Abstract
Tubulointerstitial inflammation plays an important role in the progression of diabetic nephropathy (DN), and tubular epithelial cells (TECs) are crucial promoters of the inflammatory cascade. Exchange protein activated by cAMP (Epac) has been shown to suppress the angiotensin II (Ang-II)-induced release of inflammatory cytokines in tubular cells. However, the role of Epac in TEC-mediated tubulointerstitial inflammation in DN remains unknown. We found that administering the Epac agonist 8-pCPT-2′-O-Me-cAMP (8-O-cAMP) to db/db mice inhibited tubulointerstitial inflammation characterized by macrophage infiltration and increased inflammatory cytokine release and consequently alleviated tubulointerstitial fibrosis in the kidney. Furthermore, 8-O-cAMP administration restored CCAAT/enhancer binding protein β (C/EBP-β) expression and further upregulated the expression of Suppressor of cytokine signaling 3 (SOCS3), while inhibiting p-STAT3, MCP-1, IL-6, and TNF-α expression in the kidney cortex in db/db mice. And in vitro study showed that macrophage migration and MCP-1 expression induced by high glucose (HG, 30 mM) were notably reduced by 8-O-cAMP in human renal proximal tubule epithelial (HK-2) cells. In addition, 8-O-cAMP treatment restored C/EBP-β expression in HK-2 cells and promoted C/EBP-β translocation to the nucleus, where it transcriptionally upregulated SOCS3 expression, subsequently inhibiting STAT3 phosphorylation. Under HG conditions, siRNA-mediated knockdown of C/EBP-β or SOCS3 in HK-2 cells partially blocked the inhibitory effect of Epac activation on the release of MCP-1. In contrast, SOCS3 overexpression inhibited HG-induced activation of STAT3 and MCP-1 expression in HK-2 cells. These findings indicate that Epac activation via 8-O-cAMP ameliorates tubulointerstitial inflammation in DN through the C/EBP-β/SOCS3/STAT3 pathway.
Keywords: diabetic nephropathy, Epac, 8-O-cAMP, inflammation, tubular epithelial cells, macrophages
Introduction
Diabetic nephropathy (DN) is one of the most serious microvascular complications of diabetes and is a major cause of end-stage renal disease (ESRD) [1]. Despite currently available therapies for blood pressure, glycemic control, and hyperlipidemia, a large portion of DN patients still progress to ESRD. Thus, it is important to elucidate the pathogenesis of DN and identify appropriate strategies to protect patients with DN from progressive kidney disease.
Although DN is not traditionally considered to be an inflammatory disorder, this view has changed after studies of human biopsies and animal models revealed the presence of macrophages in diabetic kidneys, even in the early stage of the disease [2]. The degree of tubulointerstitial macrophage infiltration is closely associated with renal functional decline and interstitial fibrosis [3, 4]. Notably, emerging data have shown that tubular epithelial cells (TECs) play a determining role in mediating macrophage recruitment and the subsequent inflammatory cascade in the kidneys in DN [5–7], as TECs secrete numerous inflammatory cytokines, including MCP-1, IL-6, and TNF-α [8–10]. Therefore, therapeutic strategies targeting TEC-mediated inflammation may represent a novel approach to alleviate macrophage infiltration and activation and kidney inflammation in DN.
Exchange protein activated by cAMP (Epac) is a cAMP-regulated guanine nucleotide exchange factor (GEF) that activates the small GTPase Rap [11, 12] and plays a crucial role in influencing gene expression and downregulating cytokine-mediated vascular inflammation [13]. Although Epac is particularly enriched in the kidney, especially in the renal tubular epithelium [14, 15], its role in tubular injury and the progression of DN is poorly understood. The Epac agonist 8-pCPT-2′-O-Me-cAMP (8-O-cAMP) has been shown to activate Epac without influencing the PKA pathway [16], providing a novel approach for the pharmaceutical manipulation of Epac signaling. The results of recent studies have shown that Epac activation via 8-O-cAMP exerts a protective effect in an experimental model of kidney diseases. Geurt et al. observed that 8-O-cAMP can preserve tubular epithelial cell adhesion, reduce antioxidant signaling and ultimately prevent renal failure in a mouse model of ischemia-reperfusion injury [17, 18]. Ding et al. demonstrated that the pharmacological activation of Epac using 8-O-cAMP decreased the expression of fibronectin in TGF-β1-induced tubular epithelial cell damage and alleviated renal interstitial fibrosis in a UUO model [19]. With respect to the effect of Epac activation on inflammation, 8-O-cAMP treatment has been reported to decrease angiotensin II (AngII)-induced ROS generation and inflammatory cytokine (IL-1β, IL-6, IL-8, and TNF-α) production in human renal proximal tubule epithelial (HK-2) cells [20]. In addition, the results of a subsequent study further demonstrated that the Ang II-induced expression of inflammatory cytokines was regulated by the Epac1-Rap1a-NHE3 pathway in proximal tubular cells from pig kidneys [21], indicating that Epac activation may play an inhibitory role in response to renal inflammation. However, there has been a lack of research on the pharmacological activation of Epac by 8-O-cAMP in the context of renal inflammation in DN.
Suppressor of cytokine signaling 3 (SOCS3) is an important negative regulator of the Janus kinase / signal transducers and activators of transcription (JAK/STAT3) pathway [22] in cells and has been reported to be involved in inhibiting monocyte chemoattractant protein-1 (MCP-1) upregulation and macrophage infiltration in DN [8], suggesting a renoprotective role of SOCS3. Moreover, Epac has been reported to induce SOCS3 gene expression in various types of cells, including cardiocytes [23], vascular endothelial cells [24], and retinal ganglion cells [25]. However, the correlation between Epac activation and SOCS3 in the context of proximal tubular cell inflammation in DN remains unclear.
In the present study, we assessed the effect of Epac activation by 8-O-cAMP on tubular inflammation and injury using both in vitro and in vivo models of DN. We showed that 8-O-cAMP-mediated Epac activation decreased macrophage infiltration and subsequent inflammatory cytokine production in the kidneys of db/db mice and significantly ameliorated renal tubulointerstitial fibrosis. Furthermore, the selective activation of Epac reduced high glucose (HG)-induced STAT3 activation and MCP-1 expression by increasing SOCS3 gene expression in proximal tubular cells in vitro. These results revealed the beneficial effect of Epac activation on tubular-induced inflammation in DN.
Materials and methods
Reagents
Primary antibodies against STAT3 and phosphorylated STAT3 (Tyr705) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA), while those against MCP-1, TNF-α, C/EBPβ, fibronectin, collagen I, iNOS, and F4/80 were purchased from Abcam (Cambridge, MA, UK). In addition, primary antibodies against SOCS3, IL-6, and β-actin were purchased from Proteintech (Wuhan, China). Secondary antibodies conjugated to horseradish peroxidase, Alexa Fluor 488, or Alexa Fluor 594 were obtained from Abcam. siRNAs against C/EBPβ and SOCS3 were synthesized by RiboBio, Inc. (Guangzhou, China). The SOCS3 overexpression plasmid was obtained from Sino Biological, Inc. (Peking, China). Lipofectamine 3000 was purchased from Invitrogen (Carlsbad, CA, USA).
Animal experimental design
The Animal Care and Use Committee of Second Xiangya Hospital of Central South University approved all animal procedures performed in the present study. C57BLKS db/db and C57BLKS db/m mice used for the animal experiments were purchased from Aier Matt Experimental Animal Company (Suzhou, China) and were housed in the Second Xiangya Hospital Animal Center, Central South University. All mice were provided access to standard chow and filtered tap water ad libitum. At 4 weeks of age, the mice were divided into the following three groups for the animal experiments: the db/m group (control, n = 5), the db/db vehicle treatment group (n = 5), and the db/db plus 8-O-cAMP treatment group. At week 8, db/db mice received an intraperitoneal injection (i.p.) of vehicle or 8-O-cAMP (1.47 mg/kg BW; Sigma) once every other day for 8 weeks. 8-O-cAMP was dissolved in phosphate-buffered saline (PBS) and administered intraperitoneally to db/db mice. Untreated db/db and db/m mice received identical intraperitoneal injections of PBS. The body weights and fasting blood glucose levels of mice were monitored every 2 weeks throughout the study, and at 16 weeks of age, all mice were euthanized under anesthesia induced by an i.p. injection of pentobarbital sodium (50 mg/kg BW).
Assessment of physiological features and renal function
The body weights and blood glucose levels of mice were measured every 2 weeks. Blood glucose levels in tail vein blood samples were measured using a blood glucose monitor (Boehringer Mannheim, Mannheim, Germany). Urine albumin concentrations were measured using a mouse urine albumin ELISA kit (Bethyl Laboratories, USA), and serum creatinine levels were measured using a QuantiChrom Creatinine Assay Kit (BioAssay Systems, USA) according to the manufacturer’s protocol.
Morphological analysis of kidneys
Kidney tissues were obtained from mice in the different groups, fixed in 4% paraformaldehyde (Servicebio, Wuhan, China), sliced into four-micron-thick paraffin-embedded sections and then stained with periodic acid-Schiff (PAS) and Masson’s trichrome. Glomerular or tubular damage was scored as previously described [26]: (0) no glomerular/tubular damage; (1) 25% of the glomerular/tubular area was affected; (2) 25%–50% of the glomerular/tubular area was affected; and (3) 50% of the glomerular/tubular area was affected.
Immunohistochemistry
Four-micron-thick paraffin-embedded kidney sections were used for immunohistochemistry (IHC) as previously described [27]. In brief, the sections were dewaxed, rehydrated, incubated with primary antibodies overnight at 4 °C and later incubated with horseradish peroxidase-conjugated secondary antibodies and diaminobenzidine (DAB) substrate (Servicebio, Wuhan, China). After being counterstained with hematoxylin and dehydration, the sections were mounted and analyzed with a Leica microscope. Five low-power (400×) images per kidney were quantified using Fiji ImageJ software, and a detailed, step-by-step procedure for conducting semiquantitative analysis of IHC images can be found in the reference [28].
Renal reactive oxygen species measurement and immunofluorescence analysis
Dihydroethidium (1 μM, DHE, Sigma-Aldrich) was used to assess intracellular reactive oxygen species (ROS) levels in 4-mm-thick kidney cryostat sections as previously described [29]. The mean fluorescence intensity (MFI) of DHE was calculated using ImageJ. For the immunofluorescence (IF) assay, the cells or frozen sections were fixed with 4% paraformaldehyde for 10 min at room temperature. Then, the sections were blocked with a mixture of 0.1% Triton X-100 and 5% BSA for 60 min at room temperature before being incubated with a primary antibody overnight at 4 °C. Subsequently, the cells were incubated with secondary antibodies conjugated with Alexa Fluor 488 (green) or 594 (red) for 1 h. Then, the cells or slides were counterstained with 4′,6-diamidino-2 phenylindole (DAPI), and fluorescence was visualized using an LSM 780 META laser scanning microscope (Zeiss, Thornwood, NY). The MFI values of p-STAT3 and CCAAT/enhancer binding protein β (C/EBP-β) were calculated using ImageJ. To quantify F4/80- and iNOS-positive cells, we counted the F4/80- or iNOS-positive cells in each tubulointerstitial compartment per mm2 to represent the degree of macrophage infiltration [30].
Cell culture
The human proximal tubular HK-2 cell line (ATCC) was used for in vitro studies. Time-course experiments were performed using 30 mM D-glucose (Sigma) for 0–48 h. HK-2 cells were pretreated with 8-O-cAMP (50 μM) for 5 h before exposure to high glucose (30 mM D-glucose). C/EBP-β or SOCS3 siRNA was transfected into HK-2 cells using Lipofectamine 3000 reagent for in vitro studies.
Real time PCR analysis
Total RNA was extracted from renal sections and HK-2 cells using TRIzol reagent (Takara). First-strand cDNA was prepared by two-step RT-qPCR (Takara). Relative gene expression was determined via SYBR Green quantitative real-time PCR assays on a Light Cycler 96 System (Roche). Primers for mouse or human SOCS3 and MCP-1 were synthesized by Sangon, Inc. (Shanghai, China). The cycling conditions used were as follows: denaturation at 50 °C for 2 min and 95 °C for 10 min and preincubation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s and 97 °C for 1 s. All reactions were performed in triplicate with a control, and gene expression was calculated using the 2−ΔΔCt method and normalized to ACTB. The primer sequences of each gene are shown in Table 1.
Table 1.
Primer sequences used for qRT-PCR.
| Gene | Species | Direction | Primer sequence (5′-3′) | Amplicon Size (bp) |
|---|---|---|---|---|
| CCL2 | Mouse | Forward | CCACTCACCTGCTGCTACTCATTC | 354 |
| Reverse | GTTCACTGTCACACTGGTCACTCC | |||
| SOCS3 | Mouse | Forward | CGCCCCCAGAATAGATGTAGTA | 248 |
| Reverse | GACCAAGAACCTACGCATCCA | |||
| ACTB | Mouse | Forward | GGCCCAGAGCAAGAGAGGTA | 270 |
| Reverse | CGACCAGAGGCATACAGGGA | |||
| CCL2 | Human | Forward | CCTTCTGTGCCTGCTGCTCATAG | 262 |
| Reverse | TTGCTTGTCCAGGTGGTCCATG | |||
| SOCS3 | Human | Forward | GACTGCGTGCTCAAGCTGGTG | 232 |
| Reverse | TCCGACAGAGATGCTGAAGAGTGG | |||
| ACTB | Human | Forward | GTTGCTATCCAGGCTGTGCTATCC | 284 |
| Reverse | GCCATCTCTTGCTCGAAGTCCAG |
Western blot analysis
Total proteins from db/db mouse cortex samples, HK-2 cells and controls were obtained by using radioimmunoprecipitation assay (RIPA) buffer (CoWin Biosciences, China) containing protease inhibitors and phosphatase inhibitors (CoWin Biosciences, China). A BCA protein assay kit (CoWin Biosciences, China) was used to determine the protein concentration. Proteins were separated using 8% and 12% SDS-PAGE gels and transferred to PVDF membranes, which were then probed with primary antibodies against C/EBP-β, MCP-1, TNF-α, p-STAT3, STAT3, and SOCS3. β-Actin was used as a loading control. After incubation with an appropriate secondary antibody, protein expression was measured using ImageJ, as previously described [27].
Macrophage migration assay
HK-2 cells were pretreated with or without 8-O-cAMP and incubated under high glucose (30 mM D-glucose) conditions, while HK-2 cells under basal conditions served as controls. The cell supernatants were collected after HG treatment for 24 h. THP-1 cells were cultured in the upper chambers of Transwell plates in the presence pf 100 ng/mL PMA for 48 h and then cultured in fresh complete medium in the absence of PMA for 24 h. Then, the cell supernatants derived from high glucose-induced HK-2 cells with or without 8-O-cAMP treatment were added to the lower chambers. After being cocultured for 24 h, a cotton swab was used to remove the cells on the upper surface of the membrane. Cells that migrated to the bottom surface of the membrane were fixed and stained with DAPI and then observed under a Nikon microscope.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7.0 (GraphPad, San Diego, CA, USA). Statistical differences between two groups were assessed by unpaired t tests. One-way analysis of variance (ANOVA) was used for multiple independent sample analyses. Comparisons between groups were performed with Tukey’s post hoc tests. The values are presented as the mean ± SD, and P < 0.05 was considered to indicate a significant difference.
Results
Effect of the Epac agonist 8-O-cAMP on renal function and pathological changes in the kidneys of db/db mice
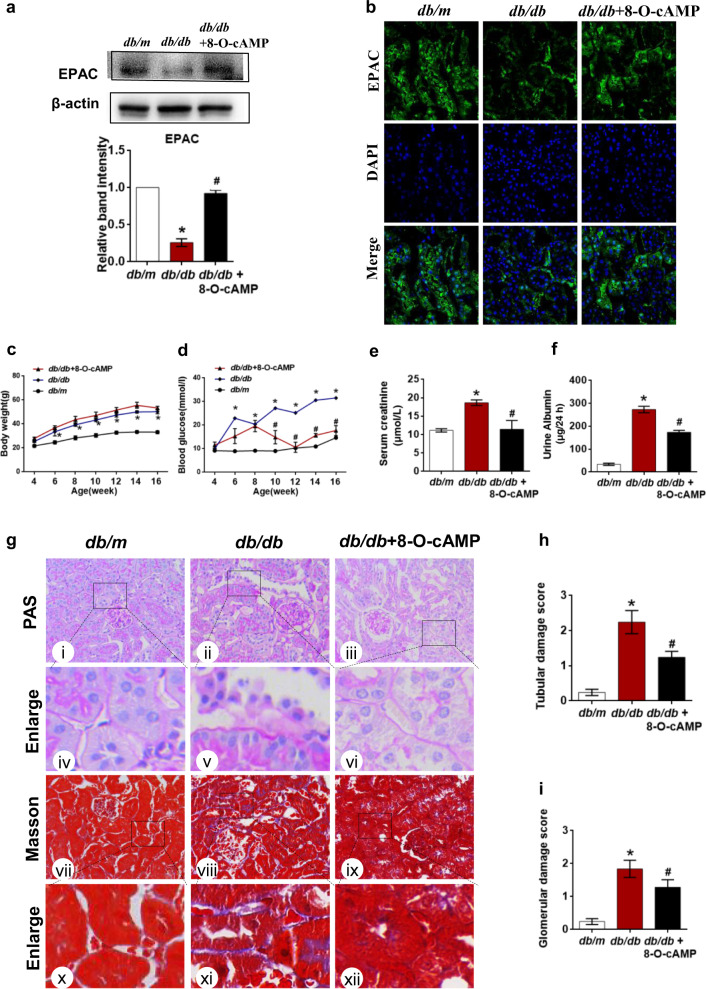
To examine the role of Epac activation in tubular damage in DN, we treated db/db mice with 8-O-cAMP by intraperitoneal injection for 8 weeks. Western blot assay and immunofluorescence staining showed that the expression of EPAC was significantly decreased in db/db mice but was restored by 8-O-cAMP treatment (Fig. 1a, b). Db/db mice exhibited higher body weights and increased blood glucose, serum creatinine, and urine albumin levels compared to those of db/m mice (Fig. 1c–f). Slight but nonsignificant increases in body weights were observed in db/db mice treated with 8-O-cAMP compared to those treated with vehicle (Fig. 1c). In contrast, blood glucose (Fig. 1d), serum creatinine (Fig. 1e) and urine albumin levels (Fig. 1f) were lower in db/db mice treated with 8-O-cAMP than in those treated with vehicle. Basement membrane thickening, tubular atrophy, mesangial matrix proliferation, and interstitial fibrosis were observed in the kidneys of db/db mice compared to db/m mice by PAS and Masson’s trichrome staining (Fig. 1g). However, 8-O-cAMP treatment ameliorated these abnormal changes (Fig. 1g). Quantitative analysis of tubular and glomerular damage scores further confirmed this trend (Fig. 1h, i). Taken together, these data indicate that the Epac activator 8-O-cAMP may have a therapeutic effect against diabetic kidney injury.
Fig. 1. Effect of the Epac-selective analog 8-pCPT-2′-O-Me-AMP on renal functional and morphological characteristics in db/db mice.
a Epac expression was measured by Western blot assays. b Immunofluorescence staining of Epac in kidney tissues. c Body weight changes in db/m, db/db and db/db mice that received 8-pCPT-2′-O-Me-cAMP (8-O-cAMP) treatment. d Blood glucose concentrations in each group. e, f The levels of serum creatinine and urine albumin. g Renal morphological analysis was assessed by PAS (i–vi) and Masson’s trichrome staining (vii–xii). h, i Quantitative analysis of tubular and glomerular damage scores in each group (n = 5); the values are the mean ± SD; *P < 0.05 vs db/m, #P < 0.05 vs db/db mice.
8-O-cAMP alleviates diabetes-induced renal interstitial fibrosis by reducing the intrarenal inflammatory response and oxidative stress in db/db mice
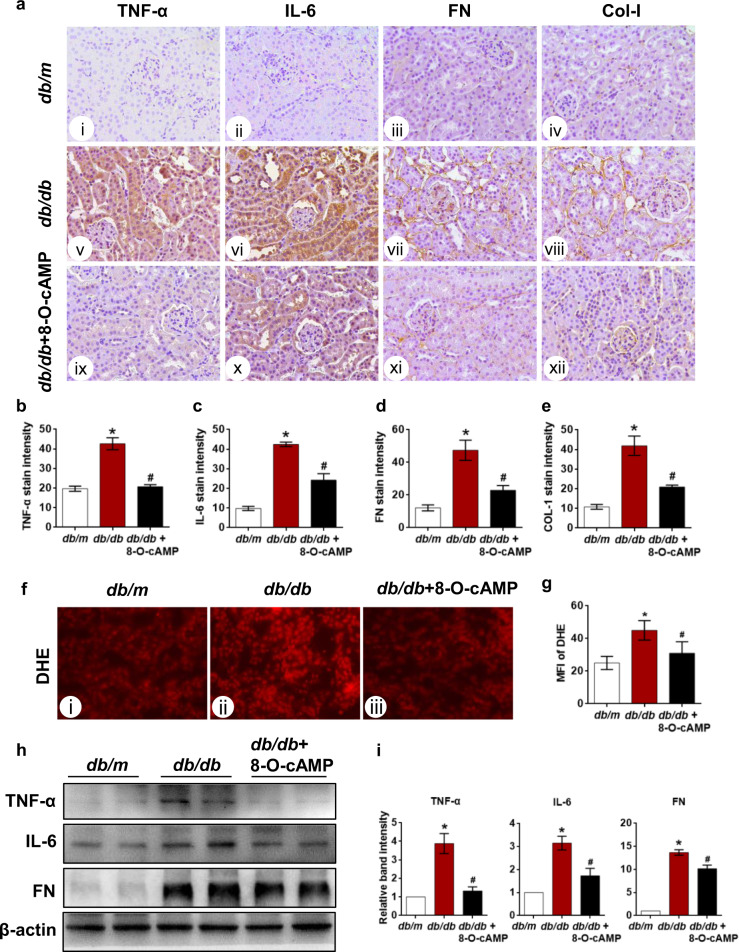
Inflammation and oxidative stress are important contributors to the initiation and development of DN. We subsequently evaluated renal inflammatory cytokine levels and ROS production in the kidneys of db/db mice treated with 8-O-cAMP. The IHC results showed notably increased TNF-α and IL-6 expression that was primarily confined to the tubular compartment of the renal cortex [Fig. 2a (v, vi vs i, ii)]. Fibronectin and Collagen I levels were also assessed. Compared to that of db/m mice, a notable increase in fibronectin and collagen I expression was observed in the tubulointerstitium of db/db mice [Fig. 2a (vii, viii vs iii, iv)]. Notably, these abnormal changes in db/db mice were partially or completely reversed by treatment with 8-O-cAMP [Fig. 2a (ix, x, xi, xii)]. The generation of tubular ROS was assessed by measuring the intensity of DHE fluorescence in the tubules of the cryostat tissue sections. Quantitative analysis of DHE staining showed that ROS production was increased in the tubular cells of kidneys from db/db mice compared with those from db/m mice (Fig. 2f, g). Consistent with the IHC findings, similar results regarding TNF-α, IL-6, and FN expression in the kidneys were determined by Western blot assay (Fig. 2h, i). These results provided compelling evidence supporting a renoprotective role of Epac activation induced by 8-O-cAMP in DN.
Fig. 2. Effect of 8-O-cAMP on the renal inflammatory response, tubulointerstitial fibrosis, and oxidative stress in db/db mice.
a Kidney sections from db/m, db/db and db/db mice treated with 8-O-cAMP were stained with TNF-α, IL-6, fibronectin (FN) and collagen 1 (Col-l) antibodies for IHC analysis (400×). b–e Quantification of the immunostaining intensities of TNF-α, IL-6, FN, and Col-l (n = 5). f Oxidative stress in kidney tissues was assessed by DHE staining. g Quantification of the mean fluorescence intensity (MFI) of DHE in tubules (n = 5). h Western blot results showing the changes in TNF-α, IL-6, and FN expression in kidney tissues. i Quantification of Western blot band intensity (n = 3). The values are the mean ± SD; *P < 0.05 vs db/m, #P < 0.05 vs db/db mice.
8-O-cAMP attenuates intrarenal macrophage infiltration in db/db mice
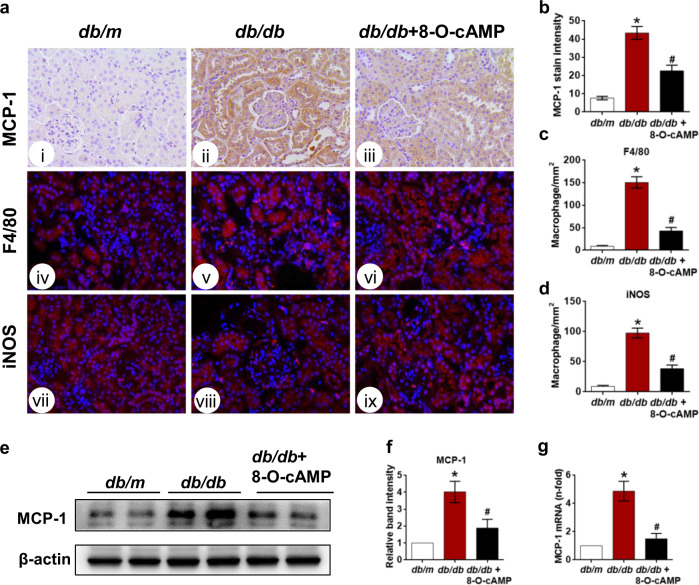
Previous studies have shown that the inflammatory response that precedes and promotes renal damage associated with diabetes largely depends on the accumulation of macrophages in renal tissues [31–33]. Furthermore, an influx of macrophages in the kidney occurs in response to the upregulation of monocyte chemotactic protein-1 (MCP-1) [5]. Therefore, we further explored the contribution of 8-O-cAMP to the anti-inflammatory response in the kidneys of db/db mice by determining its effect on MCP-1 expression and intrarenal macrophage accumulation. The IHC assay results showed that MCP-1 expression was significantly increased in the tubules of db/db mice compared to db/m mice but was inhibited by 8-O-cAMP administration (Fig. 3a, b). Moreover, the F4/80 immunofluorescence results showed that macrophage infiltration in the tubulointerstitium of db/db mice was significantly abrogated by 8-O-cAMP treatment (Fig. 3a, c). M1 macrophages are thought to play a role in worsening inflammation in DN. Interestingly, we observed an increase in the number of iNOS (a marker of M1 macrophages)-positive cells in the tubulointerstitium of db/db mice, and 8-O-cAMP treatment protected against increased numbers of M1 macrophages under diabetic conditions (Fig. 3a, d). Consistent with the IHC findings, similar results regarding MCP-1 expression were determined by Western blotting (Fig. 3e, f) and qPCR (Fig. 3g). Taken together, these results indicated that activation of Epac via 8-O-cAMP may exert anti‐inflammatory effects by reducing macrophage accumulation and activation in the diabetic kidney.
Fig. 3. Effect of the Epac agonist on MCP-1 expression and macrophage accumulation in kidney tissues.
a IHC assay assessing MCP-1 expression in the kidneys of db/m, db/db, and db/db mice treated with 8-O-cAMP (i–iii), and macrophage infiltration was visualized by F4/80 (iv–vi) and iNOS (vii–ix) immunofluorescence staining (400×). b Quantification of MCP-1 expression (n = 5). c, d Quantification of F4/80- and iNOS-positive cells per mm2 in kidney tissues (n = 5). e MCP-1 expression was evaluated by Western blotting. f Quantification of Western blot band intensities. g Relative MCP-1 mRNA expression in kidney tissues (n = 3). The values are the mean ± SD; *P < 0.05 vs db/m, #P < 0.05 vs db/db mice.
8-O-cAMP increases C/EBP-β and SOCS3 expression while inhibiting STAT3 phosphorylation
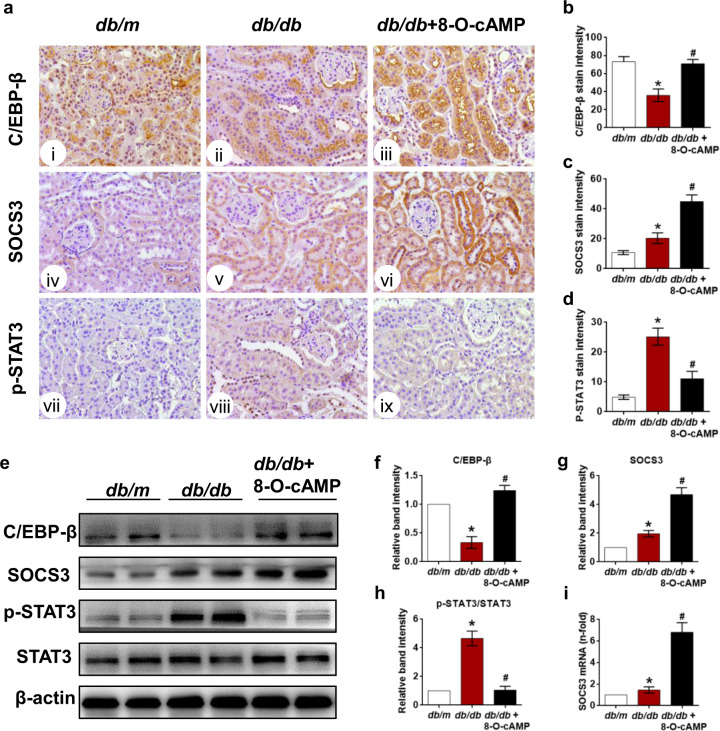
The transcription factor C/EBP-β has been reported to bind directly to the SOCS3 promoter region and initiate SOCS3 transcription [34]. We first showed that 8-O-cAMP treatment rescued the decreased expression of C/EBP-β in db/db mice compared to db/db mice (Fig. 4a, b). Considering that MCP-1 is a classical STAT3-responsive inflammatory gene and SOCS3 is a negative regulator of the STAT3 signaling pathway, we subsequently examined the effect of 8-O-cAMP treatment on the SOCS3/STAT3 axis in the kidneys of db/db mice. The IHC assay results revealed low levels of SOCS3 and phosphorylated STAT3 (Tyr705) in db/m mice, whereas these proteins were broadly distributed in db/db mice (Fig. 4a, c, d). Interestingly, 8-O-cAMP treatment further upregulated SOCS3 levels (Fig. 4a, c) but inhibited p-STAT3 levels in db/db mice (Fig. 4a, d). Similar results of SOCS3 and p-STAT3 levels were observed by Western blotting (Fig. 4e–h). Consistent with these findings, SOCS3 gene expression in db/db mice was further augmented by 8-O-cAMP treatment (Fig. 4i). These data suggest that 8-O-cAMP may increase SOCS3 expression at both the gene and protein levels by restoring C/EBP-β expression, subsequently inhibiting STAT3 phosphorylation.
Fig. 4. Effect of the Epac agonist on renal C/EBP-β, SOCS3 and p-STAT3 expression.
a The location and expression of C/EBP-β (i–iii), SOCS3 (iv–vi) and p-STAT (vii–ix) were assessed by IHC in each group (400×). b–d Quantification of C/EBP-β, SOCS3, and p-STAT expression in kidney tissues (n = 5). e The expression of C/EBP-β, SOCS3, and p-STAT (Tyr705) was measured by Western blotting. f–h Quantification of the Western blot results (n = 3). i Relative SOCS3 mRNA expression in kidney tissues (n = 3). The values are the mean ± SD; *P < 0.05 vs db/m, #P < 0.05 vs db/db mice.
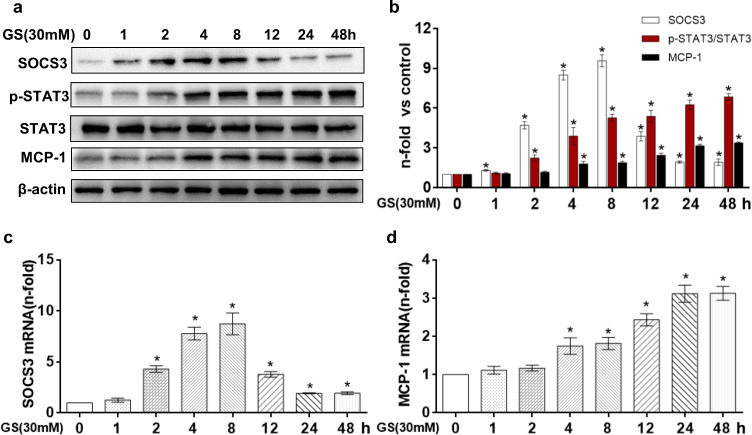
Sequential changes in SOCS3, phosphorylated STAT3, and MCP-1 levels in HK-2 cells exposed to high glucose
Western blot and qPCR results demonstrated that the HG (30 mM, D-glucose)-induced increase in SOCS3 mRNA and protein expression in HK-2 cells peaked at 8 h and then gradually decreased, although these levels remained significantly higher than those observed in the NG group of HK-2 cells (Fig. 5a–c). Unlike SOCS3, phosphorylated Tyr705 STAT3 levels increased in a time-dependent manner, peaking at 48 h following HG treatment (Fig. 5a, b). Furthermore, we assessed the expression of an established target gene of phosphorylated Tyr705 STAT3 (MCP-1) and observed that the target protein and mRNA levels were induced in a similar manner as those of phosphorylated Tyr705 STAT3 in HK-2 cells (Fig. 5a, d). Therefore, 24 h of HG treatment was used in the following experiments.
Fig. 5. Effect of high glucose on SOCS3/STAT3/MCP-1 expression in HK-2 cells.
a Western bloting results showing the changes in SOCS3, p-STAT3, and MCP-1 protein expression in HK-2 cells treated with 30 mM D-glucose at the indicated time points. b Quantification of the Western blot results (n = 3). c, d Gene expression of SOCS3 and MCP-1 was analyzed by real-time PCR (n = 3). The values are the mean ± SD. *P < 0.05 vs LG.
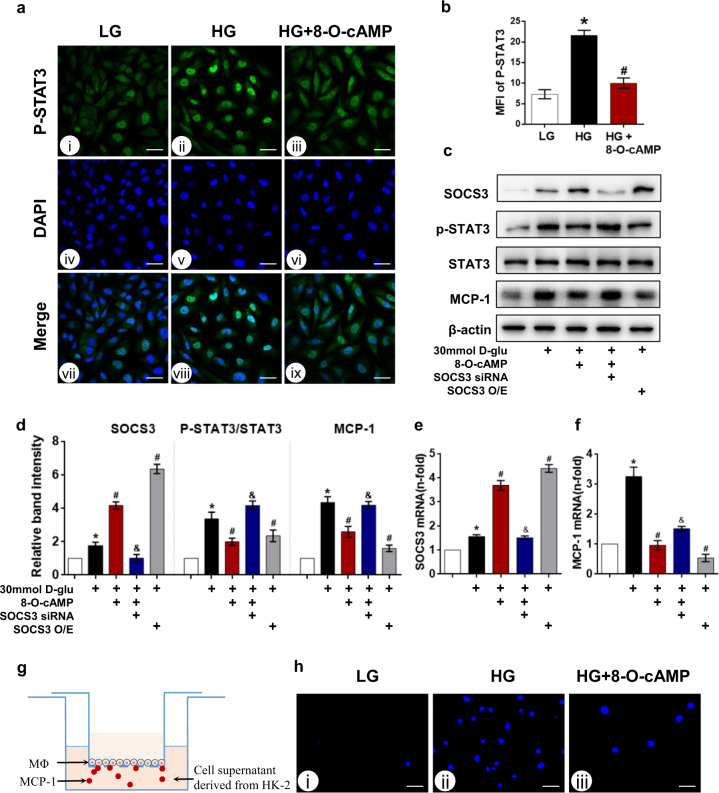
8-O-cAMP inhibits the phosphorylated Tyr705 STAT3/MCP-1 axis and decreases macrophage migration by increasing SOCS3 expression
To assess the effect of 8-O-cAMP on phosphorylated Tyr705 STAT3 levels in tubular cells exposed to HG, HK-2 cells were pretreated with 8-O-cAMP (100 μM) for 5 h before HG stimulation. The immunofluorescence assay results revealed that phosphorylated Tyr705 STAT3 expression was quite low in HK-2 cells under basal conditions (5.6 mM, D-glucose), while HG treatment resulted in the upregulation and nuclear translocation of phosphorylated Tyr705 STAT3, and this effect was largely abrogated by treatment with 8-O-cAMP (Fig. 6a, b). Moreover, 8-O-cAMP treatment did not alter total STAT3 levels in HK-2 cells. In addition, STAT3 activation increases phosphorylated STAT3 levels (p-STAT3), the activity of which is regulated by endogenous inhibitors, namely, SOCS3 [35]. To verify whether the inhibitory effect of 8-O-cAMP on p-STAT3/MCP-1 axis activation relies on SOCS3 upregulation, we transfected HK-2 cells with SOCS3 siRNA concomitant with 8-O-cAMP treatment. RNA was extracted, and lysates were collected for Western blot analysis to confirm the efficiency of the intervention. Interestingly, 8-O-cAMP treatment further increased the protein (Fig. 6c, d) and mRNA (Fig. 6e) levels of SOCS3 to ~2-fold that observed in the HG group. Moreover, the inhibitory effect of 8-O-cAMP on p-STAT3 and MCP-1 levels was abolished in HK-2 cells transfected with SOCS3 siRNA under the same experimental conditions. Furthermore, we also observed that HG-induced activation of the p-STAT3/MCP-1 axis was inhibited in HK-2 cells transfected with the SOCS3 overexpression plasmid. Taken together, these data suggest that the modulation of SOCS3 expression is sufficient to mimic Epac activation. In addition, the qPCR results demonstrated that the mRNA expression of MCP-1 was in consistent with the observed protein expression (Fig. 6f). These cell culture results indicate that 8-O-cAMP persistently induces SOCS3 expression under HG conditions, inhibiting the p-STAT3/MCP-1 axis in HK-2 cells.
Fig. 6. SOCS3 inhibits MCP-1 expression by suppressing STAT3 activation in HK-2 cells under HG conditions.
a Immunofluorescence staining of phosphorylated STAT3 in HK-2 cells incubated with HG (30 mM D-glucose) for 24 h and treated with or without 8-O-cAMP. b Quantification of the MFI of p-STAT3 (n = 3). c Western blot analysis of SOCS3, p-STAT3, and MCP-1 levels in HK-2 cells treated with or without 8-O-cAMP or transfected with SOCS3 siRNA or SOCS3 overexpression plasmid (O/E) under HG conditions. d Quantification of the Western blot results (n = 3). e, f SOCS3 and MCP-1 mRNA was analyzed by real-time PCR (n = 3). g Macrophage migration assay was conducted as schematically depicted. h Macrophage migration assay showing the migration of THP-1 macrophages incubated with medium from HG-stimulated HK-2 cells and analyzed by fluorescence microscopy after DAPI staining. Scare bar = 40 μm. The values are the mean ± SD. *P < 0.05 vs LG; #P < 0.05 vs HG; &P < 0.05 vs HG + 8-O-cAMP.
Furthermore,macrophage migration assay was performed to determine whether pretreatment of HK-2 cells with 8-O-cAMP could effectively decrease the recruitment of macrophages (Fig. 6g), as detailed in “Methods”. Using this coculture system, a notably increased number of THP-1 cells migrated from the upper to the bottom side of the membrane when THP-1 cells were exposed to hyperglycemia-stimulated HK-2 cell supernatant (Fig. 6h). In contrast, when THP-1 cells were exposed to supernatant from HK-2 cells treated with 8-O-cAMP under HG conditions, the number of migrated macrophages was significantly decreased (Fig. 6h).
8-O-cAMP increases SOCS3 expression via C/EBP-β
Subsequently, we examined the upstream regulator of SOCS3 to elucidate the molecular mechanisms by which 8-O-cAMP exerts its anti-inflammatory effects under HG conditions. The transcription factor C/EBP-β has been reported to bind directly to the SOCS3 promoter region [34]. Through IF staining, we observed that C/EBP-β was highly expressed in the nuclei of HK-2 cells under basal conditions (Fig. 7a). Moreover, HG exposure reduced the nuclear fluorescence intensity of C/EBP-β (Fig. 7a, b), which was restored by 8-O-cAMP treatment. In addition, the Western blot results substantiated these findings (Fig. 7c, d). Then, a loss-of function assay for C/EBP-β was performed via siRNA transfection to assess the direct role of C/EBP-β in the modulation of SOCS3 expression in HK-2 cells. The Western blot results showed that 8-O-cAMP treatment did not increase the protein (Fig. 7c, e) or gene (Fig. 7f) expression of SOCS3 in HK-2 cells transfected with C/EBP-β siRNA under HG conditions, suggesting that 8-O-cAMP increases SOCS3 expression at the transcriptional level, at least by restoring C/EBP-β expression.
Fig. 7. The effect of Epac activation on SOCS3 expression is mediated by C/EBP-β.
a Immunofluorescence staining of C/EBP-β in HK-2 cells incubated with HG and treated with or without 8-O-cAMP (Scale bar = 20 μm). b Quantification of the MFI of C/EBP-β (n = 3). c Western blot analysis of C/EBP-β and SOCS3 in HK-2 cells under HG conditions that were treated with 8-O-cAMP or transfected with C/EBP-β siRNA. d, e Quantification of the Western blot results (n = 3). f SOCS3 mRNA expression in HK-2 cells cultured as described in c (n = 3). The values are the mean ± SD. *P < 0.05 vs LG; #P < 0.05 vs HG; &P < 0.05 vs HG + C/EBP-β siRNA.
Discussion
In the present study, the renoprotective effect of 8-O-cAMP against tubulointerstitial injury and the underlying mechanism were assessed. We showed that 8-O-cAMP, a specific agonist of Epac, could inhibit the progression of DN by reducing oxidative stress, MCP-1 expression and macrophage infiltration, as well as by decreasing cytokine release, suggesting that 8-O-cAMP exerts multiple renoprotective effects by ameliorating renal oxidative stress, intrarenal inflammation, and fibrosis in diabetic kidneys. Mechanistically, SOCS3 was shown to promote the anti-inflammatory response via inhibition of p-STAT3/MCP-1 axis activation under HG conditions. Furthermore, 8-O-cAMP promoted SOCS3 gene transcription at least in part by restoring C/EBP-β nuclear expression in renal tubular cells. These results indicate that Epac activation by 8-O-cAMP may be a promising therapeutic strategy for the treatment of DN.
Emerging evidence has shown that inflammation characterized by macrophage infiltration plays a crucial role in the pathogenesis and progression of DN [36, 37]. The depletion of macrophages by silencing macrophage scavenger receptor-A expression was shown to alleviate STZ-induced diabetic renal damage [38]. Notably, accumulating evidence suggests that renal tubular epithelial cells act as not only the target but also the mediator of macrophage-mediated tubulointerstitial inflammation [5–7]. MCP-1, a member of the CC chemokine family that acts through its receptor CCR2 [39], is a critical factor in recruiting monocytes/macrophages into inflammatory sites [40]. Recently, Kuehn and colleagues showed that the integrity of tubular cell cilia modulates macrophage recruitment in an MCP-1-dependent manner [41]. Furthermore, the inhibition of MCP-1 expression in HK-2 cells was shown to decrease macrophage migration under HG conditions [42]. In vivo, MCP-1 deficiency has been demonstrated to alleviate STZ-induced diabetic renal damage and macrophage infiltration [36, 43]. Therefore, TEC-derived MCP‐1 plays a key role in the crosstalk between injured TECs and infiltrating macrophages under DN conditions. In this context, we observed that upregulated renal MCP-1 expression was accompanied by increased intrarenal macrophage infiltration and tubulointerstitial fibrosis in db/db mice. However, administration of the Epac agonist 8-O-cAMP dramatically reversed these abnormal alterations induced by diabetes (Figs. 1–3). Furthermore, when cultured with supernatants from HK-2 cells pretreated with 8-O-cAMP under HG conditions, a significant decrease in macrophage migration was observed, suggesting that 8-O-cAMP treatment reduces the release of inflammatory cytokines from TECs (i.e., MCP-1) and abolishes the crosstalk between injured TECs and infiltrating macrophages in tubulointerstitial inflammation (Fig. 6). However, the specific mechanism by which 8-O-cAMP reduces MCP-1 expression in tubular cells remains unknown.
Under pathological conditions, STAT3 is phosphorylated by JAK at Tyr705 and then translocates to the nucleus, where it initiates the transcription of inflammation-related genes, such as CCL2 (the gene encoding MCP-1) [16]. Previous studies have suggested that IL-6 and its receptor might be potential mediators of STAT3 activation in diabetes [44, 45]. In the present study, we observed a significant increase in IL-6 expression in the kidneys of db/db mice (Fig. 2). Consistent with this finding, Lu et al. observed that STAT3 knockdown dramatically reduced the expression of inflammatory markers, such as IL-6 and MCP-1 [46]. Moreover, the STAT3 inhibitor nifuroxazide was shown to notably decrease intrarenal macrophage infiltration and attenuate renal inflammation and fibrosis in diabetic rats [47]. These findings suggest the therapeutic potential of JAK/STAT blockade in DN [8, 48, 49]. However, studies on the relationship between STAT3 and DN have primarily focused on mesangial cells [49], and the role of STAT3 in proximal tubular cells exposed to HG conditions has not yet been elucidated. In the present study, we observed that the levels of p-STAT3 and MCP-1 were increased in the tubules of db/db mice (Fig. 4) and in HK-2 cells in a time-dependent manner after HG stimulation (Fig. 5). Moreover, 8-O-cAMP administration significantly inhibited HG-induced STAT3 phosphorylation at Tyr705 and its nuclear translocation in HK-2 cells, which consequently inhibited the transcription of CCL-2 (Fig. 6). These results suggest that 8-O-cAMP transcriptionally inhibits MCP-1 expression at least in part by preventing STAT3 phosphorylation at Tyr705.
We also investigated the molecular mechanisms by which 8-O-cAMP regulates STAT3 inactivation under hyperglycemic conditions. Epac activation has been reported to induce SOCS gene expression in various types of cells, such as cardiocytes [23], vascular endothelial cells [24] and retinal ganglion cells [25]. Consistent with these studies, we observed that Epac activation via 8-O-cAMP significantly upregulated SOCS3 gene and protein expression in db/db mice (Fig. 4) and HK-2 cells (Fig. 6). More importantly, SOCS proteins are natural inhibitors of the JAK/STAT signaling pathway and have been demonstrated to participate in the regulation of inflammatory signaling, including in the kidney [8, 22, 50, 51]. Ortiz-Muñoz et al. found JAK/STAT activation and increased SOCS3 expression in the kidneys of diabetic animal models and patients. In addition, HG activated JAK/STAT/SOCS expression. However, overexpression of SOCS reversed the HG-induced activation of JAK/STAT in tubular cells, suggesting that SOCS was an important negative regulator of JAK/STAT activation in diabetic nephropathy [8]. Consistently, we observed that the protein and mRNA expression of SOCS3 was upregulated in the kidneys of db/db mice and HG-cultured tubular cells. In addition, 8-O-cAMP increased the level of SOCS3 expression and inhibited the expression of p-STAT and inflammatory cytokines compared with those in the db/db group (Fig. 4). SOCS3 overexpression effectively prevented HG-induced STAT3 activation at Tyr705 and limited subsequent MCP-1 production in HK-2 cells. However, the inhibitory effect of 8-O-cAMP on the p-STAT3/MCP-1 axis was abolished in HK-2 cells that were transfected with SOCS3 siRNA (Fig. 6). Based on our observations and those of previous studies, a compensatory increase in SOCS3 expression exerts a negative effect on the JAK/STAT pathway and might contribute to EPAC mediated protection in tubular cells in the context of diabetes. However, we cannot exclude other mediators of Epac that target the STAT3 pathway. For example, in our present study, we also found that Epac activation ameliorated renal tubular oxidative stress in db/db mice (Fig. 2). Previous studies have demonstrated that the hyperglycemia-induced production of ROS could also activate the JAK/STAT pathway [52]. Therefore, the suppression of JAK/STAT activation by Epac may occur by inhibiting ROS production in DN, but this hypothesis requires further investigation.
Next, we assessed the role of the transcription factor C/EBP-β in Epac-induced SOCS3 expression in HK-2 cells. Our previous study showed that Rap1b, a downstream molecule of Epac, could modulate C/EBP-β transcriptional activity in DN, and studies of other cell types also showed that the activation of Epac via 8-O-cAMP regulated SOCS3 gene expression [16, 34]. Consistent with these previous studies, we first demonstrated that 8-O-cAMP treatment could restore C/EBP-β expression and promote C/EBP-β translocation to the nucleus in HK-2 cells, which increased SOCS3 expression in a C/EBP-β-dependent manner (Fig. 7). Taken together, these data suggest that C/EBP-β is an Epac-activated transcription factor that mediates the induction of the SOCS-3 gene in tubular cells. However, additional studies, such as luciferase reporter assays, should be performed to verify whether Epac activation in tubular cells leads to enhanced binding of C/EBP-β to the SOCS-3 promoter.
Although our data support an important renoprotective role of 8-O-cAMP in the progression of DN, there are several potential limitations to our study. First, Epac2a-KO mice are prone to obesity [53], and Epac activation can contribute to insulin release and glucose control in pancreatic β cells [54, 55]. In this study, it was found that the body weight only slightly increased in db/db mice treated with 8-O-cAMP, but there was no significant difference compared to that of the db/db mice group. However, the body weights of db/db mice treated with 8-O-cAMP decreased at week 16 compared to those at week 14. Second, we observed a connection between 8-O-cAMP treatment and decreased blood glucose levels in diabetic mice. One interpretation of this observation is that pancreatic β cells, where Epac is highly expressed, promote insulin secretion in response to the systematic administration of 8-O-cAMP [56]. Therefore, we cannot exclude the possibility that the beneficial effects of 8-O-cAMP on the progression of DN could be partially dependent on its glucose-lowering properties. Thus, future studies using a more appropriate mouse model are warranted to assess the renoprotective effect of 8-O-cAMP on DN. Furthermore, one cautionary note for future investigators of 8-O-cAMP is that under long-term treatment conditions, Epac exhibits a wide tissue distribution and distinguishable functions with respect to different isoforms [16]. For example, earlier published reports have hinted at the induction of cardiac arrhythmia, fibrosis, and hypertrophy in response to cardiac Epac activation [57]. Thus, localized Epac activation is expected to bypass the side effects associated with systematic Epac activation. Third, several off-target effects limit the specificity of 8-O-cAMP, such as its off-target activation of the P2Y12 receptors present in platelets [58]. With these points in mind, further research evaluating the activity of 8-O-cAMP is warranted because we anticipate that the dose, treatment period, administration route, and bioavailability could influence the therapeutic versus toxic effects.
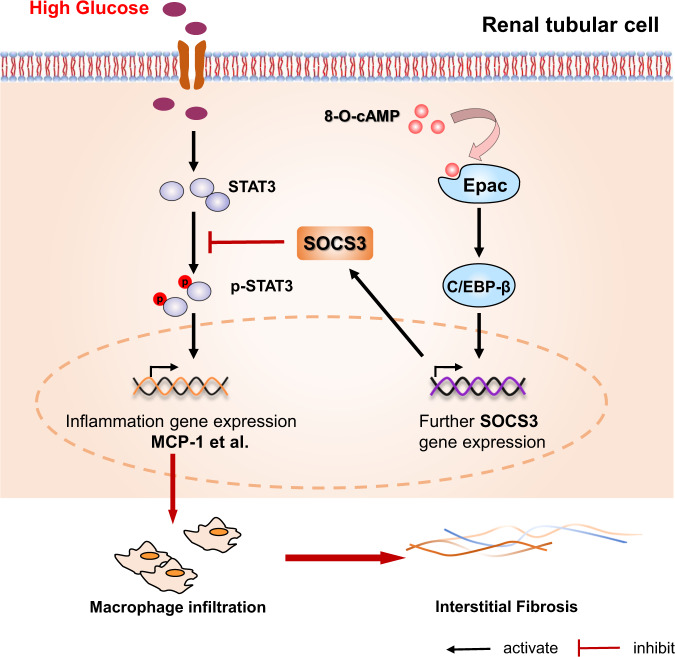
In summary, in the present study, we used in vivo and in vitro diabetes models to assess the beneficial effects of Epac activation on macrophage-mediated inflammatory injury and the mechanisms underlying these effects. Epac activation via 8-O-cAMP regulates C/EBP-β-mediated SOCS3 transcription and inhibits the p-STAT3/MCP-1 axis, improving renal macrophage infiltration and the inflammatory response, which ultimately alleviates interstitial fibrosis (Fig. 8). This finding suggests that potential applications of Epac activators (such as 8-O-cAMP) may provide a new therapeutic strategy for preventing renal inflammation injury in DN.
Fig. 8. Protective signaling pathways activated by Epac in HK-2 cells under HG conditions.
Under HG conditions, selective activation of Epac promotes C/EBP-β nuclear translocation and binding to the SOCS3 promoter, initiating SOCS3 gene expression, resulting in the suppression of STAT3 phosphorylation and subsequently decreasing STAT3-responsive inflammatory gene transcription (MCP-1, etc.), thereby attenuating hyperglycemia-induced macrophage infiltration and subsequent tubulointerstitial fibrosis.
Acknowledgements
We thank all who contributed to the present study for their time and effort. This work was supported by the National Natural Sciences Foundation of China (No. 81570658).
Author contributions
WXY, LX: Conceptualization; WXY, LX: Data curation; WXY, YL, LX: Formal analysis; LX: Funding acquisition; WXY, SMZ, YFL, HFW, JLL, XHL, MRZ, YZH: Investigation; WXY, LX: Methodology; LX: Project administration; WXY, LX: Resources; WXY, LX: Software; LX: Supervision; WXY, LX: Validation; WXY, LX: Visualization; WXY: Writing—original draft; YL, FYL, LS, LX: Writing—review and editing.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Wen-xia Yang, Yu Liu
References
- 1.Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, et al. Trends in chronic kidney disease in China. N Engl J Med. 2016;375:905–6. doi: 10.1056/NEJMc1602469. [DOI] [PubMed] [Google Scholar]
- 2.Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012:146154. doi: 10.1155/2012/146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klessens CQF, Zandbergen M, Wolterbeek R, Bruijn JA, Rabelink TJ, Bajema IM, et al. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol Dial Transpl. 2017;32:1322–9. doi: 10.1093/ndt/gfw260. [DOI] [PubMed] [Google Scholar]
- 4.Niu S, Bian Z, Tremblay A. Broad infiltration of macrophages leads to a proinflammatory state in streptozotocin-induced hyperglycemic mice. J Immunol. 2016;197:3293–301.. doi: 10.4049/jimmunol.1502494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tesch GH. Macrophages and diabetic nephropathy. Semin Nephrol. 2010;30:290–301. doi: 10.1016/j.semnephrol.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert RE. Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease. Diabetes. 2017;66:791–800. doi: 10.2337/db16-0796. [DOI] [PubMed] [Google Scholar]
- 7.Lanaspa MA, Ishimoto T, Cicerchi C, Tamura Y, Roncal-Jimenez CA, Chen W, et al. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J Am Soc Nephrol. 2014;25:2526–38. doi: 10.1681/ASN.2013080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortiz-Munoz G, Lopez-Parra V, Lopez-Franco O, Fernandez-Vizarra P, Mallavia B, Flores C, et al. Suppressors of cytokine signaling abrogate diabetic nephropathy. J Am Soc Nephrol. 2010;21:763–72. doi: 10.1681/ASN.2009060625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang W, Luo Y, Yang S, Zeng M, Zhang S, Liu J, et al. Ectopic lipid accumulation: potential role in tubular injury and inflammation in diabetic kidney disease. Clin Sci (Lond) 2018;132:2407–22.. doi: 10.1042/CS20180702. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, Chen X, Wang Y, Chen Y, Chen S, Gong W, et al. Micheliolide ameliorates diabetic kidney disease by inhibiting Mtdh-mediated renal inflammation in type 2 diabetic db/db mice. Pharmacol Res. 2019;150:104506. doi: 10.1016/j.phrs.2019.104506. [DOI] [PubMed] [Google Scholar]
- 11.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–7. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 12.de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem. 2000;275:20829–36. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- 13.Lezoualc’h F, Fazal L, Laudette M, Conte C. Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circ Res. 2016;118:881–97. doi: 10.1161/CIRCRESAHA.115.306529. [DOI] [PubMed] [Google Scholar]
- 14.Robichaux WG, Cheng X. Intracellular cAMP Sensor EPAC: physiology, pathophysiology, and therapeutics development. Physiol Rev. 2018;98:919–1053. doi: 10.1152/physrev.00025.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin Y, Stokman G, Yan K, Ramaiahgari S, Verbeek F, de Graauw M, et al. cAMP signalling protects proximal tubular epithelial cells from cisplatin-induced apoptosis via activation of Epac. Br J Pharmacol. 2012;165:1137–50. doi: 10.1111/j.1476-5381.2011.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parnell E, Palmer TM, Yarwood SJ. The future of EPAC-targeted therapies: agonism versus antagonism. Trends Pharmacol Sci. 2015;36:203–14. doi: 10.1016/j.tips.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stokman G, Qin Y, Booij TH, Ramaiahgari S, Lacombe M, Dolman ME, et al. Epac-Rap signaling reduces oxidative stress in the tubular epithelium. J Am Soc Nephrol. 2014;25:1474–85. doi: 10.1681/ASN.2013070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stokman G, Qin Y, Genieser HG, Schwede F, de Heer E, Bos JL, et al. Epac-Rap signaling reduces cellular stress and ischemia-induced kidney failure. J Am Soc Nehprol. 2011;22:859–72. doi: 10.1681/ASN.2010040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding H, Bai F, Cao H, Xu J, Fang L, Wu J, et al. PDE/cAMP/Epac/C/EBP-beta signaling cascade regulates mitochondria biogenesis of tubular epithelial cells in renal fibrosis. Antioxid Redox Signal. 2018;29:637–52. doi: 10.1089/ars.2017.7041. [DOI] [PubMed] [Google Scholar]
- 20.Fang F, Liu GC, Kim C, Yassa R, Zhou J, Scholey JW. Adiponectin attenuates angiotensin II-induced oxidative stress in renal tubular cells through AMPK and cAMP-Epac signal transduction pathways. Am J Physiol Ren Physiol. 2013;304:F1366–74. doi: 10.1152/ajprenal.00137.2012. [DOI] [PubMed] [Google Scholar]
- 21.Xie P, Joladarashi D, Dudeja P, Sun L, Kanwar YS. Modulation of angiotensin II-induced inflammatory cytokines by the Epac1-Rap1A-NHE3 pathway: implications in renal tubular pathobiology. Am J Physiol Ren Physiol. 2014;306:F1260–74. doi: 10.1152/ajprenal.00069.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–65. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 23.Jin H, Fujita T, Jin M, Kurotani R, Hidaka Y, Cai W, et al. Epac activation inhibits IL-6-induced cardiac myocyte dysfunction. J Physiol Sci. 2018;68:77–87. doi: 10.1007/s12576-016-0509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sands WA, Woolson HD, Yarwood SJ, Palmer TM. Exchange protein directly activated by cyclic AMP-1-regulated recruitment of CCAAT/enhancer-binding proteins to the suppressor of cytokine signaling-3 promoter. Methods Mol Biol. 2012;809:201–14. doi: 10.1007/978-1-61779-376-9_14. [DOI] [PubMed] [Google Scholar]
- 25.Hellstrom M, Harvey AR. Cyclic AMP and the regeneration of retinal ganglion cell axons. Int J Biochem Cell Biol. 2014;56:66–73. doi: 10.1016/j.biocel.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Zhan M, Usman IM, Sun L, Kanwar YS. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J Am Soc Nephrol. 2015;26:1304–21. doi: 10.1681/ASN.2014050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D, et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017;11:297–311. doi: 10.1016/j.redox.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowe AR, Yue W. Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: an integrated protocol. Bio-protoc. 2019;9:e3465. doi: 10.21769/BioProtoc.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie P, Sun L, Nayak B, Haruna Y, Liu FY, Kashihara N, et al. C/EBP-beta modulates transcription of tubulointerstitial nephritis antigen in obstructive uropathy. J Am Soc Nephrol. 2009;20:807–19. doi: 10.1681/ASN.2008091007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shu S, Zhu J, Liu Z, Tang C, Cai J, Dong Z. Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. EBioMedicine. 2018;37:269–80.. doi: 10.1016/j.ebiom.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You H, Gao T, Cooper TK, Brian Reeves W, Awad AS. Macrophages directly mediate diabetic renal injury. Am J Physiol Ren Physiol. 2013;305:F1719–27. doi: 10.1152/ajprenal.00141.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Awad AS, You H, Gao T, Cooper TK, Nedospasov SA, Vacher J, et al. Macrophage-derived tumor necrosis factor-α mediates diabetic renal injury. Kidney Int. 2015;88:722–33. doi: 10.1038/ki.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scurt FG, Menne J, Brandt S, Bernhardt A, Mertens PR, Haller H, et al. Systemic inflammation precedes microalbuminuria in diabetes. Kidney Int Rep. 2019;4:1373–86.. doi: 10.1016/j.ekir.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yarwood SJ, Borland G, Sands WA, Palmer TM. Identification of CCAAT/enhancer-binding proteins as exchange protein activated by cAMP-activated transcription factors that mediate the induction of the SOCS-3 gene. J Biol Chem. 2008;283:6843–53. doi: 10.1074/jbc.M710342200. [DOI] [PubMed] [Google Scholar]
- 35.Tuazon Kels MJ, Ng E, Al Rumaih Z. TNF deficiency dysregulates inflammatory cytokine production, leading to lung pathology and death during respiratory poxvirus infection. Proc Natl Acad Sci USA. 2020;117:15935–46. doi: 10.1073/pnas.2004615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- 37.Zhang MH, Feng L, Zhu MM, Gu JF, Jiang J, Cheng XD, et al. The anti-inflammation effect of Moutan Cortex on advanced glycation end products-induced rat mesangial cells dysfunction and High-glucose-fat diet and streptozotocin-induced diabetic nephropathy rats. J Ethnopharmacol. 2014;151:591–600. doi: 10.1016/j.jep.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Usui HK, Shikata K, Sasaki M, Okada S, Matsuda M, Shikata Y, et al. Macrophage scavenger receptor-a-deficient mice are resistant against diabetic nephropathy through amelioration of microinflammation. Diabetes. 2007;56:363–72. doi: 10.2337/db06-0359. [DOI] [PubMed] [Google Scholar]
- 39.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr., Broxmeyer HE, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–61. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eardley KS, Zehnder D, Quinkler M, Lepenies J, Bates RL, Savage CO, et al. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 2006;69:1189–97. doi: 10.1038/sj.ki.5000212. [DOI] [PubMed] [Google Scholar]
- 41.Viau A, Bienaime F, Lukas K, Todkar AP, Knoll M, Yakulo TA, et al. Cilia-localized LKB1 regulates chemokine signaling, macrophage recruitment, and tissue homeostasis in the kidney. EMBO J. 2018;37:e98615. doi: 10.15252/embj.201798615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Z, Guo Z, Dong J, Sheng S, Wang Y, Yu L, et al. miR-374a regulates inflammatory response in diabetic nephropathy by targeting MCP-1 expression. Front Pharmacol. 2018;9:900. doi: 10.3389/fphar.2018.00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klueh U, Czajkowski C, Ludzinska I, Qiao Y, Frailey J, Kreutzer DL. Impact of CCL2 and CCR2 chemokine/receptor deficiencies on macrophage recruitment and continuous glucose monitoring in vivo. Biosens Bioelectron. 2016;86:262–9. doi: 10.1016/j.bios.2016.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feigerlová E, Battaglia-Hsu SF. IL-6 signaling in diabetic nephropathy: from pathophysiology to therapeutic perspectives. Cytokine Growth Factor Rev. 2017;37:57–65. doi: 10.1016/j.cytogfr.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Mezzano S, Aros C, Droguett A, Burgos ME, Ardiles L, Flores C, et al. NF-kappaB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transpl. 2004;19:2505–12. doi: 10.1093/ndt/gfh207. [DOI] [PubMed] [Google Scholar]
- 46.Lu TC, Wang ZH, Feng X, Chuang PY, Fang W, Shen Y, et al. Knockdown of Stat3 activity in vivo prevents diabetic glomerulopathy. Kidney Int. 2009;76:63–71. doi: 10.1038/ki.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Said E, Zaitone SA, Eldosoky M, Elsherbiny NM. Nifuroxazide, a STAT3 inhibitor, mitigates inflammatory burden and protects against diabetes-induced nephropathy in rats. Chem Biol Interact. 2018;281:111–20.. doi: 10.1016/j.cbi.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 48.Huang JS, Chuang LY, Guh JY, Huang YJ, Hsu MS. Antioxidants attenuate high glucose-induced hypertrophic growth in renal tubular epithelial cells. Am J Physiol Ren Physiol. 2007;293:F1072–82. doi: 10.1152/ajprenal.00020.2007. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Shaw S, Amiri F, Eaton DC, Marrero MB. Inhibition of the Jak/STAT signaling pathway prevents the high glucose-induced increase in tgf-beta and fibronectin synthesis in mesangial cells. Diabetes. 2002;51:3505–9. doi: 10.2337/diabetes.51.12.3505. [DOI] [PubMed] [Google Scholar]
- 50.Tan JC, Rabkin R. Suppressors of cytokine signaling in health and disease. Pediatr Nephrol. 2005;20:567–75. doi: 10.1007/s00467-004-1766-8. [DOI] [PubMed] [Google Scholar]
- 51.Sands WA, Woolson HD, Milne GR, Rutherford C, Palmer TM. Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells. Mol Cell Biol. 2006;26:6333–46. doi: 10.1128/MCB.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marrero MB, Banes-Berceli AK, Stern DM, Eaton DC. Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am J Physiol Ren Physiol. 2006;290:F762–8. doi: 10.1152/ajprenal.00181.2005. [DOI] [PubMed] [Google Scholar]
- 53.Hwang M, Go Y, Park JH, Shin SK, Song SE, Oh BC, et al. Epac2a-null mice exhibit obesity-prone nature more susceptible to leptin resistance. Int J Obes (Lond) 2017;41:279–88.. doi: 10.1038/ijo.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chepurny OG, Kelley GG, Dzhura I, Leech CA, Roe MW, Dzhura E, et al. PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM in human islets of Langerhans. Am J Physiol Endocrinol Metab. 2010;298:E622–33. doi: 10.1152/ajpendo.00630.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kai AK, Lam AK, Chen Y, Tai AC, Zhang X, Lai AK, et al. Exchange protein activated by cAMP 1 (Epac1)-deficient mice develop β-cell dysfunction and metabolic syndrome. FASEB J. 2013;27:4122–35. doi: 10.1096/fj.13-230433. [DOI] [PubMed] [Google Scholar]
- 56.Kelley GG, Chepurny OG, Schwede F, Genieser HG, Leech CA, Roe MW, et al. Glucose-dependent potentiation of mouse islet insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM. Islets. 2009;1:260–5. doi: 10.4161/isl.1.3.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Métrich M, Morel E, Berthouze M, Pereira L, Charron P, Gomez AM, et al. Functional characterization of the cAMP-binding proteins Epac in cardiac myocytes. Pharmacol Rep. 2009;61:146–53. doi: 10.1016/s1734-1140(09)70017-9. [DOI] [PubMed] [Google Scholar]
- 58.Herfindal L, Nygaard G, Kopperud R, Krakstad C, Døskeland SO, Selheim F. Off-target effect of the Epac agonist 8-pCPT-2′-O-Me-cAMP on P2Y12 receptors in blood platelets. Biochem Biophys Res Commun. 2013;437:603–8. doi: 10.1016/j.bbrc.2013.07.007. [DOI] [PubMed] [Google Scholar]