Abstract
In this paper, we describe the synthesis and crystal structure analysis of N-acetyl-2,4-[diphenyl-3-azabicyclo[3.3.1]nonan-9-yl]-9-spiro-4′-acetyl-2′-(acetylamino)-4′,9-dihydro-[1′,3′,4′]-thiadiazole (3a) and N-acetyl- 2,4-[bis(p-methoxyphenyl)-3-azabicyclo[3.3.1]nonan-9-yl]-9-spiro-4′-acetyl-2′-(acetylamino)-4′,9-dihydro-[1′,3′,4′]-thiadiazole (3b). The title compounds 3a and 3b are characterized by 1D NMR and single crystal x-ray diffraction analysis. Non-covalent interactions in a molecule were identified by Hirshfeld surface (dnorm contacts and 2D fingerprint plot) analysis. In addition, the existence of chalcogen bond (S•••O bond) in the molecular structures (3a and 3b) are described by NCI-RDG and QTAIM analysis. NBO analysis is employed to describe the orbital interactions and electron transfer between sulfur and oxygen atoms. Molecular docking is carried out for compounds 3a and 3b with COVID-19 viral protein SARS-nCoV-2 Mpro (PDB ID: 6LU7).
Keywords: Azabicyclo[3.3.1]nonan-9-ones, Spiro thiadiazoles, Chalcogen bond, SARS-nCoV-2 Mpro, Molecular docking
Graphical Abstract
Graphical Abstract

.
1. Introduction
Spiro heterocyclic structure is a unique feature of a several natural and synthetic products that possess remarkable biological activities [1,2]. The potential use of spiro heterocycles in medicinal chemistry has been well documented owing to their prominent pharmacological properties [3]. The thiadiazole moiety is associated with a broad spectrum of biological activities such as antifungal [4], antioxidant [5], anticonvulsant, antiviral [4], plant growth regulatory [6], CNS depressant [7], and anticancer [8] activitiy. Methazolamide is a thiadiazole based drug being used to treat reduction of high pressure inside the eyes, prevents blindness, nerve damage, and vision loss and glaucoma [9]. Similarly, acetazolamide is an another thiadiazole drug candidate being used as drug for the treatment of epileptic seizure, and periodic paralysis [10].
1,3,4-thiadiazole core has been reported as an electron-deficient nature, electron-accepting ability and good thermal as well as chemical stability [11]. Thus, thiadiazole derivatives are often used to create liquid crystals due to charge-transporting ability, photoconductivity, photoluminescence and mesomorphism [12]. Therefore, development of new spiro-heterocycles having thiadiazole ring is worthwhile from the perspective of medicinal and material chemistry [13,14].
Piperidine is an another heterocyclic compound that found naturally in alkaloids, and its derivatives have good biological and pharmacological properties [15]. On the other hand, the six membered piperidine ring undergoes several conformational changes when introducing electron donating or withdrawing substituents at the nitrogen site. For example, the piperidine ring adopts chair conformation with electron donating substituent (H, CH3, CH2Ph) whereas the electron withdrawing group (heteroatom group, COR, NO) on the same site adopts boat conformation [16]. Recently, several piperidine derivatives along with the incorporation of hetero conjugate groups at the nitrogen atom of piperidine ring have been well explored by spectroscopic and X-ray analysis [17], [18], [19]. Earlier reports disclosed that the blocking of secondary nitrogen atom in the piperidine ring by electron donating or withdrawing groups, abrupt changes were observed in the ring conformations. As a result, the molecule displays different conformation such as chair, boat or twist-boat forms.
The novel coronavirus 2019 (nCoV, also called as COVID-19), is a new strain and highly contagious viral disease. The coronavirus causes a severe acute respiratory syndrome (SARS-nCoV-2), has showed to be the most deadly global health crisis since the 1918 influenza pandemic [20]. The novel virus (SARS-nCoV-2) has proven to be a worldwide unprecedented disaster that affects billions of lives across the world in many ways. Currently, there are no specific treatment options for SARS-CoV-2. Thus, it has been classified that COVID-19 is a very rare disease with a limited number of treatment options [21]. Molecular docking is the preliminary study in the drug designing, which help us to find the possible binding site between lead molecule and protein structure. For Covid-19, several docking studies have been reported to find potential antiviral drugs and vaccines [22], [23], [24], [25], [26].
In this paper, we have selected bicyclic ring system that contains both heterocyclic (piperidine) and non-heterocyclic (cyclohexane) rings based on spiro derivatives viz. N-acetyl-2,4-[diaryl-3-azabicyclo[3.3.1]nonan-9-yl]-9-spiro-4′-acetyl-2′-(acetylamino)-4′,9-dihydro-[1′,3′,4′]-thiadiazoles (3a and 3b) to describe the spectral characterization and single crystal structure analysis. Also, the DFT studies have been employed to explore the electronic properties of molecules 3a and 3b. In addition, the molecular docking is carried out to extend the scope of molecular structure with novel coronavirus causing protein.
2. Experimental
1H and 13C NMR spectra of 3a and 3b were obtained from BRUKER AMX 500 MHz FT-NMR spectrometer using CDCl3 as the NMR solvent, whereas TMS was used as an internal reference. The NMR chemical shift (δ) and coupling constants values were measured in ppm and coupling constants in Hz, respectively.
2.1. Synthesis of compounds 3a and 3b
The title compounds 3a and 3b were synthesized according to the Scheme 1 . Initially, 2,4-diaryl-3-azabicyclo[3.3.1]nonan-9-one thiosemicarbazones (2a and 2b) were synthesized according to the literature precedent [27]. The title compounds 3a and 3b were synthesized by modification of our earlier reports [28]. A mixture of 2,4-diaryl-3-azabicyclo[3.3.1]nonan-9-one thiosemicarbazone (2a or 2b, 0.5 g) and 20 mL of distilled acetic anhydride were heated at 60˚C on water bath for 6 h. After completion of the reaction, as confirmed by TLC, the reaction mixture was kept in the freezer at overnight and the separated solid was filtered off, dried and purified by column chromatography. The pure crystals of 3a and 3b for X-ray diffraction analysis were grown by slow evaporation technique using distilled ethanol.
Scheme 1.
Synthetic route for the synthesis of compounds 3a and 3b
2.1.1. N-Acetyl-2,4-[diphenyl-3-azabicyclo[3.3.1]nonan-9-yl]-5-spiro-4-acetyl-2-(acetylamino)-Δ2-1,3,4-thiadiazoline (3a)
1H NMR, (δ=ppm): 6.13 (d, 2H, H-2a & H-4a); 3.30 (d, 2H, H-1e & H-5e); 1.70 (s, 4H, amide CH3 & H-7a); 1.66 (s, 3H, H-6e, H-8e); 1.30 (d, 2H, H-6a & H-8a); 1.24 (m, 1H, H-7e); 9.67 (bs, 1H, spiro NH); 2.20, 1.90 (s, 3H, amide CH3); 7.40 & 7.51 (t & bs, 10H, aryl protons): 13C NMR (δ=ppm) CDCl3: 61.58 (C-2 & C-4); 41.38 (C-1 & C-5); 25.61 (C-6 & C-8); 18.30 (C-7); 87.28 (C-9); 27.23, 24.63 & 22.36 (amide CH3); 176.20, 174.22 & 169.55 (amide C=O); 155.60 (C=N); 142.95, 142.18 (C-2′ & C-4′); 128.53, 126.59, 125.58 (other aryl carbons).
2.1.2. N-Acetyl-2,4-[bis(p-methoxyphenyl)-3-azabicyclo[3.3.1]nonan-9-yl]-5-spiro-4-acetyl-2-(acetylamino)-Δ2-1,3,4-thiadiazoline (3b)
1H NMR, (δ=ppm): 6.11 (d, 2H, H-2a & H-4a); 3.22 (d, 2H, H-1e & H-5e); 1.75 (bs, 4H, amide CH3 & H-7a); 1.68 (m, 2H, H-6e & H-8e); 1.34 (d, 2H, H-6a & H-8a); 1.23 (m, 1H, H-7e); 9.73 (bs, 1H, spiro NH); 2.19, 1.90 (s, 6H, amide CH3); 6.91 (d, 5H, aryl protons ortho to OCH3 group; 7.41 (bs, 3H, aryl protons meta to OCH3 group); 3.82 (s, 6H, OCH3 group at phenyl group 13C NMR (δ ppm) CDCl3: 61.09 (C-2 & C-4); 41.43 (C-1 & C-5); 25.60 (C-6 & C-8); 16.78 (C-7); 87.45 (C-9); 27.37, 24.65 & 22.57 (amide CH3); 176.90, 174.01 & 169.37 (amide C=O); 155.32 (C=N); 158.21,158.14 (C-2′''' & C-4′'''); 134.94, 134.24 (C-2′ & C-4′); 126.70, 113.93 (other aryl carbons); 55.30 (OCH3 group at the phenyl group).
2.2. Single crystal X-ray analysis
Colorless rectangular crystals (0.25, 0.20, 0.20 mm for 3a and 0.20, 0.20, 0.25 mm for 3b) were used for the data collections. The single crystal X-ray diffraction data were recorded using a CCD area detector at room temperature (273 K). The data reduction and cell refinement were carried out using SAINT/XPREP (Bruker), and APEX2/SAINT, respectively. The crystal structures (3a and 3b) were solved by direct methods using SHELX-97 [29]. All the non-hydrogen atoms were treated anisotropically by the full-matrix least squares method, whereas hydrogen atom bound to the carbon atoms were constrained isotropically. However, the hydrogen atoms bound to the nitrogen atoms refined freely. Graphical molecular illustrations were done with ORTEP 3 for Windows [30]. The Crystallographic information files (CIFs) were deposited in the Cambridge Crystallographic Data Centre. The CCDC number for 3a and 3b are 796611 and 793237, respectively. CIF data of 3a and 3b can be obtained free of charge from www.ccdc.cam.ac.uk/data_request/cif.
2.3. Hirshfeld surface analysis
The Crystal Explorer 21.5 program was used to generate Hirshfeld surfaces (HS) mapped over dnorm, shape-index and curvedness plots [31], as previously studied in reference [32]. The structural input files of 3a and 3b were obtained from CIF data. The normalized intermolecular contact distance was denoted as dnorm using the following relation.
where de and di define the distance from the Hirshfeld surface to nearest outside and inside the nucleus surfaces, respectively, revdw / rivdw define the van der Waals radii of outside and inside the atoms, respectively. The value of intermolecular contacts (dnorm) such as hydrogen bonding, van der Waals radii and steric interaction are distinguished by tricolor code; red-white-blue-colors, respectively. The bright red spots on the Hirshfeld surface show the intermolecular contacts less than their van der Waals radii, whereas the blue region on the HS demonstrates intermolecular contacts longer than their van der Waals radii. However, the white spot on the HS indicates the sum of their van der Waals radii. Further, the π-π interaction between asymmetric units can be detected through the shape index mapped surface as they exhibit adjacent red-blue triangle concave.
2.4. Computational study
All quantum chemical calculations were carried out by using Gaussian 09 software with DFT method at B3LYP/6-311G (d,p) level [33]. Gauss view 5.0.9 was used to prepare and inspect the input and output files. Quantum theory of atoms in a molecule (QTAIM) was done with AIMALL software [34] whereas non-covalent interaction and radiant density gradient (NCI-RDG) were executed with Multiwin and VMD molecular visualization programmes [35]. The docking study was carried out using AutoDockTools 1.5.7 [36]. The selected protein structure for this study was obtained from Protein Data Bank (PDB ID: 6LU7). Both ligands (3a and 3b) and protein structure (6LU7) were cleaned prior to the docking using Discovery Studio 2021 Client [37]. The grid box was centered using −19.173, 10.969, and 68.039 Å along x, y, and z axes was prepared with 70 × 65 × 70 A with spacing of 0.375 Å. The genetic algorithm was employed for ligand 3a and 3b as the search parameter with 100 runs.
3. Results and Discussion
3.1. NMR analysis of 3a and 3b
NMR signals were assigned based on the signal position, multiplicity and integral values [38]. The proton and carbon NMR chemical shift values of 3a and 3b are presented in section 2.4. It is seen that, two doublets were observed at 6.13 and 3.30 ppm (for 3a) ppm and 6.11 and 3.22 ppm (for 3b) with two protons integral each should be assigned to benzylic (H-2/H-4) and bridgehead (H-1/H-5) protons, respectively. The observed chemical shift value of H-2/H-4 is deshielded about 2 ppm as compared to 2,4-diaryl-3-azabicyclo[3.3.1nonane-9-one thiosemicarbazones [27], which were reported at 4.26/4.36 ppm [27]. The large chemical shift variation should be due to incorporation of acetyl group at the nitrogen site of piperidine ring, where the acetyl group undergoes NCO bond free rotation in NMR time scale. Owing to this, the benzylic protons are deshielded significantly. Further, it was expected that broad signal for benzylic protons, as that of our earlier study on N-chloroactyl-2,6-diarylpiperidin-4-ones [17]. Contrary to this, sharp signal appeared due to perpendicular orientation of acetyl group [39].
Further, a collection of signals for H-2/H-4were observed in the upfield region from 1.24-2.73 ppm are due to signals of methylene protons of cyclohexane ring, as shown in Fig S1-S2. It is seen that, three multiplets appeared at 1.70, 1.66 and 1.30 ppm (for 3a) and 1.72, 1.60 and 1.28 ppm (for 3b). It is worth to mention that due to the anisotropy effect of C-C bond, the equatorial proton in methylene carbon of cyclohexane ring is more deshielded than its axial proton. Based on this, the deshielded multiplets at 1.70 ppm (3a), and 1.72 ppm (3b) is attributed to H-6e/H-8e protons whereas the signal at 1.66 (3a), and 1.60 ppm (3b) should be due to H-6a/H-8a protons. However, the signal 1.30 ppm (3a), 1.28 ppm (3b) with one proton integral value is due to H-7e proton. Similarly, a multiplet at 2.61 with one proton value is due to another H-7 proton (H-7a). The chemical shift value of cyclohexane methylene proton of 3a and 3b are in good agreement with the earlier report on parent compounds reported by us [27,40]. Additionally, two singlets were resonated at 2.20, 1.90 (3a) and 2.19 and 2.20 (3b) ppm with six proton integral value should be due to acetyl methyl protons. The signal at the deshielded region 9.67 ppm (3a) and 9.73 ppm (3b) with one proton integral value is ascribed to N-H proton in the side chain acetylamino group.
13C NMR spectra of compounds 3a and 3b are depicted in Fig. S3 and S4 respectively. It is seen that, two signals with equal intensity were observed at 61.58 and 41.38 ppm (for 3a) and 61.09 and 41.43 ppm (for 3b) are attributed to benzylic carbons (C2/C4) and bridgehead carbons (C1/C5), respectively. The methylene carbons of cyclohexane ring signals were observed at 25.61 and 18.30 (for 3a) and 25.60 and 16.78 ppm (for 3b). Out of which, the deshielded signal at 25.61 and 25.60 ppm in each compound can be assigned to C6/C8 carbons, whereas the shielded signals at 18.30 and 16.78 ppm in 3a and 3b, respectively, are due to C7 carbon. Further, the signals at 87.28 ppm (3a) and 87.25 ppm (3b) respectively for 3a and 3b can be assigned to C9 spiro carbon in the molecular structure.
In both compounds (3a and 3b), collection of signals appeared at 176.20, 174.22, 169.55 and 155.60 ppm (for 3a) and 176.90, 174.01, 169.37 and 155.32 ppm (for 3b). Among these, signals in the region of 176.20-169.55 ppm (for 3a) and 176.90-169.33 (for 3b) are due to amide carbonyl carbons, whereas the signal at 155.60 (for 3a) and 155.32 (for 3b) should be due to imine carbon signals. Signals at 27.23, 24.63 and 22.36 ppm (for 3a) and 27.37, 24.65 and 22.57 ppm (for 3b) are due to methyl carbon signal in acetyl group. The aryl carbon signals in both compounds were observed in the region of 158-113 ppm. The methoxy carbon signal in 3b was observed at 55.30 ppm.
3.2. Single crystal X-ray diffraction analysis
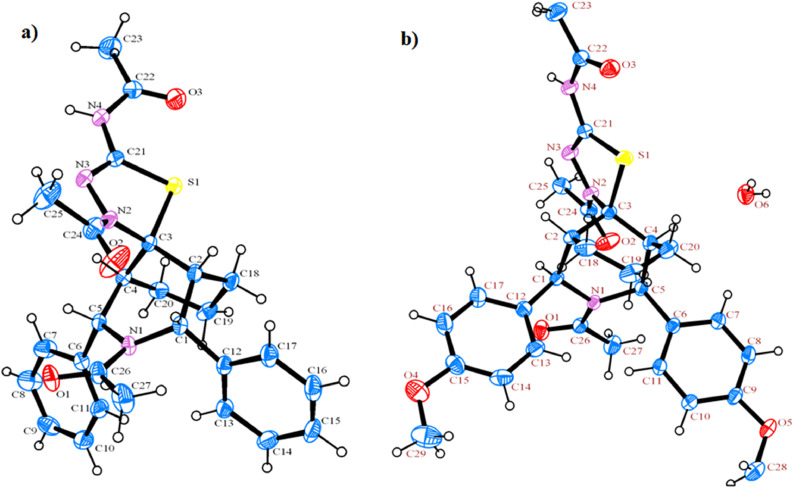
The molecular structures with atom-numbering schemes of 3a and 3b are shown in Fig. 1 a and Fig. 1b, respectively. The structure refinements and selected geometrical parameters are enumerated in Table 1 and Table 2 , respectively. The compounds 3a and 3b were crystallized into a monoclinic lattice with P21/c symmetry. Further, all the C–C and C –H bond lengths in the benzene rings were found to be in the normal range and bond angles were found to be approximately 120°. The C-C-C bond angles and C-C-C-C torsion angles of piperidine and cyclohexane rings are greatly deviated from the parent crystal structures [27]. As a result, significant changes are observed in the conformation of saturated cyclic rings.
Fig. 1.
a) ORTEP diagram of compound 3a; b) ORTEP diagram of compound 3b.
Table 1.
Single crystal XRD data collection and refinement details of 3a and 3b
| Compound 3a | Compound 3b | |
|---|---|---|
| Empirical formula | C27 H30 N4 O3 S | C29 H36 N4 O6 S |
| Formula weight | 490.61 | 568.68 |
| Temperature |
293(2) K | 293(2) K |
| Wavelength | 0.71073 A | 0.71073 A |
| Crystal system, space group | Monoclinic, P 21/c | Monoclinic, P 21/c |
| Unit cell dimensions | a = 9.8486(3) Å; b = 13.8532(3) Å; c = 19.0571(4) Å α = 90 ˚ deg. β= 101.7150(10) deg. gamma = 90 deg. |
a=17.2963(9) b=9.2931(5); c=17.6126(8) α =90.00; β= 97.5020(10) gamma = 90 deg. |
| Volume | 2545.89(11) A3 | 2806.8(2) |
| Z, Calculated density | 4, 1.280 Mg/m3 | 4, 1.346 Mg/m3 |
| Absorption coefficient | 0.163 mm−1 | 0.166 |
| F(000) | 1040 | 1208 |
| Crystal size | 0.25 × 0.20 × 0.20 mm | 0.20 × 0.20 × 0.25 mm |
| Theta range for data collection | 1.83 to 26.18˚ | 1.19 to 26.93˚ |
| Limiting indices | -12<=h<=12, -17<=k<=14, -23<=l<=17 | -22 =< h =< 21, -11 =< k =< 11, -22 =< l =< 22 |
| Reflections collected / unique | 25678 / 5073 [R(int) = 0.0338] | 29917 / 6081 [R(int) = 0.037] |
| Completeness to theta | 26.18 99.5 % | 26.93° 100 % |
| Absorption correction | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Max. and min. transmission | 0.9681 and 0.9604 | |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 5073 / 0 / 316 | 6081 / 0 / 369 |
| Goodness-of-fit on F2 | 1.040 | 1.046 |
| Final R indices [I>2sigma(I)] | R1 = 0.0798, wR2 = 0.2466 | R1 = 0.0487, wR2 = 0.1402 |
| R indices (all data) | R1 = 0.1008, wR2 = 0.2717 | R1 = 0.0707, wR2 = 0.1619 |
| Largest diff. peak and hole | 2.841 and -0.333 e.A−3 | 0.686 and -0.456 e.Å-3 |
| CCDC | 796611 | 793237 |
Table 2.
Selected geometrical parameters of 3a and 3b obtained from XRD
| Compound 3a | |||||
|---|---|---|---|---|---|
| Atoms | 3a | 3b | Atoms | 3a | 3b |
| S(1)-C(21) | 1.741(4) | 1.734(2) | N(3)-C(21) | 1.275(5) | 1.275(3) |
| S(1)-C(3) | 1.860(3) | 1.865(2) | C(5)-C(6) | 1.530(5) | 1.531(3) |
| O(1)-C(26) | 1.233(4) | 1.229(3) | C(1)-C(12) | 1.528(4) | 1.513(3) |
| N(1)-C(26) | 1.369(4) | 1.369(3) | C(1)-C(2) | 1.556(5) | 1.548(3) |
| N(1)-C(1) | 1.487(4) | 1.490(2) | C(4)-C(20) | 1.532(4) | 1.540(3) |
| N(1)-C(5) | 1.488(4) | 1.489(2) | O(2)-C(24) | 1.205(5) | 1.209(3) |
| O(3)-C(22) | 1.209(5) | 1.223(3) | C(2)-C(18) | 1.532(5) | 1.528(3) |
| N(2)-C(24) | 1.359(5) | 1.369(3) | C(19)-C(20) | 1.513(5) | 1.517(4) |
| N(2)-N(3) | 1.418(4) | 1.405(2) | C(26)-C(27) | 1.508(6) | 1.509(3) |
| N(2)-C(3) | 1.494(4) | 1.495(3) | C(22)-C(23) | 1.495(6) | 1.501(3) |
| N(4)-C(22) | 1.373(5) | 1.357(3) | N(4)-C(21) | 1.377(5) | 1.386(3) |
| C(21)-S(1)-C(3) | 87.60(16) | 88.29(9) | N(1)-C(1)-C(2) | 113.7(3) | 114.70(16) |
| C(26)-N(1)-C(1) | 117.6(3) | 112.7(2) | N(3)-C(21)-S(1) | 118.0(3) | 118.31(16) |
| C(26)-N(1)-C(5) | 113.0(3) | 117.2(2) | N(4)-C(21)-S(1) | 122.5(3) | 123.7(2) |
| C(1)-N(1)-C(5) | 124.2(3) | 124.4(2) | N(1)-C(5)-C(6) | 112.2(3) | 112.40(17) |
| C(24)-N(2)-N(3) | 115.9(3) | 114.01(17) | N(1)-C(5)-C(4) | 115.6(3) | 114.31(16) |
| C(24)-N(2)-C(3) | 132.3(3) | 132.60(17) | N(2)-C(3)-C(4) | 109.0(3) | 120.4(2) |
| N(3)-N(2)-C(3) | 111.6(3) | 113.3(2) | N(2)-C(3)-C(2) | 121.1(3) | 110.5(2) |
| C(22)-N(4)-C(21) | 123.5(3) | 125.8(2) | N(2)-C(3)-S(1) | 100.2(2) | 100.6(1) |
| C(21)-N(3)-N(2) | 109.9(3) | 110.35(17) | C(4)-C(3)-S(1) | 111.0(2) | 108.6(1) |
| N(1)-C(1)-C(12) | 114.7(3) | 113.53(16) | C(2)-C(3)-S(1) | 108.2(2) | 109.5(1) |
| C3 C2 C1 N1 | -22.05 | 41.6(2) | C3-C2-C18-C19 | 52.4 | -53.7 |
| N1-C5-C4-C3 | -39.9 | -24.6 | S1-C3-N2-N3 | 36.9 | -31.62 |
| C3-C4-C20-C19 | 61.71 | 62.17 | S-C21-N3-N2 | 4.03 | -4.01 |
To describe the conformation of piperidine and cyclohexane rings, the puckering parameters were derived for compound 3b using X-ray data. Based on the data, one of the six membered (piperidine) ring adopts boat conformation with the puckering parameters being q2 and q3 are 0.045 Å and 0.5773 Å, respectively. The total puckering amplitude QT and θ were calculated as 0.5791 Å and 4.46° respectively. However, the cyclohexane ring is preferably adopts normal chair conformation with the puckering parameters of q2 and q3 were predicted to be 0.136 Å and 0.559 Å, respectively and QT and θ were calculated to be 0.575 (2) Å, and 13.73° respectively [41]. Further, the saturated piperidine ring adopts boat conformation as suggested by the torsion angles around the bonds involving the ring atoms N1-C1-C2-C3 and N1-C5-C4-C3 were found as 22.05 and -39.9° (for 3a) and 41.6 and -24.6° (for 3b), respectively. These torsion angles suggest that the piperidine ring N1-C1-C2-C3-C4-C5 deviated significantly from the ideal value of 56˚ reported for the chair conformation of cyclohexane [16]. Therefore, the piperidine preferred to adopt boat conformation. On the other hand, the cyclohexane ring adopts chair conformation as they shows torsion angle of C3-C4-C20-C19 and C3-C2-C18-C19, were found to be -61.71 and 52.4° (for 3a) and 62.17 and -53.7° (for 3b), respectively. In both the crystal structures, the phenyl rings C2 and C4 occupy equatorial orientations as they confirmed by bond angles of C12-C1-C2, C12-C1-N1, C6-C5-C4 and C6-C5-N1 are, 112.5, 114.6, 112.1 and 112.2° (for 3a) and 111.7, 77.9, 113.7 and 112.4° (for 3b), respectively. The observed bond lengths and bond angles are in good agreement with those observed for similar bicyclic compounds [27,42].
In 3a and 3b, the five membered 1,3,4-thiadiazole ring was found to be in flattened boat conformation, as they undergo N-C=O bond free rotation by N-acetyl group. Further, the observed torsion angles of S1-C3-N2-N3/S-C21-N3-N2 are 36.9 /4.03 and -31.62/-4.01 for 3a and 3b, respectively, proved that the existence of boat form.
The crystal structures of 3a and 3b are packed by several non-bonded contacts such as C-Hδ+•••δ+H-C, S•••O, N-H•••O, C-H•••O and C-H•••Cπ interactions. Out of which, the most important contacts were found as N-H•••O and C-H•••O hydrogen bonds. The hydrogen bonding and other contacts parameters are listed in Table 3 . The solid state structure of 3a are packed in three dimension by N(4)-H(4)•••O(1) and C(16)-H(16)•••O(1), and C(17-C(21)•••C(21), hydrogen bonds with donor-acceptor distances of 2.816 and 3.513, and 3.559 Å respectively. Unlike the crystal structure 3a, 3b is packed through the water molecule. It can be seen from Table 3, C(27)-H(27) •••O(3), O(6)-H(6)•••O1, N(4)-H(3)•••O(6) are the major hydrogen bonds with donor-acceptor distances of 3.547 and 2.850, and 2.828 Å respectively.
Table 3.
Hydrogen bonding parameters of compound 3a and 3b
| Compound 3a | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Atoms | D….A | D-H | D-H….A | D-H….A | ||
| 1 | S1•••O3i | 2.71 | |||||
| 2 | N1•••O2i | 2.973 | |||||
| 3 | N4-H4•••O1ii | 2.816 | 0.86 | 2.02 | 153.37 | ||
| 4 | C23-H23A•••O1ii | 3.506 | 0.961 | 2.711 | 140.5 | ||
| 5 | C8-H8•••C14iii | 3.634 | 0.93 | 2.862 | |||
| 6 | C8-H8•••C15iii | 3.534 | 0.93 | 2.765 | |||
| 7 | C17-H17•••N4iv | 3.453 | 0.93 | 2.74 | 134.17 | ||
| 8 | C17-H17•••C21iv | 3.559 | 0.93 | 2.68 | 157.91 | ||
| 9 | C19-H19A•••C16v | 3.612 | 0.97 | 2.759 | 146.98 | ||
| 10 | C16-H16•••O1vi | 3.513 | 0.93 | 2.593 | 169.97 | ||
| Symmetry code: (i) x,y,z; (ii) -x,-1/2+y,1.5-z; (iii) 1-x,-1/2+y,1.5-z; (iv) -x,1-y,1-z; (v) 1-x,1-y,1-z; (vi) x,1.5-y,-1/2+z | |||||||
| Compound 3b | |||||||
| Entry | Atoms | D….A | D-H | D-H….A | D-H….A | ||
| 1 | S1•••O3i | 2.798 | |||||
| 2 | N1•••O2 i | 2.884 | |||||
| 3 | O6-H6•••O1 ii | 2.85 | 0.827 | 2.85 | 166.8 | ||
| 4 | N4-H3•••O6 iii | 2.828 | 0.86 | 2.828 | 177.36 | ||
| 5 | O6-H6•••O3iv | 2.877 | 0.825 | 2.877 | 165.21 | ||
| 6 | C28-H28B•••C13v | 3.551 | 0.961 | 2.764 | 139.77 | ||
| 7 | C25-H25B•••O3 vi | 3.327 | 0.961 | 2.449 | 151.67 | ||
| 8 | C29-H29A•••C10 vii | 3.471 | 0.961 | 2.874 | 121.32 | ||
| 9 | C27-H27B•••O3 viii | 3.437 | 0.961 | 2.559 | 152.22 | ||
| Symmetry code: (i) x,y,z; (ii) x,1+y,z; (iii) -x,-y,1-z; (iv) x,-1/2-y,-1/2+z; (v) 1-x,-1/2+y,1.5-z; (vi) -x,-y,1-z; (vii) 1-x,1-y,1-z; (viii) x,1/2-y,-1/2+z | |||||||
The nature and strength of intramolecular S⋅⋅⋅O chalcogen bonds play a key role in biological activity and in supramolecular recognition due to presence of sigma hole in the sulfur atom [43,44]. In both crystal structures 3a and 3b, the chalcogen interaction geometry is created motifs, which shows the S-O interaction distances of 2.710 Å with ∠C3-S1-O3 angle of 160.46˚ for 3a, whereas for 3b, the S-O distance and ∠C3-S1-O3 were found to be 2.798 Å and 156.84˚ respectively. Further, it was noted that the, the S-O interaction distance was found to be within the vander Waals radii of sum of the S and O atoms [45]. Therefore, we suggest that there is a charge transfer component to this chalcogen interaction. The bond lengths and bond angles of thiadiazole ring is similar to other previously reported crystal structures [46].
3.3. Hirshfeld surface analysis
3D Hirshfeld surface and 2D fingerprint plots of compounds 3a and 3b were obtained from Crystal Explorer 21.5 to describe the contacts of molecular surface, which have been highlighted with conventional mapping of dnorm, as shown in Fig. 2 and Fig. 3 , respectively. Due to the presence of multiple electronegative atoms and hydrogen donor group in the title compounds, the crystal structures stabilized through multiple hydrogen bonds. The dnorm plots of 3a and 3b were mapped with a colour scale range of 0.0432 au (blue) and 1.084 au (red). It can be seen from dnorm mapped surface (Fig. 2 and Fig. 3) that there are two major interactions per molecule were detected. As shown in Fig. 2, (for compound 3a), there are two intense red regions on the oxygen and nitrogen atoms in the dnorm maps, which corresponds to two O…H/H…O contacts belonging to N-H…O (where O from acetyl group and NH from acetylamino group). The donor-acceptor bond distance was found to be 2.344 Å. Further, there are three less intense red spot on the phenyl ring and acetylamino carbon atoms in the dnorm maps, corresponds to C…H/H…C contacts belonging to C-G…C. The shape index surface map shows red-blue concave; indicate the presence of π-π stacking interactions in 3a. Similarly, for compound 3b, there is a pair of reddish spot on the oxygen atom of dnorm map (Fig. 3) corresponds to H…O/O…H contacts belonging to H-O…H (where O from water and H from N-H of acetylamino group, similarly, O-H from water and O from the acetyl group). However, a weak contact was also observed by C-H…O, as shown in weak red spot on the Hirshfeld surface (Fig. 3). In addition, a weak π-π stacking interactions was detected as shown the red-blue triangle concave in shape index (Fig. S5).
Fig. 2.
Hirshfeld surface mapped over (a) dnorm (b) dnorm mapped on the Hirshfeld surface along with intermolecular interactions of compound 3a.
Fig. 3.
Hirshfeld surface mapped over (a) dnorm (b) dnorm mapped on the Hirshfeld surface along with intermolecular interactions of compound 3b.
2D-fingerprint plot analysis was executed to determine the percentage contribution of intermolecular contacts of 3a and 3b with the di and de distances scales on the graph axes for the total contacts in the range of 0.6-2.6 Å, as shown in Fig. 4 and Fig. 5 , respectively. For compound 3a (Fig. 4), the H••H/H••H and O••H/H••O close contact contribution rate were predicted as 61.6 and 16.5 % at de+di ∼ 1.1 and 0.8-1.2 Å respectively, whereas C••H/H••C and N•••H/H•••N contribution were determined as 15 and 3.8 % at de+di ∼ 1.1 and 1.0-1.6 Å, respectively. Similarly, for compound 3b (Fig.5), the H••H/H••H and O••H/H••O percentage contributions were found to be 58.6 and 23.7 % at de+di ∼ 1.0 and 0.7-1.1 Å respectively. A pair of broad spike peaks were observed for C••H/H••C and N•••H/H•••N contacts at de+di ∼ 1.1-1.6 and 1.2-1.7 Å which calculated to be 11.4 and 2.0 % respectively. In both crystals (3a and 3b), the existence of highest percentage contribution of H•••H interaction by 2D-fingerprint plots analysis is in good agreement with the single crystal X-ray analysis.
Fig. 4.
The 2D fingerprint plots for the 3a showing the most intercontacts. a) full; b) H…H/H…H (61.6%); (c) C…H/H…C (15.0%); (d) O…H/H…O (16.5%); (e) N…H/H…N (3.8%); (f) percentage contribution of various intermolecular contacts.
Fig. 5.
The 2D fingerprint plots for the 3b showing the most intercontacts. a) H…H/H…H (58.6%); (b) O…H/H…O (23.7%); (c) C…H/H…C (11.4%); (d) S…H/H…S (1.4%) e) N…H/H…N (2.0%); (f) percentage contribution of various intermolecular contacts.
3.4. QTAIM analysis
Quantum theory of atoms in a molecule (QTAIM) analysis was performed for molecular structures (3a and 3b) to understand the type of non-bonded interactions, particularly classical hydrogen and chalcogen bonds. This can be demonstrated by the bond critical point and electron density topological parameters such as electron density (ρ), positive Laplacian (∇2ρ), potential energy density (V) and local kinetic energy density (G), are summarized in Table 4 . The bond critical points (BCPs) of molecular structure 3a and 3b are shown in Fig. S6. As shown in Fig. S6, bond critical point (ρBCP) was observed between chalcogen atoms (sulfur and oxygen). The electron density ρ and positive Laplacian ∇2ρ value were calculated to be 0.017 and 0.053 (for 3a and 3b respectively, Table 4). The accumulation of electron density ρ in the interaction region and the ∇2ρ values at the S•••O chalcogen bond for compound 3a and 3b were found to be quite similar to the results from experimental charge density and quantum chemical studies on sulfa-drugs [45]. Further, the crystal structure analysis of compounds 3a and 3b show a short S•••O interaction of 2.710 and 2.798 Å with a linear C21-S1-O3 angle of 73.44 and 72.77°, respectively. However, the sum of the Van der Waals radii of sulfur and oxygen atoms are significantly shorter than S•••O interaction. Further, the linearity of the chalcogen bond angle is linked to the nature of n→σ* interactions.
Table 4.
QTAIM topological analysis of compounds 3a and 3b.
| Compound 3a | |||||||
|---|---|---|---|---|---|---|---|
| BCP # | Atoms | Rho | DelSqRho | K | V | G | DelSqV |
| 1 | S1 - O4 | 0.017166 | 0.053585 | -0.000736 | -0.01192 | 0.01266 | -0.04129 |
| 2 | H14 - O17 | 0.024281 | 0.093234 | -0.002862 | -0.01759 | 0.020447 | -0.13332 |
| 3 | C20 - H52 | 0.010473 | 0.033852 | -0.00142 | -0.00562 | 0.007043 | -0.02415 |
| 4 | C24 - H29 | 0.008146 | 0.024771 | -0.001078 | -0.00404 | 0.005114 | -0.0148 |
| 5 | H29 - H44 | 0.006226 | 0.018595 | -0.000715 | -0.00322 | 0.003934 | -0.00769 |
| 6 | H25 - H44 | 0.009692 | 0.027709 | -0.001139 | -0.00465 | 0.005788 | -0.01914 |
| Compound 3a | |||||||
| 3 | S1 - O4 | 0.017056 | 0.053269 | -0.000734 | -0.011848 | 0.012583 | -0.040829 |
| 18 | O6 - H16 | 0.023652 | 0.091046 | -0.002845 | -0.017071 | 0.019916 | -0.125536 |
| 26 | C9 - H40 | 0.010041 | 0.032541 | -0.001367 | -0.005401 | 0.006768 | -0.022363 |
| 51 | H23 - H38 | 0.009399 | 0.02765 | -0.00114 | -0.004632 | 0.005772 | -0.016634 |
| 63 | C9 - H48 | 0.008472 | 0.025686 | -0.001134 | -0.004153 | 0.005287 | -0.014533 |
| 64 | H38 - H48 | 0.007647 | 0.022341 | -0.000819 | -0.003947 | 0.004766 | -0.009975 |
In addition, other contacts such as C18-H…O17 and C13-H…O17 was observed with 0.0122 and 0.04 (for 3a) and 0.024 and 0.093 (for 3b) electron density ρ, and positive Laplacian ∇2ρ respectively (Table 4).
3.5. NCI-RDG analysis
Non-covalent interactions-reduced density gradient (NCI-RDG) method was used to explore the various attractive and repulsive forces in a molecule such as H-bond, van der Waals and steric forces. Recently, the classical chalcogen bonding interaction in a molecule has been explored widely using NCI-RDG method [47]. The scatter graph of RDG against sin(λ2)ρ exploring the type of attractive or repulsive forces that exist in the molecular structure by tri-color scheme (blue-green-red). Blue and green scattering graph at the negative sign indicates attractive forces (hydrogen and chalcogen bonding and van der Waals forces) whereas red spot on the positive sign express the repulsive forces, which mostly occurred in the rings. The following relation can be used for the detection of NCI isosurfaces in RDG using this technique.
To get deep understanding the characteristics of intramolecular chalcogen bonds and other forces in a molecules (3a and 3b), the plot of s versus electron density (sin(λ2)ρ) multiplied by the sign of second Eigen value λ2 was also investigated for compound 3a and 3b, as shown in Fig. 6 . It can be seen from Fig. 6a and 6b, the blue scattering spike at the negative sign of λ2ρ (-0.02 a.u) indicate the presence of S∙∙∙O intramolecular chalcogen bond. Further, some green peaks were lying at the negative sign of λ2ρ (-0.01 a.u) values illustrate the van der Waals and repulsive forces whereas, the red spots scattering at the positive λ2ρ demonstrates the steric interaction in the molecule 3a and 3b. Jindani et al. [48] examined the NCI-RDG analysis for thiazole molecular structure, which showed the electron density (λ2) value which is similar to the electron density of title compounds 3a and 3b.
Fig. 6.
Non-covalent interactions (NCIs) and reduced density gradient (RDG) Interactions: (a) Compound 3a; b) Compound 3b
3.6. DFT study
Density functional theory (DFT) method was employed to inspect the structural geometry (bond lengths and bond angles) and electronic parameters (EHOMO and ELUMO). To describe these, molecular structures 3a and 3b were optimized at B3LYP/6-311g(d,p) level, which are depicted in Fig. S7 and S8, respectively. The selected geometrical parameters are presented in Table S1. It can be seen from the Table S1 that the optimized bond lengths and bond angles by theoretical analysis were found to be well replicated with the experimental XRD results, as indicated by the correlation coefficient analysis, Fig. S9 [49]. However, the minor deviations were observed between theoretical and experimental results due to the differences in the molecular environment.
In the DFT method of 3a and 3b, the C11-S1 bond distance was calculated as 1.90 Å whereas the XRD value found to be 1.86 Å. This should be due to stronger interaction between sulfur and oxygen atoms in the gas phase. However, the non-bonded distance between the sulfur and oxygen in the optimized structures (3a and 3b) were estimated as 2.84 Å (3a) and 2.79 Å (3b), well less than the combined van der Waals radii of the sulfur-oxygen atoms (3.25 Å) and consistent with the previously reported S–O chalcogen bond distances [50]. The conformational analysis of two six membered rings (cyclohexane and piperidine) were described with torsion angle of the corresponding ring atoms. For 3a, the observed torsion angle for C3-C4-C20-C19 and C3-C2-C18-C19 were calculated as 52.56 and -61.06° respectively, indicates the cyclohexane ring adopts chair conformation whereas the piperidine undergoes boat conformation with an angle of 26.22 and -41.74° for N1-C1-C2-C3 and N1-C5-C4-C3 respectively. Further, the torsion angle of piperidine and cyclohexane rings are in excellent agreement with the X-ray results. Similarly, for 3b, the torsion angles of piperidine and cyclohexane rings, C3-C4-C2-C20-C19/C3-C2-C18-C19 and C3-C2-C1-N1/N1-C5-C4-C3, were predicted to be 61.53/-61.90 and 26.20/39.50 respectively.
The frontier molecular orbital (FMO) analysis is widely used to predict the molecular interactions, reactivity, charge transfer, optical and electronic properties of molecules through highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). HOMO is the electron rich molecular orbital with high energy (nucleophile) and electron donating capability whereas LUMO is the electron deficient molecular orbital with smaller energy (electrophile) and own electron accepting ability. The energy difference between HOMO-LUMO orbital is called energy gap (ΔE), which is used to describe the stability of molecules. For compounds 3a and 3b, EHOMO and ELUMO values were obtained by the DFT method using B3LYP/6-311 g(d,p) level and the 3D molecular orbital energies of the compounds 3a and 3b are depicted in Fig. 7 . From the Fig. 7, the band gap energy (ΔE) between HOMO and LUMO orbitals were found to be 5.0 and 4.65 eV, respectively for 3a and 3b. It can be further discussed that 3b shows about 0.5 eV less compared to 3a due to the presence of electron donating methoxy substituent in the phenyl group, which further increase the electron delocalization.
Fig. 7.
HOMO/LUMO energy diagram of compounds 3a and 3b
Further using the energy of HOMO-LUMO orbitals, global chemical reactivity descriptor (GCRD) such as electron affinity (EA), ionization potential (IP), Electronegativity (χ), hardness (η), softness (S) potential (μ), and electrophilicity index (ω) were calculated. The calculated GCRD parameters are presented in Table 5 . It is seen that the chemical hardness and softness were calculated to be 0.20 and 0.21 eV for 3a and 3b, respectively. Therefore, the molecule 3a and 3b have good chemical stability and strength. However, the electronegativity (χ), and chemical potential (μ), were estimated as -3.73 / -3.05 eV; and 3.73 / 3.05 eV, respectively for 3a / 3b. From the Table 5, it can be concluded that the GCRD parameters of molecule 3b is significantly lower due presence of methoxy group at the phenyl substituent.
Table 5.
Calculated energy values of 3a and 3b at B3LYP/6–311 G (d, p) method.
| Quantum Descriptors (eV) | Compound 3a | Compound 3b |
|---|---|---|
| EHOMO | -6.23 eV | -5.38 eV |
| ELUMO | -1.23 eV | -0.73 eV |
| Ionization potential (IP) | 6.23 eV | 5.38 eV |
| Electron affinity (EA) | 1.23 eV | 0.73 eV |
| Energy gap (ΔE) | 5.0 eV | 4.65 eV |
| Electronegativity (χ) | -3.73 eV | -3.05 eV |
| Chemical potential (μ) | 3.73 eV | 3.05 eV |
| Chemical hardness (η) | 2.50 eV | 2.32 eV |
| Softness (σ) | 0.20 eV | 0.21 eV |
| Electrophilicity (ω) | 2.78 eV | 2.00 eV |
The molecular electrostatic potential (MEP) of an organic molecule can be defined in terms of total charge distribution of the molecule. 3D MEP surface encompassing different color scheme between blue to red, are depicted in Fig. 8 a (for 3a) and Fig. 8b (for 3b), generated from the optimized structure at B3LYP/6-311 g(d,p) level. It is seen that red, blue and green are the three major color scheme, which define the electrostatic potential value. Further, the color scheme expands in the order of blue > green > yellow > orange > red. Out of which, the blue regions on the surface signify the positive potential corresponds to the electron deficient site and favor for nucleophilic attack, whereas the red surface region with negative potential, corresponds to electron rich regions and favor for electrophilic reactivity. The green color on the MEP surface indicates zero potential. In addition, the pale blue and yellow colors on the surface map denote the slightly electron deficient and electron rich areas, respectively. For 3a, (Fig. 8a) the red and yellow region are corresponds to the negative electrostatic potential which associated with electron rich regions (high electron density) such as electronegative groups, and targeted by electrophiles whereas the blue region indicates the positive electrostatic potential (low electron density) which found mostly on the alkyl groups and associated with nucleophilic attack. For compounds 3b, (Fig. 8b), the negative potential is dominated over the carbonyl group whereas the positive potential exists only on the N-H group.
Fig. 8.
MEP Map of the compounds 3a and 3b.
3.7. NBO analysis
As the presence of sulfur and oxygen atoms close in the molecular structure (3a and 3b), we sought to evaluate the strength of S•••O (chalcogen bonding) interaction. To do this, NBO analysis was performed to get better understanding of inter and intra molecular charge transfer and electron delocalization between filled Lewis type orbital (bonding) and empty non-Lewis type orbital (anti-bonding) of 3a and 3b using DFT method at B3LYP/6-311g(d,p) level. The 2nd order perturbation energies of 3a and 3b were used to predict the strength and type of interaction between donor and acceptor orbitals, and the interacting stabilization energy. Further, the stabilization energy E(2) is used to define the hyper conjugative interactions and charge transfers in a molecule. Larger stabilization energy provides a stronger interaction between donor and acceptor atoms. Therefore, it is concluded that the stabilization energy E(2) is closely associated with each donor and acceptor NBO orbitals which are denoted as (i) and (j), respectively. The energy delocalization between each donor NBO (i) and acceptor NBO (j) can be predicted using the given equation:
The electron delocalization and its stabilization energies E(2) of 3a and 3b are presented in Table S2-S3. Based on the results presented in Table S2 - S3, a number of σ-σ∗, π→π∗, n→π∗, and n→σ∗, intramolecular charge transfer processes observed for compounds 3a and 3b. Also, the results revealed that the significant orbital interaction between the oxygen lone pair (donor-bonding) and sulfur-carbon antibonding (acceptor) orbital (S−C σ* orbital), in 3a and 3b were calculated as 2.01 kcal/mol (O4 LP S1-C11 σ* orbital) and 1.99 kcal/mol (O4 LP S1-C17 σ* orbital, respectively.
3.8. NLO Study
Theoretical NLO study was explored for spiro thiadiazole compounds 3a and 3b by quantum chemical calculation to estimate the NLO efficiency. The relationship between NLO property and molecular structure was studied theoretically with the aid of dipole moment, polarizability and hyperpolarizability, which have been calculated using B3LYP/6-311g(d,p) level and the results are summarized in Table 6 . Based on the data presented in Table 6, the dipole moment (μ), polarizability and hyperpolarizability values were predicted to be 4.34 Debye, 8.18 × 10−24 e.s.u and 2.136 × 10−30 e.s.u. (for 3a); 3.47 Debye, 33.05 × 10−24 e.s.u and 9.145 × 10−29 e.s.u. (for 3b), respectively. The calculated first order hyperpolarizability value is significantly higher than that of urea, which has been used as the reference value for many organic compounds [26]. Therefore, the high values of first order hyperpolarizability reveal that the synthesized spiro compounds 3a and 3b have efficient NLO behavior.
Table 6.
The dipole moment (μ) (Debye), polarizability (α) and first hyperpolarizability (β) of 3a and 3b
| Parameter | 3a | 3b | Parameter | 3a | 3b |
|---|---|---|---|---|---|
| αxx | -177.40 | -175.31 | βxxx | 234.1427 | -122.88 |
| αxy | 16.37 | -4.72 | βxxy | 34.1925 | 100.94 |
| αyy | -213.46 | -243.03 | βxyy | -1.6442 | 32.70 |
| αxz | 21.40 | -19.97 | βyyy | 12.4273 | 42.42 |
| αyz | 6.44 | -11.87 | βxxz | 21.6636 | -13.68 |
| αzz | 224.12 | -251.81 | βxyz | -1.9129 | -4.78 |
| αtotal | 8.18 × 10−24 | 33.05 × 10−24 | βyyz | -19.7101 | -38.09 |
| µx | 4.18 | -1.6750 | βxzz | 11.6066 | 14.11 |
| µy | 0.32 | 1.5398 | βyzz | -14.7295 | 1.02 |
| µz | 1.1273 | -2.6231 | βzzz | 24.7215 | 0.79 |
| µtotal | 4.34 | 3.47233 | βtotal | 2.136 × 10−30 | 9.145 × 10−29 |
It should be mentioned that the NLO of organic material originates from delocalized electron density of the substituents. In this case, both 3a and 3b were found to be polar in nature and have non zero dipole moment components. However, out of 3a and 3b, compound 3b contains electron donating p-methoxy phenyl group which further increase the electron delocalization. Therefore, 3b exhibits greater (βo) values than their unsubstituted phenyl group based spiro thiadiazole 3a.
3.9. Docking study
Molecular docking has proven to be highly efficient method for screening potential drug candidates against specific disease by using computer aided design [51]. Studies of ligand binding to receptor proteins using molecular docking provide insights into the effectiveness of the interactions. Recent studies have identified that the main protease (Mpro) of SARS-CoV-2 have consist of three domain such as, domain I (residues 8–101), domain II (residues 102–184) and domain III (residues 201–303), which were similar to other coronaviruses, COVID-19 viral protein (SARS-CoV-2 Mpro) also reside a similar catalytic dyad (Cys145-His41), located in a cleft between domain I and II [52,53]. Further, these catalytic dyads of Mpro are the major protease activity [54]. It was disclosed that inhibition of Mpro catalytic dyad was found to be potential and attractive target for designing and screening of anti-coronavirus drug [55]. Also, it was reported that no specific therapeutic agents or vaccines yet to be identified to treat the infection caused by COVID-19. However, only few clinically trial antiviral drugs such as antimalarial, and anti HIV have been used as the supportive measures to treat COVID-19 infections [55,56]. Virtual screening of molecular docking provides an alternative approach to screen the potential drug candidates for the specific illness at relatively short time.
Studies have been reported various therapeutic options against the COVID-19 causing protein (SARS-CoV-2) through molecular docking studies [57,58]. The docking studies have been applied on compounds 3a and 3b with “6LU7” protein which was taken from protein data bank (PDB ID: 6LU7). To examine the protein-ligand binding affinity, 6LU7 and lead compounds (3a and 3b) were fitted to interact with the active site of amino acid residues by using autodock tools. The obtained binding interactions are shown in Fig. 9 a and Fig. 9b of 3a and 3b respectively. The docking results showed that the lead compounds 3a and 3b are formed various binding interactions including H-bond, π-cation, and π-sulfur interactions. Also, this Fig. 9 presents the best docked poses of our ligand in 6LU7. It can be seen from Fig. 9a, Compound 3a showed significant binding affinity with His41, Met49, Ser144, Cys145, and His163 residues, whereas lead compound 3b (Fig. 9b) showed interactions with His41, Met49, Pro52, Phe140, Asn142, Cys145, Glu166, and Arg188 residues of 3CLpro. Among these, His41, Ser144, Cys145, and His163 (for 3a) and Phe140, Cys145, and Glu166 (for 3b) displays conventional hydrogen bonding, whereas Met49 forms π-cation, and π-sulfur interactions with compounds 3a and 3b. “Further, it can be seen in Fig. 9, the carbonyl group of acetyl function in 3a exhibits conventional hydrogen bonding with His41, Ser144, Cys145, and His163 residues (Compound 3a). Unlike 3a, in compound 3b, sulphur atom in thiadiazole ring and amino group of acetylamino function show hydrogen bond with Phe140, and Glu166 residues. The interacting amino acid residues were compared with earlier reports [25,26,59], which showed that the residues His41 and Cys145 are the major catalytic active residues in 6LU7. Interestingly, lead compounds 3a and 3b exhibit similar binding affinity with His41 and Cys145 amino acid residues. The binding energies of 3a and 3b were calculated to be -7.90 and -7.85 kcal/mol, (Table S4) [60]. As clearly seen from Table S4, the existence of methoxy function in the phenyl group was found to be insignificant contribution towards the binding affinity”. In conclusion, the above docking results proved that the ligands 3a and 3b could be the potential lead molecule for antiviral drug against SARS-nCoV-2 Mpro.
Fig. 9.
a) and b) show two dimensional binding interaction between ligands 3a and 3b with active site of 6LU7 amino acid residues; c) and d) Compounds 3a and 3b show hydrogen bonds interactions with SARS-nCoV-2 Main protease (PDB: 6LU7) active site
4. Conclusion
Thiazole based spiro derivatives 3a and 3b were synthesized and characterized by spectroscopic and single crystal X-ray diffraction techniques. Both the NMR and XRD results proved that the bicyclic rings adopt chair and boat conformation of cyclohexane and piperdine rings, respectively. The asymmetric unit of crystal structures 3a and 3b are stabilized mainly due to chalcogen bond, whereas as the crystal packing is stabilized due to divalent hydrogen bond, C-H∙∙∙π, C-H∙∙∙O, O-H∙∙∙C, O-H∙∙∙N and H∙∙∙H interactions. 3D Hirshfeld surfaces and 2D fingerprint plots analysis were considered to visually spot the different interactions within the molecular structures. Based on the results, the structures 3a and 3b were stabilized through H…H, O…H/H…O, C…H/H…C, and N…H/H…N contacts. Further, the 2D fingerprint plot analysis revealed that conventional hydrogen bonding contribution O…H/H…O was calculated to be 16.7 and 23.5 % (respectively for 3a and 3b), which could be the strong interaction to stabilize the crystal packing. The presences of chalcogen bond in the studied compounds were identified from the NCI-RDG and QTAIM analyses. NBO analysis suggests that the intramolecular charge transfer between oxygen and sulfur and hyperconjugative interactions are dominated in the molecular structure. NLO study showed that the significant response as compared to the prototype molecule (urea). Finally, the molecular docking study was explored for the compounds 3a and 3b with COVID-19 viral protein (SARS-nCoV-2 Mpro, 6LU7). The results revealed that the various interactions strongly anchored the lead compounds to the active sites with minimum binding energy, which was calculated to be -7.90 and -7.85 kcal/mol for 3a and 3b, respectively.
CRediT authorship contribution statement
Ramachandran Rajamanickam: Methodology, Formal analysis, Data curation, Writing – review & editing. Rani Mannangatty: Methodology, Investigation, Formal analysis. Jayanthi Sampathkumar: Software, Data curation, Investigation. Kabilan Senthamaraikannan: Formal analysis, Investigation. Barathi Diravidamani: Software, Data curation, Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.molstruc.2022.132747.
Appendix. Supplementary materials
References
- 1.Natsutani I., Iwata R., Yamai Y., Ishida K., Nagaoka Y., Sumiyoshi T. Design, synthesis and evaluations of spiro-fused benzoxaborin derivatives as novel boron-containing compounds. Chem. Biol. Drug Des. 2019;93:657–665. doi: 10.1111/cbdd.13496. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 2.Moghaddam-Manesh M., Ghazanfari D., Sheikhhosseini E., Akhgar M. MgO-Nanoparticle-Catalyzed Synthesis and Evaluation of Antimicrobial and Antioxidant Activity of New Multi-Ring Compounds Containing Spiro[indoline-3,4′-[1,3]dithiine] ChemistrySelect. 2019;4:9247–9251. doi: 10.1002/slct.201900935. https://doi.org/ [DOI] [Google Scholar]
- 3.Balasubramanian S., Ramalingan C., Aridoss G., Kabilan S. Synthesis and study of antibacterial and antifungal activities of novel 8-methyl-7,9-diaryl-1,2,4,8-tetraazaspiro[4.5]decan-3-thiones. Eur. J. Med. Chem. 2005;40:694–700. doi: 10.1016/j.ejmech.2005.02.001. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 4.Chudzik B., Bonio K., Dabrowski W., Pietrzak D., Niewiadomy A., Olender A., Malodobry K., Gagoś M. Synergistic antifungal interactions of amphotericin B with 4-(5-methyl-1,3,4-thiadiazole-2-yl) benzene-1,3-diol. Sci. Rep. 2019;9:12945. doi: 10.1038/s41598-019-49425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muğlu H., Akın M., Çavuş M.S., Yakan H., Şaki N., Güzel E. Exploring of antioxidant and antibacterial properties of novel 1,3,4-thiadiazole derivatives: Facile synthesis, structural elucidation and DFT approach to antioxidant characteristics. Comput. Biol. Chem. 2022;96 doi: 10.1016/j.compbiolchem.2021.107618. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 6.AL-Quraan N.A., Al-Smadi M.L., Swaleh A.F. GABA metabolism and ROS induction in lentil (Lens culinaris Medik) plants by synthetic 1,2,3-Thiadiazole compounds. J. Plant Interact. 2015;10:185–194. doi: 10.1080/17429145.2015.1056262. [DOI] [Google Scholar]
- 7.Jatav V., Mishra P., Kashaw S., Stables J.P. CNS depressant and anticonvulsant activities of some novel 3-[5-substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur. J. Med. Chem. 2008;43:1945–1954. doi: 10.1016/j.ejmech.2007.12.003. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 8.Bahadur A., Iqbal S., Muneer S., Alsaab H.O., Awwad N.S., Ibrahium H.A. Synthesis, carbonic anhydrase enzyme inhibition evaluations, and anticancer studies of sulfonamide based thiadiazole derivatives. Bioorg. Med. Chem. Lett. 2022;57 doi: 10.1016/j.bmcl.2021.128520. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 9.Madhu Sekhar M., Nagarjuna U., Padmavathi V., Padmaja A., Reddy N.V., Vijaya T. Synthesis and antimicrobial activity of pyrimidinyl 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles. Eur. J. Med. Chem. 2018;145:1–10. doi: 10.1016/j.ejmech.2017.12.067. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 10.Matthews E., Portaro S., Ke Q., Sud R., Haworth A., Davis M.B., Griggs R.C., Hanna M.G. Acetazolamide efficacy in hypokalemic periodic paralysis and the predictive role of genotype. Neurology. 2011;77:1964. doi: 10.1212/WNL.0b013e31823a0cb6. 1960 LP–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang J., Li X., Hanif M., Zhou J., Hu D., Su S., Xie Z., Gao Y., Yang B., Ma Y. Pyridal[2,1,3]thiadiazole as strong electron-withdrawing and less sterically-hindered acceptor for highly efficient donor–acceptor type NIR materials. J. Mater. Chem. C. 2017;5:11053–11058. doi: 10.1039/C7TC03978F. [DOI] [Google Scholar]
- 12.Ghosh T., Lehmann M. Recent advances in heterocycle-based metal-free calamitics. J. Mater. Chem. C. 2017;5:12308–12337. doi: 10.1039/C7TC03502K. [DOI] [Google Scholar]
- 13.Bora D., Kaushal A., Shankaraiah N. Anticancer potential of spirocompounds in medicinal chemistry: A pentennial expedition. Eur. J. Med. Chem. 2021;215 doi: 10.1016/j.ejmech.2021.113263. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 14.Frija L.M.T., Pombeiro A.J.L., Kopylovich M.N. Building 1,2,4-Thiadiazole: Ten Years of Progress. European J. Org. Chem. 2017;2017:2670–2682. doi: 10.1002/ejoc.201601642. https://doi.org/ [DOI] [Google Scholar]
- 15.Ramachandran R., Rani M., Senthan S., Jeong Y.T., Kabilan S. Synthesis, spectral, crystal structure and in vitro antimicrobial evaluation of imidazole/benzotriazole substituted piperidin-4-one derivatives. Eur. J. Med. Chem. 2011;46:1926–1934. doi: 10.1016/j.ejmech.2011.02.036. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 16.Sethukumar A., Anand P.S., Kumar C.U., Prakasam B.A. Synthesis, stereochemical and biological studies of some N-cyclohexylcarbamoyl -2,6-diarylpiperidin-4-ones. J. Mol. Struct. 2017;1130:352–362. doi: 10.1016/j.molstruc.2016.10.035. https://doi.org/ [DOI] [Google Scholar]
- 17.Aridoss G., Balasubramanian S., Parthiban P., Kabilan S. Synthesis and NMR spectral studies of N-chloroacetyl-2,6-diarylpiperidin-4-ones. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007;68:1153–1163. doi: 10.1016/j.saa.2007.01.013. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 18.Ponnuswamy S., Sethuvasan S., Thirunavukarasu K. Synthesis, characterisation, stereochemistry and dynamic NMR studies of N-nitroso and N-formyl-t-3-isopropyl-r-2,c-6-bis(4-methoxyphenyl)piperidin-4-ones. J. Mol. Struct. 2015;1089:86–94. doi: 10.1016/j.molstruc.2015.02.045. https://doi.org/ [DOI] [Google Scholar]
- 19.Chakkaravarthy J., Muthukumaran G., Pandiarajan K. Conformational study of some N-acyl-2r,6c-diphenylpiperidin-4-one oximes using NMR spectra. J. Mol. Struct. 2008;889:297–307. doi: 10.1016/j.molstruc.2008.02.008. https://doi.org/ [DOI] [Google Scholar]
- 20.Gates B. Responding to Covid-19 — A Once-in-a-Century Pandemic? N. Engl. J. Med. 2020;382:1677–1679. doi: 10.1056/NEJMp2003762. [DOI] [PubMed] [Google Scholar]
- 21.Ali M.J., Hanif M., Haider M.A., Ahmed M.U., Sundas F.N.U., Hirani A., Khan I.A., Anis K., Karim A.H. Treatment Options for COVID-19: A Review. Front. Med. 2020;7:480. doi: 10.3389/fmed.2020.00480. https://www.frontiersin.org/article/10.3389/fmed.2020.00480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batra R., Chan H., Kamath G., Ramprasad R., Cherukara M.J., Sankaranarayanan S.K.R.S. Screening of Therapeutic Agents for COVID-19 Using Machine Learning and Ensemble Docking Studies. J. Phys. Chem. Lett. 2020;11:7058–7065. doi: 10.1021/acs.jpclett.0c02278. [DOI] [PubMed] [Google Scholar]
- 23.Khater I., Nassar A. In silico molecular docking analysis for repurposing approved antiviral drugs against SARS-CoV-2 main protease. Biochem. Biophys. Reports. 2021;27 doi: 10.1016/j.bbrep.2021.101032. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S., Sharma P.P., Shankar U., Kumar D., Joshi S.K., Pena L., Durvasula R., Kumar A., Kempaiah P., Poonam B.Rathi. Discovery of New Hydroxyethylamine Analogs against 3CLpro Protein Target of SARS-CoV-2: Molecular Docking, Molecular Dynamics Simulation, and Structure–Activity Relationship Studies. J. Chem. Inf. Model. 2020;60:5754–5770. doi: 10.1021/acs.jcim.0c00326. [DOI] [PubMed] [Google Scholar]
- 25.Rani M., Jayanthi S., Kabilan S., Ramachandran R., Synthesis Spectral, structure Crystal. Hirshfeld surface, Computational analysis, and Antimicrobial studies of Ethyl-(E)-4-(2-(2-arylidenehydrazinyl)-2-oxoethyl)piperazine-1-carboxylates. J. Mol. Struct. 2022;1252 doi: 10.1016/j.molstruc.2021.132082. https://doi.org/ [DOI] [Google Scholar]
- 26.Rajamanickam R., Sivakolunthu S., Sampathkumar J. Synthesis, crystal structure, Hirshfeld surface, DFT and docking studies of 4-[(5‑hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)(phenyl)methyl]-5-methyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one. J. Mol. Struct. 2022;1252 doi: 10.1016/j.molstruc.2021.132170. https://doi.org/ [DOI] [Google Scholar]
- 27.Ramachandran R., Rani M., Kabilan S. Synthesis, structure and conformational analysis of 2,4-diaryl-3-azabicyclo[3.3.1]nonan-9-one thiosemicarbazones and semicarbazones. J. Mol. Struct. 2010;970:42–50. doi: 10.1016/j.molstruc.2010.02.005. https://doi.org/ [DOI] [Google Scholar]
- 28.Rani M., Ramachandran R., Kabilan S. Efficient synthesis, spectral analysis and antimicrobial studies of nitrogen and sulfur containing spiro heterocycles from 2,4-diaryl-3-azabicyclo[3.3.1]nonan-9-ones. Bioorg. Med. Chem. Lett. 2010;20:6637–6643. doi: 10.1016/j.bmcl.2010.09.021. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 29.Sheldrick G.M. Univ. Gottingen; Gottingen, Ger: 1997. SHELXL-97. No Title. [Google Scholar]
- 30.Farrugia L.J. Ortep-3 for Windows. J. Appl. Cryst. 1997;30:565. [Google Scholar]
- 31.Spackman P.R., Turner M.J., McKinnon J.J., Wolff S.K., Grimwood D.J., Jayatilaka D., Spackman M.A. CrystalExplorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021;54:1006–1011. doi: 10.1107/s1600576721002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shobana D., Sudha S., Ramarajan D., Dimić D. Synthesis, crystal structure, spectral characterization and Hirshfeld surface analysis of (E)-N′-(3-ethoxy-4-hydroxybenzylidene)-4-fluorobenzohydrazide single-crystal – a novel NLO active material. J. Mol. Struct. 2022;1250 doi: 10.1016/j.molstruc.2021.131856. https://doi.org/ [DOI] [Google Scholar]
- 33.Frisch G.W.T.M.J., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Petersson G.A., Nakatsuji H., Li X., Caricato M., Marenich A., Bloino J., Janesko B.G., Gomperts R., Mennucci B., Hratchian H.P., Ortiz J.V., Izma A.F. Gaussian, Inc; Wallingford, CT, USA: 2013. Gaussian 09,Revision A.02. No Title. [Google Scholar]
- 34.AIMAll (Version 19.10.12), Todd A. Keith, TK Gristmill Software, Overland Park KS, USA, 2019
- 35.Lu T., Chen F. Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem. 2012;33:580–592. doi: 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- 36.Forli S., Huey R., Pique M.E., Sanner M.F., Goodsell D.S., Olson A.J. Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016;11:905–919. doi: 10.1038/nprot.2016.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.BIOVIA Discovery studio visualizer 4.5, Dassault Systèmes. 2016 [Google Scholar]
- 38.Silverstein Webster R.M., Francis X., Kiemle David J., Bryce David L. Spectrometric identification of organic compounds, 8th ed. 2015 [Google Scholar]
- 39.Lunazzi L., Macciantelli D., Tassi D., Dondoni A. Conformational studies by dynamic nuclear magnetic resonance. Part 17. Stereodynamic processes in hindered piperidyl-amides and -amidines. J. Chem. Soc. Perkin Trans. 1980;2:717–723. doi: 10.1039/P29800000717. [DOI] [Google Scholar]
- 40.Parthiban P., Aridoss G., Rathika P., Ramkumar V., Kabilan S. Synthesis, stereochemistry and antimicrobial studies of novel oxime ethers of aza/diazabicycles. Bioorg. Med. Chem. Lett. 2009;19:6981–6985. doi: 10.1016/j.bmcl.2009.10.042. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 41.Cremer D., Pople J.A. General definition of ring puckering coordinates. J. Am. Chem. Soc. 1975;97:1354–1358. doi: 10.1021/ja00839a011. [DOI] [Google Scholar]
- 42.Akila A., Ponnuswamy S., Shreevidhyaa Suressh V., Usha G. Synthesis, characterization and stereochemistry of N-acyl-r-2,c-4-bis(4-methoxyphenyl)-3-azabicyclo[3.3.1]nonanes. J. Mol. Struct. 2015;1093:113–118. doi: 10.1016/j.molstruc.2015.03.052. https://doi.org/ [DOI] [Google Scholar]
- 43.Carugo O., Resnati G., Metrangolo P. Chalcogen Bonds Involving Selenium in Protein Structures. ACS Chem. Biol. 2021;16:1622–1627. doi: 10.1021/acschembio.1c00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng R., Gong Z., Yan Q. Chalcogen-Bonding Supramolecular Polymers. J. Org. Chem. 2020;85:8397–8404. doi: 10.1021/acs.joc.0c00723. [DOI] [PubMed] [Google Scholar]
- 45.Thomas S.P., Jayatilaka D., Guru Row T.N. S⋯O chalcogen bonding in sulfa drugs: insights from multipole charge density and X-ray wavefunction of acetazolamide. Phys. Chem. Chem. Phys. 2015;17:25411–25420. doi: 10.1039/C5CP04412J. [DOI] [PubMed] [Google Scholar]
- 46.Umamatheswari S., Kabilan S. Spectral characterization and crystal structure of 5-spiro-(3-methyl-2,6-diphenyltetrahydropyran-4-yl)-4,5-dihydro-[1,3,4]thiadiazole. J. Mol. Struct. 2009;938:142–149. doi: 10.1016/j.molstruc.2009.09.016. https://doi.org/ [DOI] [Google Scholar]
- 47.Ibrahim M.A.A., Telb E.M.Z. σ-Hole and Lone-Pair Hole Interactions in Chalcogen-Containing Complexes: A Comparative Study. ACS Omega. 2020;5:21631–21640. doi: 10.1021/acsomega.0c02362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jindani S., Ganguly B. Exploiting the role of stereoelectronic effects to design the antagonists of the human complement C3a receptor. New J. Chem. 2021;45:9443–9455. doi: 10.1039/D1NJ00730K. [DOI] [Google Scholar]
- 49.Kavitha E., Ramarajan D., Rakić A., Dimić D., Sudha S., Nirmala P.N. Structural, spectroscopic, quantum chemical, and molecular docking investigation of (E)-N’-(2,5-dimethoxybenzylidene)picolinohydrazide. J. Mol. Struct. 2022;1253 doi: 10.1016/j.molstruc.2021.132259. https://doi.org/ [DOI] [Google Scholar]
- 50.Meille S.V, Farina A., Bezziccheri F., Gallazzi M.C. The influence of alkoxy side chains on the conformational flexibility of oligo- and polythiophenes. Adv. Mater. 1994;6:848–851. doi: 10.1002/adma.19940061109. https://doi.org/ [DOI] [Google Scholar]
- 51.Al-Salahi R., Abuelizz H.A., Ghabbour H.A., El-Dib R., Marzouk M. Molecular docking study and antiviral evaluation of 2-thioxo-benzo[g]quinazolin-4(3H)-one derivatives. Chem. Cent. J. 2016;10:21. doi: 10.1186/s13065-016-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 53.Xiaoyu X., Hongwei Y., Haitao Y., Fei X., Zhixin W., Wei S., Jun L., Zhe Z., Yi D., Qi Z., C Z.X., Ming L., Mark B., Zihe R. Structures of Two Coronavirus Main Proteases: Implications for Substrate Binding and Antiviral Drug Design. J. Virol. 2008;82:2515–2527. doi: 10.1128/JVI.02114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang H., Xie W., Xue X., Yang K., Ma J., Liang W., Zhao Q., Zhou Z., Pei D., Ziebuhr J., Hilgenfeld R., Yuen K.Y., Wong L., Gao G., Chen S., Chen Z., Ma D., Bartlam M., Rao Z. Design of Wide-Spectrum Inhibitors Targeting Coronavirus Main Proteases. PLOS Biol. 2005;3:e324. doi: 10.1371/journal.pbio.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimamoto Y., Hattori Y., Kobayashi K., Teruya K., Sanjoh A., Nakagawa A., Yamashita E., Akaji K. Fused-ring structure of decahydroisoquinolin as a novel scaffold for SARS 3CL protease inhibitors. Bioorg. Med. Chem. 2015;23:876–890. doi: 10.1016/j.bmc.2014.12.028. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nutho B., Mahalapbutr P., Hengphasatporn K., Pattaranggoon N.C., Simanon N., Shigeta Y., Hannongbua S., Rungrotmongkol T. Why Are Lopinavir and Ritonavir Effective against the Newly Emerged Coronavirus 2019? Atomistic Insights into the Inhibitory Mechanisms. Biochemistry. 2020;59:1769–1779. doi: 10.1021/acs.biochem.0c00160. [DOI] [PubMed] [Google Scholar]
- 58.Ghahremanpour M.M., Tirado-Rives J., Deshmukh M., Ippolito J.A., Zhang C.-H., Cabeza de Vaca I., Liosi M.-E., Anderson K.S., Jorgensen W.L. Identification of 14 Known Drugs as Inhibitors of the Main Protease of SARS-CoV-2. ACS Med. Chem. Lett. 2020;11:2526–2533. doi: 10.1021/acsmedchemlett.0c00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hatada R., Okuwaki K., Mochizuki Y., Handa Y., Fukuzawa K., Komeiji Y., Okiyama Y., Tanaka S. Fragment Molecular Orbital Based Interaction Analyses on COVID-19 Main Protease − Inhibitor N3 Complex (PDB ID: 6LU7) J. Chem. Inf. Model. 2020;60:3593–3602. doi: 10.1021/acs.jcim.0c00283. [DOI] [PubMed] [Google Scholar]
- 60.Milanović Ž.B., Antonijević M.R., Amić A.D., Avdović E.H., Dimić D.S., Milenković D.A., Marković Z.S. Inhibitory activity of quercetin, its metabolite, and standard antiviral drugs towards enzymes essential for SARS-CoV-2: the role of acid–base equilibria. RSC Adv. 2021;11:2838–2847. doi: 10.1039/D0RA09632F. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.