Abstract
Statement of problem.
The lack of standardization regarding the loading piston material used in fatigue tests could limit the interpretation of study findings.
Purpose.
The purpose of this in vitro study was to evaluate the effect of the piston material on the fatigue behavior of a lithium disilicate glass-ceramic.
Material and methods.
Plate-shaped, 1.2-mm-thick, lithium disilicate glass-ceramic specimens were cemented onto a dentin analog substrate with resin cement. The specimens were divided into 4 groups according to the piston material used in the fatigue test (n=30): metal, glass fiber-reinforced epoxy resin, ceramic, and human tooth. The fatigue test was performed in a mechanical cycling machine by using the boundary technique at 2 Hz in distilled water at 37 °C. The fatigue data were analyzed by using the Weibull distribution and a lifetime - inverse power law relationship. Failures were evaluated with fractography and transillumination.
Results.
The Weibull modulus (β) was similar among groups. The exponent of crack growth (n) was significantly greater for glass fiber-reinforced epoxy resin and tooth groups than for metal and ceramic; therefore, the probability of failure (Pf) of glass-ceramic specimens loaded by resin and tooth pistons depended more on load amplitude. Specimens tested with tooth showed the highest value of K (characteristic lifetime), which is an indication of greater survival. Radial crack was the only failure mode observed for all experimental groups.
Conclusions.
The piston material influenced the fatigue survival of the lithium disilicate glass-ceramic. The glass fiber-reinforced epoxy resin piston closely simulated the fatigue behavior induced by the human tooth on the evaluated glass-ceramic.
Ceramics are popular dental restorative materials because of their favorable biocompatibility and esthetics.1 However, the oral environment offers unfavorable conditions for the survival of a ceramic prosthesis, including the presence of cyclic masticatory forces and humidity.2,3
Subcritical crack growth (SCG) and cyclic fatigue have been associated with the degradation of the mechanical properties of ceramics in the oral environment.2,4 SCG involves the slow and stable growth of cracks under tension because of chemical corrosion at the crack tip in a humid environment.3,5 Intrinsic and extrinsic toughening mechanisms are associated with degradation of the mechanical properties of ceramics through cyclic fatigue.6–8 These phenomena can lead to restoration failure at lower stress levels than those reported in experimental fast fracture, and they may also affect the failure modes.2,9 Clinically, the mechanical failures of ceramic prostheses have been reported to be cracking, chipping, and catastrophic fracture.4,10–17
In order to perform clinically relevant laboratory studies, the configuration of the ceramic prosthesis should be mimicked with a multilayer structure, and the conditions of the oral environment with cyclic fatigue testing.2 However, standardization of the material, geometry, and size of the pistons used for static compressive load and cyclic fatigue mechanical tests are lacking.18–20 Different materials have been used to produce the loading pistons, including stainless steel,18,21–24 aluminum,18,19 composite resin,9,18,24 tungsten carbide,24,25 ceramic,25–28 and tooth structure.20,29 The different mechanical properties of these materials can affect the distribution and magnitude of stresses in the loaded structure, resulting in different mechanical behavior.18,20,30 In addition, most of the materials used for loading pistons are not found clinically as antagonists to ceramic restorations.
Previous studies have evaluated the type of piston material in the fracture behavior of ceramic specimens by using monotonic and fatigue tests, with controversial results being reported.18,20,24,25 Glass fiber reinforced epoxy resin (G10), aluminum, and stainless steel pistons were compared,8 with the authors concluding that metal pistons produced surface cone cracks, while the G10 piston induced radial cracks on the ceramic intaglio surface of bilayer and trilayer ceramic structures. Another study reported that the G10 piston produced a greater incidence of failures on feldspathic porcelain specimens than tungsten or stainless steel pistons.24 Moreover, a small trend has been reported toward longer lifetimes when trilayer specimens (glass/alumina/composite resin) are loaded with a glass piston compared with a tungsten piston.25 Although these studies evaluated ceramic materials under cyclic fatigue, the test arrangement and specimen configuration were different, limiting meaningful comparisons.
Few investigations have compared the mechanical behavior of ceramic specimens tested by different piston materials with the behavior induced by a human tooth.20,29,31 A study compared stainless steel, ceramic, and G10 pistons with a human tooth, reporting no significant effect of the piston material on the fracture behavior of a lithium disilicate glass-ceramic.20 However, in that study, the mechanical behavior of the ceramic specimens was assessed with a static compressive load test rather than with cyclic loading in a wet environment.
Therefore, the objective of the present study was to evaluate the effect of the loading piston material (stainless steel, glass fiber-reinforced epoxy resin (G10), ceramic, and human tooth) on the fatigue behavior of a lithium disilicate-based glass-ceramic, testing the null hypotheses that the piston material used in fatigue tests would not affect the probability of failure (Pf) or the failure mode of glass-ceramic specimens.
MATERIAL AND METHODS
The study was approved by the local Ethics in Research Committee (no. 2404475). Lithium disilicate glass-ceramic specimens were cemented onto a dentin analog substrate with resin cement and divided into 4 groups according to the piston material used for the fatigue test (n=30): M (stainless steel), R (glass fiber-reinforced epoxy resin), C (lithium disilicate glass-ceramic), and T (human tooth).
The glass-ceramic specimens were produced from prefabricated and precrystallized computer-aided design and computer-aided manufacturing (CAD-CAM) blocks (IPS e.max CAD; Ivoclar Vivadent AG) that were cut into 1.3-mm-thick slices with a diamond disk in a metallographic cutting machine (Minitom; Struers) under constant water cooling. The plate-shaped ceramic specimens were wet-flattened and polished by using a sequence of silicon carbide papers (Fertak) up to 1000 grit. The ceramic specimens were subjected to a crystallization cycle (Ivoclar Programat 5010; Ivoclar Vivadent AG) as recommended by the manufacturer. The final dimensions of the plate-shaped ceramic specimens were 1.2×10.4×12.5 mm.
Disk-shaped structures (Ø20×4 mm) were produced from a dentin analog material (glass-fiber-reinforced epoxy resin; NEMA G10; International Paper).18,32,33 The ceramic and G10 specimens were measured with digital calipers (Absolute; Mitutoyo) at 4 different points to verify the thickness and parallelism between the upper and lower surfaces.
The ceramic surface was etched with 5% hydrofluoric acid (Condac Porcelana; FGM Dental Products) for 20 seconds.20 The cementation surface of the G10 substrate was etched with 10% hydrofluoric acid (Condac Porcelana; FGM Dental Products) for 60 seconds.28 The treated surfaces of ceramic and G10 substrate were rinsed with water for 30 seconds and air-dried for 30 seconds. A silane coupling agent (Prosil; FGM Dental Products) was applied to the treated surfaces and left to evaporate for 60 seconds before air-drying for 30 seconds.
The ceramic specimens were cemented onto the G10 substrate with dual-polymerized self-adhesive resin cement (Rely X U200; 3M Dental Products) according to the manufacturer’s instructions. The resin cement was applied on the ceramic-treated surface, and the specimen was placed on the center of the treated G10 substrate. The specimen was placed in a cementation device that was used to apply a load of 7.35 N for 3 minutes. Excess cement was removed, and 2 opposing sides of the multilayer structure were photoactivated (UltraLight III Photopolymerizer, 390 mw/cm2; Sanders do Brasil Ltd.) for 20 seconds each. Then, the specimen was removed from the device, and the top surface was photoactivated for 20 seconds, resulting in a total polymerization time of 60 seconds per specimen. All specimens were stored in distilled water at 37 °C for 48 hours before fatigue testing.9,20
The pistons were produced with a Ø3-mm flat tip, simulating an occlusal contact wear facet.18,19 The M and R pistons were designed and machined with a lathe (Romi GL240; Romi Industries S.A.). The C piston was produced with a CAD-CAM system (CEREC inLab MC XL, software inLab 4.3; Dentsply Sirona). The T piston was obtained by grinding the enamel of a maxillary canine incisal edge with a diamond rotary instrument (2135F; KG Sorensen) to the standard Ø3-mm contact area.20 Thirty T pistons were used for the fatigue test, 1 for each glass-ceramic specimen. Additionally, the R pistons were remachined after each test to ensure constant geometry and dimensions.
The cyclic fatigue test was performed in a pneumatic mechanical cycling machine (Biocycle; Biopdi) by using the boundary technique at 2 Hz frequency in 37-°C distilled water.7–9,34 Ten specimens were tested simultaneously in the cycling machine. The specimens and pistons were individually fixed and aligned in the lower and upper apparatus of the cycling machine by a trained researcher (K.R.W.) to avoid premature contact during loading. The pistons remained in contact with the ceramic surface to avoid impact during the fatigue test. Thirty specimens were tested for each type of piston material (M, R, C, and T). Two lifetimes were evaluated, 100000 and 500000 cycles, each with 2 load amplitudes (L1 and L2). Ten specimens were tested in each fatigue protocol, and specimens surviving 100000 cycles were also used for the 500000 cycle profile.
Initially, 10 specimens from each group were tested for 100000 cycles with a 90-N load. The initial load was defined based on previous studies.9,20 Then, the number of specimens that failed (i) by the end of the protocol was determined, and a second load amplitude (L2) for 100000 cycles was calculated according to the following equation35:
, where i is the number of specimens that failed up to the predefined number of cycles in L1, n is the total number of specimens tested in L1 (n=10), and S is a constant selected to minimize the chance of all or none of the specimens failing at L2, which was 0.178.
A new set of 10 specimens was cycled with L2 for 100000 cycles. The surviving specimens were allowed to run through the second lifetime of 500000 cycles. Thus, L2 for 100000 cycles was used as L1 for 500000 cycles. The second load amplitude (L2) for 500000 cycles was calculated using the same equation, and a new set of 10 specimens was tested. Table 1 presents the detailed description of the fatigue protocols used.
Table 1.
Fatigue protocols and frequency of specimen failure and survival
| Group | Load (N) | Time (n. cycles)* | n | Failure - i | Survival* | |
|---|---|---|---|---|---|---|
| T | L1 | 90 | 100000 | 10 | 6 | 4 |
| L2 | 80 | 100000 → | 10 | 5 | 5 → | |
| L1 | 80 | 500000 | 2 | 3 | ||
| L2 | 70 | 500000 | 10 | 3 | 7 | |
| R | L1 | 90 | 100000 | 10 | 6 | 4 |
| L2 | 80 | 100000 → | 10 | 6 | 4 → | |
| L1 | 80 | 500000 | 1 | 3 | ||
| L2 | 70 | 500000 | 10 | 8 | 2 | |
| M | L1 | 90 | 100 000 | 10 | 8 | 2 |
| L2** | 77 | 100000 | 10 | 10 | 0 | |
| L1*** | 62 | 100000 → | 10 | 8 | 2 → | |
| L1 | 62 | 500000 | 2 | 0 | ||
| C | L1 | 90 | 100000 | 10 | 8 | 2 |
| L2 | 77 | 100000 → | 10 | 7 | 3 → | |
| L1 | 77 | 500 000 | 3 | 0 | ||
| L2 | 62 | 500000 | 10 | 10 | 0 |
C, ceramic; i, number of failed specimens; L1, first load level; L2, second load level; M, metal; n, total number of specimens; R, composite resin; T, tooth.
Arrows indicate that specimens that survived 100000 cycles allowed to run through second lifetime of 500000 cycles
All specimens failed at L2 after 100000 cycles, so not possible to use this set of specimens for next protocol.
L1 calculated for second lifetime of 500000, and test interrupted at 100000 to collect failure and survival data.
All specimens were analyzed by using blue light transillumination to determine the presence of crack(s) or fracture(s) after each predefined fatigue protocol. The failure modes investigated were radial cracks, cone cracks, and combined failures when both radial and cone cracks were found.9
The fatigue data were analyzed with an inverse power law lifetime-stress relation (IPL) and a Weibull lifetime distribution with the maximum likelihood estimation (MLE) by using a statistical software program (ALTA PRO; Reliasoft). The combined IPL-Weibull model was obtained from Pf = 1 – exp(Kσnt)1/β, where Pf is the probability of failure at time t, σ is the stress level, β is the fatigue Weibull modulus, n is the exponent of crack growth, and K is a constant used to fit the model to the data set.
The statistical model considered both failure and survival data (censored data). The fatigue test was performed with different load amplitudes and number of cycles, following the boundary technique.7–9,34,35 Data on the low and high probability of specimen failure could increase the power of the mathematical model used to predict the fatigue behavior of the experimental groups.8 The probabilities of fatigue failure (Pf) at 30 N, 60 N, and 90 N, values in the range of the masticatory force,2,4,12,16–18 were estimated based on the statistical model.
RESULTS
Table 2 presents the fatigue parameters for the glass-ceramic specimens tested with the different pistons. The Weibull modulus (β) was similar among the experimental groups, considering that the 95% confidence intervals (95% CI) overlapped. Specimens tested with T piston showed the greatest value for the characteristic lifetime parameter (K - number of cycles for 63.2% Pf), which indicates a higher survival. Specimens tested with T and R pistons showed significantly higher values of n (exponent of crack growth) than groups M and C, indicating that the Pf of specimens tested with T and R pistons was more dependent on the load amplitude.
Table 2.
Values of fatigue parameters for each experimental group: (β) Weibull modulus; (-log K) characteristic lifetime parameter (63.2% Pf); (n) exponent of subcritical crack growth. Values in parentheses are 95% confidence intervals (95% CI)
| Group | β | -log K | n |
|---|---|---|---|
| T | 2.4 (1.4, 3.9) | −43.40 (−33.03, −53.78) | 7.0 (4.6, 9.4) |
| R | 1.8 (1.3, 2.5) | −13.29 (−15.99, −10.60) | 4.0 (2.6, 5.5) |
| M | 1.8 (1.4, 2.3) | −6.97 (−8.76, −5.15) | 0.9 (−0.1, 1.9) |
| C | 1.4 (1.1, 1.8) | −6.55 (−9.34, −3.74) | 0.6 (−0.8, 2.1) |
C, ceramic; M, metal; Pf, probability of failure; R, composite resin; T, tooth.
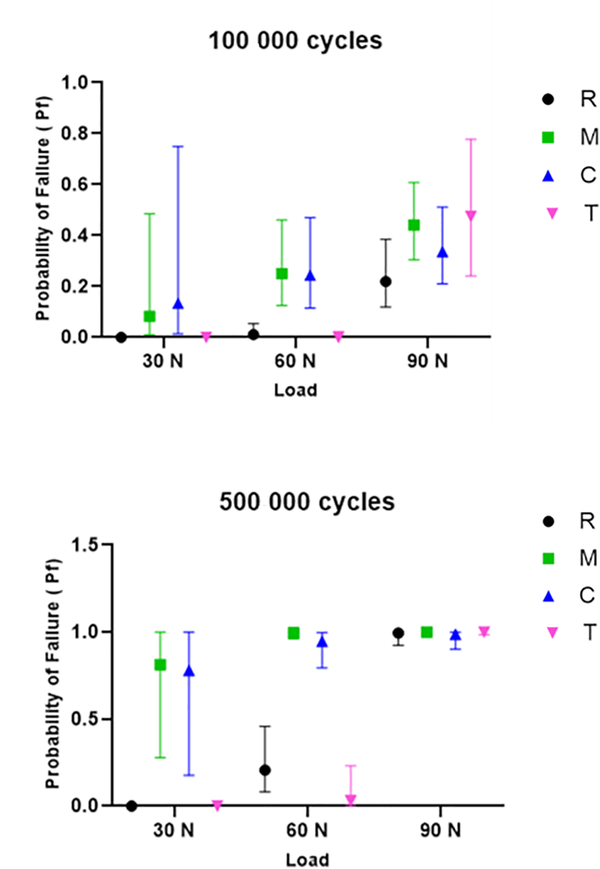
The Pf at 30 N, 60 N, and 90 N for lifetimes of 100000 and 500000 cycles were estimated by using the fatigue data (Fig. 1). Specimens tested with T and R pistons showed lower Pf than those tested with M and C pistons when predictions were performed at lower loads, including 30 N and 60 N. However, there was no significant difference among the experimental groups when the estimates were performed for a higher load, showing a similar fatigue behavior at 90 N. The Weibull graphs for 90 N, 60 N, and 30 N are presented in Figure 2. The curves for groups R and T were closer to each other and moved to the right at 30 N and 60 N, indicating a longer lifetime for the ceramic specimens tested under these conditions. The radial crack, located in the intaglio surface of the ceramic specimens, was the only failure mode observed for all experimental groups (Fig. 3). No specimens had signs of surface cracks.
Figure 1.
Probability of failure (Pf) predictions and respective confidence intervals (95% CI) for different load and cycles combinations. C, ceramic; M, metal; R, composite resin; T, tooth.
Figure 2.
Weibull graphs of failure probability versus time (number of cycles) for experimental groups at (A) 90 N, (B) 60 N, and (C) 30 N loads. Dotted lines are 95% confidence intervals (95% CI). C, ceramic; M, metal; R, composite resin; T, tooth.
Figure 3.
Cross-sectional view of ceramic layer from LD specimen tested in fatigue with metal piston. Ceramic fracture surface exposed by detaching G10 substrate, sectioning, and polishing. White arrow indicates failure origin: radial crack located at ceramic intaglio surface. Original magnification ×2.
DISCUSSION
Understanding how different types of loading pistons affect the mechanical behavior of bonded glass-ceramic structures can assist researchers in choosing the best material for their experimental designs and assist clinicians in interpreting laboratory studies. In the present investigation, 3 piston materials were evaluated: lithium disilicate-based glass-ceramic (C), a popular restorative material1,10–12; stainless steel (M), which has been the most frequently used material for loading pistons in laboratory tests18,21–24; and glass fiber-reinforced epoxy resin (R), a dentin analog composite resin material.16,18,32 A piston made of natural human tooth (T) was the control. The piston material affected the probability of fatigue failure of the resin-bonded glass-ceramic specimens, but the failure mode was similar among groups. Therefore, the null hypothesis was partially accepted. The fatigue behavior of resin-bonded glass-ceramic specimens was similar when loading pistons with similar mechanical properties were used.
Enamel and dentin have different microstructure and composition, resulting in distinct properties. Enamel is hard and brittle, while dentin is softer and more resilient.31 In the present study, the fiber-reinforced epoxy resin piston (R) produced the ceramic fatigue behavior most similar to that of the tooth piston (T). R piston has an elastic modulus of 13.1 GPa and a Poisson ratio of 0.4, in wet conditions.32 These values are similar to wet dentin, which has an elastic modulus around 21.7 GPa and a Poisson ratio of 0.3.32 Both structures have similar contact stress-strain curves and purely elastic behavior.18 The fatigue behavior of glass-ceramic specimens tested with T and R pistons was more sensitive to the load amplitude, resulting in higher n than M and C groups. Higher loads could result in material deformation for the T and R groups and can increase the stiffness of the piston tip through compaction.
Considering the characteristic lifetime parameter (K), glass-ceramic specimens survived longer in fatigue when T and R pistons were used in comparison with M and C pistons. Nevertheless, K values were greater for group T than for group R, indicating that the number of cycles to failure was greater when using the T piston; this may be explained by a more favorable stress distribution under fatigue.
C and M pistons resulted in similar fatigue parameters (β K, and n) for resin-bonded glass-ceramic specimens. For C and M groups, the Pf of the specimens was not affected by the load amplitude. The C piston has an elastic modulus of 95 GPa and Poisson ratio of 0.2530; while the M piston has an elastic modulus of 200 GPa and Poisson ratio of 0.30.20 Both materials are stiff,20 preventing the plastic deformation of the piston tip, even when fatigue testing was performed at higher load amplitudes. Moreover, the same failure mode was observed for all experimental groups, resulting in similar β-value among groups. Radial cracks were found in the intaglio surface of the ceramic specimens where tensile stresses develop when a compressive load is applied on a rigid material cemented to an elastic substrate.4,14,18 This failure mode is clinically relevant and has been associated with catastrophic fractures of glass-ceramic restorations.13 Cone cracks, reported in previous studies,9,18 were not found. The lower susceptibility to this contact type of damage may be related to the higher flexural strength and fracture toughness of the lithium disilicate glass-ceramic compared with the feldspathic porcelain and leucite-reinforced glass-ceramic tested in other studies.7,9,18,24 Additionally, the load amplitudes used in the fatigue tests were below 100 N.
A previous study reported high fracture loads for LD specimens tested with a static compressive load between 1080 N and 1568 N, but the type of piston material had no influence on their mechanical behavior.20 In the present study, the fatigue survival of ceramic specimens was similar among the experimental groups for higher loads. Differences in the fatigue behavior were only observed at low load amplitudes, which are not used in the static compressive load tests. However, low and intermittent loads are often found intraorally, indicating that fatigue testing could provide more clinically relevant data.2,4
The material for the loading piston should be chosen after considering the test methodology (static versus fatigue), load amplitude, and the properties of the evaluated restorative material. Pistons made of stiffer materials can be used in static compressive load tests, considering the great load amplitude often used in the method. Compressive load tests are a tool for estimating structural mechanical behavior but have limited clinical extrapolation. For fatigue testing, more resilient materials, including the fiber-reinforced epoxy resin evaluated in the present study, are suggested to mimic the tooth. Materials found as antagonists of the restorations in the mouth could also be used. Reasons for not using a natural tooth as a piston for in vitro tests include the difficulty in obtaining and standardizing the teeth and the occurrence of enamel cracks, chips, and tooth fractures in the presence of higher loads and longer tests.20,29
The present study used an accelerated fatigue method to optimize the test. Specimens were tested with different load amplitudes to collect high and low probability of failure data.6, 8 Failure was considered when cracks or fractures were observed after the predefined lifetime.7,8,35 The pistons were produced with a Ø3-mm flat tip to simulate an occlusal contact wear facet and to induce a more homogeneous stress distribution to the ceramic structures.18,19 The 2-Hz frequency was chosen based on the masticatory frequency, from 1 to 4 Hz.2,4,12,16–18 Fatigue was performed in 37-°C water, a simplification of the oral environment.2,4,12,16–18 Study limitations included the simplified geometry of the ceramic specimens and the absence of contact sliding during the fatigue test.2 Axial cyclic loads in the range of the masticatory force at the posterior region2,4‘12‘16–18 were applied to the ceramic specimens to characterize their fatigue behavior in a more controlled arrangement. Adding a horizontal movement could induce wear to the materials and alter the contact area between the piston and the ceramic specimen. Nevertheless, the multilayered configuration of a restoration was simulated by bonding a ceramic specimen with resin cement onto a dentin analog substrate, which also allowed the evaluation of the contribution of cement degradation in fatigue to the mechanical behavior of the structure.
CONCLUSIONS
Based on the findings of this in vitro study, the following conclusions were drawn:
The type of piston material used in the mechanical test influenced the fatigue behavior of the lithium disilicate glass-ceramic.
The fatigue behavior of the glass-ceramic structures was similar when tested with the tooth and glass fiber-reinforced epoxy resin pistons.
The probability of fatigue failure was more sensitive to load changes when tooth and epoxy resin pistons were used.
Glass-ceramic specimens survived longer lifetimes when tested with the tooth piston.
The ceramic and metal pistons induced similar fatigue behavior to the glass-ceramic specimens and they were less sensitive to the load amplitude.
CLINICAL IMPLICATIONS.
Understanding the effect of the piston material on the fatigue behavior of bonded ceramic structures can assist researchers and clinicians in designing and interpreting laboratory studies. Composite resin piston materials could more closely simulate the behavior of the human tooth when bonded glass-ceramic structures are tested under fatigue with low load amplitudes, including those observed during mastication.
Acknowledgments:
The authors thank CNPq and CAPES (Finance Code 001) for the undergraduate and graduate students’ scholarships.
Supported in part by the National Council for Scientific and Technological Development of Brazil (CNPq, research grant n. 461178/2014-1), and by the U.S. National Institutes of Health (NIH, research grant n. DE024333).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kelly JR, Benetti P. Ceramic materials in dentistry: historical evolution and current practice. Aust Dent J 2011;56 Suppl 1:84–96. [DOI] [PubMed] [Google Scholar]
- 2.Kelly JR, Cesar PF, Scherrer SS, Della Bona A, van Noort R, Tholey M, et al. ADM guidance-ceramics: fatigue principles and testing. Dent Mater 2017;33:1192–204. [DOI] [PubMed] [Google Scholar]
- 3.Salazar Marocho SM, Studart AR, Bottino MA, Bona AD. Mechanical strength and subcritical crack growth under wet cyclic loading of glass-infiltrated dental ceramics. Dent Mater 2010;26:483–90. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Sailer I, Lawn BR. Fatigue of dental ceramics. J Dent 2013;41:1135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borba M, de Araujo MD, Fukushima KA, Yoshimura HN, Cesar PF, Griggs JA, et al. Effect of the microstructure on the lifetime of dental ceramics. Dent Mater 2011;27:710–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borba M, Cesar PF, Griggs JA, Della Bona A. Step-stress analysis for predicting dental ceramic reliability. Dent Mater 2013;29:913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vicari CB, Magalhaes BO, Griggs JA, Borba M. Fatigue behavior of crystalline-reinforced glass-ceramic. J Prosthodont 2019;28:e297–e303. [DOI] [PubMed] [Google Scholar]
- 8.Ottoni R, Griggs JA, Corazza PH, Della Bona A, Borba M. Precision of different fatigue methods for predicting glass-ceramic failure. J Mech Behav Biomed Mater 2018;88:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodi E, Weber KR, Benetti P, Corazza PH, Della Bona A, Borba M. How oral environment simulation affects ceramic failure behavior. J Prosthet Dent 2018;119:812–18. [DOI] [PubMed] [Google Scholar]
- 10.Wolfart S, Eschbach S, Scherrer S, Kern M. Clinical outcome of three-unit lithium-disilicate glass-ceramic fixed dental prostheses: up to 8 years results. Dent Mater 2009;25:e63–71. [DOI] [PubMed] [Google Scholar]
- 11.van den Breemer CR, Vinkenborg C, van Pelt H, Edelhoff D, Cune MS. The clinical performance of monolithic lithium disilicate posterior restorations after 5, 10, and 15 years: a retrospective case series. Int J Prosthodont 2017;30:62–5. [DOI] [PubMed] [Google Scholar]
- 12.Belli R, Petschelt A, Hofner B, Hajto J, Scherrer SS, Lohbauer U. Fracture rates and lifetime estimations of CAD/CAM all-ceramic restorations. J Dent Res 2016;95:67–73. [DOI] [PubMed] [Google Scholar]
- 13.Scherrer SS, Lohbauer U, Della Bona A, Vichi A, Tholey MJ, Kelly JR, et al. ADM guidance-Ceramics: guidance to the use of fractography in failure analysis of brittle materials. Dent Mater 2017;33:599–620. [DOI] [PubMed] [Google Scholar]
- 14.Lawn BR, Pajares A, Zhang Y, Deng Y, Polack MA, Lloyd IK, et al. Materials design in the performance of all-ceramic crowns. Biomaterials 2004;25:2885–92. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Lee JJ, Srikanth R, Lawn BR. Edge chipping and flexural resistance of monolithic ceramics. Dent Mater 2013;29:1201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly JR. Clinically relevant approach to failure testing of all-ceramic restorations. J Prosthet Dent 1999;81:652–61. [DOI] [PubMed] [Google Scholar]
- 17.Kelly JR, Benetti P, Rungruanganunt P, Bona AD. The slippery slope: critical perspectives on in vitro research methodologies. Dent Mater 2012;28:41–51. [DOI] [PubMed] [Google Scholar]
- 18.Kelly JR, Rungruanganunt P, Hunter B, Vailati F. Development of a clinically validated bulk failure test for ceramic crowns. J Prosthet Dent 2010;104:228–38. [DOI] [PubMed] [Google Scholar]
- 19.Yi YJ, Kelly JR. Effect of occlusal contact size on interfacial stresses and failure of a bonded ceramic: FEA and monotonic loading analyses. Dent Mater 2008;24:403–9. [DOI] [PubMed] [Google Scholar]
- 20.Weber KR, Benetti P, Della Bona A, Corazza PH, Medeiros JA, Lodi E, et al. How does the piston material affect the in vitro mechanical behavior of dental ceramics? J Prosthet Dent 2018;120:747–54. [DOI] [PubMed] [Google Scholar]
- 21.Alessandretti R, Borba M, Della Bona A. Cyclic contact fatigue resistance of ceramics for monolithic and multilayer dental restorations. Dent Mater 2020;36:535–41. [DOI] [PubMed] [Google Scholar]
- 22.Mallmann F, Rosa L, Borba M, Della Bona A. Effect of screw-access hole and mechanical cycling on fracture load of 3-unit implant-supported fixed dental prostheses. J Prosthet Dent 2018;119:124–31. [DOI] [PubMed] [Google Scholar]
- 23.Borba M, Duan Y, Griggs JA, Cesar PF, Della Bona A. Effect of ceramic infrastructure on the failure behavior and stress distribution of fixed partial dentures. Dent Mater 2015;31:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miranda JS, de Carvalho RLA, de Carvalho RF, Borges ALS, Bottino MA, Ozcan M, et al. Effect of different loading pistons on stress distribution of a CAD/CAM silica-based ceramic: CAD-FEA modeling and fatigue survival analysis. J Mech Behav Biomed Mater 2019;94:207–12. [DOI] [PubMed] [Google Scholar]
- 25.Bhowmick S, Melendez-Martinez JJ, Hermann I, Zhang Y, Lawn BR. Role of indenter material and size in veneer failure of brittle layer structures. J Biomed Mater Res B Appl Biomater 2007;82:253–9. [DOI] [PubMed] [Google Scholar]
- 26.Facenda JC, Borba M, Benetti P, Della Bona A, Corazza PH. Effect of supporting substrate on the failure behavior of a polymer-infiltrated ceramic network material. J Prosthet Dent 2019;121:929–34. [DOI] [PubMed] [Google Scholar]
- 27.Coelho PG, Bonfante EA, Silva NR, Rekow ED, Thompson VP. Laboratory simulation of Y-TZP all-ceramic crown clinical failures. J Dent Res 2009;88:382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corazza PH, Duan Y, Kimpara ET, Griggs JA, Della Bona A. Lifetime comparison of Y-TZP/porcelain crowns under different loading conditions. J Dent 2015;43:450–7. [DOI] [PubMed] [Google Scholar]
- 29.Rosentritt M, Behr M, Gebhard R, Handel G. Influence of stress simulation parameters on the fracture strength of all-ceramic fixed-partial dentures. Dent Mater 2006;22:176–82. [DOI] [PubMed] [Google Scholar]
- 30.Belli R, Wendler M, de Ligny D, Cicconi MR, Petschelt A, Peterlik H, et al. Chairside CAD/CAM materials. Part 1: measurement of elastic constants and microstructural characterization. Dent Mater 2017;33:84–98. [DOI] [PubMed] [Google Scholar]
- 31.Chun KJ, Choi HH, Lee JY. Comparison of mechanical property and role between enamel and dentin in the human teeth. J Dent Biomech 2014;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merlo EG, Della Bona A, Griggs JA, Jodha KS, Corazza PH. Mechanical behavior and adhesive potential of glass fiber-reinforced resin-based composites for use as dentin analogues. Am J Dent 2020;33:310–14. [PubMed] [Google Scholar]
- 33.Clelland NL, Warchol N, Kerby RE, Katsube N, Seghi RR. Influence of interface surface conditions on indentation failure of simulated bonded ceramic onlays. Dent Mater 2006;22:99–106. [DOI] [PubMed] [Google Scholar]
- 34.Maennig W Statistical planning and evaluation of fatigue tests. A survey of recent results. Int J Fract 1975;11:123–9. [Google Scholar]
- 35.Zhang Y, Griggs JA. Evaluation of failure probability estimators for cyclic fatigue using boundary technique. J. Mater. Sci. Lett 2003;22:1775–7. [Google Scholar]