Abstract
The NCI-MATCH was designed to characterize the efficacy of targeted therapies in histology-agnostic driver mutation-positive malignancies. Sub-protocols F and G were developed to evaluate the role of crizotinib in rare tumors that harbored either ALK or ROS1 rearrangements. Patients with malignancies that progressed following at least one prior systemic therapy were accrued to the NCI-MATCH for molecular profiling, and those with actionable ALK or ROS1 rearrangements were offered participation in sub-protocols F or G, respectively. There were five patients who enrolled on Arm F (ALK) and four patients on Arm G (ROS1). Few grade 3 or 4 toxicities were noted, including liver test abnormalities, and acute kidney injury. For sub-protocol F (ALK), the response rate was 50% (90% CI 9.8–90.2%) with one complete response among the 4 eligible patients. The median PFS was 3.8 months, and median OS was 4.3 months. For sub-protocol G (ROS1) the response rate was 25% (90% CI 1.3–75.1%). The median PFS was 4.3 months, and median OS 6.2 months. Data from 3 commercial vendors showed that the prevalence of ALK and ROS1 rearrangements in histologies other than non-small cell lung cancer and lymphoma was rare (0.1% and 0.4% respectively). We observed responses to crizotinib which met the primary endpoint for ALK fusions, albeit in a small number of patients. Despite the limited accrual, some of the patients with these oncogenic fusions can respond to crizotinib which may have a therapeutic role in this setting.
Subject terms: Cancer, Cancer
Introduction
The NCI MATCH trial is an expansive National Clinical Trials Network/NCI Community Oncology Research Program effort, developed and implemented with the goal of providing a large-scale platform for the study of targeted agents in molecularly defined malignancies. With the availability of molecular testing and drugs designed to target actionable mutations, this effort has been one of the largest to provide access to treatment based on driver mutation status, rather than histology or primary site of disease. The chimeric proteins that result from gene fusions involving ALK or ROS1 are both therapeutic targets of the tyrosine kinase inhibitor crizotinib. The kinase domains of ALK and ROS1 share significant amino homology within the ATP-binding sites, resulting in high-affinity binding of crizotinib in cell-based assays. The role that ALK plays in normal physiology is poorly understood. In the wild-type state, activation of the ALK protein is thought to be potentially mediated by ligand-induced dimerization. Oncogenic activation of ALK arises from a rearranged protein in which the intact tyrosine kinase domain of ALK is fused to a variety of upstream partners resulting in constitutive activation of the ALK tyrosine kinase. This, in turn, results in activation of downstream signaling involving the MAPK, PI3K, and JAK/STAT pathways, thereby promoting increased cell growth and proliferation1.
In non-small cell lung cancer (NSCLC) EML4-ALK is one of the more common fusion events, but there are other reported variants of ALK fusion proteins that do not involve the EML4 gene such as KIF5B-ALK, STRN-ALK, HIP1-ALK, VCL-ALK, NPM-ALK, and TGF-ALK2–5. ALK fusions have been identified as drivers of oncogenesis in a variety of other malignancies6. ALK-positive anaplastic large cell lymphomas (ALCLs) represent a distinct subset of lymphomas that are associated with better outcomes in comparison to ALK-negative ALCLs. Up to 50% of ALK-positive ALCLs harbor ALK fusions, the most common of which is the t(2;5)(p23;q35) translocation, resulting in the formation of NPM-ALK. In solid tumors, ALK rearrangements including TPM4-ALK and TPM3-ALK have been identified in up to 50-75% of inflammatory myofibroblastic tumors (IMT). These fusions are transforming in various cell lines and animal models. Recently, ALK rearrangements have been found in spitzoid neoplasms, a group of melanocytic tumors including Spitz nevi, spitzoid melanomas, and atypical Spitz tumors7.
ROS1 encodes a transmembrane protein with an extracellular domain that is partially analogous to fibronectin8. Although the function of wild-type ROS1 is poorly understood, it is speculated that ROS1 might translate adhesion events to intracellular signaling because of the structural similarities to cell adhesion molecules. In NSCLC, the most common ROS1 fusion partner is CD74. Less common partners include SDC4, EZR, SLC34A2, TPM3, LIMA1, and MSN. The ROS1 proto-oncogene has been identified to be translocated in NSCLC with a frequency of 1.7% (18/107) to 2.6% (17/656)9,10. While rare, ROS1 rearrangements have also been reported in gastric cancers, glioblastomas, cholangiocarcinomas, ovarian cancers, colorectal cancers, inflammatory myofibroblastic tumors, angiosarcomas, and epithelial hemangioendotheliomas8,11,12.
Crizotinib is a first-in-class ATP-competitive small-molecule inhibitor of ALK, ROS1, and Met/hepatocyte growth factor receptor (HGFR). Crizotinib demonstrated concentration dependent inhibition of ALK kinase activity in biochemical and cell-based assays. In addition, crizotinib demonstrated growth inhibition and increased apoptosis of tumor cell lines with ALK fusion variants (EML4-ALK or NPM-ALK). Mice xenografts with ALK fusion variants also responded to crizotinib treatment in a dose dependent fashion. Based on its significant activity, crizotinib received FDA approval for ALK-positive NSCLC in 2011, for ROS1-positive NSCLC in 2016, and ALK-positive ALCL in 2021. In prior studies, common toxicities related to crizotinib included reversible visual disturbances, gastrointestinal side effects, fatigue, transaminitis and edema.
The NCI-MATCH subprotocols F and G were designed to study the activity of crizotinib in tumors other than NSCLC or ALCL with ALK and ROS1 rearrangements, respectively. Given the common therapeutic option in these subprotocols, the results of both studies were combined for this report.
Results
Patient characteristics
A total of 5 patients enrolled on sub-protocol F (ALK), with the first patient enrolled on November 24, 2015, and the last on April 24, 2019. One patient was ineligible since treatment was started before a 28-day washout following prior treatment, resulting in four analyzable patients (Supplemental Fig. 1). A total of 4 patients enrolled on sub-protocol G (ROS1), with the first patient enrolled on July 7, 2016, and the last on September 13, 2018 (Supplemental Fig. 2). Due to poor accrual, both sub-protocols were closed. Among both sub-protocols, most patients had gastrointestinal malignancies (Table 1). All but one patient had received two or more prior lines of therapy. Previously reported EML4 and GOPC fusion partners were most frequently identified for ALK and ROS1, respectively. A median of four cycles of crizotinib were administered in both sub-protocols.
Table 1.
Patient Characteristics.
| ALK fusion (n = 4) | ROS1 fusion (n = 4) | |
|---|---|---|
| Female | 3 (75%) | 2 (50%) |
| Age: median (range) | 59 (52–69) years | 54 (47–76) years |
| Race: White | 4 (100%) | 4 (100%) |
| Performance Status 0 | 3 (75%) | 2 (50%) |
| 1 | 1 (25%) | 2 (50%) |
| Number of Prior Therapies: 1 | 1 (25%) | 0 (0%) |
| 2 | 2 (50%) | 1 (25%) |
| 3 | 0 (0%) | 1 (25%) |
| >3 | 1 (25%) | 2 (50%) |
| Weight loss previous 6 months: < 5% | 4 (100%) | 4 (100%) |
| Histologies | ||
| Cholangiocarcinoma | 1 | |
| Pancreatic adenocarcinoma | 1 | |
| Colorectal adenocarcinoma | 2 | 1 |
| Ovarian adenocarcinoma | 1 | |
| Carcinoma of unknown primary | 1 | |
| Leiomyosarcoma | 1 | |
| Fusion partners | ||
| EML4 | 3 | |
| ACTG2 | 1 | |
| STRN | 1 | |
| GOPC | 4 | |
Response assessment and survival outcomes
For sub-protocol F (ALK), the response rate was 50% (90% CI 9.8–90.2%; Fig. 1) with one, ongoing complete response; the median PFS was 3.8 months, and the 6-month PFS rate was 25% (90% CI 6.0%-100%; Fig. 2). Despite the very low accrual, the null hypothesis of response rate not exceeding 5% can be rejected at the 1-sided .014 significance level, meeting the primary endpoint. The median overall survival was 4.8 months (Fig. 2; Supplemental Table 3). For sub-protocol G (ROS1), with a 25% responses rate (90% CI 1.3–75.1%; Fig. 1), the p-value for testing the null of 5% was 0.186. The median PFS was 4.3 months, and the 6-month PFS rate was 50% (90% CI 22–100 %; Fig. 2). The median overall survival was 6.2 months (Fig. 2; Supplemental Table 3).
Fig. 1. Waterfall and Swimmer plot of responses and their durations.
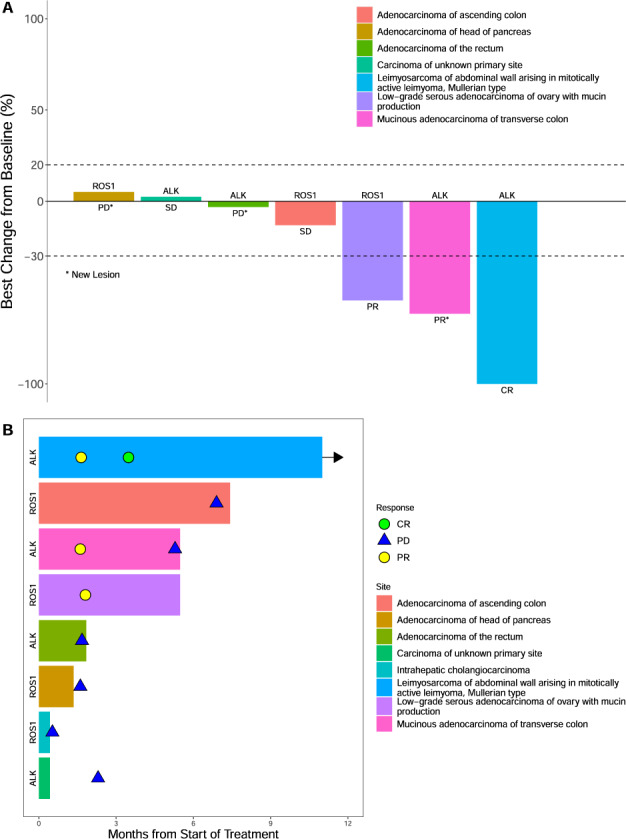
The waterfall plot shows responses for all patients who had response assessment (A n = 7). One patient on subprotocol G (ROS1) is not included as treatment was discontinued during cycle one for toxicity and response was not evaluable. The Swimmer plot shows the duration of responses for all patients (B n = 8). CR complete responses, PD progressive disease, PR partial response.
Fig. 2. Progression-free and overall survival for both sub-protocols.
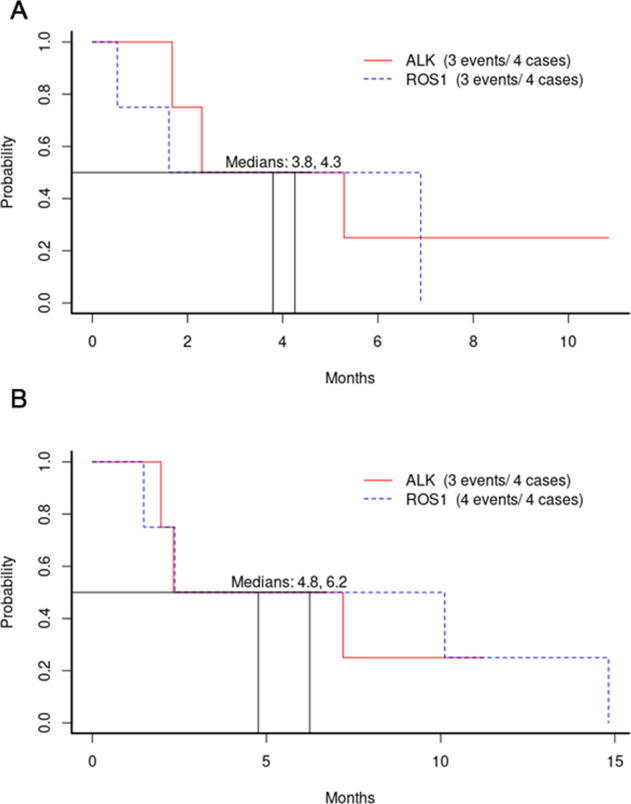
The PFS A and OS B are presented for both ALK (EAY131-F) and ROS1 (EAY131-G) sub-protocols.
Adverse events
There were very few grade 3 or 4 toxicities at least possibly related to treatment; these included liver test abnormalities, abdominal pain, and acute kidney injury. Less severe edema, hypoalbuminemia, and liver test abnormalities were also observed (Supplemental Table 4). There were two deaths on sub-protocol F attributed to disease progression or a cause that was not otherwise specified. There was one death on sub-protocol G attributed to disease progression.
Expanded molecular cohort
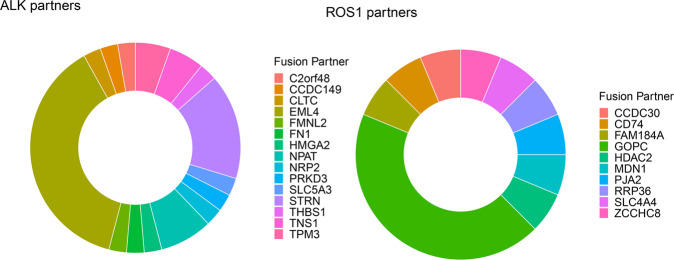
In an expanded molecular cohort of subjects compiled by three commercial vendors, the detection of ALK or ROS1 rearrangements outside of NSCLC or lymphoma was rare (Supplemental Table 5). A review of over 30,000 tumors excluding those with NSCLC or lymphoma from two of these commercial vendors identified ALK rearrangements in 1/1,000 specimens and ROS1 rearrangements in 4/10,000 specimens. One external dataset included clinical characteristics of these cases. Whereas there was a similar proportion of females (n = 18, 49%) and males (n = 19, 51%) with ALK rearrangements, there were more females (n = 10, 63%) than males (n = 6, 37%) with ROS1 rearrangements. Although there were similar median ages for patients with either rearrangement, there were younger patients with ALK rearrangements than ROS1 rearrangements (ALK median 57 years, range 25–83; ROS1 median 60.5 years, range 41–89). ALK rearrangements were most frequently seen in thyroid, colorectal, and soft tissue tumors in this cohort (Supplemental Table 6). ROS1 rearrangements were most frequently seen in glioblastoma multiforme and breast cancer (Supplemental Table 7). The most frequent ALK fusion partners were EML4 (38%) and STRN (16%) and the most frequent ROS1 fusion partner was GOPC (44%) (Fig. 3). Of the evaluable tumors, almost all with ALK or ROS1 fusions had low or intermediate TMB (96%; Supplemental Table 8) and were MSI stable (98%; Supplemental Table 9).
Fig. 3. Fusion partners for ALK and ROS1 in the expanded molecular cohort.
The most common fusion partners are shown for ALK and ROS1 from the expanded molecular cohort.
Discussion
Accrual to sub-protocols F and G was limited, reducing our ability to assess the efficacy of treatment with any precision. However, we can conclude that the true response rate for sub-protocol F exceeds the null hypothesis rate of 5%, at the 1-sided .014 significance level. We observed responses to crizotinib in patients whose tumors harbored ALK or ROS1 rearrangements outside of the FDA-approved indications for this therapy. However, the median overall survivals of 4.8 and 6.2 months suggest that the anti-tumor activity we observed was modest. Many of the reported toxicities were similar to those reported in prior trials with crizotinib. Furthermore, our rates of detection and analysis of external datasets from three commercial vendors suggest that ALK and ROS1 rearrangements are very rare outside of NSCLC.
Globally, there have been additional efforts to target either ALK or ROS1 in rare tumor types. Some of the more successful attempts have been in the pediatric population. For instance, response rates as high as 80–90% with ALK inhibitor therapy have been observed in the treatment of anaplastic large cell lymphomas which harbor ALK translocations13,14. Inflammatory myofibroblastic tumors are another rare malignancy that frequently harbor ALK or ROS1 translocations15–17. In one case, the ALK rearrangement resulted from a complex pattern of chromosomal rearrangements called chromoplexy, and that patient had durable benefit from treatment with the ALK inhibitor ceritinib18. Finally, molecular events involving ALK have also been identified in aggressive thyroid cancers and neuroblastomas19,20. While the role of ALK and ROS1 inhibitors is now established as standard of care among ALK - or ROS1 -positive NSCLC in addition to some of the rare tumors listed above, our data suggest that patients with other tumor types that harbor these mutations may also benefit from targeted therapy. The authors are not aware of other plans to develop crizotinib further for these indications.
There are several potential explanations for the modest impact on response rate and survival noted in both sub-protocols. First, this was a pre-treated population with most patients having received at least 2 lines of prior therapy. As a result, it is likely that these patients had more resistant tumors at the time of treatment. Somatic mutations that confer resistance to crizotinib are now well characterized among series of patients with NSCLC and acquired resistance mutations in ALK include L1196M, G1202R, and S1206Y21. Alternatively, these patients’ tumors may have developed bypass pathway signaling or had concurrent mutations as a result of prior therapy which limited the activity of crizotinib. One patient had a concurrent BRAF fusion, which may have been the actual oncogenic driver. Since the approval of crizotinib, more potent ALK and ROS1 inhibitors have received FDA approval such as alectinib22, brigatinib23, entrectinib24, and lorlatinib25,26. Enrollment to basket trials with some of these agents may have competed with enrollment to NCI-MATCH. At this time, it is not known if one of these newer agents would yield improved efficacy in these other tumor types with ALK or ROS1 fusions. Finally, it has been suggested that sequencing RNA might be more appropriate than DNA for the detection of fusions given the difficulties of covering all of the introns from which rearrangements can arise27. The central NCI-MATCH NGS assay did sequence fusions from RNA input, but the assay was specifically targeted for known fusions. The external laboratories use a variety of platforms but many scan introns in DNA. For that reason, there may have been some cases with novel ALK or ROS1 fusions that were not detected for inclusion in NCI-MATCH.
Our analysis of the expanded molecular cohort identified ALK or ROS1 rearrangements in many types of solid tumors. The frequencies of fusion partners did not necessarily match those reported for non-squamous NSCLC but included many of the known fusion partners. Almost all the tumors in the expanded cohort with ALK or ROS1 rearrangements had low or intermediate TMB and were microsatellite-stable, suggesting that there are few other molecular drivers in these cases.
The NCI-MATCH is ongoing with open sub-protocols. Recently, the experience performing molecular profiling on almost 6,000 patients for NCI-MATCH was reported28. Thirty subprotocols were open at some point during this screening period and eleven of them met their accrual goals of 31 eligible patients. None of the subprotocols that targeted an alteration with a prevalence <1.5% met its accrual goal during this centralized screening phase. These results highlight the difficulties of identifying eligible patients with rare molecular events for clinical trial participation despite the widespread activation of NCI-MATCH and significant engagement with community oncologists. It is possible that just-in-time clinical trial activation mechanisms could have expanded the number of potential sites with eligible patients and improved accrual to subprotocols with rare molecular events. There was a higher rate of molecular alterations and co-occurring mutations in NCI-MATCH than seen in TCGA29, which may have resulted from patient selection strategies and larger sample size for some tumor types like cholangiocarcinoma in NCI-MATCH. Many of the co-occurring mutations were in tumor-suppressor genes that have been implicated in therapeutic resistance to targeted therapies. Given the genomic complexity of many cancers and the resistance that invariably develops with most single agent targeted therapies, the NCI-ComboMATCH (EAY191)30 will test combinations of targeted therapies that are supported by robust in vivo evidence.
In spite of the low incidence of ALK and ROS1 rearrangements among adults with solid tumors other than NSCLC, we feel it is still critical to pursue comprehensive molecular testing for rare tumors and those with limited treatment options. Ongoing evaluation of newer inhibitors is needed in the rare event of an ALK or ROS1 translocation. Given rarity of these events in adult solid tumors, it will require collaboration amongst the larger oncology community to study the efficacy of these agents in this setting.
Methods
Study design and eligibility
The NCI-MATCH trial is an ongoing, nationwide clinical trial with integrated multiple independent single-arm sub-protocols, each addressing an actionable molecular alteration. Each sub-protocol aims to evaluate a single agent or combination treatment for which at least a recommended phase 2 dose has been determined.
Patients with histologically documented solid tumors, lymphomas, or myelomas whose disease had progressed following at least one line of standard systemic therapy or for whom no standard therapy exists were registered on the screening step of the NCI-MATCH protocol to undergo molecular profiling analysis on fresh tumor biopsies. The latter profiling was performed in specific Clinical Laboratory Improvement Amendments (CLIA)-accredited laboratories and consisted of next generation sequencing (NGS) with an investigational targeted gene panel, the Oncomine Ampliseq assay31. This assay was validated to detect specific targeted gene fusions with 97.67% sensitivity and 99.99% specificity. In the second phase of the trial, 30 academic and commercial laboratories that perform NGS testing were reviewed and evaluated for concordance of test results with the central NCI-MATCH laboratory assay. These laboratories were then asked to refer patients with an actionable mutation for enrollment. Patients whose tumors were found to harbor actionable ALK or ROS1 rearrangements (Supplementary Tables 1-2) were offered participation in sub-protocols F or G, respectively. The protocol allowed for subsequent treatment on other subprotocols if relevant molecular profiles were present and eligibility criteria were met. The study was initiated after approval from the Central Institutional Review board and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
Patients with NSCLC were excluded from both sub-protocols, given that the FDA previously approved crizotinib for patients with NSCLC harboring ALK or ROS1 rearrangements. Patients who had already received ALK or ROS1 inhibitors were also excluded. Patients were treated with crizotinib 250 mg twice daily on 28-day cycles. Dose reductions to 200 mg twice daily or 250 mg daily were allowed for treatment-related adverse events. Responses were assessed using revised RECIST (Response Evaluation Criteria in Solid Tumors) version 1.132, Cheson criteria for patients with lymphoma,33 and RANO criteria for patients with glioblastoma multiforme30.
Statistical considerations
The primary objective of these NCI-MATCH sub-protocols was to evaluate the proportion of patients who had objective response, defined as complete or partial response, to a targeted study agent. This proportion, termed the objective response rate and expressed as a percentage, was compared against a null benchmark value of 5%. If the observed objective response rates were ≥ 5/31 (16%), it would then be concluded that the agent is promising and worthy of further investigation. Allowing for a 10% ineligibility rate, 35 patients were to be accrued to each sub-protocol, to obtain 31 eligible patients per sub-protocol. With this design, the power was 91.8% to conclude an agent is promising if its true response rate is 25%, and the type 1 error (one-sided) was 1.8% of its true response rate is 5%. Secondary objectives included the proportion of patients who were progression-free at 6 months (PFS6), progression-free survival, and toxicity assessment.
Expanded molecular cohort
Three NGS vendors provided data on the detection of ALK and ROS1 rearrangements in the tumors of patients excluding NSCLC and lymphoma as part of their commercial testing services in an effort separate from NCI-MATCH. We used these data to see how the frequency of ALK and ROS1 rearrangements in this expanded molecular cohort compared to that detected by NCI-MATCH. Two of these vendors provided the total number of cases tested, and one vendor provided additional data on the clinical characteristics of these tumors, microsatellite instability (MSI) status, and tumor mutation burden (TMB). These cases were not necessarily included in NCI-MATCH. MSI was examined using over 7,000 target microsatellite loci and compared with the reference genome hg19 from the University of California, Santa Cruz (UCSC) Genome Browser database. The status was defined as MSI-high (MSI-H) or MSI-low/microsatellite stable (MSS). The number of microsatellite loci that were altered by somatic insertion or deletion were counted for each sample. Only insertions or deletions that increased or decreased the number of repeats were considered. Genomic variants in the microsatellite loci were detected using the same depth and frequency criteria used for mutation detection. MSI-NGS results were compared with results from over 2,000 matching clinical cases analyzed with traditional PCR-based methods. The threshold to determine MSI by NGS was determined to generate a sensitivity of >95% and specificity of >90%. TMB was measured by counting all nonsynonymous missense mutations found per tumor that had not been described previously as germline alterations [592 genes and 1.4 megabases (MB) sequenced/tumor]. Potential germline mutations were excluded by comparing data against dbSNP 137 full and 1000 Genomes Phase 3. The threshold to define TMB-high (TMB-H) was ≥17 mutations/MB and was established by comparing TMB with MSI by fragment analysis in colorectal cancer cases, based on reports of TMB having high concordance with MSI-H in colorectal cancer. TMB-intermediate was defined as ≥ 7 but <17 mutations/megabase, and TMB-low was defined as ≤6 mutations/megabase. These data were summarized and the donut charts were created with R Studio (version 1.2.5033) and tidyverse packages.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs). This work was supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: [U10CA180820, U10CA180794, UG1CA233329, UG1CA233302, UG1CA233180, UG1CA232760, UG1CA233324, UG1CA233196, UG1CA233341, and UG1CA233290]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. government. Administrative support for submission of the manuscript was provided by Bobbi Ann Jebens and Kenneth Hauer at Mayo Clinic.
Author contributions
The subprotocols were designed by A.S.M., R.M., A.T.S., C.H.L., P.M.F., and A.E.D. The statistical analysis of the clinical trial data was performed by Z.W. The analysis of the data from the commercial vendors was performed by A.S.M. This manuscript was drafted by A.S.M., Z.W., and R.M. All authors reviewed and approved this manuscript.
Data availability
The data generated or analysed from these clinical trials are included in this published article. The expanded molecular datasets generated or analysed for the current study are available from the corresponding author on reasonable request. The deidentified sequencing data from Caris Life Sciences are owned by Caris Life Sciences. Qualified researchers can apply for access to these summarized data by contacting Joanne Xiu, PhD and signing a data usage agreement. Strata Oncology will provide de-identified molecular results upon request for Strata-referred samples included in this analysis. More specifically, they will provide all prioritized variants for the ALK/ROS1 + patients, including for the fusions 5′/3′ partners, junctions and read support, as well as cancer type, age range, etc. Requests should be addresses to Dan Hovelson, PhD. Tempus has provided summary statistics, including the number of screened patients for this cohort and the count of the specific positives for post-publication replication and verification purposes (Supplemental Table 10). The protocols are available as supplementary materials.
Competing interests
Mansfield: Direct research funding: Novartis, and Verily; Honoraria to institution for participation in advisory boards: AbbVie, BeiGene, BMS, Genentech, Inc., Janssen; Travel support: Roche, and non-remunerated member of the Mesothelioma Applied Research Foundation Board of Directors. Forde: Consultant/Advisory boards: Abbvie, AstraZeneca, BMS, Janssen. Research Funding (to institution): AstraZeneca, BMS. Dr. Shaw has served as a compensated consultant or received honoraria from Achilles, Archer, Ariad/Takeda, Bayer, Blueprint Medicines, Chugai, Daiichi-Sankyo, EMD Serono, Foundation Medicine, Guardant, Ignyta, KSQ Therapeutics, Loxo Oncology, Natera, Novartis, Pfizer, Roche-Genentech, Servier, Syros, Taiho Pharmaceutical, and TP Therapeutics; received institutional research funding from Ariad, Ignyta, Novartis, Pfizer, Roche-Genentech, and TP Therapeutics; received travel support from Genentech and Pfizer, and is currently employed by and owns stock in Novartis. Drilon: Honoraria/Advisory Boards: Ignyta/Genentech/Roche, Loxo/Bayer/Lilly, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, Beigene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, MORE Health, Abbvie, 14ner/Elevation Oncology, Remedica Ltd., ArcherDX, Monopteros, Novartis, EMD Serono, Melendi; Research to Institution: Pfizer, Exelixis, GlaxoSmithKlein, Teva, Taiho, PharmaMar; Royalties: Wolters Kluwer; OTHER: Merck, Puma, Merus, Boehringer Ingelheim. Hovelson: Equity holder and employee of Strata Oncology. Tomlins: Equity holder and employee of Strata Oncology. Named as co-inventors on a patent issued to Strata Oncology related to MSI status assessment. Named as co-inventor and included in royalty streams for a patent issued to the University of Michigan regarding ETS fusions in prostate cancer that has been licensed to Hologic/Gen-Probe (sublicensed to Ventana Medical Systems) and LynxDx. Equity holder in Javelin Oncology. Previously served as a consultant to Strata Oncology and has consulted for Astellas/Medivation and Janssen. He has received research (to the University of Michigan) funding from Astellas and has received travel support from the Prostate Cancer Foundation. Hamilton: Consultant/Advisory Boards: Merck, Incyte, Bristol Myers Squibb, GSK, Loxo, Roche, Thermo Fisher Scientific, illmina, HalioDx. Research Funding to Institution: Guardant Health, CME: Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis, Peerview Institute, Paradigm Medical Communications, WebMD, MJH Life Sciences. Arteaga: reported receiving grants from Lilly, Pfizer, and Takeda; serving in an expert advisory role to Novartis, Lilly, Immunomedics, Merck, Daiichi Sankyo, Taiho Oncology, AstraZeneca, and OrigiMed outside the submitted work; holding minor stock options in Y-TRAP and Provista, and serving in the Scientific Advisory Board of the Susan G. Komen Foundation. All remaining authors have declared no conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This author contributed equally: R. Mehra.
Supplementary information
The online version contains supplementary material available at 10.1038/s41698-022-00256-w.
References
- 1.Solomon B, Wilner KD, Shaw AT. Current status of targeted therapy for anaplastic lymphoma kinase-rearranged non-small cell lung cancer. Clin. Pharm. Ther. 2014;95:15–23. doi: 10.1038/clpt.2013.200. [DOI] [PubMed] [Google Scholar]
- 2.Solomon B, Varella-Garcia M, Camidge DR. ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. J. Thorac. Oncol. 2009;4:1450–1454. doi: 10.1097/JTO.0b013e3181c4dedb. [DOI] [PubMed] [Google Scholar]
- 3.Choi YL, et al. Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer Res. 2008;68:4971–4976. doi: 10.1158/0008-5472.CAN-07-6158. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi K, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin. Cancer Res. 2009;15:3143–3149. doi: 10.1158/1078-0432.CCR-08-3248. [DOI] [PubMed] [Google Scholar]
- 5.Bronte G, et al. Farletuzumab for NSCLC: exploiting a well-known metabolic pathway for a new therapeutic strategy. Expert Opin. Investig. Drugs. 2015;24:125–132. doi: 10.1517/13543784.2015.979284. [DOI] [PubMed] [Google Scholar]
- 6.Shaw AT, Hsu PP, Awad MM, Engelman JA. Tyrosine kinase gene rearrangements in epithelial malignancies. Nat. Rev. Cancer. 2013;13:772–787. doi: 10.1038/nrc3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiesner T, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat. Commun. 2014;5:3116. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies KD, Doebele RC. Molecular pathways: ROS1 fusion proteins in cancer. Clin. Cancer Res. 2013;19:4040–4045. doi: 10.1158/1078-0432.CCR-12-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw AT, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuda H, de Figueiredo-Pontes LL, Kobayashi S, Costa DB. Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. J. Thorac. Oncol. 2012;7:1086–1090. doi: 10.1097/JTO.0b013e3182570919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cilloni D, et al. Aberrant activation of ROS1 represents a new molecular defect in chronic myelomonocytic leukemia. Leuk. Res. 2013;37:520–530. doi: 10.1016/j.leukres.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, et al. Identification of ROS1 rearrangement in gastric adenocarcinoma. Cancer. 2013;119:1627–1635. doi: 10.1002/cncr.27967. [DOI] [PubMed] [Google Scholar]
- 13.Mosse YP, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14:472–480. doi: 10.1016/S1470-2045(13)70095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosse YP, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a children’s oncology group study. J. Clin. Oncol. 2017;35:3215–3221. doi: 10.1200/JCO.2017.73.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butrynski JE, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N. Engl. J. Med. 2010;363:1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovly CM, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Disco. 2014;4:889–895. doi: 10.1158/2159-8290.CD-14-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoffski P, et al. Crizotinib in patients with advanced, inoperable inflammatory myofibroblastic tumours with and without anaplastic lymphoma kinase gene alterations (European Organisation for Research and Treatment of Cancer 90101 CREATE): a multicentre, single-drug, prospective, non-randomised phase 2 trial. Lancet Respir. Med. 2018;6:431–441. doi: 10.1016/S2213-2600(18)30116-4. [DOI] [PubMed] [Google Scholar]
- 18.Mansfield AS, et al. Chromoplectic TPM3-ALK rearrangement in a patient with inflammatory myofibroblastic tumor who responded to ceritinib after progression on crizotinib. Ann. Oncol. 2016;27:2111–2117. doi: 10.1093/annonc/mdw405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panebianco F, et al. Characterization of thyroid cancer driven by known and novel ALK fusions. Endocr. Relat. Cancer. 2019;26:803–814. doi: 10.1530/ERC-19-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki T, et al. The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res. 2010;70:10038–10043. doi: 10.1158/0008-5472.CAN-10-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katayama R, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci. Transl. Med. 2012;4:120ra117. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters S, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 23.Camidge DR, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 24.Drilon A, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:261–270. doi: 10.1016/S1470-2045(19)30690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw AT, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18:1590–1599. doi: 10.1016/S1470-2045(17)30680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw AT, et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2019;20:1691–1701. doi: 10.1016/S1470-2045(19)30655-2. [DOI] [PubMed] [Google Scholar]
- 27.Benayed R, et al. High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no mitogenic driver alteration detected by DNA sequencing and low tumor mutation burden. Clin. Cancer Res. 2019;25:4712–4722. doi: 10.1158/1078-0432.CCR-19-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flaherty KT, et al. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: national cancer institute molecular analysis for therapy choice (NCI-MATCH) J. Clin. Oncol. 2020;38:3883–3894. doi: 10.1200/JCO.19.03010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flaherty KT, et al. The molecular analysis for therapy choice (NCI-MATCH) trial: lessons for genomic trial design. J. Natl Cancer Inst. 2020;112:1021–1029. doi: 10.1093/jnci/djz245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen PY, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 31.Lih CJ, et al. Analytical validation of the next-generation sequencing assay for a nationwide signal-finding clinical trial: molecular analysis for therapy choice clinical trial. J. Mol. Diagn. 2017;19:313–327. doi: 10.1016/j.jmoldx.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Cheson BD, et al. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated or analysed from these clinical trials are included in this published article. The expanded molecular datasets generated or analysed for the current study are available from the corresponding author on reasonable request. The deidentified sequencing data from Caris Life Sciences are owned by Caris Life Sciences. Qualified researchers can apply for access to these summarized data by contacting Joanne Xiu, PhD and signing a data usage agreement. Strata Oncology will provide de-identified molecular results upon request for Strata-referred samples included in this analysis. More specifically, they will provide all prioritized variants for the ALK/ROS1 + patients, including for the fusions 5′/3′ partners, junctions and read support, as well as cancer type, age range, etc. Requests should be addresses to Dan Hovelson, PhD. Tempus has provided summary statistics, including the number of screened patients for this cohort and the count of the specific positives for post-publication replication and verification purposes (Supplemental Table 10). The protocols are available as supplementary materials.