INTRODUCTION
Arousing attention to the adverse reactions to the cardiac tissue with myocarditis or pericarditis after coronavirus disease 2019 (COVID-19) messenger RNA (mRNA) vaccine immunization have been issued in several cases or series report recently.1-3 Vast majority of events were presented after the second dose of the mRNA vaccination, or with previous COVID-19 infection preceded to the vaccine initial dose administration.3,4 In this article, we reported a case of 32-year-old male who presented to the hospital with shortness of breath and myocarditis in suspicion one week after the first mRNA-1273 COVID-19 (Moderna) vaccine dose administration, being free from previous COVID-19 infection. Raising concern that myocarditis, a rare side effect of COVID-19 vaccine, is not only tenable among few days after the second dose injection but also merit attention in the first shot vaccine recipients with delay symptoms cropped up.
CASE PRESENTATION
A previously healthy 32-year-old male, with a medical history of gouty arthritis and unmanaged dyslipidemia, presented to the emergency department with acute onset breathlessness during physical activity seven days after receiving the initial dose of the COVID-19 mRNA-1273 vaccine. The patient had local reactions with pain and swelling at the injection site initially, but no common systemic reactions, such as fever, chills, or generalized fatigue were identified. The painful swelling was spontaneously relieved in 3 days and he did not take analgesia during the time. On the sixth day after the vaccination, he had several episodes of loose stool passage. Subsequently, he developed exertional dyspnea in the morning one day after. The dyspnea lasted for one day and got worse with mild substernal chest discomfort noticed before attending the emergency department.
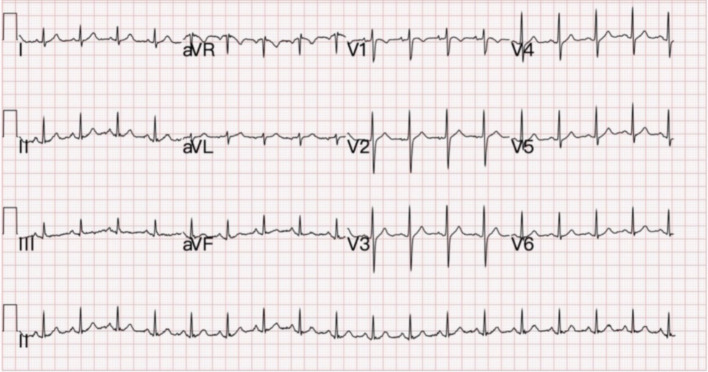
Upon presentation, his ear temperature was 36.5 °C, pulse rate hit 110 beats/min, blood pressure was 115/79 mmHg, respiratory rate was 17/min, and his peripheral oxygen saturation was 100% on room air. Electrocardiogram revealed sinus tachycardia without significant ST segment or T wave abnormality (Figure 1). Laboratory studies showed elevated cardiac troponin I (2.212 ng/mL, normal range 0.01-0.04 ng/mL), elevated creatine kinase (509 U/L, normal 30- U/L), and elevated C-reactive protein (44.47 mg/L, normal < 5.0 mg/L); yet the BNP (27.0 pg/mL) level was within normal range. Polymerase chain reaction (PCR) test on a nasal swab was negative for SARS-COV-2 viruses. Viral serology tests were negative for human parvovirus B19, adenovirus, herpes simplex virus 1 and 2, Cytomegalovirus, human immunodeficiency virus and viral hepatitis B and C.
Figure 1.
Electrocardiography (ECG) at triage shows sinus tachycardia without obvious ST-segment or T wave abnormality.
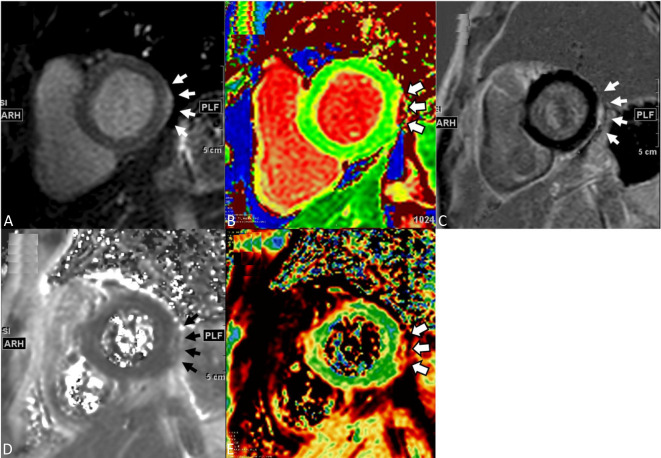
Transthoracic echocardiography was carried out which demonstrated good biventricular function without visualized regional wall motion abnormality, no significant valve disorders, and no pericardial effusion. Coronary angiogram was performed without significant epicardial coronary artery stenosis. Cardiac magnetic resonance imaging showed no regional wall motion abnormalities on cine imaging but demonstrated a small crescent area of late gadolinium enhancement and both T1 and T2 values image at the lateral wall subepicardium of left ventricle, consistent with the clinical diagnosis of acute myocarditis (Figure 2A-E). Endomyocardial biopsy was not performed due to the patient was mildly symptomatic as well as his personal will.
Figure 2.
The cardiac magnetic resonance imaging of left ventricular short-axis T1 gray-scale (A), color-coded T1 maps (B), and LGE (C) images depict a small crescent area of increased signal (white arrows) over the subepicardium of the lateral segment of left ventricle. Because of the small lesion size, both T2 gray-scale (D) and color-coded T2 maps (E) are illustrated to reassure the lesion location, focal edema and elevated T2 values (white and black arrows). LGE, late gadolinium enhancement.
During the hospitalization, anti-inflammatory agent was not prescribed but only conservative treatment with intensive monitoring, based on no signs of systemic inflammatory response or hemodynamic derangement. Clinical improvement was shortly observed in the following days, and the patient was discharged after 5 days of hospitalization. He was instructed to avoid strenuous activities for three months. Follow-up with the Cardiologist is ongoing without signs and symptoms of recurrence.
DISCUSSION
Myocarditis or pericarditis after vaccination such as smallpox and influenza have been reported;5 despite it is uncommon following immunization with mRNA-based COVID-19 vaccines on the basis of currently available data.4,6 Kim et al.2 reported 4 myocarditis cases who had received the second doses of an mRNA-based COVID-19 within five days among more than 560,000 fully vaccinated individuals in North Carolina, the U.S. in a three-month period from February to April 2021. The US Military Health System detected 23 myocarditis cases after administration of more than 2.8 million doses of mRNA-based vaccines between January and April 2021.3 Major of them presented with acute onset of chest pain between one to five days after the receipt of the second dose mRNA COVID-19 vaccine of an appropriately spaced 2-dose vaccine series.
On the other hand, the occurrence of myocarditis after initial dose of vaccine was rare and the duration from vaccination to the present of myocarditis-like symptoms varied from 2 days4 to 16 days.6 Additionally, in most of the patients who were diagnosed as myocarditis after the first dose mRNA-based vaccination shared similar experiences of previous SARS-CoV-2 infection: Jay Montgomery et al. reported 3 cases (mRNA-1273 in 2 cases; BNT162b2 in 1 case) had COVID-19 infection more than 2 months prior to vaccination,3 and Kathryn Larson et al. reported 1 case (BNT162b2) had been previously infected without the need of hospitalization.4 In our report, the previously healthy young patient developed signs and symptoms of myocarditis at the seventh day after vaccination. To our knowledge, there has been no other reported case thus far of myocarditis following after the first dose mRNA-1273 vaccine receipt, who had no prior SARS-CoV-2 infection. Although the association between the mRNA-1273 vaccine and the myocarditis could not be verified clearly, there was no evidence of other common causes unveiled. In the meantime, two cases reported in different series developed myocarditis at 7 days7 and 16 days6 respectively after the first dose of BNT162b2 vaccine without previous COVID-19 infection either, implying that former exposure was associated with the hypersensitivity response. The vulnerability and promising mechanism between myocarditis and mRNA-based COVID-19 vaccine were still not specified. Innate immunity response, multiple cytokines, and molecular mimicry between viral spike protein and an undetermined cardiac protein may serve as a possible mechanism8-10 but was undetermined.
In this report, we addressed the onset of acute myocarditis, a potential vaccine-related adverse event of which clinicians should have vigilance, might defer to a longer period (7 days or more) in patients received the first dose mRNA-based COVID-19 vaccine, especially in those without previous SARS-CoV-2 infection. Drawing an inferences that immune response actuated by the initial-dose vaccination has a longer responding time when comparing to the second one. Further investigation to unveil the association between the immunoreaction time and the vaccine receipt schedule to the development of adverse events is required. Deliberating on effectives of the vaccines at preventing COVID-19 infection and risks of sparse adverse events following vaccination; one needed to be weighted the pros and cons carefully and COVID-19 vaccination remained a high priority in establishing herd immunity.
LEARNING POINT
Acute myocarditis, a potential vaccine-related adverse event, might have a deferred onset period in patients received the first dose mRNA-based COVID-19 vaccine, especially in those without previous SARS-CoV-2 infection.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Bautista GJ, Pena OP, Bonilla F JA, et al. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19. Rev Esp Cardiol (Engl Ed) 2021;74:812–814. doi: 10.1016/j.rec.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HW, Jenista ER, Wendell DC, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6:1196–1201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US Military. JAMA Cardiol. 2021;6:1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson KF, Ammirati E, Adler ED, et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation. 2021;144:506–508. doi: 10.1161/CIRCULATIONAHA.121.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engler RJ, Nelson MR, Collins LC, Jr., et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi: 10.1371/journal.pone.0118283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu Mouch S, Roguin A, Hellou E, et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39:3790–3793. doi: 10.1016/j.vaccine.2021.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosner CM, Genovese L, Tehrani BN, et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;144:502–505. doi: 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15:586–594. doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muthukumar A, Narasimhan M, Li QZ, et al. In depth evaluation of a case of presumed myocarditis following the second dose of COVID-19 mRNA vaccine. Circulation. 2021;144:487–498. doi: 10.1161/CIRCULATIONAHA.121.056038. [DOI] [PMC free article] [PubMed] [Google Scholar]