INTRODUCTION
Prinzmetal variant angina (PVA) and cardiac syndrome X (CSX) were once introduced to describe angina pectoris in patients with normal coronary angiography and without obstructive coronary artery disease (CAD). In 1988, Cannon proposed a concept of microvascular angina (MVA) to illuminate such a clinical conundrum.1 After two decades, coronary microvascular dysfunction (CMVD) was advocated to decipher the genesis of ischemia in the anginal patients who did not have obstructive CAD.2 Ten years later, "INOCA" was coined to describe the patients presenting with "ischemia and no obstructive coronary artery disease". We reported here an 11-year follow-up of an adolescent, who presented with recurrent angina pectoris at rest and after exercise, accompanying with elevation and depression of ST-segment in 12-lead electrocardiograms (ECG) and no obstructive CAD in angiography, and necessitated hospitalization for emergency medical care since 15 years old. Thallium-201 single photon emission computed tomography-myocardial perfusion image (SPECT-MPI), performed respectively at 15 years old and at 20 years old, showed reversible and fixed perfusion defects of the apex, apical-mid-basal anterior, basal anteroseptal, apical-mid-inferior, and basal inferoseptal segments of the left ventricle. Initially, a hybrid of PVA and CSX was diagnosed. At 24.5 years old, technetium-99m sestamibi myocardial dipyridamole-stress dynamic SPECT and cardiac adenosine-stress magnetic resonance imaging (MRI) showed impaired coronary microvascular circulation, indicating presence of CMVD. According to the proposed stratification of INOCA,3 he was treated with atenolol, captopril, nifedipine, and NTG and waived of angina pectoris in the latest follow-up at 26 years old.
CASE
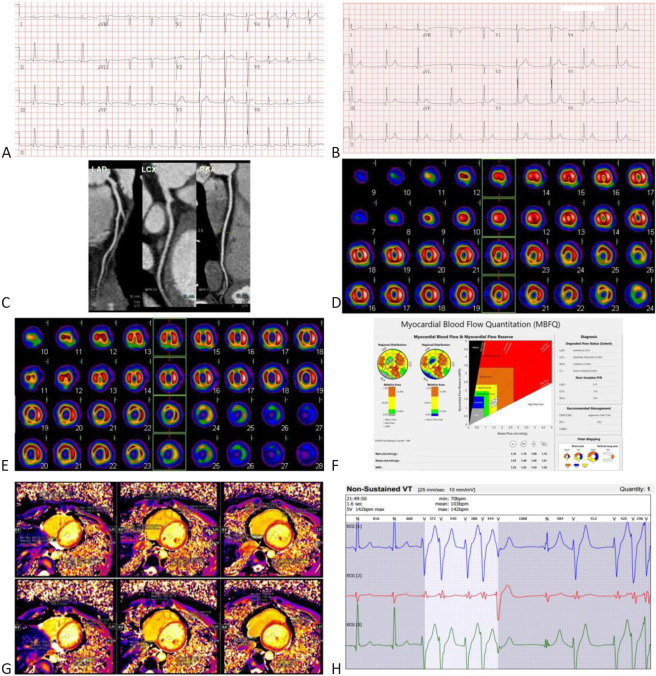
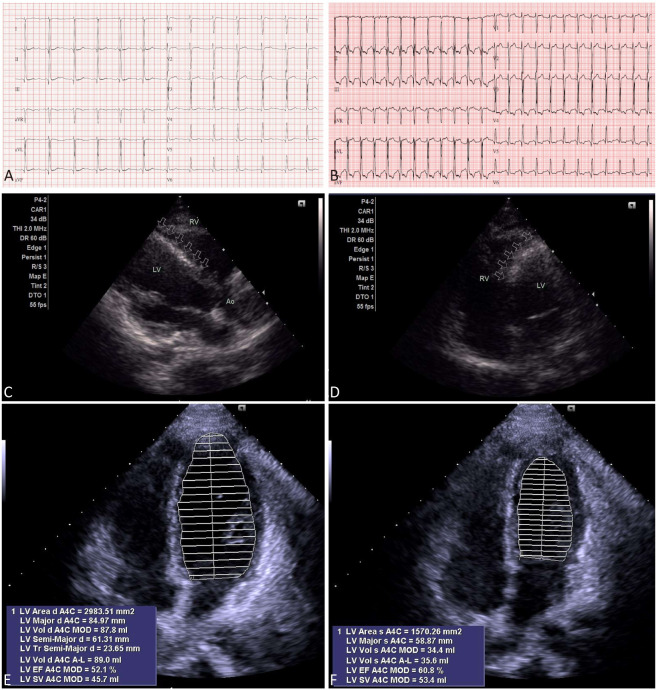
A 26-year-old man presented initially with angina pectoris at rest at 15 years old, with elevations of cardiac troponin-I (cTnI): 26.63 ng/mL (> 0.03 ng/mL) and myocardial fraction of creatine kinase mass (CK-MB): 60.1 ng/mL (> 6.3 ng/mL), and elevations of ST-segment in V1-V4 (Figure 1A). Lipid profiles were within normal limits. He had experience five more episodes of angina pectoris, both at rest and after exercise, that necessitated hospitalization for emergency medical care in the following 11 years. Neither obstructive CAD, nor myocardial bridging coronary artery was found by coronary angiography at 15 years old. Overall, he had a good response to the medical treatment of intravenous milrinone/nitroglycerin (NTG) followed by oral nefedipine/digitalis and sublingual NTG, together with regression of angina pectoris, regression of ST-segment elevation in ECG (Figure 1B), and normalization of cardiac enzymes within 2 weeks. At 19 years old, computerized tomography angiography of coronary artery showed neither obstructive CAD, nor myocardial bridging coronary artery (Figure 1C). At 20 years old, thallium-201 SPECT-MPI showed fixed myocardial perfusion defects with some reversible changes (Figure 1D). Considering a discrepancy between "improvement in symptomatology, ECG, and biochemistry" and "persistence of myocardial perfusion defects in thallium-201 SPECT-MPI", we performed another myocardial dipyridamole-stress dynamic technetium-99m sestamibi SPECT-MPI, equipped with assessment of the myocardial blood flow quantitation (MBFQ), at 24.5 years old. Serendipitously, the territories with fixed myocardial perfusion defects noted in MPI (Figure 1E) were in accordance with the territories with decreased myocardial flow reserve (MFR) and decreased stress flow (SF) by means of MBFQ (Figure 1F). Cardiac adenosine-stress MRI showed a blunted T1 reactivity (ΔT1 = [stress T1 – rest T1]/rest T1 = 0.3%-3.8%) (Figure 1G). Neither wall motion abnormalities, nor late gadolinium enhancement was found by cardiac MRI. Both myocardial technetium-99m sestamibi SPECT and cardiac MRI substantiated a diagnosis of CMVD. Meanwhile, non-sustained ventricular tachycardia was detected by a Holter ECG (Figure 1H). Before performance of treadmill ECG, a rest 12-lead ECG showed normal sinus rhythm, without ST-segment changes (Figure 2A). Angina was incidentally induced during performance of treadmill ECG, showing significant ST-segment changes (-2.80 mV to +2.30 mV) (Figure 2B). Two-dimensional echocardiography showed increased echo-density over the interventricular septum (Figure 2C, D). Using Simpsons mapping method, ejection fraction and wall motion of the left ventricle were normal over apical 4-chamber view of two-dimensional echocardiography (Figure 2E, F). According to the proposed stratification for INOCA,3 he was treated with atenolol, captopril, nifedipine, and NTG, and waived of angina pectoris in the latest follow-up at 26 years old.
Figure 1.
(A) At 15 years old, 12-lead electrocardiogram (ECG) showed elevations of ST-segments in V1-V4 at the onset of angina pectoris and ischemia and no obstructive coronary artery disease (INOCA). (B) After recovery from angina pectoris and INOCA, a follow-up 12-lead ECG showed regression of elevation of ST-segment in V1-V4. (C) At 19 years old, computerized tomography angiography of coronary artery showed non-obstructive coronary artery disease and no myocardial bridging coronary artery of the left anterior descending coronary artery (LAD), left circumflex coronary artery (LCX), and right coronary artery (RCA). (D) At 20 years old, thallium-201 single photon emission computed tomography-myocardial perfusion image (SPECT-MPI) showed fixed and some reversible perfusion defects, involving the apex, apical-mid-basal anterior, basal anteroseptal, apical-mid-inferior, and basal inferoseptal segments of the left ventricle (LV). (E) At 24.5 years old, technetium-99m sestamibi dynamic SPECT-MPI showed fixed perfusion defects involving the same territories of the LV. (F) Simultaneously, dipyridamole-stress technetium-99m sestamibi dynamic SPECT was performed to study the myocardial blood flow quantitation (MBFQ). Interestingly, myocardial flow reserve (MFR) of the LV, left anterior descending coronary artery (LAD), left circumflex coronary artery (LCX), and right coronary artery (RCA) were 1.35, 1.23, 1.53, and 1.35, respectively. Stress flow (SF) of the LV, LAD, LCX, and RCA were 1.53 mL/min/gm, 1.46 mL/min/gm, 1.65 mL/min/gm, and 1.51 mL/min/gm, respectively. Using thresholds of MFR ≤ 1.74 and/or SF ≤ 1.54 mL/min/gm, the polar map and Gould’s plot showed an 8.29% ischemia in our patient. (G) At the same time, adenosine-stress cardiac magnetic resonance images (MRI) showed a blunted T1 reactivity (ΔT1 = (stress T1 – rest T1)/rest T1 = 0.3%-3.8%). A decrease of MFR and SF (repercussion of coronary flow reserve) by dipyridamole-stress dynamic SPECT MBFQ, and a blunted T1 reactivity by adenosine-stress cardiac MRI substantiate presence of coronary microvascular dysfunction (CMVD) and meet the diagnostic criteria of microvascular angina (MVA). (H) Holter ECG showed non-sustained ventricular tachycardia.
Figure 2.
(A) Before performance of treadmill electrocardiography (ECG), a rest 12-lead ECG showed normal sinus rhythm, without ST-segment changes. (B) A 4-stage treadmill ECG showed significant ST-segment changes (-2.80 mV to +2.30 mV). (C) Parasternal long-axis view and (D) apical 4-chamber view of two-dimensional echocardiography showed increased echo-density (arrows) over the interventricular septum. Besides, the interventricular septum was found bulging from the left ventricle (LV) toward right ventricle (RV). By Simpsons method, two-dimensional echocardiography, over apical 4-chamber view, showed normal ejection fraction and wall motion of the LV, with a mean area of (E) 29.83 cm2 in diastole and (F) 15.70 cm2 in systole, respectively. This extrapolates that permanent myocardial damage could be emerged with times in patients incurring ischemia and no obstructive coronary artery disease (INOCA) due to a hibernated pathology coronary microvascular dysfunction (CMVD), however, ejection fraction and wall motion of the LV could be preserved due to a scattered distribution of myocardial ischemia in CMVD which did not interfere global contraction and metabolism.
DISCUSSION
There are several noteworthy points to be emphasized. First, either PVA or CSX was once considered to be a benign disease entity in patients presenting with angina in the absence of obstructive CAD. However, major adverse cardiac events were noted to haunt these patients.3 PVA and CSX were considered to be two distinct syndromes presenting with angina, however, there was anecdotal report of a hybrid of both syndromes.4 By a 11-year follow-up of the present patient, who had experienced six episodes of INOCA, which were intervened with both rest and exercise-induced angina, non-sustained ventricular tachycardia, and persistent and fixed myocardial perfusion defects in thallium-201 SPECT-MPI, we highlighted using dynamic technetium-99m sestamibi SPECT-MBFQ (MFR and SF) and cardiac MRI (T1 mapping) to assess the coronary microvascular circulation and to unmask the Janus face of CMVD being hibernated in the anginal patients with INOCA. Noticeably, INOCA can be encountered in adolescents and is not necessarily benign if left undiagnosed/untreated.
Second, by the routine coronary angiography, only 53% of anginal patients had obstructive CAD.3 In another words, up to 47% could be the victims of INOCA.3 There are four endotypes in the INOCA population: isolated MVA (52%), mixed MVA and vasospastic angina (VSA) (20%), isolated VSA (17%), and non-cardiac angina (11%).3 There was an increased risk of major adverse cardiac events in patients with INOCA as the times went by, thus, medical treatment should be adjusted and stratified by the functional assessment of the coronary microcirculation.3
Third, diagnosis of CMVD can be achieved through functional assessment of the coronary microcirculation.5 However, interventional diagnostic procedure, using cardiac catheterization to study coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve, is technically invasive, expertise-demanding, and time-consuming.5 Auspiciously, dynamic technetium-99m sestamibi SPECT with MBFQ6 and cardiac MRI with T1 mapping7 have been emerged as noninvasive tools to diagnose CMVD. There are endothelial cells-dependent and endothelial cells-independent coronary microcirculation, which are modulated by the interactive biological and physiological reactions.5 Endothelial cells-dependent vasoreactivity prevails in the larger arterioles (100-200 μm) and translates flow-related stimuli into vasomotor responses, namely, by vasodilatation in response to increased blood flow.5 While medium-sized arterioles (40-100 μm) are endothelial cells-independent and react predominantly to changes of the intraluminal transmural pressure which can be sensed by the stretch receptors in the smooth muscle cells, namely myogenic control, for example, by vasoconstriction in response to increased intraluminal transmural pressure.5 By dipyridamole-stress dynamic technetium-99m sestamibi SPECT-MBFQ, the SF was significantly lesser than the rest flow and MFR was significantly decreased in our patient, indicating presence of a pathology of CMVD in the distal and diseased microvasculature with decreased reactivity of vasodilatation to dipyridamole (an endothelial cells-independent agent) and coronary artery steal phenomenon, from the proximal intact microvasculature, by the adjacent healthy microvasculature which will be normally responding to dipyridamole with vasodilatation.6 Sometimes, there could be disagreement between results of MPI and MBFQ due to diffuse pathology of CMVD, with a paucity of healthy myocardium to provide a normal background reference, that may render balanced-ischemia more conspicuously unmasked by MBFQ, which will assess absolute coronary blood flow deficiency, than by MPI, which will assess relative coronary blood flow deficiency.6,8 Conspicuously, cardiac adenosine-stress MRI showed a blunted T1 reactivity in our patient, indicating presence of CMVD due to decreased reactivity of vasodilatation to adenosine (an endothelial cells-dependent agent) accompanying with coronary artery steal phenomenon.
Fourth, a decrease of MFR and SF (as repercussions of coronary flow reserve) detected by dipyridamole-stress dynamic technetium-99m sestamibi SPECT with MBFQ,6 and a blunted T1 reactivity by cardiac adenosine-stress MRI with T1 mapping7 may help substantiate a diagnosis of CMVD.9 Using thresholds of MFR ≤ 1.74 or SF ≤ 1.54 mL/min/gm,6 the polar map and the Gould’s plot showed an 8.29% ischemia of the left ventricular myocardium in our patient. By dipyridamole-stress dynamic myocardial technetium-99m sestamibi SPECT and adenosine-stress cardiac MRI, we not only disclose a hibernated pathology of CMVD that predisposed our patient to incur angina pectoris, but also decipher a discrepancy between "improvement of symptomatology, ECG changes, and cardiac enzymes" and "impairment of coronary microcirculation noted in SPECT/MRI". Such a discrepancy may reside on the fact that a scattered distribution of myocardial ischemia in patients with CMVD did not significantly interfere global contraction and metabolism.10 Left ventricular ejection fraction could be preserved initially and reduced or deteriorated later on.6
In conclusion, CMVD may be masqueraded with a Janus face of recurrent angina pectoris and INOCA in the adolescent(s), which scenario may lead to major adverse cardiac events if left undiagnosed and/or untreated. We highlighted using myocardial dipyridamole-stress dynamic technetium-99m sestamibi SPECT and cardiac adenosine-stress MRI to track down the culprit of CMVD in the anginal patients with INOCA, to whom medications can be accordingly stratified to alleviate the clinical symptoms and to decrease the risk of major adverse cardiac events.
LEARNING POINTS
1. A scenario of angina pectoris and INOCA can be encountered in the adolescents, and is not necessarily benign if left undiagnosed/untreated.
2. Myocardial dipyridamole-stress dynamic technetium-99m sestamibi SPECT (MFR and SF) and cardiac adenosine-stress MRI (T1 mapping) may be used to track down a hibernated culprit of CMVD in the anginal patients with INOCA.
FINANCIAL SUPPORT
The authors have no financial support to disclose.
AUTHOR CONTRIBUTIONS
Conceptualization: Meng-Luen Lee. Data curation: Meng-Luen Lee, Ming-Che Chang, and Chiung-Ying Liao. Investigation: Meng-Luen Lee. Resources: Meng-Luen Lee. Supervision: Meng-Luen Lee. Visualization: Meng-Luen Lee, Ming-Che Chang, and Chiung-Ying Liao. Writing – original draft: Meng-Luen Lee. Writing – review & editing: Meng-Luen Lee and Ming-Che Chang. Approval of final manuscript: all authors.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
INFORMED CONSENT
Written informed consent was obtained from the patient (at 26 years old) and his mother for the publication of the case report and the accompanying images. There is no financial assistance for this study.
REFERENCES
- 1.Cannon RO, 3rd, Epstein SE. "Microvascular angina" as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol. 1988;61:1338–1343. doi: 10.1016/0002-9149(88)91180-0. [DOI] [PubMed] [Google Scholar]
- 2.Camini PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 3.Ford TJ, Berry C. How to diagnose and manage angina without obstructive coronary artery disease: lessons from the British Heart Foundation CorMicA Trial. Interv Cardiol. 2019;14:76–82. doi: 10.15420/icr.2019.04.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yilmaz A, Hill S, Schäufele T, et al. Exercise-induced spastic coronary artery occlusion at the site of a moderate stenosis: neither Prinzmetal’s angina nor cardiac syndrome X but "Prinzmetal X". Circulation. 2010;122:e570–e574. doi: 10.1161/CIRCULATIONAHA.110.984823. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J. 2012;33:2771–282b. doi: 10.1093/eurheartj/ehs246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee ML, Chang MC, Wang YM. Ischemia and no obstructive coronary artery disease and pulmonary hypertension in a 16-year-old girl with unquenched hyperthyroidism of Graves’ disease. Acta Cardiol Sin. 2021;37:442–448. doi: 10.6515/ACS.202107_37(4).20210212A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu A, Wijesurendra RS, Liu JM, et al. Gadolinium-free cardiac MR stress T1-mapping to distinguish epicardial from microvascular coronary disease. J Am Coll Cardiol. 2018;71:957–968. doi: 10.1016/j.jacc.2017.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Motwani M, Motlagh M, Gupta A, et al. Reasons and implications of agreements and disagreements between coronary flow reserve, fractional flow reserve, and myocardial perfusion imaging. J Nucl Cardiol. 2018;25:104–119. doi: 10.1007/s12350-015-0375-1. [DOI] [PubMed] [Google Scholar]
- 9.Ong P, Camini PG, Beltrame JF, et al. Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 10.Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121:2317–2325. doi: 10.1161/CIRCULATIONAHA.109.900191. [DOI] [PubMed] [Google Scholar]