Abstract
Background
Little prospective evidence exists about whether a combination of healthy lifestyle factors is related to a considerable reduction of liver cancer risk.
Methods
Based on the prospective China Kadoorie Biobank (CKB) cohort with a total of 492,640 Chinese adults, we examined the associations of five lifestyle factors with risk of liver cancer. Low-risk lifestyle factors were defined as non-smoking, non-drinking, median or higher level of physical activity, a healthy diet, and waist-to-hip ratio (WHR) < 0.90 for men and <0.85 for women.
Results
During a median of 10.12 years of follow-up, 2529 liver cancer events were observed. There was a significant decrease in liver cancer risk with the increasing of the healthy lifestyle index scores (P < 0.001). Participants with a favourable lifestyle (4 or 5 healthy lifestyle factors) had a 43% reduced liver cancer risk compared with those with an unfavourable lifestyle (0 or 1 healthy lifestyle factor) (HR, 0.57 [95% CI, 0.47–0.68]). The cumulative protective effect of a healthy lifestyle on liver cancer appeared to be more dramatic for patients with hepatitis B surface antigen (HBsAg) positive, the individuals at high risk of liver cancer.
Conclusions
Individuals adhering to a favourable lifestyle was associated with a considerable absolute risk reduction of liver cancer.
Subject terms: Cancer epidemiology, Risk factors
Background
Liver cancer is one of the most common cancers and the fourth leading cause of cancer death worldwide [1]. Notably, the incidence of liver cancer in China accounts for >50% of the world’s burden [2]. Hepatitis B virus (HBV), diabetes and unhealthy lifestyles mainly contribute to the heavy burden of liver cancer in Chinese population [3].
A large body of evidence has been established that unhealthy lifestyle factors, such as smoking [4], alcohol consumption [5], diet [6, 7], physical inactivity [8, 9], and central obesity [10], have been linked to an elevated liver cancer risk. Notwithstanding, since many of these lifestyle behaviours often coexist, investigating the combined impact of these lifestyle factors on liver cancer risk is highly relevant. Recently, one nested case-control study has demonstrated that adherence to a healthy lifestyle defined by a combination of above modifiable factors was related to a 45% reduction in liver cancer incidence in European populations [11]. To date, there is still a lack of reliable evidence from prospective cohort studies on the protective effects of a healthy lifestyle on liver cancer. Besides, the existing evidence on the protective effect of lifestyle factors on liver cancer were mostly conducted in developed countries [4, 5, 7–9]. Unlike the European and American population, HBV infection is the most important cause of liver cancer in Chinese [12]. However, little is known whether the protective effects persist in Chinese, a population of high burden of HBV infection.
Here, based on a large cohort of 0.5 million adult Chinese—the China Kadoorie Biobank (CKB), we prospectively examined the joint association of several modifiable lifestyle factors with liver cancer risk, especially for those with HBV infection, the individuals at high risk of liver cancer.
Methods
Study population
Design, survey methods, and population characteristics of the CKB cohort have been previously described [13]. In summary, the CKB cohort was established in ten study areas geographically spread across China during 2004–2008, a total of 512,714 adults aged between 30 and 79 years were eventually enrolled. All of the participants had completed a questionnaire, physical measurements, and a written informed consent form. For each participant, a 10-ml blood sample was collected into one EDTA vacutainer (BD Hemogard™, USA). The study protocol was approved by the ethics review committee of the Chinese Centers for Disease Control and Prevention (Beijing, China) and the Oxford Tropical Research Ethics Committee, University of Oxford (Oxford, United Kingdom).
We excluded participants who had missing data or unclear hepatitis B virus surface antigen (HBsAg) test results (n = 11,732), participants with prior medical histories of cancer (n = 2578), cirrhosis or hepatitis (n = 6193), who had missing data for body mass index (BMI; n = 2). A total of 492,460 participants were included in the final analysis.
Assessment of lifestyle factors
We assessed a range of lifestyle factors in the baseline questionnaire. Information on smoking behaviour was collected, i.e., frequency, type, amount of tobacco smoked per day for ever smokers, years since quitting and the reason for quitting for former smokers. Questions about alcohol drinking behaviour included frequency, type, volume of alcohol drunk on a typical drinking day in the past 12 months and past drinking habits [14]. Participants were also asked about the common type and duration of activities in occupational, commuting, domestic, and leisure-time related domains over the past year. Based on multiplying metabolic equivalent of task (MET) values by hours spent on that activity per day, task-specific physical activity was calculated. We then summed the MET-hours for all activities to get the daily level of physical activity. Dietary habits of 12 conventional food groups in the past year were assessed by using a short qualitative food frequency questionnaire. The reproducibility of the assessment has been validated in previous studies [15–17]. Waist-to-hip ratio (WHR) was the ratio of waist circumference to hip circumference.
Definition of low-risk lifestyle
Five lifestyle factors were combined to define a low-risk lifestyle, i.e., tobacco smoking, alcohol consumption, physical activity, diet, and WHR, based on prior knowledge [18, 19]. The low-risk group was defined for the following low-risk lifestyle behaviours (Supplementary Table 1): non-smoking (never/occasional smoker, +1 score); non-drinking (abstainers or occasional drinkers, +1 score); being physically active (participants engaged in a sex-specific upper quarter of the physical activity level, +1 score); a healthy diet (participants consumed vegetables every day and fruits ≥4 days per week, +1 score); having a moderate WHR (WHR < 0.90 in men and <0.85 in women, +1 score) [20]. After dichotomising, points for the above 5 lifestyle factors were summed to obtain a healthy lifestyle score, which ranged from 0 (least healthy) to 5 (most healthy).
Assessment of covariates
Covariate information was also acquired by baseline questionnaire, i.e., sociodemographic characteristics, and personal and family medical history. Participant who reported having at least one first-degree relative (parental or siblings) with a particular disease was considered as having a family history of that disease.
Blood pressure was metered at least twice using a UA-779 digital monitor by trained staff, with the mean of two measurements used for analyses. Prevalent hypertension was defined as a systolic blood pressure (SBP) of ≥140 mmHg, or a diastolic blood pressure (DBP) of ≥90 mmHg, or self-reported diagnosis of hypertension, or self-reported taking antihypertensive medication at baseline. Weight and height were measured by calibrated instruments. Body mass index (BMI) was the ratio of weight in kilograms divided by the square of height in metres.
Random plasma glucose (RPG) was measured on-site using the SureStep Plus System (Johnson & Johnson, California, USA). For the participants measured with an abnormal RPG level (≥7.8 and <11.1 mmol/L), a fasting plasma glucose (FPG) test was subsequently executed in the next day. Previously diagnosed diabetes was defined by the question “Has a doctor ever told you that you had diabetes?”. Among positive respondents, additional information about age at diagnosis and current use of certain medications for the treatment of diabetes (e.g., insulin and metformin) and cardiovascular diseases (e.g., aspirin, lipid-lowering, and blood pressure-lowering agents) was collected. Among those without previously diagnosed diabetes, screen-detected diabetes was defined as (1) RPG ≥ 7.0 mmol/L if the time since last eating was ≥8 h; (2) RPG ≥ 11.1 mmol/L if the time since last eating was <8 hours; or (3) FPG ≥ 7.0 mmol/L on subsequent testing [21].
Baseline serum hepatitis B surface antigen (HBsAg) was tested on-site using rapid test strips (ACON Laboratories, California, USA). A participant was identified as hepatitis B virus (HBV) carrier if serum HBsAg was tested positive at baseline.
Ascertainment of outcomes
Incident outcome cases since the participants’ enrollment into the cohort were identified by means of linkage with local disease and death registries, the national health insurance system, and by active follow-up [13]. Nearly all of participants were covered by the health insurance system, which recorded details of all episodes of hospitalisation and coded examination and treatment procedures. The 10th revision of the International Classification of Diseases (ICD-10) was used to code the incident events by trained staff “blinded” to baseline information. In this study, we included liver cancer cases coded as C22, including hepatocellular carcinoma (HCC) [C22.0], intrahepatic cholangiocarcinoma (ICC) [C22.1], Hepatoblastoma [C22.2], Angiosarcoma of liver [C22.3], other sarcomas of liver [C22.4], other specified carcinomas of liver [C22.7].
Statistical analysis
Participants in the CKB cohort study were followed up from the date of baseline (2004–2008) attendance until the date of first diagnosis of a liver cancer, death, or January 1, 2017, whichever occurred first. Baseline characteristics of the analytic sample were summarised across liver cancer status as percentage for categorical variables. Cox proportional hazard regression models were used to examine the association of lifestyle categories with time to incident liver cancer and to estimate hazard ratio (HR) and 95% confidence interval (95% CI). The models were adjusted for age at baseline (years), sex (male or female), residential area (urban or rural), education level (primary school and below, or middle school and higher), HBsAg status (seropositive or seronegative), BMI (<18.5, 18.5 to <25.0, 25.0 to <30.0, and ≥30.0 kg/m2), diabetes (yes or no), and hypertension (yes or no) at baseline. The healthy lifestyle scores ranged from 0 to 5, with higher scores indicating higher adherence to a healthy lifestyle, and were subsequently categorised as favourable (4 or 5 healthy lifestyle factors), intermediate (2 or 3 healthy lifestyle factors), and unfavourable (0 or 1 healthy lifestyle factors) lifestyles. Data from the CKB cohort were analysed in Poisson regression models to estimate age-, sex-, and residential area-adjusted incidence rates of first liver cancer events per 100,000 person-years. Absolute risk was calculated as the percentage of incident liver cancer cases occurring in a given group. We calculated the numbers needed to adhere to a favourable lifestyle to prevent one liver cancer by extrapolating the differences of 10-year event rates for given groups. We conducted several sensitivity analyses to examine the robustness of our results: (1) A weighted standardised healthy lifestyle score was then derived based on β coefficients of each lifestyle factor in the Cox proportional hazards regression model with all 5 lifestyle factors and adjustment for age at baseline, sex, residential area, education level, HBsAg status, BMI, diabetes, and hypertension at baseline (Supplementary Table 1). The original binary lifestyle variables were multiplied by the β coefficients, summed. We categorised the weighted lifestyle score to three levels (favourable, intermediate, and unfavourable), where the distribution of the categories is similar with that of the three categories of the unweighted healthy lifestyle score. (2) We excluded participants who were diagnosed with liver cancer during the first 1/2/3 year of follow-up. A two-sided P value of <0.05 was considered to be statistically significant. All the analyses were performed with the use of R software, version 3.5.0 (R Project for Statistical Computing).
Results
Complete data for the present analysis were available for 492,640 participants in the CKB Study. Table 1 shows the baseline characteristics of the study population. During follow-up, 2529 liver cancer events were observed in the CKB cohort (median follow-up, 10.12 years). All of the 5 lifestyle factors were associated with the risks of liver cancer (Table 2). Multivariable-adjusted analysis showed that current or former smoking, current or former alcohol consumption, and central adiposity were associated with increased risk of liver cancer; high physical activity and a diet rich in vegetables and fruits were associated with a reduced risk of liver cancer.
Table 1.
Distribution of demographic characteristics at baseline.
| Characteristics | Total (N = 492 640) | Without liver cancer (N = 490,111) | With liver cancer (N = 2529) | Incidence rate (per 100,000 PYs) |
|---|---|---|---|---|
| Gender | ||||
| Female | 291,603 (59.19) | 290,665 (59.31) | 938 (37.09) | 32.11 |
| Male | 201,037 (40.81) | 199,446 (40.69) | 1591 (62.91) | 81.38 |
| Age at baseline, years | ||||
| <50 | 221,504 (44.96) | 220,992 (45.09) | 512 (20.25) | 22.65 |
| 50–59 | 151,365 (30.73) | 150,534 (30.71) | 831 (32.86) | 55.04 |
| 60–69 | 88,072 (17.88) | 87,245 (17.80) | 827 (32.70) | 99.25 |
| 70– | 31,699 (6.43) | 31,340 (6.39) | 359 (14.20) | 131.56 |
| Residential area | ||||
| Rural | 273,200 (55.46) | 271,769 (55.45) | 1431 (56.58) | 52.67 |
| Urban | 219,440 (44.54) | 218,342 (44.55) | 1098 (43.42) | 50.85 |
| Highest education | ||||
| Primary school and lower | 248,720 (50.49) | 247,168 (50.43) | 1552 (61.37) | 63.58 |
| Middle school and higher | 243,920 (49.51) | 242,943 (49.57) | 977 (38.63) | 40.12 |
| HBsAg test | ||||
| Seronegative | 478,917 (97.21) | 476,945 (97.31) | 1972 (77.98) | 41.58 |
| Seropositive | 13,723 (2.79) | 13,166 (2.69) | 557 (22.02) | 417.86 |
| Diabetes at baseline | ||||
| No | 463,367 (94.06) | 461,091 (94.08) | 2276 (90.00) | 49.44 |
| Yes | 29,273 (5.94) | 29,020 (5.92) | 253 (10.00) | 92.75 |
| Hypertension at baseline | ||||
| No | 318,418 (64.64) | 317,041 (64.69) | 1377 (54.45) | 43.17 |
| Yes | 174,222 (35.36) | 173,070 (35.31) | 1152 (45.55) | 68.32 |
| BMI (kg/m2) | ||||
| <18.5 | 21,197 (4.3) | 21,037 (4.29) | 160 (6.33) | 80.91 |
| 18.5–24.9 | 308,359 (62.59) | 306,740 (62.59) | 1619 (64.02) | 52.98 |
| 25–29.9 | 142,799 (28.99) | 142,148 (29.00) | 651 (25.74) | 45.80 |
| 30– | 20,285 (4.12) | 20,186 (4.12) | 99 (3.91) | 49.20 |
| WHR | ||||
| Men <0.90, women <0.85 | 204,730 (41.56) | 203,738 (41.57) | 992 (39.22) | 48.57 |
| Men 0.90–0.94, women 0.85–0.89 | 138,628 (28.14) | 137,918 (28.14) | 710 (28.07) | 51.72 |
| Men 0.95-, women 0.90 - | 149,282 (30.30) | 148,455 (30.29) | 827 (32.70) | 56.62 |
| Tobacco smoking | ||||
| Never | 334,071 (67.81) | 332,866 (67.92) | 1205 (47.65) | 36.10 |
| Former | 28,690 (5.82) | 28,415 (5.80) | 275 (10.87) | 101.38 |
| Current | 129,879 (26.36) | 128,830 (26.29) | 1049 (41.48) | 82.79 |
| Alcohol consumption | ||||
| Never | 400,367 (81.27) | 398,621 (81.33) | 1746 (69.04) | 43.92 |
| Former | 19,402 (3.94) | 19,186 (3.91) | 216 (8.54) | 118.73 |
| Current | 72,871 (14.79) | 72,304 (14.75) | 567 (22.42) | 78.85 |
| Physical activity (MET-hours/day) | ||||
| Q1 (Men < 9.6, Women <10.7) | 122,638 (24.89) | 121,748 (24.84) | 890 (35.19) | 76.08 |
| Q2 (Men 9.6–18.7, Women 10.7–16.8) | 122,969 (24.96) | 122,338 (24.96) | 631 (24.95) | 51.77 |
| Q3 (Men 18.7–32.4, Women 16.8–28.2) | 124,187 (25.21) | 123,605 (25.22) | 582 (23.01) | 46.76 |
| Q4 (Men 32.4–, Women 28.2–) | 122,846 (24.94) | 122,420 (24.98) | 426 (16.84) | 34.28 |
| Dietary pattern | ||||
| Daily vegetables and fruits ≥4 days/week | 135,614 (27.53) | 135,036 (27.55) | 578 (22.85) | 42.67 |
| Daily vegetables or fruits ≥4 days/week | 333,980 (67.79) | 332,156 (67.77) | 1824 (72.12) | 55.27 |
| Other | 23,046 (4.68) | 22,919 (4.68) | 127 (5.02) | 57.31 |
Table 2.
Multivariable-adjusted HRs (95% CIs) for incident liver cancer by lifestyle factors.
| HR(95% CI)a | P valuea | HR(95% CI)b | P valueb | HR(95% CI)c | P valuec | |
|---|---|---|---|---|---|---|
| Tobacco smoking | ||||||
| Never | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Former | 2.82 (2.47–3.21) | <0.001 | 1.27 (1.09–1.48) | 0.002 | 1.21 (1.04–1.41) | 0.015 |
| Current | 2.30 (2.12–2.50) | <0.001 | 1.44 (1.28–1.61) | <0.001 | 1.38 (1.23–1.55) | <0.001 |
| Alcohol consumption | ||||||
| Never | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Former | 2.72 (2.36–3.13) | <0.001 | 1.40 (1.21–1.63) | <0.001 | 1.31 (1.12–1.52) | <0.001 |
| Current | 1.80 (1.63–1.98) | <0.001 | 1.23 (1.10–1.36) | <0.001 | 1.16 (1.04–1.29) | 0.007 |
| Physical activity (MET-hours/day) | ||||||
| Q1 (Men < 9.6, Women <10.7) | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Q2 (Men 9.6–18.7, Women 10.7–16.8) | 0.68 (0.61–0.75) | <0.001 | 0.91 (0.82–1.01) | 0.067 | 0.91 (0.82–1.02) | 0.094 |
| Q3 (Men 18.7–32.4, Women 16.8–28.2) | 0.61 (0.55–0.68) | <0.001 | 1.00 (0.90–1.12) | 0.970 | 1.01 (0.90–1.12) | 0.930 |
| Q4 (Men 32.4–, Women 28.2–) | 0.45 (0.40–0.50) | <0.001 | 0.85 (0.75–0.96) | 0.012 | 0.82 (0.72–0.94) | 0.003 |
| Dietary pattern | ||||||
| Daily vegetables and fruits ≥4 days/week | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Daily vegetables or fruits ≥4 days/week | 1.30 (1.18–1.42) | <0.001 | 1.13 (1.02–1.24) | 0.021 | 1.01 (0.91–1.12) | 0.829 |
| Other | 1.35 (1.11–1.64) | 0.002 | 1.20 (0.99–1.46) | 0.069 | 1.14 (0.93–1.39) | 0.198 |
| WHR | ||||||
| Men <0.90, women < 0.85 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Men 0.90–0.94, women 0.85–0.89 | 1.07 (0.97–1.17) | 0.195 | 1.05 (0.95–1.15) | 0.338 | 1.11 (1.00–1.22) | 0.047 |
| Men 0.95–, women 0.90– | 1.17 (1.07–1.28) | <0.001 | 1.09 (0.99–1.19) | 0.084 | 1.16 (1.04–1.29) | 0.007 |
aUnadjusted.
bAnalyses were adjusted for age at baseline, sex, residential area.
cAnalyses were adjusted for age at baseline, sex, residential area, education level, HBV status, diabetes, hypertension at baseline, and other lifestyle factors.
When the 5 lifestyle factors were dichotomised, the low-risk factors were independently associated with a lower risk of liver cancer, except for the association of fresh vegetables and fruits consumption with liver cancer (Supplementary Table 2). Supplementary Table 3 shows the risk of incident liver cancer for combined lifestyle profiles. There was a significant decrease in liver cancer risk as the number of healthy lifestyle factors adopted increased (P < 0.001). The lowest risk of incident liver cancer was observed in participants with a health lifestyle score of 5: HR 0.39, 95% CI 0.22–0.69. We conducted analyses according to different combinations of one, two, three and four healthy lifestyle factors prevalent in at least 5% of participants compared to zero factor (Supplementary Table 4). Although none of the observed associations were as protective as the combination of five factors (Supplementary Table 3), the risk of liver cancer was comparatively low for some combinations. We found that the two factors combination of non-smoking and non-drinking was associated with a lower risk of liver cancer (HR, 0.63 [95% CI, 0.52-0.75]). The protective effect of a non-smoking and non-drinking lifestyle on liver cancer risk was similar to the effect of a combination of three healthy factors (non-smoking and non-drinking and [being physically active/a healthy diet/a moderate WHR]). Among the four factor combinations, the combination of non-smoking, non-drinking, physical activity and a moderate WHR, was associated with a lower risk of liver cancer (HR, 0.44 [95% CI, 0.32–0.61]) and the combination of non-smoking, non-drinking, a healthy diet and a moderate WHR was similarly protective (HR, 0.48 [95% CI, 0.36–0.63]).
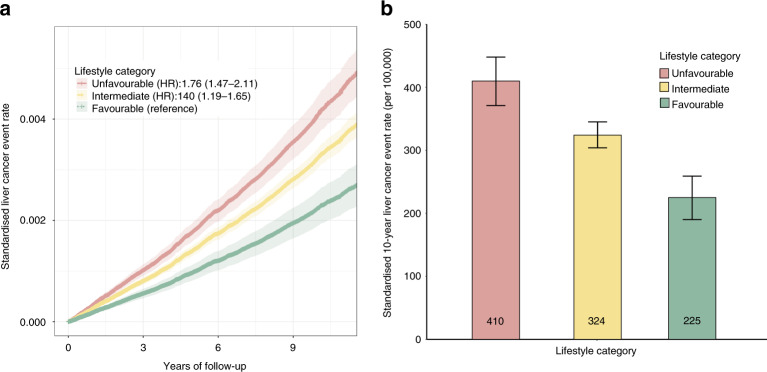
Participants were subsequently divided into three categories: favourable (4 or 5 healthy lifestyle factors), intermediate (2 or 3 healthy lifestyle factors), or unfavourable (0 or 1 healthy lifestyle factor), corresponding to a proportion of 16.67%, 64.91% or 18.42%, respectively (Table 3). Similarly, a significant gradient for liver cancer risk was observed across lifestyle categories (Fig. 1). The relative risk of incident liver cancer cases was 0.76-fold or 0.40-fold higher among participants with unfavourable lifestyle or intermediate lifestyle than among those with a favourable lifestyle (HR, 1.76 [95%CI, 1.47–2.11]; HR, 1.40 [95%CI, 1.19–1.65]; Fig. 1a). Further analysis confirmed that the benefit of a favourable lifestyle was presented for relative risk reduction of liver cancer (Fig. 1b). Participants with a favourable lifestyle (HR, 0.57 [95% CI, 0.47–0.68]) had a 43% reduced liver cancer risk compared with those with an unfavourable lifestyle (Table 3). Correspondingly, the number of participants needed to adhering to a favourable lifestyle to prevent one incident liver cancer case in 10 years was 541.
Table 3.
Multivariable-adjusted HRs (95% CIs) for incident liver cancer by lifestyle categories.
| Unfavourable lifestyle | Intermediate lifestyle | Favourable lifestyle | |
|---|---|---|---|
| No. of participants | 90 739 (18.42%) | 319 775 (64.91%) | 82,126 (16.67%) |
| No. of cases/PY | 813/873,068 | 1546/3,166,651 | 170/836,315 |
| HR (95% CI)a | 1 [Reference] | 0.80 (0.73–0.88) | 0.57 (0.47–0.68) |
| P valuea | <0.001 | <0.001 | |
| P value for trenda | <0.001 | ||
| Absolute risk, % (95% CI) | 0.90 (0.83–0.96) | 0.48 (0.46–0.51) | 0.21 (0.18–0.24) |
| Incidence rate per 100,000 PYs (95% CI)b | 48.57 (44.41–53.11) | 39.71 (37.53–42.03) | 27.82 (23.92–32.36) |
| Numbers needed -10 yearsc | 1174 | 541 | |
Lifestyle categories: Favourable lifestyle (4 or 5 healthy lifestyle factors), intermediate lifestyle (2 or 3 healthy lifestyle factors), and unfavourable lifestyle (0 or 1 healthy lifestyle factors) lifestyle.
aAdjusted for age at baseline, sex, residential area, education level, HBV status, BMI, diabetes, and hypertension at baseline.
bIncidence rate for liver cancer are adjusted for age at baseline, gender, and residential area.
cThe number needed to adhere to a healthy lifestyle to prevent one liver cancer case in 10 years.
Fig. 1. The risk of liver cancer across lifestyle categories.
a Standardised liver cancer event rates in groups of favourable (4 or 5 healthy lifestyle factors), intermediate (2 or 3 healthy lifestyle factors), and unfavourable (0 or 1 healthy lifestyle factor) lifestyle. HRs and 95% CIs were estimated using Cox proportional-hazard models with adjustment for age at baseline, sex, residential area, education level, HBV status, BMI, diabetes, and hypertension at baseline. Shaded areas are 95% CIs. b 10-Year liver cancer event rates, according to lifestyle category. Standardisation was performed to cohort-specific population averages for each covariate. The bars represent 95% CI.
To test the robustness of the findings, we examined the risk of incident liver cancer by a weighted lifestyle score. To minimise potential bias due to subclinical conditions, we performed analyses by further excluding participants whose liver cancer outcome occurred in the 1st year/2nd years/3rd years of follow-up. These sensitivity analyses did not substantially alter the risk estimates (Supplementary Table 5). Expectantly, the protective effect of healthy lifestyle was also observed when participants were grouped by baseline characteristic (Supplementary Table 6). The liver cancer outcomes were then grouped by histological subtypes. We observed the benefit of adhering to a favourable lifestyle for the risk of hepatocellular carcinoma (HR, 0.55 [95% CI, 0.45–0.66], Supplementary Table 7).
A stratification analysis was further performed to investigate the association between lifestyle categories and risk of liver cancer among groups with different HBV status (Table 4). Participants with HBV seronegative and an favourable lifestyle had a 95% reduced liver cancer risk than those participants with seropositive and a unfavourable lifestyle (HR, 0.05 [95% CI, 0.04–0.06]). Overall, compared with HBV seronegative participants, fewer number of HBV seropositive participants needed to adhere to a favourable lifestyle to prevent one incident liver cancer case in 10 years (71 vs. 556) (Supplementary Table 8).
Table 4.
Multivariable-adjusted HRs (95% CIs) for incident liver cancer by lifestyle categories according to HBsAg states.
| HBsAg status | Lifestyle catrgory | No. of participants | No. of events | PY | Incidence Rate per 100,000 PYs | HR(95%CI)a | P valuea | HR(95%CI)a | P valuea |
|---|---|---|---|---|---|---|---|---|---|
| HBsAg seropositive | Unfavourable | 2538 | 179 | 23,760 | 753.36 | 1 [Reference] | 1 [Reference] | ||
| Intermediate | 8735 | 338 | 84,843 | 398.38 | 0.91 (0.75–1.11) | 0.344 | 0.77 (0.64–0.92) | 0.005 | |
| Favourable | 2450 | 40 | 24,696 | 161.97 | 0.62 (0.43–0.90) | 0.012 | 0.51 (0.36–0.73) | <0.001 | |
| HBsAg seronegative | Unfavourable | 88,201 | 634 | 849,308 | 74.65 | 1 [Reference] | 0.08 (0.07–0.10) | <0.001 | |
| Intermediate | 311,040 | 1208 | 3081 808 | 39.20 | 0.76 (0.69–0.85) | <0.001 | 0.07 (0.06–0.08) | <0.001 | |
| Favourable | 79,676 | 130 | 811,620 | 16.02 | 0.56 (0.45–0.68) | <0.001 | 0.05 (0.04–0.06) | <0.001 |
Lifestyle categories: Favourable Lifestyle (4 or 5 healthy lifestyle factors), intermediate lifestyle (2 or 3 healthy lifestyle factors), and unfavourable lifestyle (0 or 1 healthy lifestyle factors) lifestyle.
aAdjusted for age at baseline, sex, residential area, education level, BMI, diabetes, and hypertension at baseline.
Discussion
In the present study, using a large prospective cohort of 0.5 million Chinese people, we found that adhering to a healthy lifestyle, i.e. abstaining from smoking and drinking, being physically active, eating a diet rich in vegetables and fruits, maintaining lower WHR were associated with a significantly reduced risk of liver cancer. Notably, regardless of HBV status, adherence to a healthy lifestyle was associated with a significantly decreased risk of the burden of liver cancer.
A number of prospective cohorts have been conducted to identify the association between single lifestyle factor with risk of liver cancer, such as non-smoking [22], increased consumption of fruits [23], having regular physical exercise [24]. To date, only one nested case-control study based on the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort has evaluated the combined impact of these lifestyle factors on liver cancer risk [11]. This nested case-control study conduceted a healthy lifestyle index (HLI) using 7 variables (i.e. diet, BMI, physical activity, lifetime alcohol, smoking, diabetes, and hepatitis), and estimated that the HLI were inversely associated with liver cancer risk, with the ORs for a 1-SD increase in scores equal to 0.53 (95% CI: 0.38, 0.74), which conclusions were consistent with our study. However, a limitation of the study is its small sample size (147 incident liver cancer cases and 147 matched controls). Besides, findings from European populations may not be generalisable to other populations. Herein, to the best of our knowledge, the present study was the first that comprehensively assessed the relationship between a combination of multiple lifestyle factors and risk of liver cancer and its subtypes in Chinese population.
HBV infection is the leading cause of liver cancer onset in China [12]. In the past three decades, the HBsAg prevalence rapidly declined among young people in China due to the implementation of maternal-to-infant blocks and high vaccination coverage. However, there are still 20–30 million Chinese people with chronic HBV infection, leading to a serious burden of liver cancer [25]. Antiviral therapy is the main measures taken for chronic HBV patients to prevent liver cancer onset [26]. From a novel perspective, our results showed that HBsAg carriers would obtain greater profits for preventing liver cancer when adhering to a healthy lifestyle. Our study supported public efforts that emphasise a healthy lifestyle for everyone, but the absolute risk reduction that was associated with adherence to a healthy lifestyle was greater in HBsAg carriers, who were at high risk for liver cancer in China.
It is worth noting that the central adiposity indicator—WHR, rather than the general adiposity indicator (e.g. BMI), was combined in the lifestyle index in our population. As previous prospective studies in western populations have shown that BMI is positively associated with liver cancer risk. However, the majority of prospective studies conducted in East Asia have reported no association, while in our previous association study using the CKB database, BMI has an inverse association with liver cancer risk [27]. It is possible that individuals with undetected cancer at study baseline may lose weight, thereby resulting in a false negative association of BMI with liver cancer. Besides, previous studies stated the positive associations of central adiposity with risk of liver cancer [10]. The current study showed that maintaining lower WHR had an important protective effect on liver cancer. Therefore, the indicator of WHR, instead of BMI, was combined in the lifestyle index in our study. For fresh vegetables and fruits consumption [18], epidemiological studies have suggested that increased consumption of fruits decreases the risk of liver cancer [23] and low vegetable intake was significantly associated with an increased risk of liver cancer [28]. And the important position of fresh vegetables and fruits in traditional Chinese dietary culture may lead to the insignificant regional differences in the intake of fresh vegetables and fruits in this study. In our analysis, fruit and vegetable consumption did show a protective trend against liver cancer risk. And the variables included in this study were not solely based on statistical significance. Therefore, we chose fresh vegetables and fruits as representative variables for a healthy diet.
Our study for the first time provided prospective evidence for the joint beneficial effects of multiple lifestyle factors on prevention of liver cancer in the nationally representative general population of Chinese. Sensitivity analysis was carefully executed to minimise the confounding bias by excluding participants who were diagnosed with liver cancer during the first 1/2/3 year of follow-up, or reconduceted a weighted standardised healthy lifestyle score based on β coefficients of each lifestyle factor in the Cox proportional hazards regression model; and the results remained virtually unchanged. Anyhow, our study has several limitations. First, information on antiviral treatment for HBV at baseline or during follow-up period was lacking in the CKB cohort, which precluded us from analysing the synergistic effect of lifestyle factors and antiviral treatment on liver cancer risk for CHB patients. And we cannot distinguish between HBV carrier or active infection. Second, the status of Hepatitis C virus (HCV) infection at baseline was unavailable, which prevented us from evaluating the effects of lifestyle factors on liver cancer risk in the HCV related subgroups. Third, the present study only used information on lifestyle factors collected at baseline, and could not necessarily account for the impact of long-term lifestyle patterns. Finally, lifestyle factors were self-reported, which might result in misclassification of lifestyle categories.
Conclusions
In summary, this thus far the largest prospective cohort study of Chinese adults confirmed that a substantial reduction in the burden of liver cancer could be achieved by adherence to a healthy lifestyle pattern. In light of the heavy burden of HBV infection and constrained medical resources in China, population-wide lifestyle interventions could be a cost-effective way to respond to the challenges posed by liver cancer.
Supplementary information
Acknowledgements
The most acknowledgement is to the participants in the study and the members of the survey teams in each of the 10 regional centres, as well as to the project development and management teams based at Beijing, Oxford and the 10 regional centres.
Author contributions
ZH, LL and HS contributed to the study design and supervised the whole project. CS, Chengxiao Yu contributed to the data interpretation, data analysis, and writing of the manuscript. CS and JL contributed to the study design, data collection, and data interpretation of the present analysis. MZ, Canqing Yu, YG, LY, YC, ZC, TJ, HM and GJ contributed to the study design and sample collection. All of the authors reviewed or revised the manuscript.
Funding
This study was supported by National Natural Science Foundation of China (81903382, 91846303), Natural Science Foundation of Jiangsu Province (BK20190652), grants from by the National Key R&D Program of China (2016YFC0900500, 2016YFC0900501, 2016YFC0900504), China Postdoctoral Science Foundation (General Program, 2019M651900). The CKB baseline survey and the first re-survey were supported by a grant from the Kadoorie Charitable Foundation in Hong Kong. The long-term follow-up is supported by grants from the UK Wellcome Trust (212946/Z/18/Z, 202922/Z/16/Z, 104085/Z/14/Z, 088158/Z/09/Z), and Chinese Ministry of Science and Technology (2011BAI09B01).
Data availability
Details of the CKB data are available upon reasonable request (http://www.ckbiobank.org/site/Data+Access).
Ethics approval and consent to participate
Studies based on CKB was performed in accordance with the Declaration of Helsinki. This study was approved by the ethical review committee of the Chinese Center for Disease Control and Prevention (Beijing, China) and the Oxford Tropical Research Ethics Committee, University of Oxford (UK). Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ci Song, Jun Lv, Chengxiao Yu.
Contributor Information
Zhibin Hu, Email: zhibin_hu@njmu.edu.cn.
Liming Li, Email: lmleeph@vip.163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01645-x.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Xia C, Zheng R, Zhou M, Lin C, Zeng H, et al. Disparities by province, age, and sex in site-specific cancer burden attributable to 23 potentially modifiable risk factors in China: a comparative risk assessment. Lancet Glob Health. 2019;7:e257–e269. doi: 10.1016/S2214-109X(18)30488-1. [DOI] [PubMed] [Google Scholar]
- 4.Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218–24. doi: 10.1016/j.jhep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Vandenbulcke H, Moreno C, Colle I, Knebel JF, Francque S, Serste T, et al. Alcohol intake increases the risk of HCC in hepatitis C virus-related compensated cirrhosis: a prospective study. J Hepatol. 2016;65:543–51. doi: 10.1016/j.jhep.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Zhang D, Feng N, Chen G, Liu J, Chen G, et al. Increased intake of vegetables, but not fruit, reduces risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology. 2014;147:1031–42. doi: 10.1053/j.gastro.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Ma Y, Yang W, Simon TG, Smith-Warner SA, Fung TT, Sui J, et al. Dietary patterns and risk of hepatocellular carcinoma among U.S. men and women. Hepatology. 2019;70:577–86. doi: 10.1002/hep.30362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumeister SE, Schlesinger S, Aleksandrova K, Jochem C, Jenab M, Gunter MJ, et al. Association between physical activity and risk of hepatobiliary cancers: a multinational cohort study. J Hepatol. 2019;70:885–92. doi: 10.1016/j.jhep.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Berzigotti A, Saran U, Dufour JF. Physical activity and liver diseases. Hepatology. 2016;63:1026–40. doi: 10.1002/hep.28132. [DOI] [PubMed] [Google Scholar]
- 10.Pang Y, Kartsonaki C, Guo Y, Chen Y, Yang L, Bian Z, et al. Central adiposity in relation to risk of liver cancer in Chinese adults: A prospective study of 0.5 million people. Int J cancer. 2019;145:1245–53. doi: 10.1002/ijc.32148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assi N, Gunter MJ, Thomas DC, Leitzmann M, Stepien M, Chajès V, et al. Metabolic signature of healthy lifestyle and its relation with risk of hepatocellular carcinoma in a large European cohort. Am J Clin Nutr. 2018;108:117–26. doi: 10.1093/ajcn/nqy074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Chen J, Collins R, Guo Y, Peto R, Wu F, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40:1652–66. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millwood IY, Li L, Smith M, Guo Y, Yang L, Bian Z, et al. Alcohol consumption in 0.5 million people from 10 diverse regions of China: prevalence, patterns and socio-demographic and health-related correlates. Int J Epidemiol. 2013;42:816–27. doi: 10.1093/ije/dyt078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lv J, Qi L, Yu C, Yang L, Guo Y, Chen Y, et al. Consumption of spicy foods and total and cause specific mortality: population based cohort study. BMJ. 2015;351:h3942. doi: 10.1136/bmj.h3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu C, Shi Z, Lv J, Du H, Qi L, Guo Y, et al. Major dietary patterns in relation to general and central obesity among Chinese adults. Nutrients. 2015;7:5834–49. doi: 10.3390/nu7075253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du H, Li L, Bennett D, Guo Y, Key TJ, Bian Z, et al. Fresh fruit consumption and major cardiovascular disease in China. N. Engl J Med. 2016;374:1332–43. doi: 10.1056/NEJMoa1501451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saran U, Humar B, Kolly P, Dufour JF. Hepatocellular carcinoma and lifestyles. J Hepatol. 2016;64:203–14. doi: 10.1016/j.jhep.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 19.International, W. C. R. F. Diet, nutrition, physical activity and liver cancer (2018).
- 20.Obesity. preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep. Ser 894. 2000;i-xii:1–253. [PubMed] [Google Scholar]
- 21.Bragg F, Li L, Smith M, Guo Y, Chen Y, Millwood I, et al. Associations of blood glucose and prevalent diabetes with risk of cardiovascular disease in 500 000 adult Chinese: the China Kadoorie Biobank. Diabet Med. 2014;31:540–51. doi: 10.1111/dme.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh WP, Robien K, Wang R, Govindarajan S, Yuan JM, Yu MC. Smoking as an independent risk factor for hepatocellular carcinoma: the Singapore Chinese Health Study. Br J Cancer. 2011;105:1430–5. doi: 10.1038/bjc.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandair DS, Rossi RE, Pericleous M, Whyand T, Caplin M. The impact of diet and nutrition in the prevention and progression of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2014;8:369–82. doi: 10.1586/17474124.2014.894879. [DOI] [PubMed] [Google Scholar]
- 24.Behrens G, Matthews CE, Moore SC, Freedman ND, McGlynn KA, Everhart JE, et al. The association between frequency of vigorous physical activity and hepatobiliary cancers in the NIH-AARP Diet and Health Study. Eur J Epidemiol. 2013;28:55–66. doi: 10.1007/s10654-013-9767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ. 2019;97:230–8. doi: 10.2471/BLT.18.219469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto A, Tanaka E, Rokuhara A, Kiyosawa K, Kumada H, Omata M, et al. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: A multicenter retrospective study of 2795 patients. Hepatol Res. 2005;32:173–84. doi: 10.1016/j.hepres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Pang Y, Kartsonaki C, Turnbull I, Guo Y, Chen Y, Clarke R, et al. Adiposity in relation to risks of fatty liver, cirrhosis and liver cancer: a prospective study of 0.5 million Chinese adults. Sci Rep. 2019;9:785. doi: 10.1038/s41598-018-36460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu MW, Hsieh HH, Pan WH, Yang CS, CJ CH. Vegetable consumption, serum retinol level, and risk of hepatocellular carcinoma. Cancer Res. 1995;55:1301–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Details of the CKB data are available upon reasonable request (http://www.ckbiobank.org/site/Data+Access).