by 35 cycles of denaturation at 94°C for 45 s, annealing at 61–63°C (according to each primer pair) for 30 s, extension at 72°C for 30 s, post‐extension at 72°C for 10 min, and heat preservation at 10°C.
The PCR product size was measured on an ABI 3730xl DNA Analyzer (Applied Biosystems) according to each forward primer labeled with fluorescent dyes (FAM, HEX, or TAMRA) and data were obtained with GeneMapper v.4.0 (Applied Biosystems). All samples were read at least three times to reduce artificial error.
All loci were characterized, and the full dataset (150 individuals) was analyzed for various genetic diversity indices. Based on the mitochondrial DNA phylogeny of P. mucrosquamatus (Guo et al., 2019), five populations were defined, i.e., Hainan (HN), Vietnam & Myanmar (VM), Southern China & Vietnam (SCV), Southwestern China (SWC), and Taiwan (TW). We used Micro‐Checker v.2.2.3 (Van Oosterhout et al., 2004) and FreeNA (Chapuis & Estoup, 2006) software to detect null alleles, stuttering, and large allele dropout errors that can occur during the interpretation of microsatellite allele sequences. If there is a higher frequency of null alleles, that is, if it exceeds 0.2 for population genetic analysis, and if it exceeds 0.08 for parental analysis, the locus can be discarded or the null allele can be eliminated by redesigning primers (Wen et al., 2013). Deviation from the Hardy‐Weinberg equilibrium (HWE) was tested for each locus across and within populations by Fisher's exact test (Guo & Thompson, 1992) implemented in GenePop v.4.6 (Rousset, 2008) using a Markov chain Monte Carlo (MCMC) approach with 10 00 steps and 1000 iterations. Cervus v.3.0 was used to calculate the number of alleles (N a), expected heterozygosity (H e), observed heterozygosity (H o), and polymorphic information content (PIC) of each microsatellite marker (Kalinowski et al., 2007). PGDSpider v.2.1.1.5 (Lischer & Excoffier, 2012) and GenAlEx v.6.5 (Peakall & Smouse, 2012) were used to perform conversions between different data formats.
2.4. Genetic structure
STRUCTURE v.2.3.4 (Pritchard et al., 2000) was used to infer population structure and assign individuals to subpopulations following the admixture model. What is more, we use sampling location as prior (LOCPRIOR) to assist the clustering in STRUCTURE v.2.3.4. The most likely number of genetic clusters (K) varied from K = 1 to K = 10, with a burn‐in of 100,000 and MCMC repeats of 1,000,000 with 10 iterations. Results were collated using Structure Harvester v.0.6.94 (Earl & Vonholdt, 2012) and visualized using Excel. Selection of the optimal K‐value was based on both the log‐likelihood value closest to zero and the ΔK parameter (Evanno et al., 2005). CLUMPP v.1.1.2 (Jakobsson & Rosenberg, 2007) was used to cluster repeated sampling. Distruct v.1.1 software (Rosenberg, 2004) was used to graphically display population structure. The analysis of molecular variance (AMOVA) and the coefficient of genetic differentiation among populations (F st) were performed using GenAlEx v.6.5 (Peakall & Smouse, 2012). To delineate the major ordination pattern of P. mucrosquamatus populations, a discriminant analysis of principal components (DAPC) (Jombart et al., 2010) was performed by R v3.6.1 (R Core Team, 2019) using the adegenet package (Jombart, 2008). DAPC analysis is a multivariate method used to identify and describe clusters of genetically related individuals. Genetic variation is divided into two parts: between‐group variation and within‐group variation, which maximizes the former. Linear discriminants are linear combinations of alleles that best separate clusters (Deperi et al., 2018).
2.5. Tests for sex‐biased dispersal
In total, 92 sex‐determined individuals (48 males, 44 females) from the SCV and SWC populations were used to evaluate sex‐biased dispersal. We assessed sex‐biased dispersal from three small sites in Sichuan (Mingshan, Yibin, and Zizhong) in China using a two‐sided test. With reference to Goudet (1995), Goudet et al.’s (2002), Johansson et al.’s (2008), Hofmann et al.’s (2012), and Wang et al.’s (2019) studies on sex‐biased dispersal, we choose six parameters to evaluate the sex‐biased dispersal pattern of the P. mucrosquamatus. We calculated F st (Hartl & Clarck, 1997), F is, genetic diversity (H s), relatedness (r), mean assignment index (mAIc) (Favre et al., 1997), and variance of assignment index (vAIc) for each sex separately using FSTAT v.1.2. (Goudet, 1995). Statistical significance for these indices was determined by 10,000 randomizations. We chose the unbiased Weir and Cockerham estimator to calculate F st across all populations (Weir & Cockerham, 1984), with values generally higher for the philopatric sex than the dispersing sex. F is describes how well genotype frequencies within populations fit the HWE, with values generally higher for the dispersing sex than the philopatric sex. Within‐group Hs values are also higher for the group with the greatest dispersal. In the case of sex‐biased dispersal, mAIc values should be lower for the dispersing sex than for the philopatric sex (Lampert et al., 2003). In contrast, vAIc values should be higher for the dispersing sex because members will include both residents (with common genotypes; positive values) and immigrants (with rare genotypes; negative values). In brief, higher F is, Hs, and vAIc values and lower F st, mAIc, and r values tend to be found in the dispersing sex than in the philopatric sex (Wang et al., 2019).
To further verify the results of sex‐biased dispersal, we analyze data from the 92 sex‐determined individuals and three small sites separately, we calculated and compared relatedness values between the sexes using COANCESTRY v.1.0 with five moment and two likelihood estimators (Wang, 2011).
3. RESULTS
3.1. Genetic diversity
Based on genotyping of 25 microsatellites in 150 P. mucrosquamatus individuals, 16 microsatellites were successfully optimized with polymorphic and call rates above 90% across all samples. Statistics calculated for the 16 polymorphic microsatellite loci across the sampling localities are listed in Table 2. There was no evidence of scoring error due to stuttering, and no large allele dropout was observed for any of the loci. Null alleles accounted for a certain percentage within HN, SWC, and TW populations (see Appendix S1). The null allele frequency results showed that only YM‐17 loci in HN and TW population exceeded 0.2. It may be that there are some missing sites in these two populations, but the null alleles frequency in the other three populations does not exceed 0.2. Thus, we retained this locus. What is more, the results of the Hardy‐Weinberg Equilibrium test show that some populations have 2–6 microsatellite sites deviation from the Hardy‐Weinberg, while the populations HN and TW have no loci deviate from the Hardy‐Weinberg (Appendix S2). This may be related to the widespread distribution of this species.
TABLE 2.
Sixteen microsatellite loci information
| Loci | Primer sequence (5’−3’) | Repeat motif | Size range (bp) | Tm (°C) | Labelling dye |
|---|---|---|---|---|---|
| YM‐1 | F:ATAGATGGTGGAAGGAAGGAAAG | (GAAA)9 | 112–208 | 62 | FAM |
| R:CTCAGGGTGTCCTGTTTATTGAG | |||||
| YM‐2 | F:ATATTGTTTAGGCCTCCCTGAAG | (ATGA)9 | 116–192 | 62 | HEX |
| R:CACATTTTGCCTCAACCACTTAT | |||||
| YM‐3 | F:ACTGTTAAACCACCCAGAGTCAA | (TGAA)8 | 102–188 | 63 | TAMRA |
| R:TAATTCAGGAGATTGTAGCCCAA | |||||
| YM‐4 | F:ATTCGTGGTTTTTAGTATCGCCT | (AATA)8 | 116–200 | 62 | FAM |
| R:GGAAATTTTTCCTGATTTCCAAC | |||||
| YM‐5 | F:CATTCAAAGCATCCATTTTAACC | (GGAA)8 | 118–236 | 62 | HEX |
| R:TTCTGCTGCTCTTAAATTCCTTG | |||||
| YM‐8 | F:AACCCAGGATAGGAAAGTGGTTA | (ATTC)8 | 114–190 | 62 | FAM |
| R:ATTGTCTGGGAAAGGAGATTGAT | |||||
| YM‐11 | F:AAATCCTGTTCTTTCACCAAAAA | (ATAG)8 | 86–266 | 61 | TAMRA |
| R:AGTTTCTAAAGCCATGGTGAGAT | |||||
| YM‐12 | F:TACATGGAAAGAGGGGTAATGAA | (TCAT)8 | 99–207 | 61 | FAM |
| R:CAGAAGAAAAGGTTTGACATTGG | |||||
| YM‐13 | F:GGGCCTTGTATCAACTAACACAG | (TTAT)8 | 100–188 | 63 | HEX |
| R:AGAGTTACAATGGGCAGCAAATA | |||||
| YM‐15 | F:GGTAGCTGCTCAGAGTTTGGTAA | (AGGA)8 | 142–211 | 63 | TAMRA |
| R:ATTGTGTAGCAGGCAGCTCTAGT | |||||
| YM‐17 | F:TATTGTTGAAAACCATTCCCTCA | (TATG)8 | 100–198 | 63 | FAM |
| R:GGATCCAATCCTGTAGGAAAAAT | |||||
| YM‐18 | F:GTATGCTGCTCAGAGTCCCCTA | (ATGA)8 | 144–204 | 63 | HEX |
| R:ACTGCCTTGCTGACAATCTTTT | |||||
| YM‐20 | F:CTTTTGAGAGCAAGCAACAAAAT | (GTCT)8 | 170–238 | 63 | TAMRA |
| R:AAATGGTGTCCACAACTTGAGAT | |||||
| YM‐21 | F:CATGACCTGAAAAGTCAGCATTT | (AAGA)8 | 118–240 | 62 | FAM |
| R:ATGTCCTTGCATTGGTTCATATC | |||||
| YM‐22 | F:TGCATCCTGTTAGTCACAAAAGA | (AAAC)8 | 104–168 | 62 | HEX |
| R:GCAAACATTAAAACAAGCACACA | |||||
| YM‐23 | F:ACAAATTCTGGTTTCAGCACATC | (TGAA)8 | 116–208 | 62 | TAMRA |
| R:AAATTCATGTTGTCCAAAGTTGC |
The overall level of polymorphism detected in the 16 loci was high, with total alleles of 364 and average number of alleles (Na) of 22.75 (ranging from 13 to 37). Ho varied from 0.480 (YM‐3) to 0.899 (YM‐20), with an average of 0.764. The highest He value was 0.951 (YM‐11) (average 0.891). The highest PIC value was 0.945 (YM‐11) (average 0.879). Statistics for the 16 polymorphic microsatellite loci for total dataset are listed in Table 3.
TABLE 3.
Summary statistics for 16 polymorphic microsatellite loci overall the sampling localities (N = 150). The mean number of samples analyzed (N), a number of alleles identified (N a), observed heterozygosity (H o), expected heterozygosity (H e), Polymorphic Information Content (PIC)
| Locus | N | N a | H o | H e | PIC |
|---|---|---|---|---|---|
| YM‐1 | 146 | 23 | 0.753 | 0.939 | 0.932 |
| YM‐2 | 148 | 18 | 0.804 | 0.904 | 0.892 |
| YM‐3 | 150 | 22 | 0.480 | 0.837 | 0.824 |
| YM‐4 | 150 | 18 | 0.700 | 0.792 | 0.776 |
| YM‐5 | 149 | 29 | 0.805 | 0.935 | 0.928 |
| YM‐8 | 148 | 20 | 0.743 | 0.900 | 0.888 |
| YM‐11 | 143 | 37 | 0.874 | 0.951 | 0.945 |
| YM‐12 | 140 | 23 | 0.85 | 0.882 | 0.867 |
| YM‐13 | 139 | 24 | 0.885 | 0.937 | 0.930 |
| YM‐15 | 145 | 21 | 0.793 | 0.899 | 0.887 |
| YM‐17 | 143 | 21 | 0.629 | 0.913 | 0.903 |
| YM‐18 | 147 | 17 | 0.755 | 0.887 | 0.874 |
| YM‐20 | 149 | 29 | 0.899 | 0.938 | 0.931 |
| YM‐21 | 149 | 25 | 0.859 | 0.929 | 0.921 |
| YM‐22 | 146 | 13 | 0.678 | 0.713 | 0.671 |
| YM‐23 | 144 | 24 | 0.729 | 0.909 | 0.899 |
| Average | 146 | 22.75 | 0.764 | 0.891 | 0.879 |
3.2. Population genetic structure
To analyze the genetic structure of P. mucrosquamatus populations, the coancestry relations of the populations were analyzed based on a Bayesian clustering model. The independent clustering of all samples recorded the highest ΔK value at K = 2 (Evanno et al., 2005), thus supporting the presence of two clusters (Appendix S3). The STRUCTURE bar plot also supported two genetic clusters (Figure 3). When K was 2, the genetic information of 150 samples from 5 populations came from two differential ancestral populations. At K = 2, most of the genetic information of 4 populations (HN, VM, SCV, and TW) in southern China and Myanmar Vietnam came from the same ancestral population (blue), while 1 population in southwestern China (SWC), the genetic information is mainly from another ancestral group (red). The two clusters displayed different population membership to that reported previously based on mtDNA (Guo et al., 2019), but were consistent with geographical origin. From the bar plot of various K values (K = 2–6), the majority of individuals revealed low probabilities of being assigned to any particular clusters (Appendix S4). DAPC analysis was carried out using the detected number of clusters (Figure 4). In Figure 4, Linear Discriminant 1 (LD 1) separated among the P. mucrosquamatus species (cluster 1 = HN, VM, SCV, TW populations, cluster 2 = SWC population) and Linear Discriminant 2 (LD 2) separated among P. mucrosquamatus cluster (HN, VM, SCV, TW populations). SWC population were roughly at the same level with respect to LD 2, and HN, VM and SCV, TW populations were above and below them, respectively. AMOVA of the five populations showed that 82% of the variation was found among individuals, with only 4% found among populations (see Appendix S5). The coefficient of genetic differentiation among populations (F st) was high in HN, VM, SCV, and SWC populations compared to the TW population. F st values between VM and SCV, SWC populations, and SCW with SWC population were low, suggesting low genetic differentiation among them (Appendix S6).
FIGURE 3.
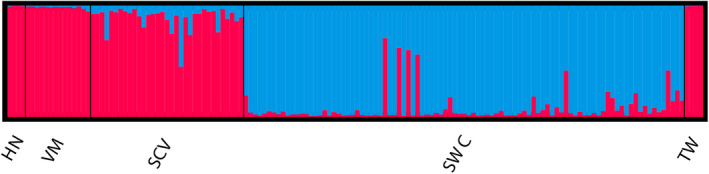
Structure diagram generated by STRUCTURE according to K = 2
FIGURE 4.
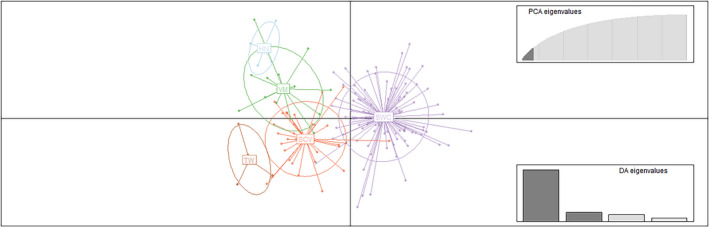
Scatter plot of the first and second principal coordinates based on the discriminant analysis of principal components (DAPC) of SSR markers. The axes represent the first two Linear Discriminants (LD). Each circle represents a cluster and each dot represents an individual. Letters represent the different populations identified by DAPC analysis
3.3. Sex‐biased dispersal in Protobothrops mucrosquamatus
For the 92 individuals, females had higher F is (female: 0.1662, male: 0.0831), H s (female: 0.8770, male: 0.8597), and vAIc values (female: 64.0346, male: 35.2241) compared to males, but lower F st, mAIc, and r values (Table 4). However, most indices did not reveal statistical significance. Analyses from the three sites (Mingshan, Yibin, and Zizhong) showed that females had higher F is (0.1113 vs. 0.0347), H s (0.8174 vs. 0.7907), and vAIc values (14.6314 vs. 12.5667) compared to males, but lower F st, mAIc, and r values (Table 5). When we examined the three sites separately, two out of seven relatedness indices were significantly higher in males than in females (p < .05) (Table 6).
TABLE 4.
Genetic differentiation (F st), inbreeding coefficient (F is), within‐site gene diversity (H s), relatedness (r), mean assignment index (mAIc), and variance of assignment index (vAIc) for the 92 individuals for females (F) and males (M) of P. mucrosquamatus
| F st | F is | H s | mAIc | vAIc | r | |
|---|---|---|---|---|---|---|
| F | 0.0273 | 0.1662 | 0.8770 | −1.1706 | 64.0346 | .0460 |
| M | 0.0321 | 0.0831 | 0.8597 | 1.2771 | 35.2241 | .0577 |
| P value | .7393 | .0012 | .1052 | .0975 | .0785 | .6250 |
p Values are from two‐sided tests.
TABLE 5.
Genetic differentiation (F st), inbreeding coefficient (F is), within‐site gene diversity (H s), relatedness (r), mean assignment index (mAIc), and variance of assignment index (vAIc) for the three sites in Sichuan, China for females (F) and males (M) of P. mucrosquamatus
| F st | F is | H s | mAIc | vAIc | r | |
|---|---|---|---|---|---|---|
| F | 0.0601 | 0.1113 | 0.8174 | −1.6936 | 14.6314 | .1033 |
| M | 0.0817 | 0.0347 | 0.7907 | 1.1547 | 12.5667 | .1467 |
| P value | .2117 | .0711 | .1699 | .0379 | .7775 | .1253 |
p Values are from two‐sided tests.
TABLE 6.
The relatedness of females and males in 92 individuals and three sites separately
|
Population |
Gender | Seven estimators | ||||||
|---|---|---|---|---|---|---|---|---|
| TrioML | Wang | LynchLi | LynchRd | Ritland | QuellerGt | DyadML | ||
| 92 individuals | Females | 0.0458 | −0.03446 | −0.02470 | −0.02214 | −0.025 | −0.02171 | 0 |
| Males | 0.0412 | −0.02291 | −0.01674 | −0.02418 | −0.0254 | −0.02297 | 0 | |
| Three sites | Females | 0.03042 | −0.04087 | −0.03680 | −0.07187 | −0.07642 | −0.07153 | 0 |
| Males | 0.03814 | −0.02410 | −0.02487 | −0.04764 | −0.04970 | −0.04786 | 0 | |
| Mingshan | Females | 0.00000 | −0.01150 | −0.00177 | −0.50003 | −0.40330 | −0.49903 | 0 |
| Males | 0.00706 | −0.00674 | −0.02015 | −0.14285 | −0.13675 | −0.14303 | 0 | |
| Zizhong | Females | 0.0225 | −0.01803 | −0.04474 | −0.16841 | −0.1673 | −0.16995 | 0 |
| Males | 0.016 | −0.00292 | −0.02008 | −0.16666 | −0.1636 | −0.16759 | 0 | |
| Yibin | Females | 0.001 | −0.07384 | −0.08568 | −0.25147 | −0.2515 | −0.25377 | 0 |
| Males | 0.0027 | −0.00468 | −0.02410 | −0.16736 | −0.1575 | −0.16912 | 0 | |
Italic means p < .05.
4. DISCUSSION
4.1. Genetic diversity and population structure
Microsatellite markers represent a powerful tool for determining the genetic diversity of populations and are widely used in vertebrate studies (e.g., Aipysurus laevis, Thermophis bailey, Leptobrachium boringii) (Hofmann et al., 2012; Lukoschek et al., 2008; Wang et al., 2019). Our research showed that these markers were detected at high levels of genetic variation within P. mucrosquamatus, with multiple alleles (N a = 22.75), high H o (0.480–0.899), and high H e (0.713–0.951) (Table 3). These results are consistent with previous findings based on mtDNA (Guo et al., 2019), but are higher than that detected using microsatellite markers in smooth snakes (Coronella austriaca) (H o = 0.357–0.507, H e = 0.418–0.601) (Pernetta et al., 2011) and olive sea snakes (Aipysurus laevis) (H o = 0.222–0.847, H e = 0.263–0.881) (Lukoschek et al., 2008) and comparable to that reported in slatey‐grey snakes (Stegonotus cucullatus) (H o = 0.62–0.84, H e = 0.55–0.83) (Dubey et al., 2008). In addition, the mean PIC (0.879) of P. mucrosquamatus was >0.5, indicating that this species was highly genetically diverse. Higher genetic diversity could be attributed to their wide regional distribution and varied habitats.
Based on genetic structure analysis, we detected two clusters in P. mucrosquamatus, different from previous mtDNA‐based findings (Guo et al., 2019) to some extent. This difference may be due to different genetic and evolutionary patterns between mtDNA and microsatellite markers. However, these two clusters displayed significant admixture, consistent with AMOVA results, which indicated variation among individuals (Appendix S5). A standard AMOVA for the 5 populations (without a hierarchy of regions) showed that 82% of the variation was located between individuals and only 4% among populations. In China, the last global glaciation, termed the Dali glaciation (DLG), occurred during 0.07–0.01 Ma (Shi & Wang, 1979). In Guo et al. (2019), three lines of evidence suggested that all defined matrilineal lineages of P. mucrosquamatus have experienced recent population expansion. The expansion of TW and VM populations was estimated to occurred about 0.03–0.04 Ma, which was close to the mid‐DLG, while the SWC population experienced a rapid expansion after the DLG (~0.005 Ma) when the temperature rose (Shi & Wang, 1979). However, the SCV population experienced an expansion before 0.07 Ma, which may have been triggered by pre‐Glacial Maximum. High temperatures.
4.2. Sex‐biased dispersal
In general, the F is, F st, r, mAIc, vAIc, and Hs parameters are indicative of sex‐biased dispersal patterns. Previous studies have shown that F st is higher for the more philopatric sex than for the more dispersing sex (Goudet et al., 2002). Members of the dispersing sex also display higher F is than the philopatric sex. Furthermore, Hs is generally higher in the group showing greater dispersal. In the case of sex‐biased dispersal, mAIc values are lower for the dispersing sex than for the philopatric sex (Lampert et al., 2003); in contrast, vAIc values are higher for the dispersing sex because members will include both residents and immigrants. Based on our total dataset, females had higher F is, H s, and vAIc values, but lower F st, r, and mAIc values than males (Tables 4 and 5), suggesting that P. mucrosquamatus snakes exhibit female‐biased dispersal. This result differs from previous studies on sex‐biased dispersal in snakes (e.g., Stegonotus cucullatus, Drymarchon couperi, Thermophis baileyi, Rhinoplocephalus nigrescens, Aipysurus laevis, Coronella austriaca, and Vipera aspis) (Dubey et al., 2008; Folt et al., 2019; Hofmann et al., 2012; Keogh et al., 2007; Lukoschek et al., 2008; Pernetta et al., 2011; Zwahlen et al., 2021). However, most indices representing sex‐biased dispersal did not differ significantly, which may be the result of incomplete sampling. Several hypotheses have been proposed for female‐dispersal in birds and mammals, including local resource competition (Greenwood, 1980), local mate competition (Dobson, 1982; Perrin & Mazalov, 2000; Rivas & Burghardt, 2005), and inbreeding avoidance (Perrin & Mazalov, 2000; Pusey, 1987). Although the true mechanism of sex‐biased dispersal is unknown in this species, we hypothesize local resource competition may better explain the dispersal pattern as females need to acquire more resources while avoiding increased competition for resources. P. mucrosquamatus is widely distributed in southeastern and southwestern China, Laos, Bangladesh, northern Vietnam, northern Myanmar, and northeastern India. It is one of the most widely distributed members in this genus, and its distribution covers different climates and vegetation types (Zhao, 2006). Maybe it has something to do with the females of this species being more inclined to dispersal.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Min Yu: Conceptualization (lead); Data curation (lead); Formal analysis (supporting); Methodology (equal); Validation (equal); Visualization (supporting); Writing – original draft (equal); Writing – review & editing (equal). Qin Liu: Conceptualization (supporting); Data curation (supporting); Formal analysis (lead); Investigation (equal); Methodology (equal); Software (lead); Visualization (equal); Writing – review & editing (equal). Ya‐yong Wu: Conceptualization (supporting); Data curation (supporting); Formal analysis (lead); Investigation (equal). Peng Guo: Conceptualization (lead); Data curation (lead); Formal analysis (supporting); Funding acquisition (supporting); Investigation (supporting); Methodology (supporting); Project administration (lead); Visualization (supporting); Writing – original draft (equal); Writing – review & editing (supporting). Kong Yang: Formal analysis (supporting); Investigation (supporting); Methodology (supporting); Software (supporting); Visualization (supporting); Writing – original draft (equal); Writing – review & editing (supporting).
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
ACKNOWLEDGMENTS
This study was supported by the Second Tibetan Plateau Scientific Expedition and Research (STEP) program (2019QZKK05010105), the National Natural Science Foundation of China (NSFC 31372152), and Sciences and Technology Department of Sichuan Province (2020YFSY0033). We would like to thank many people who helped with the collection and provision of tissue samples, including A. Malhotra, J. Vindum, D. Kizirian, R. Murphy, H. Zhao, K. Jiang, J. Hu, S. Y. Liu, M. Hou, and F. Shu.Tissues were provided by the California Academy of Sciences (CAS), American Museum of Natural History (AMNH), and Royal Ontario Museum (ROM). We thank L. M. Du for help in data analysis. The editor and two anonymous reviewers are acknowledged for their invaluable comments and corrections.
Yu, M. , Liu, Q. , Wu, Y.‐Y. , Guo, P. , & Yang, K. (2022). Genetic diversity and sex‐biased dispersal in the brown spotted pitviper (Protobothrops mucrosquamatus): Evidence from microsatellite markers. Ecology and Evolution, 12, e8652. 10.1002/ece3.8652
Min Yu and Qin Liu equally contribute to this work
Contributor Information
Peng Guo, Email: ybguop@163.com.
Kong Yang, Email: lx-yk@163.com.
DATA AVAILABILITY STATEMENT
All microsatellite genotypes for all individuals are deposited in Dryad https://datadryad.org/stash/share/Ntrk9UMZIhu7Zag5DOv0c8d1yXIsF8Fd2BJzgGtE4WA. All genetic analyses were performed with publicly available programs.
REFERENCES
- Brown, J. , Pirrung, M. , & McCue, L. A. (2017). FQC Dashboard: integrates FastQC results into a web‐based, interactive, and extensible FASTQ quality control tool. Bioinformatics, 33(19), 3137–3139. 10.1093/bioinformatics/btx373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor, T. E. (1839). Spicilegium serpentium indicorum [part 1]. Venomous serpentes. Proceedings of the Zoological Society of London, 7(1), 31–34. [Google Scholar]
- Chapuis, M. P. , & Estoup, A. (2006). Microsatellite null alleles and estimation of population differentiation. Molecular Biology and Evolution, 24(3), 621–631. 10.1093/molbev/msl191 [DOI] [PubMed] [Google Scholar]
- Corrales, C. , & Höglund, J. (2012). Maintenance of gene flow by female‐biased dispersal of black grouse Tetrao tetrix in northern Sweden. Journal of Ornithology, 153(4), 1127–1139. 10.1007/s10336-012-0844-0 [DOI] [Google Scholar]
- Costello, C. M. , Creel, S. R. , Kalinowski, S. T. , Vu, N. V. , & Quigley, H. B. (2008). Sex‐biased natal dispersal and inbreeding avoidance in American black bears as revealed by spatial genetic analyses. Molecular Ecology, 17(21), 4713–4723. 10.1111/j.1365-294x.2008.03930.x [DOI] [PubMed] [Google Scholar]
- Deperi, S. I. , Tagliotti, M. E. , Bedogni, M. C. , Manrique‐Carpintero, N. C. , Coombs, J. , Zhang, R. , Douches, D. , & Huarte, M. A. (2018). Discriminant analysis of principal components and pedigree assessment of genetic diversity and population structure in a tetraploid potato panel using SNPs. PLoS One, 13(3), e0194398. 10.1371/journal.pone.0194398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, F. S. (1982). Competition for mates and predominant juvenile male dispersal in mammals. Animal Behaviour, 30(4), 1183–1192. 10.1016/S0003-3472(82)80209-1 [DOI] [Google Scholar]
- Du, L. , Li, Y. , Zhang, X. , & Yue, B. (2012). MSDB: A user‐friendly program for reporting distribution and building databases of microsatellites from genome sequences. Journal of Heredity, 104(1), 154–157. 10.1093/jhered/ess082 [DOI] [PubMed] [Google Scholar]
- Dubey, S. , Brown, G. P. , Madsen, T. , & Shine, R. (2008). Male‐biased dispersal in a tropical Australian snake (Stegonotus cucullatus, Colubridae). Molecular Ecology, 17(15), 3506–3514. 10.1111/j.1365-294x.2008.03859.x [DOI] [PubMed] [Google Scholar]
- Earl, D. A. , & VonHoldt, B. M. (2012). STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources, 4(2), 359–361. 10.1007/s12686-011-9548-7 [DOI] [Google Scholar]
- Evanno, G. , Regnaut, S. , & Goudet, J. (2005). Detecting the number of clusters of individuals using the software structure: A simulation study. Molecular Ecology, 14(8), 2611–2620. 10.1111/j.1365-294x.2005.02553.x [DOI] [PubMed] [Google Scholar]
- Favre, L. , Balloux, F. , Goudet, J. , & Perrin, N. (1997). Female‐biased dispersal in the monogamous mammal Crocidura russula: evidence from field data and microsatellite patterns. Proceedings of the Royal Society B: Biological Sciences, 264(1378), 127–132. 10.1098/rspb.1997.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folt, B. , Bauder, J. , Spear, S. , Stevenson, D. , Hoffman, M. , Oaks, J. R. , Wood, P. L. , Jamie, R. , Jenkins, C. , Steen, D. A. , & Guyer, C. (2019). Taxonomic and conservation implications of population genetic admixture, mito‐nuclear discordance, and male‐biased dispersal of a large endangered snake, Drymarchon couperi . PLoS One, 14(3), e0214439. 10.1371/journal.pone.0214439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudet, J. (1995). FSTAT (Version 1.2): a computer program to calculate F‐statistics. Journal of Heredity, 86(6), 485–486. 10.1093/oxfordjournals.jhered.a111627 [DOI] [Google Scholar]
- Goudet, J. , Perrin, N. , & Waser, P. (2002). Tests for sex‐biased dispersal using bi‐parentally inherited genetic markers. Molecular Ecology, 11(6), 1103–1114. 10.1046/j.1365-294x.2002.01496.x [DOI] [PubMed] [Google Scholar]
- Greenwood, P. J. (1980). Mating systems, philopatry and dispersal in birds and mammals. Animal Behaviour, 28(4), 1140–1162. 10.1016/s0003-3472(80)80103-5 [DOI] [Google Scholar]
- Greenwood, P. J. , & Harvey, P. H. (1982). The natal and breeding dispersal of birds. Annual Review of Ecology and Systematics, 13(1), 1–21. 10.1146/annurev.es.13.110182.00 [DOI] [Google Scholar]
- Guerrini, M. , Gennai, C. , Panayides, P. , Crabtree, A. , Zuberogoitia, I. , Copland, A. S. , Babushkina, O. , Politi, P. M. , Giunchi, D. , & Barbanera, F. (2014). Large‐scale patterns of genetic variation in a female‐biased dispersing passerine: the importance of sex‐based analyses. PLoS One, 9(6), e98574. 10.1371/journal.pone.0098574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichoux, E. , Lagache, L. , Wanger, S. , Chaumeil, P. , Leger, P. , Lepais, O. , Lwpoittevin, C. , Malausa, T. , Revardel, E. , Salin, F. , & Petit, R. J. (2011). Current trends in microsatellite genotyping. Molecular Ecology Resources, 11(4), 591–611. 10.1111/j.1755-0998.2011.03014.x [DOI] [PubMed] [Google Scholar]
- Guo, P. , Liu, Q. , Zhu, F. , Zhong, G. H. , Che, J. , Wang, P. , Xie, Y. L. , Murphy, R. W. , & Malhotra, A. (2019). Multilocus phylogeography of the brown‐spotted pitviper Protobothrops mucrosquamatus (Reptilia: Serpentes: Viperidae) sheds a new light on the diversification pattern in Asia. Molecular Phylogenetics and Evolution, 133, 82–91. 10.1016/j.ympev.2018.12.028 [DOI] [PubMed] [Google Scholar]
- Guo, S. W. , & Thompson, E. A. (1992). Performing the exact test of Hardy‐Weinberg proportion for multiple alleles. Biometrics, 48(2), 361–372. 10.2307/2532296 [DOI] [PubMed] [Google Scholar]
- Hartl, D. L. , & Clarck, A. G. (1997). Principles of population genetics (3rd edition). Sinauer Associates. [Google Scholar]
- Hodel, R. G. J. , Segovia‐Salcedo, M. C. , Landis, J. B. , Crowl, A. A. , Sun, M. , Liu, X. X. , Gitzendanner, M. A. , Douglas, N. A. , Germain‐Aubrey, C. C. , Chen, S. C. , Soltis, D. E. , & Soltis, P. S. (2016). The report of my death was an exaggeration: a review for researchers using microsatellites in the 21st century. Applications in Plant Sciences, 4(6), 1600025. 10.3732/apps.1600025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann, S. , Fritzsche, P. , Solhøy, T. , Dorge, T. , & Miehe, G. (2012). Evidence of sex‐biased dispersal in Thermophis baileyi inferred from microsatellite markers. Herpetologica, 68(4), 514–522. 10.1655/herpetologica-d-12-00017 [DOI] [Google Scholar]
- Howard, W. E. (1960). Innate and environmental dispersal of individual vertebrates. American Midland Naturalist, 63(1), 152–161. 10.2307/2422936 [DOI] [Google Scholar]
- Jakobsson, M. , & Rosenberg, N. A. (2007). CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics, 23(14), 1801–1806. 10.1093/bioinformatics/btm233 [DOI] [PubMed] [Google Scholar]
- Johansson, H. , Surget‐Groba, Y. , & Thorpe, R. S. (2008). Microsatellite data show evidence for male‐biased dispersal in the Caribbean lizard Anolis roquet . Molecular Ecology, 17(20), 4425–4432. 10.1111/j.1365-294x.2008.03923.x [DOI] [PubMed] [Google Scholar]
- Jombart, T. (2008). adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics, 24(11), 1403–1405. 10.1093/bioinformatics/btn129 [DOI] [PubMed] [Google Scholar]
- Jombart, T. , Devillard, S. , & Balloux, F. (2010). Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genetics, 11(1), 1–15. 10.1186/1471-2156-11-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinowski, S. T. , Taper, M. L. , & Marshall, T. C. (2007). Revising how the computer program cervus accommodates genotyping error increases success in paternity assignment. Molecular Ecology, 16(5), 1099–1106. 10.1111/j.1365-294x.2007.03089.x [DOI] [PubMed] [Google Scholar]
- Keogh, J. S. , Webb, J. K. , & Shine, R. (2007). Spatial genetic analysis and long‐term mark‐recapture data demonstrate male‐biased dispersal in a snake. Biology Letters, 3(1), 33–35. 10.1098/rsbl.2006.0570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert, K. P. , Rand, A. S. , Mueller, U. G. , & Ryan, M. J. (2003). Fine‐scale genetic pattern and evidence for sex‐biased dispersal in the túngara frog, Physalaemus pustulosus . Molecular Ecology, 12(12), 3325–3334. 10.1046/j.1365-294x.2003.02016.x [DOI] [PubMed] [Google Scholar]
- Li, W. , & Godzik, A. (2006). Cd‐hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics, 22(13), 1658–1659. 10.1093/bioinformatics/btl158 [DOI] [PubMed] [Google Scholar]
- Lischer, H. E. L. , & Excoffier, L. (2012). PGDSpider: An automated data conversion tool for connecting population genetics and genomics programs. Bioinformatics, 28(2), 298–299. 10.1093/bioinformatics/btr642 [DOI] [PubMed] [Google Scholar]
- Lukoschek, V. , Waycott, M. , & Keogh, J. S. (2008). Relative information content of polymorphic microsatellites and mitochondrial DNA for inferring dispersal and population genetic structure in the olive sea snake, Aipysurus laevis . Molecular Ecology, 17(13), 3062–3077. 10.1111/j.1365-294x.2008.03815.x [DOI] [PubMed] [Google Scholar]
- Namiki, T. , Hachiya, T. , Tanaka, H. , & Sakakibara, Y. (2012). MetaVelvet: An extension of Velvet assembler to de novo metagenome assembly from short sequence reads. Nucleic Acids Research, 40(20), e155. 10.1093/nar/gks678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemesházi, E. , Szabó, K. , Horváth, Z. , & Kövér, S. (2018). Genetic structure confirms female‐biased natal dispersal in the White‐tailed Eagle population of the Carpathian basin . Acta Zoologica Academiae Scientiarum Hungaricae, 64(3), 243–257. 10.17109/AZH.64.3.243.2018 [DOI] [Google Scholar]
- Paplinska, J. Z. , Eldridge, M. D. B. , Cooper, D. W. , Temple‐Smith, P. D. M. , & Renfree, M. B. (2009). Use of genetic methods to establish male‐biased dispersal in a cryptic mammal, the swamp wallaby (Wallabia bicolor). Australian Journal of Zoology, 57(1), 65–72. 10.1071/zo09014 [DOI] [Google Scholar]
- Peakall, R. , & Smouse, P. E. (2012). GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research‐an update. Bioinformatics, 28(19), 2537–2539. 10.1093/bioinformatics/bts460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernetta, A. P. , Allen, J. A. , Beebee, T. J. C. , & Reading, C. J. (2011). Fine‐scale population genetic structure and sex‐biased dispersal in the smooth snake (Coronella austriaca) in southern England. Heredity, 107(3), 231–238. 10.1038/hdy.2011.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin, N. , & Mazalov, V. (2000). Local competition, inbreeding, and the evolution of sex‐biased dispersal. The American Naturalist, 155(1), 116–127. 10.1086/303296 [DOI] [PubMed] [Google Scholar]
- Pritchard, J. K. , Stephens, M. , & Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics, 155(2), 945–959. 10.1093/genetics/155.2.945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusey, A. E. (1987). Sex‐biased dispersal and inbreeding avoidance in birds and mammals. Trends in Ecology & Evolution, 2(10), 295–299. 10.1016/0169-5347(87)90081-4 [DOI] [PubMed] [Google Scholar]
- Qi, Y. , Yang, W. , Lu, B. , & Fu, J. (2013). Genetic evidence for male‐biased dispersal in the Qinghai toad‐headed agamid Phrynocephalus vlangaliiand its potential link to individual social interactions. Ecology and Evolution, 3(5), 1219–1230. 10.1002/ece3.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, H. , Yang, G. , Provan, J. , Liu, J. , & Gao, L. (2017). Using MiddRAD‐seq data to develop polymorphic microsatellite markers for an endangered yew species. Plant Diversity, 39(5), 294–299. 10.1016/j.pld.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Rivas, J. A. , & Burghardt, G. M. (2005). Snake mating systems, behavior, and evolution: the revisionary implications of recent findings. Journal of Comparative Psychology, 119(4), 447–454. 10.1037/0735-7036.119.4.447 [DOI] [PubMed] [Google Scholar]
- Ronce, O. (2007). How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annual Review of Ecology, Evolution, and Systematics, 38(1), 231–253. 10.1146/annurev.ecolsys.38.0912 [DOI] [Google Scholar]
- Rosenberg, N. A. (2004). Distruct: a program for the graphical display of population structure. Molecular Ecology Notes, 4(1), 137–138. 10.1046/j.1471-8286.2003.00566.x [DOI] [Google Scholar]
- Rousset, F. (2008). Genepop’007: a complete re‐implementation of the genepop software for Windows and Linux. Molecular Ecology Resources, 8(1), 103–106. 10.1111/j.1471-8286.2007.01931.x [DOI] [PubMed] [Google Scholar]
- Selkoe, K. A. , & Toonen, R. J. (2006). Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecology Letters, 9(5), 615–629. 10.1111/j.1461-0248.2006.00889.x [DOI] [PubMed] [Google Scholar]
- Shi, Y. F. , & Wang, J. T. (1979). The fluctuations of climate, glaciers and sea level since late Pleistocene in China. IAHS Publication, 131, 281–293. [Google Scholar]
- Song, Y. C. , Yang, D. D. , Zou, S. J. , Li, P. F. , Zhang, H. , Wen, H. J. , & Jiang, Z. G. (2015). Sex‐biased dispersal in naturally re‐wild Milu in the Dongting Lake Region, China. Acta Ecologica Sinica, 35(13), 4416–4424. 10.5846/stxb201408251680 [DOI] [Google Scholar]
- Trochet, A. , Courtois, E. A. , Stevens, V. M. , Baguette, M. , Chaine, A. , Schmeller, D. S. , Clobert, J. , & Wiens, J. J. (2016). Evolution of sex‐biased dispersal. The Quarterly Review of Biology, 91(3), 297–320. 10.1086/688097 [DOI] [PubMed] [Google Scholar]
- Ujvari, B. , Dowton, M. , & Madsen, T. (2008). Population genetic structure, gene flow and sex‐biased dispersal in frillneck lizards (Chlamydosaurus kingii). Molecular Ecology, 17(15), 3557–3564. 10.1111/j.1365-294x.2008.03849.x [DOI] [PubMed] [Google Scholar]
- Untergasser, A. , Cutcutache, I. , Koressaar, T. , Ye, J. , Faircloth, B. C. , Remm, M. , & Rozen, S. G. (2012). Primer3–new capabilities and interfaces. Nucleic Acids Research, 40(15), e115. 10.1093/nar/gks596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart, J. , Wang, Y. Z. , & Fu, J. Z. (2009). Historical vicariance and male‐mediated gene flow in the toad‐headed lizards Phrynocephalus przewalskii . Molecular Ecology, 18(17), 3714–3729. 10.1111/j.1365-294x.2009.04310.x [DOI] [PubMed] [Google Scholar]
- Van Oosterhout, C. , Hutchinson, W. F. , Wills, D. P. M. , & Shipley, P. (2004). Micro‐checker: Software for identifying and correcting genotyping errors in microsatellite data. Molecular Ecology Notes, 4(3), 535–538. 10.1111/j.1471-8286.2004.00684.x [DOI] [Google Scholar]
- Vangestel, C. , Callens, T. , Vandomme, V. , & Lens, L. (2013). Sex‐biased dispersal at different geographical scales in a cooperative breeder from fragmented rainforest. PLoS One, 8(8), e71624. 10.1371/journal.pone.0071624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. (2011). Coancestry: A program for simulating, estimating and analysing relatedness and inbreeding coefficients. Molecular Ecology Resources, 11(1), 141–145. 10.1111/j.1755-0998.2010.02885.x [DOI] [PubMed] [Google Scholar]
- Wang, W. , Wei, S. , Chen, M. J. , & Wu, H. (2019). Female‐biased dispersal of the Emei moustache toad (Leptobrachium boringii) under local resource competition. Asian Herpetological Research, 10(1), 26–33. 10.16373/j.cnki.ahr.180049 [DOI] [Google Scholar]
- Weir, B. S. , & Cockerham, C. C. (1984). Estimating F‐statistics for the analysis of population structure. Evolution, 38(6), 1358–1370. 10.1111/j.1558-5646.1984.tb05657.x [DOI] [PubMed] [Google Scholar]
- Wen, Y. F. , Uchiyama, K. , Han, W. J. , Ueno, S. , Xie, W. D. , Xu, G. B. , & Tsumura, Y. (2013). Null alleles in microsatellite markers. Biodiversity Science, 21(1), 117–126. 10.3724/SP.J.1003.2013.10133 [DOI] [Google Scholar]
- Zhao, E. M. (2006). Snakes of China (pp. 137–138). Anhui Science and Technology Publishing House. In Chinese. [Google Scholar]
- Zhong, G. H. , Liu, Q. , Li, C. , Peng, P. H. , & Guo, P. (2017). Sexual dimorphism and geographic variation in the Asian lance‐headed pitviper Protobothrops mucrosquamatus in the Mainland China. Asian Herpetological Research, 8(2), 118–122. 10.16373/j.cnki.ahr.160011 [DOI] [Google Scholar]
- Zwahlen, V. , Nanni‐Geser, S. , Kaiser, L. , Golay, J. , Dubey, S. , & Ursenbacher, S. (2021). Only males care about their environment: Sex‐biased dispersal in the asp viper (Vipera aspis). Biological Journal of the Linnean Society, 132(1), 104–115. 10.1093/biolinnean/blaa177 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Data Availability Statement
All microsatellite genotypes for all individuals are deposited in Dryad https://datadryad.org/stash/share/Ntrk9UMZIhu7Zag5DOv0c8d1yXIsF8Fd2BJzgGtE4WA. All genetic analyses were performed with publicly available programs.


