Abstract
Although not life-threatening, onychomycosis (a fungal infection of the nail, usually caused by a dermatophyte) constitutes an important public health problem because of its high prevalence (about 10% of the U.S. population) and associated morbidity. The disease can have certain negative consequences for patients, such as pain, and can potentially undermine work and social lives. This review discusses the etiology, classification, diagnosis, and treatment of onychomycosis. Four types of onychomycosis are recognized based on the site and pattern of fungal invasion. Dermatophyte fungi are the predominant pathogens, but yeasts (especially Candida albicans) and nondermatophyte molds may also be implicated. Accurate diagnosis requires direct microscopy and fungal culture. The differential diagnosis includes psoriasis, lichen planus, onychogryphosis, and nail trauma. Onychomycosis is more difficult to treat than most dermatophytoses because of the inherent slow growth of the nail. Older antifungal agents (ketoconazole and griseofulvin) are unsuitable for onychomycosis because of their relatively poor efficacy and potential adverse effects. Three recently developed antimycotic agents (fluconazole, itraconazole, and terbinafine) offer high cure rates and good safety profiles. In addition, the short treatment times (<3 months) and intermittent dosing schedules are likely to enhance compliance and reduce the costs of therapy.
Most cutaneous infections are the work of the homogeneous group of keratinophilic fungi known as dermatophytes. The dermatophyte Trichophyton rubrum is the major cause of tinea pedis and onychomycosis (8). After originating in West Africa, Southeast Asia, Indonesia, and Northern Australia, T. rubrum spread to Europe and North and South America in the late 19th and early 20th centuries, where it found a niche within a recently shod populace (8). Subsequent 20th century developments including wars, the modern health movement and the associated use of occlusive footwear and locker rooms, and migration of people since the invention of the jumbo jet, promoted an increased incidence of tinea pedis and onychomycosis (8).
Dermatophytoses of the fingernails and toenails, in contrast to those at other body sites, are particularly difficult to eradicate with drug treatment. This is the consequence of factors intrinsic to the nail—the hard, protective nail plate, sequestration of pathogens between the nail bed and plate, and slow growth of the nail—as well as of the relatively poor efficacy of the early pharmacologic agents.
Recent years, however, have witnessed the development of a new generation of antifungal drugs that produce impressive, long-lasting cure rates with shorter treatment times and better safety profiles than ketoconazole and griseofulvin. In this paper, current knowledge of the pathogenesis, diagnosis, and management of onychomycosis with these new agents is reviewed and evaluated.
ONYCHOMYCOSIS
Definition and Clinical Impact
“Onychomycosis” traditionally referred to a nondermatophytic infection of the nail but is now used as a general term to denote any fungal nail infection (63) (tinea unguium specifically describes a dermatophytic invasion of the nail plate). In spite of the clearly diseased appearance associated with this condition, onychomycosis is all too often regarded as merely a cosmetic problem of relatively minor importance that is hardly worth the effort to resolve. This belief may have been supported by the adverse effects and long dosing courses associated with some of the earlier antifungal agents.
In fact, onychomycosis can have significant negative effects on patients’ emotional, social, and occupational functioning and can, in addition, consume a sizable proportion of health care dollars. Affected patients may experience embarrassment in social and work situations, where they feel blighted or unclean, unwilling to allow their hands or feet to be seen. Patients may fear that they will transmit their infection to family members, friends, or coworkers, fears that can lead to diminished self-esteem and the avoidance of close relationships (55). Employment suffers if employers are reluctant to hire individuals with abnormal nails, particularly for jobs such as food handling or modelling or where interaction with the public is required. A more tangible barrier to work success is the discomfort some patients experience that prevents them from carrying out work-related tasks such as prolonged standing, writing, or typing. Finally, onychomycosis can compel workers to take periodic sick leave, a problem even for treated patients if therapy is ineffective and/or long-lasting (55). This lack of success, in turn, can cause patients to feel discouraged or even to stop treatment, resigning themselves to permanent disfigurement and discomfort.
Onychomycosis in immunocompromised patients, such as those infected with human immunodeficiency virus (HIV), can pose a more serious health problem (55). Not only does the difficult-to-treat infection serve as a constant reminder to the patient of his or her own deteriorated condition, but the possibility exists of transfer of a very high titer of fungal pathogens to another person (55).
Epidemiology and Risk Factors
Dermatophytoses of the stratum corneum, hair, and nails are common, whereas infection of the dermis and subcutaneous tissue by these agents is rare (64). Although dermatophytic infections are rarely life-threatening, their high incidence and prevalence and the associated morbidity (64) make them an important public health problem (1).
Reports concerning the prevalence of onychomycosis are conflicting, with estimates ranging from 2 to 3% of the general U.S. population (27) to 13% of the male Finnish population (40). In a recent outpatient-based, cross-sectional survey of 1,038 patients in a dermatology clinic waiting room in Cleveland, Ohio, culture-confirmed dermatophyte onychomycosis was identified in 8.7% of the total population and in 6.5 and 13.3% of the female and male subgroups, respectively (patients who presented for onychomycosis were excluded) (27). These figures are comparable to those for the general Finnish population (8.4%) (40). Several studies have shown that the prevalence of onychomycosis increases with age. For example, none of the 200 Finnish subjects who were younger than 20 years had onychomycosis but almost 24% of those aged 70 years or older had the disorder. Similarly, 28.1% of the members of the Ohio cohort aged 60 years or older were culture positive for onychomycosis, versus 1.1 and 2.9% for those aged 10 to 18 years and 19 to 30 years, respectively (27). Reasons for the age-related increase in onychomycosis may include poor peripheral circulation, diabetes, repeated nail trauma, longer exposure to pathogenic fungi, suboptimal immune function, inactivity, or the inability to cut the toenails or maintain good foot care (22, 27, 55).
As is the case among adults, prevalence rates for onychomycosis among children are quite variable: a recent review of studies of the subject in several countries outside North America lists prevalence rates varying from 0% (United States, Wales, and Finland) to 2.6% (Guatemala) (38). To learn more about the prevalence of onychomycosis among children in North America, a prospective survey was conducted of 2,500 young (≤18 years) patients and family members in Canada and the United States. Subjects’ nails were examined for signs of onychomycosis and sampled for direct microscopy and culture. Onychomycosis was diagnosed in 11 children (10 with affected toenails, and 1 with affected fingernails), indicating a prevalence of 0.44%; however, 7 of these children had been referred for treatment of onychomycosis or tinea pedis. Thus, the prevalence of onychomycosis in children with primary diagnoses other than onychomycosis or tinea pedis was 4 of 2,500, or 0.16% (37). The reasons for this 30-fold decrease in the prevalence of onychomycosis in children relative to adults may include reduced exposure to fungus because less time is spent in environments containing pathogens; faster nail growth; smaller nail surface for invasion; and lower prevalence of tinea pedis (37).
Contact with the source of the infection constitutes a risk factor; for example, Trichophyton verrucosum commonly infects the faces of farmers who lean against their cows as they milk them (64). There is no doubt that several factors unique to modern life have resulted in an increased prevalence of onychomycosis. These include the wearing of shoes, particularly fashionably tight, high-heeled shoes; the increased use by large numbers of people of damp spaces such as locker rooms and gymnasiums; the declining health of the aging American population, and the increased number of immunocompromised patients through disease (e.g., HIV infection) or therapeutic agents (e.g., immunosuppressive therapies associated with cancer or posttransplantation care, and the extensive use of broad-spectrum antibiotics) (25). Other factors that increase the risk of onychomycosis are direct trauma to the nail, including that resulting from certain tic disorders (e.g., nail biting).
DERMATOPHYTES AND ONYCHOMYCOSIS
The term “dermatophytosis” is used to describe infection by members of the genera Microsporum, Trichophyton, and Epidermophyton. The species that most often cause onychomycosis in North America and parts of Europe are T. rubrum, T. mentagrophytes, and Epidermophyton floccosum: the first two species are much more often implicated than E. floccosum (58). Infections of the skin, nail, and hair by nondermatophytic molds such as Scytalidium and Scopulariopsis are termed “dermatomycoses.” Dermatophytes account for most (90%) cases of onychomycosis of the toenails and at least 50% of fingernail infections (31). Both dermatophytes and nondermatophytes, especially Candida albicans, have been identified as sole etiologic agents of onychomycosis; however, the incidence of true mixed infections (caused by dermatophytes plus nondermatophytes) is difficult to determine accurately (58) and is discussed in detail below.
The dermatophytes are hyaline septated molds. The hyphae of these mycelial organisms penetrate the stratum corneum of the skin and nails. The fungal cells manufacture keratinolytic proteases, which provide a means of entry into living cells (39). Some dermatophytic species, which are basically soil saprophytes that have acquired the ability to digest keratinous debris in soil, have evolved to be capable of parasitizing keratinous tissues of animals (1).
The families that include many of the known keratinolytic fungi are the Arthrodermataceae and Onygenaceae in the phylum Ascomycota (52). Members of these families are homogeneous with respect to appearance, physiology, taxonomy, antigenicity, basic growth requirements, infectivity, and the diseases they cause (52). Some, such as Microsporum canis and T. mentagrophytes, have affinity for the keratin of animals and humans, whereas others are more specialized for a particular animal host (1).
Variability with respect to the causative microorganism is both geographic and, within a given region, temporal. Because organisms that cause clinically apparent disease tend to receive the most attention, pathogens whose invasion leads to hard-to-detect disease may be present in a region but are less likely to be identified (1). By contrast, pathogens that cause readily apparent signs and symptoms are likely to be identified and their prevalence is likely to be noted. Thus, because reports during the 1970s focused primarily on scalp infections, T. violaceum was the most frequently isolated dermatophyte during that decade in Europe (1) although T. tonsurans is the principal agent of tinea capitis in the United States and is emerging in Europe.
Changes over time within a region in the prevalence of particular dermatophyte species also are common: although M. audouinii and M. canis were the most common causes of scalp infection in Western and Mediterranean Europe 50 to 100 years ago, tinea capitis has declined in incidence in Western Europe and, when present, is caused primarily by M. canis (1) or T. violaceum (1). Similarly, M. audouinii and M. canis were the main causes of tinea capitis in the United States earlier in this century; this role has been taken over by T. tonsurans (1). Another change that has occurred in recent years is the growing prevalence of dermatophytoses of the foot (tinea pedis) and nails (tinea unguium) and decline in the prevalence of scalp infections (1).
CLINICAL TERMINOLOGY
As in many areas of medicine, the clinical terminology used to describe dermatophytic infections evolved in advance of accurate knowledge about causation or pathophysiology. Tinea (“a gnawing worm”) or “ringworm,” a term derived from the appearance of the characteristic skin lesions in this common dermatophytosis (64), affects the scalp (tinea capitis), glabrous skin (tinea corporis), groin (tinea cruris), nail (tinea unguium), feet (tinea pedis), beard (tinea barbae), and hand (tinea manuum). Other dermatophytoses are named for their appearance, such as tinea favosa (favus, or honeycomb-like due to T. schoenleinii) or tinea imbricata (“composed of overlapping parts”; ringworm due to T. concentricum).
ANATOMY OF THE NAIL
A review of the anatomy of the nail unit and the process of nail growth may be helpful in understanding the pathogenesis of dermatophytic fungi in the nail unit. A diagram of the nail unit is presented in Figure 1 (11). It consists of the following structures: proximal and lateral folds, cuticle, matrix, nail plate (commonly called the nail), nail bed, and hyponychium. The cuticle is the horny layer of the proximal nail fold; it consists of modified stratum corneum and protects the nail matrix from infection (12). The nail matrix is the growth center of the nail. As the nail grows, cells of the nail matrix divide, differentiate, and keratinize and are incorporated into the nail plate. The distal, visible part of the matrix looks like a “half moon” and is called the lunula. The matrix extends approximately 5 mm proximally beneath the proximal nail fold (12). The nail plate is the largest structure of the nail unit and grows by sliding forward over the nail bed, whereupon the distal end becomes free of the nail bed (44). The hyponychium, the most distal component in the nail bed, is composed of epidermis that includes a granular layer similar to that seen in plantar and volar surfaces (12). Fingernails grow at a rate of 2 to 3 mm per month, and toenails grow at a rate of 1 mm per month. Therefore, it takes about 6 months to replace a fingernail and between 12 and 18 months to replace a toenail (12). This rate of growth is often decreased in the presence of peripheral vascular disease and onychomycosis and in the elderly (12).
FIG. 1.
The nail unit. Reprinted from reference 11 with permission of the publisher.
CLASSIFICATION OF ONYCHOMYCOSIS
Four types of onychomycosis, characterized according to clinical presentation and the route of invasion, are recognized.
Distal Subungual Onychomycosis
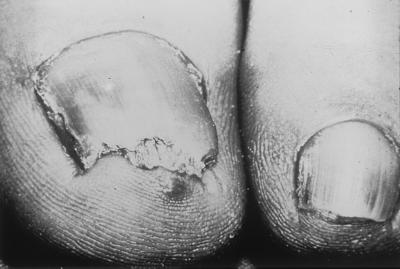
Distal subungual onychomycosis (DSO) is the most common form of onychomycosis. It is characterized by invasion of the nail bed and underside of the nail plate beginning at the hyponychium (Fig. 2). The infecting organism migrates proximally through the underlying nail matrix. Mild inflammation develops, resulting in focal parakeratosis and subungual hyperkeratosis, with two consequences: onycholysis (detachment of the nail plate from the nail bed) and thickening of the subungual region. This subungual space then can serve as a reservoir for superinfecting bacteria and molds, giving the nail plate a yellowish brown appearance (12).
FIG. 2.
Distal subungual onychomycosis. Courtesy of Gary Palmer.
DSO is usually caused by the dermatophyte T. rubrum (26, 59), although T. mentagrophytes, T. tonsurans, and E. floccosum also are known to be causative. DSO may develop on the fingernails, toenails, or both, with infection of the toenails being much more common than infection of the fingernails; in the Finnish study (40), only 2 of the 91 patients with dermatophyte-related onychomycosis of the toenails also had fingernail involvement. Toenail infections were approximately 20 times more common than fingernail infections in the Ohio cohort (27). The increased frequency of toenail in comparison to fingernail infections probably reflects the greater incidence of tinea pedis than of tinea manuum.
Proximal Subungual Onychomycosis
Proximal subungual onychomycosis (PSO) is also known as proximal white subungual onychomycosis (PWSO), a relatively uncommon subtype, and occurs when organisms invade the nail unit via the proximal nail fold through the cuticle area, penetrate the newly formed nail plate, and migrate distally (Fig. 3). The clinical presentation includes subungual hyperkeratosis, proximal onycholysis, leukonychia, and destruction of the proximal nail plate. In the United States T. rubrum is the principal causative agent of PSO.
FIG. 3.
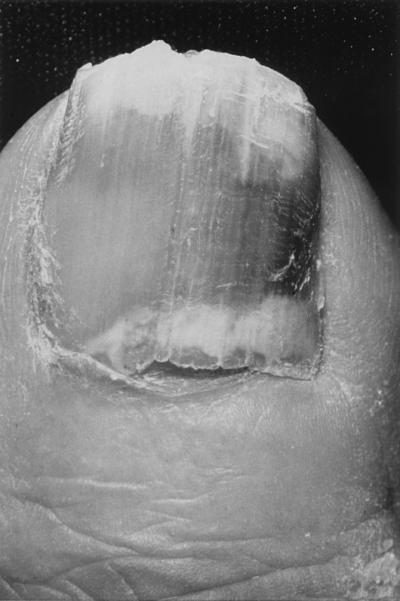
Proximal subungual onychomycosis in a patient with AIDS. Courtesy of Gary Palmer.
The pattern of growth in PSO is from the proximal nail fold on the lunula area distally to involve all layers of the nail (20). Although PSO is the most infrequently occurring form of onychomycosis in the general population, it is common in AIDS patients and is considered an early clinical marker of HIV infection (2). In one study of 62 patients with AIDS or AIDS-related complex and onychomycosis, 54 patients (88.7%) had PSO, with T. rubrum being the etiologic agent in more than half of these patients (20). In 54 patients, the feet were affected, and in 5 patients, the hands were infected; infections of both toenails and fingernails were present in 3 patients (20). Infection may also occasionally arise secondary to trauma.
White Superficial Onychomycosis
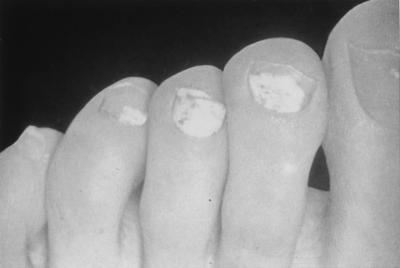
White superficial onychomycosis (WSO) is less common than DSO (estimated proportion of onychomycosis cases, 10%) (66) and occurs when certain fungi invade the superficial layers of the nail plate directly (Fig. 4). (Later, the infection may move through the nail plate to infect the cornified layer of the nail bed and hyponychium.) It can be recognized by the presence of well-delineated opaque “white islands” on the external nail plate, which coalesce and spread as the disease progresses. At this point, the nail becomes rough, soft, and crumbly (12). Inflammation is usually minimal in patients with WSO, because viable tissue is not involved (12). WSO occurs primarily in the toenails (66).
FIG. 4.
White superficial onychomycosis.
The most common etiologic agent in WSO is T. mentagrophytes (12). In addition, several nondermatophyte molds, including Aspergillus terreus, Acremonium roseogrisum (later confirmed to be Acremonium potronii), and Fusarium oxysporum, have been implicated by Zaias et al. (66).
Candida Infections of the Nail
Candida nail infections occur in patients with chronic mucocutaneous candidiasis, and are caused by C. albicans (3). The organism invades the entire nail plate. Candida spp. may cause other syndromes, including onycholysis and paronychia. These forms occur more commonly in women than in men (3) and often affect the middle finger, which may come into contact with Candida organisms that reside in the intestine or vagina (66). Candida onychomycosis can therefore be divided into three general categories. (i) Infection beginning as a paronychia (infection of the structures surrounding the nail; also called a “whitlow”), the most common type of Candida onychomycosis (54), first appears as an edematous, reddened pad surrounding the nail plate. Invasion by Candida spp., unlike dermatophytic invasion, penetrates the nail plate only secondarily after it has attacked the soft tissue around the nail (12). After infection of the nail matrix occurs, transverse depressions (Beau’s lines) may appear in the nail plate, which becomes convex, irregular, and rough and, ultimately, dystrophic (3, 12).
(ii) Patients with chronic mucocutaneous candidiasis are at risk for the second type of Candida onychomycosis, called Candida granuloma, which accounts for fewer than 1% of onychomycosis cases (12, 54, 66). This condition is seen in immunocompromised patients and involves direct invasion of the nail plate (30). The organism invades the nail plate directly and may affect the entire thickness of the nail, resulting, in advanced cases, in swelling of the proximal and lateral nail folds until the digit develops a pseudo-clubbing or “chicken drumstick” appearance (54).
(iii) Finally, Candida onycholysis can occur when the nail plate has separated from the nail bed. This form is more common on the hands than the feet (3). Distal subungual hyperkeratosis can be seen as a yellowish gray mass lifts off the nail plate. The lesion resembles that seen in patients with DSO (3).
Total Dystrophic Onychomycosis
Total dystrophic onychomycosis is used to describe end-stage nail disease, although some clinicians consider it a distinct subtype. It may be the end result of any of the four main patterns of onychomycosis. The entire nail unit becomes thick and dystrophic (65).
DIAGNOSIS OF ONYCHOMYCOSIS
The clinical presentation of dystrophic nails should alert the clinician to the possibility of onychomycosis; however, because fungi cause only about half of all nail dystrophies (30), the use of appropriate diagnostic techniques including direct microscopy and fungal culture is important to ensure correct diagnosis and treatment. The clinical appearance of the nail and the patient’s history will help differentiate fungal from nonfungal etiologies of nail dystrophies. For example, predisposing factors for onychomycosis include diabetes mellitus, older age, hyperhidrosis, onychogryphosis, nail trauma, poor peripheral circulation, and immunosuppression (12). In the presence of subungual hyperkeratosis, yellow-brown discoloration, and onycholysis, onychomycosis is likely to be present. If the patient has a history of tinea pedis, particularly moccasin type, the case for this diagnosis is even stronger (12).
Differential Diagnosis
Care should be taken to correctly identify signs and symptoms of other diseases that clinically mimic onychomycosis. These include psoriasis (the most common such disorder), lichen planus, bacterial infections, contact dermatitis, traumatic onychodystrophies, pachyonychia congenita, nail bed tumors, yellow-nail syndrome (rare), and idiopathic onycholysis. When psoriasis affects the nails, it is usually also present at other skin sites; however, in some cases nail involvement is the only sign (6). When psoriasis affects the nails, it can produce onycholysis resembling that associated with DSO (Fig. 5). A diagnosis of psoriasis is supported by the presence of fine pitting on the nail surface, the small salmon-colored “oil drop” sign of onycholysis that is not seen in onychomycosis, and fingernail involvement of both hands (28).
FIG. 5.
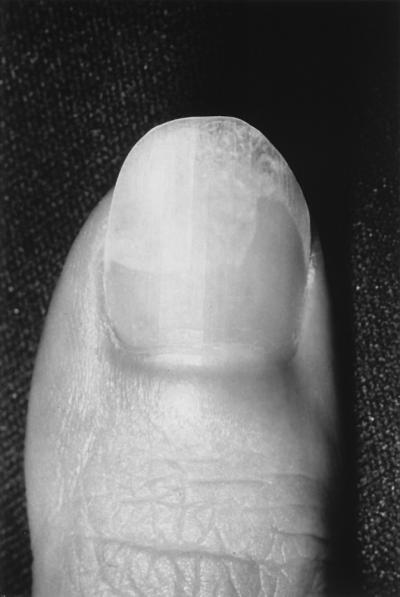
Psoriasis affecting the nail. Courtesy of C. R. Daniel III.
Approximately 10% of patients with lichen planus have abnormal nails. “Twenty-nail” dystrophy is a condition of unknown cause. Onychorrhexis (exaggerated longitudinal ridging) and “angel wing deformity,” in which the central portion of the nail is raised and the lateral portion is depressed (28), are manifestations of lichen planus. A patient with 20 dystrophic nails is unlikely to have onychomycosis. Contact dermatitis occasionally resembles onychomycosis. The correct diagnosis is facilitated by knowledge of known contactants and the presence of contact dermatitis elsewhere on the body.
Finally, repeated nail trauma can cause distal onycholysis, leading to colonization of the affected space by microorganisms that produce pigmentation of the area. If the onycholytic nail is clipped to allow examination of the nail bed, the latter will be normal if the symptoms are caused by trauma rather than onychomycosis. Nail products containing formaldehyde may also cause onycholysis. In this situation, the nails may become yellow and all exposed nails are affected. A habit tic, often manifesting as a median furrow or depression in the middle of the nail, developed from picking at the nail cuticle, may also cause abnormalities of the nail.
Collecting the Nail Specimen
The first step of the sample collection process is thorough cleansing of the nail area with alcohol to remove contaminants such as bacteria. Because the sites of invasion and localization of the infection differ in the different types of onychomycosis, different approaches, depending on the presumptive diagnosis, are necessary to obtain optimal specimens (25). The following collection techniques are recommended.
Distal subungual onychomycosis.
Because dermatophytes in patients with DSO invade the nail bed rather than the nail plate, the specimen must be obtained from the nail bed, where the concentration of viable fungi is greatest (25). The nail should be clipped short with nail clippers, and the specimen should be taken from the nail bed as proximally to the cuticle as possible with a small curet or a no. 15 scalpel blade (25, 30). If debris is insufficient, material should be obtained from the nail bed. Material should also be obtained from the underside of the nail plate, with emphasis placed on sampling from the advancing infected edge most proximal to the cuticle. This is the area most likely to contain viable hyphae and least likely to contain contaminants (25).
Proximal subungual onychomycosis.
Because the fungus invades under the cuticle before settling in the proximal nail bed while the overlying nail plate remains intact, the healthy nail plate should be gently pared away with a no. 15 scalpel blade. A sharp curet can then be used to remove material from the infected proximal nail bed as close to the lunula as possible (25, 30).
White superficial onychomycosis.
Since the infection affects the nail plate surface, a no. 15 scalpel blade or sharp curet can be used to scrape the white area and remove the infected debris.
Candida onychomycosis.
Material is needed from the proximal and lateral nail edges. If Candida onycholysis is suspected, the lifted nail bed should be scraped. Scrapings can be taken from the undersurface of the nail if insufficient debris is present in the nail bed (30).
Specimen Analysis
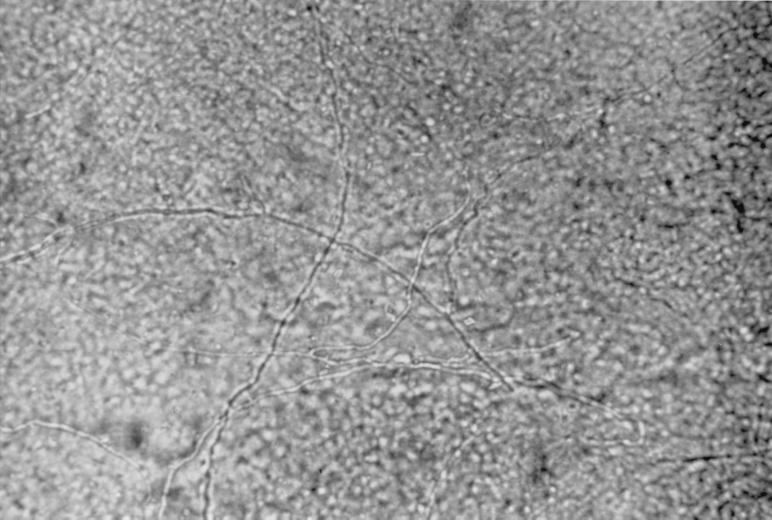
Both direct microscopy and in vitro laboratory culture of sampled material are necessary to definitively identify the etiologic agent (25). The specimen should be divided into two portions for direct microscopy and culture. It is important to understand the limitations of direct microscopy in diagnosing the cause of onychomycosis. The test serves only as a screening test for the presence or absence of fungi but cannot differentiate among the pathogens (Fig. 6). Direct microscopy is often time-consuming, because nail debris is thick and coarse and hyphae are usually only sparsely present (25). The clinician should be aware of the possibility of false-negative results, which occur at a rate of approximately 5 to 15% (26, 63). When examining a KOH preparation, it is important to observe the hyphae closely to determine if they are typical of dermatophyte fungi or have features of nondermatophyte molds or yeasts. A final caveat concerns the common practice of treating nail infections on the basis of a microscopic preparation alone without culturing the putative pathogen. Although direct microscopy can provide clues about the identity of the microorganism, careful matching of microscopic and culture results is necessary for the clinician to be confident of the diagnosis (9). Almost half of all specimens taken from onychomycotic nails fail to yield a pathogen in culture. In onychomycosis, direct microscopy is the most efficient screening technique (26, 63).
FIG. 6.
Potassium hydroxide preparation of a nail specimen showing onychomycosis.
The specimen can be mounted in a solution of 20 to 25% KOH or NaOH mixed with 5% glycerol, heated to emulsify lipids (1 h at 51 to 54°C), and examined under ×40 magnification. An alternative formulation consists of 20% KOH and 36% dimethyl sulfoxide (63). The specimen may be counterstained with chitin-specific Chlorazol black E to accentuate hyphae that are present; this is of particular value if the number of fungal elements is small. This stain is especially useful because it does not stain likely contaminants such as cotton or elastic fibers, which can help prevent false-positive identifications (25). Parker blue-black ink also can be added to the KOH preparation to improve visualization, but this stain is not chitin specific.
Culture is the only method by which the causative microorganism can be identified. Caution should be used in analyzing culture results, because nails are nonsterile and fungal and bacterial contaminants may obscure the nail pathogen (63). Specimens should be plated on two different media: a primary medium that is selective against most nondermatophytic molds and bacteria, and a secondary medium that allows such growth. Cycloheximide inhibits the growth of nondermatophytes and is incorporated into media such as dermatophyte test medium or Sabouraud peptone-glucose agar (Emmons’ modification) with cycloheximide, available under a variety of names (Mycobiotic [Difco Laboratories, Detroit, Mich.] and Mycosel [BBL, Cockeysville, Md.]) (63). Cycloheximide-free media that are commonly used include Sabouraud’s glucose agar, Littman’s oxgall medium, and inhibitory mold agar (Sabouraud’s glucose agar with the addition of antibiotics) (25). If growth occurs on both types of media, the infective agent is probably a dermatophyte, whereas growth only on the cycloheximide-free medium indicates that the infective agent may be a nondermatophyte such as Scopulariopsis brevicaulis, Scytalidium dimidiatum, or Scytalidium hyalinum.
However, growth of a nondermatophyte alone from a specimen that has tested positive for fungi on direct microscopy does not prove conclusively that the infective agent is a nondermatophyte (63). The last three decades have seen unequivocal documentation of the role of nondermatophytes as causal agents in onychomycosis (59). The most common yeast that is involved is C. albicans (58). Of the nondermatophytic filamentous fungi, agents implicated in onychomycosis include members of Scopulariopsis (particularly S. brevicaulis) and Scytalidium (the two most common genera), which are both thought to digest keratin in vivo, as well as members of the genera Alternaria, Aspergillus, Acremonium, and Fusarium (59). Many of these nondermatophyte fungi invade the nail unit directly and cause WSO. Other nondermatophytic fungi that can cause onychomycosis include Onychocola canadensis, Pyrenochaeta unguis-hominis, and Botryodiplodia theobromae (Table 1) (57, 59).
TABLE 1.
Major causes of onychomycosis
| Dermatophyte fungi |
| Epidermophyton floccosum |
| Trichophyton mentagrophytes |
| Trichophyton rubrum |
| Nondermatophyte fungi |
| Acremonium |
| Alternaria species |
| Aspergillus species |
| Botryodiplodia theobromae |
| Fusarium species |
| Onycochola canadensis |
| Pyrenochaeta unguis-hominis |
| Scytalidium dimidiatum scopulariopsis species |
| Scytalidium hyalimum |
| Yeast |
| Candida albicans |
Part of the difficulty in evaluating the role of nondermatophyte fungi cultured from the nail arises because the same fungi that can be laboratory contaminants are also occasionally found to be pathogens (58). Reference laboratories should provide data on whether the isolated fungus was a likely pathogen or an unlikely one. All dermatophytes should be considered pathogens. All other isolated organisms are probably laboratory contaminants unless KOH or microscopy indicates they have the atypical frondlike hyphae associated with nondermatophyte molds or if the same organism is repeatedly isolated. Potential pathogens associated with onychomycosis are listed in Table 1.
To increase the predictive power of a diagnosis of nondermatophytic invasion of a nail, Summerbell (58) suggested that nonfilamentous nondermatophytes identified in nail tissue be categorized as one of the following: contaminant (species growing in culture from dormant propagules on the nails); normal mammalian surface commensal organism; transient saprobic colonizer (colonizer of accessible surface molecules but noninvasive); persistent secondary colonizer (colonizer of material infected by a dermatophyte but incapable of remaining after the dermatophyte is eliminated); successional invader (species that can cause infection after gaining entry into a nail via the disruption caused by a primary pathogen); or primary invader (able to infect and cause onychomycosis in a previously uncolonized nail). Such an analysis has the value of identifying for treatment only nondermatophytic infections that are truly invasive.
As an additional confirmatory technique, definitive identification of nondermatophytic invasion in nails may require the isolation of the agent from successive specimens from the infected region (63). If the infective pathogen is a dermatophyte, subsequent cultures will most probably grow out the dermatophyte itself, a second contaminant unrelated to the first, or no growth at all. Some investigators believe that claims of an increasing proportion of mixed infections in onychomycosis are exaggerated and have gone so far as to state that nondermatophyte molds and yeasts are usually contaminants secondary to dermatophyte onychomycosis and that their presence need not affect treatment outcome (31).
ANTIFUNGAL SUSCEPTIBILITY TESTING
Part of the diagnosis and treatment strategy might include fungal susceptibility testing. Susceptibility testing for antifungal drugs was virtually unknown in the 1980s but is now the focus of interest of the Subcommittee for Antifungal Susceptibility Testing, established in 1982 by the National Committee for Clinical Laboratory Standards (51). The impetus for standardized antifungal susceptibility testing was provided by reports of suboptimal interlaboratory reproducibility; studies showed extensive interlaboratory variation in the absolute MIC of antifungal drugs in the absence of standardized testing methods (51). The Subcommittee began with Candida and Cryptococcus species and, after years of work, developed a broth-based method for testing yeast susceptibility that yields an interlaboratory reproducibility level comparable to that obtained for antibacterial agent testing (51).
By using these susceptibility testing methods, attempts were made to establish interpretive breakpoints above which a given MIC is associated with a known (and unacceptable) probability of treatment failure in candidal infections (51). Tentative breakpoints were established for fluconazole (excluding therapy for C. krusei) and itraconazole (for mucosal candidal infections only) and are now under review (51). Work is now in progress by a National Committee for Clinical Laboratory Standards working group to develop susceptibility testing methods for antifungal agents in dermatophytic infections (10). Susceptibility testing in infections such as onychomycosis becomes more clinically relevant when a choice of effective agents exists, as it does now. In addition, susceptibility testing can help distinguish relapse (reinfection by the same agent) from reinfection (by a new agent) and provide evidence about whether the fungus itself is responsible for treatment failure (10).
ANTIMYCOTIC AGENTS USED TO TREAT ONYCHOMYCOSIS
As can be imagined, treatment of onychomycosis has been attempted throughout the ages, but success has been limited until the current decade. Because of the perception that a superficial cause was to blame for the unsightly lesions, the earliest remedies were topical agents. Topical agents in wide use for treating localized dermatophytic infections include the imidazoles (ketoconazole, econazole, and oxiconazole), the allylamines (naftifine and terbinafine hydrochloride), and the pyridone ciclopirox olamine (18). However, these topical drugs are generally ineffective against fungal infections of the nails due to their inability to penetrate the entire nail unit and eradicate the infection. Only recently, when the fungal nature of these locally invasive infections was appreciated, have systemically active drugs been developed for onychomycosis (7).
It is most fortunate that the efficacy of systemic antimycotics has improved in pace with the increase in incidence of serious systemic fungal infection. In the Western world, this increase in infection is the consequence of wider use of chemotherapy and radiation therapy and the rising incidence of HIV infection, among other factors (7). Limitations of earlier antimycotic agents—narrow therapeutic windows, drug-resistant strains, long treatment courses—have been largely overcome by the use of the newer drugs (fluconazole, itraconazole, and terbinafine). Also, the long period required for growth of the nail necessitates prolonged treatment times, posing problems for compliance and threatening overall efficacy.
Limitations of Traditional Antifungal Agents
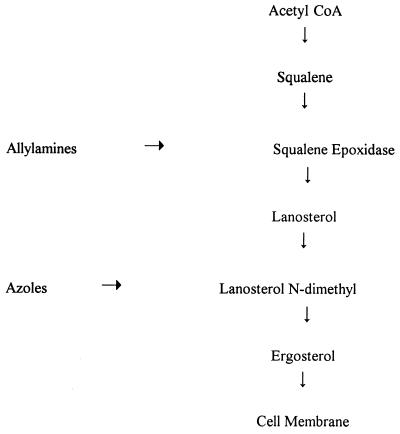
The kingdom of fungi comprises more than 100,000 species of yeasts and molds. They are eukaryotic organisms with a true nucleus and nuclear membrane and with a cell wall that contains chitin. It is the ergosterol in the cell wall of yeasts and molds, however, that constitutes the fungal cell’s Achilles heel; all currently prescribed antifungal agents act in one way or another to inhibit ergosterol synthesis (Fig. 7). The azole drugs include the older imidazole ketoconazole and the new triazoles itraconazole and fluconazole. Griseofulvin, the mainstay of onychomycosis treatment for decades, is active against growing hyphae and may also affect nucleic acid synthesis and arrest fungal cell mitosis in metaphase (13).
FIG. 7.
Synthetic pathway for ergosterol biosynthesis and inhibition by allylamines and imidazoles. CoA, coenzyme A. Reprinted from reference 24 with permission of the publisher.
Griseofulvin.
Griseofulvin represented a promising advance in antifungal therapy when it first became available for clinical use nearly 40 years ago (12). However, its effectiveness in onychomycosis proved a disappointment, since its spectrum of activity is limited to dermatophytes only and a prolonged duration of therapy is required for maximal efficacy (13). Griseofulvin appears to exert its fungistatic effect within the nail matrix, forming a so-called barrier. As a result, only the newly formed nail plate is cleared of the invading organism. Griseofulvin does not persist in the nail plate at therapeutic levels after the end of oral dosing to effect a long-lasting cure, resulting in the need for ongoing treatment as the nail grows out—a period that may last for more than 1 year (25). Poor compliance with long-term therapy as a result of the side effects and the slow and incomplete clearance of dystrophic nails led to success rates as low as 3 to 38%, with recurrence of infection often observed (25). Additionally, the cost of protracted daily therapy with griseofulvin was prohibitive.
Griseofulvin is generally well tolerated, with the most common side effects being associated with hypersensitivity (skin rashes, urticaria; rarely, angioneurotic edema and epidermal necrolysis), which occur in as many as 7% of patients (28, 50). Serious adverse effects such as hepatotoxicity, leukopenia, thrombocytopenia, or anemia are rarely reported. More common side effects of griseofulvin therapy include headache, nausea, and photosensitivity, which are observed in approximately 8 to 15% of patients. Porphyria, hepatocellular failure, and a history of sensitivity to griseofulvin are contraindications to the use of this drug. Griseofulvin affects the efficacy of oral contraceptives and decreases the activity of warfarin-type anticoagulants; if given with barbiturates, its own absorption is decreased. The effects of alcohol are potentiated if it is coadministered with griseofulvin (28, 50).
Ketoconazole.
Ketoconazole, developed in the 1980s, was the first orally active imidazole with a relatively broad spectrum of activity against dermatophytes, some yeasts, and several molds (28, 43). However, the long-term use of oral ketoconazole in onychomycosis, which is necessary to effect improvement or cure, is limited by the occurrence of side effects and significant drug interactions (28). The reported incidence of hepatotoxicity of 1 in 10,000 probably represents underreporting, and fatalities have occurred (43). Patients who take ketoconazole on a long-term basis are obliged to undergo regular liver function tests. Potentially serious drug interactions between ketoconazole and drugs metabolized by the cytochrome P-450 system, as well as other drugs (warfarin, rifampin, isoniazid, cyclosporine, terfenadine, cisapride, and others), have been observed. Other adverse effects of ketoconazole therapy include hypersensitivity reactions, nausea, vomiting, headache, abdominal pain, pruritus, and fever (43). Even in the absence of the new antifungal agents, the relatively poor safety and efficacy data for ketoconazole in long-term regimens limit the use of this agent in onychomycosis.
Advantages of Newer Antifungal Agents
A comparison of some key characteristics of older (griseofulvin and ketoconazole) and newer (itraconazole, fluconazole, terbinafine) antifungal drugs is presented in Table 2. The azoles and terbinafine block the ergosterol synthesis pathway at different points, a difference with implications for these drugs’ efficacy and side effects (Fig. 7). Itraconazole and fluconazole inhibit the formation of ergosterol at the demethylation step, where lanosterol is converted to 14-dimethyl lanosterol. This demethylation depends on activation of the cytochrome P-450 system; one of the N atoms in the five-member azole ring binds to the heme iron of cytochrome P-450, resulting in the inhibition of the P-450-dependent enzyme 14α-demethylase, whose normal function is to mediate the synthesis of ergosterol (15). However, because cytochrome P-450 enzymes are involved in a variety of synthetic processes (the formation of testosterone and other steroids) and detoxification (ethanol), the potential exists for certain reversible side effects related to inactivation of this system at doses as high as those that are needed in ketoconazole treatment for onychomycosis (24). Compared with ketoconazole, itraconazole and fluconazole bind more weakly to mammalian cytochrome P-450 enzymes; however, these newer drugs retain a high affinity for fungal P-450 enzyme sites. The consequences of this include a decreased probability of side effects related to steroid synthesis and a lower risk of hepatotoxicity (24).
TABLE 2.
Characteristics of five antifungal drugsa
| Variable | Characteristic for:
|
||||
|---|---|---|---|---|---|
| Griseofulvin | Ketoconazole | Itraconazole | Fluconazole | Terbinafine | |
| Systemic administration | Oral | Oral | Oral | Oral or intravenous | Oral |
| Related concerns or problems | Tolerance, treatment length | Toxicity, interactions | Interactions | Interactions, Candida resistance | Adverse events |
| Affinity for keratin | Low | High | High | High | High |
| Excretion in sweat | High | High | Minimal | High | None |
| Excretion in sebum | Limited | Limited | High | Limited | High |
| Drug concnb | P = S | P = S | S > P | S > P | P = S |
| Time to reach stratum corneum | Epidermis within 8 h | Unknown | Within 24 h | Within 24 h | Within 8 h |
| Appropriate for skin | Yes | Yes | Yes | Yes | Yes |
| Appropriate for onychomycosis | No | No | Yes | Yes | Yes |
Adapted from reference 7 with permission of the publisher.
P, plasma; S, skin.
The allylamines inhibit ergosterol formation earlier in the pathway than the imidazoles, at the point where squalene is converted to squalene epoxide, a step that does not require cytochrome P-450. Thus, side effects associated with cytochrome P-450-mediated actions, such as steroidogenesis, do not occur. The resulting intracellular accumulation of squalene exerts a disruptive effect on the fungal cell membrane, a step that is likely to be fungicidal, whereas the ergosterol deficiency is probably fungistatic (24).
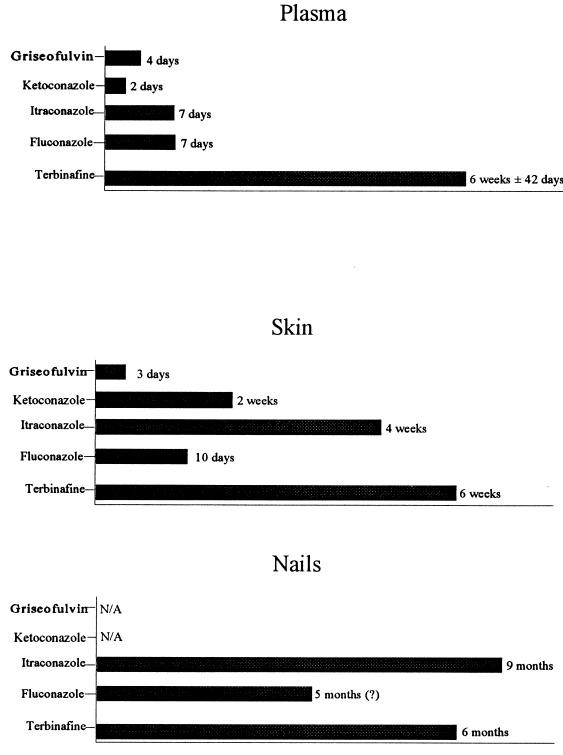
A brief description of each agent, including pharmacokinetic data, follows. Because the antifungal agent must be present at the infected site in therapeutic concentrations long enough to effect a cure, it is important to understand the persistence of these drugs in plasma, skin, and nails. Figure 8 compares griseofulvin, ketoconazole, itraconazole, fluconazole, and terbinafine with respect to persistence in the nail unit (16).
FIG. 8.
Persistence of oral antifungal drugs in plasma, skin, and nails. Reprinted from reference 16 with permission of the publisher.
Fluconazole.
Fluconazole, a hydrosoluble bis-triazole drug, was developed in the 1990s and is available for oral and intravenous usage. Fluconazole has high bioavailability (>90%) because of its low molecular weight and high water solubility. With only 11% binding to plasma proteins, the majority of the ingested dose circulates in free form. Concentrations of 1.0 μg/ml in serum are attained after a single 50-mg dose of fluconazole, with peak levels in plasma in healthy volunteers of 1.9 and 6.7 μg/ml after doses of 100 and 400 mg, respectively. Continued administration results in a peak concentration in plasma that is 2.5 times higher than that seen after a single dose. The long half-life (22 to 37 h) allows once-daily dosing; complete elimination from systemic circulation occurs within 1 week. Fluconazole is metabolically stable and is excreted almost unchanged in the urine, with 91% recovered in the urine and 2% recovered in the feces. The fate of the remaining 7% is unknown. The health status of the patient may influence fluconazole pharmacokinetics; for example, old age and renal disease are associated with an increase in the t1/2 (16).
Figure 8 shows the persistence of fluconazole in plasma, skin, and nails. The compound has limited affinity for tissues but is detectable in the skin within 3 h of initial therapy. In nails, diffusion from the nail bed appears to be a key route of penetration. In studies with male volunteers, fluconazole concentrations in nails on days 1 and 14 of treatment (50 mg/day) were 1.3 and 1.8 μg/g, respectively (16). A long-term study (33) found fluconazole levels of approximately 2 μg/g in healthy and diseased nails after 6 months of treatment. The drug persists in nails for approximately 5 months after discontinuation of therapy (Fig. 8).
Fluconazole has demonstrated good activity against dermatophyte fungi and many Candida spp. The side effects of fluconazole include headache, nausea, and gastrointestinal upset; these are generally infrequent. Because it inhibits the cytochrome P-450 enzyme system, fluconazole has some potentially significant drug interactions. It should not be coadministered with oral hypoglycemic agents, phenytoin, cyclosporine, rifampin, theophylline, or terfenadine. Fluconazole is not currently approved in the United States for use in onychomycosis.
Studies of fluconazole in onychomycosis show high cure rates but a need for long treatment times. For example, in one study of 20 patients with toenail onychomycosis (all 20 patients) and fingernail onychomycosis (4 patients), 150 mg of fluconazole per week and 40% urea ointment were administered for an average of 9.3 months. At the end of treatment, 92% of affected nails were mycologically cured, with 83% of toenails and 100% of fingernails remaining so at the last follow-up visit (45).
Two recent placebo-controlled trials of fluconazole at three dose levels (150, 300, and 450 mg) showed high efficacy and good tolerability in patients with DSO. In the study of 362 patients with onychomycosis of the toenail, once-weekly dosing with fluconazole at any of the three doses resulted in a clinical success rate between 80 and 90%. The mean time to clinical success was 6 to 7 months (56). Mycologic cure rates at 6 months were 53, 59, and 61% for the three dose levels, respectively, versus 16% for placebo (56). The incidence and severity of adverse events were similar for fluconazole- and placebo-treated patients. In the study of fluconazole in fingernail onychomycosis (21), 349 patients were randomized and evaluated after 6 months of treatment. Clinical cure rates were 76, 85, and 90% for patients treated with 150, 300, and 450 mg, respectively, versus 3% for patients treated with placebo. Again, the rate of adverse events was similar for patients regardless of the treatment group and were reported by 33, 39, 35, and 34% of patients in the 150-, 300-, and 450-mg group and the placebo group, respectively. In both studies, the most common treatment-related adverse event was headache (reported by 6% of fluconazole patients in both studies, and by 2 and 8% of patients with toenail and fingernail infections, respectively).
Itraconazole.
The broad-spectrum, synthetic antifungal triazole itraconazole was approved in 1995 for the treatment of onychomycosis and is available in capsule form and as an oral solution. Itraconazole represents an advance beyond earlier therapies due to its broad spectrum of activity, its high affinity for keratin (16), and its pharmacokinetic profile. Itraconazole is rapidly absorbed, with peak levels attained within 4 h. It is subject to extensive first-pass metabolism and has a maximal oral bioavailability of about 56%. Peak levels in plasma of 0.127 and 0.272 μg/ml are reached after a single oral dose of 100 or 200 mg, respectively. Steady-state concentrations are reached after 10 to 14 days of dosing and are three to four times higher than after a single dose. Itraconazole has a high affinity for proteins, and 99.8% of the circulating compound is bound to plasma proteins. However, the distribution of itraconazole in tissues is considerably higher, and the tissue/plasma concentration ratio ranges from 1:1 in the brain to 25:1 in fat (16). Itraconazole is rapidly eliminated from the systemic circulation within 7 to 10 days and is excreted in the urine (35%) and feces (54%).
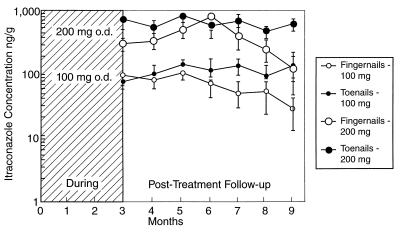
Itraconazole reaches the site of infection within 24 h of administration and can be detected in the distal nail plate as soon as 1 month after the beginning of therapy (46). The rapid diffusion into the distal nail indicates that itraconazole penetrates the nail unit via the nail bed, which is confirmed by the twofold-higher drug levels in subungual nail material than in distal nail clippings of a patient treated with this drug for 1 month (16). An early analysis of the kinetics of itraconazole penetration demonstrated the persistence of high drug levels in fingernails and toenails after the cessation of therapy (Fig. 9) (53). As can be seen in Fig. 8, itraconazole persists in the nail for longer than fluconazole or terbinafine and can be detected for as long as 9 months (16). Mean maximal itraconazole levels in toenails in two published studies were 149 ng/g after a 100-mg dose and 99 ng/g after a 200-mg dose; the corresponding values for levels in fingernails were 111 and 80 ng/g, respectively (16). Concentrations in toenails remain elevated longer than do those in fingernails after cessation of therapy. By contrast, levels of itraconazole in plasma decrease rapidly and the compound is completely eliminated 1 week after a pulse dose (16).
FIG. 9.
Concentration of itraconazole in distal nail clippings after treatment. Reprinted from reference 53 with permission of the publisher.
Although initially indicated for the treatment of onychomycosis due to dermatophytes, itraconazole may also be effective in nondermatophytic infections. For example, one study involved 36 patients with toenail onychomycosis caused by nondermatophyte molds alone or in combination with dermatophytes. Patients received itraconazole as continuous therapy or as a one-week pulse regimen. At 12 months follow-up, clinical and mycologic cures were seen in 88% of the patients whose infections were caused by a single mold (17).
Evidence from several clinical trials supports the effectiveness of itraconazole in onychomycosis (17, 38, 48). In three multicenter, randomized, double-blind, placebo-controlled studies, a total of 214 patients received either itraconazole (200-mg capsules once daily) or a matching placebo for 12 weeks; this was followed by a 9-month follow-up period (29). The patients had onychomycosis of the toenail confirmed by direct microscopy and culture, with at least 25% involvement of the entire nail bed of a great toe (29). Patients with total dystrophic nail bed disease were also included. T. rubrum was the most common organism isolated. Results in all three studies showed significantly greater improvement in patients treated with itraconazole than in those treated with placebo. Mean severity scores, investigator assessment, clinical success ratings, mean length of unaffected nail, mean nail growth, and mycologic cure rates were all significantly greater in the itraconazole group. Clinical success was achieved by 65% of itraconazole-treated patients versus 3% of placebo-treated patients, while mycologic cure was achieved by 54 and 6% of patients, respectively. The relapse rate among patients who received itraconazole was 21%; because no patients who received placebo achieved overall success, a relapse rate could not be computed for this group. However, it is difficult to determine if relapse was a result of reinfection or recurrence of the original infection, because molecular strain typing of pathogens was not performed.
Evidence from research concerning the pharmacokinetics of itraconazole in the nail has been used to help develop optimal dosing strategies for treatment of onychomycosis (17). Due to its rapid penetration into the nail and prolonged presence in the nail after discontinuation of drugs, the treatment course with itraconazole can be reduced to only one-week intervals. Shorter dosing regimens have advantages in terms of cost and a greater likelihood that patients will comply with treatment, compared with longer regimens (49). Consequently, itraconazole has been evaluated in intermittent dosing or “pulse therapy” regimens. Pulse therapy with itraconazole consists of dosing for 1 week (pulse) per month for a set number of months (17, 49). Examples of regimens that have been evaluated for safety and efficacy in randomized trials include a three- and a four-pulse regimen with doses of 200 mg of itraconazole twice daily (17), a three-pulse regimen only with 200 mg of itraconazole twice daily (38), and a two-pulse regimen with 200 mg of itraconazole twice daily (49).
The two-pulse regimen was evaluated in a randomized, placebo-controlled, multicenter study of 73 patients with onychomycosis of the fingernail confirmed by direct microscopy and culture. Patients received either 200 mg of itraconazole twice daily or placebo for the first 7 days of each month for two consecutive months. The patients were followed up for an additional 19 weeks (total study time, 24 weeks). Significantly more itraconazole-treated than placebo-treated patients achieved clinical success (77 versus 0%), mycologic success (73 versus 13%), and overall success (68 versus 0%) during the study, and no patient who received itraconazole experienced a clinical or mycologic relapse during follow-up.
Itraconazole is generally well tolerated, with fewer than 7% of treated dermatology patients reporting side effects. The most common side effects are in the category of nuisance side effects such as nausea, gastrointestinal distress, and headache. Hepatic reactions are rare. Because it inhibits the cytochrome P-450 enzyme system, itraconazole should not be taken with terfenadine, astemizole, simvastatin, lovastatin, midazolam, triazolam, or cisapride (42). Additionally, drugs with narrow therapeutic windows, such as coumarin, may require plasma monitoring.
Terbinafine.
The allylamine antifungal agent terbinafine is effective against dermatophytes and some molds but has less activity in C. albicans infections. It is strongly lipophilic and is well distributed in the skin, fat, and nails. It binds strongly to plasma proteins but also has high affinity for tissues. Oral terbinafine is well absorbed, with 70 to 80% of the ingested dose being absorbed. Maximal levels of terbinafine are attained within 2 h. Terbinafine undergoes extensive hepatic metabolism, with excretion being primarily (>70%) in the urine. Maximal levels in plasma after a single oral dose of 250 or 500 mg of terbinafine are 0.9 and 1.7 to 2.0 μg/ml, respectively. Steady-state levels are attained after 10 to 14 days of treatment, and low levels of terbinafine in plasma can be measured for as long as 6 weeks after cessation of therapy (16).
Terbinafine appears to diffuse into the nail plate via the nail bed and nail matrix, as evidenced by its appearance in distal nail clippings 7 days after administration (16). Because terbinafine concentrations in the nail do not increase during treatment, it is likely that the drug penetrates via both the nail bed and nail matrix rather than by incorporation into matrix cells (16). Therapeutic levels of terbinafine can be detected in the nails as long as 6 months after discontinuation of treatment (28).
Terbinafine administered for 12 months was initially shown to effect cure rates as high as 80% in dermatophytic infections of the toenails (34), with relapse rates of 20% and minimal side effects. Because mycologic cure was observed to occur early in these studies, shorter treatment times were evaluated in subsequent trials. In one study, 250 mg of terbinafine or placebo per day was administered to 85 patients with mycologically proven dermatophyte onychomycosis of the toenails (75 patients) or fingernails (10 patients) for a duration of 12 weeks, with long-term follow-up of 36 weeks. Results for toenail infections showed rates of mycological cure at follow-up of 82% among terbinafine-treated patients and 12% among placebo-treated patients (34). Cure rates of fingernail infections were 71% in terbinafine-treated patients at follow-up. All the results showed a statistically significant advantage for terbinafine over placebo. Other studies concurred in finding a 12-week treatment period to be optimal (62).
An even shorter treatment regimen was evaluated in a recent randomized, double-blind study involving 152 patients with toenail onychomycosis, of whom 25 also had fingernail onychomycosis (62). Two regimens were compared for safety and efficacy: one with 250 mg of terbinafine per day for 6 weeks, and the other with 250 mg of terbinafine per day for 12 weeks (60). Patients were evaluated at 6, 12, 18, 24, and 36 weeks after the study began; those in the 6-week group received placebo pills for the last 6 weeks of the study (60). Clinical response rates for toenail infections (more than 4 mm of normal nail outgrowth, sum of signs and symptoms) were similar for the two treatment groups. However, overall efficacy, as represented by a “cure” variable that took into account both clinical and mycologic results, was not equivalent in the two groups; patients who received 6 weeks of treatment had an overall cure rate of 45.9%, in contrast to those who received 12 weeks of treatment, who had an overall cure rate of 58.9%. A small number of patients (25 patients) also had fingernail onychomycosis. All of these patients in the 6-week treatment group showed significant improvement at week 24. The authors concluded that terbinafine treatment lasting 6 weeks is not sufficient to effect a cure in toenail onychomycosis, although fingernail onychomycosis appears to respond well to such a regimen. At present, the recommended duration of treatment with 250 mg of terbinafine per day is 6 weeks for fingernail infections and 12 weeks for toenail infections (47a).
Terbinafine was evaluated in 65 patients with onychomycosis caused by a nondermatophyte (culture-confirmed C. albicans, C. parapsilosis, or S. brevicaulis) at a regimen of 250 mg of terbinafine per day for as long as 48 weeks. Results showed clinical cure rates for disease due to the three microorganisms of 54, 63, and 37%, respectively; mycologic cure rates for C. albicans and C. parapsilosis were 70 and 85%, respectively. However, adverse events were reported by 43% of the patients (47). Terbinafine was also evaluated in a randomized study of 118 patients with dermatophytic onychomycosis and secondary colonization by nondermatophytic molds or yeasts. The patients received 250 mg of terbinafine per day for 12 weeks or, if needed, an additional 12 weeks beginning at week 28 after the onset of treatment. An overall mycologic cure rate of 94% was seen by week 48; 80% of the patients remained mycologically negative at 2 years follow-up (32).
The safety profile of terbinafine is generally favorable, with most side effects being categorized as minor. Clinically significant drug interactions are limited to cimetidine and rifampin. The most commonly reported adverse reactions involve headache, gastrointestinal symptoms, liver test abnormalities, and rash (47a). In addition to nuisance side effects, which are similar to those associated with itraconazole, other side effects have been reported with terbinafine, including cholestasis (23), rash (36), taste disturbance (19), possible vision disturbance (35), and hematologic abnormalities (19).
ANTIFUNGAL THERAPY
Comparative Trials of Antifungal Agents
Oral itraconazole and oral terbinafine are clearly superior to griseofulvin in treating onychomycosis, with cure rates in the range of 70% generally reported (53, 66). Whether taken for 1 week of every month or daily for 12 weeks, the new antifungal agents offer advantages over griseofulvin in terms of cost, compliance, and drug exposure.
Although there are no comparative trials that include fluconazole, several recent trials compared itraconazole and terbinafine in patients with dermatophytic infections of the nails (4, 61). In an open, randomized study of 53 patients with toenail onychomycosis, similar cure rates were obtained in 27 patients taking 200 mg of itraconazole per day and 26 patients taking 250 mg of terbinafine per day for 3 months. The clinical and mycologic cure rates for itraconazole- and terbinafine-treated patients were 60.9 and 64.7%, with improvement in the remaining patients (4).
A more recent study (61) found no treatment-related differences in 63 patients with onychomycosis of the toenails (60 patients) or fingernails (3 patients) who were randomly assigned to receive 250 mg of terbinafine per day (group 1), 500 mg of terbinafine per day for 1 week every month (group 2), or 400 mg of itraconazole per day for 1 week every month (group 3). Cure rates in this study after 4 months of treatment in these three treatment groups were statistically similar, averaging approximately 80% for toenail onychomycosis. Ten months after the beginning of treatment, the mycologic cure rates remained high. None of the intergroup differences were statistically significant. All patients with fingernail infections only were mycologically cured at the end of the 2-month treatment period (two patients received 250 mg of terbinafine, one received 400 mg of itraconazole).
A study by De Backer et al. (14) compared 186 patients with pedal onychomycosis who were treated for 12 weeks with 250 mg of terbinafine daily and 186 patients who were treated for 12 weeks with 200 mg of itraconazole per day. The mycologic cure rates were 73% for terbinafine and 48% for itraconazole, and the clinical cure rates were 76% for terbinafine and 50% for itraconazole. Both drugs were well tolerated (14). In a third comparative study by Brautigam et al. (5), 86 patients each took 250 mg of terbinafine daily or 200 mg of itraconazole daily. The mycological cure rates were 81% for terbinafine and 63% for itraconazole (5). A recently published study by Honeyman et al. (41) compared 200 mg of oral itraconazole per day (85 patients) and 250 mg of terbinafine per day (82 patients) for 4 months. The clinical cure rates at the 12th month were 57.8% in the terbinafine group and 62.9% in the itraconazole group. The mycological cure rates at the 12th month were 84.3 and 95.3% for itraconazole and terbinafine, respectively. Actual clinical experience with itraconazole and terbinafine indicates that the cure rates are similar for these two agents.
Adjuncts to Systemic Therapy
Although the use of topical agents alone or in combination with oral therapies has been disappointing, topical agents may be useful in preventing the relapse of chronic tinea pedis, which often accompanies onychomycosis. Concomitant use of topical therapy with the newer oral antifungal agents may result in a more rapid cure, a possibility that will be evaluated in clinical trials.
Although nail surgery is useful in some cases of onychomycosis, it is a painful and disfiguring process and should be limited to one or only a few nails if used at all. Paronychia associated with pain, contraindications to the use of systemic antifungal agents, and the presence of drug-resistant pathogens are conditions that could favor the use of surgery. Such cases may require a combination of surgery, systemic, and topical approaches.
Prevention of Relapse after Treatment
Even with apparently optimal diagnosis and treatment, one in five onychomycosis patients is not cured by current therapies. This is a considerable improvement over previous cure rates of around 20% (with griseofulvin). The reasons for the 20% failure rate include inaccurate diagnosis; misidentification of the pathogen; and the presence of a second disorder, such as psoriasis (10). In some cases, characteristics of the nails, such as slow growth or excessive thickness, make treatment difficult. The presence of a high fungal inoculum and/or drug-resistant microorganisms is particularly challenging. Host-related factors such as a compromised immune system (typically seen in individuals infected with HIV), diabetes mellitus, or peripheral vascular disease may also impede success (10).
In addition to specific drug therapies, some patients may benefit from several hygiene measures that may prevent relapse. Patients should wear thong sandals in public showers and slippers in hotel rooms and should rest shoes periodically to limit exposure to infectious fungi. Unfortunately, some people seem to have a genetic predisposition to fungal infections, and these people may experience relapses despite careful care and effective therapy (67). Finally, all patients should be urged to comply with the treatment protocol; poor compliance continues to be a problem, particularly with long treatment courses.
Selection of an Appropriate Therapy
Two general categories of concern are important in the context of selection of appropriate therapy: disease-oriented and patient-oriented factors. Selection of an agent depends, first and foremost, on the accurate identification of the infecting organism. However, of considerable importance, too, are patient-related factors that may influence decision-making.
In the case of infection by a dermatophyte, itraconazole and terbinafine provide excellent efficacy and safety profiles. Both can be used in regimens of short duration. Patient-related factors of concern include the likelihood of compliance, which may be increased by shorter regimens in some patients and by more consistent (nonintermittent) regimens in others. Onychomycosis due to Candida infections can be effectively treated by itraconazole or fluconazole. Reports of drug-resistant Candida species in onychomycosis have not appeared, although this has been documented for fluconazole in long-term treatment of oral candidiasis in immunocompromised patients. In the case of infection caused by other nondermatophytes, itraconazole, alone among the new agents, has broad spectrum of activity. Further studies of newer antimycotics, both singly and in combination, will be valuable in identifying ways to improve onychomycosis therapy.
Clinical Management of Treated Patients
Although low, the possibility exists of hepatic injury during therapy with the newer antifungal agents, and it should remain a consideration. Liver function tests at baseline and periodically during therapy should be performed for patients receiving continuous therapy with terbinafine, fluconazole, or itraconazole. Patients receiving terbinafine are also advised to undergo baseline and periodic complete blood counts as well. Pulse therapy with itraconazole does not require laboratory monitoring. The recommendations for intermittent therapy with fluconazole are unknown. In all cases, patients should be educated so that they can recognize and report signs of drug-related adverse reactions, including jaundice, upper abdominal tenderness, malaise, dark urine, pale stools, fatigue, nausea, and vomiting.
Educating Patients about Their Role in Treatment
The importance of instilling realistic expectations in patients with onychomycosis can hardly be overemphasized. Patients should be told that, just as onychomycosis developed gradually over several months or years, a cure is not likely to be achieved overnight. In fact, particularly for toenail infections, treatment may require several months. The positive aspect of modern treatments is that a cure is probable and side effects are rare. Immunocompromised patients face a particularly difficult situation with respect to compliance, because cure may take even longer in their cases.
Patients with onychomycosis often need to break old habits and learn new, healthier habits to achieve an optimal therapeutic response and prevent reinfection. Appropriate nail care should be explained and demonstrated to the patient, and the importance of factors such as wearing loose shoes, keeping nails short, and stopping a range of potentially risky behaviors (going barefoot in public places, wearing open sandals, wearing other people’s shoes, and exposing feet or hands to damp environments) should be stressed. Antifungal powders should be used once a week to help keep shoes free from pathogens. Individuals with tinea pedis are at increased risk for onychomycosis and may need specific instruction to avoid its development. In addition to the above instructions, patients should be advised to be on the alert for recurrence of infection.
Physician support staff can also play a role in educating and supporting the patient. Every effort should be made to empathize with the frustration that patients may feel during prolonged treatment regimens and to reinforce patient compliance with the prescribed plan. Patients on pulse regimens may benefit from a calendar that clearly delineates drug “on” days and weeks, dates for laboratory testing, and follow-up office visits.
CONCLUSION
Although regional and temporal variability exists among the microorganisms that are pathogenic in onychomycosis, this disease is caused primarily by dermatophytes. After decades of frustration and disappointment with this stubborn infection, dermatologists and other clinicians now have access to drugs with high cure rates and excellent safety profiles. Moreover, short treatment times increase patient compliance, reduce treatment costs, and allow patients to feel hopeful that their unsightly infections will be ended.
Perhaps the most important task of the clinician is accurate diagnosis of the causal agent. Direct microscopy and culture are both necessary to ensure this. Selection of an optimal antifungal drug whose spectrum of activity encompasses the infecting microorganism can proceed only with accurate diagnosis.
In the last decade, there have been significant advances in the development of effective and safe drugs for onychomycosis. What remains to be achieved? Unfortunately, onychomycosis is likely to remain a disease of modern civilization. The environmental conditions that foster it, longer life expectancies, and the increasing numbers of immunocompromised individuals have combined to increase its prevalence. Against this trend are the new antifungal drugs itraconazole, terbinafine, and fluconazole. But perhaps the single area most deserving of our attention in the near future is that of improving diagnostic methods. Diagnostic methodology and fungal susceptibility testing lag behind therapeutic advances. We should turn our attention to these problems.
REFERENCES
- 1.Aly R. Ecology and epidemiology of dermatophyte infections. J Am Acad Dermatol. 1994;31:S21–S25. doi: 10.1016/s0190-9622(08)81262-5. [DOI] [PubMed] [Google Scholar]
- 2.Aly, R., and T. Berger. 1996. Common superficial fungal infections in patients with AIDS. Clin. Infect. Dis. 22(Suppl. 2):S128–S132. [DOI] [PubMed]
- 3.André J, Achten G. Onychomycosis. Int J Dermatol. 1987;26:481–490. doi: 10.1111/j.1365-4362.1987.tb02287.x. [DOI] [PubMed] [Google Scholar]
- 4.Arenas R, Dominguez-Cherit J, Fernandez L M. Open randomized comparison of itraconazole versus terbinafine in onychomycosis. Int J Dermatol. 1995;34:138–143. doi: 10.1111/j.1365-4362.1995.tb03600.x. [DOI] [PubMed] [Google Scholar]
- 5.Brautigam M, Nolting S, Schoff R E, Weidinger G for the Seventh Lamisil German Onychomycosis Study Group. Randomized double blind comparison of terbinafine and itraconazole for treatment of toenail tinea infection. Br Med J. 1995;311:919–922. doi: 10.1136/bmj.311.7010.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodell R T, Elewski B E. Superficial fungal infections: errors to avoid in diagnosis and treatment. Postgrad Med. 1997;101:279–287. doi: 10.3810/pgm.1997.04.209. [DOI] [PubMed] [Google Scholar]
- 7.Brody N. Cutaneous fungal infections: innovative treatment schedules with systemic agents. Int J Dermatol. 1995;34:284–289. doi: 10.1111/j.1365-4362.1995.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 8.Charif M A, Elewski B E. A historical perspective on onychomycosis. Dermatol Ther. 1997;3:43–45. [Google Scholar]
- 9.Clayton, Y. M., and R. J. Hay. 1993. Epidemiology of fungal skin and nail disease: roundtable discussion held at Dermatology 2000, Vienna, 17 May 1993. Br. J. Dermatol. 130(Suppl. 4):9–11. [DOI] [PubMed]
- 10.Clinical Courier. New strategies for the effective management of superficial fungal infections. Clin Courier. 1997;16:2–3. [Google Scholar]
- 11.Clinical Courier. Superficial fungal infections: diagnosis and management of onychomycosis. Clin Courier. 1994;12:2. [Google Scholar]
- 12.Cohen J L, Scher R K, Pappert A S. The nail and fungus infections. In: Elewski B, editor. Cutaneous fungal infections. New York, N.Y: Igaku-Shoin Inc.; 1992. pp. 106–122. [Google Scholar]
- 13.Davies R R. Griseofulvin. In: Speller D C E, editor. Antifungal chemotherapy. Chichester, England: John Wiley & Sons Ltd.; 1980. pp. 149–182. [Google Scholar]
- 14.De Backer, P., P. De Keyser, C. De Vroey, and E. Lesaffre. 1996. A 12-week treatment for dermatophyte toe onychomycosis: terbinafine 250 mg/day vs. itraconazole 200 mg/day—a double-blind comparative trial. Br. J. Dermatol. 134(Suppl. 46):16–17. [DOI] [PubMed]
- 15.Decroix J, Fritsch P, Picoto A, Thrülimann W, Degreef H. Short-term itraconazole versus terbinafine in the treatment of superficial dermatomycosis of the glabrous skin (tinea corporis or cruris) Eur J Dermatol. 1997;7:353–357. [Google Scholar]
- 16.De Doncker P. Pharmacokinetics of oral antifungal agents. Dermatol Ther. 1997;3:46–57. [Google Scholar]
- 17.De Doncker P, Decroix J, Pierard G E, Roelant D, Woesternborghs R, Jacqmin P, Odds F, Heremans A, Dockx P, Roseeuw D. Antifungal pulse therapy for onychomycosis: a pharmacokinetic and pharmacodynamic investigation of monthly cycles of 1-week pulse therapy with itraconazole. Arch Dermatol. 1996;132:34–41. doi: 10.1001/archderm.132.1.34. [DOI] [PubMed] [Google Scholar]
- 18.Del Rosso J Q. Advances in the treatment of superficial fungal infections: focus on onychomycosis and dry tinea pedis. J Am Osteopath Assoc. 1997;97:339–345. doi: 10.7556/jaoa.1997.97.6.339. [DOI] [PubMed] [Google Scholar]
- 19.Del Rosso J Q, Gupta A K. Oral antifungal agents: recognition and management of adverse reactions. Today’s Ther Trends. 1997;15:75–84. [Google Scholar]
- 20.Dompmartin D, Dompmartin A, Deluol A M, Grosshans E, Coulaud J P. Onychomycosis and AIDS: clinical and laboratory findings in 62 patients. Int J Dermatol. 1990;29:337–339. doi: 10.1111/j.1365-4362.1990.tb04755.x. [DOI] [PubMed] [Google Scholar]
- 21.Drake, L., D. Babel, D. M. Stewart, P. Rich, M. R. Ling, D. Breneman, R. K. Sher, A. G. Martin, D. M. Pariser, R. J. Pariser, C. N. Ellis, D. Friedman, H. I. Katz, C. J. McDonald, J. Muglia, R. C. Savin, G. Webster, B. E. Elewski, J. J. Leyden, A. D. Monroe, E. H. Tschen, J. M. Hanifin, M. R. Morman, J. L. Shupack, N. Levine, N. J. Lowe, W. F. Bergfeld, C. Camisa, D. S. Feingold, N. Konnikov, R. B. Odom, R. Aly, and D. L. Greer. A placebo-controlled, randomized, double-blind trial of once-weekly fluconazole (150, 300, or 450 mg) in the treatment of distal subungual onychomycosis of the fingernail. J. Am. Acad. Dermatol., in press. [DOI] [PubMed]
- 22.Drake L A, Dinehart S M, Farmer E R, Goltz R W, Graham G F, Hordinsky M K, Lewis C W, Pariser D M, Skouge J W, Webster S B, Whitaker D C, Butler B, Lowery B J. Guidelines of care for superficial mycotic infections of the skin: onychomycosis. J Am Acad Dermatol. 1996;34:116–121. doi: 10.1016/s0190-9622(96)80136-8. [DOI] [PubMed] [Google Scholar]
- 23.Dwyer C M, White M I, Sinclair T S. Cholestatic jaundice due to terbinafine. Br J Dermatol. 1997;136:968–981. doi: 10.1111/j.1365-2133.1997.tb03954.x. [DOI] [PubMed] [Google Scholar]
- 24.Elewski B E. Mechanisms of action of systemic antifungal agents. J Am Acad Dermatol. 1993;28:S28–S34. doi: 10.1016/s0190-9622(09)80305-8. [DOI] [PubMed] [Google Scholar]
- 25.Elewski B E. Clinical pearl: diagnosis of onychomycosis. J Am Acad Dermatol. 1995;32:500–501. doi: 10.1016/0190-9622(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 26.Elewski B E. Large scale epidemiological study of the causal agents of onychomycosis: mycological findings from the multicenter onychomycosis study of terbinafine. Arch Dermatol. 1997;133:1317–1318. [PubMed] [Google Scholar]
- 27.Elewski B E, Charif M A. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172–1173. . (Letter.) [PubMed] [Google Scholar]
- 28.Elewski B E, Hay R J. Update on the management of onychomycosis: highlights of the third annual international summit on cutaneous antifungal therapy. Clin Infect Dis. 1996;23:305–313. doi: 10.1093/clinids/23.2.305. [DOI] [PubMed] [Google Scholar]
- 29.Elewski B E, Scher R K, Aly R, Daniel III R, Jones H E, Odom R B, Zaias N, Jacko M L. Double-blind, randomized comparison of itraconazole capsules vs. placebo in the treatment of toenail onychomycosis. Cutis. 1997;59:217–220. [PubMed] [Google Scholar]
- 30.Elewski B E, Rinaldi M G, Weitzman I. Diagnosis and treatment of onychomycosis. a clinician’s handbook. Califon, N.J: Gardiner-Caldwell SynerMed; 1995. [Google Scholar]
- 31.Ellis D H, Watson A B, Marley J E, Williams T G. Non-dermatophytes in onychomycosis of the toenails. Br J Dermatol. 1997;136:490–493. [PubMed] [Google Scholar]
- 32.Ellis, D. H., J. E. Marley, A. B. Watson, and T. G. Williams. 1997. Significance of non-dermatophyte molds and yeasts in onychomycosis. Dermatology 194(Suppl. 1):40–42. [DOI] [PubMed]
- 33.Fraki J, Heikkilä H T, Kero M O, Kuokkanen K E, Oksman R O, Rantanen T T, Saari S S, Sten M L, Stubb S H A, Uggeldahl P E. Dermatology 2000 (symposium) 1991. Fluconazole in the treatment of onychomycosis: an open, non-comparative study with oral 150 mg fluconazole once weekly, abstr. 14. [Google Scholar]
- 34.Goodfield, M. J. D. 1992. Short-duration therapy with terbinafine for dermatophyte onychomycosis: a multicentre trial. Br. J. Dermatol. 126(Suppl. 39):33–35. [DOI] [PubMed]
- 35.Gupta A K. The development of green vision in association with terbinafine therapy. Arch Dermatol. 1996;132:845–846. doi: 10.1001/archderm.132.7.845. [DOI] [PubMed] [Google Scholar]
- 36.Gupta A K, Kopstein J B, Shear N H. Hypersensitivity reaction to terbinafine. J Am Acad Dermatol. 1997;36:1018–1019. doi: 10.1016/s0190-9622(97)80293-9. [DOI] [PubMed] [Google Scholar]
- 37.Gupta A K, Sibbald R G, Lynde C W, Hull P R, Prussick R, Shear N H, De Doncker P, Daniell III C R, Elewski B E. Onychomycosis in children: prevalence and treatment strategies. J Am Acad Dermatol. 1997;36:395–402. doi: 10.1016/s0190-9622(97)80215-0. [DOI] [PubMed] [Google Scholar]
- 38.Havu V, Brandt H, Heikkilä H, Hollne A, Oksman R, Rantanen T, Saari S, Stubb S, Turjanmaa K, Piepponen T. A double-blind, randomized study comparing itraconazole pulse therapy with continuous dosing for the treatment of toe-nail onychomycosis. Br J Dermatol. 1997;136:230–234. [PubMed] [Google Scholar]
- 39.Hay R J. Fungal skin infections. Arch Dis Child. 1992;67:1065–1067. doi: 10.1136/adc.67.9.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heikkalä H, Stubbs S. The prevalence of onychomycosis in Finland. Br J Dermatol. 1995;133:699–701. doi: 10.1111/j.1365-2133.1995.tb02741.x. [DOI] [PubMed] [Google Scholar]
- 41.Honeyman J F, Talarico F S, Arruda L H F, Pereira A C, Jr, Santamaria J R, Souza E M, Woscoff A, Amorim F R, de la Parra C R, Enokihara M Y, Gavazoni M F, Gubelin H W, Rosa S P, Turini M A G, Vitale M A. Itraconazole versus terbinafine (LAMISIL®): which is better for the treatment of onychomycosis? J Eur Acad Dermatol Venereol. 1997;9:215–221. [Google Scholar]
- 42.Janssen Pharmaceutica. Itraconazole package insert. Titusville, N.J: Janssen Pharmaceutica; 1997. [Google Scholar]
- 43.Janssen Pharmaceutica. Ketoconazole package insert. Titusville, N.J: Janssen Pharmaceutica; 1997. [Google Scholar]
- 44.Junquiera L C, Carneiro J, Kelley R O. Basic histology. 8th ed. Stamford, Conn: Appleton & Lange; 1995. pp. 353–355. [Google Scholar]
- 45.Kuokkanen K, Alava S. Fluconazole in the treatment of onychomycosis caused by dermatophytes. J Dermatol Treat. 1993;3:115–117. [Google Scholar]
- 46.Matthieu L, De Doncker P, Cauwenbergh G, Woestenborghs R, van de Velde V, Janssen P A, Dockx P. Itraconazole penetrates the nail via the nail matrix and the nail bed: an investigation in onychomycosis. Clin Exp Dermatol. 1991;16:374–376. doi: 10.1111/j.1365-2230.1991.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 47.Nolting, S., M. Brautigam, and G. Weidinger. 1994. Terbinafine in onychomycosis with involvement by non-dermatophytic fungi. Br. J. Dermatol. 130(Suppl. 43):16–21. [DOI] [PubMed]
- 47a.Novartis Pharmaceuticals Corporation. Terbinafine package insert. E. Hanover, N.J: Novartis Pharmaceuticals Corp.; 1997. [Google Scholar]
- 48.Odom R, Daniel III C R, Aly R. A double-blind, randomized comparison of itraconazole capsules and placebo in the treatment of onychomycosis of the toenail. J Am Acad Dermatol. 1996;35:110–111. doi: 10.1016/S0190-9622(96)90519-8. [DOI] [PubMed] [Google Scholar]
- 49.Odom R B, Aly R, Scher R K, Daniel III C R, Elewski B E, Zaias N, DeVillez R, Jacko M, Oleka N, Moskovitz B L. A multicenter-placebo-controlled, double-blind study of intermittent therapy with itraconazole for the treatment of onychomycosis of the fingernail. J Am Acad Dermatol. 1997;36:231–235. doi: 10.1016/s0190-9622(97)70286-x. [DOI] [PubMed] [Google Scholar]
- 50.Ortho Diagnostics. Griseofulvin package insert. Raritan, N.J: Ortho Dermatologic Division; 1997. [Google Scholar]
- 51.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L for the Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 52.Rippon J W. Medical mycology: the pathogenic fungi and the pathogenic actinomycetes. 3rd ed. Philadelphia, Pa: The W. B. Saunders Co.; 1988. pp. 169–275. [Google Scholar]
- 53.Roseeuw D, De Doncker P. New approaches to the treatment of onychomycosis. J Am Acad Dermatol. 1993;29:S45–S50. doi: 10.1016/s0190-9622(08)81837-3. [DOI] [PubMed] [Google Scholar]
- 54.Scher R K. Diseases of the nails. In: Conn H, editor. Current therapy. Philadelphia, Pa: The W. B. Saunders Co.; 1990. pp. 736–742. [Google Scholar]
- 55.Scher R K. Onychomycosis: a significant medical disorder. J Am Acad Dermatol. 1996;35(Part 2):S2–S5. doi: 10.1016/s0190-9622(96)90061-4. [DOI] [PubMed] [Google Scholar]
- 56.Scher, R. K., D. Breneman, P. Rich, R. C. Savin, D. S. Feingold, N. Konnikov, J. L. Shupack, S. Pinnell, N Levine, N. J. Lowe, R. Aly, R. B. Odom, D. L. Greer, M. R. Morman, A. D. Monroe, E. H. Tschen, B. E. Elewski, and E. B. Smith. A placebo-controlled, randomized, double-blind trial of once-weekly fluconazole (150 mg, 300 mg, or 450 mg) in the treatment of distal subungual onychomycosis of the toenail. J. Am. Acad. Dermatol., in press. [DOI] [PubMed]
- 57.Sigler L, Congly H. Toenail infection caused by Onychocola canadensis gen. et sp. nov. J Med Vet Mycol. 1990;28:405–17. [PubMed] [Google Scholar]
- 58.Summerbell, R. C. 1997. Epidemiology and ecology of onychomycosis. Dermatology 194(Suppl. 1):32–36. [DOI] [PubMed]
- 59.Summerbell R C, Kane J, Krajden S. Onychomycosis, tinea pedis, and tinea manuum caused by non-dermatophytic filamentous fungi. Mycoses. 1989;32:609–619. doi: 10.1111/j.1439-0507.1989.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 60.Tausch I, Brautigam M, Weidinger G, Jones T C the Lagos V Study Group. Evaluation of 6 weeks treatment of terbinafine in tinea unguium in a double-blind trial comparing 6 and 12 weeks therapy. Br J Dermatol. 1997;136:737–742. [PubMed] [Google Scholar]
- 61.Tosti A, Piraccini B M, Stinchi C, Venturo N, Bardazzi F, Colombo M D. Treatment of dermatophyte nail infections: an open randomized study comparing intermittent terbinafine therapy with continuous terbinafine treatment and intermittent itraconazole therapy. J Am Acad Dermatol. 1996;34:595–600. doi: 10.1016/s0190-9622(96)80057-0. [DOI] [PubMed] [Google Scholar]
- 62.Van der Schroeff, J. G., P. K. S. Cirkel, M. B. Crijns, T. J. A. Van Kijk, F. J. Govaert, D. A. Groeneweg, D. J. Tazelaar, R. F. E. DeWitt, and J. Wuite. 1992. A randomized treatment duration-finding study of terbinafine in onychomycosis. Br. J. Dermatol. 126(Suppl. 39):36–39. [DOI] [PubMed]
- 63.Weitzman I, Summerbell R C. The dermatophytes. Clin Microbiol Rev. 1995;8:240–259. doi: 10.1128/cmr.8.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winn W C., Jr . Mycotic diseases. In: Henry J B, editor. Clinical diagnosis and management. Philadelphia, Pa: The W. B. Saunders Co.; 1996. pp. 1210–1251. [Google Scholar]
- 65.Zaias N. Onychomycosis. Arch Dermatol. 1972;105:263–274. [PubMed] [Google Scholar]
- 66.Zaias N, Glick B, Rebell G. Diagnosing and treating onychomycosis. J Fam Pract. 1996;42:513–518. [PubMed] [Google Scholar]
- 67.Zaias N, Tosti A, Rebell G, Morelli R, Bardazzi F, Bieley H, Zaiac N, Glick B, Paley B, Allevato M, Baran R. Autosomal dominant pattern of distal subungual onychomycosis caused by Trichophyton rubrum. J Am Acad Dermatol. 1996;34(2 Pt 1):302–304. doi: 10.1016/s0190-9622(96)80142-3. [DOI] [PubMed] [Google Scholar]